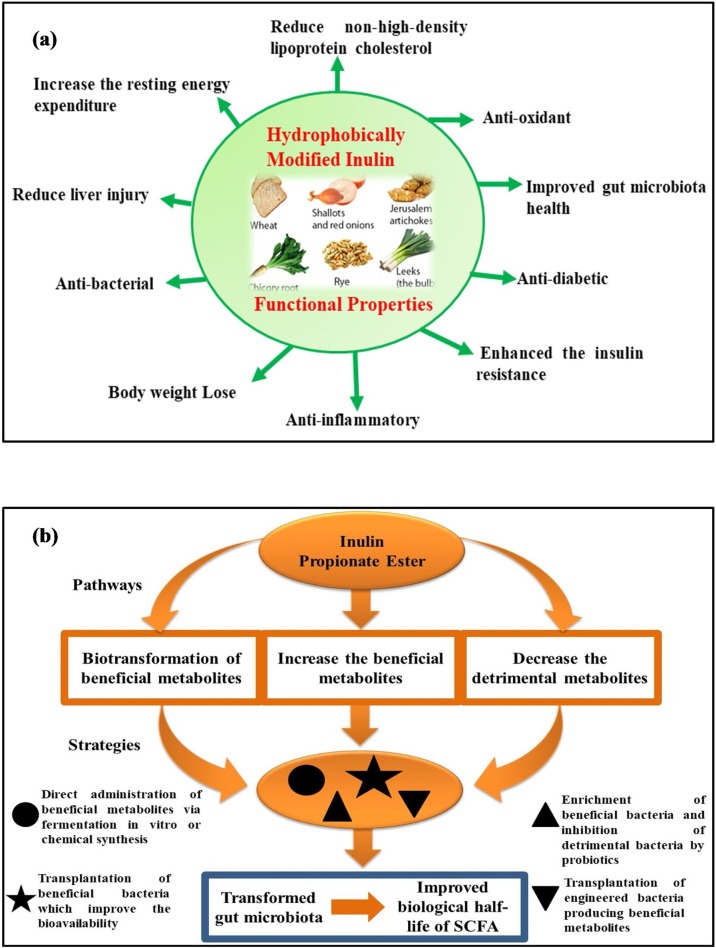
Graphical abstract
Keywords: Encapsulation, Hydrophobically modified inulin, Controlled release, Emulsions, Gut microbiota
Highlights
-
•
Inulin is a substance found in a wide variety of fruits, vegetables, and herbs.
-
•
Inulin was modified by physical and chemical means to improve functionality.
-
•
HMI has been used in the stability of emulsions and suspensions.
-
•
SCFAs inulin esters have transformed the gut microbiota and improved the bioavailability of SCFAs.
-
•
HMI based bioconjugates, hydrogel, and nanomicelles were used as a controlled release of drugs and vaccines.
Abstract
Over the past few years, hydrophobically modified inulin (HMI) has gained considerable attention due to its multitudinous features. The targeted release of drugs remains a subject of research interest. Moreover, it is important to explore the properties of short-chain fatty acids (SCFAs) inulin esters because they are less studied. Additionally, HMI has been used to stabilize various dispersion formulations, which have been observed to be safe because inulin is generally recognized as safe (GRAS). However, the results regarding HMI-based dispersion products are dispersed throughout the literature. This comprehensive review is discussed the possible limitations regarding SCFAs inulin esters, real food dispersion formulations, and HMI drugs. The results revealed that SCFAs inulin esters can regulate the human gut microbiota and increase the biological half-life of SCFAs in the human body. This comprehensive review discusses the versatility of HMI as a promising excipient for the production of hydrophobic drugs.
1. Introduction
Inulin, which was discovered as a fructan-type oligosaccharide, is distributed in more than 36,000 vegetables and herbs. Jerusalem artichokes, leeks, oats, onion, and garlic are abundant sources of inulin, while it is obtained commercially from members of the Asteraceae family, such as chicory (Afinjuomo et al., 2019; Kokubun, Ratcliffe, & Williams, 2018). Inulin is made up of d-fructose units that are linked by β-2,1 glycosidic bonds with a wide range of degrees of polymerization (between 2 and 60) and commonly combined with a glucose residue at the terminus (López-Molina et al., 2015). In addition, inulin has received generally recognized as safe (GRAS) status by the Food and Drug Administration (FDA) due to its several outstanding properties including biodegradability, renewability, nontoxicity, etc., compared to those of many other polysaccharides (Afinjuomo et al., 2019). It is an undigested polysaccharide and is classified as a dietary fiber that escapes small intestinal digestion but is degraded (partial or complete) by colonic microbiota. Its degradation subsequently produces short-chain fatty acids (SCFAs), which may improve human health (Tripodo and Mandracchia, 2019). Inutec®SP1 is a commercially available graft copolymer that is synthesized by the reaction of inulin with dodecyl isocyanate in an aprotic solvent to obtain inulin dodecyl carbamate (a; 2009b; Exerowa et al., 2007; Gotchev, Kolarov, Levecke, Khristov, & Exerowa, 2007; Nestor et al., 2007; Stevens, Meriggi, Peristeropoulou et al., 2001) and has been widely used to provide steric stabilization for various dispersions, improve the biological half-life of SCFAs and control the release of drugs (Chambers et al., 2019; Tadros, 2017; Tripodo et al., 2019; Tripodo et al., 2015a; Tripodo et al., 2015b). Moreover, various types of inulin derivatives have been produced by the reaction of inulin with fatty acid methyl esters (FAMEs), fatty acid chlorides, alkyl epoxides, and alkyl isocyanates (Exerowa and Platikanov, 2009a; Exerowa et al., 2009b; Gochev et al., 2011; Khristov & Czarnecki, 2010; Stevens, Meriggi, & Booten, 2001a; Stevens et al., 2001b). In consideration of environmental issues with the development of industrialization, a green methodology has been established to synthesize different types of inulin derivatives with varying alkenyl chain lengths and varying degrees of substitution (DSs) by using alkenyl succinic anhydrides in an aqueous environment under mild alkaline conditions (Han, Ratcliffe, & Williams, 2015; Han, Ratcliffe, & Williams, 2017; Kokubun, Ratcliffe, & Williams, 2013; Kokubun, Ratcliffe, & Williams, 2015; Kokubun et al., 2018; Morros, Levecke, & Infante, 2010; Morros, Levecke, & Infante, 2011). It was revealed that these types of inulin derivatives adsorbed at the liquid-liquid interface, solid-liquid interface, and air-water interface and produced micellar-like structures in the solution above a critical concentration. Moreover, the HMI derivatives contributed excellent encapsulation efficiency, reaching up to 100 %, and near-spherical drug-loaded micellar aggregates of ∼250 nm, resulting in prolonged drug and vaccine release in the human body (Han et al., 2020; Kesharwani, Dachineni, Bhat, & Tummala, 2019; Walz, Hagemann, Trentzsch, Weber, & Henle, 2018a). The commercially available Inutec®SP1 has been widely used for the targeted release of anticancer drugs, particularly paclitaxel (Muley, Kumar, El Kourati, Kesharwani, & Tummala, 2016). Furthermore, Tripodo, Chlapanidas et al. (2015a) and Tripodo, Pasut et al. (2015) prepared INVITE bioconjugates with varying DS and designed a drug delivery system based on mesenchymal stromal cells (MSCs) for the therapy of neurodegenerative diseases, which obtained practical achievements regarding the drug delivery profile. In addition, HMI such as amine derivatives have been grafted with biotin, retinoic acid, and vitamin E to produce mucoadhesive micelles, which exhibit transcorneal permeation properties, as well as long-circulating carriers for receptor-mediated targeted drug delivery (Di Prima et al., 2017; Mandracchia et al., 2018). Studies have revealed that inulin serves as a promising transporter for colonic drug delivery because it is not digested in the stomach and small intestine (Wang et al., 2019). SCFAs play an important physiological role in combating colon-related diseases and altering gut microbiota compositions. Moreover, the amount of SCFAs can be regulated exogenously and endogenously (Xu, Zhu, Li, & Sun, 2020; Zhu et al., 2018). It was confirmed that the therapeutic value of exogenously administered SCFAs is limited due to the minimum biological half-life (Polyviou et al., 2016). Experimental studies have revealed that SCFA inulin esters, mainly inulin propionate ester (IPE), can enhance the biological half-life of SCFAs, which improves overall human health. In addition, a number of inulin derivatives have been widely produced by using different anhydrides to improve the antimicrobial abilities and antioxidant activities of inulin (Chen, Mi, Li, Dong, & Guo, 2020; Ren et al., 2012). HMI derivatives have been shown to minimize plant fungi that damage fruits and vegetable crops (6–48 %) worldwide, particularly in developing countries (Chen et al., 2018, 2019b; Li, Qiu, Tan, Gu, & Guo, 2017; Tripodo et al., 2019). Similarly, hydrophobic inulin derivatives have been shown to exhibit greater antioxidant activity than native inulin (Chen et al., 2017, 2019b). Based on the scientific literature, it has been observed that HMI derivatives were more studied as emulsion stabilizers than as foam and wetting film stabilizers. Furthermore, reports on the application of modified inulin in real food dispersions are scarce. Likewise, HMI derivative-mediated vaccines have been less elucidated than their drug counterparts. The functional properties of SCFA inulin esters have shown excellent findings, but systematic knowledge is scattered throughout the literature. Therefore, this comprehensive review summarizes the recent information on the use of HMI in the controlled release of drugs and functional foods as well as antimicrobial abilities and antioxidant activities. Additionally, the evaluation of emulsions, suspensions, and wetting films has been deeply discussed in this article.
2. Chemistry and synthesis of HMI
2.1. Background
Over the past few decades, keen attention has been paid to the chemical modification of inulin, which depends on the charge of the final products. Thus, Stevens, Meriggi, Booten et al. (2001) described the chemical modification of inulin, which was a great leap forward to develop novel industrial products. The chemical modification of inulin is classified into three types: anionic, cationic, and neutral modification (Stevens, Meriggi, Booten et al., 2001; Rogge and Stevens, 2004). Moreover, high-performance liquid chromatography (HPLC), Raman spectroscopy, nuclear magnetic resonance [(NMR) H-NMR, C-NMR] spectroscopy and Fourier transform infrared (FTIR) spectroscopy have been used to reveal the changes in the inulin conformation structure. The significant difference in the chemical modification of inulin can be divided according to the type of reaction medium and reaction conditions, and the most important is the anhydride type, which is combined on the inulin backbone. Initially, the graft copolymer, i.e., Inutec®SP1, has been synthesized commercially by using dodecyl isocyanate in an aprotic solvent (which can not donate protons) to obtain inulin dodecyl carbamate (Fig. 1 a). This graft copolymer has widely been used as an emulsifier due to the multipoint attachment of its particles or droplets and high degree of hydration, with more than 97 % purity of the end product (Exerowa et al., 2007, 2009b; Exerowa and Platikanov, 2009a; Gotchev et al., 2007; Nestor et al., 2007; Stevens, Meriggi, Booten et al., 2001; Tadros, 2017). Consequently, several authors have documented the modification of inulin by esterification, etherification, and carboxymethylation using fatty acid methyl esters (FAMEs), fatty acid acyl chlorides, alkyl epoxides, and alkyl isocyanates or by alkenyl succinic anhydrides, mainly in organic solvents and environmentally friendly aqueous solvents (Exerowa et al., 2009b; Exerowa and Platikanov, 2009a; Morros, Infante, & Pons, 2012, 2011; Morros et al., 2010a; Morros, Levecke, & Infante, 2010; Nestor et al., 2007; Stevens, Meriggi, Booten et al., 2001, 2001b; Gotchev et al., 2011; Khristov & Czarnecki, 2010; Zhu et al., 2018; Hartzell, Maldonado-Gómez, Yang, Hutkins, & Rose; Han et al., 2015, 2017; Kokubun et al., 2013). Thus, it is important to divide the chemical modification reactions of inulin based on the types of anhydride and types of reaction medium, which will be discussed below.
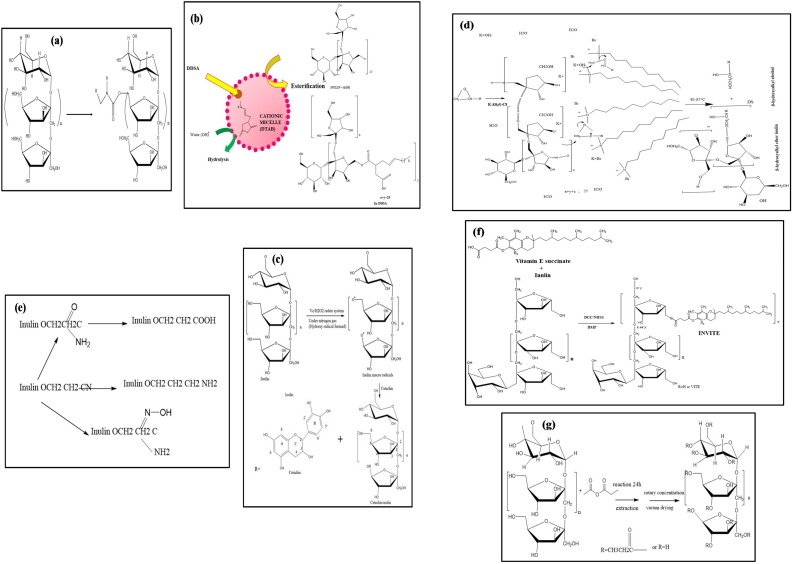
Fig. 1.
(b) Esterification of inulin, (a) Modification of Inutec®SP1 from inulin, (c) Coupling of inulin with catechin, (d) Synthesis of β-hydroxydodecyl inulin ether, (e) Synthesis of cyanoethyl inulin and its derivatives, (f) Synthesis of inulin vitamin E bioconjugates (g) Synthesis of inulin propionate ester.
2.2. Modification of inulin in aqueous solvent
There has been surging interest in modifying inulin in environmentally friendly solvents because environmentalists have expressed enormous concerns about the environmental impacts of chemical processes, particularly in the development of industrialization. A number of research groups have developed strategies to modify inulin ethers and inulin esters by using water as a solvent in the presence of different catalysts. However, in this organic chemical reaction, the production yield may be affected because two different chemical species participate in the chemical reaction, which have different polarities, such as a hydrophilic polymer and a hydrophobic reactant. Therefore, the rate of the reaction is essential to obtain specific end products with high DSs. For this purpose, different types of basic and acidic catalysts, including sodium hydroxide, potassium carbonate, 4-(dimethylamino) benzene, 4-(dimethylamino) pyridine, 4- (dimethylamino) benzaldehyde, ion-exchange resins, stearoyl chloride, acrylonitrile, sodium acetate, etc., were used to enhance the chemical reaction rate. Moreover, the basic ion-exchange resin could be used to obtain high DSs compared with the fundamental catalyst because it is not essential to neutralize before reaction.
2.3. Production of inulin esters in aqueous solvent
Recently, an environmentally friendly approach was used to synthesize novel inulin derivatives by the reaction of inulin with alkenyl succinic anhydrides [(octenyl succinic anhydride (OSA) and dodecenyl succinic anhydride (DDSA)] in an aqueous solution under mild alkaline conditions (Fig. 1b) (Kokubun et al., 2013). Overall, this environmentally friendly mechanism has exhibited excellent reaction efficiency ranging from 59 to 95 % for OSA-inulin derivative and DDSA-inulin derivative (Kokubun et al., 2013). Moreover, the results revealed that the reaction efficiency was too high for the OSA-inulin derivative compared to the DDSA-inulin derivative. Normally, the high reaction efficiency is very desirable. Furthermore, the same research group has produced different types of HMI derivatives (Han et al., 2017) and alkenylated inulin samples (OSA, DDSA, TDSA, HDSA, and ODSA) (Han et al., 2015). The former derivatives were synthesized using fatty acid acyl chlorides with varying alkyl chain lengths (C10-C16), while the latter samples were formed using alkenyl succinic anhydrides (ASAs) with a wide range of alkenyl chain lengths (C8-C18) in aqueous solution. Both types of compounds were characterized by NMR spectroscopy and FTIR spectroscopy, and the DS was calculated under the same reaction conditions (temperature, time) and with the same chemicals, washing steps, and end-product drying steps. The findings revealed that the alkenylated inulin samples were successfully modified with a high degree of substitution; thus, they can be used for the encapsulation of β-carotene as a natural biomaterial for pharmaceutical, nutraceutical, and personal care applications. Moreover, the DS was observed to decrease with increasing amounts of fatty acid acyl chlorides. Morros et al. (2011) found approximately similar results regarding the reaction efficiency, reaction time, and DS of pure and end products. The authors prepared DDSA-inulin derivative and OSA-inulin derivative through ASA in environmentally friendly surfactant aqueous media and aqueous media, respectively. The results revealed that the reaction time was noticeably decreased up to 1 h, obtaining a 65 % reaction efficiency using cationic surfactants such as dodecyl trimethylammonium bromide (DTAB). The profound differences in reaction efficiency may be due to the utilization of different anhydrides, catalysts, and experimental conditions. Polyviou et al. (2016) synthesized inulin propionate ester (IPE) by reaction of inulin with propionic anhydride in aqueous solution, with up to 70 % yield and a 1.25 % degree of esterification. Moreover, Liu, Lu, Kan, Wen and Jin (2014) grafted inulin with catechin by hydrogen peroxide and ascorbic acid in aqueous medium, which was utilized as a functional ingredient for patients with liver disease and diabetes (Fig. 1c).
2.4. Production of inulin ethers in aqueous solvent
In etherification, the fundamental catalyst typically used is sodium hydroxide, which is added in a sufficient amount to perform the chemical reaction and promote the hydroxylation of inulin. Initially, Tomecko and Adams (1923) described the etherification of inulin by the reaction of inulin with epichlorohydrin in basic aqueous solution. Later, Remon, Duncan and Schacht (1984) developed a method to explore inulin ethers by reacting allyl bromide in aqueous medium. However, the reaction efficiency was too low in these aqueous solutions. Therefore, evidence-based studies have focused on producing a neutral hydrophobic β-hydroxyalkyl inulin ether in environmentally friendly aqueous media with a high DS. It was confirmed that by using alkyl epoxides such as ethylene and propylene oxide, the reaction efficiency could be improved up to 70 %, while butyl epoxide or 1,2-hexyl epoxide exhibited less reaction efficiency, at most 40 %, owing to their lower solubility in the solution (Morros et al., 2010a). It is important to emphasize that the reaction efficiency is directly proportional to the solubility of the alkyl epoxides. However, insoluble alkyl epoxides have shown a partial response to hydrophobic effects that depend on the alkyl chain lengths of the epoxides, which was not able to modify the required amount of solubilizer. Further, the reaction efficiency was found to be low in water–isopropyl alcohol mixtures with long-chain epoxides, such as C12 and C14. Quite the reverse, Morros et al. (2010b) also formed hydrophobic β-hydroxyalkyl inulin ether in an aqueous reaction medium consisting of 1 M KOH and 40 % inulin at 80 °C. The authors described that the nonionic surfactant β-hydroxydodecyl inulin ether had no effects on the etherification reaction, although cationic surfactants such as DTAB and hexadecyltrimethylammonium bromide (CTAB) noticeably improved the reaction efficiency up to 50 % as described in Fig. 1d. The findings have stated that during the etherification of inulin, the reaction efficacy was dependent on the types and nature of the surfactants, particularly in an aqueous environment using 1,2-dodecylepoxide. Moreover, β-hydroxydodecyl inulin ethers including InEC8, InEC12, and InEC14 were synthesized using 1,2-alkylepoxides, namely, 1,2-octylepoxide, 1,2-dodecylepoxide, and 1,2-tetradecylepoxide, respectively, in aqueous media, and their properties were compared with those of commercially available Inutec®N25 and Inutec®SPI. Potassium hydroxide and DTAB were introduced as an excellent micellar-like catalyst. The catalyst reduced the reaction time and increased the total reaction yield and reaction efficiencies, i.e., 80 % and 50 %, respectively, from 4 to 24 h (Morros et al., 2012). These outstanding inulin ethers have found use in several industrial applications, such as in pharmaceuticals as a stabilizing agent for aqueous solutions that contain poorly soluble molecules or as carriers for water-insoluble substances.
2.5. Production of cyanoethyl inulin ether in aqueous solvent
The cyanoethylation of polysaccharides is a dynamic approach that has been prevalent in the last few decades (Tripodo and Mandracchia, 2019; Verraest, Peters, Kuzee, Raaijmakers, & van Bekkum, 1997). Cyanoethylated starch was used in the textile industry due to its high dispersing and emulsifying properties. However, the modified starch resulted in high-viscosity solutions and exhibited low solubility, significantly decreasing the applicability. It was believed that inulin showed lower solution viscosities and exquisite solubility due to its low molecular weight (Verraest et al., 1997). Hence, the cyanoethylation of inulin has been performed by reaction of inulin with Michael-type addition in an analogous manner, mainly in an aqueous environment, by using stearoyl chloride and acrylonitrile as a catalyst (Fig. 1e) (Stevens, Meriggi, Booten et al., 2001, 2001b). Cyanoethyl inulin and its derivatives showed multiple industrial applications, including as a crystallization inhibitor for calcium carbonate, in detergent formulations and as a dispersing agent. Nevertheless, 3-amino-3-oxopropyl and carboxyethyl cyanoethyl inulin derivatives can be mixed and substantially used as hair fixatives, metal ion carriers, and dispersing agents. It is also observed that when the cyanoethyl inulin derivatives exhibit a low DS (viz., DS < 1.5), they are soluble in water, while when the products exhibit a high DS (viz., DS > 1.5), they are insoluble in water. Thus, it was concluded that an appropriate DS is critical to determine the quality of nonionic polymeric surfactants. It is well known that inulin can be reduced to avoid intense color formation and side chain products before further modification. The reduction process is completed by employing many reducing agents, such as primary amine, sodium borohydride, and molecular hydrogen, or by electrochemical reduction (Stevens, Meriggi, Booten et al., 2001; Stevens et al., 2001b).
2.6. Modification of inulin in organic solvent
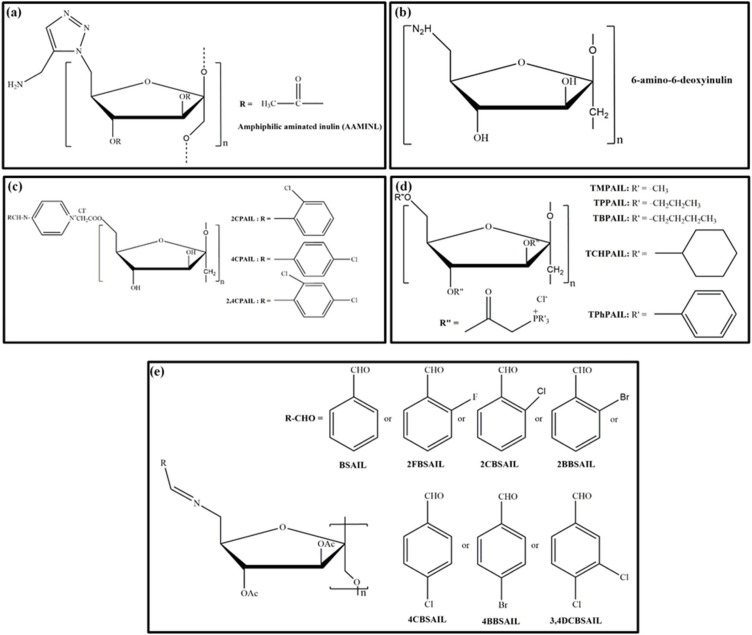
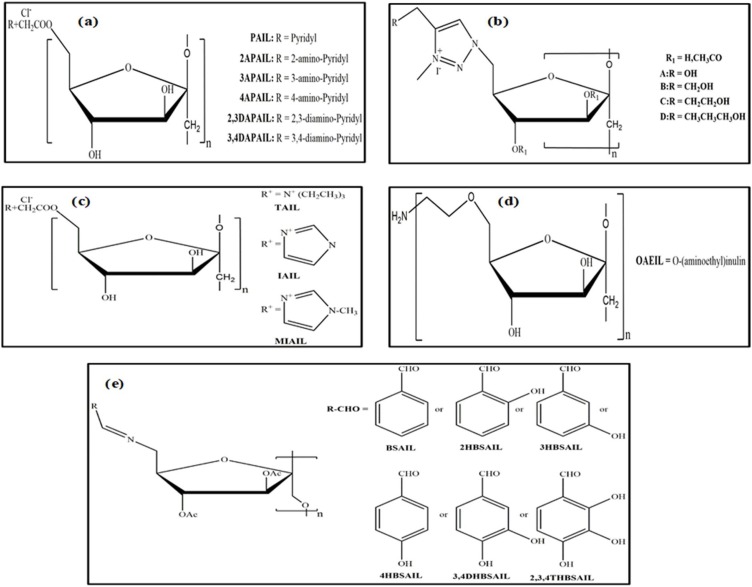
In the early 19th century, most scientists focused on the preparation of triacetyl inulin by the reaction of native inulin with pyridine at 40−140 °C (Haworth & Streight, 1932). As a result, a good amount of unpurified end product was obtained, ranging from 57 to 99 %, whereas the amount of purified end product obtained ranged from 73 % to 80 %. However, this modification may not be applicable on an industrial scale due to the low rate of the chemical reaction, which makes the process expensive and time-consuming. Therefore, in 1932, Haworth and Streight produced acetylated inulin using methyl alcohol and obtained high amounts of the purified end product (approximately 95 %). Recently, Hartzell, Maldonado-Gómez, Yang, Hutkins, & Rose (2013) synthesized butyrylated, propionylated, and acetylated inulin derivatives by reaction of inulin with dimethylsulfoxide 1-methylimidazole and acetic anhydride in pyridine solvent. It was noted that a foamy precipitate was produced during the production of propionylated inulin, whereas it was not observed during the formation of butyrylated and acetylated inulin. This phenomenon occurred due to the high concentrations of unreacted acid and depolymerization of the inulin units, particularly in the aqueous environment. It was confirmed that inulin is extremely susceptible to acid hydrolysis (Courtin et al., 2009), which may affect the DS of the end product. However, Zhu et al. (2018) synthesized propionylated inulin by the reaction of inulin with propionic anhydride using pyridine as the solvent (Fig. 1f). Further, the authors evaluated the effect of the anhydride ratio, inulin concentration and temperature on the DS of IPE. The findings revealed that the DS was high with increasing propionic anhydride ratio, while it was decreased as the temperature and concentration of inulin increased. Moreover, Tripodo et al. (2019) synthesized inulin vitamin E (INVITE) bioconjugates and INVITE succinic anhydride (INVITESA) by reaction of inulin with vitamin E and succinic anhydride, respectively, in fluorescein isothiocyanate (FTIC) and dimethylformamide (DMF) as mentioned in Fig. 1g. The H-NMR and FTIR studies confirmed that with sufficient DS, polymeric micelles were produced upon water dispersion. Ren, Liu, Dong and Guo (2011) synthesized O-aminoethyl inulin in water, NMP, and benzene using NaOH, Et3N, and AlCl3 as catalysts, respectively. The inulin derivative produced in NMP/Et3N exhibited better yield or reaction efficiency than other inulin derivatives produced in water/NaOH. To date, a few groups have also synthesized 6-azido-6-deoxy-3,4-di-O-acetyl inulin (AAIL), 6-bromo-6-deoxy-3,4-di-O-acetyl inulin (BAIL), and chloracetyl inulin (CAIL) to improve their antimicrobial abilities and antioxidant activities as illustrated in Fig. 3, Fig. 4 (Chen et al., 2018; Chen, Hao, Ting, Li, & Gao, 2019, 2019b, Chen et al., 2020; Guo et al., 2014; Hu et al., 2014). Subsequently, a number of functional groups such as aminopyridine, benzaldehydes, aromatic aldehydes, quaternary ammonium salts, triphenylphosphonium salts and trialkylphosphonium salts have been grafted onto the backbone of inulin with the addition of organic solvents. A series of inulin derivatives were conveniently produced, and their chemical structures were characterized by FTIR, C-NMR, and H-NMR spectroscopy. The results showed that the chemical structures of inulin derivatives differed in number and substitution position on the hydroxyl phenolic groups on the aromatic and benzene aldehydes as well as quaternary ammonium salts, triphenylphosphonium salts and trialkylphosphonium salts. Moreover, Dong et al. (2014) formed amphiphilic aminated inulins via click chemistry by introducing triazolyl functional groups and evaluated their chemical structure by C-NMR and FTIR spectroscopy. To the best of our knowledge, this is the first study to modify inulin via click chemistry. In this mechanism, first, a 6-Br inulin derivative was synthesized by the reaction between the primary hydroxyl group of inulin with N-bromosuccinimide (NBS) and triphenylphosphine (Ph3P). Afterwards, the secondary hydroxyl group of the 6-Br inulin derivative was reacted with acetic anhydride; as a result, the amphiphilic aminated inulin was used as a potential biomaterial. Further advancement in the development of HMI derivative techniques is essential, which should be unique, convenient, relatively less expensive, and environmentally friendly owing to the increased demands of modified natural products.
Fig. 3.
Reported Inulin derivatives for antimicrobial activity.
Fig. 4.
Reported Inulin derivatives for antioxidant activity.
2.7. Stabilization of dispersions by HMI
Many industrial products are composed of dispersions including liquid/liquid (emulsions) and solid/liquid (suspensions) dispersions. These dispersions require stabilization against coalescence and flocculation, which is needed to produce an energy barrier between two particles to ultimately prevent them from coming into close proximity, where the van der Waals attraction is large (Tadros, 2017). The two basic mechanisms of stabilization are reported to include steric and electrostatic stabilization. Electrostatic stabilization works to provide charge separation and production of electric double layers whose extension is influenced by valency and electrolyte concentration. This stabilization mechanism of dispersions is commonly known as 'Deryaguin-Landau-Verwey-Overbeek' (DLVO theory or colloid stability theory). However, electrostatic stabilization of dispersions has not been commonly used due to the high electrolyte concentration, which can destabilize industrial products. Evidence based studies revealed that ionic emulsifiers in solutions do not easily adsorb at the liquid/liquid and solid/liquid interfaces (Tadros, 2017). Thus, nonionic surfactants have gained considerable attention due to the excellent stabilization properties of dispersions at high temperature or at high electrolyte concentrations and against high volume fractions, which is also frequently referred to as steric stabilization. It is essential to specify that destabilization difficulties may occur using customary surfactants even in the presence of nonionic stabilizers due to a reduction in the thickness of the adsorbed layer. As a result, coalescence and flocculation are observed in such dispersions (Tadros, 2011). This instability can be avoided by the utilization of graft (BAn) and block (A-B or A-B-A) nonionic copolymers owing to their considerable physical properties and specific chemical structure (A and B chains). The A chain is referred to as the stabilizing chain (usually with a molar mass > 1000 Daltons), which is hydrophilic and should be soluble in the medium and strongly solvated based on its molecular Flory-Huggins interaction parameter χ (< 0.5), whereas the B chain is considered the "anchor" chain, which is hydrophobic in the medium and highly adsorbed on the surface of droplets or particles (Tadros, 2003).
2.8. Stabilization of emulsions by HMI
The steric stabilization of oil-in-water (O/W) emulsions has been achieved through nonionic surfactants, namely, HMI, at higher concentrations of electrolyte and different temperatures, as summarized in Table 1 . Recently, O/W emulsions were prepared by mixing MCT oil and DDSA, OSA, and Inutec®SP1 solution in a mixer for 3 min at 24,000 rpm. Subsequently, the emulsification properties were evaluated by using zeta potential (Zetasizer) and droplet size measurements (Mastersizer) at various pH values and up to 21 days of storage at room temperature and 50 °C. The zeta potential of the OSA-inulin derivative was observed to increase (-4.8 mV to -60.8 mV) with increasing pH (1.9 to 9.7), whereas that of the DDSA-inulin derivative was found to increase (2.2 mV–55.5 mV) within a pH range of 1.8–10.2. The results of droplet size as a function of time and temperature were assessed for the DDSA-inulin derivative, OSA-inulin derivative, and Inutec®SP1, and it was observed that the DDSA-inulin derivative showed the greatest emulsification properties (smaller droplet size) of the three compounds (Kokubun et al., 2018). The same research group studied the emulsification properties of OSA-inulin derivative and DDSA-inulin derivative in the presence of electrolytes and during storage. It was also revealed that the ∼2 % DDSA-inulin derivative exhibited a smaller droplet size and produced stronger medium-chain triglyceride emulsions than the OSA-inulin derivative and Inutec®SP1 (Kokubun et al., 2015). The high emulsion stability of OSA-inulin derivative and DDSA-inulin derivative has been achieved due to the significantly shorter inulin chain and formation of electrostatic repulsive forces owing to the presence of carboxylate ions in the head group. Tadros, Vandamme, Booten, Levecke and Stevens (2004) also found similar droplet sizes that were stable, and there was no oil separation for one year against extreme temperature (up to 50 °C) and a particular concentration of NaCl and MgSO4 (1 mol dm−3). The stability of the Inutec®SP1 emulsion was also evaluated through cloud-point measurements. There was no sign of cloudiness up to 100 °C for the emulsion containing 1 mol dm−3 NaCl and MgSO4. In contrast, the polyethylene glycol surfactant did not demonstrate that ability and exhibited coalescence and flocculation in the solution. The difference in zeta potential among OSA-inulin derivative, DDSA-inulin derivative, and Inutec®SPI was due to the absence or presence of the ionic group.
Table 1.
Stabilization of O/W and W/O emulsions using various types of nonionic polymeric surfactants.
| HMI Type | Derivatization of Inulin | Emulsion Preparation | Major Findings | References |
|---|---|---|---|---|
| Inulin coded Fibruline®DS2, Inutec®SP1. | Synthesized in aqueous solution under alkaline conditions using OSA and DDSA. OSA-1, OSA-2, and DDSA-1 had ∼1–2 alkenyl chains per molecule, whereas DDSA-2 had ∼5 alkenyl chains per molecule. | O/W emulsions were prepared by mixing 1.5 g of MCT oil with a certain percentage of DDSA-inulin, OSA-inulin, and Inutec®SP1 solution. The emulsion stability has been measured using Mastersizer and Zetasizer systems. | DDSA-inulin had much better emulsification properties than OSA-inulin and Inutec®SP1. | Kokubun et al. (2015),(2018) |
| Inulin, coded Inutec H25P, Tween 20. | Successfully synthesized in deionized water under alkaline conditions using ASA. The following inulin derivatives were found: OSA, DSA, DDSA, TDSA, HDSA, ODSA. | O/W emulsions (15 %, w/w) were prepared by mixing 1.5 g of oil and 8 g of a 2.5 % aqueous solution of alkenyl succinylated inulin and 0.5 g of water to reach 2.35 %. | On the basis of droplet size and CAC, all ASA-inulin samples could stabilize the O/W emulsion. | Han et al. (2015) |
| Inulin coded Fibruline®DS2 | Synthesized in aqueous solution under alkaline conditions using acyl chlorides. The inulin derivatives (DS2C10, DS2C12, DS2C14, DS2C16) | 15 % w/w, O/W emulsions were prepared by adding 8.5 g of 1.5 % acylated inulin solution (C10, C12, and C14) to 1.5 g of MCT contained in a 20 mL tube. 15 % W/O emulsions were prepared by adding 3 mL of the C16 modified inulin solution at varying concentrations (0.5−1.5 %) to 17 mL of MCT. Droplet size was measured by laser diffraction and Mastersizer | The droplet size decreased with increasing alkyl length and stabilized the O/W emulsions with DS2C10, DS2C12, and DS2C14, inulin derivatives. DS2C16 was unable to form O/W emulsions; however, it could form W/O emulsions. | Han et al. (2017) |
| Inutec®SP1 | Inutec®SP1 was formed by carbamoylation using dodecyl isocyanate in an aprotic solvent to obtain inulin dodecyl carbamate. | HMI emulsions were prepared using three stages. Added DDW in HMI, stirred for 1 h, and added olive oil ((olive oil 2 % w/w: HMI 0.4 % w/w in DDW). β-lg stabilized emulsions were prepared as control emulsions (i.e., olive oil 2 % w/w: β-lg 0.4 % w/w in DDW). | HMI has great potential to stabilize the emulsion against various pH values, CaCl2 levels and gastric conditions. | Meshulam et al. (2014) |
| Inutec®SP1, PS-80 | Inutec®SP1 was formed by carbamoylation using dodecyl isocyanate in an aprotic solvent to obtain inulin dodecyl carbamate. | Stock O/W nanoemulsions was prepared by weighing the oil phase, consisting of cinnamaldehyde and/or HOSO (5 % w/w) and aqueous surfactant solution (0.5–5 % w/v). Sodium azide (0.02 % w/v) was added to all aqueous solutions, except for the samples for antimicrobial activity tests. | HMI could be stabilized the nanoemulsion at the high salt concentration (2 M) and high temperature up to 90 °C. PS-80 did not promote any physical instability but exhibited an increase in the droplet size. | Doost et al. (2018) |
| Inutec® SP1, Tween 80 | Inutec®SP1 was formed by carbamoylation using dodecyl isocyanate in an aprotic solvent to obtain inulin dodecyl carbamate. | Stock emulsions were prepared by mixing 5 % w/w of an oil phase (containing oregano EO and/or HOSO) and 95 % w/w aqueous solution containing 0.5 % w/v of surfactant. The creaming stability was evaluated by Lumifuge® 116 stability analyzer | HMI could stabilize the EO emulsions with 50 % HOSO in the lipid phase. At 4 °C, no variation in droplet size was found for up to two weeks. However, the formed nanoemulsion were not stable at a high salt concentration | Doost et al. (2017) |
| Inutec®SP1 Inutec®N 25 Span 80. | Inutec®SP1 was formed by carbamoylation using dodecyl isocyanate in an aprotic solvent to obtain inulin dodecyl carbamate. | Most emulsions consisted of a 50/50 (v/v) ratio oil in water, but in some cases, other ratios were used. The polymeric surfactant concentration was varied between 0.25 and 2 % (w/v) based on the oil phase. The emulsion was evaluated with optical microscopy. | No oil separation occurred for one year. No emulsions at 1 mol dm−3 showed any sign of flocculation or coalescence up to 50 °C. | Tadros et al. (2004) |
| Inutec®SP1 Inutec®N 25 | Synthesized employing methyl esters and acyl phosphonates. | O/W emulsions with a 50/50 (v/v) ratio were prepared on a 50 mL scale; 0.5 g of the inulin surfactant (2 %) was dissolved in 25 mL of demineralized water or 1 M MgSO4, to which 25 mL of Isopar M oil was added. | A long acyl chain length enables strong physical stability of emulsions for more than one year—even at 50 °C. Most stable emulsions were obtained with the dodecanoyl and octadecanoyl inulin derivatives | Rogge et al. (2007) |
OSA = octenyl succinic anhydride, DDSA = dodecenyl succinic anhydride, TDSA = tetradecenyl succinic anhydride, HDSA = hexadecenyl succinic anhydride, ODSA = octadecenyl succinic anhydride, O/W = oil-in-water, W/O = water-in-oil, DDW = double-distilled water, HOSO = high oleic sunflower oil, EO = essential oil.
The OSA-inulin derivative and DDSA-inulin derivative contain an ionic group that dissociates from alkaline succinic anhydride as the pH increases; on the other hand, Inutec®SP1 lacks an ionic group (Nestor et al., 2005) and thus does not exhibit similar trends. The increase in the zeta potential of Inutec®SP1 is due to the adsorption of the molecules on the surface of oil droplets, which can be formed because of covalent attachment of hydrophobic chains to the modified inulin (Liu, Sun, Li, Liu, & Xu, 2006; Xin et al., 2013). Nestor et al. (2005) and Stevens, Meriggi, Peristeropoulou et al. (2001) found that Inutec®SPI droplet aggregation prevented steric repulsive forces produced at the interfaces of carbohydrate moieties. Moreover, Khristov and Czarnecki (2010) and Gotchev et al. (2011) revealed that the size of the loops of inulin molecules at the interface depended on the alkyl chains attached to it. Furthermore, the experimental results regarding the stabilization of emulsions motivated the interrogation of O/W and W/O emulsions, which were prepared through varying concentrations of oils (Han et al., 2017). The stability of the emulsions was evaluated for several inulin derivatives, which were synthesized with various alkyl chain lengths (C10-C16). The inulin derivatives including DS2C10, DS2C12, and DS2C14 were able to stabilize O/W emulsions, whereas DS2C16 did not stabilize O/W emulsions at either 25 °C or 50 °C. It is important to mention that inulin derivatives such as DS2C16 were only able to stabilize the W/O emulsions. The results obtained from photomicrographs were unambiguous; the droplet size dramatically decreased (∼8 μm to ∼1 μm) as the DS2C16 concentration increased (0.5 %–1.5 %). In a preliminary study, the authors also observed the properties of the emulsions immediately after preparation and after 21 days of storage. The emulsion properties of the alkenyl succinylated inulin derivatives (C8-C18) were explained, and Tween 20 was used for comparison. The findings of this work demonstrated that except Tween 20 and the C8-alkenyl succinylated inulin derivative, all the inulin derivatives noticeably stabilized the O/W emulsions. In addition, a slight variation occurred in the droplet size after storage for 21 days (Han et al., 2015).
As is known, foods are the prime energy source for humans and help to prevent diseases and live a healthy life. Consequently, it is important to evaluate the stability of emulsions against intestinal lipolysis and gastric proteolysis because the complex sequences of biochemical and physical processes in the human body alter the stability of both O/W and W/O emulsions. Experimental results have shown that HMI increases the stability and functionality of emulsions within a range of CaCl2 concentrations (0−40 mM) and pH values (2.0 < pH < 10.0) compared to protein-based emulsions. Moreover, the emulsions were exposed to intestinal digestion and in vitro gastric conditions, as well as blended human bile (0–25 mg/ml). According to the results, it was proved that the emulsions were stabilized under the dynamic conditions of the human intestine and exhibited improved intestinal lipolysis (Meshulam, Slavuter, & Lesmes, 2014). It was concluded that HMI can be used in a multifunctional emulsion system as a potential bioactive compound in the colon microbiome and upper gastrointestinal tract (GIT). Moreover, cinnamaldehyde (CA) nanoemulsions were prepared using HMI and polysorbate (PS) 80 for comparison with a range of surfactant concentrations from 0.5 to 5 % (w/w). The findings of this study suggested that the droplet size was noticeably decreased with increasing surfactant concentration of both PS 80 and HMI. However, the HMI emulsions showed excellent stability against high temperature (90 °C) and high salt content (2 M), whereas PS 80 resulted in a drastic increase in the droplet size of nanoemulsions, indicating that its emulsions were less stable than those with HMI (Doost, Dewettinck, Devlieghere, & Van der Meeren, 2018). The same authors also reported a stability study of oregano essential oil emulsions composed of two nonionic emulsifiers, Tween 80, and Inutec®SP1. The results established that compared with Tween 80Inutec®SP1 provides more stable emulsions even in the presence of high salt concentration, high temperature, and acidic conditions. The high emulsion stability in the presence of Inutec®SP1 is due to the alkyl groups of Inutec®SP1, which attaches to the oil surface by a strong anchor, while its hydrophilic backbone (polyfructose) is soluble in an aqueous environment and is expected to remain hydrated. Thus, Inutec®SP1 is a potential candidate to provide a steric barrier by using polyfructose chain loops and is stable against strong coalescence and flocculation in the presence of salt (Tadros, 2017). On the other hand, the low stability of the emulsion when using Tween 80 was due to the interaction of monovalent ions with the emulsifier polar head groups; as a result, the Tween 80 head groups may have become dehydrated, eventually producing flocculation and coalescence. Moreover, instability has been reported to occur due to a lower adsorption affinity of the emulsifier (Tween 80) for the oil phase (Van Haute et al., 2016). The authors also revealed that Inutec®SP1 can decrease the rate of Ostwald ripening owing to strong adsorption by multipoint attachment at the O/W emulsion interface. Additionally, this compound increases the Gibbs dilatational elasticity, which reduces the diffusion of oil molecules from smaller to larger droplets (Tadros, 2011). The authors concluded that this type of emulsion is beneficial in the production of marinades on an industrial scale (Doost, Sinnaeve, De Neve, & Van der Meeren, 2017). The application of modified inulin in real food system is very limited. Normally, the HMI have used to stabilize the model emulsions or suspensions to prepare various industrial formulations. However, the native inulin has widely reported in the literature as a bulking agent, sucrose replacer in confectionary products, and fat replacer in dairy products. Recently, Kiumarsi, Majchrzak, Yeganehzad, Jäger and Shahbazi (2020) have prepared low-calorie chocolate by using different levels of dodecenyl modified inulin and to stabilize the particle phase dispersed in a fat-based solid suspension. The finding revealed that the intermediate and highest levels of HMI (50 % to 100 % modified inulin replace with sucrose) have provided more stable chocolates which were free from any strong coalescence, flocculation, fat crystals, and fat blooming upon storage. Moreover, these levels of HMI can also delay the undesirable appearance and deterioration of textural properties during storage. It was concluded that, this research work is a great leap forward to use bio-surfactants in the development of low-calorie chocolate and can reduce the production cost of the product (Kiumarsi et al., 2021). Furthermore, Rogge, Stevens, Colpaert, Levecke and Booten (2007) focused on the variation in synthesis procedures of inulin derivatives that exhibited different stability characteristics, which could be described by the impurities in the emulsions. For example, multiple inulin derivatives have formed by employing methyl esters and acyl phosphonates. In the procedure with methyl esters, two reactions were introduced, for instance, NaOMe in NMP or NaH in DMSO. The DMSO method with NaH resulted in moderate emulsion stability, whereas NaOMe in NMP presented outstanding emulsion stability for up to one year. However, the lowest emulsion stability was observed in the presence of inulin hexanoate, with the maximum time reaching two days. Moreover, a few inulin derivatives prepared with long acyl chain lengths have proven the strong physical stability of emulsions for more than one year—even at 50 °C. The most stable emulsions were obtained using dodecanoyl and octadecanoyl inulin derivatives. From the above studies, it was concluded that the inulin-based nonionic emulsifier should be considered as an exceptional emulsion-stabilizing compound. However, determining the emulsion stability is a key requirement to explore the stability of films that form between emulsion droplets or particles, which will be discussed below.
2.9. Stabilization of foam and emulsion films by HMI
As described above, nonionic polymeric surfactants have been applied for the stabilization of foam and O/W emulsion films as well as wetting films in an aqueous environment (Exerowa and Platikanov, 2009a; Exerowa et al., 2009b, 2009c). Moreover, the stability of films has been tested against various types of electrolytes (Na2SO4, NaCl, and Mg2SO4) with varying concentrations at constant capillary pressure (45–50 kPa) and specific concentrations of polymers using the thin liquid film–pressure balance technique and microinterferometric technique of Scheludko–Exerowa, as reported in Table 2 . It is important to emphasize that the steric repulsion in the graft copolymers was mostly due to loop-to-loop interactions, whereas that in the block copolymers was due to brush-to-brush interactions at the O/W interface. Thus, Exerowa, Platikanov, Levecke and Tadros (2009d) observed the effect of block (A-B -A) and graft copolymers [(ABn) (Inutec®SP1)] on the stabilization of foam, O/W emulsion, and wetting films. The findings revealed that graft copolymers (ABn) showed higher stability than block copolymers. This stability was obtained due to the formation of a Newton black film (NBF) at a lower disjoining pressure (0.5 kPa) in the presence of graft copolymer. It was proved that the transition from electrostatic to steric stabilization occurred successfully. Moreover, Exerowa, Gotchev et al. (2009c) synthesized HMI derivatives including HMI-A, HMI-B, and HMI-C for the stabilization of emulsions in comparison with Inutec®SP1 against a constant polymer concentration (2 × 10−5 mol dm−3) and various NaCl concentrations. The results established that the equivalent film thickness was noticeably reduced as the NaCl concentration increased, reaching 2 × 10−5 mol dm-3. This study also elucidated that the transition from electrostatic to steric stabilization is possible due to the lower capillary pressure (up to 36) and high DS for synthesized inulin derivatives. Moreover, high DSs and NaCl concentrations (up to 2 mol dm−3) can result in the formation of NBFs. Furthermore, a reduction in disjoining pressure-equivalent film thickness isotherms at a transition point occurred with increasing DS, which also indicated the transition from electrostatic to steric stabilization. The outcomes of these two studies are in agreement with those of Exerowa et al., who reported the performance of Inutec®SP1, which stabilized foam films (Exerowa, Kolarov, Pigov, Levecke, & Tadros, 2006) and O/W emulsion films (Exerowa et al., 2007) at constant capillary pressure, i.e., 50 Pa and 36 Pa, respectively. It was observed that the equivalent film thickness significantly decreased up to 11 nm for emulsion films and 16 nm for foam films with increasing NaCl concentrations and established NBFs, giving a layer thickness of the Inutec®SP1 loops of ∼3.6 nm. In contrast, the disjoining pressure-equivalent film thickness isotherms demonstrated that the foam films were not stable at a certain capillary pressure of approximately 1 × 103 Pa. Therefore, it is important to find a suitable foam film stabilizer at higher capillary pressure and electrolyte concentrations. Gochev et al. (2011) stabilized foam and O/W emulsion films by the thin liquid film–pressure balance technique using four different types of graft copolymers with varying DSs. The results showed that the foam film thickness was gradually decreased with an increase in the disjoining pressure. Different HMI derivatives have shown different trends, revealing that the foam films were unstable at 8 kPa for 2-HMI and 150 Pa for 3-HMI, whereas in the case of 0.5-HMI and Inutec®SP1, the foam film was stable at 100 kPa. On the other hand, the O/W emulsion films were also stabilized by HMI derivatives and developed NBFs up to 45 kPa, which showed the high stability of O/W emulsion films (Gochev et al., 2011).
Table 2.
Stabilization of wetting, foam and O/W emulsion films using various types of nonionic polymeric surfactants.
| Surfactant Type | Research Methodology | Major Findings | References |
|---|---|---|---|
| Inutec®SP1 | Microinterferometric technique of Scheludko–Exerowa was used to measure the stability of foam films at a constant concentration of Inutec®SP1 (2 × 10−5 mol dm-3) and at several NaCl concentrations (1 × 10-4 to 2 mol dm-3). | The film thickness was significantly decreased with increasing NaCl concentration, which indicated the stability of the foam film. At 1 × 10−2 mol dm-3 NaCl, the film thickness remained constant at approximately 16 nm. | Exerowa et al. (2006) |
| Inutec®SP1 | The microinterferometric method for investigation of thin liquid films described in the monograph of Exerowa-Kruglyakov against a constant concentration of Inutec®SP1 (2 × 10−5 mol dm-3) and at quite a few NaCl concentrations. | The O/W emulsion film thickness was approximately 11 nm. The film thickness could be decreased at critical NaCl concentration 5 × 10−2 mol dm-3. | Exerowa et al. (2007) |
| Inutec®SP1, Block and Triblock copolymeric surfactant | The microinterferometric thin liquid pressure balance experimental technique was used to calculate the stability of O/W emulsion films against varying NaCl concentrations | Emulsions using Inutec®SP1 should be more stable than those using Pluronics ABA copolymers, in particular at high electrolyte concentrations. | Exerowa, Gotchev, Gotchev et al. (2009b) |
| HMI-A, HMI-B, HMI-C, Inutec®SP1 | The microinterferometric technique of Scheludko–Exerowa was used to identify the stability of O/W emulsion films at a constant concentration of surfactants (2 × 10−5 mol dm-3) and multiple concentrations of NaCl. HMI-A, HMI-B, and HMI-C were prepared by changing the DS. Thus, one would expect the loop size to decrease as follows: HMI-A > INUTEC®SP1 >HMI-B >HMI-C. | The film thickness was markedly decreased with increasing NaCl concentration at a certain level (5 × 10−2 mol dm−3). In all cases, these NBFs are very stable and have a constant thickness up to the highest possible measured capillary pressure of 45 kPa. With the polymeric surfactant possessing the highest DS, the transition to an NBF of thickness 7 nm occurs even at a low capillary pressure of 36 Pa. With a reduction in DS, the loop size increases, and the transition to an NBF of 7 nm occurs at a higher capillary pressure of 0.5 kPa | Exerowa, Gotchev et al. (2009c) |
| Inutec®SP1 | The microinterferometric technique of Scheludko–Exerowa was designed to find the stability of O/W emulsion films against different types of electrolytes (Na2SO4 NaCl and Mg2SO4). | The film thickness significantly decreased and produced NBFs in all types of electrolyte at a specific capillary pressure, with no observed influence of electrolyte types on the equivalent film thickness, the formation of NBF and disjoining pressure-equivalent film thickness isotherms. | Gotchev et al. (2007) |
| Inutec®SP1 | The microinterferometric technique of Scheludko–Exerowa was used to measure the stability of wetting films produced on a hydrophilic silica surface. The stability was evaluated against different Inutec®SP1 concentrations in the presence or absence of Na2SO4 and NaCl. | The equilibrium film thickness varied with increasing electrolyte and polymeric surfactant concentrations. The reduction pattern in the equilibrium film thickness can be observed at 10−1 mol dm−3 NaCl, 10−6 mol dm−3 Inutec®SP1 and 10−2 or 1 mol dm−3 Na2SO4. | Nedyalkov et al. (2007) |
| Inutec®SP1, HMI-B, EFKA-4550 | The microinterferometric technique of Scheludko–Exerowa was used to measure the stability of wetting films against different types of polymeric surfactants at varying DSs. | The wetting films were stable at (Øw ≤25°) for Inutec®SP1 and (Øw ≤20°) for HMI-B. The general trend of change of h with CEFKA is similar to that obtained for Inutec®SP1 and HMI-B. | Nedyalkov et al. (2010) |
| Inutec®SP1 | The microinterferometric method for investigating the wetting and O/W emulsion films described in the monograph of Exerowa-Kruglyakov both in aqueous solution and in the presence of different electrolytes (NaCl, Na2SO4, and MgSO4) concentrations have been studied. | Emulsion and wetting films could be stabilized using HMI surfactants in the presence of all different types of electrolytes at varying concentrations. | Exerowa, Platikanov et al. (2009d) |
| Inutec®SP1, 0.5HMI, 2HMI 3HMI. | The thin liquid film–pressure balance technique has been used to measure the stability of foam and O/W emulsion films against different types of polymeric surfactants that are synthesized by altering the DS. Thus, one would expect the inulin loop size to decrease as follows: 0.5HMI > Inutec®SP1 > 2HMI > 3HMI. | NBFs have been found in all types of inulin-based surfactants and had the same thickness of approximately 7 nm. Foam films are unstable at 8 kPa for 2HMI and 150 Pa for 3HMI, whereas for 0.5HMI and Inutec®SP1, the foam films are stable at 100 kPa. Due to the formation of NBFs, the O/W emulsion films are more stable, up to 45 kPa in all HMI synthesized derivatives. | Gotchev et al., 2011. |
O/W = oil-in-water, NBF = Newton black film, HMI-A, HMI-B, HMI-C and 1HMI, 2HMI, 3HMI = Different types of inulin derivatives with different DSs. DS = degree of substitution.
In general, NBFs were found in all types of inulin-based surfactants, exhibiting almost similar thickness levels reaching 7 nm. Further, the experimental data revealed that Inutec®SP1 is an excellent candidate for the stabilization of O/W emulsion films and wetting films in the presence of different kinds of electrolytes (NaCl, Na2SO4, and Mg2SO4), even at higher concentrations, with a constant temperature of 22 °C. It was observed that the film thickness was significantly decreased and that NBFs were produced in all types of electrolytes, and there was no influence of electrolyte types on the equivalent film thickness, the formation of NBF, and disjoining pressure-equivalent film thickness isotherms (Exerowa et al., 2009b; Gotchev et al., 2007). Nedyalkov, Alexandrova, Platikanov, Levecke and Tadros (2010) arranged three different types of nonionic polymeric surfactants [HMI derivatives, Inutec®SP1, and hydrophobically modified polyacrylate (EFKA)] with varying DSs for the stabilization of wetting films obtained on hydrophobic solid surfaces. The empirical outcomes have shown some common features using different types of nonionic polymeric surfactants. It has been demonstrated that the equivalent film thickness dramatically decreased due to a reduction in the hydrophobicity (Øw ≤25°) and concentrations (CSP1≥10−4 mol dm−3) of surfactants. In addition, the wetting films should be unstable and rupture at low concentrations and elevated DSs (Øw >25° and CSP1 <10−4 mol dm−3) of surfactants. For example, HMI-B stabilized the wetting films at low hydrophobicity (Øw ≤20°) and high concentrations (CHMI-B ≥ 5 × 10−5 mol dm−3). For the remaining systems (Øw >20° and CHMI-B <5 × 10−5 mol dm−3), the wetting films were ruptured and unstable. Moreover, the equilibrium film thickness of wetting films was measured against three EFKA-4550 aqueous solution concentrations (5 × 10−5, 7.5 × 10−5, and 10−4 mol dm−3) with three different DS Øw values (60°, 70°, and 80°). Different results were observed, which showed that with a lower degree of hydrophobicity (Øw = 60° and Øw = 70°), the wetting films were less stable than those with the highest degree of hydrophobicity (Øw = 80°) under a wide range of EFKA concentrations (5 × 10−5–10−4 mol dm−3). However, the results showed that the equilibrium film thickness decreased with increasing concentrations of CEFKA at varying degrees of hydrophobicity. The same tendencies were also observed concerning the degree of hydrophobicity at constant concentrations of EFKA-4550 polymeric surfactants, which promoted strong stabilization of the films. In contrast, different concentrations of Inutec®SP1 were also proposed for the stabilization of wetting films obtained on a hydrophilic silica surface in the presence or absence of Na2SO4 and NaCl electrolytes (Nedyalkov, Alexandrova, Platikanov, Levecke, & Tadros, 2007). A decreasing trend in equilibrium film thickness was observed with increasing polymeric and electrolyte concentrations at a particular level, i.e., 10−1 mol dm−3 for NaCl, 10−6 mol dm−3 for Inutec®SP1 and 10−2 or 1 mol dm−3 for Na2SO4. However, in the case of the Na2SO4 electrolyte, the film thickness showed a weak dependence on the Inutec®SP1 concentration. From the above studies, it was revealed that stable symmetric and asymmetric thin liquid films were obtained using HMI derivatives and graft copolymers. Moreover, the NBF involves a short-range force that can help in the stability of emulsions and foam films against a wide range of electrolyte concentrations and at higher capillary pressures due to the strongly hydrated loops and brushes, which provide steric stabilization. Thus, there is no doubt that the formation of NBFs is a critical phenomenon, necessitating further exploration of their nature and development of a unique approach, which would contribute to the stabilization of emulsion and foam films. Apart from the abovementioned reports, thin liquid films from aqueous solutions stabilized by HMI derivatives and graft copolymers are limited, and their quantitative research is scarce.
2.10. Stabilization of suspension by HMI
In a previous section, we described the stabilization of emulsions by biocompatible polymeric surfactants, i.e., HMI. Consequently, there is a surging interest in exploring the stabilization of suspensions through nonionic polymeric surfactants. Thus, several types of latexes such as butyl acrylate, polystyrene (PS) and poly-(methyl methacrylate) (PMMA) have been developed by emulsion polymerization using potassium persulfate as an initiator, which was determined by turbidimetry measurements and expressed in terms of critical coagulation concentration (CCC) against different types of electrolytes (Table 3 ). In a significant study, Nestor et al. (2005) prepared emulsion polymerization of PS and PMMA particles using an optimum ratio of polymer and monomer. It was reported that Inutec®SP1 is the best option to stabilize the suspensions owing to an increase in its CCC with an increasing Inutec®SP1 amount even at a high concentration of CaCl2. However, the latex particles prepared without surfactants showed a low CCC value of approximately 0.0175 – 0.05 mol dm−3. The superior Inutec®SP1 was shown to stabilize the latex particles at up to 20 % monomer content, with a relatively low ratio of surfactant/monomer of approximately 0.002. Esquena et al. (2003) also reported similar results and prepared PS and PMMA particles using surfactant-free and Inutec®SP1 emulsion polymerization, respectively. The CCC values of the three types of electrolytes were 0.0004 mol dm−3 for Al2(SO4)3, 0.375 mol dm−3 for NaCl, and 0.007 mol dm−3 for CaCl2. As mentioned in the previous report, Inutec®SP1 can remarkably improve the stability of latex particles due to the higher CCC above a critical polymer concentration, producing a hydrated layer with a thickness of almost 4 nm.
Table 3.
Stabilization of suspensions by using nonionic polymeric surfactants.
| Surfactant Type | Latex Particles | Research Methodology | Major Findings | References |
|---|---|---|---|---|
| Inutec®SP1 | PMMA and PS | The PMMA and PS particles were prepared using an optimum ratio of polymer/monomer of approximately 0.001 for PMMA and 0.0033 for PS with a constant ratio of initiator/monomer of approximately 0.00125. The stability of these latex suspensions was determined by turbidimetry measurements and expressed in terms of CCC using different types of electrolytes. | Inutec®SP1 was a suitable polymeric surfactant to stabilize the latex particles such as PMMA and PS at 20 % monomer content, with a lower ratio of surfactant/monomer of approximately 0.002, due to the increase in CCC when using a higher concentration of CaCl2. | Nestor et al. (2005) |
| Inutec®SP1 | PMMA and PS | The PS and PMMA were prepared using surfactant-free emulsion polymerization and by the addition of Inutec®SP1, respectively. The stability of these latex suspensions was determined by CCC using Al2(SO4)3, NaCl and CaCl2 as electrolytes. | HMI can markedly improve the stability of latex particles due to the higher CCC above a critical polymer concentration and produced a hydrated layer with a thickness of almost 4 nm. | Esquena et al. (2003) |
| Inutec®SP1, Synperonic A, Synperonic NP | PS | PS was formed using emulsion polymerization of Inutec®SP1, Synperonic A, and Synperonic NP. The stability was measured using AFM in the presence of water and varying Na2SO4 concentrations. | For 5 wt % latex, the Inutec®SP1 concentration was kept constant at 0.0165 wt %, and the initiator concentration was also kept constant at 0.0125 wt %, whereas the cosurfactant concentration was varied between 0.1 and 0.5 wt %. | Nestor et al. (2008) |
| Inutec®NRA | VNR | The stability of VNR using HMI was determined by measuring the CCC of calcium nitrite. The adsorption values of steric repulsive force were also studied and determined through dynamic light scattering and zeta potential measurements | The CCC of vulcanized natural rubber particles significantly increased with increasing HMI concentrations in up to 0.002 M calcium nitrite. In other cases, flocs are produced in the suspensions. The dynamic light scattering and zeta potential experiments revealed that HMI could stabilize the latex particles. | Singh et al. (2014) |
| Inutec NRA | PMMA/BuA | The stability of latex particles has been identified against KBr electrolyte concentrations and in the presence of water. The adhesion and elastic properties were also measured using AFM. | The findings revealed that these latexes had a polydispersity index of 1.05 and diameter of 118 nm, with stable suspensions up to 0.2 mol dm−3. The adsorbed surfactant films showed outstanding elastic characteristics, and their adhesion force and elastic modulus was markedly maintained in the presence of up to 0.05 mol dm−3 Na2SO4. | Obiols-Rabasa et al. (2017) |
PMMA = polymethyl methacrylate, PS = polystyrene, AFM = atomic force microscopy, VNR = vulcanized natural rubber, BuA = butyl acrylate, CCC = critical coagulation concentration.
The high stability of these latex particles depends on the production of hydrated tails and loops and ample adsorption of Inutec®SP1 on the latex particles. In 2008, Nestor et al. measured the steric repulsive forces of these latexes, which were adsorbed on glass spheres and plates, by atomic force microscopy (AFM) in the presence of water and varying Na2SO4 concentrations. In the force-distance curve, it was found that the repulsion interactions persisted even against a higher concentration of Na2SO4. Moreover, the layer thickness was significantly decreased from 10 nm to 3 nm with increasing electrolyte concentration from 0.3 mol dm−3 to 1.5 mol dm−3. The findings of this report were consistent with those of Obiols-Rabasa et al. (2017), who decided to prepare PMMA/BuA (butyl acrylate) latex particles in the presence of HMI. The findings revealed that these latexes had a polydispersity index of 1.05 and diameter of 118 nm, and the suspensions were proven to be stabilized against KBr electrolyte concentration (up to 0.2 mol dm−3) and water. Moreover, the adhesion and elastic properties were measured by AFM. The measurement results demonstrated that the adsorbed surfactant films showed outstanding elastic and adhesion characteristics, and their adhesion force and elastic modulus were maintained at Na2SO4 concentrations up to 0.05 mol dm−3. This result was an outstanding contribution that confirmed the strong repulsion and steric interactions due to the elastic behavior of the latexes, as mentioned earlier. Furthermore, the adhesion force did not depend on electrolyte concentrations, which confirms the smooth deposition of latex particles on a flat substrate for use in coating applications. Recently, Singh, Esquena, Solans, Booten and Tadros (2014) stabilized vulcanized natural rubber using HMI determined by measuring the CCC of calcium nitrite. The CCC of vulcanized natural rubber particles significantly increased as the HMI concentration increased; however, high concentrations of calcium nitrite above 0.002 M produced flocs that were observed through optical micrographs. Furthermore, the adsorption conformation in response to the steric repulsive force was evaluated by light scattering and zeta potential measurements, which confirmed that the HMI could improve the colloidal stability of latex particles. Thus, during the stability of latex particles, three types of stability regions can be observed, namely, a stable dispersion region, coagulation region, and weak flocculation region. The development of a more uniform layer of latex was achieved in the weak flocculation region, which has been utilized in the glove manufacturing industry. It is essential to mention that flocculation appeared gradually in the suspensions and showed dramatic behavior, which made it hard to estimate the real CCC values. Thus, the polyethylene oxide does not provide steric stabilization for suspensions at extreme electrolyte concentrations compared to HMI.
2.11. Critical aggregation behavior of HMI
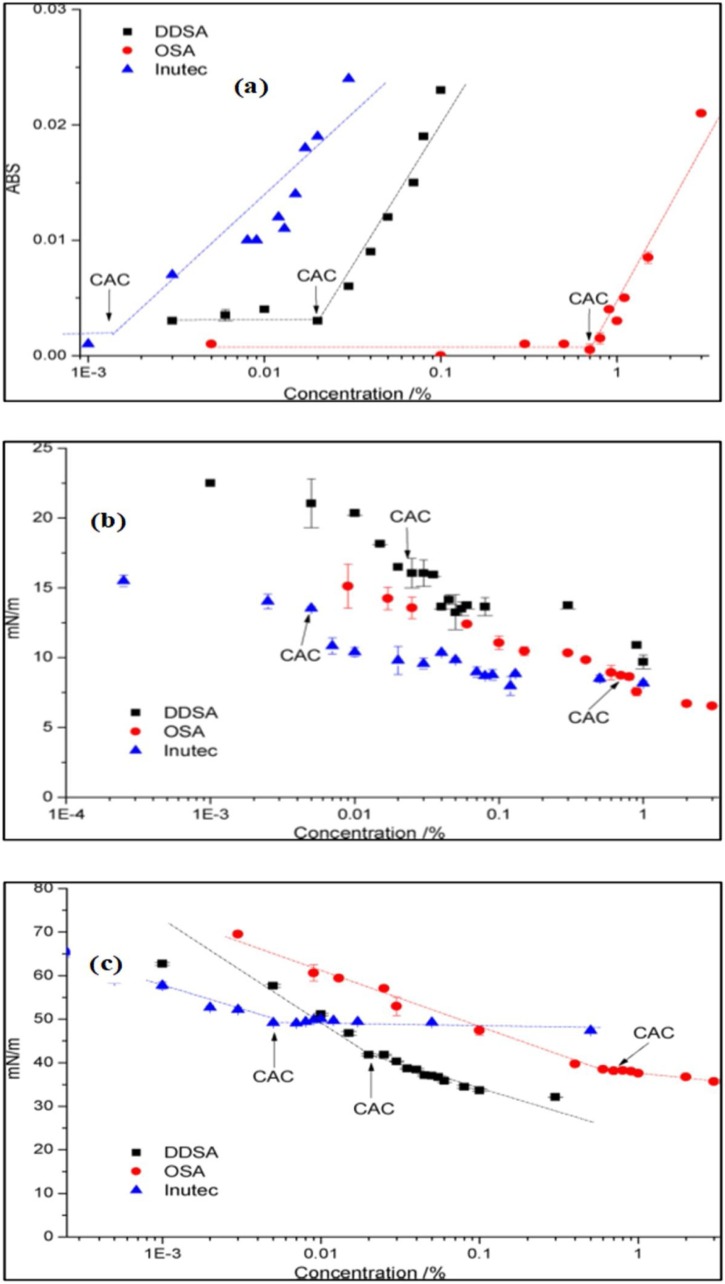
Determining the aggregation behavior of nonionic polymers is a meticulous process, and its importance in drug delivery and nanotechnological systems is undeniable. It is important to mention that the critical micelle concentration (CMC) is a point at which hydrophobic polymers self-assemble into substantial globular aggregates, although the critical aggregation concentration (CAC) measures the concentration at which premicellar aggregates emerge. Moreover, the CAC is an attractive parameter for tuning the formation of micellar-like structures by one or more self-assembling polymer chains and is determined using light scattering spectroscopy, UV/vis spectrometry, self-diffusion coefficients and steady-state fluorescence quenching (Han et al., 2017). Furthermore, the hierarchy of surfactants has been expressed at three levels, namely, precipitates (>500 nm), flocks (<100 nm), and aggregates (<20 nm) (Morros et al., 2012). It is noted that the solubilization of HMI depends on the CAC or CMC value; for example, a smaller value indicates the excellent solubilization properties of HMI derivatives in the colloidal system. Han et al. (2017) recently documented the CAC value of synthesized inulin derivatives through surface tension and dye solubilization measurements. The results showed that Sudan IV dye dissolved in the hydrophobic region of the derivatives, confirming that the absorbance value of esterified inulins increased above a critical concentration; as a result, a micellar-like structure was formed, as summarized in Fig. 2 a. Moreover, the surface tension is measured by using the Du Nouy ring method and is expressed as a function of concentration. The surface tension was found to be low (45 mN/m) for DS2C10 and high (62 mN/m) for DS2C14. The surface tension of esterified inulins dramatically decreased as the alkyl chain length increased, and one would expect that this behavior may be due to the position of chain attachment and varying DSs. Moreover, this effect has been elucidated based on the interplay between the intramolecular and intermolecular interactions of nonionic polymeric surfactants in solutions and at the air-water interface. It was concluded that the amphiphilic inulin derivatives succeeded in forming micellar-like aggregates in the solutions.
Fig. 2.
(a) Absorbance at varying concentrations in the presence of Sudan IV, (b) Interfacial tension at the oil/water interface as a function of concentration, (c) surface tension as a function of the concentration of OSA, DDSA-inulin derivatives, and Inutec®SP1. Reprinted with permission from Elsevier.
Likewise, the same group reported that the ASA inulin derivatives also produced micellar-type aggregates, with successful dissolution of the tested dye (Han et al., 2015). The abovementioned findings are in agreement with the results of Kokubun et al. (2013). The authors reported the CAC values of OSA and DDSA inulin derivatives in comparison with Tween 20 and ASA-inulin samples using dynamic light scattering, dye solubilization, conductivity, and surface tension measurements. The results revealed that the CAC value of DDSA decreased from 12 to 6 % as the amount of hydrophobic dye increased. Moreover, the surface tension ranged from ∼35–40 mN/m using concentrations of 0.05 % and 0.6 % for DDSA and OSA inulin derivatives, respectively, as shown in Fig. 2c. In contrast, Tween 20 and ASA exhibited less interaction with the hydrophobic dye than OSA and DDSA. In addition, the conductivity results were not presentable because the authors did not find inflexion of the head groups, which did not pack close together. Recently, another study reported the surface tension values of Inutec®SPI, DDSA, and OSA, which were 49 mN/m, 42 mN/m, and 38 mN/m, with noted inflexions of 0.0025 %, 0.020 %, and 0.70 %, respectively. Though it is not surprising that the CAC values for DDSA and OSA were higher regarding Inutec®SPI, OSA exhibited higher CAC values than DDSA (Kokubun et al., 2018). Moreover, the results regarding dye solubilization were consistent with the surface tension values. Nestor et al. (2005) and Srinarong et al. (2011) presented similar tendencies concerning surface tension that decreased with increasing concentration, i.e., 0.00035 % and 0.009 % from ∼68 to ∼45 mN/m and ∼55 mN/m, respectively, for Inutec®SP1. Moreover, the surface excess (air/water interface) reached 1.44 nm2, 0.74 nm2, and 0.87 nm2 for OSA, DDSA, and Inutec®SP1, respectively (Kokubun et al., 2018).
In contrast, Stevens, Meriggi, Peristeropoulou et al. (2001) documented surface excess values of approximately 0.9 nm2 for Inutec®SP1. Furthermore, the interfacial tension at the interface between aqueous solutions of the abovementioned inulin derivatives and MCT oil was graphed as a function of concentration and found to be reduced as the concentration increased; however, no evident inflexion was observed (Fig. 2b). This effect may be due to the heterogeneous nature of the modified inulin samples (Kokubun et al., 2015). However, the interfacial tension has been reported to be approximately 13 mN/m, 16 mN/m, and 8 mN/m for the Inutec®SPI, DDSA, and OSA inulin derivatives, respectively. Although the value was almost close to that of Stevens, Meriggi, Peristeropoulou et al. (2001), it was 6.8 mN/m for Inutec®SPI at the Isopar/M oil/water interface. Furthermore, Morros et al. (2012) synthesized HMIs including InEC8, InEC12, and InEC14 and discussed the surface tension compared to that with Inutec®SP1. In all cases, the surface tension was decreased, reaching 72.0 mN m−1 at 1 mM (∼0.5 % (w/w)) for water, but drastically was reduced to approximately 66 mN m−1 for 10 % inulin solution, whereas it was nearly 40 mN m−1 for the InEC8 derivative and between 30 and 20 mN m−1 for InEC14, InEC12, and Inutec®SP1. The results indicated the correlation between surface tension reduction, concentration, and equilibrium. Archetypal equilibration times can last for more than two hours, with reductions in surface tension up to 20 mN m−1. The documented findings showed that the HMI derivatives are attractive contenders, with equilibrium surface tension values as low as 30 mN m−1. Another study described the CMC of commercially available Inutec®SP1, which was used for encapsulation of anticancer drugs. The emission spectrum was measured at 375 nm (I1) and 384 nm (I3), whereas the excitation wavelength was fixed at 334 nm. Accordingly, the CMC was measured by taking the midpoint of the Inutec®SP1 concentration at which the relative fluorescence intensity ratio of I3/I1 was varied. The CMC of Inutec®SP1 reached 27.8 μg/mL, which made it possible to stabilize the O/W emulsions, films, and foams (Muley et al., 2016). This result was consistent with a previous report in which inulin was used for film formation (Kurečič, Smole, & Stana-Kleinschek, 2013). Moreover, Tripodo, Chlapanidas et al. (2015), (2015b) prepared inulin bioconjugates to improve the drug delivery profile with the targeted site. The findings displayed outstanding CAC values, which were obtained through pyrene for three different types of inulin bioconjugates, namely, INVITE-1, INVITE-2, and INVITE-3, reaching 22.3 × 10−3, 9.1 × 10−3, and 2.4 × 10−3 mM, respectively. In the same year, different authors reported different CAC values for inulin bioconjugates. The results revealed excellent CAC values, with the experiments performed using the pyrene and curcumin approach as a hydrophobic probe. As expected, the CAC values for INVITE-1, INVITE-2, and INVITE-3 were approximately 7.5 × 10−2, 6 × 10−2, and 3.8 × 10−2 mM, respectively. In contrast, with a fluorescence spectroscopy method, the CAC value reached 2.4 × 10−2, 1.6 × 10−2, and 2.5 × 10−2 mM for the INVITE-1, INVITE-2, and INVITE-3 bioconjugates, respectively (Mandracchia, Tripodo, Latrofa, & Dorati, 2014). Recently, the CAC value was determined for the inulin-ethylenediamine-retinoic acid (INU-RDA-RA) copolymer by a spectrofluorimetric approach using pyrene in double-distilled water and two different buffer solutions, namely, HEPES and DPBS, at pH 7.4. The CAC value was expressed in terms of molar concentration and weight concentration. The determined CAC values for DPBS, HEPES, and double-distilled water were found to be 0.073, 0.185, and 0.290 mg/mL, respectively. However, in terms of molar concentration, the self-assembling micelles obtained were obtained in 44.343 M double-distilled water, 1.116 M DPBS and 28.287 M HEPES. These interesting outcomes proved that the CAC value strongly depends on the ionic strength of the external medium and the pH of the buffer solutions (Di Prima et al., 2019). These results were consistent with those of Di Prima et al. (2017), who found the CAC values for the same inulin derivatives with a similar method, reaching 0.136 mg/mL. In contrast, the CAC for the formation of self-assembling inulin-LA conjugate micelles was demonstrated to be 0.0669 mg/ml (Wang et al., 2018). In 2014, Licciardi, Scialabba, Sardo, Cavallaro, & Giammona reported the self-assembled micelle structure of graft copolymers including inulin-ceramide and inulin-ceramide PEG2000 in water. The results revealed that the CAC values were very consistent for both inulin ceramide and inulin-ceramide PEG2000, achieving 6 × 10−2 and 5 × 10−2 mg/mL, respectively. By contrast, the CAC value was measured by determining the crossover point of two straight lines, which reached 3.0 × 10−4 g/L in an aqueous environment, which means that the formation of nanoparticles occurred (Zhang et al., 2014). It is important to mention that the HMI derivatives showed more ability to form a micellar-like structure than HMP derivatives, with CAC values ranging from 24.5 × 10−2 to 24 × 10−2 mg/mL in the different solutions (Wang et al., 2012; Wu et al., 2014; Zhu et al., 2011). This exceptional performance of HMI derivatives regarding aggregation behavior can enhance the research interest in exploring notable critical aggregation values.
2.12. Antimicrobial properties of HMI
As a type of organic polysaccharide, inulin plays imperative roles in any living creature. It has been rapidly gaining great attention due to its increased applications as a biomaterial attributable to its biodegradability, low immunogenicity, high availability, and biocompatibility (Apolinário et al., 2014; Tziveleka, Ioannou, & Roussis, 2019). Recently, HMI has been extensively used in the biomedical field owing to its physicochemical properties, surfactant abilities, encapsulation properties, and physiological functions (Xu et al., 2019; Yu, Shen, Song, & Xie, 2018). As a result, HMI derivatives have been developed utilizing different techniques to enhance antimicrobial and antioxidant activities (Fig. 3, Fig. 4 ) (Gupta, Jangid, Pooja, & Kulhari, 2019; Sardo et al., 2015). Schiff base reactions represent a systematic mechanism to develop modified inulin derivatives, with several applications in photochromic materials, food preservatives, analytical chemistry, synthetic medicine, protein delivery, and tissue engineering as well as wound healing and self-healing (Ansari & Bhat, 2019; Anush, Vishalakshi, Kalluraya, & Manju, 2018; Berhanu et al., 2019; Wang, Yuan, Li, Li, & Jiang, 2016; Suflet et al., 2015). This approach was reported to increase the various bioactivities of modified polysaccharides such as antimicrobial, anticancer, and antioxidant activities compared to those of the native polysaccharides (Antony, Arun, & Manickam, 2019; Kenawy et al., 2019; Nematidil, Sadeghi, Nezami, & Sadeghi, 2019). Therefore, six types of inulin derivatives have been developed through Schiff base chemistry, and their antifungal activities have been demonstrated against Phomopsis asparagi, Botrytis cinerea, and Fusarium oxysporum f. sp. cucumerium Owen. The findings showed that all modified inulin derivatives had the potential to degrade the fungi due to their broad-spectrum antifungal activity. Thus, at 1.6 mg/mL, the inhibitory rates of 3-HBSAIL were excellent, i.e., 82 %, 93 %, and 83 %, against Phomopsis asparagi, Botrytis cinerea, and Fusarium oxysporum f. sp. cucumerium Owen, respectively (Chen et al., 2020). Another study performed by the same group, who prepared seven inulin derivatives with aromatic Schiff bases, found complete inhibition of the growth of plant pathogens such as Fusarium oxysporum f., (sp. cucumerium Owen, sp. Niveum), Phomopsis asparagi and Botrytis cinerea at 1.0 mg/ml (Chen, Mi et al., 2019). Moreover, the inhibitory indices of 3,4DCBSAIL were 100 % at 1.0 mg/mL, and Botrytis cinerea showed more sensitivity to all inulin derivatives; F. oxysporum f. sp. niveum was more vulnerable to derivatives containing chlorine; and, F. oxysporum f. sp. cucumerium Owen was more easily degraded by the derivatives containing bromine. In preliminary research, Chen et al. (2018) prepared five significant inulin derivatives through chemical modification with quaternary phosphonium salts, namely, triphenylphosphonium and trialkylphosphonium salts. Their antifungal activity was investigated against plant-based fungi, such as Phomopsis asparagi, Fusarium oxysporum, and Colletotrichum lagenarium, by hyphal measurements in vitro. The inulin derivative modified by triphenylphosphine (TPhPAIL) showed exquisite antifungal activity at 1.0 mg/mL, with inhibitory values of 78.8 % for Phomopsis asparagi, 80.0 % for Colletotrichum lagenarium and 87.4 % for Fusarium oxysporum. Likewise, the OSA-inulin derivative has also been used to improve the antibacterial potential; the findings revealed that the inhibition indices increased as the concentration of the OSA-inulin derivative increased, with minimum inhibitory concentrations (MICs) of approximately 0.5 % for E. coli and 1 % for S. aureus (w/v) (Zhang et al., 2015). Additionally, Guo et al. (2014) reported the antifungal spectrum of inulin derivatives prepared by Schiff bases against 3 types of phytopathogens determined through hypha measurement in vitro. The results showed that all the developed inulin derivatives, particularly dichlorobenzylideneamino pyridyl acetyl inulin chloride, inhibited the activity of the phytopathogens Fusarium oxysporum, Colletotrichum lagenarium and Phomopsis asparagi, with inhibitory rates of approximately 43 %, 67 %, and 47 %, respectively, at 1.0 mg/mL. Another research group synthesized triazole (4a˗4d) and triazolium (5a-5d) inulin derivatives and tested them against the plant pathogens C. lagenarium and Gibberella zeae (Li, Qiu, Tan, and Gu, 2017). The findings suggested that the triazolium derivatives at 1.0 mg/ml had excellent antifungal indices, ranging from 45.31 % to 57.93 % for C. lagenarium and 43.10 %–82.56 % for Gibberella zeae. The results revealed that the antifungal ability of triazolium may be largely attributed to the alkylation of the 1,2,3-triazole moiety. The substantial effect of the triazolium inulin derivatives may be due to their cationic nature, which interacts with anionic components on the cell wall of fungi.
In contrast, 'click chemistry' is an attractive platform for the chemical modification of inulin, with a significant inhibitory rate of approximately 58 % against Staphylococcus aureus at 1 mg/ml (Dong et al., 2014). The reports, as mentioned earlier, were almost consistent with the findings of Ren et al. (2012), who synthesized the 6-amino-6-deoxy-inulin derivative using expedient chemical manipulation to broaden the applications of this presently underutilized biodegradable and environmentally benign resource. As a result, the antifungal characteristics were determined through in vitro hypha measurements, which demonstrated exceptional antifungal activities of approximately 53.3 % and 60.1 % against Fusarium oxysporum sp. Cucumis sativus L and Cladosporium cucumerinum (Ell.) et Arthur, respectively, at 1000 μg/mL. Moreover, HMI and PS 80 were used to develop a cinnamaldehyde (CA) nanoemulsion and to evaluate the antibacterial activity against E. coli (EC) and Staphylococcus aureus (SA) in the presence of different concentrations of long-chain triglycerides, i.e., high oleic sunflower oil [(HOSO)-(0-50 %)]. It is significant to mention that the difference in the MIC values was nonsignificant (P < 0.05) for the studied surfactants in the presence of various HOSO concentrations. In contrast, regarding the described MIC outcomes, an Ostwald ripening (OR) inhibitor did not cause a substantial difference in the inhibition zone diameter (IZD) of SA and EC. The IZD values of pure oil (4.2 mg) noticeably increased from 24 to 44 mm due to the entrapment of CA in nanoemulsions, and as a result, the antibacterial properties of CA were augmented. This effect may be attributed to the higher bioavailability of the CA molecules, as there was more exchange area when the CA was encapsulated inside small nanoemulsion droplets. However, the IZD values against SA and EC were 24 and 18 mm, respectively, implying that CA nanoemulsions are more efficient in inhibiting SA. Nevertheless, this hypothesis needs to be explored further. These results suggest that only a low amount of CA essential oil is needed to obtain pronounced antibacterial activity, which is important from an industrial and consumer point of view. In other words, it appeared that in the microdilution assay, the addition of sunflower oil did not reduce the antibacterial activity of the CA nanoemulsions, which was in agreement with the results obtained from well diffusion assay experiments, revealing that the amount of essential oil plays a major role in the antibacterial activity (Doost et al., 2018). Furthermore, Tripodo et al. (2019) prepared two inulin-based micelles (viz., INVITE-SA-RIF and INVITE-RIF) and tested them against three types of gram-positive bacteria and two types of gram-negative bacteria. Free RIF was utilized as a control treatment. The experimental findings revealed that compared to INVITE-RIF, INVITE-SA-RIF possessed higher antibacterial activity, reaching values of 0.013, 9.00 and 18.00 μg/mL against S. aureus, S. pyogenes, and M. smegmatis, respectively. The results obtained from these studies have significant implications for the formulation of nanoemulsion-based delivery systems and functional foods as natural antimicrobial agents.
2.13. Antioxidant activity of HMI
Reactive oxygen species (ROS), which generally consist of nonradical and free radical molecules, are vital for the normal functioning of all living cells in the human body. Moreover, if the level of ROS is exceeded in the human body, it can lead to the destruction of several cellular functions by disrupting various aspects of biomolecules, including DNA, enzymes, and RNA (Wojtunik-Kulesza, Oniszczuk, Oniszczuk, & Waksmundzka-Hajnos, 2016). In the case of biomaterials, antioxidant activity is essential, and it reduces the risk of inflammation by the viable release of antioxidant substances. In general, the results regarding the antioxidant activity are divided into two parts: (ⅰ) in vivo antioxidant activity and (ⅱ) in vitro antioxidant activity. However, the in vivo antioxidant activity depends on the in vitro antioxidant activity. In the past few years, there has been surging interest in determining the antioxidant activity of HMI using different spectrophotometric methods, such as reductive ability, superoxide anion radical, DPPH radical, and hydroxyl radical scavenging assays. Hydroxyl radicals are very strong oxidative free radicals that can cause cell death by damaging pyrimidines and purines in DNA (Chen, Mi et al., 2019). DPPH radicals are very persistent nitrogen-centered free radicals by virtue of their steric and conjugation barrier effects. Additionally, superoxide anion free radicals are a type of free radical formed during the metabolic process of all living organisms. Consequently, these free radicals attack biological macromolecules, further act as a precursor of hydrogen peroxide and hydroxyl radicals, and as a result, damage cell function and structure. In other words, the superoxide anion is one of the most destructive molecules for aerobic life owing to its toxic nature and large production (Chen, Mi et al., 2019; Liochev, 2013). The reduction ability of polysaccharides, including inulin, is also a prevalent mechanism corresponding to antioxidant activity (Chen et al., 2020). The reductive mechanism of inulin is dynamic, producing K4Fe(CN6) when using the oxidant K3Fe(CN6), which consequently reacts with Fe(III) to form Fe4[Fe(CN)6]3, resulting in an exceptional visible absorption at 700 nm. Thus, the reductive capacity of inulin derivatives and native inulin have been measured by using UV–vis spectrophotometry. The results revealed the robust reductive ability of the sample due to the massive formation of Fe4[Fe(CN)6]3, exhibiting a remarkable absorbance at approximately 700 nm. From this background, Chen et al. (2020) emphatically assessed the antioxidant activity of various types of inulin derivatives (Schiff bases) using the abovementioned methods. Compared to that of native inulin, the antioxidant activity of all inulin derivatives was effectively increased. The documented results demonstrated that the 3-HBSAIL-inulin derivative at 1.6 mg/ml significantly scavenged DPPH and hydroxyl radicals. Moreover, 2,3,4-THBSAIL and 3,4-DHBSAIL-inulin derivatives at 1.6 mg/ml exhibited exquisite antioxidant activity toward DPPH and superoxide radicals, with scavenging indices of approximately 100 % and 90 %, respectively. These two inulin derivatives also exhibited excellent antioxidant activity, even at a low concentration, i.e., at 0.1 mg/mL. In a deep analysis, it was found that scavenging ability against hydroxyl radicals decreased as the number of phenolic hydroxyl groups on the benzene ring decreased, in the order 2,3,4-THBSAIL> 3,4-DHBSAIL> 4-HBSAIL> 3-HBSAIL, with scavenging rates ranging from 79 % to 100 %. The same trend was also observed in the case of DPPH and superoxide radicals, with the order 3-HBSAIL> 4-HBSAIL> 2-HBSAIL > BSAIL. The results further proved the importance of phenolic hydroxyl groups in the case of radical scavenging ability. Moreover, the position of the phenolic hydroxyl groups on the benzene ring influences the scavenging ability. In general, the meta-position is more advantageous. It is worth mentioning that the number of phenolic hydroxyl groups did not influence the two inulin derivatives 2,3,4-THBSAIL and 3,4-DHBSAIL regarding superoxide and DPPH radical scavenging ability. The results of the reductive ability were nearly in agreement with the findings of DPPH and superoxide radicals. The inulin derivatives such as 2,3,4-THBSAIL and 3,4-DHBSAIL demonstrated better reductive abilities of approximately 3.9 and 3.7, respectively, at 1.6 mg/mL. Moreover, the 4-HBSAIL 3-HBSAIL, 2-HBSAIL, and BSAIL inulin derivatives showed better reductive ability than native inulin. Accordingly, the reductive ability of inulin derivatives is affected by the numbers of phenolic hydroxyl groups on the benzene ring.
The same group found the antioxidant activity of inulin derivatives that were prepared with quaternary ammonium salts. The findings showed that 1.6 mg/ml 2-imidazoleacetyl inulin chloride (IAIL) had great scavenging rates of approximately 67.8 % for superoxide radicals and 86.7 % for hydroxyl radicals compared to those of the 2-triethylamine acetyl inulin chloride (TAIL) and 2-(1-methylimidazole) acetyl inulin chloride (MAIL) derivatives. In general, the results demonstrated that imidazole and quaternary ammonium salt enhanced the antioxidant activity of inulin derivatives compared with native inulin. The results also revealed that the antioxidant rates may be increased due to the hydrophobic moiety of the prepared inulin derivatives (Chen et al., 2017). The profound alteration of IAIL and MAIL regarding antioxidant ability may be due to the substitution of 1-H by ethyl groups in the imidazole molecule. Moreover, a novel series of inulin derivatives were synthesized by using 1,2,3-triazole quaternization, and their antioxidant activity was assessed at different concentrations (Li et al., 2017). The DPPH, hydroxyl, and superoxide radical scavenging activity of the inulin derivative triazolium (5a to 5d) was better than that of the triazoles (4a to 4d). It is important to mention that the 5a to 5d and 4a to 4d inulin derivatives were characterized by H-NMR spectroscopy. The antioxidant ability was reported in the order of 5a–5d (IC50 0.16–0.32 mg/mL) < 4a–4d (IC50 0.34–0.59 mg/mL) < inulin. Moreover, another research group elucidated the antioxidant activity of native inulin and modified inulin through DPPH, hydroxyl, and superoxide radical scavenging activity assays. The inulin derivatives, i.e., 4-APAIL and 3,4-DAPAIL, exhibited exquisite antioxidant activity up to 80 % at 0.4 mg/mL, reaching 85 % at 1.6 mg/mL. The results suggested that the number of amino groups on pyridine significantly affected the antioxidant activity of inulin derivatives against the aforementioned radical scavenging models (Hu et al., 2014). Furthermore, Ren et al. (2011a) and Ren, Liu, Dong and Guo (2011) observed the antioxidant activity of O-aminoethyl and N-aminoethyl inulin derivatives by using superoxide and hydroxyl radical scavenging activity assays. The results revealed that the inulin derivatives demonstrated excellent antioxidant activity compared to unmodified inulin (Fig. 4). In addition, the modified inulin derivatives presented an average hydroxyl radical scavenging activity of approximately 35 %, whereas the superoxide radical scavenging activity ranged from 72.08 to 83.74 % at varying concentration levels (0.1−1 mg/mL) and DSs (0.14, 0.20, 0.54, 0.70, and 0.76). The antioxidant activity of modified inulin could be improved with increasing DS. Furthermore, it is noted that the attached N—H2 group might be conducive to the superoxide radical scavenging ability. Thus, the antioxidant activity is a significant property describing the functional attributes of HMI, which will be presented below.
2.14. Functional properties of HMI
The development of functional foods and their acceptance by consumers have escalated the demands of producing healthy food products. These functional foods contain a variety of health compounds, specifically polyphenols, whose ingestion is beneficial to the prevention of certain conditions, including hyperuricemia, hepatic injury, cancer, cardiovascular diseases (CVDs) and oxidative stress (Manach, Scalbert, Morand, Rémésy, & Jiménez, 2004; Mehmood et al., 2019, 2020). Thus, several studies have focused on the modification of inulin and the production of SCFA inulin esters. It is important to note that these esters have transformed the gut microbiota and improved the biological half-life of SCFAs due to the biotransformation of beneficial metabolites and increase the beneficial metabolites in the gut microbiota as reported in Fig. 5 b (Flint, Scott, Louis, & Duncan, 2012; Polyviou et al., 2016). It was found that the biological life cycle of SCFAs is shallow in the human gut microbiota, which has been well documented in various studies, reaching 13.5 min (Daniel et al., 1989). Further, SCFAs participate in energy metabolism, strengthen immunity, and help stimulate anorectic gut hormones (Bjerkeng, Storebakken, & Wathne, 1999). Several findings have confirmed that the utilization of drugs for the treatment of hepatic injury may be imperfect and exhibits partial therapeutic effects due to the variability of humans. Henceforth, there has been surging interest in exploring advanced strategies that can ameliorate hepatic injury risk, diabetes mellitus, and management of body weight (Fig. 5a). Interestingly, bioavailable propionates have been found to promote the release of gastrointestinal hormones, such as glucagon in the form of peptide-1 and peptide YY (PYY), to lower body weight and regulate appetite. Furthermore, these compounds were associated with reduced lipid and cholesterol levels. Similar to propionate, acetate can reduce appetite and induce apoptosis in colorectal cancer cells by increasing the amount of anorectic gut hormones including glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), and it also inhibits the production of TNF-alpha, which mediates hyperinsulinemia (Freeland & Wolever, 2010; Frost et al., 2014). The basic mechanism behind lowering the glycemic index is to impede the absorption of glucose using hydrolyzing enzymes, such as α-amylase and α-glucosidase.
Fig. 5.
(a) Functional properties of HMI, (b) Improvement of bioavailability of short-chain fatty acids.
Like acetate and propionate, butyrate plays an important physiological role in reducing the risk of colon cancer by taking advantage of the substantial functions of the colonic epithelium (Pingitore et al., 2017; Roshanravan et al., 2017). Recently, Zhu et al. (2018) prepared a propionate ester using pyridine and propionic anhydride. Inulin ester augmented the amount of SCFAs, reaching 117.2 mmol/mL compared to that of native inulin at approximately 90.2 mmol/mL, which resulted in the proliferation of Bifidobacterium to trigger an increased production of propionate during fermentation over 48 h in an in vitro model. As expected, the inulin ester had a high DS, approximately 2.86, due to the use of pyridine as a catalyst as well as a specific temperature and anhydride concentration. The results are consistent with the study of Hartzell et al. (2013), in which three important inulin esters, namely, butylated, acetylated, and propionylated, were synthesized using 1-methylimidazole and dimethylsulfoxide. These inulin esters, i.e., butylated, propionylated, and acetylated, showed relatively low DS values of approximately 0.371, 0.313, and 0.152, respectively. This phenomenon may be attributed to the differences in the catalyst, whereas Zhu et al. (2018) found higher DSs using pyridine as a catalyst. According to the study, it was proved that SCFA inulin esters, viz., acetylated, propionylated, and butylated, increased the SCFA profile to approximately 19.7, 20.0, and 24.1 mmol/100 mg, respectively, which could modulate the gut microbiota, particularly after 24 h of fermentation in an in vitro model. The inulin derivatives can be obtained using copolymerization techniques and have been grafted with catechin by hydrogen peroxide and ascorbic acid to perform as antidiabetic and hepatoprotective agents (Liu et al., 2014; Liu, Lu, Wen, Kan, & Jin, 2015). In this study, the grafting ratio was approximately 124.8 mg CAE/g, which was confirmed by FTIR analysis. The findings demonstrated that compared to native inulin, catechin-grafted inulin prominently decreased the inhibitory activity of α-amylase and α-glucosidase. These results reflected the potential of catechin-grafted inulin in the development of a novel effective antidiabetic agent. Moreover, catechin-grafted inulin significantly affected the levels of serum aspartate transaminase, alanine transaminase, alkaline phosphatase, and malondialdehyde. However, it increased the levels of hepatic superoxide dismutase, catalase, and glutathione peroxidase, resulting in a reduction in liver injury.
The liver is an integral part of the human body and performs critical functions such as the elimination of toxins and xenobiotics. Accordingly, it was a major relief that catechin-grafted inulin could be used to exterminate harmful toxins. Furthermore, Polyviou et al. (2016) developed inulin propionate ester (IPE) using propionic anhydride with 0–61 % wt propionate, i.e., (IPE-0 to IPE-61). IPE was tested through batch fecal fermentation (in vitro) and in a controlled, randomized, crossover study (in vivo). Native inulin was used as a control, and ad libitum food intake (kcal) was compared after 7 days with IPE-27 or IPE-54 supplementation (10 g/day all treatments). The results indicated that IPE-27 and IPE-34 prominently increased the propionate levels in an in vitro model, whereas in an in vivo model, IPE-27 exhibited excellent findings, i.e., decreased food intake, confirming that propionate plays a significant role in appetite modulation in the colon. The outcomes of this study were consistent with those of Chambers et al. (2015), who prepared IPE and proved that IPE inhibited food ingestion in adults by stimulating the release of GLP-1 and PYY from human colonic cells. Moreover, the stable isotope technique revealed the release of propionate from IPE. This result indicated that more than 80 % of propionate was released in the colon, suggesting that only a small quantity of esterified propionate was enzymatically degraded in the small intestine. Thus, the optimum levels of SCFAs, particularly propionate in the colon, can regulate body weight management on a large scale. However, the optimum percentage of SCFAs in the human gut microbiota is still unknown (Xu et al., 2020). Additionally, the physiological impact of IPE was evaluated on 21 obese or healthy overweight humans. For this purpose, IPE was added to food products, such as fruit smoothies and bread rolls. The findings showed that IPE-food products dramatically regulated appetite and augmented resting energy expenditure (REE), whereas the results regarding metabolic and hormone analysis were nonsignificant. To date, this is perhaps the first study concerning the direct addition of IPE into palatable food products in order to obtain practical results due to its lack of side effects on the GIT (Byrne et al., 2019).
The same research group has explored the effect of IPE and inulin on insulin sensitivity, systemic inflammatory responses, gut microbiota, and plasma metabolome in obese adults. It is important to emphasize that the molar and total percentages of SCFAs were insignificant in fasting and stool serum. However, IPE and inulin diet intervention markedly enhanced insulin resistance, reaching 1.23 and 1.17, respectively, compared to 1.59 for cellulose using homeostatic model assessment 2. A similar trend was observed in adipose tissue insulin resistance and was found to be approximately 6.5, 6.3, and 8.3 mmol/L×μU/mL for IPE, inulin, and cellulose, respectively. In addition, IPE and inulin altered the bacterial strains in the gut microbiome at the order, class, and species levels. However, there was no difference observed at the phylum level. For example, compared to cellulose supplementation, IPE supplementation enhanced the amount of Fusicatenibacter saccharivorans, B. xylanisolvens and B. uniformis, whereas it decreased the amount of B. faecale, Prevotella copri, and A. hadrus. Furthermore, the IPE diet intervention distinctly increased the IgG indices, which were observed to be 10.29 g/L, higher than those with cellulose (up to 9.89 g/L). The IL-8 levels were also increased with increasing supplementation of IPE, inulin, and cellulose, with values of approximately 5.86, 8.05, and 8.89 pg/mL, respectively. It is important to point out that the results regarding the systemic inflammatory response were significantly different between IPE and inulin diet intervention (Chambers et al., 2019). Likewise, Malkova et al. (2020) reported the physiological impact of 4-week IPE diet intervention under a normal exercise training schedule on plasma satiety hormones (viz., PYY and GLP-1) and body weight management. In total, 20 healthy overweight women volunteers contributed to this study and were divided into two groups, i.e., EX/placebo and EX/IPE. The results revealed that the EX/IPE group had decreases in body fat mass from 37.7 to 36.9 % and body weight from 77.3 to 76.6 kg. This effect was achieved due to increased intra-abdominal fat oxidation. The abovementioned in vivo studies have documented that supplementation with 10 g/day and 20 g/day IPE or IPE-food products is essential to manage body weight and body fat mass by reducing ad libitum energy intake and improving fat oxidation. Moreover, IPE diet intervention could increase the REE, but further research is required to understand whether IPE has the potential to enhance the REE. It has been confirmed that IPE supplementation also regulates appetite by anorectic gut hormones. Therefore, the essential mechanism for IPE appetite reduction remains to be elucidated. It is well known that the oral administration of SCFAs is unstable and unpalatable as a dietary mediation strategy. However, other pathways such as encapsulation of the duodenal supply are conceivable, but whether large or small intestinal SCFAs facilitate the aforementioned outcomes in a physiologically identical manner remains unknown. Moreover, valerate and caproate inulin esters remain to be studied. Valerate and caproate also have essential health effects but have drawn less attention. These two SCFAs are beneficial for glucose and lipid metabolism and reduce non-high-density lipoprotein cholesterol levels. They were also shown to increase the microbial abundance of Coprococcus spp. and Prevotella spp (Tap et al., 2015; Zhao et al., 2017).
2.15. Encapsulation properties of HMI
Microencapsulation is a robust technology involving the physical entrapment of delicate elements in a homogeneous or heterogeneous matrix, leading to their protection. This technology comprises several types of methodologies, for instance, freeze-drying, spray-cooling, spray-chilling, spray-drying, coacervation, polymerization, and evaporation. Most of them are solvent-based and involve expensive manufacturing and purification steps to obtain the desired product. According to the targeted properties of the substance, application, and material, the development of novel formulations requires different encapsulation or particle formation techniques. Nevertheless, the most frequently applied technology is 'spray-drying', which is substantially due to the cost-effectiveness and equipment availability (Beirão-da-Costa et al., 2013; Walz et al., 2018a). The formation of micellar aggregates by HMI derivatives has attracted much interest in recent years (Kokubun et al., 2018). Several studies have been conducted on the encapsulation of HMI for drug delivery and release, as summarized in Table 4 . Walz, Hagemann et al. (2018a) studied the encapsulation of dexpanthenol with inulin alone and acetylated as well as propionylated inulin by spray-drying. By the esterification of acetic anhydride and propionic anhydride with free hydroxyl groups, inulin was chemically modified. The yields of inulin alone, acetylated inulin and propionylated inulin were 78 %, 82 %, and 60 %, respectively, indicating that acetylated inulin had the maximum yield among them. Additionally, the particles displayed a great encapsulation efficiency of approximately 100 % for all polymeric materials. In another study published that year, the same group scrutinized the degradation of modified inulin as a prospective encapsulation material for the release of mesalamine and colon targeting (Walz et al., 2018a). Encapsulation of mesalamine with inulin and acetylated inulin was accomplished by spray-drying, and analysis of the release behavior was performed. The encapsulation efficiency of inulin was higher, i.e., 109 % ± 10 %, than that of AcIn (84 % ± 5 %). In addition, the particle yield was 82 % for inulin and 87 % for acetylated inulin. HMI, i.e., octenyl- and dodecenyl succinic anhydride derivatives (OSA- and DDSA-) of inulin have also been manufactured; their properties such as solution and interfacial properties were compared with those of a commercially available alkylated inulin, Inutec®SP1, along with the study of their emulsification as well as encapsulation properties (Kokubun et al., 2018). The encapsulation of beta-carotene with HMI was performed using the solvent evaporation method. Encapsulation using anionic succinylated derivatives had amazing benefits in the controlled release of beta-carotene in comparison with commercial nonionic Inutec®SP1. The DDSA-inulin sample was more effective in encapsulation, with a higher degree of modification than OSA-inulin and Inutec®SP1. These materials exhibit promising applicability in the encapsulation of active compounds, including antimicrobials, drugs, vitamins, aromas, etc. Furthermore, the drug delivery application of HMI-based doxorubicin (DOX) and paclitaxel (PTX) micelles for breast cancer treatment was studied to assess the transport mechanisms (Kesharwani et al., 2019). INT micelles encapsulated with doxorubicin (DOX) and paclitaxel (PTX) were produced by a thin-film hydration method. INT-micelles represent an exclusive delivery system for one or more chemotherapeutic agents, with very high drug encapsulation efficiency (89.5 % with DOX and 76.6 % for PTX).
Table 4.
Encapsulation Properties of HMI.
| HMI Type | Substances encapsulated with HMI | Encapsulation/Fabrication Method | Encapsulation Efficiency | Major Findings | References |
|---|---|---|---|---|---|
| OSA- and DDSA- derivatives of inulin. | Beta-carotene | Solvent evaporation | N/A | The DDSA (2) sample was more effective than the OSA-inulin and Inutec SP1 samples for encapsulating and releasing beta-carotene. | Kokubun et al. (2018) |
| Ac-In and Prop-In inulin. | Dexpanthenol | Spray-drying | 100 % for both | Ac-In and Prop-In inulin are more effective at encapsulating dexpanthenol than inulin is. | Walz et al., 2018a |
| AcIn inulin. | Mesalamine | Spray-drying | 109 % ± 10 % and 84 % ± 5 % | Mesalamine was encapsulated with high encapsulation efficiency along with high enzymatic degradability | Walz et al., 2018b |
| Lauryl carbamate derivative of inulin (Inutec SP1®, INT) | PTX and Doxorubicin | Thin-film hydration for INT-Micelles | 76.6 ± 11.23 % for INT-P, 89.5 ± 4.6 % for INT-D, 89.9 ± 3 % for INT-DP for DOX, and 48.6 ± 0.8 % for PTX | The efficiency of drug encapsulation is very high for INT micelles with a clathrin-mediated endocytosis pathway | Kesharwani et al. (2019) |
| OSA-inulin | Beta-carotene | Freeze-drying | N/A | Beta-carotene was solubilized within the micelles ranging from 12 −25 mg/g of OSA-inulin | Han et al. (2020) |
| Lauryl carbamate derivative of inulin (Inutec SP1®, INT) | PTX | Thin film hydration and solvent evaporation for INT-micelles | 95.66 ± 2.25 % | PTX-loaded INT micelles demonstrated outstanding drug encapsulation efficiency and drug loading with in vivo antitumor activity | Muley et al. (2016) |
| INVITE | CUR and CLX | dialysis method for INVITE micelles | 37.1 ± 1.5 for INVITE-CUR and 52.3 ± 1.3 for INVITE-CLX | Encapsulation of CUR and CLX in INVITE micelles boosted the water solubility of CUR and CLX and Encapsulation efficiency of INVITE-CLX was higher than that of INVITE-CUR | Mandracchia et al. (2016) |
| RGD-peptide conjugated inulin | EPB | Modified nanoprecipitation and dialysis for inulin-ibuprofen conjugates | 81.3 % | The RGD-conjugated EPB-loaded nanoparticles exhibit excellent encapsulation efficiency and antitumor efficacy | Zhang et al. (2016) |
OSA = octenyl succinic anhydrides, DDSA = dodecenyl succinic anhydrides, CUR = curcumin, EPB = epirubicin, CLX = celecoxib, PTX = paclitaxel.
Han et al. (2020) investigated how changes in pH trigger drug release, applying octenyl-succinylated inulin for the encapsulation and release of hydrophobic compounds. OSA-inulin particles were fabricated with beta-carotene by freeze-drying. The encapsulated beta-carotene was quickly released in the small intestinal fluid at pH 7 from freeze-dried particles but was not released after introduction into gastric fluid at pH 2.5. The results showed the potential application of OSA-inulin in the encapsulation and targeted delivery of hydrophobic drug molecules. Paclitaxel (PTX), a prevailing anticancer drug applied to treat a comprehensive range of cancers, has been utilized to synthesize paclitaxel (PTX)-loaded micelles; it was found that the lauryl carbamate derivative of the polyfructose natural polymer inulin (Inutec®SP1) self-assembled with micelles with almost 96 % encapsulation efficiency, exhibiting improved in vitro and in vivo antitumor activity and proving its potential in drug delivery systems for numerous types of cancers (Muley et al., 2016). Further, Mandracchia et al. (2016) explored the antiangiogenic activity of encapsulated curcumin or celecoxib in micelles based on an INVITE amphiphilic polymer and observed that the encapsulation efficiency for both curcumin and celecoxib was 37.1 ± 1.5 and 52.3 ± 1.3, respectively, with significant antiangiogenic potential, demonstrating their ability as antiangiogenic drug-loaded INVITE-based micelles for targeted anticancer therapy. The ability of nanoparticle drug delivery systems to improve antitumor efficiency and alleviate toxicity has attracted cumulative attention in cancer treatment. Accordingly, Zhang et al. (2016) synthesized RGD-peptide coupled inulin-ibuprofen nanoparticles for targeted delivery of encapsulated epirubicin. The encapsulation efficiency of EPB and loading contents were calculated to be 81.3 % and 8.1 %, respectively, with increased tumor inhibition and decreased systemic toxicity. It was expected that the encapsulation efficiency of OSA- and DDSA-inulin derivatives is similar to that of graft and block copolymers; however, the encapsulated properties of graft and block copolymers remain to be studied. Here, it is important to stipulate that there is still a plethora of research needed for the optimization of encapsulation methodologies for several inulin derivatives to augment the encapsulation efficiency, which would serve as a tool to design novel encapsulated drugs or vaccines in controlled delivery systems.
2.16. Advantages of HMI in drug delivery systems
Natural biomolecule-based drug delivery systems have recently emerged as a novel approach to protect, release or encapsulate hydrophobic therapeutics or bioactive compounds or drugs to increase their biological potency. These macromolecules comprise proteins, including zein, gelatin, etc., polysaccharides including inulin, starch, chitosan, etc., and lipids, including lipid nanocarriers such as lipid-drug conjugates (LDCs), solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs) (Lu et al., 2019; Chen, Miao, Campanella, Jiang, & Jin, 2016; Shah et al., 2019). Due to their many favorable characteristics such as cost-effectiveness, nontoxicity, nonreactivity, availability at a large scale, biodegradability and biocompatibility, the most well-known choice for targeted nutraceuticals or drug delivery systems is polysaccharides.
Polysaccharides also possess physicochemical characteristics, offering suitable sites for chemical modification as needed and permitting informal fabrication of particles and hydrogels for delivery or release purposes. These compounds also promote adapted cellular physiology, which is responsible for several aforementioned properties. Overall, polysaccharides are the paramount choice for the creation of drug delivery vehicles (Barclay, Day, Petrovsky, & Garg, 2019).
Recently, cellulose derivatives have commonly been used as essential ingredients in the manufacturing of cosmetics, production of functional foods, and formulation of pharmaceutical products (Abbaspoor, Ashrafi, & Abolfarsi, 2019). Moreover, many other considerable examples, such as dextran (Anirudhan, 2016), hyaluronic acid (Huang & Chen, 2019; Huang et al., 2019; Tripodo et al., 2015c), chitosan (Sahariah & Masson, 2017), pullulan (Alhaique, Matricardi, Di Meo, Coviello, & Montanari, 2015), and starch (Chen, Hao et al., 2019), have widely been applicable in the field of pharmaceuticals. However, these compounds have some potential disadvantages as drug delivery vehicles including their mixed molecular weights, variable chemistry, lack of solubility in most organic solvents, and slow enzymatic degradation, which makes it hard to precisely define the delivery vehicle (Barclay et al., 2019).
The fact that inulin is not digested or absorbed by humans in the small intestine makes this polymer an alluring transporter for gastrointestinal drug delivery. Thus, inulin has been demonstrated to be a versatile substance for application as a drug vehicle. Moreover, inulin may serve as a perfect model for microbially activated drug delivery to the colon, which leads to sole applications such as identification of kidney function and colonic targeting, where metabolization by microbiota present in the colon has been utilized (López-Molina et al., 2015).
Compared with other saccharides, inulin varies in terms of the type of glycosidic bond between monomers as well as molecular weight. It possesses a higher molecular weight than saccharides, which is relevant to a lower solubility, along with a higher glass transition and melting temperature as well as higher viscosity. Inulin also has high molecular flexibility because of its (2→1) linked d-fructosyl backbone in comparison with other saccharides (Kokubun et al., 2018; López-Molina et al., 2015). Reducing groups of saccharides are undesired for various pharmaceutical applications. Therefore, inulin is more suitable as an excipient than other saccharides when there is concern about reducing groups (Mensink, Frijlink, van der Voort Maarschalk, & Hinrichs, 2015). It also received generally recognized as safe (GRAS) status by the Food and Drug Administration (FDA) due to its several outstanding properties including biodegradability, renewability, nontoxicity, etc., compared to many other polysaccharides as described in Fig. 6 (Afinjuomo et al., 2019).
Fig. 6.
Major advantages of hydrophobically modified inulin in drugs and vaccines.
Over the past few years, hydrophobically modified polysaccharides (HMPs), especially hydrophobically modified inulins (HMIs), have been gaining greater attention in numerous drug delivery systems owing to their capability to produce self-assembling micelles and alleviate safety concerns (Kesharwani et al., 2019; Kokubun et al., 2018). Polymeric micelles have also received substantial interest as versatile drug delivery platforms. Micelles are self-assembling colloidal particles comprising two chief parts, namely, a hydrophilic shell and a hydrophobic core, which play a key role in the pharmacokinetic behavior of the delivery system. A hydrophilic shell interacts with aqueous biological fluids, while the hydrophobic core acts as a repository for poorly water-soluble drugs (Kesharwani et al., 2019).
The application of inulin alone as a hydrophobic drug delivery vehicle is inadequate due to its high water solubility. For its application as a gastrointestinal drug carrier material, the physicochemical characteristics of inulin can be altered by the replacement of hydroxyl groups with hydrophobic functional groups. Thus, inulin derivatives have been manufactured to acquire suitable systems for various applications including hydrogels, surfactants, microspheres, etc. (Sun et al., 2018; Walz, Hirth, & Weber, 2018b). Recently, inulin has been fabricated with many hydrophobic functional groups including methyl esters, fatty acid chlorides, alkyl epoxides, alkyl isocyanates, etc., in organic solvents to obtain several hydrophobic derivatives. HMI has numerous benefits, such as biodegradability, biocompatibility, renewability, and strong stability at extreme electrolyte concentrations and under highly acidic conditions as well as extreme temperatures (Doost et al., 2018).
2.17. Importance of hydrophobic drugs
Drug targeting can be defined as the targeted delivery of a drug to the site of action. The reproducible and continuous release rate of the pharmaceutical or targeted compound is the benefit of drug targeting, which helps to prevent overdose and in turn alleviates the side effects and drug toxicity. In the recent few decades, an upsurge in the global frequency of colonic diseases has resulted in the increased urgency for operational local treatment of colonic diseases, for instance, Crohn’s disease, ulcerative colitis, amebiasis, colonic cancer, colorectal cancer (CRC), inflammatory bowel disease (IBD), etc., for more effective and safer drug therapies. There is an extreme need for targeted drug delivery into the colon for local treatment of a range of bowel diseases and colonic pathologies through the systemic delivery of protein and peptide drugs (Walz et al., 2018b; Lee et al., 2020; Han et al., 2020; Philip & Philip, 2010).
Colon targeting has developed increasing interest over the past few decades because of its ability to treat colon-specific diseases with fewer side effects. Colon-targeted drug delivery systems, in addition to contemporary delivery, are advantageous for improving the bioavailability of drugs that are at risk of enzymatic or acidic disruption in the upper gastrointestinal (GI) tract, specifically macromolecules such as proteins and peptides, because of lower protease activity in the colon. This approach of transporting a hydrophobic drug into the lower intestinal tract might emerge as an effective plan to achieve local drug release and a targeted therapy for various intestinal diseases (Walz et al., 2018b; Mandracchia et al., 2018).
2.18. HMI-mediated targeted delivery/release of drugs
For decades, there has been rising interest in inulin-coated metallic nanoparticles, inulin-based hydrogels, inulin-based nanomicelles, and inulin-conjugated polymeric nanoparticles for drug delivery applications (Fig. 7 ). Inulin has been chemically modified to obtain new photocrosslinkable derivatives. UV-photocrosslinking of inulin derivatives resulted in the formation of hydrogels that were applied for the drug delivery of ibuprofen (Tripodo, Pitarresi, Palumbo, Craparo, & Giammona, 2005). Inulin was derivatized with methacrylic anhydride (MA) to acquire four INU-MA derivatives photocrosslinkable by UV irradiation. Further, one of the derivatives INU MA1 was derivatized with succinic anhydride (SA) to obtain an INU-MA1-SA derivative, which was again crosslinked by UV irradiation. Then, the model drug ibuprofen was loaded by immersion into INU-MA1 and INU-MA1-SA hydrogels, and from these matrices, release studies were carried out in gastrointestinal fluids. The INU-MA1-SA hydrogel showed pH-dependent swelling and high resistance to acidic degradation. The drug delivery profile of both INUMA1 and INU-MA1-SA hydrogels was studied. Compared to the INU-MA1 hydrogel, the INU-MA1-SA hydrogel showed little release of the drug in gastric fluid and a high release in simulated intestinal fluid based on swelling and degradation data. In contrast, the INU-MA1 hydrogel acted as a drug delivery system after oral administration, even though the release of the drug was not dependent on alterations in physiological pH. HMIs such as Inutec®SP1 present a safe, low-cost, and natural alternative to broadly used PEG-modified polymers for the formulation of micellar delivery systems for paclitaxel. Inutec®SP1 possesses outstanding tensioactive properties and is an emulsifier in the pharmaceutical industry. Thus, for micellar delivery of paclitaxel, it has been utilized as an amphiphilic carbohydrate polymer through the intravenous route. The paclitaxel (PTX)-embedded micelles showed high drug encapsulation efficiency (95.66 ± 2.25 %) and loading (8.69 ± 0.22 %); moreover, they displayed low toxicity and exceptional hemo-compatibility toward cultured cells as well as the continued release of PTX and greater anticancer efficiency in vitro in mouse melanoma cells (B16F10) and equivalent in vivo antitumor activity in a B16F10 allograft mouse model (Muley et al., 2016). The chemical modification of inulin results in a decline in enzymatic degradation capability by enzymes, which can be expressed by the colon microbial flora. Degradation studies of modified inulin as a potential encapsulation material and release of mesalamine in the colon have been conducted. Different degrees of substitution of acetylated inulin were obtained. Microparticles synthesized from inulin and acetylated inulin were loaded with the colon-specific drug mesalamine by spray-drying. Acetylated inulin microparticles presented less burst release of mesalamine than inulin particles within 6 h, followed by a continuous drug release phase (Walz et al., 2018a). Further, the applicability of inulin as a drug vehicle system for dexpanthenol in particles has been explored. By esterification of free hydroxyl groups with propionic anhydride and acetic anhydride, chemically modified inulin was prepared using a spray-drying technique, resulting in smooth and spherical particles. Dexpanthenol (1 %) was encapsulated, and release behavior studies were conducted. Overall, chemically modified inulin derivatives showed a longer drug release; i.e., after 24 h, acetylated inulin particles released 60 % of the drug, and only 10 % of the drug was released by propionylated inulin. On the other hand, inulin particles released 100 % dexpanthenol after 6 h (Walz, Hirth et al., 2018). For the transport of the highly hydrophobic drug celecoxib, INVITE-SA, a pH-sensitive micelle prepared from a succinylated inulin-vitamin E polymer, has been proven to be the best choice for targeted site-specific intestinal drug delivery. Mandracchia et al. (2018) synthesized pH-sensitive inulin-based nanomicelles INVITE-SA for intestinal site-specific and controlled release of celecoxib. The resulting INVITE-SA micelles were nanosized, with a pronounced pH-dependent release profile. The micelles were stabilized against acidic hydrolysis, and drug release was strongly dependent on the pH. At pH 1.2 in PBS, only 1 % of the drug was released after 2 h in PBS; however, at pH 6.8 in PBS, a controlled and quick release occurred for nearly 10 h.
Fig. 7.
Schematic demonstration of drug-loaded various inulin (INU)-based drug delivery carriers (a) Inulin coated metallic nanoparticles, (b) Inulin-based nanomicelles, (c) Inulin conjugated polymeric nanoparticles, (d) Inulin-based hydrogel.
Inulin-based micelles embedded with curcumin or celecoxib, which are highly hydrophobic drugs, display prominent antiangiogenic activity (Mandracchia et al., 2016). This study was the first to report angiogenesis suppression triggered by CLX-loaded polymeric micelles. CUR or CLX were introduced to INVITE micelles by the dialysis method. Not only CUR-loaded but also CLX-loaded INVITE micelles showed notable antiangiogenic activity, as proven by in vivo CAM experiments. Additionally, there was a rise in the water solubility of CUR and CLX by using this INVITE nanotechnology. These results have opened the doors in regenerative medicine as well as anticancer or diabetic maculopathy therapy based on the antiangiogenesis strategy. For cancer therapy, the inulin-based glutathione-receptive delivery system was found to be productive in colorectal cancer and promoted the growth and development of useful commensal microbiota in the gut. Inulin esterified with lipoic acid and a novel delivery system for tanshinone IIA for the treatment of colorectal cancer in vitro were established. It was observed that in tumor cells, the drug-loaded CR micelles discharged the loaded drug along with the addition of 10 mM DTT, and the release of tanshinone IIA in the system was highly receptive to glutathione (Wang et al., 2018). Further, a rifampicin (RIF)-loaded antituberculosis drug delivery system was developed based on two inulin derivatives (viz., INVITE and INVITE-SA) for treatment against M. tuberculosis or other bacterial infections. RIF was incorporated in INVITE or INVITE-SA micelles by dialysis. INVITE-SA-RIF possessed higher antibacterial activity against gram-positive bacteria than INVITE-RIF. For up to seven days, both INVITE and INVITE-SA released approximately 80 % w/w RIF, and no verified differences between the INVITE and INVITE-SA drug release profiles were observed (Tripodo et al., 2019). During the last decade, regarding deteriorating pathologies of the retina, corticosteroid therapy has arisen as a propitious treatment. Nevertheless, it is essential to discover an alternative promising ocular delivery system that can release corticosteroids very effectively. An amphiphilic derivative of inulin (INU-EDA-RA) was synthesized by fabrication with ethylenediamine (EDA) and retinoic acid (RA) to form micelles in aqueous media using the solvent casting method. Three corticosteroid drugs, viz., dexamethasone (DEX), triamcinolone (T), and triamcinolone acetonide (TA), loaded with INU-EDA-RA micelles were selected for the treatment of degenerative pathologies of the retina. It was observed that INU-EDA-RA micelles quickly released a high percentage of the entrapped drug based on the drug release profiles. Owing to the mucoadhesive properties, capability to release encapsulated drugs, and suitable particle size, this drug delivery system is ideal for ocular drug delivery (Di Prima et al., 2017).
For the delivery of single or amalgamated therapeutics in breast cancer treatment, HMI-based micelles are inexpensive, efficient, and safe alternatives. Nanomicelles of lauryl carbamate HMI (Inutec SP1) used to transport a combination of chemotherapeutic drugs (PTX and DOX) for breast cancer treatment were synthesized by the thin-film hydration technique. The drug encapsulation efficiency was found to be very high with INT nanomicelles (89.5 % with DOX and 76.6 % for PTX). At pH 7.4, the in vitro drug release from the micelles was constant for more than 72 h, and PTX was released at a lower rate than DOX (approximately 50 %) from INT-D within the initial 24 h because PTX is more hydrophobic than DOX (Kesharwani et al., 2019). Very recently, octenyl-succinylated inulin (OSA-inulin) particles produced by freeze-drying were studied for the entrapment and release of beta-carotene. Beta-carotene was easily dissolved in the hydrophobic cores of the micelles, and alterations in pH activated its release. When administered into gastric fluid at pH 2.5, the encapsulated beta-carotene was not released from the freeze-dried particles. Conversely, it was readily released in small intestinal fluid at pH 7 (Han et al., 2020). The ability of mesenchymal stromal cells (MSCs), when administered intravenously, to discharge a massive amount of bioactive molecules with immunomodulatory properties makes them a great drug repository that can travel especially to damaged tissues; this capability has been utilized by Tripodo, Chlapanidas et al. (2015a) to design a drug delivery system based on MSCs loaded with curcumin-INVITE (inulin-d-α-tocopherol succinate bioconjugates) micelles for the treatment of neurodegenerative diseases. This study established that curcumin-loaded micelles achieved maximum concentration-dependent loading in MSCs in a few minutes and were able to substantially release the entrapped drug, proving the viability of this approach for the therapy of selected neurodegenerative diseases. The same authors also published another study in the same year, using the same INVITE micelles as nanocarriers for effective intravenous injection of curcumin to develop the biopharmaceutical characteristics of hydrophobic drugs (Tripodo et al., 2015b). The authors prepared INVITE bioconjugates with different degrees of derivatization, i.e., INVITE 1, 2, and 3, and further evaluated their drug release profile. INVITE 3MC was able to release 42 % curcumin in PBS at pH 7.4 in 48 h and 53 % at pH 5.5, while INVITE 2MC released 23 % curcumin in PBS at pH 7.4 in 48 h and 33 % at pH 5.5. In the case of INVITE 1 MC, 15 % of curcumin was released in PBS at pH 7.4 and 25 % at pH 5.5. It was observed that curcumin release for all of the INVITE micelles at pH 5.5 was ≈10 % higher than that at pH 7.4 and was controlled by penetration through the cellular membrane.
The synthesis of self-assembling micelles based on amphiphilic inulin graft copolymers for anticancer model drug doxorubicin delivery was reported by Licciardi, Scialabba, Sardo, Cavallaro and Giammona (2014). The micelles based on two graft copolymers, INU-ceramide and INU ceramide-PEG2000, were loaded with the drug doxorubicin, and its release was studied in different media; the micelles were able to release DOXO in the complete form for a longer time without burst release with 16−17 wt % of drug loading. A study was conducted to assess whether cinnamoylated inulin is inhibited by the enzyme inulinase to reveal its potential in colonic drug delivery. Microspheres of cinnamoylated derivatives of inulin were synthesized in the presence of Tween 20, and it was found that these microspheres were proficiently hydrolyzed by the inulinase enzyme and were successfully embedded with MTX. The MTX release by these microspheres was controlled, and the observation was made that MTX release from the microspheres was triggered by enzymatic hydrolysis of the polymer by inulinase (López-Molina et al., 2015). Small interfering RNAs (siRNAs) have also been found to possess therapeutic value for many human diseases such as cancer, metabolic diseases, neurodegenerative diseases, and cardiovascular diseases in comparison with traditional drugs, which epitomize an evolving model for the treatment of many human diseases. Sardo et al. (2015) designed a novel inulin-diethylenetriamine (Inu-DETA)-based siRNA transporting system that effectively delivered functional siRNAs and was highly cytocompatible with no cytotoxicity, opening the way for the treatment of these aforementioned diseases. Magnetic nanoparticles represent an advanced group of nanocarrier materials that can deliver target anticancer drugs, and this ability of magnetic nanoparticles has been utilized by Scialabba et al. (2014). The authors designed a novel self-assembly based on inulin-polymer (PEGylated squalene grafted inulin amphiphile)-coated superparamagnetic iron oxide nanoparticles (SPIONs) for targeted cancer therapy, which were able to self-organize into nanocarriers to deliver the drug doxorubicin. The nanoparticles liberated doxorubicin in the intact form for a prolonged time without a first burst effect and displayed higher anticancer activity than the free drug, exhibiting excellent magnetic targeting. Further, self-assembling core-shell-type nanostructures of enzyme-sensitive inulin-dehydropeptide conjugates were analyzed for targeted delivery of ornidazole as a remedy for gastrointestinal diseases (Shivhare et al., 2018). Dehydrophenylalanine was introduced in the peptide to stabilize it over a broad spectrum of pH values and proteases. In addition to drug delivery, the effect of the inulinase enzyme on inulin peptide degradation has been studied, and it was concluded that the discharge was controlled in the presence of the inulinase enzyme, where ∼93 % of ornidazole was liberated. The semicrystalline form of inulin, i.e., delta inulin or AdvaxT, induces the development of cellular as well as humoral immune responses to a wide spectrum of antigens after interacting with the immune system. This property of nanostructured delta inulin particles to boost the immunogenicity of injected protein antigens has been utilized as a vaccine adjuvant (Wang et al., 2017). It was observed that the administered delta inulin particles were transported to the liver as well as secondary lymphoid tissue after being primarily taken up by macrophages, hydrolyzed to be dispersed into the bloodstream and finally eliminated into the urine. Furthermore, delta inulin was modified by coating doxorubicin on its nanostructured surface for application in targeted drug delivery for anticancer therapy (Wang et al., 2019). The authors used the property of delta inulin in which it is endocytosed by monocytes to design a platform to transport doxorubicin to lymphoid organs. The study showed that 2.48 ± 0.12 % w/w doxorubicin was loaded onto the surface of the delta inulin. The drug release profile revealed pH-dependent controlled drug discharge in artificial lysosomal fluid (ALF), reflecting the applicability of delta inulin-doxorubicin particles for the treatment of cancer.
2.19. HMI mediated targeted delivery/release of vaccines
Vaccines have been considered to be one of the most vital scientific breakthroughs for the prevention and treatment of numerous infectious diseases. For several diseases, including cancer, AIDS, Ebola, malaria, influenza, etc., the unavailability of current vaccine technologies is attributable to their inability to adequately stimulate both cellular and humoral immune responses at safe doses. Hence, before any severe pandemic or epidemic disease outbreaks such as COVID-19, there is a medical necessity to investigate new vaccine adjuvants or technologies on an immediate basis (Gallovic et al., 2016; Kumar, Kesharwani, Kuppast, Bakkari, & Tummala, 2017). A novel pathogen simulating a vaccine transporting system was developed by targeting specific signaling pathways of the innate immune system. To overcome alum's numerous inadequacies, chemically modified inulin, Ace-IN, was used to provide numerous characteristics that have benefits for a vaccine delivery vehicle when encapsulated into microparticles (MPs), playing dual roles as an immune-stimulatory adjuvant and antigen delivery vehicle (Gallovic et al., 2016). Naturally occurring inulin polysaccharides have been chemically modified to produce acid-sensitive hydrophobic microparticles (MPs) (acetylated inulin, Ace-IN) by oil-in-water emulsions followed by solvent evaporation. Texas Red-labeled OVA (TR-OVA) antigen was encapsulated in Ace-IN MPs using the W/O/W homogenization procedure. At pH 7.4, TR-OVA was released slowly from Ace-IN MPs, with just 20 % release at 168 h, while after optimization, 100 % release of OVA occurred in only 16 h at pH 7.4. Moreover, higher production of anti-OVA IgG antibody levels was identified when mice were immunized with Ace-IN MPs embedded with ovalbumin (OVA) antigen. To target antigen-presenting cells (APCs), a unique particle-based pathogen-mimicking vaccine delivery system (PMVDS) was designed by Kumar et al. (2017) using inulin acetate (InAc), which triggered innate immunity. PMVDS delivered improved, prolonged antigen delivery to APCs very efficiently and concurrently as an immune-adjuvant, activating Toll-like receptor-4 (TLR-4) on APCs to release cytokines. The release of the OVA antigen was controlled to less than 25 % of the total embedded antigen and was constant for a more extended period than the control. This technology has broad applications in developing a new generation of vaccines against both intracellular and extracellular pathogens. The encapsulated hydrophobic inulin-loaded drugs have been much better studied than vaccines, which may be due to the wider availability of drugs as therapeutic compounds. However, it is imperative to develop new vaccines loaded with natural materials, such as hydrophobic inulin.
2.20. HMI-mediated nanoparticle-based targeted delivery/release of drugs
Currently, nanotechnology is at the cutting edge of drug delivery and pharmaceutical research. Recently, due to their noteworthy superiority in increasing antitumor efficiency and attenuating toxicity, especially in cancer treatment, nanoparticle-based drug delivery systems have attracted growing attention. When the formulation is meticulously injected or entrapped, the nanoparticles gradually release the anticancer drugs inside solid tumors with the appropriate sizes and surface properties. Due to the subcellular and nanoscale size, this nanoparticle drug delivery system can simply permeate deeply through tissues and delicate capillaries (Kesharwani et al., 2019; Zhang et al., 2014). The application of methylprednisolone-loaded ibuprofen-modified inulin-based nanoparticles prepared by self-assembly for drug delivery in the treatment of spinal cord injury was studied. The synthesis of ibuprofen-modified inulin was achieved by in situ activation of the carboxylic acid with N,N′-carbonyldiimidazole through a direct esterification linkage. Methylprednisolone-loaded nanoparticles did not display evident cytotoxic effects when assessed against RSC-96 cells. The drug encapsulation and loading amounts were found to be 91.2 ± 1.2 % and 14.9 ± 0.8 %, respectively. A drug release study showed that approximately 94.9 % of the loaded methylprednisolone was released from the nanoparticles within 96 h (Zhang et al., 2014).
Two years later, the same research group employed RGD peptide-modified inulin-ibuprofen nanoparticles for targeted delivery of epirubicin, which was used against several types of cancers. For targeted drug delivery, RGD-coupled EPB-based nanoparticles were fabricated by the self-assembly of inulin-ibuprofen polymer and in situ entrapment of EPB. It was observed that the RGD-coupled EPB-loaded nanoparticles increased cellular uptake and lowered cytotoxicity. More importantly, they exhibited better tumor growth suppression and decreased systemic toxicity. The EPB release exhibited a speedy burst release profile; the EPB release profile at pH 5.0 was found to have a slower release speed, with approximately 67 % of the total EPB released before 24 h in comparison with that at pH 7.4, where 87 % of the EPB was released from EPB-loaded nanoparticles after 48 h (Zhang et al., 2016). Redox-sensitive nanoparticles coupled with 4-aminothiophenol-carboxymethyl inulin (ATP-CMI) were prepared for the specific delivery of budesonide (BDS) to the swollen mucosa in inflammatory bowel diseases. The ATP-CMI-based nanoparticles (NPs) were obtained by embedding 4-aminothiophenol onto carboxymethyl inulin (CMI). The NPs displayed a high release rate (80 wt %) in GSH containing 20 mM GSH. In contrast, GSH-free media showed a low release rate (45 wt %) (Sun et al., 2018). Currently, it is essential to seek a way to boost the transcorneal entry of drugs to effectively treat chronic ocular diseases. Di Prima et al. (2019) recently developed an inulin-based mucoadhesive PEGylated self-assembling nanoparticle INU-EDA-RA-PEG drug delivery system for improved transcorneal penetration of corticosteroids. INU-EDA-RA-PEG was utilized to synthesize self-assembling nanoparticles and corticosteroid-loaded self-assembling nanoparticles by the film rehydration technique. The self-assembling nanoparticles demonstrated suitable particle size values, mucoadhesiveness, and cytocompatibility and were capable of loading and discharging a high quantity of triamcinolone (T), dexamethasone (DEX), and triamcinolone acetonide (TA). As a result, the inulin-based self-assembling nanoparticles displayed substantial potential for ocular topic drug delivery.
3. Conclusion and future outlook
Inulin is a popular natural polysaccharide owing to its (ⅰ) high molecular flexibility, (ⅱ) easy availability, (ⅲ) high biodegradability, biocompatibility, (ⅵ) low toxicity, and (ⅴ) nonreactogenicity. In other words, its modification is very easy and tends to be used to provide steric stabilization for various dispersion formulations. Moreover, hydrophobic inulin has a wide range of functions as a targeted drug delivery vehicle in the human body, encompassing (ⅰ) a range of targeting approaches appropriate for gastrointestinal fate, (ⅱ) a potential prospect to enhance the biological half-life of loaded therapeutics, and (ⅲ) improvement of the circulation of phagocyte cells that ingest damaging particles, dead cells, and bacteria. One drawback of this natural polysaccharide as a drug excipient is that it is hard to define the structure of this polysaccharide regarding chemistry and molecular weight owing to its environmental and seasonal variations. The above-emphasized disadvantage of inulin in drug vehicles is that it is not a permanent solution for all self-healing drug applications. Notwithstanding, the advantages of inulin more often outweigh the disadvantages, and thus, in the future, it is expected to be utilized on a large scale in pharmaceutical science in drug vehicles through normal biological and physical processes. It is an enticing fact that the derivatization of inulin can enhance the functionalities in a single simple system and can further provide magnificent solutions to the intricate problems facing encapsulated hydrophobic inulin-mediated drugs. It is clear that these meticulous studies have promoted the application of hydrophobic inulin in various drugs and even in vaccines in the future.
Authors contributions
The author Muhammad Usman: Data curation, Writing- Original draft preparation. Prasanna Jagannath Patil: Reviewing, Editing, and Writing. Arshad Mehmood and Junaid Haider: Visualization, Reviewing and Preparation of figures. Muhammad Bilal and Shabbir Ahmad: Reviewing, Editing and Visualization. Chengnan Zhang and Xiuting Li: Conceptualization and Supervision. I would like to express my sincere gratitude to my Prof. Xiuting Li for the continuous support during review article writing regarding motivation, patience, and immense knowledge.
Declaration of Competing Interest
The authors confirm that they have no known competing financial interests or personal relationship that could have appeared to influence the manuscript.
Acknowledgments
The authors wish to express their deep gratitude and appreciation for the support obtained from the National Key Research and Development Program of China (2017YFD0400206).
References
- Abbaspoor S., Ashrafi A., Abolfarsi R. Development of self-healing coatings based on ethyl cellulose micro/nano-capsules. Surface Engineering. 2019;35(3):273–280. [Google Scholar]
- Afinjuomo F., Barclay T.G., Song Y., Parikh A., Petrovsky N., Garg S. Synthesis and characterization of a novel inulin hydrogel crosslinked with pyromellitic dianhydride. Reactive & Functional Polymers. 2019;134:104–111. [Google Scholar]
- Alhaique F., Matricardi P., Di Meo C., Coviello T., Montanari E. Polysaccharide-based self-assembling nanohydrogels: An overview on 25-years research on pullulan. Journal of Drug Delivery Science and Technology. 2015;30:300–309. [Google Scholar]
- Anirudhan T.S. Dextran based nanosized carrier for the controlled and targeted delivery of curcumin to liver cancer cells. International Journal of Biological Macromolecules. 2016;88:222–235. doi: 10.1016/j.ijbiomac.2016.03.040. [DOI] [PubMed] [Google Scholar]
- Ansari R.M., Bhat B.R. Copper (II) Schiff base-graphene oxide composite as an efficient catalyst for Suzuki-Miyaura reaction. Chemical Physics. 2019;517:155–160. [Google Scholar]
- Antony R., Arun T., Manickam S.T.D. A review on applications of chitosan-based Schiff bases. International Journal of Biological Macromolecules. 2019 doi: 10.1016/j.ijbiomac.2019.02.047. [DOI] [PubMed] [Google Scholar]
- Anush S.M., Vishalakshi B., Kalluraya B., Manju N. Synthesis of pyrazole-based Schiff bases of Chitosan: Evaluation of antimicrobial activity. International Journal of Biological Macromolecules. 2018;119:446–452. doi: 10.1016/j.ijbiomac.2018.07.129. [DOI] [PubMed] [Google Scholar]
- Apolinário A.C., de Lima Damasceno B.P.G., de Macêdo Beltrão N.E., Pessoa A., Converti A., da Silva J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydrate Polymers. 2014;101:368–378. doi: 10.1016/j.carbpol.2013.09.081. [DOI] [PubMed] [Google Scholar]
- Barclay T.G., Day C.M., Petrovsky N., Garg S. Review of polysaccharide particle-based functional drug delivery. Carbohydrate Polymers. 2019 doi: 10.1016/j.carbpol.2019.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirão-da-Costa S., Duarte C., Bourbon A.I., Pinheiro A.C., Januário M.I.N., Vicente A.A.…Delgadillo I. Inulin potential for encapsulation and controlled delivery of Oregano essential oil. Food Hydrocolloids. 2013;33(2):199–206. [Google Scholar]
- Berhanu A.L., Mohiuddin I., Malik A.K., Aulakh J.S., Kumar V., Kim K.H. A review of the applications of Schiff bases as optical chemical sensors. TrAC Trends in Analytical Chemistry. 2019 [Google Scholar]
- Bjerkeng B., Storebakken T., Wathne E. 1999. Cholesterol and short-chain fatty acids in diets for Atlantic salmon Salmo salar (L.): Effects on growth, organ indices, macronutrient digestibility, and fatty acid composition. [Google Scholar]
- Byrne C.S., Chambers E.S., Preston T., Tedford C., Brignardello J., Garcia-Perez I.…Frost G.S. Effects of inulin propionate ester incorporated into palatable food products on appetite and resting energy expenditure: A randomised crossover study. Nutrients. 2019;11(4):861. doi: 10.3390/nu11040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers E.S., Viardot A., Psichas A., Morrison D.J., Murphy K.G., Zac-Varghese S.E.…Blundell J.E. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers E.S., Byrne C.S., Morrison D.J., Murphy K.G., Preston T., Tedford C.…Reynolds C.J. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut. 2019;68(8):1430–1438. doi: 10.1136/gutjnl-2019-318424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Miao M., Campanella O., Jiang B., Jin Z. Biological macromolecule delivery system for improving functional performance of hydrophobic nutraceuticals. Current Opinion in Food Science. 2016;9:56–61. [Google Scholar]
- Chen Y., Zhang J., Tan W., Wang G., Dong F., Li Q., Guo Z. Antioxidant activity of inulin derivatives with quaternary ammonium. Starch‐Stärke. 2017;69(11–12) [Google Scholar]
- Chen Y., Tan W., Li Q., Dong F., Gu G., Guo Z. Synthesis of inulin derivatives with quaternary phosphonium salts and their antifungal activity. International Journal of Biological Macromolecules. 2018;113:1273–1278. doi: 10.1016/j.ijbiomac.2018.03.055. [DOI] [PubMed] [Google Scholar]
- Chen Y., Mi Y., Li Q., Dong F., Guo Z. Synthesis of Schiff bases modified inulin derivatives for potential antifungal and antioxidant applications. International Journal of Biological Macromolecules. 2020;143:714–723. doi: 10.1016/j.ijbiomac.2019.09.127. [DOI] [PubMed] [Google Scholar]
- Chen Y., Hao Y., Ting K., Li Q., Gao Q. Preparation and emulsification properties of dialdehyde starch nanoparticles. Food Chemistry. 2019;286:467–474. doi: 10.1016/j.foodchem.2019.01.188. [DOI] [PubMed] [Google Scholar]
- Chen Y., Mi Y., Sun X., Zhang J., Li Q., Ji N., Guo Z. Novel inulin derivatives modified with Schiff bases: Synthesis, characterization, and antifungal activity. Polymers. 2019;11(6):998. doi: 10.3390/polym11060998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel P., Brazier M., Cerutti I., Pieri F., Tardivel I., Desmet G.…Chany C. Pharmacokinetic study of butyric acid administered in vivo as sodium and arginine butyrate salts. Clinica Chimica Acta. 1989;181(3):255–263. doi: 10.1016/0009-8981(89)90231-3. [DOI] [PubMed] [Google Scholar]
- Di Prima G., Saladino S., Bongiovì F., Adamo G., Ghersi G., Pitarresi G., Giammona G. Novel inulin-based mucoadhesive micelles loaded with corticosteroids as potential transcorneal permeation enhancers. European Journal of Pharmaceutics and Biopharmaceutics. 2017;117:385–399. doi: 10.1016/j.ejpb.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Di Prima G., Bongiovì F., Palumbo F.S., Pitarresi G., Licciardi M., Giammona G. Mucoadhesive PEGylated inulin-based self-assembling nanoparticles: In vitro and ex vivo transcorneal permeation enhancement of corticosteroids. Journal of Drug Delivery Science and Technology. 2019;49:195–208. [Google Scholar]
- Dong F., Zhang J., Yu C., Li Q., Ren J., Wang G.…Guo Z. Synthesis of amphiphilic aminated inulin via “click chemistry”and evaluation for its antibacterial activity. Bioorganic & Medicinal Chemistry Letters. 2014;24(18):4590–4593. doi: 10.1016/j.bmcl.2014.07.029. [DOI] [PubMed] [Google Scholar]
- Doost A.S., Sinnaeve D., De Neve L., Van der Meeren P. Influence of non-ionic surfactant type on the salt sensitivity of oregano oil-in-water emulsions. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2017;525:38–48. [Google Scholar]
- Doost A.S., Dewettinck K., Devlieghere F., Van der Meeren P. Influence of non-ionic emulsifier type on the stability of cinnamaldehyde nanoemulsions: A comparison of polysorbate 80 and hydrophobically modified inulin. Food Chemistry. 2018;258:237–244. doi: 10.1016/j.foodchem.2018.03.078. [DOI] [PubMed] [Google Scholar]
- Esquena J., Domínguez F.J., Solans C., Levecke B., Booten K., Tadros T.F. Stabilization of latex dispersions using a graft copolymer of inulin based surfactants. Langmuir. 2003;19(25):10463–10467. [Google Scholar]
- Exerowa D., Platikanov D. Thin liquid films from aqueous solutions of non-ionic polymeric surfactants. Advances in Colloid and Interface Science. 2009;147:74–87. doi: 10.1016/j.cis.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Exerowa D., Kolarov T., Pigov I., Levecke B., Tadros T. Interaction forces in thin liquid films stabilized by hydrophobically modified inulin polymeric surfactant. 1. Foam films. Langmuir. 2006;22(11):5013–5017. doi: 10.1021/la0600301. [DOI] [PubMed] [Google Scholar]
- Exerowa D., Gotchev G., Kolarov T., Khristov K., Levecke B., Tadros T.F. Interaction forces in thin liquid films stabilized by hydrophobically modified inulin polymeric surfactant. 2. Emulsion films. Langmuir. 2007;23(4):1711–1715. doi: 10.1021/la062820g. [DOI] [PubMed] [Google Scholar]
- Exerowa D., Gotchev G., Kolarov T., Kristov K., Levecke B., Tadros T. Oil-in-water emulsion films stabilized by polymeric surfactants based on inulin with different degree of hydrophobic modification. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2009;334(1–3):87–91. [Google Scholar]
- Exerowa D., Gotchev G., Kolarov T., Kristov K., Levecke B., Tadros T. Comparison of oil-in-water emulsion films produced using ABA or ABn copolymers. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2009;335(1–3):50–54. [Google Scholar]
- Exerowa D., Platikanov D., Levecke B., Tadros T. Emulsion and wetting films stabilized by hydrophobically modified inulin polymeric surfactant. Journal of Dispersion Science and Technology. 2009;30(6):789–794. [Google Scholar]
- Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nature Reviews Gastroenterology & Hepatology. 2012;9(10):577. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- Freeland K.R., Wolever T.M. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-α. The British Journal of Nutrition. 2010;103(3):460–466. doi: 10.1017/S0007114509991863. [DOI] [PubMed] [Google Scholar]
- Frost G., Sleeth M.L., Sahuri-Arisoylu M., Lizarbe B., Cerdan S., Brody L.…Carling D. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nature Communications. 2014;5(1):1–11. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallovic M.D., Montjoy D.G., Collier M.A., Do C., Wyslouzil B.E., Bachelder E.M., Ainslie K.M. Chemically modified inulin microparticles serving dual function as a protein antigen delivery vehicle and immunostimulatory adjuvant. Biomaterials Science. 2016;4(3):483–493. doi: 10.1039/c5bm00451a. [DOI] [PubMed] [Google Scholar]
- Gochev G., Petkova H., Kolarov T., Khristov K., Levecke B., Tadros T.F., Exerowa D. Effect of the degree of grafting in hydrophobically modified inulin polymeric surfactants on the steric forces in foam and oil-in-water emulsion films. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2011;391(1–3):101–104. [Google Scholar]
- Gotchev G., Kolarov T., Levecke B.T.T., Khristov K., Exerowa D. Interaction forces in thin liquid films stabilized by hydrophobically modified inulin polymeric surfactant. 3. Influence of electrolyte type on emulsion films. Langmuir. 2007;23(11):6091–6094. doi: 10.1021/la7002673. [DOI] [PubMed] [Google Scholar]
- Guo Z., Li Q., Wang G., Dong F., Zhou H., Zhang J. Synthesis, characterization, and antifungal activity of novel inulin derivatives with chlorinated benzene. Carbohydrate Polymers. 2014;99:469–473. doi: 10.1016/j.carbpol.2013.08.044. [DOI] [PubMed] [Google Scholar]
- Gupta N., Jangid A.K., Pooja D., Kulhari H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. International Journal of Biological Macromolecules. 2019 doi: 10.1016/j.ijbiomac.2019.03.188. [DOI] [PubMed] [Google Scholar]
- Han L., Ratcliffe I., Williams P.A. Self-assembly and emulsification properties of hydrophobically modified inulin. Journal of Agricultural and Food Chemistry. 2015;63(14):3709–3715. doi: 10.1021/acs.jafc.5b00333. [DOI] [PubMed] [Google Scholar]
- Han L., Ratcliffe I., Williams P.A. Synthesis, characterisation and physicochemical properties of hydrophobically modified inulin using long-chain fatty acyl chlorides. Carbohydrate Polymers. 2017;178:141–146. doi: 10.1016/j.carbpol.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Han L., Hu B., Ratcliffe I., Senan C., Yang J., Williams P.A. Octenyl-succinylated inulin for the encapsulation and release of hydrophobic compounds. Carbohydrate Polymers. 2020 doi: 10.1016/j.carbpol.2020.116199. [DOI] [PubMed] [Google Scholar]
- Hartzell A.L., Maldonado-Gómez M.X., Yang J., Hutkins R.W., Rose D.J. In vitro digestion and fermentation of 5-formyl-aminosailcylate-inulin: A potential prodrug of 5-aminosalicylic acid. Bioactive Carbohydrates and Dietary Fibre. 2013;2(1):8–14. [Google Scholar]
- Haworth W.N., Streight H.R.L. The acetylation and methylation of inulin. Helvetica Chimica Acta. 1932;15(1):609–615. [Google Scholar]
- Hu Y., Zhang J., Yu C., Li Q., Dong F., Wang G., Guo Z. Synthesis, characterization, and antioxidant properties of novel inulin derivatives with amino-pyridine group. International Journal of Biological Macromolecules. 2014;70:44–49. doi: 10.1016/j.ijbiomac.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Huang G., Chen J. Preparation and applications of hyaluronic acid and its derivatives. International Journal of Biological Macromolecules. 2019;125:478–484. doi: 10.1016/j.ijbiomac.2018.12.074. [DOI] [PubMed] [Google Scholar]
- Huang L., Shen M., Morris G.A., Xie J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends in Food Science & Technology. 2019;92:1–11. [Google Scholar]
- Kenawy E.R., Ali S.S., Al-Etewy M., Sun J., Wu J., El-Zawawy N. Synthesis, characterization and biomedical applications of a novel Schiff base on methyl acrylate-functionalized chitosan bearing p-nitrobenzaldehyde groups. International Journal of Biological Macromolecules. 2019;122:833–843. doi: 10.1016/j.ijbiomac.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Kesharwani S.S., Dachineni R., Bhat G.J., Tummala H. Hydrophobically modified inulin-based micelles: Transport mechanisms and drug delivery applications for breast cancer. Journal of Drug Delivery Science and Technology. 2019;54 [Google Scholar]
- Khristov K., Czarnecki J. Emulsion films stabilized by natural and polymeric surfactants. Current Opinion in Colloid & Interface Science. 2010;15(5):324–329. [Google Scholar]
- Kiumarsi M., Majchrzak D., Yeganehzad S., Jäger H., Shahbazi M. Comparative study of instrumental properties and sensory profiling of low-calorie chocolate containing hydrophobically modified inulin. Part 1: Rheological, thermal, structural and external preference mapping. Food Hydrocolloids. 2020;104 [Google Scholar]
- Kiumarsi M., Majchrzak D., Jäger H., Song J., Lieleg O., Shahbazi M. Comparative study of instrumental properties and sensory profiling of low-calorie chocolate containing hydrophobically modified inulin. Part II: Proton mobility, topological, tribological and dynamic sensory properties. Food Hydrocolloids. 2021;110 [Google Scholar]
- Kokubun S., Ratcliffe I., Williams P.A. Synthesis, characterization and self-assembly of biosurfactants based on hydrophobically modified inulins. Biomacromolecules. 2013;14(8):2830–2836. doi: 10.1021/bm4006529. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Ratcliffe I., Williams P.A. The emulsification properties of octenyl-and dodecenyl-succinylated inulins. Food Hydrocolloids. 2015;50:145–149. [Google Scholar]
- Kokubun S., Ratcliffe I., Williams P.A. The interfacial, emulsification and encapsulation properties of hydrophobically modified inulin. Carbohydrate Polymers. 2018;194:18–23. doi: 10.1016/j.carbpol.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Kumar S., Kesharwani S.S., Kuppast B., Bakkari M.A., Tummala H. Pathogen-mimicking vaccine delivery system designed with a bioactive polymer (inulin acetate) for robust humoral and cellular immune responses. Journal of Controlled Release. 2017;261:263–274. doi: 10.1016/j.jconrel.2017.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurečič M., Smole M.S., Stana-Kleinschek K. Use of polysaccharide based surfactants to stabilize organically modified clay particles aqueous dispersion. Carbohydrate Polymers. 2013;94(1):687–694. doi: 10.1016/j.carbpol.2013.01.085. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Bajracharya R., Min J.Y., Han J.W., Park B.J., Han H.K. Strategic approaches for colon targeted drug delivery: An overview of recent advancements. Pharmaceutics. 2020;12(1):68. doi: 10.3390/pharmaceutics12010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Qiu L., Tan W., Gu G., Guo Z. Novel 1, 2, 3-triazolium-functionalized inulin derivatives: Synthesis, free radical-scavenging activity, and antifungal activity. RSC Advances. 2017;7(67):42225–42232. [Google Scholar]
- Licciardi M., Scialabba C., Sardo C., Cavallaro G., Giammona G. Amphiphilic inulin graft co-polymers as self-assembling micelles for doxorubicin delivery. Journal of Materials Chemistry B. 2014;2(27):4262–4271. doi: 10.1039/c4tb00235k. [DOI] [PubMed] [Google Scholar]
- Liochev S.I. Reactive oxygen species and the free radical theory of aging. Free Radical Biology & Medicine. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Liu W., Sun D., Li C., Liu Q., Xu J. Formation and stability of paraffin oil-in-water nano-emulsions prepared by the emulsion inversion point method. Journal of Colloid and Interface Science. 2006;303(2):557–563. doi: 10.1016/j.jcis.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Liu J., Lu J.F., Kan J., Wen X.Y., Jin C.H. Synthesis, characterization and in vitro anti-diabetic activity of catechin grafted inulin. International Journal of Biological Macromolecules. 2014;64:76–83. doi: 10.1016/j.ijbiomac.2013.11.028. [DOI] [PubMed] [Google Scholar]
- Liu J., Lu J.F., Wen X.Y., Kan J., Jin C.H. Antioxidant and protective effect of inulin and catechin grafted inulin against CCl4-induced liver injury. International Journal of Biological Macromolecules. 2015;72:1479–1484. doi: 10.1016/j.ijbiomac.2014.09.066. [DOI] [PubMed] [Google Scholar]
- López-Molina D., Chazarra S., How C.W., Pruidze N., Navarro-Perán E., García-Cánovas F.…Rodríguez-López J.N. Cinnamate of inulin as a vehicle for delivery of colonic drugs. International Journal of Pharmaceutics. 2015;479(1):96–102. doi: 10.1016/j.ijpharm.2014.12.064. [DOI] [PubMed] [Google Scholar]
- Lu X., Chen J., Guo Z., Zheng Y., Rea M.C., Su H.…Miao S. Using polysaccharides for the enhancement of functionality of foods: A review. Trends in Food Science & Technology. 2019 [Google Scholar]
- Malkova D., Polyviou T., Rizou E., Gerasimidis K., Chambers E.S., Preston T.…Morrison D.J. Moderate intensity exercise training combined with inulin-propionate ester supplementation increases whole body resting fat oxidation in overweight women. Metabolism. 2020;104 doi: 10.1016/j.metabol.2019.154043. [DOI] [PubMed] [Google Scholar]
- Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: Food sources and bioavailability. The American Journal of Clinical Nutrition. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Mandracchia D., Tripodo G., Latrofa A., Dorati R. Amphiphilic inulin-D-α-tocopherol succinate (INVITE) bioconjugates for biomedical applications. Carbohydrate Polymers. 2014;103:46–54. doi: 10.1016/j.carbpol.2013.11.056. [DOI] [PubMed] [Google Scholar]
- Mandracchia D., Tripodo G., Trapani A., Ruggieri S., Annese T., Chlapanidas T.…Ribatti D. Inulin based micelles loaded with curcumin or celecoxib with effective anti-angiogenic activity. European Journal of Pharmaceutical Sciences. 2016;93:141–146. doi: 10.1016/j.ejps.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Mandracchia D., Trapani A., Perteghella S., Sorrenti M., Catenacci L., Torre M.L.…Tripodo G. pH-sensitive inulin-based nanomicelles for intestinal site-specific and controlled release of celecoxib. Carbohydrate Polymers. 2018;181:570–578. doi: 10.1016/j.carbpol.2017.11.110. [DOI] [PubMed] [Google Scholar]
- Mehmood A., Zhao L., Wang C., Nadeem M., Raza A., Ali N., Shah A.A. Management of hyperuricemia through dietary polyphenols as a natural medicament: A comprehensive review. Critical Reviews in Food Science and Nutrition. 2019;59(9):1433–1455. doi: 10.1080/10408398.2017.1412939. [DOI] [PubMed] [Google Scholar]
- Mehmood A., Zhao L., Wang C., Hossen I., Raka R.N., Zhang H. Correction: Stevia residue extract increases intestinal uric acid excretion via interactions with intestinal urate transporters in hyperuricemic mice. Food & Function. 2020;11(3):2764. doi: 10.1039/d0fo90011g. [DOI] [PubMed] [Google Scholar]
- Mensink M.A., Frijlink H.W., van der Voort Maarschalk K., Hinrichs W.L. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohydrate Polymers. 2015;134:418–428. doi: 10.1016/j.carbpol.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Meshulam D., Slavuter J., Lesmes U. Behavior of emulsions stabilized by a hydrophobically modified inulin under bio-relevant conditions of the human gastro-intestine. Food Biophysics. 2014;9(4):416–423. [Google Scholar]
- Morros J., Levecke B., Infante M.R. Chemical hydrophobic modification of inulin in aqueous media: Synthesis of β-hydroxyalkyl ethers of inulin. Carbohydrate Polymers. 2010;81(3):681–686. [Google Scholar]
- Morros J., Levecke B., Infante M.R. Synthesis of β-hydroxyalkyl ethers of inulin in aqueous surfactant media. Carbohydrate Polymers. 2010;82(4):1168–1173. [Google Scholar]
- Morros J., Levecke B., Infante M.R. Hydrophobically modified inulin from alkenyl succinic anhydride in aqueous media. Carbohydrate Polymers. 2011;84(3):1110–1116. [Google Scholar]
- Morros J., Infante M.R., Pons R. Surface activity and aggregation of pristine and hydrophobically modified inulin. Soft Matter. 2012;8(44):11353–11362. [Google Scholar]
- Muley P., Kumar S., El Kourati F., Kesharwani S.S., Tummala H. Hydrophobically modified inulin as an amphiphilic carbohydrate polymer for micellar delivery of paclitaxel for intravenous route. International Journal of Pharmaceutics. 2016;500(1–2):32–41. doi: 10.1016/j.ijpharm.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Nedyalkov M., Alexandrova L., Platikanov D., Levecke B., Tadros T. Wetting films on a hydrophilic silica surface obtained from aqueous solutions of hydrophobically modified inulin polymeric surfactant. Colloid and Polymer Science. 2007;285(15):1713–1717. [Google Scholar]
- Nedyalkov M., Alexandrova L., Platikanov D., Levecke B., Tadros T. Wetting films from aqueous solutions of polymeric surfactants on hydrophobic solid surface. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2010;354(1–3):22–27. [Google Scholar]
- Nematidil N., Sadeghi M., Nezami S., Sadeghi H. Synthesis and characterization of Schiff-base based chitosan-g-glutaraldehyde/NaMMTNPs-APTES for removal Pb2+ and Hg2+ ions. Carbohydrate Polymers. 2019;222 doi: 10.1016/j.carbpol.2019.114971. [DOI] [PubMed] [Google Scholar]
- Nestor J., Esquena J., Solans C., Levecke B., Booten K., Tadros T.F. Emulsion polymerization of styrene and methyl methacrylate using a hydrophobically modified inulin and comparison with other surfactants. Langmuir. 2005;21(11):4837–4841. doi: 10.1021/la047018y. [DOI] [PubMed] [Google Scholar]
- Nestor J., Esquena J., Solans C., Luckham P.F., Musoke M., Levecke B.…Tadros T.F. Interaction forces between particles stabilized by a hydrophobically modified inulin surfactant. Journal of Colloid and Interface Science. 2007;311(2):430–437. doi: 10.1016/j.jcis.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Nestor J., Esquena J., Solans C., Levecke B., Booten K., Tadros T.F. Emulsion polymerization of styrene using mixtures of hydrophobically modified inulin (polyfructose) polymeric surfactant and nonionic surfactants. Journal of Applied Polymer Science. 2008;108(2):811–815. [Google Scholar]
- Obiols-Rabasa M., Oncins G., Sanz F., Tadros T.F., Solans C., Levecke B.…Esquena J. Investigation of the elastic and adhesion properties of adsorbed hydrophobically modified inulin films on latex particles using Atomic Force Microscopy (AFM) Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2017;524:185–192. [Google Scholar]
- Philip A.K., Philip B. Colon targeted drug delivery systems: A review on primary and novel approaches. Oman Medical Journal. 2010;25(2):79. doi: 10.5001/omj.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingitore A., Chambers E.S., Hill T., Maldonado I.R., Pingitore A., Chambers E.S.…Persaud S.J. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes, Obesity & Metabolism. 2017;19:257–265. doi: 10.1111/dom.12811. [DOI] [PubMed] [Google Scholar]
- Polyviou T., MacDougall K., Chambers E.S., Viardot A., Psichas A., Jawaid S.…Zac‐Varghese S.E.K. Randomised clinical study: Inulin short‐chain fatty acid esters for targeted delivery of short‐chain fatty acids to the human colon. Alimentary Pharmacology & Therapeutics. 2016;44(7):662–672. doi: 10.1111/apt.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remon J.P., Duncan R., Schacht E. Polymer-drug combinations: Pinocytic uptake of modified polysaccharides containing procainamide moieties by rat visceral yolk sacs cultured-itin vitro. Journal of Controlled Release. 1984;1(1):47–56. [Google Scholar]
- Ren J., Liu J., Dong F., Guo Z. Highly efficient synthesis and antioxidant activity of O-(aminoethyl) inulin. Carbohydrate Polymers. 2011;83(3):1240–1244. [Google Scholar]
- Ren J., Liu J., Dong F., Guo Z. Synthesis and hydroxyl radicals scavenging activity of N-(aminoethyl) inulin. Carbohydrate Polymers. 2011;85(1):268–271. [Google Scholar]
- Ren J., Wang P., Dong F., Feng Y., Peng D., Guo Z. Synthesis and antifungal properties of 6-amino-6-deoxyinulin, a kind of precursors for facile chemical modifications of inulin. Carbohydrate Polymers. 2012;87(2):1744–1748. [Google Scholar]
- Rogge T.M., Stevens C.V. Facilitated synthesis of inulin esters by transesterification. Biomacromolecules. 2004;5(5):1799–1803. doi: 10.1021/bm049869q. [DOI] [PubMed] [Google Scholar]
- Rogge T.M., Stevens C.V., Colpaert A., Levecke B., Booten K. Use of acyl phosphonates for the synthesis of inulin esters and their use as emulsion stabilizing agents. Biomacromolecules. 2007;8(2):485–489. doi: 10.1021/bm060592z. [DOI] [PubMed] [Google Scholar]
- Roshanravan N., Mahdavi R., Alizadeh E., Jafarabadi M.A., Hedayati M., Ghavami A.…Ostadrahimi A. Effect of butyrate and inulin supplementation on glycemic status, lipid profile and glucagon-like peptide 1 level in patients with type 2 diabetes: A randomized double-blind, placebo-controlled trial. Hormone and Metabolic Research. 2017;49(11):886–891. doi: 10.1055/s-0043-119089. [DOI] [PubMed] [Google Scholar]
- Sahariah P., Masson M. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules. 2017;18(11):3846–3868. doi: 10.1021/acs.biomac.7b01058. [DOI] [PubMed] [Google Scholar]
- Sardo C., Farra R., Licciardi M., Dapas B., Scialabba C., Giammona G.…Cavallaro G. Development of a simple, biocompatible and cost-effective Inulin-Diethylenetriamine based siRNA delivery system. European Journal of Pharmaceutical Sciences. 2015;75:60–71. doi: 10.1016/j.ejps.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Scialabba C., Licciardi M., Mauro N., Rocco F., Ceruti M., Giammona G. Inulin-based polymer coated SPIONs as potential drug delivery systems for targeted cancer therapy. European Journal of Pharmaceutics and Biopharmaceutics. 2014;88(3):695–705. doi: 10.1016/j.ejpb.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Shah M.K., Khatri P., Vora N., Patel N.K., Jain S., Lin S. Lipid nanocarriers: Preparation, characterization and absorption mechanism and applications to improve oral bioavailability of poorly water-soluble drugs. Biomedical applications of nanoparticles. 2019:117–147. [Google Scholar]
- Shivhare K., Garg C., Priyam A., Gupta A., Sharma A.K., Kumar P. Enzyme sensitive smart inulin-dehydropeptide conjugate self-assembles into nanostructures useful for targeted delivery of ornidazole. International Journal of Biological Macromolecules. 2018;106:775–783. doi: 10.1016/j.ijbiomac.2017.08.071. [DOI] [PubMed] [Google Scholar]
- Singh M., Esquena J., Solans C., Booten K., Tadros T.F. Influence of hydrophobically modified inulin (INUTEC NRA) on the stability of vulcanized natural rubber latex. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2014;451:90–100. [Google Scholar]
- Srinarong P., Hämäläinen S., Visser M.R., Hinrichs W.L., Ketolainen J., Frijlink H.W. Surface-active derivative of inulin (Inutec® SP1) is a superior carrier for solid dispersions with a high drug load. Journal of Pharmaceutical Sciences. 2011;100(6):2333–2342. doi: 10.1002/jps.22471. [DOI] [PubMed] [Google Scholar]
- Stevens C.V., Meriggi A., Booten K. Chemical modification of inulin, a valuable renewable resource, and its industrial applications. Biomacromolecules. 2001;2(1):1–16. doi: 10.1021/bm005642t. [DOI] [PubMed] [Google Scholar]
- Stevens C.V., Meriggi A., Peristeropoulou M., Christov P.P., Booten K., Levecke B.…Tadros T.F. Polymeric surfactants based on inulin, a polysaccharide extracted from chicory. 1. Synthesis and interfacial properties. Biomacromolecules. 2001;2(4):1256–1259. doi: 10.1021/bm015570l. [DOI] [PubMed] [Google Scholar]
- Suflet D.M., Popescu I., Pelin I.M., Nicolescu A., Hitruc G. Cationic curdlan: Synthesis, characterization and application of quaternary ammonium salts of curdlan. Carbohydrate Polymers. 2015;123:396–405. doi: 10.1016/j.carbpol.2015.01.050. [DOI] [PubMed] [Google Scholar]
- Sun Q., Luan L., Arif M., Li J., Dong Q.J., Gao Y.…Liu C.G. Redox-sensitive nanoparticles based on 4-aminothiophenol-carboxymethyl inulin conjugate for budesonide delivery in inflammatory bowel diseases. Carbohydrate Polymers. 2018;189:352–359. doi: 10.1016/j.carbpol.2017.12.021. [DOI] [PubMed] [Google Scholar]
- Tadros T. Interaction forces between particles containing grafted or adsorbed polymer layers. Advances in Colloid and Interface Science. 2003;104(1–3):191–226. doi: 10.1016/s0001-8686(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Tadros T. Interaction forces between adsorbed polymer layers. Advances in Colloid and Interface Science. 2011;165(2):102–107. doi: 10.1016/j.cis.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Tadros T. Stabilisation of dispersions using a graft copolymer of hydrophobically modified polyfructose. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2017;519:11–19. [Google Scholar]
- Tadros T.F., Vandamme A., Booten K., Levecke B., Stevens C.V. Stabilisation of emulsions using hydrophobically modified inulin (polyfructose) Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2004;250(1–3):133–140. [Google Scholar]
- Tap J., Furet J.P., Bensaada M., Philippe C., Roth H., Rabot S.…Fontaine E. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environmental Microbiology. 2015;17(12):4954–4964. doi: 10.1111/1462-2920.13006. [DOI] [PubMed] [Google Scholar]
- Tomecko C.G., Adams R. The allyl ethers of various carbohydrates. Journal of the American Chemical Society. 1923;45(11):2698–2701. [Google Scholar]
- Tripodo G., Mandracchia D. Inulin as a multifaceted (active) substance and its chemical functionalization: From plant extraction to applications in pharmacy, cosmetics and food. European Journal of Pharmaceutics and Biopharmaceutics. 2019 doi: 10.1016/j.ejpb.2019.05.011. [DOI] [PubMed] [Google Scholar]
- Tripodo G., Pitarresi G., Palumbo F.S., Craparo E.F., Giammona G. UV‐photocrosslinking of inulin derivatives to produce hydrogels for drug delivery application. Macromolecular Bioscience. 2005;5(11):1074–1084. doi: 10.1002/mabi.200500134. [DOI] [PubMed] [Google Scholar]
- Tripodo G., Perteghella S., Grisoli P., Trapani A., Torre M.L., Mandracchia D. Drug delivery of rifampicin by natural micelles based on inulin: Physicochemical properties, antibacterial activity and human macrophages uptake. European Journal of Pharmaceutics and Biopharmaceutics. 2019;136:250–258. doi: 10.1016/j.ejpb.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Tripodo G., Chlapanidas T., Perteghella S., Vigani B., Mandracchia D., Trapani A.…Marazzi M. Mesenchymal stromal cells loading curcumin-INVITE-micelles: A drug delivery system for neurodegenerative diseases. Colloids and Surfaces B: Biointerfaces. 2015;125:300–308. doi: 10.1016/j.colsurfb.2014.11.034. [DOI] [PubMed] [Google Scholar]
- Tripodo G., Pasut G., Trapani A., Mero A., Lasorsa F.M., Chlapanidas T.…Mandracchia D. Inulin-d-α-tocopherol succinate (INVITE) nanomicelles as a platform for effective intravenous administration of curcumin. Biomacromolecules. 2015;16(2):550–557. doi: 10.1021/bm501616e. [DOI] [PubMed] [Google Scholar]
- Tripodo G., Trapani A., Torre M.L., Giammona G., Trapani G., Mandracchia D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. European Journal of Pharmaceutics and Biopharmaceutics. 2015;97:400–416. doi: 10.1016/j.ejpb.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Tziveleka L.A., Ioannou E., Roussis V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydrate Polymers. 2019 doi: 10.1016/j.carbpol.2019.04.074. [DOI] [PubMed] [Google Scholar]
- Verraest D.L., Peters J.A., Kuzee H.C., Raaijmakers H.W., van Bekkum H. Distribution of substituents in O-carboxymethyl and O-cyanoethyl ethers of inulin. Carbohydrate Research. 1997;302(3–4):203–212. [Google Scholar]
- Walz M., Hagemann D., Trentzsch M., Weber A., Henle T. Degradation studies of modified inulin as potential encapsulation material for colon targeting and release of mesalamine. Carbohydrate Polymers. 2018;199:102–108. doi: 10.1016/j.carbpol.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Walz M., Hirth T., Weber A. Investigation of chemically modified inulin as encapsulation material for pharmaceutical substances by spray-drying. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2018;536:47–52. [Google Scholar]
- Wang X.H., Tian Q., Wang W., Zhang C.N., Wang P., Yuan Z. In vitro evaluation of polymeric micelles based on hydrophobically-modified sulfated chitosan as a carrier of doxorubicin. Journal of Materials Science Materials in Medicine. 2012;23(7):1663–1674. doi: 10.1007/s10856-012-4627-1. [DOI] [PubMed] [Google Scholar]
- Wang H., Yuan H., Li S., Li Z., Jiang M. Synthesis, antimicrobial activity of Schiff base compounds of cinnamaldehyde and amino acids. Bioorganic & Medicinal Chemistry Letters. 2016;26(3):809–813. doi: 10.1016/j.bmcl.2015.12.089. [DOI] [PubMed] [Google Scholar]
- Wang L., Barclay T., Song Y., Joyce P., Sakala I.G., Petrovsky N., Garg S. Investigation of the biodistribution, breakdown and excretion of delta inulin adjuvant. Vaccine. 2017;35(34):4382–4388. doi: 10.1016/j.vaccine.2017.06.045. [DOI] [PubMed] [Google Scholar]
- Wang D., Sun F., Lu C., Chen P., Wang Z., Qiu Y.…Duan J. Inulin based glutathione-responsive delivery system for colon cancer treatment. International Journal of Biological Macromolecules. 2018;111:1264–1272. doi: 10.1016/j.ijbiomac.2018.01.071. [DOI] [PubMed] [Google Scholar]
- Wang L., Song Y., Parikh A., Joyce P., Chung R., Liu L.…Garg S. Doxorubicin-loaded delta inulin conjugates for controlled and targeted drug delivery: Development, characterization, and in vitro evaluation. Pharmaceutics. 2019;11(11):581. doi: 10.3390/pharmaceutics11110581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtunik-Kulesza K.A., Oniszczuk A., Oniszczuk T., Waksmundzka-Hajnos M. The influence of common free radicals and antioxidants on development of Alzheimer’s Disease. Biomedecine & Pharmacotherapy. 2016;78:39–49. doi: 10.1016/j.biopha.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Wu M., Guo K., Dong H., Zeng R., Tu M., Zhao J. In vitro drug release and biological evaluation of biomimetic polymeric micelles self-assembled from amphiphilic deoxycholic acid–phosphorylcholine–chitosan conjugate. Materials Science and Engineering: C. 2014;45:162–169. doi: 10.1016/j.msec.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Xin X., Zhang H., Xu G., Tan Y., Zhang J., Lv X. Influence of CTAB and SDS on the properties of oil-in-water nano-emulsion with paraffin and span 20/Tween 20. Colloids and surfaces A: Physicochemical and Engineering Aspects. 2013;418:60–67. [Google Scholar]
- Xu Y., Wu Y.J., Sun P.L., Zhang F.M., Linhardt R.J., Zhang A.Q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. International Journal of Biological Macromolecules. 2019 doi: 10.1016/j.ijbiomac.2019.03.213. [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhu Y., Li X., Sun B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends in Food Science & Technology. 2020 [Google Scholar]
- Yu Y., Shen M., Song Q., Xie J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydrate Polymers. 2018;183:91–101. doi: 10.1016/j.carbpol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li Y., Wang C., Li G., Zhao Y., Yang Y. Synthesis of methylprednisolone loaded ibuprofen modified inulin based nanoparticles and their application for drug delivery. Materials Science and Engineering: C. 2014;42:111–115. doi: 10.1016/j.msec.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang Y.W., Zhang H., Yang Q., Wang H., Zhang G. Preparation, characterization and antibacterial activity of octenyl succinic anhydride modified inulin. International Journal of Biological Macromolecules. 2015;78:79–86. doi: 10.1016/j.ijbiomac.2015.03.067. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li G., Gao M., Liu X., Ji B., Hua R.…Yang Y. RGD-peptide conjugated inulin-ibuprofen nanoparticles for targeted delivery of Epirubicin. Colloids and Surfaces B: Biointerfaces. 2016;144:81–89. doi: 10.1016/j.colsurfb.2016.03.077. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Liu J., Hao W., Zhu H., Liang N., He Z.…Chen Z.Y. Structure-specific effects of short-chain fatty acids on plasma cholesterol concentration in male syrian hamsters. Journal of Agricultural and Food Chemistry. 2017;65(50):10984–10992. doi: 10.1021/acs.jafc.7b04666. [DOI] [PubMed] [Google Scholar]
- Zhu H., Liu F., Guo J., Xue J., Qian Z., Gu Y. Folate-modified chitosan micelles with enhanced tumor targeting evaluated by near infrared imaging system. Carbohydrate Polymers. 2011;86(3):1118–1129. [Google Scholar]
- Zhu X., Jia C., Meng X., Xing M., Yi Y., Gao X. Synthesis, characterization of inulin propionate ester, and evaluation of its in vitro effect on SCFA production. Starch‐Stärke. 2018;70(9–10) [Google Scholar]