Abstract
Objective
To describe clinical effectiveness of belimumab for systemic lupus erythematosus (SLE) in real-world practice in Argentina.
Methods
This retrospective, observational study analysed medical record data of patients with SLE treated with belimumab in 15 centres in Argentina. Primary endpoint: overall clinical response (assessed on a scale similar to the 6-point Physician Global Assessment) at months 6, 12, 18 and 24, all versus index (belimumab initiation). Secondary endpoints: improvement in disease activity (SELENA-SLEDAI), SLE manifestations, and corticosteroid dose change.
Results
Records for 81 patients (91% female) were analysed. Clinical improvements were reported for 95%, 95%, 98% and 100% patients at 6, 12, 18, and 24 months post index, respectively. Mean SELENA-SLEDAI score decreased from 11.21 at index to 4.76, 3.77, 3.86 and 2.17 at 6, 12, 18, and 24 months post index, respectively. Number of flares decreased from 1.05 at index to 0.21, 0.09, 0.22 and 0.30 at 6, 12, 18, and 24 months post index, respectively. Mean corticosteroid dose was 14.59 mg/day at index, and 6.45, 5.18, 5.17 and 4.78 mg/day at 6, 12, 18, and 24 months post index, respectively.
Conclusions
Real-world patients with SLE treated with belimumab in Argentina demonstrated clinical improvements and reductions in corticosteroid dose.
Keywords: Belimumab, systemic lupus erythematosus, real-world, steroid sparing, disease activity, observational study
Introduction
Systemic lupus erythematosus (SLE) is a multisystem, chronic, autoimmune disease with diverse clinical and laboratory manifestations.1 The incidence of SLE varies between countries, and has been found to be higher in Latin America, at 4.7–8.7/100,000 person-years,2 compared with the USA and Europe, where incidences of 5.1/100,000 and 2.2–5.0/100,000 person-years have been reported, respectively.3 A study in Buenos Aires, Argentina, has estimated the incidence of SLE at 6.3/100,000 person-years, and prevalence at 58.6/100,000.4
SLE is typically managed by a combination of therapies including corticosteroids, antimalarials, non-steroidal anti-inflammatory drugs, and immunosuppressive agents.5 The combination of chronic remitting-relapsing course of SLE with flares of disease activity, and toxicity of associated medications, result in an increased risk of long-term organ damage and poorer health-related quality of life of patients with SLE, and present a substantial burden to healthcare systems.6,7 In Latin America, studies performed by GLADEL (Grupo Latino Americano De Estudio del Lupus) demonstrated that in patients with SLE, the risk of damage accrual increases with each flare, regardless of flare severity and independently of other known risk factors, such as gender, age at diagnosis, disease duration, high disease activity, previous damage, the presence of antiphospholipid antibodies and antiphospholipid syndrome, high-dose corticosteroids and immunosuppressants, non-Caucasian race/ethnicity, and socio-economic factors.8,9
Since 2011, belimumab, a new generation biologic therapy, has become available for adults with SLE10,11 and children 5–17 years of age with childhood-onset SLE.12 Belimumab is a human, immunoglobulin 1λ monoclonal antibody that binds and antagonises the biological activity of circulating B-cell activating factor (BAFF), also known as B-lymphocyte stimulator (BLyS), which is elevated in patients with SLE and promotes abnormal B-cell activation and differentiation.13–15 Short-term (up to 76 weeks) efficacy and safety of belimumab have been demonstrated in four large Phase 3 clinical trials,16–19 and these safety and efficacy profiles have recently been shown to be maintained up to 7 years of belimumab treatment.20 Currently, belimumab is indicated as add-on therapy in adult patients with active, autoantibody-positive SLE despite standard therapy (ST)11 and with a high degree of disease activity (e.g. positive anti-dsDNA and low complement levels).10
The real-world utilisation of belimumab has been evaluated in several countries, through the global OBSErve (evaluation Of use of Belimumab in clinical practice SEttings) programme. These retrospective, observational studies, conducted so far in the USA, Germany, Canada, Spain and Switzerland, described the reality of SLE care and clinical benefits of long-term belimumab treatment in every day clinical practice.21–26 Overall, patients with SLE treated with belimumab showed clinical improvements in various manifestations of SLE, and a reduction in steroid use and healthcare resource utilisation (HCRU).
In addition, two large prospective studies of belimumab are currently ongoing: a 5-year prospective observational registry, which aims to collect long-term information regarding side effects and effectiveness of belimumab when given in combination with other ST in adults with active, autoantibody-positive SLE (SABLE study; NCT01729455), and a randomised, double-blind, placebo-controlled 52-week study to assess adverse events (AEs) of special interest in adults with active, autoantibody-positive SLE receiving belimumab (BASE study; NCT01705977).
Similar to other OBSErve studies,21–26 this study aims to investigate the patterns of belimumab use for SLE management, its clinical effectiveness, and health outcomes in patients with SLE who received belimumab as part of their ST for up to 2 years in clinical practice settings in Argentina.
Materials and methods
Study design
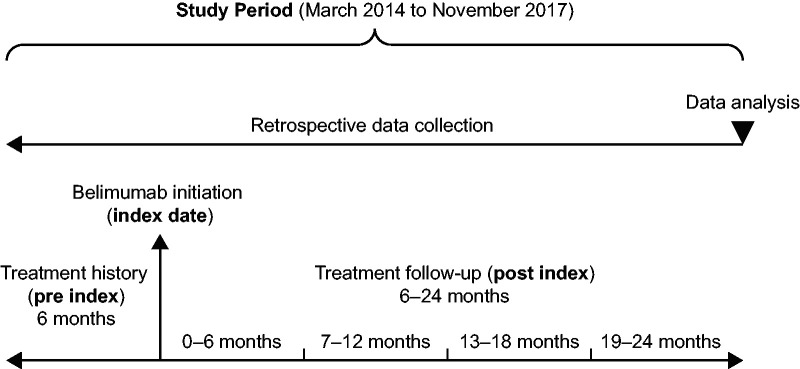
This was a Phase 4, multicentre, observational cohort study (GSK study 201282), designed to retrospectively collect real-world information from patient medical records on the long-term outcomes of belimumab use in patients with SLE. Data were collected at six time points: 6 months prior to belimumab initiation, at belimumab initiation (index date), and at 6, 12, 18 and 24 months after treatment initiation (Figure 1). The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.27
Study sites and physicians
Physicians (rheumatologists or internal medicine physicians) who, at the time of recruitment, managed/treated ≥10 patients with SLE, had ≥5 years of experience in treating patients with SLE and had treated at least two patients with belimumab plus ST, and were currently treating at least one patient with belimumab plus ST, were invited to participate in the study. Physicians completed the site feasibility questionnaire, which gathered general information about the participating medical centre, including geographic location, experience (years) in treating SLE and the number of patients with SLE currently managed.
Patient inclusion and exclusion criteria
Physicians enrolled all patients from their practices who fulfilled the following inclusion criteria: adults ≥18 years of age with a confirmed diagnosis of SLE (according to the 82/97 American College of Rheumatology criteria and/or Systemic Lupus International Collaborating Clinics criteria for SLE), who received belimumab as part of their usual SLE care, and for whom documented follow-up medical records are available for at least 6 months after belimumab initiation, or who received at least one dose of belimumab before discontinuing. To avoid selection bias, all patients with different exposures to belimumab, including those who received at least one dose of belimumab as part of their usual SLE care, but discontinued its use for any reason, were included. Patients who received belimumab as part of a clinical study were excluded. Physicians extracted data on treatment, clinical outcomes and HCRU from patient medical records and collated onto case report forms. If available, data were collected from other sources such as recent medical consultations, with the aim of gathering as much information as possible.
Objectives
The primary objective was to describe the overall clinical response in patients with SLE after 6, 12, 18 and 24 months of treatment with belimumab, in clinical practice in Argentina. Secondary objectives were to describe characteristics of patients (reasons for initiation and discontinuation of belimumab, use of disease assessment scales), use of concomitant medications, change in Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI) score, number and severity of SLE flares, HCRU and impact on unemployment. Although safety was not an objective of this study, AEs were reported to the study sponsor within 24 hours.
Endpoints
The primary endpoint was physician-assessed, overall clinical response to belimumab treatment based on medical records, reported as a percentage of patients with a clinical improvement at 6, 12, 18 and 24 months from index. The primary endpoint was assessed on a scale similar to the 6-point, Physician’s Global Assessment (PGA) scale, and categorised as worse, no improvement, and improvement of <20%, 20–49%, 50–79% and ≥80%. Secondary endpoints, analysed after 6, 12, 18 and 24 months of belimumab treatment, included: change in the severity of arthritis and rash (assessed on a scale similar to the 6-point PGA scale, and categorised as worse, no improvement, and improvement of <20%, 20–49%, 50–79% and ≥80%); number of painful and swollen joints; reasons for initiation and discontinuation of belimumab; use of disease assessment scales (SELENA-SLEDAI, PGA, British Isles Lupus Activity Group [BILAG], Systemic Lupus Activity Measure [SLAM], European Consensus Lupus Activity Measurements [ECLAM], Fatigue Severity Scale [FSS]) as part of regular SLE management; treatment patterns of concomitant medications, particularly corticosteroids: dose increase, reduction, and discontinuation; change in SELENA-SLEDAI score from index; number and severity of disease flares; HCRU and employment status.
Statistical analyses
Given the descriptive nature of the study, no formal sample size calculations were performed. The aim was to include approximately 20 physicians and 70–80 patient medical records for the extraction of anonymised data. Descriptive statistics, such as counts and percentages, and confidence intervals (CI) of 95% were used to analyse categorical data, and mean, median, standard deviation (SD), minimum and maximum were used to analyse continuous data. For non-Gaussian distribution of data, median and interquartile range were used. Statistical tests were used to summarise inferential comparisons between subgroup endpoints when relevant strata of patients were observed. All time point comparisons were performed against index. No analyses of loss-to-follow-up, incomplete case documentation, or deviations from the target study population were performed. No imputation for missing data was used.
Results
Participating sites
Between March 2014 and November 2017, 15 centres, located in Buenos Aires, Concordia, Formosa, General Roca, La Plata, Mendoza, Pergamino, Rosario and Santa Fe, participated in the study; each site recruited between 2 and 12 patients. The centres had a mean (SD) of 17 (7.5) years of experience in treating SLE, and the average number of patients with SLE currently managed was 93 per centre.
Patient demographics and baseline characteristics
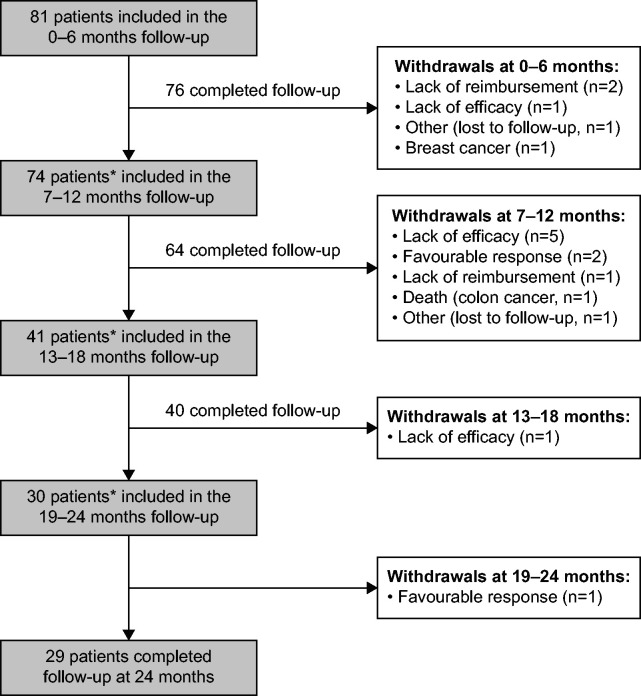
Data were available post index at 0–6 months (n = 81), 7–12 months (n = 74), 13–18 months (n = 41) and 19–24 (n = 30); the reasons for patient withdrawal in each 6-monthly period are listed in Figure 2. One death (due to colon cancer) and one serious AE (breast cancer) were reported during the study period; neither was considered related to belimumab (Figure 2).
Figure 2.
Patient disposition and withdrawals.
*Patients did not receive belimumab long enough to be included in the next follow-up period/physicians withdrew from the next follow-up period (n = 2, 7–12 months; n = 23, 13–18 months; n = 10, 19–24 months)
Figure 1.
Study design.
At index, mean (SD) age was 42 (12) years, and 74 (91%) patients were female. Duration of SLE >5 years was reported for 49 (60%) patients; 70 (86%) patients had moderate to severe SLE, 59 (73%) had a persistent disease activity, and 22 (27%) experienced an exacerbation. Lupus nephritis was reported for 12 (15%) patients, 11 (92%) of whom were in remission. The most frequently observed clinical manifestations of SLE at index were arthritis and rash, reported for 52 (64%) and 48 (59%) patients, respectively. Hypocomplementemia, high anti-double stranded (ds)DNA, and SELENA-SLEDAI >10 were reported for 58 (72%), 49 (60%) and 38 (47%) patients, respectively. In addition, 10 (12%) and 4 (5%) patients experienced hypertension and dyslipidaemia, respectively. At index, 74 (91%) of patients received steroids, and 60/74 (81%) at a dose ≥7.5 mg/day (Table 1).
Table 1.
Baseline patient demographics and disease characteristics.
| Patient characteristics | At index (N = 81) |
|---|---|
| Female, n (%) | 74 (91) |
| Age, years, mean (SD) | 42 (12) |
| Duration of SLE, n (%) | |
| <1 year | 2 (2) |
| 1–5 years | 29 (36) |
| 6–10 years | 17 (21) |
| >10 years | 32 (40) |
| Unknown | 1 (1) |
| SLE severity at index, n (%) | |
| Mild | 10 (12) |
| Moderate | 61 (75) |
| Severe | 9 (11) |
| Unknown | 1 (1) |
| SLE activity at index, n (%) | |
| Persistent activity | 59 (73) |
| Exacerbation | 22 (27) |
| High disease activity subgroups, n (%) | |
| Hypocomplementemia | 58 (72) |
| High anti-dsDNA | 49 (60) |
| Steroid dose ≥7.5 mg/day | 60 (74) |
| SELENA-SLEDAI >10 | 38 (47) |
| SLE clinical manifestations at index, n (%) | |
| Arthritis | 52 (64) |
| Mild | 4 (8) |
| Moderate | 45 (86) |
| Severe | 3 (6) |
| Rash | 48 (59) |
| Mild | 14 (29) |
| Moderate | 32 (67) |
| Severe | 2 (4) |
| Alopecia | 27 (33) |
| Othera | 33 (41) |
| Fatigue | 32 (40) |
| Mucosal ulcers | 27 (33) |
| Lupus nephritis | 12 (15) |
| Headache | 12 (15) |
| Fever | 10 (12) |
| Vasculitis | 10 (12) |
| Comorbidities, n (%) | |
| Hypertension | 10 (12) |
| Dyslipidaemia | 4 (5) |
| Diabetes | 1 (1) |
| Renal disease not related to SLE | 1 (1) |
| Coronary artery disease | 1 (1) |
| Asthma/COPD | 1 (1) |
| Concomitant medications at index, n (%) | |
| Corticosteroids | 74 (91) |
| Antimalarials | 70 (86) |
| Azathioprine | 22 (27) |
aIncludes: pleurisy (n = 7, 9%), thrombocytopenia (n = 6, 7%), pericarditis (n = 5, 6%), myositis (n = 3, 4%), proteinuria (n = 3, 4%), organic brain syndrome (n = 2, 2%), seizure (n = 2, 2%), peripheral neuropathy (n = 2, 2%), psychosis (n = 1, 1%), urinary casts (n = 1, 1%), haematuria (n = 1, 1%).
COPD: coronary obstructive pulmonary disease; SD: standard deviation; SELENA-SLEDAI: Safety of Estrogens in Lupus National Assessment-SLE Disease Activity Index; SLE: systemic lupus erythematosus.
Physician’s evaluation of overall clinical response
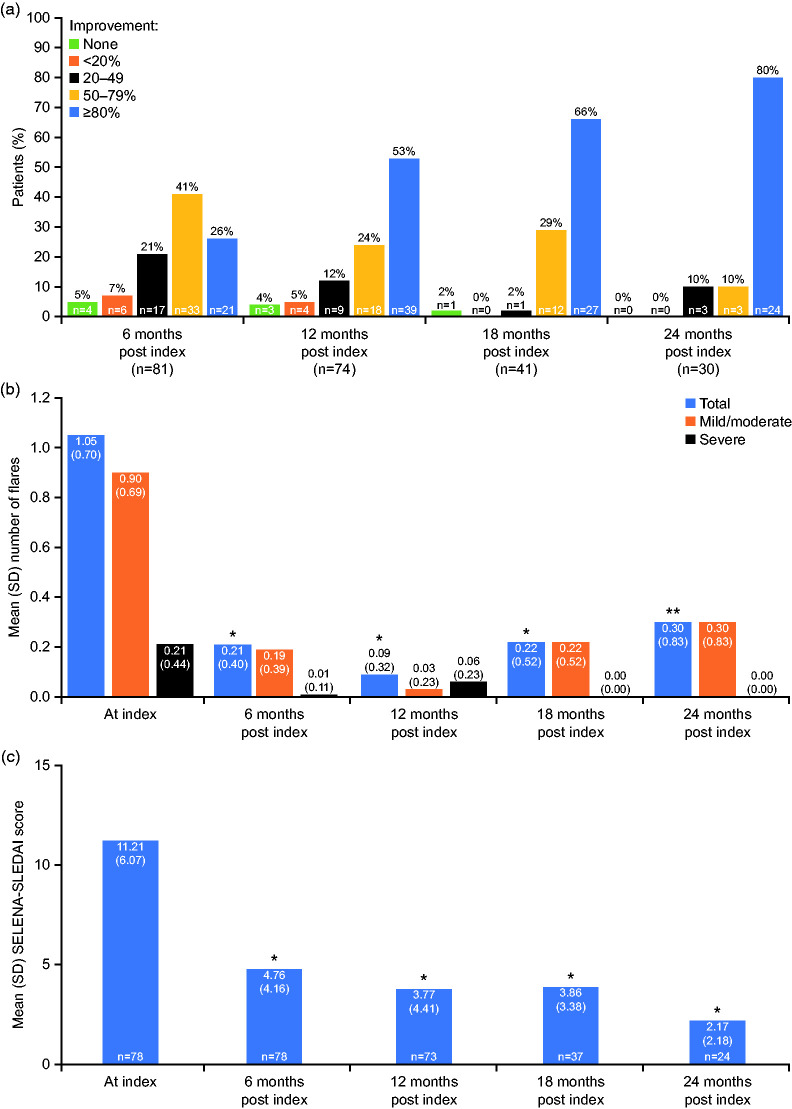
Using a PGA-like scale, physicians reported an improvement of ≥80% in overall clinical condition for 21/81 (26%), 39/74 (53%), 27/41 (66%) and 24/30 (80%) patients at 6, 12, 18 and 24 months post index, respectively, with 33/81 (41%), 18/74 (24%), 12/41 (29%) and 3/30 (10%) patients experiencing an improvement of 50–79%. An improvement of 20–49% was reported for 17/81 (21%), 9/74 (12%), 1/41 (2%) and 3/30 (10%) patients and an improvement of <20% for 6/81 (7%), 4/74 (5%), 0/41 (0%) and 0/30 (0%) patients at 6, 12, 18, and 24 months post index, respectively. No change was reported for 4/81 (5%), 3/74 (4%), 1/41 (2%), and 0/30 (0%) patients at 6, 12, 18, and 24 months post index, respectively (Figure 3(a)). Worsening was reported for 1/74 (1%) patient at 12 months, and no patients at 6, 18, and 24 months post index.
Figure 3.
Clinical outcomes in patients with SLE after 6, 12, 18 and 24 months of belimumab therapy. (a) Physician evaluation of overall clinical response, (b) mean number and severity of flares, (c) mean SELENA-SLEDAI score.
P-value represents change versus index; *p < 0.001; **p = 0.001.
SELENA-SLEDAI: Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index; SD: standard deviation; SLE: systemic lupus erythematosus.
Patient characteristics
Arthritis, one of the most common clinical manifestations of SLE, improved by ≥80% in 25/52 (48%), 34/49 (69%), 24/26 (92%) and 17/21 (81%) patients at 6, 12, 18, and 24 months post index, respectively. Further, 15/52 (29%), 10/49 (20%), 0/26 (0%) and 3/21 (14%) patients experienced an improvement of 50–79%, and 8/52 (15%), 3/49 (6%), 0/26 (0%) and 0/21 (0%) an improvement of 20–49% at 6, 12, 18, and 24 months post index. An improvement of <20% was observed in 3/52 (6%), 2/49 (4%), 0/26 (0%) and 1/21 (5%) of patients, 1/52 (2%), 0/49 (0%), 1/26 (4%) and 0/21 (0%) had no improvement and no patients experienced worsening in arthritis during the treatment period.
The reduction in mean (SD) number of painful and swollen joints observed over the study period was significant (p < 0.001) for each 6-month period versus index. At index, 7.4 (4.9) and 5.4 (4.1) painful and swollen joints were reported, respectively, which decreased to 2.4 (3.5) and 1.0 (2.0) at 6 months, 1.1 (2.9) and 0.5 (1.5) at 12 months, 1.0 (2.2) and 0.3 (0.8) at 18 months, and 0.3 (0.8) and 0.2 (0.5) at 24 months post index.
Patients also experienced considerable improvements in rash, with 22/48 (46%), 29/46 (63%), 11/22 (50%) and 14/18 (78%) reporting an improvement of ≥80%, further 6/48 (13%), 8/46 (17%), 9/22 (41%) and 4/18 (22%) an improvement of 50–79%, and 13/48 (27%), 3/46 (7%), 2/22 (9%) and 0/18 (0%) an improvement of 20–49%, at 6, 12, 18 and 24 months post index, respectively. Physicians reported an improvement of <20% in 4/48 (8%), 5/46 (11%), 0/22 (0%) and 0/18 (0%) patients; 2/48 (4%), 1/46 (2%), 0/22 (0%) and 0/18 (0%) patients had no improvement at 6, 12, 18 and 24 months post index; worsening in rash was reported for 1/48 (2%) patient at 6 months post index.
At index, the mean (SD) number of flares was 1.05 (0.70). This decreased significantly to 0.21 (0.40), 0.09 (0.32), 0.22 (0.52) (all p < 0.001) and 0.30 (0.83) (p = 0.001) at 6, 12, 18, and 24 months post index, respectively. The mean number of severe flares also decreased over the study period, from 0.21 (0.44) at index, to 0.01 (0.11), 0.06 (0.23), 0 (0) and 0 (0) at 6, 12, 18, and 24 months post index, respectively (Figure 3(b)).
Belimumab was prescribed to patients with uncontrolled SLE disease based on physicians’ clinical decision. Main reasons for belimumab initiation included ineffective previous treatment, reported by 62 (77%) patients, the intent to reduce the corticosteroid dose (n = 47, 58%), worsening condition (n = 40, 49%), previous treatment not tolerated (n = 8, 10%) and maculopathy (n = 6, 5%).
Patients received a mean (SD) intravenous (IV) belimumab dose of 10 (1.0) mg/kg monthly, following the loading dose on Days 0, 14 and 28. The cumulative rate of belimumab discontinuation (number of patients discontinuing belimumab at any time from index to 24 months post index, divided by the number of patients who entered the study) was 21% (n = 17/81). Five (6%) patients discontinued belimumab in the first 6 months post index, with a further 10 (12%), 1 (1%) and 1 (1%), discontinuing at 7–12, 13–18 and 19–24 months post index, respectively. Seven (41%) patients discontinued belimumab due to lack of efficacy; 1 patient in the first 6 months post index, and 5 patients at 7–12 months and 1 patient at 13–18 months post index. Favourable response to treatment (patients withdrew due to achieving disease control; n = 3/17, 18%), high cost/lack of reimbursement (n = 3/17, 18%), cancer (n = 2/17, 12%) and other (n = 2/17, 12%), were reported as further reasons for discontinuation (Figure 2). In addition to the 17 patients who discontinued belimumab, 35 patients did not receive belimumab long enough to be included in the next follow-up period or physicians withdrew from the next follow-up period (n = 2, 7–12 months; n = 23, 13–18 months; n = 10, 19–24 months) (Figure 2).
The use of existing disease assessment tools during the study varied between the participating sites. SELENA-SLEDAI was the most frequently used scale, used by 29 (36%) physicians. PGA was used by 3 (4%), and BILAG by 2 (2%) physicians; none of the physicians used SLAM, ECLAM or FSS scales. No data on the use of the disease assessment tools were available for the remaining 47 (58%) physicians.
Disease activity assessment: SELENA-SLEDAI score
SELENA-SLEDAI scores were available for 78 patients at index and 6 months post index, and for 73, 37 and 24 patients at 12, 18, and 24 months post index. In these patients, mean (SD) SELENA-SLEDAI score decreased significantly from 11.21 (6.07) at index to 4.76 (4.16), 3.77 (4.41), 3.86 (3.38) and 2.17 (2.18) at 6, 12, 18, and 24 months post index, respectively (all p < 0.001) (Figure 3(c)).
Concomitant medications
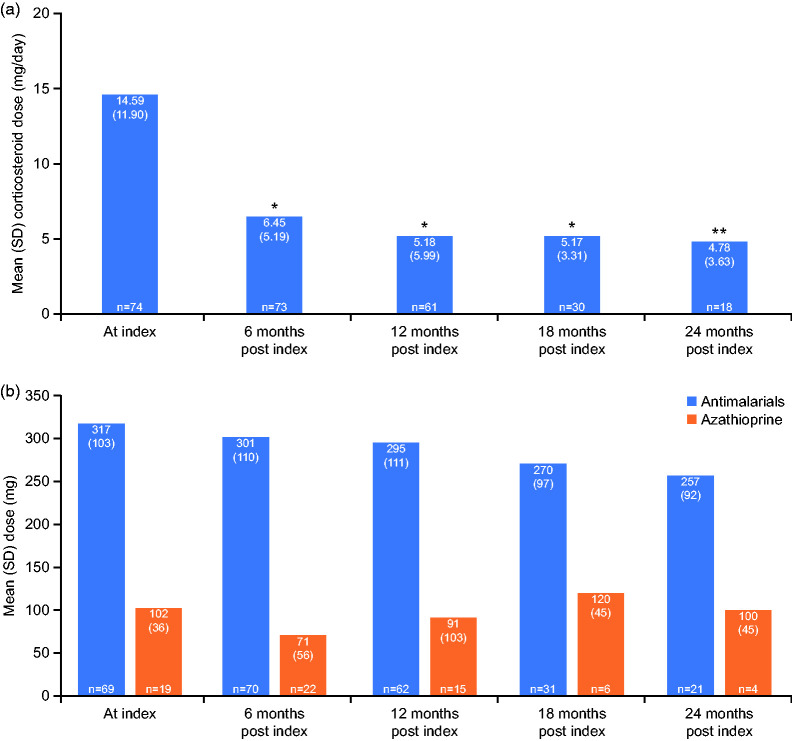
At index, 74 patients were receiving corticosteroids; 73, 61, 30 and 18 patients received corticosteroids at 6, 12, 18, and 24 months post index, respectively. Patients experienced significant reductions in mean (SD) corticosteroid dose during the treatment period, from 14.6 (11.90) mg/day at index, to 4.78 (3.63) mg/day (p = 0.01) at 24 months post index (Figure 4(a)).
Figure 4.
Change in mean dose of corticosteroids (a) and antimalarials and azathioprine (b) over the study period.
P-value represents change versus index; *p < 0.001; **p = 0.01.
SD: standard deviation.
Overall, 11/73 (15%) patients discontinued corticosteroids during the first 6 months of treatment, with further 12/61 (20%), 5/30 (17%) and 3/18 (17%) discontinuing at 12, 18, and 24 months post index. The cumulative rate of corticosteroid discontinuation (number of patients discontinuing corticosteroids at any time from index to 24 months post index, divided by the number of patients who entered the study medicated with corticosteroids) was 42% (n = 31/74).
At index, 70 (86%) patients received antimalarials, and 22 (27%) were prescribed azathioprine. No significant changes in antimalarial and azathioprine doses were observed over the study period (Figure 4(b)). Cumulative rates of discontinuation of antimalarials and azathioprine were 3% (n = 2/70) and 45% (n = 10/22), respectively.
Healthcare resource utilisation
While no significant changes were observed in the utilisation of healthcare resources, a trend towards fewer hospital admissions over time was observed compared with index, with 11 (14%) patients hospitalised at index, and 5/81 (6%), 2/72 (3%), 1/41 (2%) and 1/30 (3%) at 0–6, 7–12, 13–18 and 19–24 months post index, respectively. Similarly, fewer emergency room visits were reported post index, with 0.30 visits at index, and 0.16, 0.11, 0.05 and 0.20 visits at 0–6, 6–12, 12–18 and 18–24 months post index, respectively. The number of planned rheumatologist visits slightly increased from 3.49 at index to 3.78, 3.40, 3.68 and 3.87 at 0–6, 7–12, 13–18 and 19–24 months post index, respectively. A slight increase in the number of laboratory tests was reported over the study period (Table 2).
Table 2.
Healthcare resource utilisation and laboratory use over the belimumab treatment period.
| At index |
Months post index |
||||
|---|---|---|---|---|---|
| 0–6 | 7–12 | 13–18 | 19–24 | ||
| Hospital admissions, n (%)a | 11 (14) | 5 (6) | 2 (3) | 1 (2) | 1 (3) |
| Emergency room visits, mean (SD) | 0.30 (0.67) | 0.16 (0.43) | 0.11 (0.45) | 0.05 (0.21) | 0.20 (0.80) |
| Planned rheumatologist visits, mean/6 months | 3.49 | 3.78 | 3.40 | 3.68 | 3.87 |
| Laboratory use, mean/6 months | |||||
| Anti-dsDNA antibody | 1.36 | 1.19 | 1.29 | 1.58 | 1.66 |
| Complement | 1.63 | 1.68 | 1.67 | 1.69 | 1.93 |
| Haemoglobin | 1.99 | 2.19 | 1.92 | 2.30 | 2.73 |
| Creatinine | 1.75 | 1.88 | 1.73 | 2.16 | 2.40 |
| Liver enzymes | 1.69 | 1.85 | 1.75 | 2.11 | 2.40 |
aP-value after treatment versus index: p = 0.166 (0–6 months), p = 0.052 (7–12 months), p = 0.058 (13–18 months and 19–24 months).
dsDNA: double-stranded DNA; SD: standard deviation.
Impact on unemployment
No significant changes in the employment status were observed over the study period, with 7/81 (9%), 7/81 (9%), 5/75 (7%), 3/37 (8%) and 3/30 (10%) patients unemployed at index, and at 0–6, 7–12, 13–18 and 19–24 months post index, respectively.
Discussion
The retrospective OBSErve Argentina cohort study was conducted to provide an insight into the clinical benefits of belimumab in routine clinical practice in Argentina. The study investigated the clinical outcomes, patient characteristics and HCRU in patients with SLE treated with belimumab for up to 24 months in clinical practice. Over the 24-month treatment period, and as early as 6 months after treatment initiation, patients had an overall improvement in disease activity, with a reduced SELENA-SLEDAI score, marked improvements in arthritis and rash, and reduced number and severity of disease flares.
Furthermore, a steroid-sparing effect of belimumab therapy was observed in the study, with patients having their mean corticosteroid dose reduced to less than 7.5 mg/day as early as 6 months after belimumab IV initiation, and an overall rate of corticosteroid discontinuation (over 24 months) of 42%. Minimising corticosteroid use is a crucial goal in the management of SLE, as prolonged treatment with high-dose (>7.5 mg/day) corticosteroids is associated with significant toxicity and can lead to considerable organ damage.28,29 A steroid-sparing effect of belimumab treatment has been suggested in post hoc analyses of a subgroup of patients from the Phase 3 belimumab clinical trials, and in the long-term continuation study of BLISS-76.20,30
The effectiveness of belimumab demonstrated in this study supports the findings of similar studies conducted under the OBSErve programme in the USA, Canada, Germany, Spain and Switzerland,21,23–26 which reported dose reductions of concomitant corticosteroid medication and clinical improvements with belimumab therapy.
Arthritis and skin rashes are amongst the most prominent complaints in patients with SLE, and also among Argentinian patients.6,31 These manifestations, combined with frequent disease flares, have been recognised as factors that significantly impact health-related quality of life and patient’s ability to work.29,31 Here, reductions in the number and severity of disease flares, and the number of swollen and painful joints, which are prominent manifestations of arthritis, were observed following belimumab treatment. However, these improvements in SLE manifestations did not have an impact on patient unemployment, which did not change over the study period.
Belimumab is a long-term treatment, and although a favourable clinical response has been observed as early as 8 weeks,32 it is recommended that patients are treated for a minimum of 6 months before belimumab discontinuation is considered.10 Here, one-fifth (n = 17/81, 21%) of patients discontinued belimumab over the 2-year treatment period, with only 6% discontinuing in the first 6 months. Fewer than half of patients who discontinued belimumab did so due to lack of belimumab efficacy, and nearly 20% terminated their treatment because they achieved a favourable response and disease control. It should be noted that, although belimumab was generally prescribed to patients with uncontrolled disease, some reasons for belimumab initiation captured in this study were not specified as indications for belimumab treatment in the prescribing information.10,11
In addition, HCRU remained stable during the study, although patients required fewer SLE-related hospital admissions and emergency room admissions during belimumab treatment period. The number of scheduled rheumatologist visits slightly increased during belimumab therapy; however, this may be explained by the belimumab IV dosing regimen, which requires monthly infusions at the clinic.12 The reported mean number of scheduled visits was fewer than six, which suggests that not all scheduled visits for belimumab infusions were documented. Other OBSErve studies also reported reductions in the utilisation of healthcare resources with belimumab treatment.21,22,24
SELENA-SLEDAI was used during the study by approximately one-third of physicians, with other assessment scales rarely or never used in routine SLE management. The lack of a consistent use of measures of disease activity was also reported by the OBSErve studies conducted in the USA and Canada.21,25 Thus, a standardised disease assessment and monitoring practice in routine SLE care is required, and a need for development of appropriate consensus recommendations has previously been highlighted.29,33,34
This study has some limitations. The study population comprised patients who received belimumab treatment shortly after its approval, in selected clinical centres in Argentina; extrapolation of these results to other patients with SLE should be performed with caution. Medical records and the use of health resources represent treatment patterns of physicians participating in the study and may be different from those of non-participating physicians. The primary endpoint was also based on the individual, subjective clinical judgement of the treating physician, following retrospective review of medical records. In addition, this retrospective observational study is subject to the potential biases inherent to this type of an epidemiological study and did not include a control group to compare belimumab treatment with other standard SLE therapies. Finally, the relatively small study sample of 81 patients warrants cautious interpretation of data.
In conclusion, this first observational study of the clinical effectiveness of belimumab among patients with SLE in Argentina provides further evidence to support the use of belimumab in clinical practice. The key finding of this study, a reduction of corticosteroid use, has important clinical implications given the health impact of prolonged steroid use in SLE.28,29 The steroid-sparing effect and overall clinical improvements support the findings of belimumab clinical trials. As part of a wider OBSErve programme, the study provides further understanding of belimumab use and effectiveness in an every-day clinical practice in Argentina in comparison with other participating countries. The findings may prove useful in planning future treatment strategies for patients with SLE.
Acknowledgements
The authors would like to express their gratitude for Dr Juan Carlos Barreira (Hospital Británico de Buenos Aires, Argentina), Roberto Baez (Policonsultorios Holos, General Roca, Rio Negro, Argentina) and Dr Miguel Angel Paez (Centro Médico Dharma, Mendoza, Argentina) for their contributions to the recruitment of patients and collection of data for this study. The authors would like to acknowledge Marcelo Gonzalez Della Valle, Maria Laura Cattaneo and Raul Bozzo (IC Research Group, Buenos Aires, Argentina) for their contribution to the analysis of data. Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors’ comments, grammatical editing and referencing) was provided by Gosia Carless, PhD, of Fishawack Indicia Ltd, UK, funded by GlaxoSmithKline (GSK).
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MI-R and GS are employees of GSK and hold stocks and shares in the company. BAPE discloses participating in a GSK advisory board and a GSK-sponsored lecture.
The following authors have nothing to disclose, AB, AMC, CC, GC, AE, HF, MAG, SM, PM, SPR and EJV.
Funding
The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study (201282) was funded by GSK, Uxbridge, UK.
Research ethics and patient consent
The study was conducted according to the consensus ethical principles of the Declaration of Helsinki and Guidelines for Good Pharmacoepidemiology Practices and was approved by each applicable Ethics Committee/Institutional Review Board in Argentina. The study was performed in compliance with national regulations for observational studies (resolution number 1480/11 of the Ministry of Health in Argentina). Written informed consent was obtained from one site (CEMIC, Buenos Aires, Argentina), as deemed necessary by the local ethics committee. In accordance with local laws for the protection of personal data and the resolution 1480/11 of the Ministry of Health of the Nation, the remaining sites were not required to obtain patient consent, as anonymised patient data were collected retrospectively from patient medical records.
Availability of study materials
All data relevant to the study are included in the manuscript.
ORCID iD
References
- 1.D'Cruz DP. Systemic lupus erythematosus. BMJ 2006; 332: 890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scolnik M, Soriano ER. Epidemiology of lupus in Latin America. Lupus Open Access 2016; 01: 110. [Google Scholar]
- 3.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus 2006; 15: 308–318. [DOI] [PubMed] [Google Scholar]
- 4.Scolnik M, Marin J, Valeiras SM. Incidence and prevalence of lupus in Buenos Aires, Argentina: a 11-year health management organisation-based study. Lupus Sci Med 2014; 1: e000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertsias G, Ioannidis JP, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics. Ann Rheum Dis 2008; 67: 195–205. [DOI] [PubMed] [Google Scholar]
- 6.Zhu TY, Tam LS, Lee VW, Lee KK, Li EK. Relationship between flare and health-related quality of life in patients with systemic lupus erythematosus. J Rheumatol 2010; 37: 568–573. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez D, Kirou KA. What causes lupus flares? Curr Rheumatol Rep 2016; 18: 14. [DOI] [PubMed] [Google Scholar]
- 8.Ugarte-Gil MF, Acevedo-Vásquez E, Alarcón GS, et al. The number of flares patients experience impacts on damage accrual in systemic lupus erythematosus: data from a multiethnic Latin American cohort. Ann Rheum Dis 2015; 74: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 9.Ugarte-Gil MF, Wojdyla D, Pastor-Asurza CA, et al. Predictive factors of flares in systemic lupus erythematosus patients: data from a multiethnic Latin American cohort. Lupus 2018; 27: 536–544. [DOI] [PubMed] [Google Scholar]
- 10.EMA. Benlysta summary of product characteristics, www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002015/WC500110150.pdf (2011, accessed November 2018).
- 11.FDA. Benlysta prescribing information, www.accessdata.fda.gov/drugsatfda_docs/label/2017/761043lbl.pdf (2011, accessed November 2018).
- 12.FDA. FDA approves first treatment for pediatric patients with lupus, www.fdagov/news-events/press-announcements/fda-approves-first-treatment-pediatric-patients-lupus (2019, accessed May 2019).
- 13.Baker KP, Edwards BM, Main SH, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum 2003; 48: 3253–3265. [DOI] [PubMed] [Google Scholar]
- 14.Frieri M, Heuser W, Bliss J. Efficacy of novel monoclonal antibody belimumab in the treatment of lupus nephritis. J Pharmacol Pharmacother 2015; 6: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001; 166: 6–10. [DOI] [PubMed] [Google Scholar]
- 16.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011; 63: 3918–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377: 721–731. [DOI] [PubMed] [Google Scholar]
- 18.Stohl W, Schwarting A, Okada M, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol 2017; 69: 1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F, Bae SC, Bass D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis 2018; 77: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furie RA, Wallace DJ, Aranow C, et al. Long-term safety and efficacy of belimumab in patients with systemic lupus erythematosus: a continuation of a seventy-six-week phase III parent study in the United States. Arthritis Rheumatol 2018; 70: 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins CE, Dall'Era M, Kan H, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med 2016; 3: e000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortés J, Andreu JL, Calvo J, García-Aparicio AM, Coronell CG, Díaz-Cerezo S. Evaluation of use of belimumab in clinical practice settings (observe study) in Spain: health resource utilization and labour absenteeism. Value in Health 2014; 17: A534. [DOI] [PubMed] [Google Scholar]
- 23.Cortes JMC, Andreu JL, Calvo J, et al. Evolution of patients with systemic lupus erythematous treated with belimumab in clinical practice settings. Arthritis Rheumatol 2014; 66: S291. [Google Scholar]
- 24.Schwarting A, Schroeder JO, Alexander T, et al. First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in Germany: results from the OBSErve Germany study. Rheumatol Ther 2016; 3: 271–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touma Z, Sayani A, Pineau CA, et al. Belimumab use, clinical outcomes and glucocorticoid reduction in patients with systemic lupus erythematosus receiving belimumab in clinical practice settings: results from the OBSErve Canada study. Rheumatol Int 2017; 37: 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Kempis J, Duetsch S, Reuschling N, et al. Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland. Swiss Med Wkly 2019; 149: w20022. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 28.Gladman DD, Urowitz MB, Rahman P, Ibanez D, Tam LS. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol 2003; 30: 1955–1959. [PubMed] [Google Scholar]
- 29.van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 2014; 73: 958–967. [DOI] [PubMed] [Google Scholar]
- 30.van Vollenhoven RF, Petri MA, Cervera R, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis 2012; 71: 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caprarulo C, Spindler A, Graf C, et al. Registro argentino de pacientes con lupus eritematoso sistémico (relessar). Reporte preliminar. In: 48th Argentinian Congress of Rheumatology, Mar del Plata, Argentina, 26–29 September 2015. Paper no. PO014 (0235).
- 32.Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum 2012; 64: 2328–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strand V, Gladman D, Isenberg D, Petri M, Smolen J, Tugwell P. Endpoints: consensus recommendations from OMERACT IV. Lupus 2000; 9: 322–327. [DOI] [PubMed] [Google Scholar]
- 34.Strand VG, Isenberg D, Petri M, Smolen J and Tugwell P. Outcome measures to be used in clinical trials in systemic lupus erythematosus. J Rheumatol 1999; 26: 490–497. [PubMed] [Google Scholar]