Version Changes
Revised. Amendments from Version 1
The updated article provides additional sources where necessary to further highlight the roles of galectin-3 in the innate immune system. Additionally, recent evidence is included that validates the S1-NTD of SARS-CoV2 as a promising therapeutic target. We hope this updated text to be a more detailed review with enhanced readability compared to the prior copy.
Abstract
The pandemic brought on by the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has become a global health crisis, with over 22 million confirmed cases and 777,000 fatalities due to coronavirus disease 2019 (COVID-19) reported worldwide. The major cause of fatality in infected patients, now referred to as the “Cytokine Storm Syndrome” (CSS), is a direct result of aberrant immune activation following SARS-CoV2 infection and results in excess release of inflammatory cytokines, such as interleukin (IL)-1, tumor necrosis factor α (TNF-α), and IL-6, by macrophages, monocytes, and dendritic cells. Single cell analysis has also shown significantly elevated levels of galectin 3 (Gal-3) in macrophages, monocytes, and dendritic cells in patients with severe COVID-19 as compared to mild disease. Inhibition of Gal-3 reduces the release of IL-1, IL-6, and TNF-α from macrophages in vitro, and as such may hold promise in reducing the incidence of CSS. In addition, Gal-3 inhibition shows promise in reducing transforming growth factor ß (TGF-ß) mediated pulmonary fibrosis, likely to be a major consequence in survivors of severe COVID-19. Finally, a key domain in the spike protein of SARS-CoV2 has been shown to bind N-acetylneuraminic acid (Neu5Ac), a process that may be essential to cell entry by the virus. This Neu5Ac-binding domain shares striking morphological, sequence, and functional similarities with human Gal-3. Here we provide an updated review of the literature linking Gal-3 to COVID-19 pathogenesis. Dually targeting galectins and the Neu5Ac-binding domain of SARS-CoV2 shows tentative promise in several stages of the disease: preventing viral entry, modulating the host immune response, and reducing the post-infectious incidence of pulmonary fibrosis.
Keywords: COVID-19, galectin, cytokines; ARDS, fibrosis, sialic acid, galectin-3
Introduction
Galectin 3 (Gal-3) is a carbohydrate-binding protein that exhibits pleiotropic effects throughout the body, including the modulation of apoptosis, cell migration and adhesion, angiogenesis, tumorigenesis, and post-injury remodeling ( Chen & Kuo, 2016; Elola et al., 2018; Nangia-Makker et al., 2018). It is most highly expressed in myeloid cells (macrophages, dendritic cells, neutrophils, and monocytes), as well as epithelial cells, endothelial cells, and fibroblasts ( Diaz-Alvarez & Ortega, 2017). When secreted by myeloid cells, Gal-3 and other galectins can act as modulators of cytokine expression by immune cells, and also as orchestrators of the damage associated molecular pattern (DAMP) system ( Sato et al., 2009). Recent discoveries specific to viral infections have begun to shed light on its role as well ( Wang et al., 2019). For example, in HIV and HTLV, Gal-3 serves as an attachment factor that facilitates viral entry into T-cells ( Wang et al., 2019). HIV infection also induces further Gal-3 expression through activation of NF-kB dependent pathways ( Okamato et al., 2019). Secreted Gal-3 then mediates a number of deleterious effects. In particular, Gal-3 has been shown during infection to induce a dysregulated pattern expression of pro-inflammatory cytokine expression via the JAK/STAT1, ERK, and AKT signaling pathways ( Nita-Lazar et al., 2015). The cytokine profile observed includes tumor necrosis factor α (TNFα), interleukin (IL)-1β, and IL-6, among others ( Nita-Lazar et al., 2015). Gal-3 is also a known agonist of toll like receptor 4 (TLR4) and nuclear factor kappa beta (NF-kB) dependent pathways, which are well characterized and potent inducers of inflammation during infection ( Yip et al., 2017; Zhou et al., 2018). Patients suffering from severe coronavirus disease 2019 (COVID-19) show highly elevated levels of Gal-3, TNFα, IL-1β, and IL-6, as compared to those with moderate disease ( De Biasi et al., 2020; Wang et al., 2020a). Inhibition of Gal-3 significantly reduces the levels of these cytokines, and so may show promise in reducing inflammatory sequelae associated with COVID-19 ( De Biasi et al., 2020; Kalfaoglu et al., 2020; Liu et al., 2020).
The continued lack of an effective standard of care for treating patients with COVID-19 has brought on an urgent need to identify effective therapies. In a prior review article, we had discussed promising indications for Gal-3 targeted therapy in the treatment of COVID-19, with the goal of inspiring further research on the topic ( Caniglia et al., 2020). In recent months, however, a substantial amount of new evidence has emerged that further links Gal-3 to severe COVID-19 infection. As such, the authors see a need to achieve two aims in this review: highlighting novel discoveries to expand upon previously discussed treatment indications, and to detail a further potential role for anti-galectin therapy in reducing post-infectious pulmonary fibrosis. This article may be particularly useful for immunologists studying COVID-19, as well as any researchers with a structural or functional focus on galectins.
SARS-CoV2: host cell attachment and entry
A critical step prior to viral infection is the entry of the virus into host cells, a process that in severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) is mediated by the S1 subunit of the spike protein ( Blaas, 2016; Zhai et al., 2020). Within coronaviridae, it is commonplace to refer to the S1 protein as consisting of two distinct regions: the C-terminal domain (CTD) and N-terminal domain (NTD) ( Li, 2016). In most cases, the CTD binds peptide receptors and the NTD binds sugar receptors ( Li, 2016). The main entry mechanism of SARS-CoV2 has been shown to be via the CTD binding to angiotensin converting enzyme receptor 2 (ACE2) receptors ( Wang et al., 2020b). Until recently, the role of the NTD has been largely overlooked. A study from Baker et al. has shown evidence that SARS-CoV2 also binds N-acetylneuraminic acid (Neu5Ac), with this interaction being mediated by the NTD of the S1 subunit ( Baker et al., 2020). This is the first in vitro evidence of this occurring, although several prior bioinformatics and modeling studies have hypothesized that a Neu5Ac binding site exists, with one suggesting its affinity for Neu5Ac (0.88) is only slightly lower than that of influenza hemagglutinin (0.94) ( Alban et al., 2020; Behloul et al., 2020; Fantini et al., 2020; Kim, 2020; Milanetti et al., 2020; Robson, 2020). Binding of sialic acids by the NTD is the main entry mechanism in several other coronaviruses known to infect humans, most notably members of the bovine coronavirus family ( Li, 2015). Additionally, the closely related middle eastern respiratory syndrome coronavirus (MERS-CoV) has been shown to exhibit a dual attachment model similar to SARS-CoV2, where the CTD binds a peptide receptor and the NTD binds sialic acids ( Li et al., 2017). Depletion of sialic acids with neuraminidase inhibitors prevented MERS-CoV infection of Calu-3 human airway cells, indicating that NTD-targeted therapies may be effective in preventing cell entry by coronaviruses possessing this function ( Li et al., 2017). Additionally, a neutralizing antibody against the SARS-CoV2 S1-NTD has been shown to completely inhibit cell entry by the virus ( Chi et al., 2020). This indicates the NTD region is essential for viral entry and a promising therapeutic target ( Chi et al., 2020). The dual mechanism by which SARS-CoV2 may enter host cells is seen in Figure 1.
Figure 1. A dual attachment model for SARS-CoV2.
Evidence has shown that a pocket in the NTD of SARS-CoV2 is capable of binding N-acetylneuraminic acid (Neu5Ac). This strongly supports a dual attachment model for SARS-CoV2, where NTD-Neu5Ac interactions facilitate initial host cell recognition by the virus and stabilize its entry via ACE2 receptors.
The binding of Neu5Ac may also explain the greater infectivity of SARS-CoV2 as compared to SARS-CoV ( Alban et al., 2020). While the CTD of SARS-CoV2 has been shown to exhibit higher affinity for ACE2 receptors than that of SARS-CoV, this is likely insufficient to fully explain the marked disparity in transmissibility ( Tai et al., 2020). The NTD of SARS-CoV2 has been rigorously analyzed and compared to both human galectins and the NTD of other coronaviruses ( Behloul et al., 2020). Behloul et al. found that while SARS-CoV2 and SARS-CoV share 74.75% similarity in the CTD, they exhibit just 52.69% similarity in the NTD region ( Behloul et al., 2020). This is particularly noteworthy when viewed together with the findings that despite SARS-CoV2 being able to bind Neu5Ac in vitro, the same domain on SARS-CoV did not exhibit this ability ( Baker et al., 2020). Modeling studies comparing the NTD of SARS-CoV2 and SARS-CoV have led to the same conclusion ( Behloul et al., 2020). The far greater abundance of Neu5Ac in the human body as compared to ACE2 receptors, particularly at common viral entry points such as the nasopharynx and oral mucosa, may explain the high transmissibility of SARS-CoV2 ( Barnard et al., 2019).
Several studies to date have referred to the “galectin fold” present on the NTD of coronaviruses ( Behloul et al., 2020; Li, 2016; Li et al., 2017; Peng et al., 2011; Peng et al., 2012; Tortorici et al., 2019). The structures of Gal-3 and the S1-NTD of betacoronaviridae are so similar, in fact, that it is hypothesized that coronaviruses incorporated a host galectin gene into their genome (and then the NTD) at some point in their evolution ( Caniglia et al., 2020; Li, 2015). Structural analysis comparing the SARS-CoV2 NTD to Gal-3 resulted in a Z-score of 6 (p < 0.00001), indicating a high degree of similarity between the structures ( Behloul et al., 2020). In fact, human Gal-3 was shown to be equally similar to SARS-CoV2 NTD as the NTD of NL63-CoV and infectious bronchitis coronavirus, accounting for both sequence and structure ( Behloul et al., 2020). Given the high degree of structural and promising sequence similarity (12%) of the NTD with Gal-3, it may be possible that existing Gal-3 inhibitors possess dual-binding capabilities ( Behloul et al., 2020). Such a mechanism shows promise in reducing viral entry to host cells ( Milanetti et al., 2020).
Gal-3 in severe infection: promoting immunologic sequelae of COVID-19
The major cause of death in patients infected with SARS-CoV and MERS-CoV infection was found to be the “Cytokine Storm Syndrome” (CSS), and this is likely to be the case in COVID-19 as well ( Channappanavar & Perlman, 2017; Zhang et al., 2020). CSS develops due to hyper-activation of macrophages, monocytes, and dendritic cells, which are stimulated to release a variety of inflammatory mediators including IL-1, IL-6, and TNF-α ( Zhang et al., 2020). This in turn leads to systemic organ dysfunction that may result in death ( England et al., 2020). Notably, a study of nearly 4,000 patients has found the levels of IL-1, IL-6, and TNF-α to be significantly elevated in the sera of patients suffering from severe COVID-19 as compared to those with mild disease ( Wang et al., 2020a). Similar findings were reported in a cohort of over 1,5000 patients, where serum IL-6 and TNF-α were found to be independent predictors of disease severity and mortality in COVID-19 ( Del Valle et al., 2020). This data speaks to the urgency of identifying therapeutics to reduce the incidence of CSS ( Del Valle et al., 2020; Wang et al., 2020a).
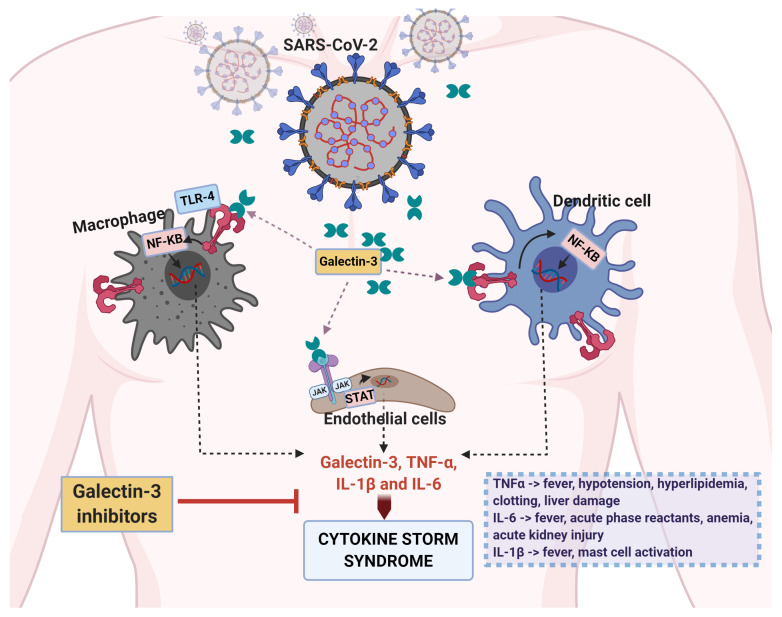
There is a plethora of evidence that makes Gal-3 a promising target to achieve this aim. First, the most concerning sequelae of CSS is evolution to acute respiratory distress syndrome (ARDS), a condition which often leads to respiratory failure despite proactive measures such as mechanical ventilation and intubation ( Vabret et al., 2020). Elevated serum levels of Gal-3 are significantly associated with worse outcomes and lower survival in patients suffering from ARDS ( Xu et al., 2017). Additionally, significantly elevated levels of Gal-3 have been shown in the serum of patients suffering from severe COVID-19 as compared to those with mild disease ( De Biasi et al., 2020). On a cellular level, Gal-3 was shown to be most elevated in immune cells during severe COVID-19 ( Kalfaoglu et al., 2020) The highest levels of Gal-3 were seen in infected macrophages, monocytes, and dendritic cells, the very cells responsible for initiating CSS ( Liu et al., 2020). A pathway through which Gal-3 may contribute to the development of CSS is detailed in Figure 2.
Figure 2. Gal-3 may amplify the cytokine storm syndrome associated with severe COVID-19.
During severe SARS-CoV2 infection, increased plasma concentrations of Gal-3 are observed in circulating macrophages, monocytes, and dendritic cells. When secreted, Gal-3 can then agonize TLR4 receptors on their surfaces and induce the release of inflammatory cytokines such as IL-1, IL-6, and TNF-α. This process also results in the secretion of further Gal-3, resulting in a positive feedback loop that may contribute to the development of CSS.
Several studies to date have shown the effects of anti-Gal-3 therapy on cytokine release ( Chen et al., 2015; Chen et al., 2018; Ren et al., 2019; Yip et al., 2017). Significant reductions in IL-1, IL-6, and TNF-α secretion by dendritic cells has been observed upon silencing of Gal-3 ( Chen et al., 2015). In models of traumatic brain injury and spinal cord injury, treatment with anti-Gal-3 antibodies and the Gal-3 inhibitor GB1107, respectively, both led to significant reductions in the systemic levels of IL-1, IL-6, and TNF-α ( Ren et al., 2019; Yip et al., 2017). Gal-3 K/O has also been shown to decrease both NF-kB activation and HIV viral replication in infected cells ( Okamato et al., 2019). Lastly, in mice infected with H5N1 influenza virus, Gal-3 K/O led to a significant reduction of IL-1ß secretion by macrophages and improved survival rate as compared to controls ( Chen et al., 2018). These findings are due to Gal-3’s known role as an alarmin of the innate immune system, triggering the release of inflammatory cytokine, such as TNF-α and IL-6 from monocyte-derived cells during infection or other inflammatory insults ( Mishra et al., 2013; Yip et al., 2017). The enhanced secretion of cytokines likely occurs through TLR4/NF-kB mediated pathways ( Yip et al., 2017; Zhou et al., 2018). With all this information taken together, Gal-3 inhibition shows promise in reducing the incidence and symptoms of CSS.
Gal-3 post-infection: pathologic fibrosis
It is well known that persistent viral infections are a risk factor for the subsequent development of pulmonary fibrosis ( Sheng et al., 2020). A study found that tests for SARS-CoV2 RNA in the serum of infected individuals did not become negative until a median of 24 days post-symptom onset, with some individuals remaining positive even greater than a month from the beginning of symptoms ( Gombar et al., 2020). Additionally, in a cohort of recovered COVID-19 patients in Italy, 87.4% reported persistent symptoms, most notably fatigue and dyspnea, at an average of 60.3 days post-infection ( Carfi et al., 2020). This indicates that for some, persistent post-viral inflammation may result in deleterious changes such as pulmonary fibrosis ( Crisan-Dabija et al., 2020). Findings such as these have led to the question of whether or not anti-fibrotic therapy would be beneficial for such patients ( George et al., 2020).
In SARS-CoV infection, particularly in patients who suffered from ARDS, marked pulmonary fibrosis was found in a cohort of patients following prolonged infection ( Ye et al., 2007). Though long term outcomes remain to be seen, lung tissue in the acute phase of COVID-19 shows similar changes ( Xu et al., 2020a). Following a 24 hour incubation of SARS-CoV2, human airway cells showed upregulation of ACE2, vascular endothelial growth factor (VEGF), connective tissue growth factor (CTGF), fibronectin (FN), and transforming growth factor ß (TGF-ß), a molecular signature highly similar to that of patients with diagnosed pulmonary fibrosis ( Xu et al., 2020a). It is believed that a large number of COVID-19 patients will go on to develop pulmonary fibrosis, and that these changes are mediated by a number of cytokines including TGF- ß, IL-1, IL-6, and TNF-α ( Delpino & Quarleri, 2020).
The role of Gal-3 as a mediator of lung fibrosis has long been studied since the discovery that its levels are elevated in alveolar macrophages following lung injury ( Kasper & Hughes, 1996; Nishi et al., 2007). Higher levels of Gal-3 have now been extensively associated with the development of restrictive lung diseases ( Ho et al., 2016). Following cellular stress, the secretion of Gal-3 by macrophages upregulates TGF-ß receptors on fibroblasts and myofibroblasts ( Henderson et al., 2008). This in turn activates these cells, initiating the formation of granulation tissue (via collagen deposition) that is eventually remodeled to a fibrous scar ( Henderson et al., 2008; Mackinnon et al., 2012). This Gal-3 mediated pathway is widespread throughout the body and fundamental to the development of fibrotic change in the liver, kidneys, and heart as well ( Hara et al., 2020). Gal-3 mediated fibrosis often has deleterious effects; for example, pathologic scar formation is the likely explanation for serum Gal-3’s utility as an independent predictor of mortality and heart failure post-myocardial infarction ( Asleh et al., 2019). The mechanism by which Gal-3 may contribute to post-infectious pulmonary fibrosis in COVID-19 patients can be seen in Figure 3.
Figure 3. Gal-3 contributes to a pro-fibrotic microenvironment in COVID-19.
During SARS-CoV2 infection, transcriptional upregulation of VEGF, TGF-ß, and fibronectin (FN) is seen in the pulmonary epithelium, creating a pro-fibrotic microenvironment. Secretion of Gal-3 by macrophages contributes to fibrosis by increasing the expression of TGF-ß receptors on the surface of fibroblasts. The fibroblasts and myofibroblasts are then activated by TGF-ß mediated signaling, stimulating the deposition of extracellular matrix and collagen that leads to fibrotic damage. Cytokines induced by Gal-3 expression such as IL-1, IL-6, and TNF-α further accelerate this process.
Gal-3 inhibitors show promise in limiting fibrotic change following lung injury. In a model of adenovirus induced lung injury, Gal-3 K/O mice showed significant reductions in lung fibrosis and ß-catenin activation, indicating the beneficial effects were mediated via interruption of TGF-ß signaling ( Mackinnon et al., 2012). Treatment with the drug TD139 showed significant reductions in these parameters as well following bleomycin-induced pulmonary fibrosis ( Mackinnon et al., 2012). This drug (now referred to as GB0139) was well tolerated in phase I/IIa trials in the treatment of idiopathic pulmonary fibrosis (IPF) and is now in phase IIb trials ( Saito et al., 2019). An additional indication for this drug may be in reducing the post-viral development of pulmonary fibrosis ( Mackinnon et al., 2012). The drug TD139 has recently begun phase II trials for the treatment of COVID-19, the first clinical trial of a galectin inhibitor in COVID-19 to date ( University of Edinburgh DEFINE trial, 2020).
Conclusions and future directions
In summary, Gal-3 is a lectin that exhibits a pleiotropic role in mediating the acute and chronic consequences of infection and inflammation. Multiple studies have shown Gal-3 to be highly upregulated in patients suffering from severe COVID-19 ( De Biasi et al., 2020; Kalfaoglu et al., 2020; Liu et al., 2020). On a cellular level, Gal-3 is most highly expressed in monocytes, macrophages, and dendritic cells during severe COVID-19 infection ( Liu et al., 2020). CSS complicated by the development of ARDS is the major cause of fatality in COVID-19 patients ( Xu et al., 2020b; Zhai et al., 2020; Zhang et al., 2020). This process is chiefly mediated by the release of IL-1, IL-6, and TNF-α from macrophages, monocytes, and dendritic cells ( Zhang et al., 2020). Gal-3 inhibition has been shown to reduce the release of these cytokines from immune cells ( Chen et al., 2015; Ren et al., 2019; Yip et al., 2017). Additionally, high Gal-3 is directly associated with worse outcomes and lower survival in ARDS patients ( Xu et al., 2017).
A key domain in the spike protein exhibits a high degree of morphological and sequence similarity to human Gal-3 ( Behloul et al., 2020). This NTD has been shown to bind Neu5Ac in vitro, an interaction that likely explains the high infectivity of SARS-CoV2 and may be essential for cell entry ( Alban et al., 2020; Barnard et al., 2019; Baker et al., 2020). Inhibitors of Gal-3 that target regions of structural overlap with the NTD may possess dual binding capabilities, exhibiting a novel mechanism by which to inhibit viral entry ( Milanetti et al., 2020).
Lastly, pulmonary fibrosis has been observed following SARS-CoV infection and is likely to be a major complication in survivors of COVID-19 that is cytokine-mediated ( Delpino & Quarleri, 2020; Xu et al., 2020b; Ye et al., 2007). Among other mediators, elevated levels of TGF-ß have been observed following SARS-CoV2 infection ( Xu et al., 2020a). Gal-3 secreted by macrophages during injury promotes the upregulation of TGF-ß receptors, leading to fibroblast activation and collagen deposition ( Delpino & Quarleri, 2020). Gal-3 inhibition has been shown to reduce adenovirus-induced lung fibrosis, and an inhibitor is currently in Phase IIb clinical trials for IPF treatment ( Mackinnon et al., 2012; Saito et al., 2019). The indications for targeting Gal-3 in the treatment of COVID-19 are widespread. Processes directly mediated or affected by Gal-3 have been shown to be deleterious in several stages of the disease process. As such, Gal-3 represents a highly promising target for COVID-19 treatment that should urgently be investigated.
Literature search methodology
Eligibility criteria
This review consists of original studies that provided information about SARS-CoV2, Gal-3, or Gal-3 inhibitors. Compiled results from both in vivo, in vitro, and clinical studies were used for analysis. Studies with only an abstract or no full-text available were excluded from the review.
Search methodology
To retrieve primary literature, electronic searches were performed on PubMed and Google Scholar. A list of search terms can be seen in Table 1.
Table 1. Search strategy for our literature review.
| Database | Search Queries |
|---|---|
| PubMed |
On SARS-CoV2: ‘COVID-19 symptoms’ ‘SARS-CoV2 AND cytokine release syndrome’ ‘SARS-CoV2 entry
mechanism’ ‘SARS-CoV2 AND galectins’ ‘SARS-CoV2 S1-NTD’ ‘SARS-CoV2 spike protein’ ‘SARS-CoV2 cytokine storm syndrome’ ‘SARS-CoV2 sialic acids’ ‘SARS-CoV2 fibrosis’ On β-coronaviruses: ‘MERS-CoV entry mechanism’ On Galectin-3: ‘Galectins’ ‘Galectin-3’ ‘Galectin-3 AND cytokines’ ‘Galectin-3 AND inflammation’ ‘Galectin-3 AND viruses’ ‘Galectin-3 AND viral infection’ ‘Galectin-3 AND fibrosis’ On Galectin-3 Inhibitors: ‘Galectin-3 inhibitors’ ‘TD139’ ‘TD139 pulmonary fibrosis’ |
|
Google
Scholar |
On SARS-CoV2: ‘COVID-19,’ ‘COVID-19 symptoms’ ‘SARS-CoV2 fibrosis’
On Galectin-3: ‘Galectins’ ‘Galectin-3 cytokines’ ‘Galectin-3 fibrosis’ On Galectin-3 Inhibitors: ‘Galectin-3 inhibitors’ ‘TD139’ ‘Gal-3 clinical trials’ |
Risk of bias
To minimize the risk of error, all authors involved assessed the cited studies for quality. To discuss important claims in the article, including that SARS-CoV2 binds sialic acids with the S1-NTD, that Gal-3 is upregulated in human immune cells, and Gal-3 inhibitors’ ability to reduce fibrosis, multiple sources were included. Additionally, the use of open-ended searches ensured that an accurate profile of results was obtained on the topics discussed.
Data availability
No data are associated with this article.
Acknowledgments
The authors thank Mark Linder Walk for the Mind, Illinois Neurological Institute, OSF foundation, Peoria, IL, and KB Strong Foundation, Washington, IL for their support. The authors thank Erika Sung for help in the formatting the manuscript.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved]
References
- Alban TJ, Bayik D, Otvos B, et al. : Glioblastoma Myeloid-Derived Suppressor Cell Subsets Express Differential Macrophage Migration Inhibitory Factor Receptor Profiles That Can Be Targeted to Reduce Immune Suppression. Front Immunol. 2020;11:1191. 10.3389/fimmu.2020.01191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asleh R, Enriquez-Sarano M, Jaffe AS, et al. : Galectin-3 Levels and Outcomes After Myocardial Infarction: A Population-Based Study. J Am Coll Cardiol. 2019;73(18):2286–2295. 10.1016/j.jacc.2019.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AN, Richards SJ, Guy CS, et al. : The SARS-COV-2 Spike Protein Binds Sialic Acids, and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device. ChemRxiv.Preprint.2020. 10.26434/chemrxiv.12465680.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard KN, Wasik BR, LaClair JR, et al. : Expression of 9- O- and 7,9- O-Acetyl Modified Sialic Acid in Cells and Their Effects on Influenza Viruses. mBio. 2019;10(6):e02490–19. 10.1128/mBio.02490-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behloul N, Baha S, Shi R, et al. : Role of the GTNGTKR motif in the N-terminal receptor-binding domain of the SARS-CoV-2 spike protein. Virus Res. 2020;286:198058. 10.1016/j.virusres.2020.198058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaas D: Viral entry pathways: the example of common cold viruses. Wien Med Wochenschr. 2016;166(7–8):211–226. 10.1007/s10354-016-0461-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniglia JL, Guda MR, Asuthkar S, et al. : A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ. 2020;8:e9392. 10.7717/peerj.9392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A, Bernabei R, Landi F, et al. : Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324(6):603–605. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R, Perlman S: Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Kuo PL: The Role of Galectin-3 in the Kidneys. Int J Mol Sci. 2016;17(4):565. 10.3390/ijms17040565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SS, Sun LW, Brickner H, et al. : Downregulating galectin-3 inhibits proinflammatory cytokine production by human monocyte-derived dendritic cells via RNA interference. Cell Immunol. 2015;294(1):44–53. 10.1016/j.cellimm.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Wang SF, Weng IC, et al. : Galectin-3 Enhances Avian H5N1 Influenza A Virus-Induced Pulmonary Inflammation by Promoting NLRP3 Inflammasome Activation. Am J Pathol. 2018;188(4):1031–1042. 10.1016/j.ajpath.2017.12.014 [DOI] [PubMed] [Google Scholar]
- Chi X, Yan R, Zhang J, et al. : A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science.Retrieved September 08, 2020.2020;369(6504):650–655. 10.1126/science.abc6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan-Dabija R, Pavel CA, Popa IV, et al. : 'A chain only as strong as its weakest link': an up-to-date literature review on the bidirectional interaction of pulmonary fibrosis and COVID-19. J Proteome Res.[published online ahead of print, 2020 Sep 3].2020. 10.1021/acs.jproteome.0c00387 [DOI] [PubMed] [Google Scholar]
- De Biasi S, Meschiari M, Gibellini L, et al. : Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 2020;11(1):3434. 10.1038/s41467-020-17292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpino MV, Quarleri J: SARS-CoV-2 Pathogenesis: Imbalance in the Renin-Angiotensin System Favors Lung Fibrosis. Front Cell Infect Microbiol. 2020;10:340. 10.3389/fcimb.2020.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle DM, Kim-Schulze S, Huang HH, et al. : An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020. 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elola MT, Ferragut F, Mendez-Huergo SP, et al. : Galectins: Multitask signaling molecules linking fibroblast, endothelial and immune cell programs in the tumor microenvironment. Cell Immunol. 2018;333:34–45. 10.1016/j.cellimm.2018.03.008 [DOI] [PubMed] [Google Scholar]
- England JT, Abdulla A, Biggs CM, et al. : Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev. 2020;100707. 10.1016/j.blre.2020.100707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J, Di Scala C, Chahinian H, et al. : Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55(5):105960. 10.1016/j.ijantimicag.2020.105960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George PM, Wells AU, Jenkins RG: Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807–815. 10.1016/S2213-2600(20)30225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombar S, Chang M, Hogan CA, et al. : Persistent detection of SARS-CoV-2 RNA in patients and healthcare workers with COVID-19. J Clin Virol. 2020;129:104477. 10.1016/j.jcv.2020.104477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A, Niwa M, Noguchi K, et al. : Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules. 2020;10(3):389. 10.3390/biom10030389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Mackinnon AC, Farnworth SL, et al. : Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172(2):288–298. 10.2353/ajpath.2008.070726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JE, Gao W, Levy D, et al. : Galectin-3 Is Associated with Restrictive Lung Disease and Interstitial Lung Abnormalities. Am J Respir Crit Care Med. 2016;194(1):77–83. 10.1164/rccm.201509-1753OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfaoglu B, Almeida-Santos J, Adele Tye C, et al. : T-cell hyperactivation and paralysis in severe COVID-19 infection revealed by single-cell analysis. BioRxiv.Preprint.2020. 10.1101/2020.05.26.115923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper M, Hughes RC: Immunocytochemical evidence for a modulation of galectin 3 (Mac-2), a carbohydrate binding protein, in pulmonary fibrosis. J Pathol. 1996;179(3):309–316. [DOI] [PubMed] [Google Scholar]
- Kim CH: SARS-CoV-2 Evolutionary Adaptation toward Host Entry and Recognition of Receptor O-Acetyl Sialylation in Virus-Host Interaction. Int J Mol Sci. 2020;21(12):4549. 10.3390/ijms21124549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Alvarez L, Ortega E: The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediators Inflamm. 2017;2017(9247574):10. 10.1155/2017/9247574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F: Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev Virol. 2016;3(1):237–261. 10.1146/annurev-virology-110615-042301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F: Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol. 2015;89(4):1954–1964. 10.1128/JVI.02615-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Hulswit RJG, Widjaja I, et al. : Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc Natl Acad Sci U S A. 2017;114(40):E8508–E8517. 10.1073/pnas.1712592114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu A, He J, et al. : Single-cell analysis reveals macrophage-driven T-cell dysfuntion in severe COVID-19 patients. MedRxiv.Preprint.2020. 10.1101/2020.05.23.20100024 [DOI] [Google Scholar]
- Mackinnon AC, Gibbons MA, Farnworth SL, et al. : Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med. 2012;185(5):537–546. 10.1164/rccm.201106-0965OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanetti E, Miotto M, Di Rienzo L, et al. : In-silico evidence for two receptors based strategy of SARS-CoV2. BioRxiv.Preprint.2020. 10.1101/2020.03.24.006197 [DOI] [Google Scholar]
- Mishra BB, Li Q, Steichen AL, et al. : Galectin-3 functions as an alarmin: pathogenic role for sepsis development in murine respiratory tularemia. PLoS One. 2013;8(3):e59616. 10.1371/journal.pone.0059616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangia-Makker P, Hogan V, Raz A: Galectin-3 and cancer stemness. Glycobiology. 2018;28(4):172–181. 10.1093/glycob/cwy001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi Y, Sano H, Kawashima T, et al. : Role of galectin-3 in human pulmonary fibrosis. Allergol Int. 2007;56(1):57–65. 10.2332/allergolint.O-06-449 [DOI] [PubMed] [Google Scholar]
- Nita-Lazar M, Banerjee A, Feng C, et al. : Galectins regulate the inflammatory response in airway epithelial cells exposed to microbial neuraminidase by modulating the expression of SOCS1 and RIG1. Mol Immunol. 2015;68(2 Pt A):194–202. 10.1016/j.molimm.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamato M, Hidaka A, Toyama M, et al. : Galectin-3 is involved in HIV-1 expression through NF-κB activation and associated with Tat in latently infected cells. Virus Res. 2019;260:86–93. 10.1016/j.virusres.2018.11.012 [DOI] [PubMed] [Google Scholar]
- Peng G, Sun D, Rajashankar KR, et al. : Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc Natl Acad Sci U S A. 2011;108(26):10696–10701. 10.1073/pnas.1104306108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Xu L, Lin YL, et al. : Crystal structure of bovine coronavirus spike protein lectin domain. J Biol Chem. 2012;287(50):41931–41938. 10.1074/jbc.M112.418210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Liang W, Sheng J, et al. : Gal-3 is a potential biomarker for spinal cord injury and Gal-3 deficiency attenuates neuroinflammation through ROS/TXNIP/NLRP3 signaling pathway. Biosci Rep. 2019;39(12): BSR20192368. 10.1042/BSR20192368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B: Bioinformatics studies on a function of the SARS-CoV-2 spike glycoprotein as the binding of host sialic acid glycans. Comput Biol Med. 2020;122:103849. 10.1016/j.compbiomed.2020.103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Alkhatib A, Kolls JK, et al. : Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J Thorac Dis. 2019;11(Suppl 14):S1740–S1754. 10.21037/jtd.2019.04.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, St-Pierre C, Bhaumik P, et al. : Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol Rev. 2009;230(1):172–187. 10.1111/j.1600-065X.2009.00790.x [DOI] [PubMed] [Google Scholar]
- Sheng G, Chen P, Wei Y, et al. : Viral Infection Increases the Risk of Idiopathic Pulmonary Fibrosis: A Meta-Analysis. Chest. 2020;157(5):1175–1187. 10.1016/j.chest.2019.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W, He L, Zhang X, et al. : Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613–620. 10.1038/s41423-020-0400-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici MA, Walls AC, Lang Y, et al. : Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol. 2019;26(6):481–489. 10.1038/s41594-019-0233-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Edinburgh: Rapid Experimental Medicine for COVID-19 (DEFINE).In: ClinicalTrials.gov [cited 2020 Aug 6]. Reference Source [Google Scholar]
- Vabret N, Britton GJ, Gruber C, et al. : Immunology of COVID-19: Current State of the Science. Immunity. 2020;52(6):910–941. 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jiang M, Chen X, et al. : Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020a;108(1):17–41. 10.1002/JLB.3COVR0520-272R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Wu L, et al. : Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020b;181(4):894–904.e9. 10.1016/j.cell.2020.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Lin CY, Chang MR, et al. : The role of galectins in virus infection - A systemic literature review. J Microbiol Immunol Infect. 2019; S1684-1182(19)30149-5. 10.1016/j.jmii.2019.09.005 [DOI] [PubMed] [Google Scholar]
- Xu J, Xu X, Jiang L, et al. : SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir Res. 2020a;21(1):182. 10.1186/s12931-020-01445-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Li X, Huang Y, et al. : The Predictive Value of Plasma Galectin-3 for Ards Severity and Clinical Outcome. Shock. 2017;47(3):331–336. 10.1097/SHK.0000000000000757 [DOI] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, et al. : Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020b;8(4):420–422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Zhang B, Xu J, et al. : Molecular pathology in the lungs of severe acute respiratory syndrome patients. Am J Pathol. 2007;170(2):538–545. 10.2353/ajpath.2007.060469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip PK, Carrillo-Jimenez A, King P, et al. : Galectin-3 released in response to traumatic brain injury acts as an alarmin orchestrating brain immune response and promoting neurodegeneration. Sci Rep. 2017;7:41689. 10.1038/srep41689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P, Ding Y, Wu X, et al. : The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55(5):105955. 10.1016/j.ijantimicag.2020.105955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu Z, Li JW, et al. : Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Chen X, Hu Q, et al. : Galectin-3 activates TLR4/NF-kappaB signaling to promote lung adenocarcinoma cell proliferation through activating lncRNA-NEAT1 expression. BMC Cancer. 2018;18(1):580. 10.1186/s12885-018-4461-z [DOI] [PMC free article] [PubMed] [Google Scholar]