Abstract
An essential element in the pursuit of value-based health care is provider payment reform. This article aims to identify and analyze payment initiatives comprising a specific manifestation of value-based payment reform that can be expected to contribute to value in a broad sense: (a) global base payments combined with (b) explicit quality incentives. We conducted a systematic review of the literature, consulting four scientific bibliographic databases, reference lists, the Internet, and experts. We included and compared 18 initiatives described in 111 articles/documents on key design features and impact on value. The initiatives are heterogeneous regarding the operationalization of the two payment components and associated design features. Main commonalities between initiatives are a strong emphasis on primary care, the use of “virtual” spending targets, and the application of risk adjustment and other risk-mitigating measures. Evaluated initiatives generally show promising results in terms of lower spending growth with equal or improved quality.
Keywords: value-based health care, accountable care, provider payments, global payments, pay-for-performance
Introduction
Worldwide, the interest in value-based health care (VBHC) is growing rapidly. In many developed countries there is public recognition that waste and inefficiency can be reduced, while quality and health outcomes can be improved (Berwick & Hackbarth, 2012). Encouraging health care providers to deliver high-value care is thus a focal point in health policy.
An essential element in the pursuit of VBHC is provider payment reform. The reason for this is twofold. First, financial incentives in general, convincingly show to substantially influence provider behavior (Gaynor, Rebitzer, & Taylor, 2004; McGuire, 2000, 2011; Robinson, 2001). For example, physicians paid on a fee-for-service (FFS) basis, tend to provide more care compared with capitated and salaried physicians (Gosden et al., 2000). Second, predominant payment methods—in particular FFS—are not well aligned with value (Christianson & Conrad, 2011; Ellis & Miller, 2008; Jegers, Kesteloot, de Graeve, & Gilles, 2002; Robinson, 2001). Specifically, paying providers separately and per activity encourages overprovision, maintains fragmentation, discourages prevention, and does not stimulate high-quality care. Since working toward VBHC, while leaving financial incentives for low-value care intact would clearly be counterproductive, there is consensus that VBHC and payment reform should go hand-in-hand.
Over the past decade, there has been much experimentation with various types of value-based payment (VBP) models. In this regard, both “value” and “VBP” are defined and operationalized in different ways. According to Berwick, Nolan, and Whittington (2008), high-value care requires pursuit of the “triple aim”: limiting per capita cost of care, improving individual patient experience, and improving population health. Porter (2009, 2010) provides a more general description of value, namely, the best health outcomes achieved per dollar spent. Conrad (2015) defines value as maximum health benefit (operationalized as health outcomes, processes of care, and patient experience) at minimum cost. A commonality in these definitions is that value is considered a multidimensional concept, comprising not only high quality and integration of care but also cost-consciousness and good health outcomes, which in turn require prevention.
Regarding VBP reform, emphasis is primarily on developing and implementing bundled-payment models for specific conditions or treatments as well as pay-for-performance (P4P) models that explicitly reward specific, measurable aspects of value (Chee, Ryan, Wasfy, & Borden, 2016; Roland & Campbell, 2014; Ryan, Krinsky, Maurer, & Dimick, 2017). Examples of the former are the Bundled Payment for Care Improvement Initiative and the Acute Care Episode Demonstration, both implemented in U.S. Medicare. Examples of the latter are the Hospital Value-Based Purchasing Program in U.S. acute care hospitals and the Quality and Outcomes Framework in the U.K. primary care sector. Although bundled payment and P4P could contribute to improvement of specific value dimensions, other important dimensions are unlikely to be strongly affected. Bundled payment mainly stimulates cost-conscious behavior and coordination, regarding the services pertaining to the condition or treatment in question (Stokes et al., 2018). P4P, by design, only focuses on aspects of value that can be explicitly measured using indicators, which are typically aspects of clinical quality. In other words, both types of VBP adopt a relatively narrow definition of value and are not well-suited for simultaneously incentivizing the multiple value dimensions as defined in the literature.
If payment reform is to substantially contribute to value in a broad sense, more profound reform of current payment models is likely to be required. Indeed, there is growing recognition in the literature as well as in practice that VBP models be designed in such a manner that incentives for high-value care stretch beyond the level of conditions or treatments. In addition, these models should not only stimulate measurable aspects of high-quality care but also cost-conscious behavior, well-coordinated care, and prevention (Peikes et al., 2018; Quentin et al., 2018; Scott, Liu, & Yong, 2018). Arguably, this can be realized by combining two payment components: (a) global base payments and (b) explicit quality incentives (Cattel, Eijkenaar, & Schut, 2020; see in section “The Rationale of Global Base Payments in Combination With Explicit Quality Incentives” for a justification). Over the past years, payment reform initiatives adopting these two components have been gaining ground, for example, in the shape of accountable care organizations (ACOs). To date, however, these initiatives have not been systematically identified and described.
New Contribution
Prior literature reviews investigating VBP reform mainly focused on bundled payment and P4P initiatives, which adopt a relatively narrow definition of value (Conrad, Grembowski, Hernandez, Lau, & Marcus-Smith, 2014; Mendelson et al., 2017; Milstein & Schreyögg, 2016; Scott et al., 2018). A comprehensive overview of VBP initiatives aiming at improving value in a broad sense via global base payments combined with explicit quality incentives is lacking. Currently, it is unclear how these initiatives are being designed and to what extent they are effective in improving value. In this article, we aim to fill this gap by systematically identifying and analyzing VBP initiatives comprising these two payment components. Specifically, we (a) describe the design features of these initiatives and (b) assess the extent to which initiatives have been successful in improving value. In doing so, we aim to provide policy makers, payers, and health care providers insight in promising and practically feasible modalities of VBP reform. In turn, this could support additional innovation, facilitate future model comparison, and ultimately contribute to VBHC. The integration of non-U.S. initiatives is especially valuable to stimulate international comparisons and shared learning.
This article proceeds as follows. The next section presents a framework of a VBP model comprising global base payments and explicit quality incentives, which will be used to systematically describe and compare identified initiatives. “Search Strategy and Selection Procedure” elaborates on the strategy followed while conducting this systematic literature review, and “Search Results” presents the results. “Discussion” reflects on the main findings and provides an overall conclusion.
Conceptual Framework
Recent papers have attempted to explicate the relationship between what a health care system ideally pursues in terms of value and what is required in terms of the design of provider payment systems (e.g., Cattel et al., 2018; Eijkenaar, 2013a; Scott et al., 2018). After reviewing existing descriptions of value and arguments used in the societal debate on what stakeholders in health care ideally aim for, we conclude that value is a multidimensional concept. The commonality in all descriptions is that value encompasses not only high-quality care, but also multidisciplinary coordination, cost-conscious behavior, and prevention (Berwick et al., 2008; Conrad, 2015; Donabedian, 1988; Eijkenaar & Schut, 2015; Institute of Medicine, 2001; Porter, 2009, 2010; Stokes et al., 2018). Based on a comprehensive synthesis of the payment incentive literature, Cattel et al. (2018) conclude that a combination of global base payments with explicit quality incentives seems well-suited to stimulate all these value dimensions simultaneously. The next section briefly elaborates on the rationale of such a two-component model.
The Rationale of Global Base Payments in Combination With Explicit Quality Incentives
The first component of a VBP model that stimulates value in a broad sense is a substantial global base payment. In essence, global payments are a form of bundled payment, with the bundle being constructed at a higher level than at the level of conditions or treatments. This addresses the shortcomings of lower level forms of bundled payment mentioned in the Introduction. The second component is a relatively low-powered P4P payment that explicitly rewards some measurable aspects of value.
Any provider payment system will at least consist of a base component that is not directly linked to providers’ measured performance. The reason is that many aspects of value, such as well-coordinated care and many health outcomes, are difficult or impossible to measure and attribute. While important, these aspects can thus not “explicitly” be accounted for in the payment contract (Eggleston, 2005; Holmstrom & Milgrom, 1991). The base payment can be designed in such a manner that it “implicitly” incentivizes aspects of value that cannot be adequately measured and thus not stimulated through explicit incentives (see section “Design of Global Base Payments and Explicit Quality Incentives”). Designing the base payment as a global payment facilitates cost-consciousness and well-coordinated care across the full continuum of care, with a focus on whole persons instead of on separate conditions or treatments.
Global base payments transfer financial risk from payer to provider. A possible danger is that providers become exposed to too much financial risk. As a result, they may be inclined to skimp on quality or act too aggressively in attempts to reduce spending by underproviding necessary but expensive services. These concerns, which are not just theoretical (Frakt & Mayes, 2012; Robinson, 2001), can be mitigated by supplementing the global base payment with risk-sharing arrangements and explicit quality incentives. Risk sharing results in a situation in which providers are being held accountable for only a share of savings/losses realized under the global base payment. Explicit quality incentives may trigger providers to give sufficient attention to value aspects that are unlikely to be incentivized by the global base payment but may be prone to quality skimping or underprovision (Eijkenaar, 2013b). These incentives should be relatively low-powered to prevent a disproportionate focus on rewarded tasks (Campbell, Reeves, Kontopantelis, Sibbald, & Roland, 2009; Mullen, Frank, & Rosenthal, 2010; Steel, Maisey, Clark, Fleetcroft, & Howe, 2007). In addition, high-powered explicit incentives may have a negative effect on physicians’ intrinsic motivation (Eijkenaar, 2013b; Wynia, 2009).
Empirical work supports the theoretical rationale of a two-component VBP model. Vlaanderen et al. (2019), for example, conclude that using explicit incentives for (outcome) quality paired with global base payments seems preferred over using explicit quality incentives alone.
Design of Global Base Payments and Explicit Quality Incentives
In this review, we analyze VBP initiatives combining global base payments with explicit quality incentives in terms of design and impact on value. For this purpose, we use two existing conceptual frameworks: one for the global base payment (Cattel et al., 2018) and one for the explicit quality incentives (Eijkenaar, 2013a). Although other frameworks made important contributions to the VBP literature, they are not suited for thoroughly describing and comparing key design features of payment models adopting the two-component structure described above. Shortell, Wu, Lewis, Colla, and Fisher (2014), for example, established a taxonomy to classify and understand early ACOs using eight general attributes that are not all related to payment design. In another article, Stokes et al. (2018) proposed a typology of payment models for integrated care. Since the focus of that article is specifically on incentives and facilitators for integrated care, it is also not suitable for the purpose of our review.
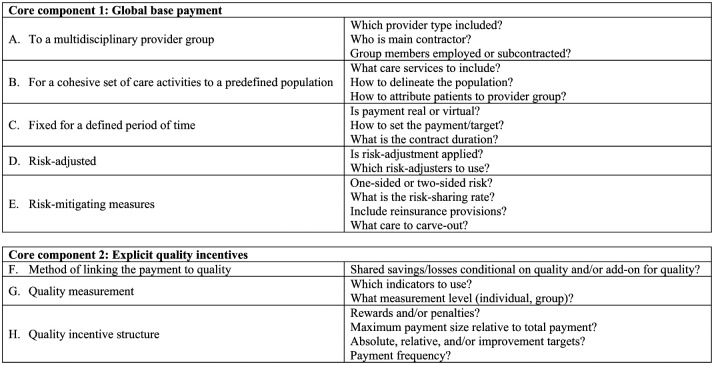
Figure 1 summarizes design features and issues regarding both payment components, which we briefly discuss below. First, providing the global base payment to a multidisciplinary provider group fosters coordination across the continuum of care (Anderson & Weller, 1999; Berenson, 2010; Burwell, 2015; Mehrotra & Hussey, 2015). Financial barriers between providers and sites are removed, resulting in more flexibility in the resource deployment (Cutler & Ghosh, 2012; Mechanic & Altman, 2009; H. D. Miller, 2009). Generally, a main contractor is responsible for administering and distributing the payment and employing and/or subcontracting individual providers (Anderson & Weller, 1999).
Figure 1.
Core components and associated design features of a VBP model combining global base payments with explicit quality incentives.
Note. Based on Cattel et al. (2018) and Eijkenaar (2013a). VBP = value-based payment.
Second, a global base payment pertains to a comprehensive set of care services for a predefined population of individuals. By adopting a person-based rather than a condition-based approach, incentives for prevention and cost-conscious behavior are strengthened. Another advantage is that cost-shifting becomes more difficult and is even impossible if the payment applies to the full continuum of care (Busse & Stahl, 2014; Hussey, Ridgely, & Rosenthal, 2011; Ridgely, de Vries, Bozic, & Hussey, 2014). The population can be delineated in various ways, for example, based on provider and/or payer affiliation. Attribution of this population to the provider group can be done prospectively or retrospectively.
Third, providing a payment that is fixed for a defined period of time stimulates cost-conscious behavior because it transfers financial risk to providers (Conrad, 2015; Frakt & Mayes, 2012; Jegers et al., 2002; H. D. Miller, 2009; Robinson, 2001). The payment can be determined in various ways, including based on historical spending and on average per capita spending in the region. The payment can be implemented as a “real” payment that actually replaces existing payment systems or as a “virtual” spending target with end-of-period reconciliation with claims. Regarding the contract period, in principle multiyear contracts seem preferable over short-term contracts because they provide room for earning back investments in value improvement. In addition, multiyear contracts signal mutual trust and prevent costly effort on “overwriting” complex, short-term contracts (Christianson & Conrad, 2011; Marques & Berg, 2011; Shortell, 2013; Silberberg, 1990). In practice, however, multiyear contracting could be difficult, especially in settings with high rates of beneficiary “churn.”
Finally, to realize better effects on the different value dimensions, theory recommends risk-adjusting the base payment and applying risk-mitigating measures. Risk adjustment prevents providers from being unfairly penalized for caring for a disproportionate share of high-risk individuals and from being incentivized to select favorable risks (Iezzoni, 2003; Rose, Zaslavsky, & McWilliams, 2016). Adopting risk-mitigating measures protects providers against excessive financial risk due to large random shocks in spending. Several options are available to bring financial risk to appropriate levels, including using one- or two-sided risk contracts (i.e., sharing upside risk only or also downside risk), varying the risk-sharing rate, adding reinsurance provisions, and carving out specific high-cost services from the contract.
The second component of a two-component VBP model is a payment explicitly linked to quality. Three main design features are of relevance: the method used to link payment to quality, quality measurement, and the quality incentive structure (Eijkenaar, 2013a). Regarding the method for linking payment to quality, the payment can either be applied as “add-on” to the global base payment or the provider share of realized savings/losses can be made conditional on aggregated quality scores. Regarding quality measurement, indicators could reflect “technical” quality (structures, processes, and outcomes) and/or patient-reported quality. Finally, the incentive structure concerns choices with regard to rewards versus penalties, incentive size relative to the total payment, type of quality targets, and payment frequency. Although each choice has advantages and disadvantages, prior literature suggests that using relatively low-powered rewards (Deci, Koestner, & Ryan, 1999; Eijkenaar, 2013a; Holmstrom & Milgrom, 1991; Moscucci et al., 2005; Shen, 2003), limiting the time lag between care delivery and payment (Conrad & Perry, 2009; Frederick, Loewenstein, & O’Donoghue, 2002; Thaler, 1981), and using absolute quality targets (Conrad & Perry, 2009; Rosenthal & Dudley, 2007; Young et al., 2007) is most likely to be effective in stimulating desired behavior.1
Method
Search Strategy and Selection Procedure
Complying with the Cochrane Handbook for systematic reviews (Higgins & Green, 2011), we conducted a systematic review of the literature on VBP initiatives written in English or Dutch and published between January 2000 and April 2017. We included articles/documents describing VBP initiatives that
have been implemented in developed countries;
combine global base payments with explicit quality incentives;
involve payments to multidisciplinary provider groups; and
involve payment for the provision of cohesive sets of care activities to predefined populations.
Consequently, we excluded initiatives that have not been implemented as well as initiatives that have adopted payment models without clearly discernable global base payments and/or explicit quality incentives, that are targeted at individual providers, and/or that are organized around specific conditions or treatments.
We mainly focused on articles published in peer-reviewed scientific journals. However, we did not exclude unpublished studies, reports, or policy briefs beforehand, because they may still describe initiatives meeting our inclusion criteria. Our main focus was on articles/documents describing VBP initiatives; the absence of a quantitative evaluation was not an exclusion criterion. Insofar available, however, we included studies describing quantitative effects on value, but only if published in peer-reviewed scientific journals and if the research approach corresponds to a difference-in-differences, interrupted-time series, randomized controlled trial, or systematic review design.
In identifying eligible VBP initiatives, we consulted four sources: (a) scientific bibliographic databases, (b) reference lists, (c) the Internet, and (d) experts publishing in the field of VBHC and/or VBP. We started our review by searching four bibliographic databases on April 12, 2017: Embase, Medline, Web of Science, and Cochrane Central. We used the same search terms for each database, while taking into account database-specific requirements (see Appendix A, available online). In consultation with an information specialist of the library of the Erasmus Medical Centre in Rotterdam, we developed the search strings using a combination of the terms value-based payment and care provider. After removal of duplicates, we independently screened the titles and abstracts of all articles yielded by the search and assessed their potential eligibility for inclusion. We compared initially included articles and resolved discrepancies by discussion. In a second round of screening, the first author retrieved full texts and assessed each article on eligibility.
Next, we examined reference lists of included articles/documents resulting from the database search and used forward citation tracking to identify additional VBP initiatives. Together with the database search, this resulted in a preliminary list of initiatives. To gather additional information on these initiatives and identify potentially relevant other initiatives, we searched Google and websites of relevant organizations, including the Centers for Medicare and Medicaid Services (CMS) and health insurers. Last, we consulted experts (see Appendix B, available online) to validate our preliminary list of initiatives and to suggest additional initiatives, if any. Importantly, we consulted the four sources in an iterative process. For example, if we encountered an initiative via reference screening that was not identified based on the database search using the original search string, we used initiative-specific key words to search the databases again and obtain additional articles/documents.
Analysis and Synthesis
For each identified VBP initiative, we extracted data on (a) general characteristics, (b) key design features with regard to the global base payment and the explicit quality incentives, and (c) effects on value. Regarding the general characteristics, we recorded the name of the initiative, setting, year of implementation, main contracting entities, and availability of a quantitative evaluation. We analyzed the results concerning the two payment components according to the design features shown in Figure 1. Finally, for initiatives that were evaluated, we recorded the design of quantitative studies, the effects on the applicable value dimensions, and information on the magnitude and statistical significance of effects. Because of heterogeneity in study design and outcome measures used, formal meta-analysis was not possible. Therefore, we present the results narratively.
We extracted relevant information using three standardized extraction forms. In case of inconsistencies among articles/documents describing the same initiative, we used information from the article/document with the most recent publication date. After completion of the extraction forms, we summarized the information in three compressed tables with key results only.
Results
Search Results
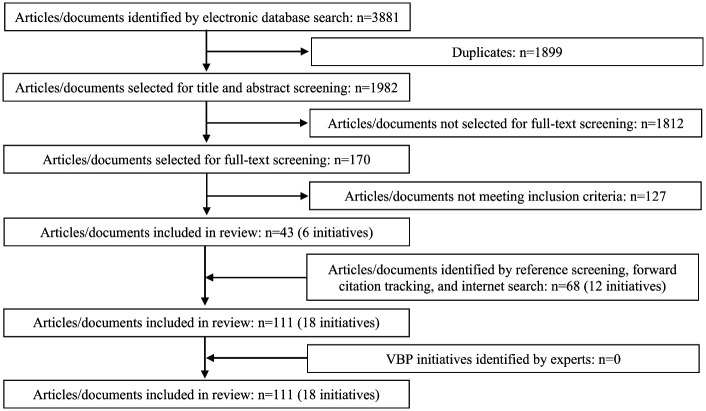
Applying our search string in the four databases resulted in 3,881 hits (Embase = 1,215; Medline Ovid = 1,403; Web of Science = 1,160; Cochrane Central = 103). After removing duplicates and examining titles and abstracts, we retrieved full texts of 170 potentially relevant articles/documents, which were screened in detail by the first author. Of these, we included 43 articles/documents describing six VBP initiatives. Based on reference screening, forward citation tracking, and searching the Internet, we added 68 articles/documents describing another 12 VBP initiatives. Since expert consultation did not result additional initiatives or articles/documents, we included a total of 111 articles/documents in the review (see Appendix C, available online), representing 18 VBP initiatives (Figure 2).
Figure 2.
Flow diagram of steps taken in the systematic review.
The 18 included initiatives represent approximately 15% of all payment reform initiatives that we identified in our search (N = 126). More than 40% of all identified initiatives pertain to payment models comprising only one of the two components. Generally, these models are “traditional” P4P initiatives without global base payments. Examples are the hospital Value-Based Purchasing Program and the Programs for All-inclusive Care for the Elderly. In almost 25% of the cases, we excluded initiatives because they use alternative payment models that do not fit our inclusion criteria. Examples are models where providers receive a case rate for an episode of care related to a specific condition or treatment or separate fees for coordinating patient care (e.g., the Acute Care Episode Demonstration and the Cigna Collaborative Accountable Care Model).
Despite fitting our inclusion criteria, we excluded two initiatives—the Physician Group Practice Demonstration and the Pioneer ACO Model—because they are precursors of current models that are included (#14, 15). Experiences and lessons learned in these “early versions” were used to (re)design current models and in that sense, we still indirectly incorporated these two initiatives in our review (CMS, 2018).
For the remaining excluded cases, insufficient information was available to determine whether the payment model consisted of the two payment components and/or to describe the design of these components. Examples are the Medica Patient Choice Model, the Rhode Island Health System Transformation Model, and the Medicaid ACO Learning Collaborative in New York, Vermont, and Washington, respectively.
Description of General Characteristics
Table 1 summarizes the general characteristics of the 18 identified VBP initiatives. The initiatives were implemented in four different countries: 15 in the United States, 1 in Spain, 1 in Germany, and 1 in the Netherlands. Most VBP models are regional initiatives, with four initiatives having been implemented nationally (#3, 14, 15, 18). Seven initiatives were initiated by public payers, nine by private payers, and two by public–private partnerships. Of the seven public initiatives, three are U.S. Medicare programs (#14, 15, 18), and four are U.S. Medicaid programs (#1, 8, 12, 16). Five initiatives have been formally evaluated on their impact on spending and/or quality.
Table 1.
General Characteristics of Identified VBP Initiatives.
| Name initiative | Country | Setting | Year of implementation | Contracting entities | Evaluated on impact on value |
|---|---|---|---|---|---|
| 1. Accountable Care Collaborative Program | USA, Colorado | Public (Medicaid) | 2011 | CMS + the State of Colorado + Regional Accountable Entities | No |
| 2. Advocate care | USA, Greater Chicago area | Private | 2011 | Private health insurer + private group of physicians | No |
| 3. Aetna’s Shared Savings Model | USA, nationwide | Private | 2011 | Private health insurer + integrated health systems | No |
| 4. Alternative Quality Contract | USA, Massachusetts | Private | 2009 | Private health insurer + ACOs | Yes, spending and quality |
| 5. Alzira Model | Spain, Valencia | Public–private partnership | 2003 | The regional health ministry + private contractor who owns a hospital | No |
| 6. Anthem WellPoint ACO Arrangement | USA, California | Private | 2011 | Private health insurer + health care delivery systems | No |
| 7. CalPERS Sacramento ACO Program | USA, California | Public–private partnership | 2010 | Private health insurer + public pension fund + large, independent physician association + hospital system | No |
| 8. Coordinated Care Organizations | USA, Oregon | Public (Medicaid) | 2012 | CMS + the State of Oregon + coordinated care organizations | No |
| 9. Dutch Shared Savings Program | The Netherlands, Twente region | Private | 2014 | Private health insurer + multispecialty primary care provider groups | No |
| 10. Gesundes Kinzigtal | Germany, Kinzigtal region | Private | 2005 | Two statutory private health insurers + physician network that concluded a contract with health management company | Yes, only quality |
| 11. Horizon BCBS New Jersey ACO Pilot | USA, North of Atlantic City, New Jersey | Private | 2010 | Private health insurer + large, multispecialty medical group | No |
| 12. Integrated Health Partnership Demonstration Project | USA, Minnesota | Public (Medicaid) | 2013 | CMS + the State of Minnesota + health care delivery systems | No |
| 13. Medica Shared Savings Model | USA, Minnesota | Private | 2009 | Private health insurer + integrated health systems and physician clinics | No |
| 14. Medicare Shared Savings Program | USA, nationwide | Public (Medicare) | 2012 | CMS + ACOs | Yes, spending and quality |
| 15. Next Generation ACO Model | USA, nationwide | Public (Medicare) | 2016 | CMS + ACOs | No |
| 16. Partners for Kids Program | USA, Ohio | Public (Medicaid) | 2012 | CMS + five Medicaid Managed Care Plans + large pediatric ACO | Yes, spending and quality |
| 17. ProvenHealth Navigator | USA, Pennsylvania | Private | 2006 | Private health insurer + Patient-centered medical homes | Yes, only spending |
| 18. Independence at Home | USA, nationwide | Public (Medicare) | 2012 | CMS + primary care practices | No |
Note. ACO = accountable care organization; BCBS = Blue Cross Blue Shield; CalPERS = The California Public Employees’ Retirement System; CMS = Centers for Medicare and Medicaid Services; VBP = value-based payment.
Key Design Features of Identified VBP Initiatives
Table 2 summarizes the initiatives’ key design features. In sections “Key Design Features of the Global Base Payment” and “Key Design Features of the Explicit Quality Incentives,” these findings are summarized and synthesized for the global base payment and the explicit quality incentives, respectively. The structure of these sections mirrors Figure 1.
Table 2.
Key Design Features of Identified VBP Initiatives.
| Name initiative | Multidisciplinary provider group | Cohesive set of care activities for a predefined population | Fixed payment for a defined period of time | Risk adjustment and risk-mitigating measures | Explicit quality incentives criteria |
|---|---|---|---|---|---|
| a. Main contractor b. Providers in the group c. Employed or subcontracted |
a. Healthcare
services b. Population c. Attribution method |
a. Virtual or real, current main payment
system b. Setting the payment or target c. Contract duration |
a. Risk adjustment b. One-sided or two-sided risk c. Risk-sharing rate d. Reinsurance provisions e. Carve-outs |
a. Link payment and quality b. Quality measures c. Level of measurement/payment d. Rewards or penalties e. Maximum payment size relative to total payment/target, except when denoted otherwise f. Absolute or relative targets g. Payment frequency |
|
| 1. Accountable Care Collaborative Program | a. Regional accountable care entity (e.g., community partnerships and insurers), responsible for developing provider networks. | a. Regular Health First Colorado benefit package: medical care, long-term care, and behavioral health. | a. Virtual, FFS and PMPM payment for coordination and case management. | a. N/A | a. P4P and savings conditional on achieving quality thresholds. |
| b. Formal networks of PCPs and informal networks of specialists, hospitals, and social services. | b. All Medicaid beneficiaries in the region are automatically enrolled. | b. N/A | b. One-sided risk | b. Eight key performance indicators: total cost of care, emergency department visits for conditions that could be prevented with primary care, wellness visits, members receiving behavioral health services/prenatal care/dental care services, rates of overweight/obesity, use of electronic consultations, and agreements with specialists. | |
| c. N/A | c. Attribution to PCP and corresponding regional accountable care entity based on prior utilization. If a patient did not use care, they are to select a PCP. | c. One-year contract, with possibility to renew contract annually for up to 4 years. | c. N/A | c. Payments to regional accountable care entity and PCPs | |
| d. N/A | d. Rewards | ||||
| e. No carve-outs | e. 5% of behavioral health capitation | ||||
| f. Improvement and meeting | |||||
| g. Quarterly | |||||
| 2. Advocate care | a. Private physician group that partners with not-for-profit multihospital integrated health system. | a. Full continuum of care | a. Virtual, FFS | a. Yes, using DxCG software | a. P4P and savings conditional on achieving quality thresholds. |
| b. Numerous care sites, including integrated children’s hospitals, acute care hospitals, and home care providers. Provider groups consists of solo, group, single- and multispecialty practices. | b. Fully insured and self-insured commercial PPO members receiving care from the provider group at least 2 times during 2 years. No minimum size. | b. Benchmark is the projected average medical cost trend in the market (i.e., BCBS Illinois’ PPO network). | b. Two-sided risk | b. 116 measures of clinical quality (i.e., preventive care, acute care processes, and outcomes), patient safety, and patient satisfaction. | |
| c. Both (employed and independent) | c. Prospective attribution based on prior utilization (claims from previous 2 years). | c. Three-year contract | c. Up to 50% | c. N/A | |
| d. N/A, Cost are not truncated | d. Rewards and penalties (i.e., lower unit price in next year if quality has declined). | ||||
| e. Some high-cost services such as transplantation. | e. 10% | ||||
| f. Maintain quality baseline during year 1; thereafter negotiated improvements. | |||||
| g. Quarterly, with annual reconciliation. | |||||
| 3 Aetna’s Shared Savings Model | a. Variety of health systems (e.g., independent physician associations, multispecialty physician groups, and multispecialty physician groups with contracted hospitals). | a. Full continuum of care | a. Virtual, payment system varies by health systems. | a. N/A | a. Savings conditional on meeting efficiency thresholds and set of clinical quality measures. Whether P4P as add-on is used is unclear. |
| b. N/A | b. Varies by health system | b. N/A | b. One-sided risk | b. Clinical quality measures and thresholds related to other domains (e.g., avoidable inpatient admissions and ER visits). | |
| c. N/A | c. In some cases prospective attribution based on enrolment with an ACO. In other cases retrospective attribution based on the plurality of utilization in the completed year. | c. N/A | c. Up to 50% | c. N/A | |
| d. N/A | d. N/A | ||||
| e. N/A | e. N/A | ||||
| f. N/A | |||||
| 4. Alternative Quality Contract | a. ACOs | a. All medical services BCBS pays for; full continuum of care. | a. Virtual, FFS | a. Yes, using DxCG software. Health status is measured concurrently. | a. P4P and risk-sharing rates depend on passing quality gates. |
| b. Variety of primary and specialty providers (e.g., physicians, hospitals, post-acute care facilities). Each ACO is required to include a PCP. | b. BCBS members with a HMO/POS policy. Minimum population size of 5,000. | b. Spending target is negotiable. Historical PMPM spending in the population of the group’s PCP serves as a starting point and spending is trended forward using a negotiated annual growth rate. | b. Two-sided risk | b. 64 measures: 32 in ambulatory setting (i.e., HEDIS clinical process and intermediate outcome measures, and patient experience measures) and 32 in hospital setting (i.e., process measures for specific diseases/treatments, patient safety indicators, and patient experience measures). In total, 47 process, 5 outcomes for diabetes, hypertension, and cardiovascular disease, and 12 patient experience measures. | |
| c. Physicians are either employed or independent; for other providers information N/A. | c. Prospective attribution based on affiliation with PCP whom enrolees designate each year. | c. Five-year contract | c. Negotiated, 50% to 100% | c. Payment to ACO | |
| d. Mandatory reinsurance, unit cost corridor, and in some cases overall cost trend corridor. | d. Rewards | ||||
| e. Behavioral health services | e. 10% | ||||
| f. Passing predefined “gates” and year-to-year performance | |||||
| g. Annually | |||||
| 5. Alzira Model | a. Private contractor who owns a hospital, consisting of health insurer, 3 regional savings banks, and 2 construction companies. | a. Primary and specialty care | a. Real, annual capitation paid to main contractor. | a. N/A | a. P4P, no link between quality and savings. |
| b. Numerous care sites (e.g., health centers, outpatient clinics, and a hospital). | b. Health zones of Alzira | b. N/A, updated according to the yearly growth rate in the Valencian health budget. | b. Two-sided risk | b. Quality and safety targets, including indicators for processes, clinical outcomes, and patient experience. | |
| c. Hospital physicians and about half of the PCPs are employed and paid salary. Others are public employees or civil servants. | c. Prospective attribution to primary health center based on geographical catchment area. | c. 15-year contract, extendable to 20 years | c. Up to 7.5% | c. N/A | |
| d. N/A | d. Rewards | ||||
| e. N/A | e. Negotiated, up to 20% between €6.000 and €24.000 per year. Percentage and amount also include on-call payments. | ||||
| f. N/A | |||||
| g. N/A | |||||
| 6. Anthem WellPoint ACO Arrangement | a. Health care delivery systems (e.g., integrated health systems and independent practice associations in private practice). | a. The full continuum of medical services | a. Virtual, FFS and care management fee | a. N/A | a. Savings conditional on meeting quality thresholds and efficiency criteria. |
| b. Multiple care sites for a broad spectrum of care services (e.g., primary and specialty care, laboratory, physical therapy, radiology, pharmacy, and urgent care). | b. Minimum population size of 15,000 | b. N/A | b. One-sided risk | b. Clinical quality measures and measures related to other domains (e.g., avoidable ER visits or all-cause readmissions), specific to physician care and hospital care. | |
| c. Attribution is prospective and based on prior utilization in the past 2 years. To be attributed to a provider group, a patient should have received at least 50% of their care with this group. | c. Five-year contract | c. 50% | c. N/A. | ||
| d. Caps on high-cost cases and stop-loss reinsurance | d. Not applicable | ||||
| e. Transplants | e. Not applicable | ||||
| f. Improvement and attainment | |||||
| g. N/A | |||||
| 7. CalPERS Sacramento ACO Program | a. Large, independent physician association for primary and specialized care and a not-for-profit hospital system. | a. The full continuum of care | a. Virtual, hospital receives FFS payment and physician group receives capitation budget and pays individual providers FFS. | a. Yes, based on “case complexity” | a. P4P and savings conditional on maintaining or improving quality. |
| b. Multiple care sites for primary and specialty care. | b. Blue Shield HMO members in the Sacramento area. | b. PMPM cost target for specific cost categories. Information on how targets are set N/A. | b. Two-sided risk | b. Quality, utilization, and patient satisfaction measures. | |
| c. N/A | c. N/A | c. Multi-year contract, information on exact duration N/A. | c. Depends on partner’s ability to influence particular costs category. Hospital system: up to 50%. Independent physician association: up to 33.3%. | c. N/A | |
| d. Stop-loss reinsurance | d. Rewards | ||||
| e. N/A | e. Unclear, but top-performing physicians have earning potential of 150% of Medicare rates. | ||||
| f. N/A | |||||
| g. N/A | |||||
| 8. Coordinated Care Organizations | a. CCOs, that is, networks of physical, mental, and dental care providers linked to publicly funded health programs. | a. Full continuum of care, including services for physical health, behavioral health, oral health, mental health, and addiction. | a. Real, CCOs receive PMPM payment | a. Yes, information on which variables are used N/A | a. P4P and savings conditional on quality metrics. |
| b. A broad range of primary and specialty providers. | b. All Medicaid beneficiaries in the region are automatically enrolled. | b. Unclear, adjusted according to historical growth rate. | b. Two-sided risk | b. 17 measures on preventive care, access, patient satisfaction, chronic illness management, behavioral health, maternal care, overuse, and electronic health record adoption and use. | |
| c. N/A, Each CCO must decide how to contract providers. PCPs usually paid capitation; specialty care providers receive less frequently capitated budget. | c. N/A | c. N/A | c. Full financial risk: 100% | c. Payment to CCOs | |
| d. Mandatory reinsurance | d. Rewards | ||||
| e. Mental health drugs, long-term care, case management, and public health. | e. Approximately 2% to 3% | ||||
| f. Achievement of benchmark metric or improving performance relative to the State’s benchmark. | |||||
| g. N/A | |||||
| 9. Dutch Shared Savings Program | a. A multidisciplinary primary care provider group. | a. All medical services for which health insurer provides coverage under both mandatory and supplementary benefits packages. | a. Virtual, PCPs are paid salary or combination of capitation, FFS, bundled payment, and P4P. | a. Yes, adjusted for demographics and socioeconomic status (concurrently) and morbidity (prospectively). | a. Savings conditional on overall quality score. In case performance has declined more than 5% during the year, the overall quality score is insufficient to be eligible for sharing any savings. |
| b. Provider group is led by primary care physicians and comprises nurse practitioners, physician assist, pharmacists, and physiotherapists. | b. Individuals who take up health insurance from the pilot insurer. | b. Historical spending in the past 3 years (with larger weights attached to more recent years), updated using a growth rate based on spending in a control group of randomly sampled nonparticipating providers in the region, and adjusted for periodic effects (e.g., inflation). | b. One-sided risk | b. 41 measures in 4 domains: patient satisfaction, chronic care, drug prescription behavior, and practice management. | |
| c. N/A | c. Attribution based on enrolment with PCP | c. N/A | c. Confidential risk rate. | c. Measurement at provider group level | |
| d. Cost cap at €22.500 ($25.376) per patient per year | d. Not applicable | ||||
| e. Dental care services | e. Not applicable | ||||
| f. Absolute performance and improvement relative to prior year | |||||
| g. N/A | |||||
| 10. Gesundes Kinzigtal | a. Physician network (including local independent primary care physicians, specialists, and hospitalists) that concluded a contract with a health management company specialized in the management of integrated care. | a. Care across all health service sectors and indications. Noticeable focus on preventive programs and health promotion. | a. Virtual, FFS | a. Yes, age, sex, and morbidity, based on German risk-equalization model. | a. Payment similar to P4P and savings depending on quality. |
| b. Multidisciplinary teams including PCPs, specialists, hospitals, nursing homes, ambulatory agencies, psychotherapists, physiotherapists, and social workers. | b. Individuals living in the Kinzigtal region who have an insurance policy with 1 of the 2 insurers. | b. Spending target determined by combining the German “standardized norm costs” and spending during a reference period prior to the start of the initiative. | b. One-sided risk | b. Information on specific measures N/A, but clinical outcome measures and patient satisfaction included. | |
| c. N/A | c. N/A | c. Unlimited contract | c. N/A | c. Measurement at individual provider level | |
| d. N/A | d. Variable performance-related rewards (i.e., an add-on payment to encourage coordination, rewards for activities such as participating in the electronic health record, and hourly rates for participating in certain project groups). | ||||
| e. Dental care services | e. 10% | ||||
| f. N/A | |||||
| g. N/A | |||||
| 11. Horizon BCBS New Jersey ACO Pilot | a. Multispecialty medical group | a. Full continuum of care | a. Virtual, FFS | a. Yes, information on which variables are used N/A | a. P4P and savings conditional on meeting quality threshold. |
| b. Primary care, specialty care, ancillary services, and some ambulatory and surgery services. | b. Patients with a commercial self-insured PPO policy. | b. N/A | b. Two-sided risk | b. Variety of HEDIS measures regarding quality of care, diabetes, cardiovascular disease, oncology, and (over)weight assessment. | |
| c. N/A | c. Retrospective attribution based on percentage of total visits. | c. 2-year contract | c. Negotiated, but specific percentages N/A | c. N/A | |
| d. Outliers are eliminated | d. Rewards | ||||
| e. N/A | e. N/A | ||||
| f. Reward if provider is in top-10% of best performers | |||||
| g. N/A | |||||
| 12. Integrated Health Partnership Demonstration Project | a. Integrated delivery systems (e.g., multispecialty provider network or not-for-profit medical practice group). | a. All Medicaid services | a. Real, population-based payment | a. Yes, age, sex, and diagnostic information using Johns Hopkins Adjusted Clinical Groups tool. | a. Savings conditional on total quality score; losses do not depend on quality. |
| b. Provider groups deliver full scope of primary care services, coordinate with specialty providers and hospitals, and partner with community organizations and social service agencies. | b. Medicaid enrollees in Minnesota (children and adults). Minimum population size applies to Track 2 participants (i.e., 2,000 patients). | b. Negotiable. Prior year’s spending is starting point and trended forward using an expected trend rate. | b. One-sided risk in year 1, thereafter two-sided risk | b. Measures of care quality (nationally accepted indicators for e.g., screening and patient safety; weight 70%), health information technology (weight 20%), and pilot measures (based on populations served; weight 10%). | |
| c. N/A | c. Retrospective attribution based on plurality of utilization (>1 visit with provider affiliated with the program), using a 24-month look-back period. | c. 1-year contract that renews annually during 3 years | c. 25% in year 1 and 2, thereafter 50%. Up to an agreed maximum savings/losses threshold. | c. N/A | |
| d. Cost cap at $200.000 per patient per year. | d. N/A | ||||
| e. Dental care services, transportation, personal care services in home care, long-term care, and residential mental health. | e. N/A | ||||
| f. In Year 1 only reporting. Thereafter, relative thresholds (i.e., being at least in 30th percentile for State or Medicaid average rates) and improvement during the years. | |||||
| g. Annually | |||||
| 13. Medica Shared Savings Model | a. Integrated health systems and physician clinics. | a. Full continuum of care | a. Virtual, FFS with withholds or prospective adjustments for the risk and reward pool. | a. Yes, age, sex, and diagnostic information using Johns Hopkins Adjusted Clinical Groups tool. | a. P4P and savings conditional on quality. |
| b. A broad range of primary and specialty care (e.g., primary care clinics, inpatient care providers, and home care providers). | b. Medica’s members enrolled in fully insured and self-insured PPOs and some members enrolled in commercially insured HMOs. Minimum population size of 15,000 to 20,000 member-months or 1,250 to 1,667 patients. | b. Spending target in comparison to a peer group | b. One-sided risk | b. Measures of quality, patient experience, provider collaboration, and utilization among practices, according to Minnesota Community Measurement Program focusing on prevention, chronic care, and utilization. | |
| c. N/A | c. Retrospective attribution based on claims (attribution in case of receiving >50% of primary care services from the group) with 1 year look-back. | c. N/A | c. Up to 50% | c. N/A | |
| d. Cost cap at $250.000 or $500.000 per patient per year. | d. Rewards and penalties | ||||
| e. Behavioral health and dental care services. | e. 2%-8% | ||||
| f. Attainment and improvement | |||||
| g. Annually | |||||
| 14. Medicare Shared Savings Program | a. Medicare ACOs | a. The full set of services furnished under Medicare Parts A and B. | a. Virtual, FFS | a. Yes, using the CMS-HCC model. Initially prospectively, but retrospectively adjusted. | a. Savings depend on overall quality score. Minimum savings rate and minimum losses rate that must at least be met to qualify for shared savings or repay shared losses. |
| b. ACO professionals (i.e., physicians and certain nonphysician practitioners). Involvement of PCP is mandatory. | b. Medicare FFS beneficiaries. Minimum population size of 5,000. | b. Historical spending in the past 3 years (with larger weights attached to more recent years), trended forward by the national growth rate. | b. ACOs can choose to accept one-sided risk (Track 1) or two-sided risk (Tracks 2 and 3). | b. Four quality domains: Patient/caregiver experience, care coordination/patient safety, preventive health, and at risk population. | |
| c. N/A | c. Attribution is based on where patients have received the plurality of primary care services in that year. Track 1 and 2: prospective attribution, with retrospective reconciliation. Track 3: prospective attribution. | c. At least three-year contract | c. Track 1 (50% of savings), Track 2 (60% of savings and 40% to 60% of losses), Track 3 (70% of savings and 40% to 75% of losses). Maximum share of savings payment capped at 10% (Track 1), 15% (Track 2), and 20% (Track 3) of spending target. | c. N/A | |
| d. Expenditures capped at 99th percentile of expenditure distribution | d. N/A | ||||
| e. N/A | e. N/A | ||||
| f. Attainment and improvement, relative to national Medicare FFS and Medicare Advantage percentiles. | |||||
| g. Annually | |||||
| 15. Next Generation ACO Model | a. ACOs that are experienced in coordination care for defined populations. | a. All services covered by Medicare Part A or Part B. | a. Both possible. Virtual, FFS or FFS and PMPM payment. Real, PMPM payment equal to percentage FFS reduction or capitation. | a. Yes, using the CMS-HCC model. Initially prospectively, but retrospectively adjusted. | a. Share of savings is conditional on quality; losses are independent. In addition, the quality score is used in determining the discount applied to the spending target. |
| b. Participants (i.e., PCPs aligned with ACO), preferred providers (e.g., specialists, hospitals, home health facilities), and all other Medicare providers (no formal link between these providers and the model). | b. Medicare FFS beneficiaries | b. Historical spending trended forward by the national growth rate and Medicare geographic pricing factors. | b. Two-sided risk | b. 31 measures on 4 domains with equal weights: patient/caregiver experience, care coordination/patient safety, preventive health, and population at-risk of chronic diseases. | |
| c. N/A | c. Prospective attribution based on claims using provider lists, supplemented with possibility for beneficiaries to confirm a care relationship with an ACO. | c. Three-year contract, extendable to 5-year contract | c. Type A: Performance Years 1 and 3 80% and Performance Years 4 and 5 85%. Type B: 100%. Maximum share of savings payment is capped at 15% of spending target. | c. N/A | |
| d. Expenditures capped at 99th percentile of expenditure distribution. | d. N/A | ||||
| e. N/A. | e. N/A | ||||
| f. Attainment and improvement, relative to national Medicare FFS and Medicare Advantage percentiles. | |||||
| g. N/A | |||||
| 16. Partners for Kids Program | a. Pediatric ACO | a. All Medicaid care | a. Virtual. Three payment mechanisms: (a) FFS + P4P for independent providers contracted as member, (b) FFS for community providers not contracted as member, and (c) capitation for the academic personal from NCH. | a. Yes, age and sex | a. P4P, no link between quality and savings. P4P for contracted providers, not for non-members and hospital physicians. |
| b. Academic medical center with multiple facilities (NCH), primary and specialty physician practice groups and advanced practice professionals. | b. All Medicaid beneficiaries aged 0-18 years in central and south eastern Ohio. | b. N/A | b. Two-sided risk | b. Selection of HEDIS measures (n = 14), number of Medicaid members accepted per physician, completion of Maintenance of Certification program, and being recognized as PCMH. | |
| c. N/A | c. N/A | c. N/A | c. Full financial risk: 100% | c. Payment at provider group level | |
| d. N/A | d. Rewards | ||||
| e. N/A | e. N/A | ||||
| f. N/A | |||||
| g. N/A | |||||
| 17. ProvenHealth Navigator | a. Patient-centered medical homes (i.e., reengineered primary care practices) owned by private health insurer or private independent physician practices. | a. N/A | a. Virtual, FFS | a. Yes, information on which variables are used N/A. | a. P4P and savings conditional on meeting quality targets. |
| b. Medical home teams composed of PCPs, teams of specialists, physician’s assistants, nurses, case managers, pharmacists, social workers, and community health assistants. | b. Adult commercial population | b. Spending in the past 2 years, adjusted for medical cost inflation. | b. One-sided risk | b. Shared savings conditional on 10 measures regarding chronic illnesses, preventive care, care transition, patient/professional experience, and continuous improvement. For P4P, a more comprehensive set of HEDIS-measures is used. | |
| c. N/A | c. N/A | c. N/A | c. 50% | c. Measurement at primary care practices level. Payments split between providers and practice. | |
| d. N/A | d. Rewards | ||||
| e. N/A | e. 9% | ||||
| f. Improve and maintain quality | |||||
| g. Annually | |||||
| 18. Independence at home | a. Single primary care practices, other multidisciplinary teams or consortia (multiple primary care within a region) that are led by physicians or nurse practitioners (in total 14). | a. Care across all settings | a. Virtual, FFS | a. Yes, using the CMS-HCC and CMS ESRD model. To reflect functional impairment, frailty factors are used. | a. Savings conditional on meeting at least 3 of the 6 quality targets and surpassing savings threshold of 5%. |
| b. Physicians, nurses, physician assistants, pharmacists, social workers, and other staff required to deliver complete range of primary care services in home setting. | b. High-cost, frail Medicare beneficiaries with multiple chronic conditions and functional dependencies (e.g., feeding and walking). Minimum population size of 200. | b. Medicare FFS Parts A and B expenditures that would have been incurred by beneficiaries in the absence of the initiative, trended forward using set annual growth rate. | b. One-sided risk | b. Shared savings depending on proportion of 6 quality measures met: rates of emergency department and inpatient admissions for ambulatory care–sensitive conditions, 30-day readmission rate, contact with and visits to beneficiaries within 48 hours of hospital admission and discharge, completed medication reconciliation, and documentation of patient preferences. | |
| c. N/A | c. Attribution based on enrolment with PCP | c. Five-year contract | c. Ranging from 50% to 80%, with higher shares with higher quality. | c. Practice/consortium level | |
| d. Expenditures capped at 99th percentile of expenditure distribution. | d. N/A | ||||
| e. Claims associated with hurricane Sandy were not included. Indirect and graduate medical education and disproportionate share hospital payments excluded. | e. N/A | ||||
| f. N/A | |||||
| g. N/A |
Note. ACO = accountable care organization; BCBS = Blue Cross Blue Shield; CCO = coordinated care organization; CMS-HCC = Centers for Medicare and Medicaid Services’ hierarchical condition category risk-adjustment model; ER = emergency room; FFS = fee-for-service; HEDIS = Healthcare Effectiveness Data and Information Set; HMO = health maintenance organization; N/A = not available; NCH = Nationwide Children’s Hospital; PCP = primary care provider/physician; PMPM = per member per month; POS = point-of-service; PPO = preferred provider organization; P4P = pay-for-performance; VBP = value-based payment.
Key Design Features of the Global Base Payment Multidisciplinary Provider Group
In most initiatives, large, multispecialty provider groups act as main contractor. Typically, these groups comprise different types of physicians, other health care professionals (e.g., nurses, nurse practitioners, physician assistants, case managers, and social workers), and facilities such as hospitals, labs, and outpatient clinics. Although generally a broad range of provider types is involved, all initiatives have a particularly strong focus on substitution to primary care, which becomes evident from the explicit and central role of primary care physicians (PCPs) in all initiatives. We were unable to determine whether individual providers are being employed or subcontracted by the main contractor.
Within each group, providers are jointly accountable for the care for the attributed population with regard to quality and spending. Often, the groups are referred to as ACOs (#4, 10, 14, 15, 16), although terminology varies. Across the 18 initiatives, different types of provider groups take on the role of main contractor. Examples are groups of independent practices that have united themselves into organized networks (e.g., #9), multispecialty group practices that usually have a strong link with hospitals (e.g., #7), and integrated delivery systems including hospitals and a range of other care services like home health care, skilled nursing care, and physician services (e.g., #8). Note that within the same initiative, multiple group types may take on the role of main contractor (e.g., #6).
Cohesive set of care activities to a predefined population
Typically, the payment covers virtually the full continuum of primary and specialized medical services and prescription drugs, covered by the relevant benefit package. Information was lacking for #17. In some initiatives (e.g., #1, 8), the payment even covers a broader scope than medical care services only, including behavioral health care and long-term care. In case of the Medicare Shared Savings Program (#14), the Next Generation ACO Model (#15), and the Independence at Home Demonstration (#18), the payment covers the full set of services furnished under Medicare Parts A and B, including, among other services, inpatient care, physician care, outpatient care, skilled nursing facility care, home health agency care, hospice care, and durable medical equipment. Prescription drugs covered under Medicare Part D are not included in the payment of these initiatives.
Commercial initiatives (#2, 3, 4, 6, 9, 10, 11, 13, 17) often use payer affiliation, geographical catchment areas, or a combination of both as a ground for delineating the population. For example, the Alternative Quality Contract (AQC) (#4) only includes Blue Cross Blue Shield of Massachusetts’ members with a health maintenance organization (HMO) or point-of-service policy. The four Medicaid initiatives (#1, 8, 12, 16), automatically enroll all Medicaid beneficiaries in the region in the program. For the three Medicare initiatives (#14, 15, 18) the population consists of Medicare FFS beneficiaries (i.e., age 65 years and older), with the Independence at Home Demonstration (#18) focusing on the most expensive and frailest elders. One initiative (#16) delineates the population based on age, since the focus is on children only. Six of the 18 initiatives (#4, 5, 12, 13, 14, 18), impose a minimum population size per provider group to reduce the influence of stochastic variation (e.g., 5,000 in #4).
Information on the method used to attribute the population to provider groups was not available for five initiatives (#7, 8, 10, 16, 17). Of the other 13 initiatives, 6 use prospective attribution based on prior utilization (#1, 2, 6), affiliation with a provider group or PCP practice (#4, #9, #18), or region (#5). In contrast, three initiatives (#11, 12, 13) retrospectively attribute populations based on the plurality of utilization in the completed year. The three remaining initiatives (#3, 14, 15) use a mixture of assignment methods, depending on, for example, the specific financial risk “tracks” provider groups may opt for.
Fixed payment for a defined period of time
Fourteen initiatives incorporate “virtual” spending targets by building risk-sharing arrangements on the existing payment modality, most often a FFS-chassis. Three initiatives (#5, 8, 12) actually replaced existing payment systems with “real” global base payments in the shape of per-member-per-month (PMPM) payments. The remaining initiative (#15) uses both modalities; depending on the “track” chosen, providers are confronted with a “virtual” spending target or a “real” PMPM payment.
Information on the method for setting the payment/target was unavailable for eight initiatives (#1, 3, 5, 6, 7, 8, 11, 16). In 6 of the 10 other initiatives, historical spending in the prior year(s) is the basis for the payment/target. Advocate Care (#2) and the Medica Shared Savings Model (#13) use relative cost benchmarks as targets, that is, the average medical cost trend in the relevant market and the total cost of care of a peer group, respectively. The Independence at Home Demonstration (#18) uses Medicare FFS Part A and B expenditures that would have been incurred by beneficiaries in the absence of the initiative as the spending target. Gesundes Kinzigtal (#10) uses a combination of the German “standardized norm cost” (i.e., the average cost across all insurers, risk-adjusted using the German risk-equalization formula) for the specific provider group and spending during a reference period prior to the start of the initiative as a spending target. In nine initiatives, spending targets are trended forward using annual growth rates (#4, 5, 8, 9, 12, 14, 15, 16, 18).
Most initiatives rely on multiyear contracts, although information was missing for six initiatives. One initiative (#7) assumes a multiyear contract but does not specify the exact duration. Nine initiatives apply a contract of 2 to 5 years (#1, 2, 4, 6, 11, 12, 14, 15, 18), one initiative administers a 15-year contract that is extendable to 20 years (#5), and one initiative even applies an unlimited contract (#10), although the precise content of this contract is unclear.
Risk adjustment
In 14 initiatives, the payment/target is adjusted to the risk profile of the attributed population. For the other four initiatives (#1, 3, 5, 6), it was unclear whether or not risk adjustment is being applied. Among the initiatives using risk adjustment, information on the specific variables used is available for 11 initiatives. In one of these (#16), the risk-adjustment model includes only demographic information, while 10 other initiatives (#2, 4, 7, 9, 10, 12, 13, 14, 15, 18) use rather sophisticated models including demographic, socioeconomic, and diagnoses-based morbidity information. Typically, initiatives adopt existing “off-the-shelf” algorithms, originally developed in the context of risk adjustment for health plan payment. For example, the Medicare Shared Savings Program (#14) uses the CMS Hierarchical Condition Category (HCC) risk-adjustment model (Pope et al., 2004). This model funnels diagnostic codes into diagnoses and ranks them into condition categories, representing conditions with similar cost patterns.
Risk-mitigating measures
In eight initiatives providers accept upside risk only (#1, 3, 6, 9, 10, 13, 17, 18), while in eight other initiatives providers also assume downside risk (#2, 4, 5, 7, 8, 11, 15, 16). In the remaining two initiatives, provider groups are free to choose either a one-sided or two-sided contract (#14), or groups are accountable for upside risk only in the first year, and downside risk as well from the second year onward (#12). In initiatives in which providers also assume downside risk, the provider share of savings is larger compared with initiatives in which providers assume upside risk only. For example, in the Medicare Shared Savings Program (#14), providers assuming only upside risk receive 50% of accrued savings, while providers assuming both upside and downside risk receive 60% of savings.
With regard to the risk-sharing rate, information is available for 14 initiatives; for the other 4 initiatives, rates are not available/confidential (#1, 9, 10, 11). Risk-sharing rates for providers exceed 50% in six initiatives (#4, 8, 14, 15, 16, 18), while all other initiatives use a rate of maximally 50%. For example, in the Alzira Model (#5) the risk rate is maximally 7.5%, whereas this rate is 50% in the Anthem WellPoint ACO Arrangement (#6). One initiative (#7) adjusts the risk-sharing rate according to provider groups’ ability to influence cost in a particular category. For example, if a provider group is considered not to have any influence over mental health care utilization, the financial risk for this group in this particular domain is zero. For initiatives #12, 14, and 15, the risk-sharing rate increases over time. Typically, in two-sided contracts, the sharing rates for savings are higher than for losses.
The majority of identified VBP contracts include reinsurance provisions, although information is lacking for seven initiatives (#1, 2, 3, 5, 10, 16, 17). The AQC (#4), for example, applies overall cost trend corridors to protect provider groups against significant trends that affect the complete market. Another example is the Dutch Shared Savings Program (#9), in which providers are protected against high-cost cases by means of a cap of €22.500 (about $25.500) per patient per year. Finally, in all but one (#1) of the 10 initiatives for which information is available, some specific high-cost services are carved-out from the payment contract. Examples are dental care services (#9, 10, 12, 13), transplants (#2, 6, 12), behavioral health services and drugs (#4, 8, 12, 13), and long-term care (#8, 12). The Medicare initiatives (#14, 15, 18) exclude prescription drugs furnished under Medicare Part D from the payment.
Key Design Features of the Explicit Quality Incentives
Method of linking payment to quality
Across the 18 initiatives, we observe three main modalities of linking payment to quality. The most common modality (#1, 2, 4, 7, 8, 10, 11, 13, 15, 17) applies quality incentives as add-on payment in combination with a system in which the provider share of realized savings/losses depends on quality. In the AQC (#4), for example, providers passing higher “quality gates” receive both a higher bonus and a larger share of savings (or a smaller share of losses). In the second modality, savings/losses also depend on quality but there is no direct add-on payment for high quality scores (#3, 6, 9, 12, 14, 18). The last modality only involves add-on payments (#5, 16).
Quality measurement
The initiatives use a broad range of indicators. Clinical quality indicators are adopted most frequently (e.g., #16), although many initiatives incorporate other domains such as patient experience (e.g., #14), patient safety (e.g., #12), and avoidable hospital admissions (e.g., #3). Most initiatives predominantly use measures of process quality, with few initiatives also using outcome measures (e.g., #2). Often, the indicator set is based on a selection of nationally accepted measures (e.g., HEDIS [Healthcare Effectiveness Data and Information Set] measures in #11).
For 10 initiatives (#2, 3, 5, 6, 7, 11, 12, 13, 14, 15), we were unable to determine the level of measurement or payment. The remaining initiatives measure quality at the level of individual providers (#10) or provider groups (#1, 4, 8, 9, 16, 18). One initiative splits the savings between individual providers and the relevant group practice (#17).
Quality incentive structure
Among the 12 initiatives that implemented add-on payments for quality, eight initiatives only use rewards (#1, 4, 5, 7, 8, 11, 16, 17), while three also use penalties (#2, 13, 15). Information for #10 is missing. The maximum size of the add-on payment relative to the total payment is 10% (#2, 4, 10), but typically lower (e.g., 2% to 3% for #8 and 2% to 8% for #13). An exception is the Alzira Model in Spain (#5) in which the maximum payment size is 20%, although this percentage also includes on-call payments for providers. For initiatives #7, 11, and 16, information on payment size is lacking.
Across the 15 initiatives for which information is available, providers are typically rewarded for both achieving absolute targets and improving over time or relative to other providers. For example, in the Medicare Shared Savings Program (#14), providers share in realized savings only if they attain certain quality levels and show improvement relative to national Medicare FFS and Medicare Advantage. With regard to payment frequency, five initiatives pay on an annual basis (#4, 12, 13, 14, 17) and two on a quarterly basis (#1, 2). Information is lacking for other initiatives.
Effects on Value
Table 3 presents information on the effects on value of the five VBP initiatives that have been evaluated. For these initiatives, only effects on quality and spending are available (yet). In total, we included 24 studies, 20 of which pertain to either the AQC (#4) or the Medicare Shared Savings Program (#14). Partners for Kids (#16) was evaluated in two studies, while both Gesundes Kinzigtal (#10) and ProvenHealth Navigator (#17) were each evaluated in one study.
Table 3.
Effects of Five Identified VBP Initiatives That Have Been Formally Evaluated.
| Name initiative | References | Study design | Effects on resource use/spending | Effects on quality |
|---|---|---|---|---|
| 4. Alternative Quality Contract (AQC) | 1. Afendulis et al. (2014) | 1. DiD analyses of drug spending and utilization between 2006 and 2010. | 1. No significant effect on drugs utilization. | |
| 2. Barry et al. (2015) | 2. DiD analyses of probability of mental health service use, spending, HEDIS metrics for diabetes and cardiovascular conditions using 2006-2011 data. | 2. Intervention group is slightly less likely (−1.41%; p < .05) to use mental health services. No significant change in mental health spending, but a 1% annual decline in total health care spending for mental health services users. | 2. No significant improvements for diabetes or cardiovascular disease among enrollees with co-occurring mental health care use. For two measures (nephropathy monitoring and retinal exams) nonmental health users appear to have benefited more than mental health care users (annual change in probability of −2.90%; p < .01, and −2.57%; p < .05). | |
| 3. Chien et al. (2014) | 3. DiD analyses of quality and spending between 2006 and 2010 for children aged 0 to 21 years, including children with special health care needs (CSHCN). | 3. No significant effect on spending trends. | 3. Significant, positive effect on pediatric preventive care quality measures tied to P4P (+1.8% for CSHCN and +1.2% for non-CSHCN; p < .001). No significant changes for measures not tied to P4P. | |
| 4. Huskamp et al. (2016) | 4. DiD analyses of tobacco cessation service use using 2006-2011 data. | 4. Significant increases rates of tobacco cessation treatment use for the overall population (+0.13%; p < .0001). | ||
| 5. McWilliam, Landon, and Chernew (2013) | 5. DiD analyses of spending and quality between 2007 and 2010 for elderly FFS Medicare beneficiaries in Massachusetts served by 11 provider organizations entering the AQC in 2009 or 2010 versus beneficiaries served by other providers. | 5. Significant reductions in spending for Medicare beneficiaries in intervention (change of −$99 or −3.4% relative to an expected quarterly mean of $2.895; p = .02). | 5. Significant improvements of some measures (e.g., 3.1% for low-density lipoprotein cholesterol testing [p < .001] and 2.5% for cardiovascular disease [p < .001]), but no differential change for others. | |
| 6. Sharp et al. (2013) | 6. DiD analyses of emergency department (ED) visits using 2006-2009 data. | 6. No significant effect on ED use. | ||
| 7. Song et al. (2011) | 7. DiD analyses of spending and quality using 2006-2009 data. | 7. Smaller spending increase for intervention group, that is, $15.51 less per quarter (−1.9%; p = .007). | 7. Improved quality for chronic conditions in adults (p < .001) and pediatric care (p = .001) after 1 year, but not for adult preventive care. | |
| 8. Song et al. (2012) | 8. DiD analyses of spending using 2006-2010 data for the 2009 and 2010 intervention cohort. | 8. Savings of $22.58 over 2 years (−2.8%; p = .04). | 8. Improvements in measures for chronic care management (+3.7%; p < .001), adult preventive care (+0.3%; p = .008), and pediatric care (+0.3%; p < .001). | |
| 9. Song et al. (2013) | 9. DiD analyses of spending and utilization of several categories of medical technologies and quality using 2006-2010 data | 9. Higher use of colonoscopies for the intervention group in the first 2 years of the contract (+5.2%;p = .04). Decreases in spending on cardiovascular services in the first 2 years (−7.4%; p = .02), and on imaging services (−6.1%; p < .001). No effect in orthopedics | ||
| 10. Song et al. (2014) | 10. DiD analyses of spending and unadjusted DiD analyses for ambulatory process quality and outcome measures during the first 4 years (2009-2012) of the initiative for the 2009, 2010, 2011, and 2012 cohorts using 2006-2012 data | 10. Over the 4-year period lower spending growth for the intervention group (6.8% for the 2009 cohort; p < .001). The 2010/2011/2012 cohorts had savings of 8.8% (p < .001), 9.1% (p < .001), and 5.8% (p = .04) | 10. Measures of chronic disease management increased by 3.9%, and unadjusted performance in adult preventive care and pediatric care increased by 2.7% and 2.4% (p values are unavailable) compared to the HEDIS national average. The five outcome measures for patients with diabetes, patients with coronary artery disease, and patients with hypertension improved compared to the national and regional HEDIS scores (size of the effect and p values unavailable). | |
| 11. Song et al. (2017) | 11. DiD analyses of spending and quality using 2006-2012 data for enrollees in areas with lower and higher socioeconomic status. Outcome measures were measured only after the intervention | 11. No significant differences in spending between areas with lower versus higher socioeconomic status | 11. Process measures improved +1.2% per year more among individuals living in areas with lower versus higher socioeconomic status (p < .001). No significant differences in outcome measures. | |
| 12. Stuart et al. (2017) | 12. DiD analyses of substance use disorder service use, spending, and three HEDIS-based performance measures related to substance use disorder using 2006-2011 data | 12. No sizeable changes | 12. No sizeable changes. | |
| 10. Gesundes Kinzigtal | 1. Pimperl et al. (2017) | 1. Quasi-experimental design using propensity score matched control to evaluate the effect on population health using 2005-2013 data. Control group is a random sample of all members of the two insurers in the region Baden-Wurttemberg of 18 years and older. | Not available | 1. For the ACO intervention group age at time of death is on average 1.4 years higher compared to the control group but not significant, 639 fewer years of potential life were lost compared to the control group (p < .05), and the estimated survival time is approximately 7 days higher for beneficiaries participating in the program (significant; p value unavailable). |
| 14. Medicare Shared Savings Program | 1. Borza et al. (2019) | 1. DiD analyses of hospital readmission after common surgical procedures using 2010-2014 data. | 1. Significant reduction in readmissions for hospitals in the program (−0.52%; p = .021). | |
| 2. Busch, Huskamp, and McWilliams (2016) | 2. DiD analyses of mental health care spending, utilization, and quality using 2008-2013 data. | 2. No significant changes in mental health care spending and utilization. | 2. No significant changes in quality metrics. | |
| 3. Colla et al. (2016) | 3. DiD analyses of spending and high-cost institutional use using 2009-2013 data. | 3. Modest reductions in total spending (−1.3%; p < .001). Hospital and ED use reduced significantly by 1.3 (p < .05) and 3.0 (p < .01) events per 1,000 beneficiaries per quarter. | ||
| 4. Herrel et al. (2016) | 4. DiD analyses of 30-day mortality, complications, readmissions, and length of stay for patients undergoing a major surgical resection for various types of cancer using 2011-2013 data. | 4. No significant effect on perioperative outcome measures. | ||
| 5. McWilliam, Landon, Chernew, and Zaslavsky (2014) | 5. DiD analyses of patient experience using 2010-2013 data. | 5. Improvements in some patients experience measures (e.g., effect size for reports of timely access to care is 2.1 standard deviation of the ACO-level distribution, adjusted for trends; p = .02), but not (significantly) in others (e.g., overall ratings of care and physicians). | ||
| 6. McWilliam, Hatfield, Chernew, Landon, and Schwartz (2016) | 6. DiD analyses of spending and quality using 2009-2013 data. | 6. Significant reductions in spending for the 2012 cohort (−1.4%; p = .02), but not for the 2013 cohort. | 6. No significant differences in quality or use of low-value services for the majority of measures. | |
| 7. McWilliams et al. (2017) | 7. DiD analyses of post-acute spending and utilization using 2009-2014 data. | 7. Significant reductions in post-acute spending (−9.0%; p = .003 for 2012 ACO cohort and smaller for the 2013 and 2014 cohort). | ||
| 8. Winblad et al. (2017) | 8. DiD analyses of all-cause rehospitalizations from skilled nursing facilities using 2007-2013 data. | 8. Significant reduction in rehospitalization rate (−0.994%;p < .01). | ||
| 16. Partners for Kids Program | 1. Gleeson et al. (2016) | 1. DiD analyses of pediatric performance of primary care physicians using 2010-2013 data. | 1. Significant improvements in 8 of the 14 HEDIS measures for preventive care, chronic care, and acute care primary care services for the group of Nationwide Children Hospital physicians compared to incentivized physicians (“traditional” P4P). ORs favored the intervention group mainly in the immunization measures (range of OR of 0.34 with CI of [0.31, 0.37] for hepatitis vaccine to 0.86 with CI of [0.78, 0.95] for meningococcal vaccine). | |
| 2. Kelleher et al. (2015) | 2. Observational study of spending, growth rates, and quality using 2008-2013 data. Results for the PFK group is compared to Ohio Medicaid FFS and Ohio managed care (MC). | 2. Compared to both control groups, PMPM spending was significantly lower in 2008, and grew at a rate of $2.40 per year compared to $16.15 per year in the FFS group (p < .001) and $6.47 per year (p < .121) in the MC group. | 2. Significant improvement for gastroenteritis admission rate (−0.05 events/1,000; p = .000), pediatric quality acute composite (−0.03 events/1,000; p = .018), and pediatric quality overall composite (−0.05 events/1,000; p = .046). Significant declines in quality regarding diabetes short-term admission rates (+0.02 events/1,000; p = .027) and perioperative hemorrhage or hematoma rates (+3.99 events/1,000; p = .048). No significant differences on 10 other measures. | |
| 17. ProvenHealth Navigator | 1. Gilfillan et al. (2010) | 1. DiD analyses of hospital admissions, readmission rates, and the total cost of care using 2005-2008 data for Medicare Advantage patients at 11 intervention sites and 75 control groups | 1. Significant reduction in hospital admissions (−18%; p < .01) and readmissions (−36%; p = .02). Total cost of care decreased 7% (not significant) | Not available |
Note. VBP = value-based payment; DID = difference-in-differences; ACO = accountable care organization; PFK = Partners for Kids; FFS = fee-for-service; HEDIS = healthcare effectiveness data and information set; OR = odds ratio; CI = confidence interval.
Typically, studies adopted a difference-in-differences design investigating the effects of the initiative on both spending/resource use and quality of care. Initiative #10 has only been evaluated on its impact on quality and #17 only on its impact on spending. Usually, studies compared enrollees attributed to providers participating in the initiative with comparable enrollees attributed to providers not participating in the initiative, using pre- and post-intervention longitudinal data. Below, we summarize the main findings of the evaluation studies separately for the AQC, the Medicare Shared Savings Program, and the three other initiatives.
Alternative Quality Contract
Using 3 years of pre-intervention data and 4 years of post-intervention data, Song, Rose, and Safran (2014) investigated the impact of the AQC on medical spending growth and quality of care for the general population of Massachusetts AQC enrollees. The authors found that spending growth was significantly lower in the first 4 years of the contract for the four cohorts under study (2009-2012) compared with control states. For the 2009 cohort, for example, 6.8% savings were realized over the 4-year period (p < .001), mainly as a result of lower prices and volumes in the outpatient facility setting. Similar results were found for the other three cohorts. For the 2009 cohort, savings first exceeded quality incentive payments and investments in, for example, information technology in 2012. Regarding quality, Song et al. compared scores on 18 measures of ambulatory care processes and five outcome measures for chronic diseases to New England and national HEDIS averages. Quality improvements were generally significantly larger for the AQC cohorts. Two earlier studies conducted by largely the same researchers (Song et al., 2011; Song et al., 2012) found similar results regarding both spending and quality.
Nine other studies explored the effects on spending on and utilization of specific services and the effects in specific populations. McWilliams et al. (2013) found significant reductions in spending for FFS Medicare beneficiaries served by provider organizations in the AQC compared with beneficiaries served by providers not in the contract, suggesting a positive spillover effect. Uptake of tobacco cessation treatment slightly increased in the AQC population (Huskamp et al., 2016). Song, Fendrick, Safran, Landon, and Chernew (2013) provide evidence that providers participating in the contract used lower priced facilities and services more often than providers outside the contract. Barry et al. (2015), however, show that mental health care delivery was not meaningfully affected in the first years of the AQC. In addition, other studies did not find significant differences in pharmaceutical spending and utilization, pediatric health care spending or utilization, emergency department use, and substance use disorder treatment between intervention and control groups (Afendulis et al., 2014; Chien et al., 2014; Sharp, Song, Safran, Chernew, & Fendrick, 2013; Stuart et al., 2017). Finally, Song, Rose, Chernew, and Safran (2017) found no significant changes in spending between enrollees in the AQC in areas with lower and higher socioeconomic status.
With regard to quality, one study (Chien et al., 2014) found small but significant positive effects on pediatric preventive care measures, but no effects for diabetes, cardiovascular disease, and HEDIS measures related to substance use (Barry et al., 2015; Stuart et al., 2017). Two other studies (McWilliams et al., 2013; Song et al., 2017) observed a positive change for some measures—such as annual rates of low-density lipoprotein cholesterol and adult preventive care—but not for others.
Medicare Shared Savings Program
Eight studies evaluated the effect of the Medicare Shared Savings Program on spending/utilization and/or quality. Of the four studies evaluating the impact on spending/utilization, three found significant reductions relative to the control groups. Specifically, McWilliams et al. (2016) and Colla et al. (2016) found reductions in total spending of approximately 1% compared with beneficiaries served by providers not participating in the program. McWilliams et al. (2017) show a 9% reduction in post-acute spending and Colla et al. (2016) found a decrease of hospitalizations and emergency department visits of 1.3 and 3 events per 1,000 beneficiaries per quarter, respectively. One study (Busch et al., 2016) found no significant changes in spending and utilization of mental health care.
Of the six studies reporting on the impact on quality, three studies found insignificant effects (Busch et al., 2016; McWilliams et al., 2016; Winblad, Mor, McHugh, & Rahman, 2017). The three remaining studies found small but significant reductions of hospital readmissions after common surgical procedures (Borza et al., 2019) and significant improvements of some patient experience measures (McWilliams et al., 2014). Finally, Winblad et al. (2017) demonstrate a significant reduction of 1% in rehospitalization rates from skilled nursing facilities compared with the control group.
Other Initiatives
Four different studies evaluated Gesundes Kinzigtal, Partners for Kids Program, and ProvenHealth Navigator. Kelleher et al. (2015) demonstrate lower PMPM spending in the Partners for Kids Program compared with Ohio Medicaid FFS (p < .001) and Ohio Managed Care (p = .121) populations. A study investigating the effects of the ProvenHealth Navigator (Gilfillan et al., 2010) found that the number of hospital admissions and readmissions reduced by 18% (p < .01) and 36% (p = .02), respectively, although total cost of care did not change.
Regarding quality, three studies mainly found positive or null effects as a result of participation in the particular program relative to the control group. For example, Pimperl et al. (2017) show improvements for Gesundes Kinzigtal enrollees in potential years of life lost and estimated survival time, but found no significant effect in average age at time of death. In contrast, one study (Kelleher et al., 2015) provides evidence of significant declines in quality for 2 of the 15 measures used in the Partners for Kids Program: diabetes short-term admission rates and perioperative hemorrhage or hematoma rates.
Discussion
Summary and Discussion of Main Findings
In this article, we systematically identified and analyzed 18 VBP initiatives aiming at improving value in a broad sense. Specifically, our focus was on initiatives combining global base payments with payments explicitly linked to quality. Our analysis has resulted in a comprehensive overview of the possibilities in terms of operationalization of the two payment components and associated design features. Six main findings merit further discussion.
First, although all identified initiatives share the same two payment components, they differ considerably in the exact operationalization thereof. Specifically, we observed heterogeneity in the degree of risk sharing, the method of attributing populations to provider groups, the sophistication of the risk-adjustment methodology, and the way in which payment is linked to quality. Reasonable explanations for this heterogeneity are local preferences and contextual differences among settings. For example, in a setting in which providers lack experience with bearing downside risk, payers may choose to start with transferring upside risk only, allowing providers to gain this experience. After an adaption period, incentives for cost-conscious behavior can be intensified by transferring some downside risk as well.
Second, 15 of the 18 initiatives have been implemented in the United States. In part, this may be due to the adopted language restriction in this review. Another potential explanation can be found in the specific structure and history of the U.S. health care system. Specifically, it is likely that essential preconditions for a successful introduction of VBP are better fulfilled in the United States than in other countries, enabling a jump-start of VBP in the United States. Collaborative networks of multidisciplinary providers that are able and willing to take on the role of risk-bearing accountable group are historically embedded in the U.S. health care system (Enthoven, 2009). This might be partly the result of the integrated delivery systems that gained traction in the 1980s.
A third noteworthy finding is the strong reliance on primary care in all initiatives, which is evident from the explicit and central role of PCPs. In the Dutch Shared Savings Model, for example, groups of PCPs are accountable for the full continuum of primary and specialized care services. As gatekeepers, Dutch PCPs have at least some control over both primary and specialist care, legitimating their role as main contractor. The central focus on primary care across all initiatives is consistent with the global trend toward primary care–oriented systems. This trend is understandable given the many studies showing that areas with higher ratios of PCPs to population are associated with better health outcomes and lower total cost of health services compared with other areas (Starfield, Shi, & Macinko, 2005).
Fourth, the majority of identified initiatives adopt spending targets with risk-sharing arrangements built on existing (FFS) payment systems. This finding is consistent with the recommendation derived from a major VBP initiative in California to start with “virtual” targets and shift to “real” prospective payments at a later stage (Williams & Yegian, 2014). Virtual payments can potentially realize the same goal as real payments, without the regulatory and administrative burdens of replacing current payment and billing systems that could disrupt momentum. In addition, initially testing the model using virtual payments offers the possibility of developing a reliable benchmark from which the fixed payment level can be reasonably negotiated (Williams & Yegian, 2014). However, the incentives emanating from virtual payments may be perceived as weaker than those from real prospective payments (Struijs, Hayen, & van der Swaluw, 2018). Thus, although virtual payments can be a practical first step, moving away from FFS should remain a priority (De Bakker et al., 2012; Williams & Yegian 2014).
Fifth, most initiatives apply some form of risk adjustment and incorporate risk-mitigating measures in their payment contracts. This contributes to fairness in payment, reduced incentives for risk selection, and protection against excessive random variation in spending. Apparently, the importance of these two VBP design features is not only recognized in theory (Ash & Ellis, 2012; Cattel et al., 2018) but also in practice. Regarding risk adjustment, initiatives typically use existing diagnoses-based algorithms that were originally developed in the context of health plan payment. Although this may be an efficient and pragmatic approach that could serve its purpose in the short run, in the longer run it seems preferable to customize the risk-adjustment algorithm to the specific purpose of paying providers (Ash & Ellis, 2012). This may be particularly relevant to prevent the introduction of new perverse incentives such as for manipulating the diagnoses-based morbidity information used in the risk-adjustment formula to maximize payment (Geruso & Layton, 2015; Landon & Mechanic, 2017; Markovitz et al., 2019).
Finally, our results indicate that VBP models as defined here have the potential to improve value and contribute to the provision of VBHC. Regarding the five initiatives that have been evaluated, studies generally demonstrate similar or reduced spending growth and equal or improved quality. In this respect, it is noteworthy that the Medicare Shared Savings Program excludes prescription drugs from the VBP contract. Since prescription drugs account for a substantial proportion of total health care spending, it is possible that this initiative did not fully reach its potential for value improvement.
Our findings are consistent with results found for ACOs in the United States that indicate no association between ACO implementation and worsened health outcomes (Kaufman, Spivack, Stearns, Song, & O’Brien, 2019). In addition. Our findings correspond well with the results of a recent review of outcome-based P4P initiatives, which found favorable effects only when P4P was combined with global base payments (Vlaanderen et al., 2019). Conversely, our findings are in contrast with results from prior reviews on the effects of P4P, which did not find convincing evidence for P4P being (cost-)effective in improving value when the underlying, flawed base payment system is left intact (e.g., Eijkenaar, Emmert, Scheppach, & Schöffski, 2013; Mendelson et al., 2017; Vlaanderen et al., 2019). A possible explanation for the latter is that P4P typically concerns a relatively small part of the total provider payment, whereas initiatives included in this article focus on reform of the total payment system. Finally, our finding that quality does at least not seem to have deteriorated, suggests that quality—as operationalized by the chosen indicators—did not suffer from the adopted global base payments in VBP. This is in contrast with the widespread concern about the use of capitation payments in the context of HMOs (Dudley & Luft, 2001; R. H. Miller & Luft, 1997).
Limitations and Implications
Our findings should be interpreted in the light of several limitations. First, as any systematic review, this study suffers from publication bias. Second, it is possible that we missed relevant VBP initiatives as a consequence of our search strategy, specifically the restriction to articles/documents written in English or Dutch. In addition, we excluded multiple potentially relevant initiatives due to insufficient information. For example, we expect that long-standing integrated delivery systems such as Kaiser Permanente and Cleveland Clinic also adopt relevant VBP models, but since specific information on the payment structure is lacking, we could not include them. Overall, maximally twice as much VBP initiatives could have been included in this review, had sufficient information been available. Third, we were not always able to describe all relevant design features of each included initiative. In particular, information was often unavailable on the attribution methods, methods of setting the payment/target, internal payment contracts, contract duration, risk-mitigating measures, and quality incentive structure. Fourth, the overrepresentation of U.S. initiatives limits the generalizability of our findings to other settings. Finally, our findings regarding the effects on value are based studies evaluating only 5 of the 18 initiatives, with 20 of the 24 included evaluation studies pertaining to 2 initiatives: The Alternative Quality Contract and the Medicare Shared Savings Program. Moreover, the effects found in these studies are unlikely to reflect the impact of payment reform exclusively. This is because VBP is typically part of a broader approach to value improvement including other interventions that are implemented simultaneously, like structured performance feedback and public reporting.
In addition to the implications mentioned in the section “Summary and Discussion of Main Findings,” the results of this review have two other implications for research and policy. First, from both a research and a policy perspective, the design of VBP models are ideally documented more carefully in the future. Furthermore, it is important that VBP implementation goes hand-in-hand with rigorous evaluation. This is expected to result in important insights with regard to VBP design and the link with effectiveness, enabling others to learn from prior experiences. As this review shows, few initiatives have been subject to rigorous evaluation. Hence, little is still known about the effects in general, let alone about the impact of specific design choices on value. Moreover, the long-term impact of VBP is often not assessed, even though the gains from specific interventions such as investments in prevention are expected to emerge only after a longer period of time. The only two initiatives for which effects in the longer run are available confirm this statement. For example, net savings were generated only after 4 years in the AQC (Song et al., 2014).
Second, policy makers pursuing VBHC should keep in mind that although payment reform is an invaluable element in this process, it is not the only relevant factor. Other financial and nonfinancial interventions on both the supply- and demand-side of the market are likely to be important for the success of VBHC as well. Examples are a joint IT-infrastructure, physician leadership, performance monitoring with structured feedback, and public reporting (McClellan, McKethan, Lewis, Roski, & Fisher, 2010; Phipps-Taylor & Shortell, 2016; Robinson, 2001; Shortell & Casalino, 2010). Consistent with the recommendation by Roland and Campbell (2014) that P4P needs to be combined with other improvement strategies to produce sustained improvements, implementing VBP while disregarding other relevant factors is unlikely to materially affect value. The successful AQC, for example, embraced a multifaceted improvement strategy by offering technical support for participating provider groups parallel to the intervention of payment reform (Chernew, Mechanic, Landon, & Safran, 2011). The role of other value-adding aspects and the interplay with VBP is an interesting avenue for future research.
Conclusion
In the coming years, VBP models stimulating value in a broad sense will likely continue to gain ground, as the quest toward VBHC proceeds. This article demonstrates that VBP models consisting of global base payments combined with explicit quality incentives are operationalized in practice in various ways. In addition, our results show that this particular VBP model has the potential to improve value and contribute to VBHC. Going forward, this article may serve as inspirational material for those interested in developing new or improving on existing VBP models.
Supplemental Material
Supplemental material, Appendix for Value-Based Provider Payment Initiatives Combining Global Payments With Explicit Quality Incentives: A Systematic Review by Daniëlle Cattel and Frank Eijkenaar in Medical Care Research and Review
Acknowledgments
The authors are grateful to Erik Schut, two anonymous reviewers, and Tamara Konetzka for their helpful comments on earlier drafts of this article. In addition, our sincere thanks to Wichor Bramer for assistance with developing the search string and to Richard Heijink, Lieven Annemans, Maria Trottmann, Thomas McGuire, and Noaki Ikegami for validating our list of included VBP initiatives.
The VBP model as described in this section shows similarities with the global capitation payment model traditionally used by Health Maintenance Organizations (HMOs). In both models, provider groups receive a fixed payment for the provision of a comprehensive set of care activities for a predefined population, with the goal to increase efficiency by shifting financial risk to providers (Frakt & Mayes, 2012). However, both models differ in two important respects, specifically meant to address the concerns that were often raised against HMOs and global capitation: underprovision and quality skimping (section “The Rationale of Global Base Payments in Combination With Explicit Quality Incentives”; Frakt & Mayes, 2012). First, under VBP, providers and payer share financial risk, while HMOs typically use full capitation models that involve much more financial risk for providers. Second, under VBP, total compensation is partly dependent on quality performance, while in HMOs this was often not the case or only to a relatively limited extent (Frakt & Mayes, 2012). Thus, the VBP model takes advantage of the benefits of traditional capitation, while trying to avert its main disadvantages.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Daniëlle Cattel  https://orcid.org/0000-0001-6759-7874
https://orcid.org/0000-0001-6759-7874
References
- Afendulis C., Fendrick M., Song Z., Landon B. E., Safran D. G., Mechanic R. E., Chernew M. E. (2014). The impact of global budgets on pharmaceutical spending and utilization: early experience from the Alternative Quality Contract. Inquiry, 51, 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G. F., Weller W. E. (1999). Methods of reducing the financial risk of physicians under capitation. Archives of Family Medicine, 8, 149-155. [DOI] [PubMed] [Google Scholar]
- Ash A. S., Ellis R. P. (2012). Risk-adjusted payment and performance assessment for primary care. Medical Care, 50, 643-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry C. L., Stuart E. A., Donohue J. M., Greenfield S. F., Kouri E., Duckworth K., . . . Huskamp H. A. (2015). The early impact of the “Alternative Quality Contract” on mental health service use and spending in Massachusetts. Health Affairs, 34, 2077-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson R. A. (2010). Shared savings program for accountable care organizations: A bridge to nowhere? American Journal of Managed Care, 16, 721-726. [PubMed] [Google Scholar]
- Berwick D. M., Hackbarth A. D. (2012). Eliminating waste in US health care. JAMA Journal of the American Medical Association, 307, 1513-1516. [DOI] [PubMed] [Google Scholar]
- Berwick D. M., Nolan T. W., Whittington J. (2008). The triple aim: Care, health, and cost. Health Affairs, 27, 759-769. [DOI] [PubMed] [Google Scholar]
- Borza J., Oerline M. K., Skolarus T. A., Norton E. C., Dimick J. B., Jacobs B. L., . . . Hollenbeck B. K. (2019). Association between hospital participation in Medicare Shared Savings Program accountable care organizations and readmission following major surgery. Annals of Surgery, 269, 873-878. doi: 10.1097/SLA.0000000000002737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell S. M. (2015). Setting value-based payment goals: HHS efforts to improve U.S. health care. New England Journal of Medicine, 372, 897-899. [DOI] [PubMed] [Google Scholar]
- Busch A. B., Huskamp H. A., McWilliams J. M. (2016). Early efforts by Medicare accountable care organizations have limited effect on mental illness care and management. Health Affairs, 25, 1247-1256. [DOI] [PubMed] [Google Scholar]
- Busse R., Stahl J. (2014). Integrated care experiences and outcomes in Germany, the Netherlands, and England. Health Affairs, 33, 1549-1558. [DOI] [PubMed] [Google Scholar]
- Campbell S. M., Reeves D., Kontopantelis E., Sibbald B., Roland M. (2009). Effects of pay for performance on the quality of primary care in England. New England Journal of Medicine, 361, 368-378. [DOI] [PubMed] [Google Scholar]
- Cattel D., Eijkenaar F., Schut F. T. (2020). Value-based provider payment: Towards a theoretically preferred design. Health Economics, Policy and Law, 15, 94-112. doi: 10.1017/S1744133118000397 [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. (2018). Next Generation ACO Model. Retrieved from https://innovation.cms.gov/initiatives/Next-Generation-ACO-Model/
- Chee T. T., Ryan A. M., Wasfy J. H., Borden W. B. (2016). Current state of value-based purchasing programs. Circulation, 133, 2197-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernew M. E., Mechanic R. E., Landon B. E., Safran D. G. (2011). Private-payer innovation in Massachusetts: The “Alternative Quality Contract.” Health Affairs, 30, 51-61. [DOI] [PubMed] [Google Scholar]
- Chien A. T., Song Z., Chernew M. E., Landon B. E., McNeil B. J., Safran D. G., Schuster M. A. (2014). Two-year impact of the Alternative Quality Contract on pediatric health care quality and spending. Pediatrics, 133, 96-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson J. B., Conrad D. (2011). Provider payment and incentives. In Glied S., Smith P. (Eds.), The Oxford handbook of economics (pp. 624-648). New York, NY: Oxford University Press, Belknap Press. [Google Scholar]
- Colla C. H., Lewis V. A., Kao L. S., O’Malley A. J., Chang C. H., Fisher E. S. (2016). Association between Medicare accountable care organization implementation and spending among clinically vulnerable beneficiaries. Journal of the American Medical Association Internal Medicine, 176, 1167-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad D. A. (2015). The theory of value-based payment incentives and their application to health care. Health Services Research, 50, 2057-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad D. A., Grembowski D., Hernandez S. E., Lau B., Marcus-Smith M. (2014). Emerging lessons from regional and state innovation in value-based payment reform: balancing collaboration and disruptive innovation. Milbank Quarterly, 92, 568-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad D. A., Perry L. (2009). Quality-based financial incentives in health care: Can we improve quality by paying for it? Annual Review of Public Health, 30, 357-371. [DOI] [PubMed] [Google Scholar]
- Cutler D. M., Ghosh K. (2012). The potential for cost savings through bundled episode payments. New England Journal of Medicine, 366, 1075-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bakker D. H., Struijs J. N., Baan C. B., Raam J., de Wildt J. E., Vrijhoef H. J., Schut F. T. (2012). Early results from adoption of bundled payment for diabetes care in the Netherlands show improvement in care coordination. Health Affairs, 31, 426-433. [DOI] [PubMed] [Google Scholar]
- Deci E. L., Koestner R., Ryan R. M. (1999). A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychological Bulletin, 125, 627-668. [DOI] [PubMed] [Google Scholar]
- Donabedian A. (1988). The quality of care: How can it be assessed? JAMA Journal of the American Medical Association, 260, 1743-1748. [DOI] [PubMed] [Google Scholar]
- Dudley R. A., Luft H. S. (2001). Managed care in transition. New England Journal of Medicine, 344, 1087-1092. [DOI] [PubMed] [Google Scholar]
- Eggleston K. (2005). Multitasking and mixed systems for provider payment. Journal of Health Economics, 24, 211-223. [DOI] [PubMed] [Google Scholar]
- Eijkenaar F. (2013. a). Key issues in the design of pay-for-performance programs. European Journal of Health Economics, 14, 117-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkenaar F. (2013. b). Pay-for-performance for healthcare providers: Design, performance measurement, and (unintended) effects (Doctoral dissertation). Erasmus University, Rotterdam, Netherlands. [Google Scholar]
- Eijkenaar F., Emmert M., Scheppach M., Schöffski O. (2013). Effects of pay-for-performance in health care: A systematic review of systematic reviews. Health Policy, 10, 115-130. [DOI] [PubMed] [Google Scholar]
- Eijkenaar F., Schut F. T. (2015). Uitkomstenbekostiging in de zorg: een (on)begaanbare weg? [Outcome funding in health care: An (un) passable road?]. Rotterdam, Netherlands: Erasmus University. [Google Scholar]
- Ellis R. P., Miller M. M. (2008). Provider payment methods and incentives. In Heggenhougen H. K., Quah S. R. (Eds.), International encyclopaedia of public health (pp. 395-402). Amsterdam, Netherlands: Elsevier. [Google Scholar]
- Enthoven A. C. (2009). Integrated delivery systems: The cure for fragmentation. American Journal of Managed Care, 15 (10 Suppl.), S284-S290. [PubMed] [Google Scholar]
- Frakt A. B., Mayes R. (2012). Beyond capitation: How new payment experiments seek to find the “sweet spot” in amount of risk providers and payers bear. Health Affairs, 31, 1951-1958. [DOI] [PubMed] [Google Scholar]
- Frederick S., Loewenstein G., O’Donoghue T. (2002). Time discounting and time preference: A critical review. Journal of Economic Literature, 40, 351-401. [Google Scholar]
- Gaynor M., Rebitzer J. B., Taylor L. J. (2004). Physician incentives in health maintenance organizations. Journal of Political Economy, 112, 915-931. [Google Scholar]
- Geruso M., Layton T. (2015). Upcoding: Evidence from Medicare on squishy risk adjustment (NBER Working Paper No. 21222). Retrieved from http://www.nber.org/papers/w21222.pdf [DOI] [PMC free article] [PubMed]
- Gilfillan R. J., Tomcavage J., Rosenthal M. B., Davis D. E., Graham J., Roy J. A., . . . Steele G. D. (2010). Value and the medical home: effects of transformed primary care. American Journal of Managed Care, 16, 607-614. [PubMed] [Google Scholar]
- Gleeson S., Kelleher K., Gardner W. (2016). Evaluating a pay-for-performance program for Medicaid children in an accountable care organization. JAMA Pediatrics, 170, 259-266. [DOI] [PubMed] [Google Scholar]
- Gosden T., Forland F., Kristiansen I. S., Sutton M., Leese B., Giuffrida A., . . . Pedersen L. (2000). Capitation, salary, fee-for-service and mixed systems of payment: Effects on the behaviour of primary care physicians. Cochrane Database on Systematic Reviews, (3), CD002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrel L. A., Norton E. C., Hawken S. R., Ye Z., Hollenbeck B. K., Miller D. C. (2016). Early impact of Medicare accountable care organizations on cancer surgery outcomes. Cancer, 122, 2739-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Green S. (Eds.). (2011). Cochrane handbook for systematic reviews of interventions. London, England: Wiley. [Google Scholar]
- Holmstrom B., Milgrom P. (1991). Multitask principal-agent analyses: Incentive contracts, asset ownership, and job design. Journal of Law, Economics, & Organization, 7, 24-52. [Google Scholar]
- Huskamp H. A., Greenfield S. F., Stuart E. A., Donohue J. M., Duckworth K., Kouri E. M., . . . Barry C. L. (2016). Effects of global payment and accountable care on tobacco cessation service use: An observational study. Journal of General Internal Medicine, 31, 1134-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey P. S., Ridgely M. S., Rosenthal M. B. (2011). The PROMETHEUS bundled payment experiment: Slow start shows problems in implementing new payment models. Health Affairs, 30, 2116-2124. [DOI] [PubMed] [Google Scholar]
- Iezzoni L. I. (ed.). (2003). Risk adjustment for measuring health care outcomes. Chicago: Health Administration Press. [Google Scholar]
- Institute of Medicine. (2001). Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Jegers M., Kesteloot K., de Graeve D., Gilles W. (2002). A typology for provider payment systems in health care. Health Policy, 60, 255-273. [DOI] [PubMed] [Google Scholar]
- Kaufman B. G., Spivack B. S., Stearns S. C., Song P. H., O’Brien E. C. (2019). Impact of accountable care organizations on utilization, care, and outcomes: A systematic review. Medical Care Research and Review, 76, 255-290. doi: 10.1177/1077558717745916 [DOI] [PubMed] [Google Scholar]
- Kelleher K. J., Cooper J., Deans K., Carr P., Brilli R. J., Allen S., Gardner W. (2015). Cost savings and quality of care in a pediatric accountable care organization. Pediatrics, 135, e582-e589. [DOI] [PubMed] [Google Scholar]
- Landon B. E., Mechanic R. E. (2017). The paradox of coding: Policy concerns raised by risk-based provider contracts. New England Journal of Medicine, 377, 1211-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A. A., Hollingsworth J. M., Ayanian J. Z., Norton E. C., Moloci N. M., Yan P. L., Ryan A. M. (2019). Risk adjustment in Medicare ACO program deters coding increases but may lead ACOs to drop high-risk beneficiaries. Health Affairs, 38, 253-261. doi: 10.1377/hlthaff.2018.05407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques R. C., Berg S. (2011). Public-private partnership contracts: A tale of two cities with different contractual arrangements. Public Administration, 89, 1585-1603. [Google Scholar]
- McClellan M., McKethan A. N., Lewis J. L., Roski J., Fisher E. S. (2010). A national strategy to put accountable care into practice. Health Affairs, 29, 982-990. [DOI] [PubMed] [Google Scholar]
- McGuire T. G. (2000). Physician agency. In Culyer A. J., Newhouse J. P. (Eds.), Handbook of health economics (pp. 461-536). Amsterdam, Netherlands: Elsevier Science. [Google Scholar]
- McGuire T. G. (2011). Physician agency and payment for primary medical care. In Glied S., Smith P. (Eds.), The Oxford handbook of health economics (pp. 602-623). New York, NY: Oxford University Press. [Google Scholar]
- McWilliams J. M., Gilstrap L. G., Stevenson D. G., Chernew M. E., Huskamp H. A., Grabowski D. C. (2017). Changes in postacute care in Medicare Shared Savings Program. Journal of the American Medical Association Internal Medicine, 177, 518-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams J. M., Hatfield L. A., Chernew M. E., Landon B. E., Schwartz A. (2016). Early performance of accountable care organizations in Medicare. New England Journal of Medicine, 374, 2357-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams J. M., Landon B. E., Chernew M. E. (2013). Changes in health care spending and quality for Medicare beneficiaries associated with a commercial ACO contract. JAMA Journal of the American Medical Association, 310, 829-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams J. M., Landon B. E., Chernew M. E., Zaslavsky A. M. (2014). Changes in patients’ experiences in Medicare accountable care organizations. New England Journal of Medicine, 371, 1715-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanic R. E., Altman S. H. (2009). Payment reform options: Episode payment is a good place to start. Health Affairs, 28, w262-w271. [DOI] [PubMed] [Google Scholar]
- Mehrotra A., Hussey P. (2015). Including physicians in bundled hospital care payments: Time to revisit an old idea? JAMA Journal of the American Medical Association, 313, 1907-1908. [DOI] [PubMed] [Google Scholar]
- Mendelson A., Kondo K., Damberg C., Low A., Motúapuaka M., Freeman M., . . . Kansagara D. (2017). The effects of pay-for-performance programs on health, health care use, and processes of care: A systematic review. Annals of Internal Medicine, 166, 341-353. [DOI] [PubMed] [Google Scholar]
- Miller H. D. (2009). From volume to value: Better ways to pay for health care. Health Affairs, 28, 1418-1428. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Luft H. S. (1997). Does managed care lead to better or worse quality of care? Health Affairs, 16, 7-25. [DOI] [PubMed] [Google Scholar]
- Milstein R., Schreyögg J. (2016). Pay for performance in the inpatient sector: A review of 34 P4P programs in 14 OECD countries. Health Policy, 120, 1125-1140. [DOI] [PubMed] [Google Scholar]
- Moscucci M., Eagle K. A., Share D., Smith D., De Franco A. C., O’Donnell M., . . . Brown D. L. (2005). Public reporting and case selection for percutaneous coronary interventions: An analysis from two large multicenter percutaneous coronary intervention databases. Journal of the American College of Cardiology, 45, 1759-1765. [DOI] [PubMed] [Google Scholar]
- Mullen K. J., Frank R. G., Rosenthal M. B. (2010). Can you get what you pay for? Pay-for-performance and the quality of healthcare providers. RAND Journal of Economics, 41, 64-91. [DOI] [PubMed] [Google Scholar]
- Peikes D., Dale S., Ghosh A., Taylor E. F., Swankoski K., O’Malley A. S., . . . Brown R. S. (2018). The Comprehensive Primary Care Initiative: Effects on spending, quality, patients, and physicians. Health Affairs, 37, 890-899. [DOI] [PubMed] [Google Scholar]
- Phipps-Taylor M., Shortell S. M. (2016). More than money: Motivating physician behavior change in accountable care organizations. Milbank Quarterly, 94, 832-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimperl A., Schulte T., Mühlbacher A., Rosenmöller M., Busse R., Groene O., . . . Hildebrandt H. (2017). Evaluating the impact of an accountable care organization in population health: The quasi-experimental design of the German Gesundes Kinzigtal. Population Health Management, 20, 239-248. [DOI] [PubMed] [Google Scholar]
- Pope G. C., Kautter J., Ellis R. P., Ash A. S., Avanian J. Z., Iezzoni L. I., . . . Robst J. (2004). Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financing Review, 25, 119-141. [PMC free article] [PubMed] [Google Scholar]
- Porter M. E. (2009). A strategy for health care reform: Toward a value-based system. New England Journal of Medicine, 361, 109-112. [DOI] [PubMed] [Google Scholar]
- Porter M. E. (2010). What is value in health care? New England Journal of Medicine, 363, 2477-2481. [DOI] [PubMed] [Google Scholar]
- Quentin W., Geissler A., Wittenbecher F., Ballinger G., Berenson R., Bloor K., . . . Busse R. (2018). Paying hospital specialists: Experiences and lessons from eight high-income countries. Health Policy, 122, 473-484. [DOI] [PubMed] [Google Scholar]
- Ridgely M. S., de Vries D., Bozic K. J., Hussey P. S. (2014). Bundled payment fails to gain a foothold in California: The experiment of the IHA bundled payment demonstration. Health Affairs, 33, 1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. C. (2001). Theory and practice in the design of physician payment incentives. Milbank Quarterly, 79, 149-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland M., Campbell S. (2014). Successes and failures of pay for performance in the United Kingdom. New England Journal of Medicine, 370, 1944-1949. [DOI] [PubMed] [Google Scholar]
- Rose S., Zaslavsky A. M., McWilliams J. M. (2016). Variation in accountable care organization spending and sensitivity to risk adjustment: Implications for benchmarking. Health Affairs, 35, 440-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M. B., Dudley R. A. (2007). Pay-for-performance: Will the latest payment trend improve care? JAMA Journal of the American Medical Association, 297, 740-744. [DOI] [PubMed] [Google Scholar]
- Ryan A. M., Krinsky S., Maurer K. A., Dimick J. B. (2017). Changes in hospital quality associated with hospital value-based purchasing. New England Journal of Medicine, 376, 2358-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A., Liu M., Yong J. (2018). Financial incentives to encourage value-based health care. Medical Care Research and Review, 75, 3-32. [DOI] [PubMed] [Google Scholar]
- Sharp A. L., Song Z., Safran D. G., Chernew M. E., Fendrick M. A. (2013). The effect of bundled payment on emergency department use: Alternative quality contract effects after year one. Academic Emergency Medicine, 20, 961-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. (2003). Selection incentives in a performance-based contracting system. Health Services Research, 38, 535-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortell S. M. (2013). Bridging the divide between health and health care. JAMA Journal of the American Medical Association, 309, 1121-1122. [DOI] [PubMed] [Google Scholar]
- Shortell S. M., Casalino L. P. (2010). Implementing qualifications criteria and technical assistance for accountable care organizations. JAMA Journal of the American Medical Association, 303, 1747-1748. [DOI] [PubMed] [Google Scholar]
- Shortell S. M., Wu F. M., Lewis V. A., Colla C. H., Fisher E. S. (2014). A taxonomy of accountable care organizations for policy and practice. Health Services Research, 49, 1883-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg E. (1990). The structure of economics: A mathematical analysis. San Francisco, CA: McGraw-Hill. [Google Scholar]
- Song Z., Fendrick A. M., Safran D. G., Landon B. E., Chernew M. E. (2013). Global budgets and technology-intensive medical services. Healthcare (Amsterdam, Netherlands), 1, 15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Rose S., Chernew M. E., Safran D. G. (2017). Lower- versus higher-income populations in the Alternative Quality Contract: Improved quality and similar spending. Health Affairs, 36, 74-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Rose S., Safran D. G. (2014). Changes in health care spending and quality 4 years into global payment. New England Journal of Medicine, 371, 1704-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Safran D. G., Landon B. E., He Y., Ellis R. P., Mechanic R. E., . . . Chernew M. E. (2011). Health care spending and quality in year 1 of the Alternative Quality Contract. New England Journal of Medicine, 365, 909-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Safran D. B., Landon B. E., Landrum M. B., He Y., Mechanic R. E., . . . Chernew M. E. (2012). The Alternative Quality Contract in Massachusetts, based on global budgets, lowered medical spending and improved quality. Health Affairs, 31, 1885-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starfield B., Shi L., Macinko J. (2005). Contribution of primary care to health systems. Milbank Quarterly, 83, 457-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel N., Maisey S., Clark A., Fleetcroft R., Howe A. (2007). Quality of clinical primary care and targeted incentive payments: an observational study. British Journal of General Practice, 57, 449-454. [PMC free article] [PubMed] [Google Scholar]
- Stokes J., Struckmann V., Kristensen S. R., Fuchs S., van Ginneken E., Tsiachristas A., . . .Sutton M. (2018). Towards incentivizing integration: A typology of payments for integrated care. Health Policy, 122, 963-969. [DOI] [PubMed] [Google Scholar]
- Struijs J., Hayen A., van der Swaluw K. (2018, April 25). When designing bundled payments, don’t ignore the lessons of behavioural economics [Web log post]. Retrieved from https://www.healthaffairs.org/do/10.1377/hblog20180420.640240/full/
- Stuart E. A., Barry C. L., Donohue J. M., Greenfield S. F., Duckworth K., Song Z., . . . Huskamp H. A. (2017). Effects of accountable care and payment reform on substance use disorder treatment: Evidence from the initial three years of the Alternative Quality Contract. Addiction, 112, 124-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler R. H. (1981). Some empirical evidence on dynamic inconsistency. Economic Letters, 8, 201-207. [Google Scholar]
- Vlaanderen F. P., Tanke M. A., Bloem B. R., Faber M. J., Eijkenaar F., Schut F. T., Jeurissen P. P. T. (2019). Design and effects of outcome-based payment models in healthcare: A systematic review. European Journal of Health Economics, 20, 217-232. doi: 10.1007/s10198-018-0989-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T., Yegian J. (2014, August 5). Bundled payment: Learning from our failure [Web log post]. Retrieved from https://www.healthaffairs.org/do/10.1377/hblog20140805.040596/full/
- Winblad U., Mor V., McHugh J. P., Rahman M. (2017). ACO-affiliated hospitals reduced rehospitalizations from skilled nursing facilities faster than other hospitals. Health Affairs, 36, 67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynia M. K. (2009). The risks of rewards in health care: How pay-for-performance could threaten, or bolster, medical professionalism. Journal of General Internal Medicine, 24, 854-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G. J., Meterko M., Beckman H., Baker E., White B., Sautter K. M., . . . Burgess H. A. (2007). Effects of paying physicians based on their relative performance for quality. Journal of General Internal Medicine, 22, 872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix for Value-Based Provider Payment Initiatives Combining Global Payments With Explicit Quality Incentives: A Systematic Review by Daniëlle Cattel and Frank Eijkenaar in Medical Care Research and Review