Abstract
Background:
To analyze demography, clinical signs, and survival of intensive care patients diagnosed with nonocclusive mesenteric ischemia (NOMI) and to evaluate the effect of a local intra-arterial prostaglandin therapy.
Methods:
Retrospective observational study screening 455 intensive care patients with acute arterial mesenteric perfusion disorder in a tertiary care hospital within the past 8 years. Lastly, 32 patients with NOMI were enrolled, of which 11 received local intra-arterial prostaglandin therapy. The diagnosis of NOMI was based on the clinical presentation and established biphasic computed tomography criteria. Clinical and biochemical data were obtained 24 hours before, at the time, and 24 hours after diagnosis.
Results:
Patients were 60.5 (49.3-73) years old and had multiple comorbidities. Most of them were diagnosed with septic shock requiring high doses of norepinephrine (NE: 0.382 [0.249-0.627] μg/kg/min). The Sequential Organ Failure Assessment (SOFA) score was 18 (16-20). A decrease in oxygenation (Pao 2/Fio 2), pH, and bicarbonate and an increase in international normalized ratio, lactate, bilirubin, leucocyte count, and NE dose were early indicators of NOMI. Median SOFA score significantly increased in the last 24 hours before diagnosis of NOMI (16 vs 18, P < .0001). Overall, 28-day mortality was 75% (81% nonintervention vs 64% intervention cohort; P = .579). Median SOFA scores 24 hours after intervention increased by +5% in the nonintervention group and decreased by 5.5% in the intervention group (P = .0059).
Conclusions:
Our data suggest that NOMI is a detrimental disease associated with progressive organ failure and a high mortality. Local intra-arterial prostaglandin application might hold promise as a rescue treatment strategy. These data encourage future randomized controlled trials are desirable.
Keywords: shock, intestinal failure, nonocclusive mesenteric ischemia, sepsis
Background
Nonocclusive mesenteric ischemia (NOMI) was first reported by Ende in 1958 in patients with severe heart failure1 and accounts for 5% to 15% of acute mesenteric ischemia.2 The NOMI is characterized by the absence of embolic or atherosclerotic thrombotic occlusion of the mesenteric arteries in combination with functional vasoconstriction of the splanchnic arterial vessels, leading to a progressive intestinal ischemia.2 Mortality ranges between 50% and 93%.3,4 Case reports and small retrospective case series described the occurrence of NOMI especially after cardiac surgery5–10 and heart failure6,11 but also in a variety of other acute critical illnesses.11–24
Besides the clinical difficulties in diagnosing the disease, only a small number of risk factors that predict onset or outcome of NOMI have been identified. The typical NOMI candidate is an elderly patient with cardiovascular and/or renal disease, who has an additional life-threatening event (eg, cardiovascular surgery, sepsis, shock) and who requires vasopressor therapy.25,26
Compared to the well-known symptoms of an occlusive arterial mesenteric ischemia, the symptoms of NOMI are variable and unspecific.27 Abdominal pain can be absent or is not reported due to sedation.28 Therefore, NOMI is often diagnosed in advanced stages. However, early diagnosis would be of utmost importance to avoid intestinal ischemia with bacterial translocation and subsequent “intestinal-driven” multi-organ failure (MOF) or potentially lethal complications such as bowel necrosis and perforation.26,28
So far there is no established causal therapy for NOMI.27,29 Case series demonstrated clinical improvement in splanchnic blood flow through selective angiography of the superior mesenteric artery (SMA) and local application of papaverine, an acetyl cholinesterase inhibitor.26,30 Oral administration of phosphodiesterase inhibitors in non-intensive care patients was also attempted.31 Intra-arterial perfusion with the prostacyclin iloprost was studied in patients with NOMI following cardiopulmonary bypass and showed positive effects.32 Mitsuyoshi et al described in a small series of 9 patients the success of local prostaglandin E1 administration, which resulted in splanchnic vasodilation without systemic effects.33 All previous studies, including the one by Mitsuyoshi et al, included almost exclusively NOMI cases in patients following cardiothoracic surgery. It was uncertain whether previous observations would be transferable to medical intensive care patients. Here, we identified 32 medical and surgical intensive care patients, making it the largest study describing NOMI and the effect of local prostaglandin E1 therapy in a mixed cohort.
The aim of the present study was to analyze demography, clinical signs, and survival of a mixed cohort of intensive care patients diagnosed with NOMI and to describe the effects of local intra-arterial prostaglandin treatment on organ function and outcome.
Methods
Study Population
This was a retrospective single-center observational study performed in a tertiary care hospital from June 2010 to January 2018. We screened 455 intensive care patients diagnosed with an acute mesenteric perfusion disorder based on the German DRG-coding (no. K55.0). Included were patients on all surgical and medical intensive care unit (ICU) environments of our center. Specifically, the initial screening was done by the controlling personnel of our center employing a search for the specific DRG coding number for acute mesenteric perfusion disorders. All yielded results were then carefully evaluated by a sixth-year critical care fellow in means of a thorough and complete review of the individual medical charts for accuracy of diagnosis and presence of the adequate imaging diagnostics. Patients with an occlusive mesenteric perfusion disorder or mesenteric venous thrombosis were excluded. We finally enrolled 32 patients based on clinical suspicion and with a computed tomography (CT)-based diagnosis of NOMI, of which 11 had received local intra-arterial prostaglandin therapy. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Diagnostic Criteria for NOMI: Inclusion of Patients Into Retrospective Analysis
Patient records were analyzed if they fulfilled the diagnostic criteria for NOMI defined as (i) clinical suspicion, (ii) exclusion of thrombotic or embolic mesenteric artery occlusion, and (iii) radiographic signs suggesting diagnosis of NOMI (such as vasospasm of the SMA and its branches indicated by reduced vessel diameter and contrast) on biphasic contrast-enhanced CT scan34,35 and digital subtraction angiography (DSA; if additional angiograph was performed). Images were acquired using a 64-row scanner (GE LightspeedVCT; GE-Healthcare, Chalfont St. Giles, United Kingdom) or a dual-source CT (Somatom Force, Siemens, Forchheim, Germany) with a reconstructed slice thickness of 1 mm. The specific employed imaging protocol consisted of an arterial and venous phase of the entire abdomen with threshold-based bolus triggering in the aorta. The same CT imaging scanner was employed during the study period. The original radiographic report on CT imaging was independently reviewed by a separate experienced radiology attending for accuracy of the initial diagnosis.
Intervention: Intra-Arterial Prostaglandin E1 Application
Twenty-one patients did receive standard intensive medical care (nontreatment group), while 11 patients additionally received local intra-arterial prostaglandin therapy (treatment group). Standard medical care was defined as optimal supportive therapy of sepsis and shock by means of early and aggressive volume resuscitation and broad parenteral anti-infective therapy, as repeatedly recommended in all subsequent guidelines of the “Surviving Sepsis campaign.”36 Additionally, all patients with the clinical diagnosis of ileus received gastric decompression by insertion of a nasogastric tube. The decision to perform intra-arterial therapy was always made in an interdisciplinary approach, including both the intensivist and the interventional radiologist.
In the treatment group, patients with NOMI confirmatory computed tomography angiography (CTA) were transported to the angiographic suite. Of the 11 patients receiving intervention, 7 had the intervention performed immediately after CT diagnosis of NOMI, while 4 of the 11 patients had intervention at a later time point at the same day with a median time difference of 171 minutes (103-220 minutes) between diagnosis and intervention. Vascular access was achieved through the common femoral artery and a 4F hemostasis sheath (Avanti+, Cordis, Miami Lakes, Florida) was placed. A diagnostic catheter (Radifocus, Glidecath Cobra2; Terumo Europe, Leuven, Belgium) was advanced in the SMA. Angiography was obtained to verify the correct catheter position. A bolus of 20 μg of prostaglandin E1 (Alprostadil; UCB Pharma GmbH, Monheim, Germany) was slowly infused in the SMA over 10 minutes. Subsequently, another angiography documented the early treatment response. The sheath and the catheters were fixed, labeled, and attached to a continuous infusion drip of prostaglandin at a dose of 60 to 80 μg/24 hours depending on the patient weight and following previous reported dosing instructions.37–39 The duration of prostaglandin infusion was based on the individual course and continued until clinical improvement or death. Clinical improvement was defined as a combination of clinical observations determined by the primarily treating intensivist. Criteria of clinical improvement were hemodynamic improvement (indicated by reduction of norepinephrine (NE) dose by at least 20% compared to baseline), improvement of organ dysfunction (indicated by any reduction of Sequential Organ Failure Assessment [SOFA] score), improvement of bowel ischemia (indicated by a significant reduction of lactate concentrations), and resolution of paralytic ileus (indicated by regular bowel movements).
Data Collection
Data were collected using electronic medical records, including the patient data monitoring system m-life. The SOFA scores were calculated according to the description by Vincent et al.40 Organ failure was defined as an organ-specific SOFA score of equal or greater than 2.
Statistical Analysis
We used GraphPad Prism 7 (La Jolla, California), IBM SPSS Statistics (version 25.0 IBM Corp, Armonk, New York), and STATA (version 13.0, StataCorp, College Station, Texas) for data analysis and graph generation. Categorical variables are shown as numbers (n) and percentages (%). Continuous variables are shown as median and 25% to 75% quartiles, unless indicated otherwise. Variables were checked for normal distribution using the D’Agostino-Pearson omnibus normality test and the Shapiro-Wilk normality test. For comparisons, Fisher exact test, χ2 test, Mann-Whitney U test, Wilcoxon matched pairs signed rank test, and 2-sided paired t test were used accordingly. Univariate and multivariate logistic regressions were conducted, and receivers operating characteristic (ROC) curves were generated to determine thresholds of continuous parameters. Afterward, these thresholds were used to compare survival using log-rank test. All reported P values are 2 sided unless otherwise indicated; P values <.05 were considered statistically significant.
Results
Cohort Characterization
From June 2010 to March 2018, a total of 32 patients (out of 455 with acute mesenteric ischemia) were diagnosed with NOMI. All patients received standard medical therapy for septic shock41 combined with the placement of a gastric tube to decompress the stomach. Of the 32patients, 11 (34%) received local intra-arterial prostaglandin therapy in addition. A flowchart in accordance to the consort statement is shown in Supplemental Figure 1. Prostaglandin infusion was carried out as long as reasonable until clinical improvement for 3.4 ± 3 days. Except for local hematoma at the groin puncture side as a minor complication, <2% of the procedure was safe. In particular, no systemic hemodynamic side effects were observed. The 2 cohorts receiving standard medical therapy only (no intervention) and additional local intra-arterial prostaglandin application (intervention) were largely comparable in terms of demographic and clinical parameters (Table 1). The body mass index of the intervention group was slightly higher. Overall, 94% of all patients were diagnosed with sepsis and 87% with septic shock. The patients required high doses of NE (0.382 [0.249-0.627] μg/kg/min). Sepsis usually showed a severe clinical course indicated by high incidences of mechanical ventilation with 96.8%, renal replacement therapy 78.1%, and MOF 96.9%. Organ failure was defined as an organ-specific SOFA score of equal to or higher than 2. The median SOFA score was 18.16–20
Table 1.
Demographic and Clinical Characteristics at Diagnosis.a
| Category | All, N = 32 | No Intervention, n = 21 | Intervention, n = 11 | P |
|---|---|---|---|---|
| Age, years | 60.5 (49.3-73) | 61 (48-74) | 60 (54-73) | .85 |
| Sex, n (%) | .72 | |||
| Male | 17 (53.1) | 12 (57.1) | 7 (63.6) | |
| Female | 15 (46.9) | 9 (42.9) | 4 (36.4) | |
| Weight, kg | 75 (65-90) | 70 (62-86.5) | 85 (75-90) | .04 |
| Height, m | 1.7 (1.65-1.78) | 1.7 (1.65-1.74) | 1.76 (1.59-1.8) | .44 |
| BMI, kg/m2 | 25.3 (23.7-28.8) | 24.2 (23.5-27.7) | 27.8 (25.4-29.4) | .03 |
| Comorbidities, n (%) | ||||
| Adipositas | 7 (21.9) | 4 (19) | 3 (27.3) | .59 |
| Hypertension | 19 (59.4) | 12 (57.1) | 7 (63.6) | .72 |
| Diabetes | 8 (25) | 5 (23.8) | 3 (27.3) | .83 |
| COPD | 2 (6.3) | 1 (4.8) | 1 (9.1) | .63 |
| Heart insufficiency | 16 (50) | 10 (47.6) | 6 (54.5) | .71 |
| CAD | 12 (37.5) | 7 (33.3) | 5 (45.5) | .5 |
| CABG | 8 (25) | 5 (23.8) | 3 (27.3) | .83 |
| PTCA | 5 (15.6) | 2 (9.5) | 3 (27.3) | .19 |
| CKD | 14 (43.8) | 8 (38.1) | 6 (54.5) | .37 |
| Chronic renal replacement therapy | 1 (3,1) | 1 (4.8) | 0 (0) | .46 |
| Immunosuppression | 9 (28.1) | 6 (28.6) | 3 (27.3) | .94 |
| Reason for admission, n (%) | .17 | |||
| Surgical | 17 (53.1) | 13 (61.9) | 4 (36.4) | |
| Medical | 15 (46.9) | 8 (38.1) | 7 (63.6) | |
| Sepsis present, n (%) | 30 (94) | 19 (90) | 11 (100) | .29 |
| Side of infection, n (%) | ||||
| Pulmonary | 13 (40.6) | 7 (33.3) | 6 (54.5) | .25 |
| Abdomen | 10 (31.3) | 8 (38.1) | 2 (18.2) | .25 |
| Urogenital | 1 (3.1) | 0 (0) | 1 (9.1) | .16 |
| Soft tissue | 3 (9.4) | 2 (9.5) | 1 (9.1) | .97 |
| Endocarditis | 1 (3.1) | 1 (4.8) | 0 (0) | .46 |
| Mixed | 1 (3.1) | 1 (4.8) | 0 (0) | .46 |
| Nonidentified | 1 (3,1) | 0 (0) | 1 (9.1) | .16 |
| Identified pathogen, n (%) | ||||
| Gram positive | 6 (18.8) | 4 (19) | 2 (18.2) | .95 |
| Gram negative | 9 (28.1) | 6 (28.6) | 3 (27.3) | .94 |
| Fungi | 2 (6.3) | 0 (0) | 2 (18.2) | .04 |
| Viral | 2 (6.3) | 0 (0) | 2 (18.2) | .04 |
| Mixed | 3 (9.4) | 3 (14.3) | 0 (0) | .19 |
| Nonidentified | 8 (25) | 6 (28.6) | 2 (18.2) | .52 |
| GCS, n (%) | .63 | |||
| 3 points | 30 (94) | 20 (95.2) | 10 (90.9) | |
| 15 points | 2 (6) | 1 (4.8) | 1 (9.1) | |
| SOFA score points | 18 (16-20) | 18 (16-20) | 18 (16-20) | .56 |
| Norepinephrine, n (%) | 28 (87.5) | 19 (90.5) | 11 (100) | .29 |
| Norepinephrine dose, μg/kg/min | 0.382 (0.249-0.627) | 0.37 (0.273-0.661) | 0.489 (0.187-0.598) | .64 |
| Dobutamine, n (%) | 6 (18.8) | 5 (23.8) | 2 (18.2) | .95 |
| Dobutamine dose, μg/kg/min | 3.2 (1.837-5.238) | 3.491 (1.806-5.453) | 3.2 (1.96-4.44) | .99 |
| Epinephrine, n (%) | 8 (25) | 7 (33.3) | 2 (18.2) | .52 |
| Epinephrine dose, μg/kg/min | 0.194 (0.096-0.472) | 0.233 (0.148-0.709) | 0.076 (0.042-0.109) | .43 |
| Mechanical ventilation, n (%) | 31 (96.8) | 20 (95.2) | 11 (100) | .63 |
| Oxygenation index (Pao 2/Fio 2) | 165.5 (95.3-226) | 188 (105.5-304.5) | 150 (87-197) | .21 |
| Renal replacement therapy, n (%) | 25 (78.1) | 16 (76.2) | 9 (81.8) | .59 |
| Organ failure, n (%) | ||||
| Respiratory | 27 (84.4) | 16 (76.2) | 11 (100) | .08 |
| Coagulation | 15 (46.9) | 11 (52.4) | 4 (36.4) | .39 |
| Liver | 24 (75) | 16 (76.2) | 8 (72.7) | .83 |
| Cardiovascular | 30 (93.8) | 19 (90.5) | 11 (100) | .29 |
| Neurological | 30 (93.8) | 20 (95.2) | 10 (90.9) | .63 |
| Renal | 27 (84.4) | 17 (81) | 10 (90.9) | .46 |
| Multiorgan failure, n (%) | ||||
| 2 | 0 (0) | 0 (0) | 0 (0) | |
| 3 | 3 (9.4) | 1 (4.8) | 2 (18.2) | .22 |
| 4 | 4 (12.5) | 3 (14.3) | 1 (9.1) | .67 |
| 5 | 15 (46.9) | 11 (52.4) | 4 (36.4) | .39 |
| 6 | 9 (28.1) | 5 (23.8) | 4 (36.4) | .45 |
Note. Bold numbers highlight significant values.
Abbreviations: BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; GCS, Glasgow Coma Scale; PTCA, percutaneous transluminal coronary angioplasty; SOFA, Sequential Organ Failure Assessment.
aDescription of the whole patient cohort (n = 32) and subgroups of patients, who received standard supportive medical treatment (no intervention, n = 22) as well as additional local intra-arterial prostaglandin therapy (intervention, n = 11). Demographic and clinical characteristics at the time of biphasic computed tomography (CT)-based diagnosis of NOMI are given. Values are presented as median (25%-75% interquartile range) or if categorical as numbers and percentages.
Clinical and Biochemical Parameters Before, at, and After Diagnosis of NOMI
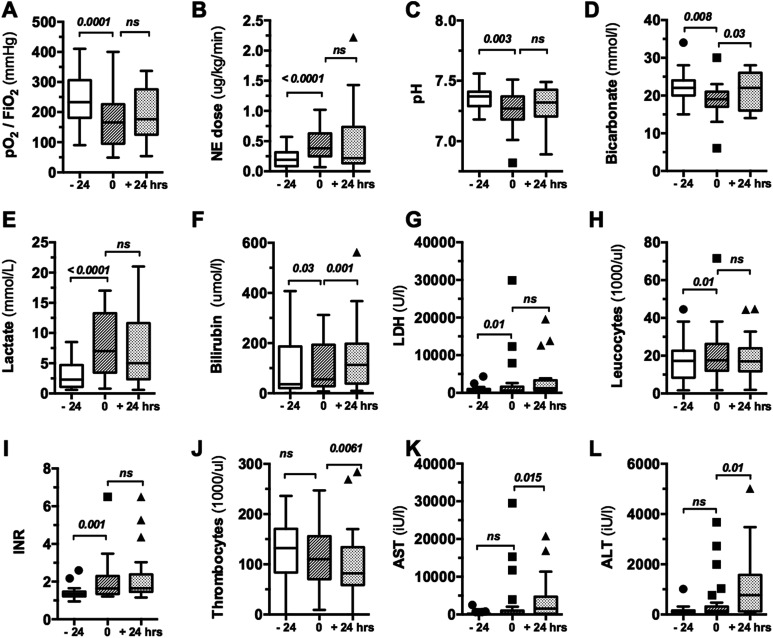
Figure 1 shows clinically relevant parameters during the disease course (−24 hours, 0 hour, and +24 hours) in the overall cohort. Several parameters changed during the development of NOMI (ie, comparison −24 hours vs 0 hour), including decreases in oxygenation index (Pao 2/Fio 2), pH, and bicarbonate as well as increases in international normalized ratio (INR), lactate, bilirubin, leucocyte count, and NE dose. The SOFA score increased in the last 24 hours before diagnosis of NOMI (P < .0001, Supplemental Figure 2A). Meanwhile other parameters, such as platelet count and transaminases (aspartate aminotransferase and alanine aminotransferase), were significantly altered only in the later disease course (ie, 0 hour vs +24 hours). The oxygenation index decreased early (−24 hours: 233 [181-306] vs 0 hour: 166 [95-226], P = .0001; Figure 1A), while at the same time NE dose increased significantly (−24 hours: 0.19 [0.089-0.315] vs 0 hour: 0.374 [0.222-0.608] μg/kg/min, P < .0001; Figure 1B). Creatinine kinase, C-reactive protein, and procalcitonin concentrations were not significantly altered during disease course (data not shown).
Figure 1.
Clinical and biochemical data obtained at various time points in relation to NOMI diagnosis. Box and whisker blots showing clinical and biochemical routine parameters 24 hours before (−24), at the time (0), and 24 hours after diagnosis of NOMI (+24 hours) for both treatment and nontreatment groups. Both a decrease in oxygenation index (A), arterial pH (C), and bicarbonate concentration (D) and an increase in lactate (E), bilirubin (F), LDH (G), leucocyte count (H), INR (I), and required norepinephrine dose (B) were an significant early indicator of NOMI while a significant decrease in thrombocyte count (J) and transaminases (K, L) was observed later. INR indicates international normalized ratio NOMI indicates nonocclusive mesenteric ischemia.
Progressive Organ Failure
At 24 hours prior to the diagnosis of NOMI, the majority of patients (69%) had MOF, in particular respiratory failure (53%), coagulation disorders (22%), liver failure (44%), cardiovascular failure (66%), neurological dysfunction (66%), and renal failure (63%). At the time of NOMI diagnosis, the respective organ failure rates had increased to 84%, 47%, 75%, 94%, 93%, and 84%, respectively. The overall incidence of MOF increased during the time course of NOMI, as indicated by the fact that 69%, 97%, and 88% of the patients had MOF at 24 hours before (named −24 hours), at (named 0), and 24 hours postdiagnosis of NOMI (named +24 hours). Severity of MOF increased over time as well (Supplemental Figure 2B).
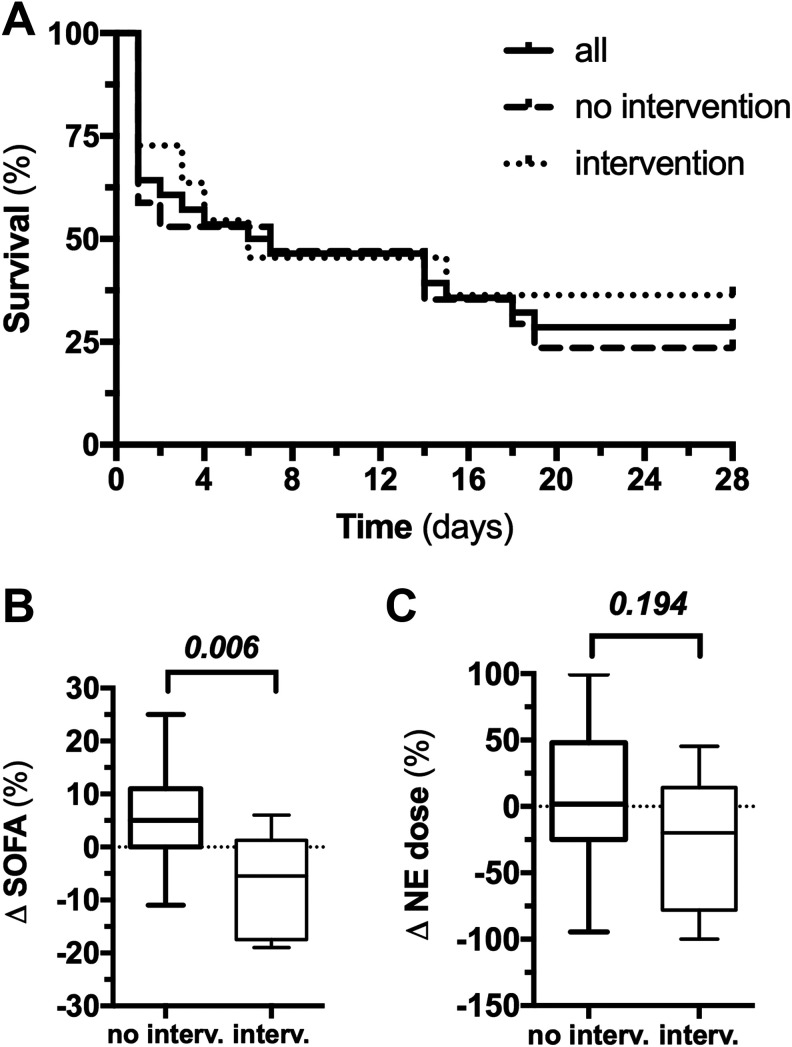
Twenty-Eight-Day Mortality and Effect of Intervention
The overall 28-day in-hospital mortality was 75%: 81% in the nonintervention and 64% in the intervention group (risk-ratio intervention: 0.76; 95% confidence interval: 0.28-2.02, P = .579; Figure 2A). Organ function significantly improved in the intervention group compared to the nonintervention group—median delta-SOFA scores (0 hour vs +24 hours postdiagnosis) were +5% (0%-11%) in the nonintervention and −5.5% (−17.5% to +1.25%) in the intervention group (P = .006; Figure 2B). The NE dose was slightly reduced in the intervention group (P = .194; Figure 2C). Local prostaglandin E1 application was not associated with systemic side effects.
Figure 2.
A 28-day survival and effect of intervention on survival, organ dysfunction, and shock. Kaplan-Meier graphs showing the 28-day survival course in the overall cohort, patients with standard supportive medical therapy only (no intervention), and additional local intra-arterial prostaglandin therapy (intervention) showing an observed mortality of 75%, 81%, and 64%, respectively (A). Organ function significantly improved in the intervention group compared to the nonintervention group with median delta-SOFA scores of +5% and −5.5% (P = .0059), respectively (B). At the same time, vasopressor requirement was slightly reduced in the intervention group, indicated by a median delta norepinephrine (NE) requirement of +1.9% and −19.8% for the nonintervention and intervention group, respectively (P = .194) (C).
Computed Tomography Angiography Imaging Characteristics at Diagnosis and DSA Characteristics Before and After Intervention
Supplemental Table 1 shows CTA characteristics of all patients at diagnosis of NOMI. These characteristics including semiquantitative mesenteric vessel width and vessel contrast, quantitative mesenteric vessel diameter at diverse locations, and several different secondary signs of bowel ischemia have been suggested previously34 to be of specific and sensitive value for the diagnosis NOMI. As Supplemental Table 1 shows, 100% of arterial and 86.6% of venous mesenteric vessel width were described as barely definable or at least narrow. Quantitative mesenteric vessel diameters measured at different locations were indicated as well. Established secondary signs of bowel ischemia, such as bowel wall edema, thickening, and hypoenhancement, as well as bowel loop distention and mesenteric edema, were found in 40% to 86.7% of the patients, respectively. Indicators of advanced bowel ischemia with disruption of the bowel barrier function such as pneumatosis intestinalis and portal venous gas were observed in 13.3% and 10%, respectively. Atherosclerotic lesions of the SMA were present in 10% of the patients.
Moreover, we could demonstrate by DSA imaging in the subset of patients receiving local intra-arterial prostaglandin therapy that already the initial prostaglandin bolus was able to reverse mesenteric vessel spasm.42 Supplemental Table 2 demonstrates that constriction of mesenteric vessels and reflux of contrast medium into the aorta as a sign of the increased mesenteric vascular resistance were effectively reduced by local intra-arterial prostaglandin infusion.
Predictors of Mortality
Survivors (n = 8) and nonsurvivors (n = 24) were comparable in most demographic and clinical parameters (Table 2). Nonsurvivors showed a higher prevalence of diabetes (33% vs 0%, P = .059) and required higher NE doses at the time of diagnosis (0.455 [0.321-0.69] vs 0.172 [0.073-0.321] µg/kg/min, P = .002). Nonsurvivors had at the time of diagnosis significantly higher lactate levels (2.1 vs 8.7 mmol/L, P = .033) and lower thrombocyte counts (90 [70-130] vs 220 [86-396.5]/nL, P = .001) than survivors. Of the 32 patients, 13 (40.6%) underwent emergency surgery. Of these patients, 3 (23.1%) survived, and of the 19 patients, who did not undergo surgery, 5 (26.3%) survived. Abdominal surgery had no significant influence on survival as shown in Table 2. Of the 8 surviving patients, 4 (50%) were receiving additional prostaglandin therapy, while of the 24 nonsurvivors, only 7 (29%) obtained additional intervention. However, multivariate regression analysis for demographic, clinical, or biochemical parameter did not identify predictors of 28-day mortality.
Table 2.
Demographic, Clinical, and Biochemical Characteristics for Nonsurviving and Surviving Patients.a
| Category | Deceased, n = 24 | Alive, n = 8 | P |
|---|---|---|---|
| Age, years | 60 (47.8-73.5) | 63.5 (51-73) | .72 |
| Sex, n (%) | .84 | ||
| Male | 14 (58.3) | 5 (62.5) | |
| Female | 10 (41.7) | 3 (37.5) | |
| BMI, kg/m2 | 25.2 (23.9-28.7) | 25.4 (23.5-29) | .78 |
| Comorbidities, n (%) | |||
| Adipositas | 6 (25) | 1 (12.5) | .46 |
| Hypertension | 13 (54.2) | 6 (75) | .3 |
| Diabetes | 8 (33.3) | 0 (0) | .06 |
| COPD | 2 (8.3) | 0 (0) | .4 |
| Heart insufficiency | 11 (45.8) | 5 (62.5) | .41 |
| CAD | 8 (33.3) | 4 (50) | .4 |
| CABG | 6 (25) | 2 (25) | .99 |
| PTCA | 5 (20.8) | 0 (0) | .16 |
| CKD | 11 (45.8) | 3 (37.5) | .68 |
| Chronic renal replacement therapy | 1 (4.2) | 0 (0) | .56 |
| Immunosuppression | 7 (29.2) | 2 (25) | .82 |
| Reason for admission, n (%) | .54 | ||
| Surgical | 12 (50) | 5 (62.5) | |
| Medical | 12 (50) | 3 (37.5) | |
| Need for abdominal surgery/bowel resection, n (%) | 10 (41.7) | 3 (37.5) | .84 |
| Sepsis present, n (%) | 22 (91.7) | 8 (100) | .4 |
| Side of infection, n (%) | |||
| Pulmonary | 10 (41.7) | 3 (37.5) | .84 |
| Abdomen | 8 (33.3) | 2 (25) | .66 |
| Urogenital | 0 (0) | 1 (12.5) | .08 |
| Soft tissue | 2 (8.3) | 1 (12.5) | .73 |
| Endocarditis | 1 (4.2) | 0 (0) | .56 |
| Mixed | 0 (0) | 1 (12.5) | .08 |
| Nonidentified | 1 (4.2) | 0 (0) | .56 |
| Identified pathogen, n (%) | |||
| Gram positive | 4 (16.7) | 2 (25) | .6 |
| Gram negative | 5 (20.8) | 4 (50) | .11 |
| Fungi | 1 (4.2) | 1 (12.5) | .4 |
| Viral | 2 (8.3) | 0 (0) | .4 |
| Mixed | 3 (12.5) | 0 (0) | .29 |
| Nonidentified | 7 (29.2) | 1 (12.5) | .35 |
| SOFA score points | 18 (17-20) | 15 (12-18.8) | .09 |
| Norepinephrine, n (%) | 23 (95.8) | 7 (87.5) | .4 |
| Norepinephrine dose, μg/kg/min | 0.455 (0.321-0.69) | 0.172 (0.073-0.321) | .002 |
| Oxygenation index (Pao 2/Fio 2) | 165 (95-226) | 145 (91-233) | .69 |
| Renal replacement therapy, n (%) | 20 (83.3) | 5 (62.5) | .22 |
| Organ failure, n (%) | |||
| Respiratory | 20 (83.3) | 7 (87.5) | .78 |
| Coagulation | 13 (54.2) | 2 (25) | .15 |
| Liver | 19 (79.2) | 5 (62.5) | .35 |
| Cardiovascular | 22 (91.7) | 8 (100) | .4 |
| Neurological | 23 (95.8) | 7 (87.5) | .4 |
| Renal | 21 (87.5) | 6 (75) | .4 |
| pH | 7.26 (7.16-7.37) | 7.29 (7.2-7.4) | .4 |
| Bicarbonate, mmol/L | 19 (17-21) | 21 (16-22) | .49 |
| Lactate, mmol/L | 8.7 (5.2-14.1) | 2.1 (1.3-9.3) | .03 |
| CK, IU/L | 727 (240-1870) | 223 (50-573) | .1 |
| LDH, U/L | 660 (364-2177) | 663 (459-747) | .9 |
| AST, U/L | 479 (112-1515) | 147 (42-469) | .23 |
| ALT, U/L | 159 (98-471) | 123 (43-205) | .41 |
| Bilirubin, μmol/L | 65.5 (37-193.3) | 35 (10-231) | .32 |
| Leucocytes, 1000/μl | 18 (13.1-25.9) | 15.6 (8.3-27.5) | .51 |
| CRP, mg/L | 104.5 (52-256.3) | 234 (106.3-279) | .19 |
| PCT, μg/L | 7.1 (3.5-13.8) | 16.6 (7-27.3) | .23 |
| Thrombocytes, 1000/μl | 90 (70.3-130.3) | 219.5 (86-396.5) | .001 |
| INR | 1.59 (1.33-2.3) | 1.79 (1.49-2.73) | .56 |
Note. Bold numbers highlight significant results.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CK, creatinine kinase; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; GCS, Glasgow Coma Scale; INR, international normalized ratio; LDH, lactate dehydrogenase; PCT, procalcitonine; PTCA, percutaneous transluminal coronary angioplasty; SOFA, Sequential Organ Failure Assessment.
aDescription of patients, who died (deceased, n = 24) and those who survived (alive, n = 8). Values are presented as median (25%-75% interquartile range) or if categorical as numbers and percentages.
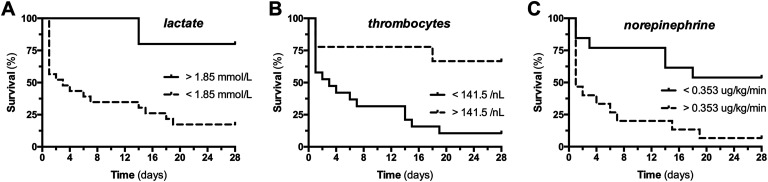
Receiver operating characteristics were analyzed for the abovementioned parameters. The NE dose, lactate level, and thrombocyte count at the time of diagnosis showed significant areas under the ROC curve (AUC) for survival (AUC NE, 0.855, P = .003; lactate, 0.755, P = .033; thrombocytes, 0.773, P = .074; Table 3). Thereafter, theoretical cutoff values were calculated and later used in a Kaplan-Meier model to visualize clinical outcome in 2 groups stratified above and below these calculated cutoff value (Table 3 and Figure 3).
Table 3.
ROC Analysis and Optimal Cutoff Values Predicting Survival in NOMI.a
| Characteristic | ROC | Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| AUC | P | Cutoff | Sensitivity (%) | Specificity (%) | Above Cutoff (%) | Below Cutoff (%) | P | |
| Norepinephrine requirement | 0.855 | .003 | 0.353 | 72.7 | 87.5 | 5.9 | 53.8 | .001 |
| Lactate | 0.755 | .03 | 1.850 | 95.8 | 50 | 14.8 | 80 | .01 |
| Thrombocytes | 0.773 | .07 | 141.5 | 80 | 86.7 | 66.7 | 8.7 | .002 |
Abbreviations: NOMI, nonocclusive mesenteric ischemia; ROC, receiver operating characteristic.
aArea under the curve and corresponding P values for ROC analysis of norepinephrine requirement, lactate concentration, and thrombocyte count at baseline are given. Optimal cutoff values for predicting 28-day survival and their sensitivities and specificities and 28-day percentage survival for cohorts above and below the cutoff values are indicated.
Figure 3.
A 28-day survival dependent on clinical baseline parameters. Kaplan-Meier graphs showing the 28-day survival course of patient groups below and above the optimal cutoff values for (A) lactate concentration, (B) platelet counts, and (C) norepinephrine requirement.
Discussion
This study evaluated clinical data on risk factors, clinical signs, and survival of 32 intensive care patients diagnosed with NOMI based on clinical presentation and CT findings. Moreover, the effect of local continuous intra-arterial prostaglandin application in this mixed surgical and medical ICU cohort was assessed.
About half of the patients in the present study were nonsurgical and sepsis was present in over 90% of these patients. Sepsis as risk factor for NOMI has not been described so far probably since the vast majority of the earlier described cohorts included surgical patients.30,33,38,43,44 Of the 32 patients investigated, 10 (31.3%) developed NOMI following major cardiac surgery. This is in line with previous observations that cardiac surgery is a major risk factor for the development of NOMI. However, the majority of NOMI cases in the present series were unrelated to cardiac surgery. With regard to sepsis severity, we found that patients were in need of high doses of vasopressors and had manifest MOF at the time of NOMI diagnosis (SOFA 17.1 ± 4.5). Consistently, it has been reported recently that reduced cardiac output and a high SOFA score were independent predictors for the emergence of NOMI.4
Early diagnosis of NOMI is challenging, consecutively lowering potential therapeutic success. In particular, clinical and biochemical parameters to reliably diagnose NOMI are still lacking.28 In this study, we could demonstrate by comparing a set of parameters (1) before, (2) at, and (3) after diagnosis of NOMI that parameters associated with organ dysfunction such as oxygenation index, NE requirement, and SOFA score changed in the process of establishing the diagnosis, while other parameters, mainly reflecting severe tissue injury such as creatinine kinase and transaminases, changed rather late during disease course. For the intensive care specialist, this is of no major surprise since these former unspecific parameters have earlier been reported to be associated with progressive multi-organ dysfunction.45 These observations strongly suggest that rapidly developing novel organ failure (combined with an abdominal clinic) that is not explained by other obvious reasons should raise the possibility of NOMI and guide to the early initiation of imaging diagnostics.
The diagnosis of NOMI still relies on the combination of initial clinical suspicion and adequate imaging including both CTA and DSA techniques, for which characteristics of NOMI have been proposed.34,42 We could demonstrate that most of our patient cohort did fulfill both semiquantitative and quantitative characteristics of NOMI. Quantitative mesenteric vessel diameters measured at different locations of the SMA and the superior mesenteric vein showed mean values that are comparable or even lower than reported previously in patients with NOMI46: proximal SMA 5.5 mm (vs 5.85 mm as reported by Kammerer et al), middle SMA 4.2 mm (vs 4.52 mm), distal SMA 1.9 mm (vs 2.56 mm), and SMV 9.7 mm (vs 9.2 mm). Mesenteric vessel spasm led to intestinal hypoperfusion as indicated by various indirect criteria, such as bowel wall edema, thickening, and hypoenhancement, as well as bowel loop distention and mesenteric edema. At the same time, indicators of advanced bowel ischemia with perforation requiring surgical treatment were seen only very rarely. The existence of mesenteric vessel sclerosis in only 10% of our patients confirms the nonocclusive character of mesenteric ischemia in patients with NOMI. Interestingly, DSA imaging demonstrated reversibility of vessel spasm following prostaglandin administration.
The 28-day mortality in our series was very high (75% in the entire cohort), which is consistent with a recent study describing 90-day mortality rates of up to 90%.4
Previous case series describing the employment of a local intra-arterial prostaglandin application included 3 to 9 patients.47–50 The so far largest case series of 9 patients included almost exclusively patients who had undergone cardiovascular surgery.33 In this series, the reported mortality was 31%, that is, better than in our series (31% vs 77%). This study with 32 patients of heterogeneous surgical and medical background adds further evidence for a possible beneficial effect of this intervention. We observed a survival improvement of 17.4% (19% vs 36.4%), which was not statistically significant. However, any survival benefit from prostaglandin treatment needs to be placed in the context of the general low power of this study. Given the lack of robust clinical trials, it remains unclear whether local prostaglandin treatment confers a survival benefit in patients with NOMI. Still, organ dysfunction as indicated by longitudinal changes in SOFA scores improved in the intervention compared to the nonintervention group. This observation suggests that local prostaglandin perfusion might have a beneficial role not restricted to the intestinal system. Reperfusion of the ischemic gut might improve the intestinal barrier, thereby positively affecting bacterial translocation and consequently disrupting an otherwise deadly viscous circle. This concept has been previously coined as remote organ injury.51–53
In terms of safety, it is important to mention that we did not see a worsening of shock during or after the intervention that would theoretically been possible due to (1) the ischemia–reperfusion injury and (2) a systemic effect of the prostaglandin. This lack of systemic vasodilatory effects is probably attributable to a high clearance of prostaglandin E1 especially by the lungs as described earlier.54–56
Although this is the largest study analyzing local prostaglandin treatment in NOMI so far, it was still too small to identify independent prognostic factors for survival employing multivariate regression analysis. However, we observed that NE dose, lactate, and platelet count at the time of NOMI diagnosis significantly differed between patients who survived the next 28 days and those who did not. It is intuitive that a higher NE dose as the main trigger of intestinal vasoconstriction, a higher lactate as a marker of increasing tissue hypoxemia, and a lower platelet count as a general indicator of sequential progressive organ failure are major indicators of higher mortality in NOMI. Based on this finding, we performed AUC analysis to calculate optimal cutoff values to identify patients at high risk for unfavorable outcome.
This study has important limitations—mostly, its retrospective and nonrandomized nature and the small sample size. Further, we used established CT morphological changes of mesenteric vessel architecture to diagnose NOMI. However, NOMI may reflect a microvascular pathology37 on the mucosal capillary level that might require a more sophisticated panel of parameters to make the correct diagnosis than solely CT morphology. For a future randomized controlled trail, one might therefore consider additional criteria of intestinal dysfunction or a monitoring tool of local perfusion (ie, microcirculation). The ROC data have not been validated in an additional NOMI cohort and therefore need to be interpreted with caution. Given the retrospective nature of this study, the decision to initiate prostaglandin treatment was uncontrolled and certainly influenced by patient- and doctor-specific characteristics—we therefore cannot exclude the presence of a selection bias. In our series, surgical intervention was associated with a poor outcome, but we cannot exclude the possibility that earlier surgery might have provided better results. However, our data suggest that early pharmacological approaches to improve bowel perfusion before the development of advanced bowel ischemia may hold more promise than surgical treatment.
Conclusions
In conclusion, NOMI is a difficult-to-diagnose disease entity associated with progressive MOF and a high mortality not only in multimorbid surgical patients but also in medical intensive care patients. Several variables such as NE dose, platelet count, and serum lactate might help to identify patients at high risk who could benefit from early intervention. Local intra-arterial prostaglandin application holds promise as a rescue treatment to reperfuse the ischemic gut and to ultimately improve remote multi-organ dysfunction. A controlled randomized clinical trial is desirable.
Supplemental Material
Supplemental_material for A Retrospective Analysis of Nonocclusive Mesenteric Ischemia in Medical and Surgical ICU Patients: Clinical Data on Demography, Clinical Signs, and Survival by Klaus Stahl, Markus Busch, Sabine K. Maschke, Andrea Schneider, Michael P. Manns, Jan Fuge, Olaf Wiesner, Bernhard C. Meyer, Marius M. Hoeper, Jan B. Hinrichs and Sascha David in Journal of Intensive Care Medicine
Footnotes
Authors’ Note: The need for ethical approval was waived due to retrospective nature of analysis. All data were anonymized before analysis. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The data sets used and analyzed during the current study, are available from the corresponding author on reasonable request. Klaus Stahl collected clinical data from the PDMS and generated the figures for publication. Sabine K. Maschke, Bernhard C. Meyer, and Jan B. Hinrichs performed local intra-arterial prostaglandin intervention. Markus Busch, Olaf Wiesner, and Andrea Schneider recruited patients. Michael P. Manns, Jan B. Hinrichs, Marius M. Hoeper, Jan Fuge, Klaus Stahl, and Sascha David and interpreted data, wrote the manuscript, and calculated statics. Sascha David had the original idea for this trial and wrote the proposals. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Klaus Stahl, MD  https://orcid.org/0000-0002-4833-6035
https://orcid.org/0000-0002-4833-6035
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ende N. Infarction of the bowel in cardiac failure. N Engl J Med. 1958;258(18):879–881. [DOI] [PubMed] [Google Scholar]
- 2. Clair DG, Beach JM. Mesenteric Ischemia. N Engl J Med. 2016;374(10):959–968. [DOI] [PubMed] [Google Scholar]
- 3. Bassiouny HS. Nonocclusive mesenteric ischemia. Surg Clin North Am. 1997;77(2):319–326. [DOI] [PubMed] [Google Scholar]
- 4. Soussi S, Taccori M, De Tymowski C, et al. Risk factors for acute mesenteric ischemia in critically ill burns patients—a matched case–control study. Shock. 2019;51(2):153–160. [DOI] [PubMed] [Google Scholar]
- 5. Piton G, Paquette B, Delabrousse E, Capellier G. Transient portal venous gas associated with reversible non-occlusive mesenteric ischemia: a case report. Int J Surg Case Rep. 2017;37:76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samura T, Toda K, Yoshioka D, et al. Non-occlusive mesenteric ischemia in a patient with left ventricular assist device implantation. J Artif Organs. 2017;20(3):277–279. [DOI] [PubMed] [Google Scholar]
- 7. Meguro K, Ishizaki J, Yanagisawa T, Koitabashi T, Kitamura T, Ako J. Non-occlusive mesenteric ischemia accompanied by aortic regurgitation after transcatheter aortic valve implantation. Cardiovasc Interv Ther. 2017;32(4):425–429. [DOI] [PubMed] [Google Scholar]
- 8. Goleanu V, Alecu L, Lazar O. Acute mesenteric ischemia after heart surgery. Chirurgia (Bucur). 2014;109(3):402–406. [PubMed] [Google Scholar]
- 9. Schutz A, Eichinger W, Breuer M, Gansera B, Kemkes BM. Acute mesenteric ischemia after open heart surgery. Angiology. 1998;49(4):267–273. [DOI] [PubMed] [Google Scholar]
- 10. Fukada Y, Hoshino J, Katahira S, Hirota M, Gyoten T, Isomura T. . Non-occlusive mesenteric ischemia after off-pump coronary artery bypass grafting [in Japanese]. Kyobu Geka. 2012;65(7):534–537. [PubMed] [Google Scholar]
- 11. Versyck G, de Gheldere C, Vanclooster P. Non-occlusive mesenteric ischemia: two case reports and a short review of the literature. Acta Chir Belg. 2018;118(6):392–397. [DOI] [PubMed] [Google Scholar]
- 12. Kaneko T, Kumagai J, Homma Y. Fatal non-occlusive mesenteric ischemia after laparoscopic radical nephrectomy in a hemodialysis patient. Int J Urol. 2014;21(10):1071. [DOI] [PubMed] [Google Scholar]
- 13. Qureshi SS, Neve RS, Raina SA, Mistry RC. Fatal non-occlusive mesenteric ischemia after esophagectomy. J Cancer Res Ther. 2010;6(1):112–113. [DOI] [PubMed] [Google Scholar]
- 14. Yamane H, Fukuda N, Nishino K, et al. Non-occlusive mesenteric ischemia after splenic metastasectomy for small-cell lung cancer. Intern Med. 2015;54(7):743–747. [DOI] [PubMed] [Google Scholar]
- 15. Hirota M, Inoue K, Kimura Y, et al. Non-occlusive mesenteric ischemia and its associated intestinal gangrene in acute pancreatitis. Pancreatology. 2003;3(4):316–322. [DOI] [PubMed] [Google Scholar]
- 16. Murono K, Ishihara S, Kawai K, et al. Non-occlusive mesenteric ischemia localized in the transverse colon: a case report. Surg Case Rep. 2017;3(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quiroga B, Verde E, Abad S, et al. Detection of patients at high risk for non-occlusive mesenteric ischemia in hemodialysis. J Surg Res. 2013;180(1):51–55. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka A, Ito Y, Sugiura Y, Sezaki R. Non-occlusive mesenteric ischemia in a hemodialysis patient. Intern Med. 2011;50(5):523. [DOI] [PubMed] [Google Scholar]
- 19. Brener ZZ, Bergman M, Ohm HK, Winchester JF. Acute non-occlusive mesenteric ischemia of the small bowel in a patient started on hemodialysis: a case report. Cases J. 2008;1(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gocho N, Aoki E, Okada C, et al. Non-occlusive mesenteric ischemia with diabetic ketoacidosis and lactic acidosis following the administration of a sodium glucose co-transporter 2 Inhibitor. Intern Med. 2016;55(13):1755–1760. [DOI] [PubMed] [Google Scholar]
- 21. Bawany MZ, Nawras A, Youssef WI, Sodeman T. The unusual suspect: a case of non-occlusive mesenteric ischemia in a patient with cirrhosis. Gastroenterol Res. 2010;3(5):232–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinez R, Rogers A, Numanoglu A, Rode H. Fatal non-occlusive mesenteric ischemia and the use of propranolol in paediatric burns. Burns. 2016;42(4):e70–e73. [DOI] [PubMed] [Google Scholar]
- 23. Anderson JE, Brown IE, Olson KA, Iverson K, Cocanour CS, Galante JM. Non-occlusive mesenteric ischemia in patients with methamphetamine use. J Trauma Acute Care Surg. 2018;84(6):885–892. [DOI] [PubMed] [Google Scholar]
- 24. Martinez-Vieira A, Camacho-Ramirez A, Diaz-Godoy A, et al. Bowel ischaemia and cocaine consumption; case study and review of the literature. Rev Esp Enferm Dig. 2014;106(5):354–358. [PubMed] [Google Scholar]
- 25. Groesdonk HV, Klingele M, Schlempp S, et al. Risk factors for nonocclusive mesenteric ischemia after elective cardiac surgery. J Thorac Cardiovasc Surg. 2013;145(6):1603–1610. [DOI] [PubMed] [Google Scholar]
- 26. Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur Radiol. 2002;12(5):1179–1187. [DOI] [PubMed] [Google Scholar]
- 27. Lock G, Scholmerich J. Non-occlusive mesenteric ischemia. Hepatogastroenterol. 1995;42(3):234–239. [PubMed] [Google Scholar]
- 28. Kolkman JJ, Mensink PB. Non-occlusive mesenteric ischaemia: a common disorder in gastroenterology and intensive care. Best Pract Res Clin Gastroenterol. 2003;17(3):457–473. [DOI] [PubMed] [Google Scholar]
- 29. Bailey RW, Bulkley GB, Hamilton SR, Morris JB, Haglund UH. Protection of the small intestine from nonocclusive mesenteric ischemic injury due to cardiogenic shock. Am J Surg. 1987;153(1):108–116. [DOI] [PubMed] [Google Scholar]
- 30. Klotz S, Vestring T, Rotker J, Schmidt C, Scheld HH, Schmid C. Diagnosis and treatment of nonocclusive mesenteric ischemia after open heart surgery. Ann Thorac Surg. 2001;72(5):1583–1586. [DOI] [PubMed] [Google Scholar]
- 31. Murthy KA, Kiran HS, Cheluvaraj V, Bhograj A. Non-occlusive mesenteric ischemia and the role of cilostazol in its management. J Pharmacol Pharmacother. 2012;3(1):68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bomberg H, Groesdonk HV, Raffel M, et al. Vasopressin as therapy during nonocclusive mesenteric ischemia. Ann Thorac Surg. 2016;102(3):813–819. [DOI] [PubMed] [Google Scholar]
- 33. Mitsuyoshi A, Obama K, Shinkura N, Ito T, Zaima M. Survival in nonocclusive mesenteric ischemia: early diagnosis by multidetector row computed tomography and early treatment with continuous intravenous high-dose prostaglandin E(1). Ann Surg. 2007;246(2):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kammerer S, Schuelke C, Berkemeyer S, et al. The role of multislice computed tomography (MSCT) angiography in the diagnosis and therapy of non-occlusive mesenteric ischemia (NOMI): could MSCT replace DSA in diagnosis? PLoS One. 2018;13(3):e0193698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perez-Garcia C, de Miguel Campos E, Fernandez Gonzalo A, et al. Non-occlusive mesenteric ischaemia: CT findings, clinical outcomes and assessment of the diameter of the superior mesenteric artery. Br J Radiol. 2018;91(1081):20170492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levy MM, Evans LE, Rhodes A. . The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925–928. [DOI] [PubMed] [Google Scholar]
- 37. Stockmann H, Roblick UJ, Kluge N, et al. Diagnosis and therapy of non-occlusive mesenteric ischemia (NOMI) [in German]. Zentralbl Chir. 2000;125(2):144–151. [PubMed] [Google Scholar]
- 38. Ernst S, Luther B, Zimmermann N, et al. Current diagnosis and therapy of non-occlusive mesenteric ischemia [in German]. Rofo. 2003;175(4):515–523. [DOI] [PubMed] [Google Scholar]
- 39. Schindler G, Bruch HP. [The current status of the diagnosis and therapy of nonocclusive intestinal ischemia (NII)]. Rofo. 1991;155(2):123–127. [DOI] [PubMed] [Google Scholar]
- 40. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On Behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 41. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925–928. [DOI] [PubMed] [Google Scholar]
- 42. Minko P, Stroeder J, Groesdonk HV, et al. A scoring-system for angiographic findings in nonocclusive mesenteric ischemia (NOMI): correlation with clinical risk factors and its predictive value. Cardiovasc Intervent Radiol. 2014;37(3):657–663. [DOI] [PubMed] [Google Scholar]
- 43. Huwer H, Winning J, Straub U, Isringhaus H, Kalweit G. Clinically diagnosed nonocclusive mesenteric ischemia after cardiopulmonary bypass: retrospective study. Vascular. 2004;12(2):114–120. [DOI] [PubMed] [Google Scholar]
- 44. Eris C, Yavuz S, Yalcinkaya S, et al. Acute mesenteric ischemia after cardiac surgery: an analysis of 52 patients. ScientificWorldJournal. 2013;2013:631534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. [DOI] [PubMed] [Google Scholar]
- 46. Biraima AM, Genoni M, Winkler MB, Bruhlmann W, Turina MI. Mesenteric ischemia after a cardiac operation: arteriosclerotic versus vasospastic etiology. J Cardiovasc Surg (Torino). 2002;43(1):87–89. [PubMed] [Google Scholar]
- 47. Kamimura K, Oosaki A, Sugahara S, Mori S. Survival of three nonocclusive mesenteric ischemia patients following early diagnosis by multidetector row computed tomography and prostaglandin E1 treatment. Intern Med. 2008;47(22):2001–2006. [DOI] [PubMed] [Google Scholar]
- 48. Kanzaki T, Koide M, Kunii Y, Watanabe K, Maeda T, Okamoto T. . [Successful management of nonocclusive mesenteric ischemia after aortic valve replacement;report of a case]. Kyobu Geka. 2015;68(2):137–140. [PubMed] [Google Scholar]
- 49. Weiss G, Lippert H, Meyer F. Successful management of non-occlusive mesenteric ischemia (NOMI) – case report. Pol Przegl Chir. 2012;84(4):214–218. [DOI] [PubMed] [Google Scholar]
- 50. Luckner G, Jochberger S, Mayr VD, et al. Vasopressin as adjunct vasopressor for vasodilatory shock due to non-occlusive mesenteric ischemia. Anaesthesist. 2006;55(3):283–286. [DOI] [PubMed] [Google Scholar]
- 51. Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131(3):241–4. [DOI] [PubMed] [Google Scholar]
- 52. Puleo F, Arvanitakis M, Van Gossum A, Preiser JC. Gut failure in the ICU. Seminars in respiratory and critical care medicine. Semin Respir Crit Care Med. 2011;32(5):626–638. [DOI] [PubMed] [Google Scholar]
- 53. Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon. 2012;10(6):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iijima T, Ohishi F, Tatara T, Iwao Y. Effect of continuous infusion of prostaglandin E1 on hepatic blood flow. J Clin Anesth. 2001;13(4):250–254. [DOI] [PubMed] [Google Scholar]
- 55. Golub M, Zia P, Matsuno M, Horton R. Metabolism of prostaglandins A1 and E1 in man. J Clin Invest. 1975;56(6):1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Awas JA, Soteriou MC, Drougas JG, Stokes KA, Roberts LJ, II, Pinson CW. Plasma prostaglandin E1 concentrations and hemodynamics during intravenous infusions of prostaglandin E1 in humans and swine. Transplantation. 1996;61(11):1624–1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_material for A Retrospective Analysis of Nonocclusive Mesenteric Ischemia in Medical and Surgical ICU Patients: Clinical Data on Demography, Clinical Signs, and Survival by Klaus Stahl, Markus Busch, Sabine K. Maschke, Andrea Schneider, Michael P. Manns, Jan Fuge, Olaf Wiesner, Bernhard C. Meyer, Marius M. Hoeper, Jan B. Hinrichs and Sascha David in Journal of Intensive Care Medicine