Abstract
Background
Chronic lymphocytic leukemia (CLL) is a heterogeneous disease, which is attributed to differences in the genetic characteristics of the leukemic clone. We studied the genomic profile of 52 treatment-naïve CLL patients.
Methods
Genetic analysis was performed by multiplex ligation-dependent probe amplification (MLPA) using the SALSA P038 Probemix (MRC Holland, Amsterdam), which contains probes for 2p (MYCN,ALK,REL), 6q, 8p (TNFRSF10A/B), 8q (EIF3H,MYC), 9p21 (CDKN2A/B), 10q (PTEN), 11q (ATM, RDX, PPP2R1B, CADM1), chromosome 12, 13q14 (RB1, DLEU1/2/7, KCNRG, MIR15A), 14q, 17p (TP53) and chromosome 19, and for NOTCH1 7541-7542delCT, SF3B1 K700E, and MYD88 L265P mutations.
Results
The median age was 65 years (malefemale=21). The median hemoglobin, total leuko- cyte, and platelet counts were 12.4 g/dL, 57.7×109/L, and 176.5×109/L, respectively. At least one genetic abnormality was observed in 34 (65%) patients. The most common abnormality was del(13q14) (deleted DLEU2 and DLEU1/RB1 genes), which was observed in 22 (42%) cases, followed by trisomy 12 [7 (13%) cases]. Del(11q) (deleted ATM, RDX11/PPP2R1B-4) and del(17p) (deleted TP53) were present in 5 (10%) and 2 (4%) cases, respectively. 19p13.2 (CDKN2D-2) amplification and NOTCH1 mutation were found in one case each.
Conclusion
Genetic abnormalities are commonly (65%) observed in CLL patients. Del(13q), which is associated with DLEU2 and DLEU1/RB1 gene deletion, was the most common. Compared with other abnormalities, del(11q) and del(17p) patients presented with cytopenia and higher Binet stage, while those with del(13q14) had a longer time to first treatment.
Keywords: Chronic lymphocytic leukemia, Genetic abnormality, Multiplex ligation-dependent probe amplification
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is a disease with variable clinical history and survival ranging from only a few months to over 15 years. The reason that some patients experience a benign stable disease course over many years while others have a progressive disease has been attributed to differences in the genetic characteristics of the leukemic clone [1].
The heterogeneity in CLL stems from the complex interplay of different intrinsic and extrinsic mechanisms, making CLL a prototype for cancers in which tumor initiation and progression are linked to a combined effect of these mechanisms. The cell-intrinsic factors include more general cancer-associated mechanisms, e.g., activation of oncogenes (e.g. NOTCH1) and/or elimination of tumor suppressor genes (e.g., TP53 and ATM), with certain aberrations, most notably those disrupting the TP53 gene, strongly influencing disease outcome [2].
Disease-related factors have been identified to further classify CLL into biologically and clinically distinct subtypes. Recently identified disease parameters, such as the presence of mutations involving the TP53, ATM, NOTCH1, SF3B1, and MYD88 genes and an unmutated IGHV gene, impact survival outcomes and can help categorize CLL into clinically distinct subtypes [3]. Genomic aberrations in CLL have been reported as independent predictors of disease progression (Dohner hierarchial classification) and survival, and have implications for the design of risk-adapted treatment strategies [4]. Therefore, information regarding the genetic aberrations has produced clinically useful information that can aid in risk stratification, deciding therapy, and prognostication of CLL patients.
Incidence of CLL exhibits distinct patterns reflecting racial disparity. SEER revealed a fivefold increased risk of CLL in African-Americans compared to Caucasians, which is suggested to be due to either inherent race-related genetic susceptibility or differences in environmental factors between African-Americans and Caucasians in the US, or possibly due to some combination of both [5]. Variable risk of acquiring CLL across different races supports the hypothesis of race-related genetic predisposition. This also reflects unknown environmental influences possibly interacting with genetic factors. South Asian CLL patients have been reported to have more rapid disease progression than Caucasians [5].
CLL presents at a younger age in Indian patients. The presence of geographical and genetic variations in CLL patients has been reported and may be responsible for this variable clinical characteristic in Indian CLL patients [6]. Given that the incidence and disease manifestations are affected by the geographic and genetic factors, we performed genomic profiling on our group of CLL patients.
Genomic aberrations were analyzed in this study by multiplex ligation-dependent probe amplification (MLPA). MLPA is a variation of a polymerase chain reaction based technique and can simultaneously detect copy number changes, DNA methylation, and point mutations of up to 50 genomic DNA sequences in a single experiment [7]. Previous studies have demonstrated that MLPA is an acceptable technique to detect genomic aberrations in CLL and exhibits good correlation with FISH results in CLL patients [8-11]. Additionally, MLPA has the advantage of being faster and more cost-effective than FISH. While FISH provides information for only a limited number of genomic targets at the same time, MLPA can detect copy number alterations and point mutations simultaneously in multiple target regions.
MATERIALS AND METHODS
Fifty-two consecutive, newly diagnosed and treatment-naïve CLL patients were included in the study.
Nine healthy adult subjects (controls) were also enrolled in the study for the purpose of obtaining normal reference DNA samples. The study was approved by the Institutional Ethics Review Board and informed consent was obtained from all participants (patients and controls) in accordance with the Declaration of Helsinki. CLL was diagnosed per the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria [12]. Diagnostic criteria included the presence of clonal B-lymphocytosis (≥5×109/L) in peripheral blood (persistent for at least 3 months), with typical CLL immunophenotype.
Molecular genetic testing
Peripheral blood samples collected in 3 mL EDTA anticoagulated tubes were used for genetic testing.
DNA was extracted using the QIAmp DNA Blood Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA purity and concentration was measured spectrophotometrically using a Nanodrop 2000 (Thermo Fisher Scientific Inc., Waltham, MA, USA), and a 260/280 ratio between 1.8-1.9 was considered pure.
MLPA
Extracted DNA was analyzed by MLPA using the SALSA MLPA Probemix P038 CLL from MRC Holland (Amsterdam, The Netherlands). This Probemix kit detects the status of 13q14 deletion (RB1/DLEU/MIR15A; 10 probes), 11q22-3 deletion (ATM; 7 probes), 17p13 deletion (TP53; 8 probes), and the presence of trisomy 12 (10 probes). Additionally, three probes specific for the NOTCH1 7541-7542delCT, SF3B1 K700E, and MYD88 L265P mutations, which only generate a signal when the particular mutation is present, are included in the same kit.
Peripheral blood samples from healthy donors were used as reference controls. At least three controls were used per run (up to 21 test samples). For every subsequent seven test samples, one additional control was taken.
Brief methodology for MLPA [9]
MLPA consists of following steps:
DNA denaturation: The optimal amount (50–100 ng) of genomic DNA from patients and reference controls is denatured at 98°C for 5 minutes, followed by cooling to 25°C before removing the tubes from the thermocycler.
Hybridization reaction: 3 μL hybridization master mix (1.5 μL MLPA buffer+1.5 μL Probemix) is added to each sample tube containing denatured DNA and incubated at 60°C for 16–20 hours (overnight).
Ligation reaction: 1 μL Ligase-65 enzyme and 32 μL ligase master mix (consisting of 26 μL ultrapure water, 3 μL Ligase Buffer A, and 3 μL Ligase Buffer B) is added to each reaction tube while in the thermocycler. Samples are incubated for 15 minutes at 54°C (for ligation) and the Ligase-65 enzyme is subsequently heat inactivated at 98°C for 5 minutes, followed by cooling to 20°C before removing the tubes from the thermocycler.
PCR amplification: 10 μL master mix (7.5 μL ultrapure water+2 μL SALSA PCR primer mix+0.5 μL SALSA Polymerase) is added to each tube at room temperature. The tubes are then placed in the thermocycler (35 cycles of amplification at 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 60 seconds; followed by incubation at 72°C for 20 min).
Fragment separation by capillary electrophoresis: Fragments were separated using the fragment analysis program on an ABI-3500 (Applied Biosystems, Foster City, CA, USA) analyzer. For each sample, the injection mixture, consisting of 0.7 μL PCR product, 0.2 μL Applied Biosystems GeneScan 500 LIZ size standard, and 9 μL HiDi formamide, is added to plate. The plate is sealed and heated for 3 minutes at 98°C, then cooled for 2 minutes to 4°C before it is placed on the analyzer.
Analysis using Coffalyser.Net: A test DNA sample without copy number abnormalities exhibits an identical peak pattern to that of the reference samples, i.e., the final probe ratios were determined to be close to 1.0. This final probe ratio is also called the Dosage Quotient (DQ). Coffalyser.Net software calculates the DQ for each probe in each sample. DQ values between 0.80 and 1.20 were considered to be normal (identical to reference samples). DQ values less than 0.8 were considered to be deletions, and DQ values greater than 1.2 were considered to be amplifications. For heterozygous deletions, the DQ was ∼0.5. DQ values near 0.0 were considered to be homozygous/biallelic deletions.
RESULTS
The study prospectively included 52 newly diagnosed treatment- naïve cases of CLL. There was male predominance (male:female ratio=2:1), with a median age of 65 years (range, 43–88 yr).
The median hemoglobin, total leukocyte count, absolute lymphocyte count, and platelet count was 12.4 g/dL, 57.7×109/L, 52.0×109/L, and 176.5×109/L, respectively. The distribution of cases according to Rai stage was: stage 0, 31%; stage I, 21%; stage II, 23%; stage III, 15%; and stage IV, 10%. The details of the clinico-hematological parameters are summarized in Table 1.
Table 1.
Baseline parameters of CLL patients (N=52).
| Male, N (%) | 35 (67.3%) |
| Female, N (%) | 17 (32.7%) |
| Male:female | 2:1 |
| Median age (range), yr | 65 (43–88) |
| <60 yr, N (%) | 15 (28.8%) |
| ≥60 yr, N (%) | 37 (71.2%) |
| Incidental lymphocytosis | 9 (17%) |
| Fatigue | 19 (37%) |
| B-symptoms | 7 (13.5%) |
| Hepatomegaly | 14 (27.0%) |
| Splenomegaly, N (%) | 20 (38.5%) |
| Lymphadenopathy, N (%) | 28 (53.8%) |
| <3 groups involved | 09 (32.1%) |
| 3 or more groups involved | 19 (67.9%) |
| ECOG performance status | |
| 0, N (%) | 16 (30.8%) |
| 1, N (%) | 28 (53.8%) |
| 2/3, N (%) | 08 (15.4%) |
| Hemoglobin, median (g/dL) | 12.4 (5.0–16.6) |
| Total leucocyte count, median (×109/L) | 57.7 (18.0–307.8) |
| Absolute lymphocyte count, median (×109/L) | 52.0 (7.6–292.4) |
| Platelet count, median (×109/L) | 176.5 (46.0–449.0) |
| Smudge cells, median (%) | 32 (5–126) |
| Lactate dehydrogenase (LDH) (U/L) | |
| Within normal range, N (%) | 31 (59.6%) |
| Elevated (>1×upper limit of normal), N (%) | 16 (30.8%) |
| Missing data, N (%) | 5 (9.6%) |
| β2-microglobulin (mg/L), median | 4.7 (0.89–16.5) |
| Within normal range, N (%) | 4 (7.7%) |
| Elevated, N (%) | 27 (51.9%) |
| Missing data, N (%) | 21 (40.4%) |
| Modified Rai stage | |
| Low, N (%) | 16 (30.8%) |
| Intermediate, N (%) | 23 (44.2%) |
| High, N (%) | 13 (25.0%) |
| Binet stage | |
| A, N (%) | 29 (55.8%) |
| B, N (%) | 10 (19.2%) |
| C, N (%) | 13 (25.0%) |
| Atypical immunophenotypic features in different combinations identified in the study cohort | |
| CD23 weak or absent expression, N (%) | 7 (13.5%) |
| Bright CD20 expression, N (%) | 5 (9.6%) |
| Bright expression of SIgλ/SIgκ, N (%) | 12 (23.1%) |
Two patients were positive upon direct antiglobulin test (DAT) and were diagnosed with autoimmune hemolytic anemia. Of these two cases, one showed 3+ IgG positivity and the other had 4+ IgG positivity on poly-specific gel card-based DAT.
Flow cytometry-based immunophenotyping was performed in all cases. CLL diagnosis was confirmed by the presence of typical immunophenotypic features associated with CLL. However, 19 cases (36%) exhibited some aberrancies known to occur in CLL immunophenotype. The shift from the usual immunophenotype is mentioned in Table 1.
Genetic abnormalities
Among the 52 cases, a majority (34 cases, 65%) had one or more cytogenetic abnormality detected by MLPA. Eighteen patients (35%) lacked copy number variations in any of the probes in the Probemix. Twenty-eight cases (54%) revealed a sole cytogenetic anomaly, the details of which can be found in Table 2. Six cases had an additional genetic abnormality. Among the somatic mutations, only one case exhibited NOTCH1 c.7541_7542delCT mutation.
Table 2.
Cytogenetic abnormalities and somatic mutations detected by MLPA (N=52).
| 13q14 deletion, N (%) | 22 (42.3%) |
| Trisomy 12, N (%) | 7 (13.4%) |
| 11q (ATM) deletion, N (%) | 5 (9.6%) |
| 17p (TP53) deletion, N (%) | 2 (3.8%) |
| No abnormality, N (%) | 18 (34.6%) |
| Two abnormalities, N (%) | 6 (11.5%) |
| NOTCH1 c.7541_7542delCT mutation | 1 (1.9%) |
| MYD88 L265P mutation | 0 (0.0%) |
| SF3B1 K700E mutation | 0 (0.0%) |
Among the 22 cases with 13q14 deletion, both type 1 and type 2 deletions were detected. The minimally deleted region (MDR), including the Deleted in Lymphocytic Leukemia-2 gene (DLEU2) (containing mir-15a/mir-16-1), was deleted in all cases, along with a portion of the DLEU1 gene. Type 1 deletions involving only DLEU/mir-15/mir-16 were detected in 14 cases (64%). The larger type 2 deletion, which also involved the RB1 gene, was observed in the remaining 8 cases (36%). In the five cases harboring 11q deletion, multiple probes targeting different ATM gene sections revealed that varying lengths of the ATM gene were deleted. The neighboring RDX-11 gene was also deleted in two of the five cases, and the PPP2R1B-4 gene, which is located next to RDX-11 gene locus, was also deleted in one of these cases.
Six cases exhibited more than one cytogenetic abnormalities (Table 3). Two cases with 13q14 deletion also had Cyclin Dependent Kinase Inhibitor 2D gene (CDKN2D-2; located at 19p13.2) gain/amplification. One case with 13q14 deletion also had 17p13 deletion. CDKN2D-2 deletion was also detected in one case with trisomy 12. The presence of trisomy 12 was also detected in one case that also had 13q14 deletion (type 2). Another case with trisomy 12 exhibited heterozygous NOTCH1 c.7541_7542delCT mutation. MYD88 L265P and SF3B1 K700E point mutations were not detected in any of the 52 cases.
Table 3.
Brief details of the cases with two concurrent genetic abnormalities.
| Case No. | Genetic abnormalities detected | Modified Rai/Binet stage | Management | Time to treatment |
|---|---|---|---|---|
| 5 | Trisomy 12+CDKN2D-2(19p13.2) deletion | Intermediate/B | Chemotherapy | 1 mo |
| 15 | 13q14.2-3 deletion+CDKN2D-2(19p13.2) gain | Low/A | Observation | - |
| 21 | 13q14.2-3 deletion+trisomy 12 | High/C | Observation | - |
| 39 | Trisomy 12+NOTCH1(p.P2514*fs) mutation | Intermediate/B | Chemotherapy | 2 mo |
| 53 | 13q14.2-3 deletion+CDKN2D-2(19p13.2) gain | High/C | Chemotherapy | 3.5 mo |
| 76 | 13q14.3 deletion+17p13.1 deletion | Low/A | Lost to follow-up |
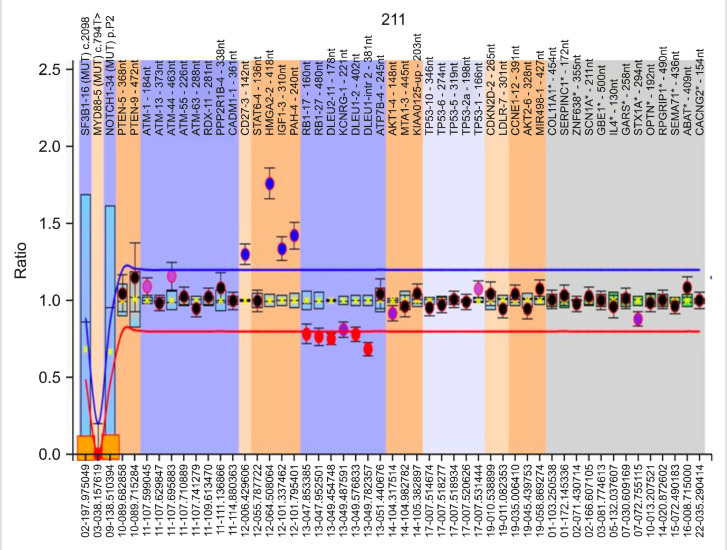
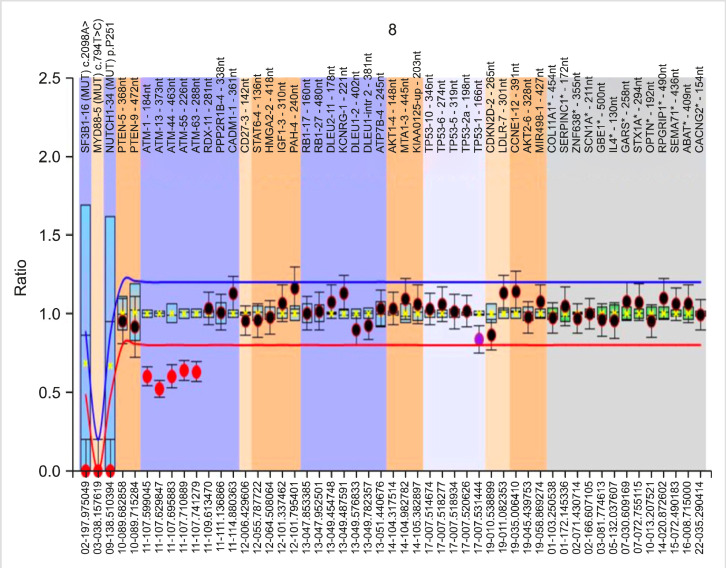
Figs. 1, 2 show representative images of a case with trisomy 12 and 13q14 deletion, along with a case with 11q (ATM) deletion.
Fig. 1.
Peak patterns from a CLL case with trisomy 12 (dosage quotient, -1.5) and heterozygous 13q14 deletion (dosage quotient, -0.7).
Fig. 2.
Peak patterns from a CLL case with heterozygous 11q (ATM) deletion (dosage quotient, -0.6).
Correlation of cytogenetic abnormalities with clinico-hematological parameters
Of all the variables, only hemoglobin concentration, Binet stage, and presence of elevated LDH level were statistically significantly correlated with cytogenetic abnormalities. Cases with high risk cytogenetics, del(11q), and del(17p) had a lower mean hemoglobin concentration compared to cases in other cytogenetic groups. This indicates that cases with high risk cytogenetics presented more often with cytopenia in the study cohort (Table 4).
Table 4.
Correlation of clinical and laboratory variables with cytogenetic abnormalities.
| Parameter | Del (13q) | Trisomy 12 | Del (11q) | Del (17p) | Normal | P |
|---|---|---|---|---|---|---|
| Age (yr), mean | 65.4 | 64.2 | 66.6 | 72.0 | 62.9 | 0.804 |
| Sex | 0.641 | |||||
| Male | 12 | 05 | 04 | 01 | 13 | |
| Female | 09 | 01 | 01 | 01 | 05 | |
| Hemoglobin (g/dL), mean | 12.1 | 12.1 | 8.6 | 11.5 | 12.9 | 0.011 |
| ALC (×109/L), mean | 88.2 | 85.6 | 113.9 | 61.6 | 43.6 | 0.178 |
| Platelet count (×109/L), mean | 173.6 | 179.0 | 199.6 | 166.5 | 192.7 | 0.935 |
| Smudge cells (%), mean | 31.3 | 19.3 | 40.8 | 12.5 | 37.9 | 0.243 |
| Modified Rai stage | 0.062 | |||||
| Low | 06 | 01 | 01 | 01 | 07 | |
| Int./high | 15 | 05 | 04 | 01 | 11 | |
| Binet stage | 0.025 | |||||
| A | 13 | 02 | 01 | 01 | 12 | |
| B/C | 08 | 04 | 04 | 01 | 06 | |
| LDH (47 cases) | 0.012 | |||||
| Normal | 14 | 01 | 01 | 00 | 15 | |
| Elevated | 05 | 05 | 02 | 01 | 03 |
Treatment and follow-up details
The median follow-up period (FUP) of the study cohort was 5.5 months (range, 0–18 mo). Seventeen cases were lost to follow-up following the primary diagnosis. Of the remaining 35 cases, 9 (25.7%) were offered treatment. Of these nine cases, five were treated with Bendamustine plus rituximab, three were treated with chlorambucil plus rituximab, and one was treated with chlorambucil plus prednisolone. Lenalidomide was used for maintenance therapy. Twenty-six cases (74.3%) remained under observation with regular follow-up visits and a ‘watch and wait’ policy was followed.
None of the treated cases and cases under observation died or had documented disease progression or transformation at the end of the study period.
Details of all cases with cytogenetic abnormalities in relation to treatment and follow-up are out-lined in Table 5.
Table 5.
Cases with cytogenetic abnormalities - details of staging, treatment and follow-up.
| Case No. | Age/sex | Cytogenetic abnormality |
Modified Rai staging | Binet staging | Treatment | Time to treatment | Follow-up period |
|---|---|---|---|---|---|---|---|
| 4 | 57/M | Del (13q) | High | C | 6 cycles of BR, achieved CR, followed by 5 cycles of Len maintenance | 5 mo | 18 mo |
| 5 | 63/M | Trisomy 12 | Intermediate | B | 6 cycles of BR, attained PR, now on observation | 1 mo | 18 mo |
| 6 | 51/F | Del (13q) | Intermediate | A | - | - | Lost to follow-up |
| 8 | 63/F | Del (11q) | High | C | Observation | - | 2 mo |
| 11 | 57/M | Del (11q) | High | C | - | - | Lost to follow-up |
| 15 | 80/M | Del (13q) | Low | A | Observation | - | 18 mo |
| 19 | 55/F | Del (13q) | Low | A | Observation | - | 6 mo |
| 21 | 48/M | Del (13q)+Trisomy 12 | High | C | Observation | - | 18 mo |
| 22 | 60/F | Del (13q) | Low | A | - | - | Lost to follow-up |
| 23 | 70/M | Del (17p) | Intermediate | B | 2 cycles of BR, then 6 cycles of R-Len, achieved PR, now Len maintenance | 0.2 mo | 16 mo |
| 24 | 74/M | Del (13q) | Intermediate | A | Observation | - | 16 mo |
| 34 | 57/M | Trisomy 12 | Intermediate | A | Observation | - | 14 mo |
| 35 | 50/F | Del (13q) | Low | A | - | - | Lost to follow-up |
| 36 | 67/M | Del (11q) | High | C | 3 cycles of R-Clb | 2 mo | 4 mo |
| 39 | 69/M | Trisomy 12+NOTCH1 mutation | Intermediate | B | 6 cycles of R-Clb, achieved PR, now 4 cycles of Len maintenance | 2 mo | 13 mo |
| 40 | 62/M | Del (13q) | Intermediate | B | 4 cycles of BR | 7.5 mo | 13 mo |
| 42 | 73/M | Del (13q) | High | C | - | - | Lost to follow-up |
| 44 | 80/M | Del (11q) | Low | A | - | - | Lost to follow-up |
| 45 | 66/M | Del (11q) | High | C | - | - | Lost to follow-up |
| 50 | 70/M | Del (13q) | Intermediate | A | Observation | - | 12 mos |
| 51 | 63/M | Trisomy 12 | Low | A | Observation | - | 12 mo |
| 52 | 62/F | Del (13q) | Intermediate | A | Observation | - | 5 mo |
| 53 | 65/M | Del (13q) | High | B | 6 cycles of BR, achieved PR | 3.5 mo | 12 mo |
| 56 | 65/F | Del (13q) | High | C | 9 cycles of R-Clb | 1.5 mo | 11 mo |
| 57 | 65/M | Del (13q) | Intermediate | A | Observation | - | 11 mo |
| 61 | 68/F | Del (13q) | Low | A | Observation | - | 10 mo |
| 65 | 66/F | Trisomy 12 | High | C | 5 cycles of Clb-Pred completed | 2.5 mo | 9 mo |
| 68 | 80/F | Del (13q) | Low | A | Observation | - | 9 mo |
| 71 | 86/M | Del (13q) | Intermediate | A | - | - | Lost to follow-up |
| 72 | 67/M | Trisomy 12 | Intermediate | C | Observation | - | 8 mo |
| 74 | 50/F | Del (13q) | High | C | Observation | - | 8 mo |
| 76 | 74/F | Del (13q)+Del (17p) | Low | A | - | - | Lost to follow-up |
| 77 | 78/M | Del (13q) | High | C | - | - | Lost to follow-up |
| 78 | 75/M | Del (13q) | Intermediate | A | - | - | Lost to follow-up |
Abbreviations: BR, bendamustine plus rituximab; Clb-Pred, chlorambucil+prednisolone; CR, complete response; Len, lenalidomide; PR, partial response; R-Clb, rituximab+chlorambucil; R-Len, rituximab+lenalidomide.
Correlation between cytogenetic abnormalities and disease progression
Univariate and multivariate logistic regression analyses were performed to study the effect of various clinico-hematological parameters on the time to treatment (<8 mo). Given the limited number of patients with 11q and 17p deletions, cytogenetic abnormalities were partitioned into three risk groups. The low risk group consisted of 13q deletion and no detected abnormality (normal); the intermediate risk group had trisomy 12; and the high-risk group included 11q and 17p deletions.
Univariate analysis revealed that hemoglobin concentration, presence of organomegaly, involvement of three or more lymph node groups, modified Rai stage, Binet stage, LDH and β2- microglobulin levels, and cytogenetic risk group were significantly correlated with a shorter time to treatment (Table 6). However, multivariate logistic regression found only elevated LDH and β2-microglobulin levels, and the involvement of three or more lymph node groups to be statistically significant (P<0.05) (Table 7). This indicates that, in our cohort, primarily patients with bulky disease/ symptomatic lymphadenopathy required earlier initiation of chemotherapy in accordance with the established iwCLL guidelines.
Table 6.
Univariate logistic regression analysis showing the impact of variables on time to treatment.
| Parameter | P |
|---|---|
| Age≥60 yr | 0.133 |
| Sex, male | 0.334 |
| Hemoglobin<10 g/dL | 0.044 |
| Absolute lymphocyte count>50×109/L | 0.319 |
| Platelet count<100×109/L | 0.594 |
| Hepatomegaly | 0.015 |
| Splenomegaly | 0.068 |
| Lymph node groups involved≥3 | <0.001 |
| Modified Rai stage (low vs. intermediate/high) | 0.029 |
| Binet stage (A vs. B/C) | <0.001 |
| ECOG performance status (0–1 vs. 2–4) | 0.383 |
| Lactate dehydrogenase level, normal/elevated | 0.006 |
| β2-microglobulin level, normal/elevated | <0.001 |
| CD38 expression≥30% | 0.383 |
| Cytogenetic risk group (low vs. intermediate/high) | 0.015 |
Table 7.
Multivariate logistic regression analysis showing the impact of variables on time to treatment.
| Parameter | P |
|---|---|
| Hemoglobin<10 g/dL | 0.315 |
| Hepatomegaly (present/absent) | 0.303 |
| Splenomegaly (present/absent) | 0.584 |
| Lymph node groups involved≥3 | <0.001 |
| Modified Rai stage (low vs. intermediate/high) | 0.082 |
| Binet stage (A vs. B/C) | 0.058 |
| Lactate dehydrogenase level (normal/elevated) | 0.035 |
| β2-microglobulin level (normal/elevated) | 0.025 |
| Cytogenetic risk group (low vs. intermediate/high) | 0.194 |
DISCUSSION
CLL is a clinically heterogeneous disease; while some patients undergo rapid disease progression, others have an indolent course and survive for decades. The environmental and genetic factors have been implicated for this variation [6].
Up to 80% of CLL cases possess chromosomal alterations detected by molecular cytogenetics. In our study, we identified chromosomal alterations in 65% of the patients. The incidence of 13q deletion, trisomy 12, and 11q deletion did not deviate from previous reports, except for 17p deletion [4, 13, 14]. Our study cohort had a relatively lower incidence (4%) of 17p deletion compared to both Western and Indian studies. This difference is most likely due to the smaller study cohort and patient selection. However, 17p deletion incidence as low as 2–4% has been previously reported [8].
Using multiple probes per locus in the MLPA Probemix, we revealed that the size of the 13q14 deletion is heterogeneous. Of the 22 cases with 13q14 deletion, the RB1 gene was deleted in only eight cases. Ouillette et al. [15] referred to these as type 2 deletions, whereas those that did not include the RB1 gene were referred to as type 1 deletions. The significance of large 13q14 deletions is emphasized by the involvement of multiple genomic loci, including miR15a/16-1 microRNAs and RB1 genes that are involved in key biological pathways, such as cell cycle control and NF-κB signaling [15-17]. In our study, the DLEU locus was included in all cases with 13q14 deletion. This genetic locus encodes for mir-15a/mir16-1 microRNAs, which function as tumor suppressors. Although, biallelic deletions are reported to occur in nearly one-fourth of CLL cases with 13q deletion, only one case in our cohort exhibited biallelic deletion [18]. The remaining del(13q) cases were heterozygous (monoallelic) deletions.
Six of the 34 cases also had additional cytogenetic abnormalities. The presence of multiple genetic aberrations has been well documented in CLL and contributes to disease heterogeneity [16]. One case with trisomy 12 also carried a NOTCH1 mutation. Trisomy 12 is associated with the presence of NOTCH1 mutations, potentially explaining the relatively poorer prognosis of trisomy 12 cases compared to del(13q) cases [19].
Three cases also exhibited gain or loss of the CDKN2D-2 gene locus. Al Zaabi et al. [8] detected the gain of the 19p13.2 CDKN2D locus in 14 patients using MLPA. This was accompanied by loss of the LDLR gene, which is located in same chromosomal region, indicating the presence of trisomy 19. However, in our study, the two cases with CDKN2D-2 gains (heterozygous) were not associated with abnormalities detected by other probes directed at the 19p13 region. Small intragenic deletions of the 19p13.2 CDKN2D locus detected by MLPA has also been reported by Abdool et al. [20]. However, these abnormalities should be confirmed by additional testing.
The median time to first treatment is reported to be shorter in CLL patients with high risk cytogenetics (11q and 17p deletion) [21, 22]. In our study, univariate regression analysis revealed that cytogenetic risk was significantly correlated with earlier initiation of therapy. However, in a multivariate regression model, the association between time to treatment and cytogenetic risk was not statistically significant. Therefore, a longer study period utilizing a larger study cohort would be required to better analyze the impact of cytogenetics on time to treatment in our population.
A better understanding of the underlying genetic abnormalities in CLL patients will help improve risk stratification and provide new targets for future therapies, especially with regards to personalized medicine. There is limited literature detailing the cytogenetic profiles of Indian CLL patients. This study extends the data further, providing insights into the distribution of genetic abnormalities among CLL patients in the Indian population.
Footnotes
This study was supported in part by Intramural Research Grant (No. 71/2-Edu-16/1952) from Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Authors’ Disclosures of Potential Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Zenz T, Mertens D, Küppers R, Döhner H, Stilgenbauer S. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev. 2010;10:37–50. doi: 10.1038/nrc2764. [DOI] [PubMed] [Google Scholar]
- 2.Sutton LA, Rosenquist R. The complex interplay between cell-intrinsic and cell-extrinsic factors driving the evolution of chronic lymphocytic leukemia. Semin Cancer Biol. 2015;34:22–35. doi: 10.1016/j.semcancer.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Vollbrecht C, Mairinger FD, Koitzsch U, et al. Comprehensive analysis of disease-related genes in chronic lymphocytic leukemia by multiplex PCR-based next genera-tion sequencing. PLoS One. 2015;10:e0129544. doi: 10.1371/journal.pone.0129544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 5.Ries LAG, Harkins D, Krapcho M, et al. SEER cancer statistics review, 1975-2004. National Cancer Institute; Bethesda, MD: 2007. [Accessed March 2, 2020]. https://seer.cancer.gov/archive/csr/1975_2004/ [Google Scholar]
- 6.Gunawardana C, Austen B, Powell JE, et al. South Asian chronic lymphocytic leukaemia patients have more rapid disease progression in comparison to White patients. Br J Haematol. 2008;142:606–9. doi: 10.1111/j.1365-2141.2008.07226.x. [DOI] [PubMed] [Google Scholar]
- 7.Hömig-Hölzel C, Savola S. Multiplex ligation-dependent probe amplification (MLPA) in tumor diagnostics and prognostics. Diagn Mol Pathol. 2012;21:189–206. doi: 10.1097/PDM.0b013e3182595516. [DOI] [PubMed] [Google Scholar]
- 8.Al Zaabi EA, Fernandez LA, Sadek IA, Riddell DC, Greer WL. Multiplex ligation-dependent probe amplification versus multiprobe fluorescence in situ hybridization to detect genomic aberrations in chronic lymphocytic leukemia: a tertiary center experi-ence. J Mol Diagn. 2010;12:197–203. doi: 10.2353/jmoldx.2010.090046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coll-Mulet L, Santidrián AF, Cosialls AM, et al. Multiplex ligation-dependent probe amplification for detection of genomic alterations in chronic lymphocytic leukaemia. Br J Haematol. 2008;142:793–801. doi: 10.1111/j.1365-2141.2008.07268.x. [DOI] [PubMed] [Google Scholar]
- 10.Buijs A, Krijtenburg PJ, Meijer E. Detection of risk-identifying chromosomal abnor-malities and genomic profiling by multiplex ligation-dependent probe amplification in chronic lymphocytic leukemia. Haematologica. 2006;91:1434–5. [PubMed] [Google Scholar]
- 11.Alhourani E, Rincic M, Othman MA, et al. Comprehensive chronic lymphocytic leu-kemia diagnostics by combined multiplex ligation dependent probe amplification (MLPA) and interphase fluorescence in situ hybridization (iFISH) Mol Cytogenet. 2014;7:79. doi: 10.1186/s13039-014-0079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–60. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 13.Sindelárová L, Michalová K, Zemanová Z, et al. Incidence of chromosomal anomalies detected with FISH and their clinical correlations in B-chronic lymphocytic leukemia. Cancer Genet Cytogenet. 2005;160:27–34. doi: 10.1016/j.cancergencyto.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Patkar N, Rabade N, Kadam PA, et al. Immunogenetics of chronic lymphocytic leu-kemia. Indian J Pathol Microbiol. 2017;60:38–42. doi: 10.4103/IJPM.IJPM_466_16. [DOI] [PubMed] [Google Scholar]
- 15.Ouillette P, Erba H, Kujawski L, Kaminski M, Shedden K, Malek SN. Integrated genomic profiling of chronic lymphocytic leukemia identifies subtypes of deletion 13q14. Cancer Res. 2008;68:1012–21. doi: 10.1158/0008-5472.CAN-07-3105. [DOI] [PubMed] [Google Scholar]
- 16.Ouillette P, Fossum S, Parkin B, et al. Aggressive chronic lymphocytic leukemia with elevated genomic complexity is associated with multiple gene defects in the response to DNA double-strand breaks. Clin Cancer Res. 2010;16:835–47. doi: 10.1158/1078-0432.CCR-09-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouillette P, Collins R, Shakhan S, et al. Acquired genomic copy number aberrations and survival in chronic lymphocytic leukemia. Blood. 2011;118:3051–61. doi: 10.1182/blood-2010-12-327858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg R, Wierda W, Ferrajoli A, et al. The prognostic difference of monoallelic versus biallelic deletion of 13q in chronic lymphocytic leukemia. Cancer. 2012;118:3531–7. doi: 10.1002/cncr.26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balatti V, Bottoni A, Palamarchuk A, et al. NOTCH1 mutations in CLL associated with trisomy 12. Blood. 2012;119:329–31. doi: 10.1182/blood-2011-10-386144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdool A, Donahue AC, Wohlgemuth JG, Yeh CH. Detection, analysis and clinical validation of chromosomal aberrations by multiplex ligation-dependent probe ampli-fication in chronic leukemia. PLoS One. 2010;5:e15407. doi: 10.1371/journal.pone.0015407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramer P, Hallek M. Prognostic factors in chronic lymphocytic leukemia-what do we need to know? Nat Rev Clin Oncol. 2011;8:38–47. doi: 10.1038/nrclinonc.2010.167. [DOI] [PubMed] [Google Scholar]
- 22.Delgado J, Espinet B, Oliveira AC, et al. Chronic lymphocytic leukaemia with 17p deletion: a retrospective analysis of prognostic factors and therapy results. Br J Haematol. 2012;157:67–74. doi: 10.1111/j.1365-2141.2011.09000.x. [DOI] [PubMed] [Google Scholar]