Key Points
Question
Is early noninvasive cardiac testing (NIT) after an emergency department (ED) evaluation for acute coronary syndrome more effective than not testing to reduce the risk of death or acute myocardial infarction (MI) within 30 days?
Findings
In a cohort study of 79 040 adults presenting to the ED with chest pain who had MI ruled out, early NIT was associated with a small (0.4%) but significant decrease in the absolute composite risk of death or MI. The number needed to treat was 250.
Meaning
Early NIT may reduce the risk of death or MI, but its value is questionable for most patients seen in the ED.
This retrospective cohort study evaluates the effectiveness of early noninvasive cardiac testing in patients presenting to an emergency department with chest pain.
Abstract
Importance
Professional guidelines recommend noninvasive cardiac testing (NIT) within 72 hours of an emergency department (ED) evaluation for suspected acute coronary syndrome. However, there is inexact evidence that this strategy reduces the risk of future death or acute myocardial infarction (MI).
Objective
To evaluate the effectiveness of early NIT in reducing the risk of death or acute MI within 30 days.
Design, Setting, and Participants
This retrospective, multicenter cohort study within the Kaiser Permanente Southern California integrated health care delivery system compared the effectiveness of early noninvasive cardiac testing vs no testing in patients with chest pain and in whom acute MI was ruled out who presented to an ED from January 2015 to December 2017. Patients were followed up for up to 30 days after emergency department discharge.
Exposures
Noninvasive cardiac testing performed within 3 days of an ED evaluation for suspected acute coronary syndrome.
Main Outcomes and Measures
The primary outcome was composite risk of death or acute MI, within 30 days of an ED discharge.
Results
A total of 79 040 patients were evaluated in this study, of whom 57.7% were female. The mean (SD) age of the cohort was 57 (16) years, and 16 164 patients (21%) had completed early NIT. The absolute risk of death or MI within 30 days was low (<1%). Early NIT had the minor benefit of reducing the absolute composite risk of death or MI (0.4% [95% CI, −0.6% to −0.3%]), and, separately, of death (0.2% [95% CI, −0.2% to −0.1%]), MI (−0.3% [95% CI, −0.5% to −0.1%]), and major adverse cardiac event (−0.5% [95% CI, −0.7% to −0.3%]). The number needed to treat was 250 to avoid 1 death or MI, 500 to avoid 1 death, 333 to avoid 1 MI, and 200 to avoid 1 major adverse cardiovascular event within 30 days. Subgroup analysis revealed a number needed to treat of 14 to avoid 1 death or MI in the subset of patients with elevated troponin.
Conclusions and Relevance
Early NIT was associated with a small decrease in the risk of death or MI in patients admitted to the ED with suspected acute coronary syndrome, but this clinical strategy may not be optimal for most patients given the large number needed to treat.
Introduction
Acute coronary syndrome (ACS) is high-risk manifestation of coronary atherosclerosis, which includes ST-segment elevation myocardial infarction, non–ST-segment elevation myocardial infarction, and unstable angina. Acute coronary syndrome is the leading cause of worldwide mortality and morbidity.1,2 The majority of patients with ACS present to emergency departments (EDs) with chest pain, and chest pain is the second most frequent reason for all US ED visits, accounting for more than 7 million annual encounters.3 However, only a minority (1%-13%) of these visits are related to ACS. Accurate diagnosis is challenging and fraught with high medical and legal risks.4,5 The missed ACS rate after an ED evaluation has been reported as high as 2% to 4% and is associated with doubled mortality.6,7,8,9 Additionally, missed ACS is the top reason for medical malpractice claims against ED physicians, which encourages increased testing.10,11
The American Heart Association/American College of Cardiology guidelines recommend noninvasive cardiac stress testing (NIT) before discharge or within 72 hours of discharge, after serial electrocardiogram (ECG) and troponin biomarkers have excluded acute myocardial infarction (MI) in patients with suspected ACS (class IIA recommendation).12,13,14 This approach is recommended for even low-risk patients and is the ED standard of care in the US.12,14 The 2015 European Society of Cardiology guidelines recommend an NIT (preferably with imaging) for inducible ischemia, during admission or shortly after discharge, for patients with no recurrence of chest pain, normal ECG, and normal cardiac troponin levels, but with suspected ACS.15 The National Institute for Health and Care Excellence has questioned European Society of Cardiology guidelines because stress testing has relatively low sensitivity and specificity for diagnosing coronary artery disease (CAD) in patients with suspected troponin-negative ACS.16
Patients with suspected ACS are often hospitalized to facilitate early NIT. Evaluation of suspected ACS is the top reason for US short-stay (<48 hours) inpatient and observation admissions and accounts for more than $3 billion in hospital costs per year.17,18,19,20 However, there is no evidence that early NIT benefits patients.2,21,22,23 Recent data suggest that current use of early NIT increases rates of invasive coronary angiography and revascularization without reducing risk of MI.2,24 However, these studies used administrative data and are limited by lack of mortality data, lack of clinically relevant information such as cardiac biomarkers, and potential for unmeasured confounding.2,24,25
We evaluated the effect of early NIT in a large representative cohort of people presenting to the ED with suspected ACS in one of the largest integrated health care delivery systems in the US. The objective of this study was to evaluate the effectiveness of early NIT in reducing the primary outcome of all-cause death or MI within 30 days of ED encounter.
Methods
Study Design, Population, and Settings
This retrospective cohort study was conducted in the member population of Kaiser Permanente Southern California (KPSC), an integrated health care organization with more than 7500 physicians, 15 medical centers, and 231 medical offices. The KPSC system provides comprehensive health care to more than 4.6 million racially and socioeconomically diverse members residing within 7 counties of Southern California. Health care at KPSC is coordinated through regionwide electronic medical records (EMRs) that capture detailed information on care provided to members at outpatient visits and during inpatient stays, as well as pharmacy, immunization, imaging, and laboratory services received at KPSC-owned and KPSC-contracting facilities. The research database also includes administrative claims for members of the system that capture any out-of-network clinical care and patient outcomes.
Hospitals in the KPSC system provide care to more than 1 million patients in the ED per year (study sites ranging from approximately 25 000 to 95 000 ED visits per year). Of these ED visits, approximately 80% are among health plan members. All sites use the same troponin lab assay (Access AccuTnI+3 [Beckman Coulter]) as well as a uniform threshold of 0.5 ng/mL MI or higher and a 0.04 to 0.5 ng/mL elevated risk cutoff. Physicians in the ED can order NIT as part of the evaluation and discharge plan of patients with suspected ACS.
The study was approved by the KPSC Institutional Review Board. The Institutional Review Board granted a waiver/exemption from the requirement of obtaining informed consent from study participants owing to use of deidentified data.
Inclusion and Exclusion Criteria
This study included all KPSC members 18 years or older who visited an ED for chest pain between January 1, 2015, and December 1, 2017, at 13 EDs operated by KPSC. To ensure complete capture of comorbidity and outcomes, all included patients were required to have continuous health plan enrollment in the 12 months prior to and for at least 30 days postdischarge from their ED visit. Emergency department encounters were included in the study if a valid troponin biomarker assay result was available for that encounter.
Patients were excluded from the study if they (1) had MI identified during the ED encounter, (2) had an initial troponin level greater than 0.5 ng/mL, (3) had coronary revascularization procedure performed before NIT, (4) were transferred from another hospital, (5) died in the ED, (6) were in hospice status, or (7) had a documented do not resuscitate order in the EMR.
Outcomes, Exposure, and Covariates Measurement
Outcomes
The primary outcome was the composite risk of 30-day MI or all-cause death. Death data were obtained from KPSC administrative records, EMRs, and claims for out-of-network deaths. These data were supplemented with California State death files and Social Security Administration records for out-of-state deaths.
The secondary outcome of this study was 30-day incidence of revascularization by percutaneous coronary intervention or coronary artery bypass grafting. The 30-day incidences of MI and death independently were also measured as secondary outcomes.
The 30-day time frame is consistent with ED ACS research guidelines, as longer time frames are unlikely to affect ED decision-making.26 Additionally, we defined major adverse cardiac event (MACE) as the composite outcome of all-cause death, MI, or revascularization within 30 days.
Exposure
The exposure was performance of NIT within 3 days of the ED visit. Noninvasive stress testing included any of the following: stress ECG, stress echocardiogram, stress myocardial perfusion, or coronary computed tomography angiogram that were identified by Current Procedural Terminology codes or EMR order entry.
Covariates
Covariates included patient demographic information and clinical history (Table 1). Age, sex, and race were obtained from the health plan’s administrative records. Clinical data were obtained from the EMR. Comorbidities and cardiac risk factors were defined using laboratory values, diagnostic or procedure codes, and the Elixhauser comorbidity index. The details on the procedure and diagnostic codes have been described elsewhere.5,27,28 Body mass index, calculated as weight in kilograms divided by height in meters squared, was measured from ED intake documentation or the most recently available visit, while smoking and family history of CAD/stroke were self-reported EMR fields. Those with a history of percutaneous coronary intervention or coronary artery bypass grafting were considered to have had prior coronary vascularization. Initial troponin level was dichotomized, with a value below 0.04 ng/mL indicating a normal result and results between 0.04 and 0.5 ng/mL representing an elevated ACS risk. Additionally, using pharmacy prescription records, we identified patients on active antidiabetic, anticoagulant, antihyperlipidemia, and antihypertension treatment in the 90 days prior to their ED encounter.
Table 1. Descriptive Statistics of the Cohort’s Demographics and Clinical Characteristics.
| Characteristic | No. (%) | P value of mean differencesa | ||
|---|---|---|---|---|
| Total cohort (N = 79 040) | No early NIT (n = 62 876) | Early NIT (n = 16 164) | ||
| Outcomes, within 30 d | ||||
| Acute MI or deathb | 344 (0.4) | 296 (0.5) | 48 (0.3) | .003 |
| Death | 143 (0.2) | 134 (0.2) | 9 (0.1) | <.001 |
| MI | 209 (0.3) | 170 (0.3) | 39 (0.2) | .52 |
| Coronary revascularization | 209 (0.3) | 143 (0.2) | 66 (0.4) | <.001 |
| MACE | 355 (0.4) | 306 (0.5) | 49 (0.3) | .002 |
| Demographics and clinical characteristics | ||||
| Age, mean (SD), y | 57.1 (16.3) | 55.7 (16.8) | 62.4 (12.6) | <.001 |
| ≥65 | 27 441 (34.7) | 20 221 (32.2) | 7220 (44.7) | <.001 |
| Female | 45 586 (57.7) | 36 782 (58.5) | 8804 (54.5) | <.001 |
| White | 40 787 (51.6) | 31 822 (50.6) | 8965 (55.5) | <.001 |
| Active/passive smoker | 5663 (7.2) | 4562 (7.3) | 1101 (6.8) | .051 |
| BMI, mean (SD), kg/m2 | 30.0 (6.88) | 30.0 (6.95) | 30.0 (6.60) | .83 |
| Overweight or obese | 60 191 (76.2) | 47 595 (75.7) | 12 596 (77.9) | <.001 |
| Elevated troponin (0.04-0.5) | 2854 (3.6) | 2085 (3.3) | 769 (4.8) | <.001 |
| CAD | 13 987 (17.7) | 10 877 (17.3) | 3110 (19.2) | <.001 |
| Stroke | 2006 (2.5) | 1595 (2.5) | 411 (2.5) | .97 |
| PTCA or CABG in prior year | 1008 (1.3) | 859 (1.4) | 149 (0.9) | <.001 |
| Family history | ||||
| CAD | 26 337 (33.3) | 20 526 (32.6) | 5811 (36) | <.001 |
| Stroke | 14 472 (18.3) | 11 507 (18.3) | 2965 (18.3) | .90 |
| Medications | ||||
| Antidiabetic | 12 493 (15.8) | 9423 (15) | 3070 (19) | <.001 |
| Antihyperlipidemia | 23 947 (30.3) | 17 880 (28.4) | 6067 (37.5) | <.001 |
| Antihypertension | 33 673 (42.6) | 25 580 (40.7) | 8093 (50.1) | <.001 |
| Anticoagulant | 7459 (9.4) | 5902 (9.4) | 1557 (9.6) | .49 |
| Elixhauser comorbidity index, mean (SD) | 3.6 (2.98) | 3.5 (3.04) | 3.7 (2.73) | <.001 |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CABG, coronary artery bypass graft; CAD, coronary artery disease; MACE, major adverse cardiovascular event; MI, myocardial infarction; NIT, noninvasive cardiac testing; PTCA, percutaneous transluminal coronary angioplasty.
χ2 or analysis of variance tests.
A total of 8 patients had MI and died subsequently. They have not been counted twice in the composite outcome.
Statistical Analysis
Evaluation of the association of early NIT with primary and secondary outcomes using an observational study design is challenging due to the nonrandomized assignment (selection bias) to treatment (ie, early NIT) as well as heterogeneity of the effect of NIT on outcomes observed in the diverse sample of patients seen in the ED.29 We used Rubin’s potential outcomes framework to evaluate the treatment effect of early NIT with primary and secondary outcomes.30 The treatment effect was estimated relaxing the restrictive assumption of unconfoundedness, by using generalized method of moments-based residual inclusion instrumental variables (IV) techniques.31,32 Models were adjusted for sociodemographic and clinical covariates. To intuitively understand IV analysis, we can consider the variation in the receipt of treatment (ie, early NIT) to have 2 parts: the part that is not confounded and the part that is correlated with the error (bad variation or confounding by indication).33,34 Instrumental variables analysis isolates and retains only the unconfounded variation in the treatment and disregards the bad variation. Instrumental variables models generate this quasiexperimental variation through excluded (from the outcome model) instruments that predict receipt of the treatment but are not related to prognosis.33,34 We used (1) each KPSC medical center’s practice pattern for NIT within 72 hours and (2) day of the week of the ED encounter as 2 excluded instruments to isolate the good variation.27 We postulated that weekend-related scheduling delays make it less likely that stress testing can be completed within 72 hours if the order was placed on a weekend.23 Each medical center’s practice pattern was calculated as the percentage of patients who have suspected ACS receiving NIT in the 1 year prior to the ED date of each included cohort case with suspected ACS. The medical center’s practice pattern synthesizes consensus, experience, and training of the ED professional staff, the medical center’s protocol/policies, and the infrastructure available to support early NIT. The calculation of the medical center’s practice pattern based on presenting patient’s ED encounter date made it dynamic and allowed this study to capture changes over time at the same medical center based on changes to any system or human capital factors.
We provide estimates of the first stage IV treatment selection model (eTable 1 in the Supplement) as well as statistical tests to evaluate the validity of the IV modeling assumptions (eTable 2 in the Supplement). We report the number needed to treat (NNT) as the inverse of the adjusted absolute risk reduction (Table 2).
Table 2. Absolute Risk, Risk Reduction, and Number Needed to Treat (NNT).
| Outcome | Adjusted risk, mean (SE) | Risk reduction, early NIT adjusted risk − control adjusted risk, mean (95% CI)a | P value | NNT absolute risk reduction | |
|---|---|---|---|---|---|
| No early NIT (n = 62 876) | Early NIT (n = 16 164) | ||||
| Death or MI | 0.005 (0.0008) | 0.0008 (0.0004) | −0.004 (−0.006 to −0.003)b | <.001 | 1/0.004 = 250b |
| Death | 0.0019 (0.0003) | 0.00013 (0.00005) | −0.002 (−0.002 to −0.001)b | <.001 | 1/0.002 = 500b |
| Acute MI | 0.003 (0.0009) | 0.0007 (0.0003) | −0.003 (−0.005 to −0.001)b | .004 | 1/0.003 = 333b |
| Coronary revascularization | 0.004 (0.002) | 0.002 (0.002) | −0.002 (−0.006 to 0.003) | .45 | NAc |
| MACE | 0.006 (0.001) | 0.0008 (0.0003) | −0.005 (−0.007 to −0.003)b | <.001 | 1/0.005 = 200b |
Abbreviations: MACE, major adverse cardiovascular events; MI, myocardial infarction; NA, not applicable; NIT, noninvasive cardiac testing.
All models adjusted for age, sex, race, smoking, body mass index (calculated as weight in kilograms divided by height in meters squared), self and family history of coronary artery disease, initial troponin, antidiabetic medication, anticoagulant medication, antihyperlipidemia medication, antihypertension medication, and Elixhauser comorbidities.
Statistically significant difference.
Differences in event rates are not statistically significant at α = 0.05, and the 95% CI contains zero.
In sensitivity analysis, we analyzed the data using doubly robust inverse probability of treatment weighted and regression adjusted models assuming the unconfoundedness requirement was not violated (Table 3). Additionally, we report the association of treatment of early NIT in subgroups of patients with high cardiac risk (Table 4). All hypothesis tests were 2 sided with significance set at α=0.05. Stata, version 15 (Stata Corp LLC), was used for data analysis.
Table 3. Sensitivity Analysis Inverse Probability Weighted Models.
| Outcome | Adjusted risk, mean (SE) | Risk reduction, early NIT adjusted risk − control adjusted risk, mean (95% CI) | P value | NNT absolute risk reduction | |
|---|---|---|---|---|---|
| No early NIT (control) (n = 62 876) | Early NIT (treated) (n = 16 164) | ||||
| Death or MIa | 0.005 (0.0003) | 0.003 (0.0005) | −0.002 (−0.003 to −0.001)b | .001 | 1/0.002 = 500b |
| Deathc | 0.005 (0.0003) | 0.003 (0.0004) | −0.002 (−0.003 to −0.001)b | <.001 | 1/0.002 = 500b |
| Acute MIa | 0.003 (0.0002) | 0.002 (0.0004) | −0.001 (−0.002 to 0.0003) | .22 | NAd |
| Coronary revascularizationa | 0.003 (0.0002) | 0.003 (0.0004) | 0.001 (−0.0002 to 0.002) | .13 | NAd |
| MACEa | 0.005 (0.0003) | 0.003 (0.0005) | −0.002 (−0.003 to −0.001)b | .001 | 1/0.002 = 500b |
Abbreviations: CAD, coronary artery disease; MACE, major adverse cardiovascular events; MI, myocardial infarction; NIT, noninvasive cardiac testing; NNT, number needed to treat.
Doubly robust inverse probability weighting model models with regression adjustment for age, sex, race, smoking, body mass index (calculated as weight in kilograms divided by height in meters squared), self and family history of CAD, initial troponin, antidiabetic medication, anticoagulant medication, antihyperlipidemia medication, antihypertension medication, and Elixhauser comorbidities.
Statistically significant differences.
Estimate based on inverse probability weighting model without regression adjustment since one or more covariates perfectly predicted death.
Difference in event rates are not statistically significant at α = 0.05 and the 95% CI contains zero.
Table 4. Effect of Early Noninvasive Cardiac Testing (NIT) on Death or Acute Myocardial Infarction (MI) in High Cardiac Risk Subgroup.
| Subgroups | No. | Adjusted risk, mean (SE) | Risk reduction, early NIT adjusted risk − control adjusted risk, mean (95% CI)a | P value | NNT absolute risk reduction | |
|---|---|---|---|---|---|---|
| No early NIT (control) | Early NIT (treatment) | |||||
| Age >65 y | 27 169 | 0.01 (0.002) | 0.005 (0.006) | −0.005 (−0.016 to 0.007) | .43 | NAc |
| Sex | ||||||
| Female | 44 612 | 0.004 (0.001) | 0.001 (0.001) | −0.004 (−0.007 to −0.0006)b | .018 | 1/0.004 = 250b |
| Male | 31 605 | 0.007 (0.001) | 0.001 (0.001) | −0.005 (−0.007 to −0.003)b | <.001 | 1/0.005 = 200b |
| Quit smoking | 22 711 | 0.01 (0.004) | 0.002 (0.002) | −0.008 (−0.016 to −0.0004)b | .04 | 1/0.008 = 125b |
| Active/passive smoker | 5596 | 0.005 (0.001) | 0.001 (0.0004) | −0.004 (−0.006 to −0.002)b | .001 | 1/0.004 = 250b |
| Obese | 32 728 | 0.004 (0.001) | 0.0005 (0.0001) | −0.003 (−0.006 to −0.0013)b | .002 | 1/0.003 = 333b |
| CAD | 13 883 | 0.015 (0.002) | 0.007 (0.009) | −0.008 (−0.03 to 0.01) | .42 | NAc |
| Elevated troponin | 2828 | 0.07 (0.03) | 0.006 (0.002) | −0.07 (−0.12 to −0.013)b | .015 | 1/0.07 = 14b |
| Family history of CAD | 25 695 | 0.007 (0.002) | 0.001 (0.0002) | −0.006 (−0.01 to −0.0004)b | .033 | 1/0.006 = 167b |
| Medication | ||||||
| Antidiabetic | 12 413 | 0.009 (0.002) | 0.0012 (0.0009) | −0.008 (−0.011 to −0.004)b | <.001 | 1/0.008 = 125b |
| Antihypertension | 33 367 | 0.009 (0.002) | 0.001 (0.0004) | −0.008 (−0.012 to −0.004)b | <.001 | 1/0.008 = 125b |
| Antihyperlipidemia | 23 758 | 0.009 (0.002) | 0.002 (0.002) | −0.007 (−0.013 to −0.001)b | .027 | 1/0.007 = 143b |
| Anticoagulation | 7431 | 0.01 (0.002) | 0.003 (0.002) | −0.009 (−0.015 to −0.002)b | .007 | 1/0.009 = 111b |
Abbreviations: CAD, coronary artery disease; MACE, major adverse cardiovascular events; NNT, number needed to treat.
Except for each subgroup stratification variable, models adjusted for age, sex, race, smoking, BMI, self and family history of CAD, initial troponin, antidiabetic medication, anticoagulant medication, antihyperlipidemia medication, antihypertension medication, and Elixhauser comorbidities.
Statistically significant differences.
Difference in event rates are not statistically significant at α = 0.05 and the 95% CI contains zero.
Results
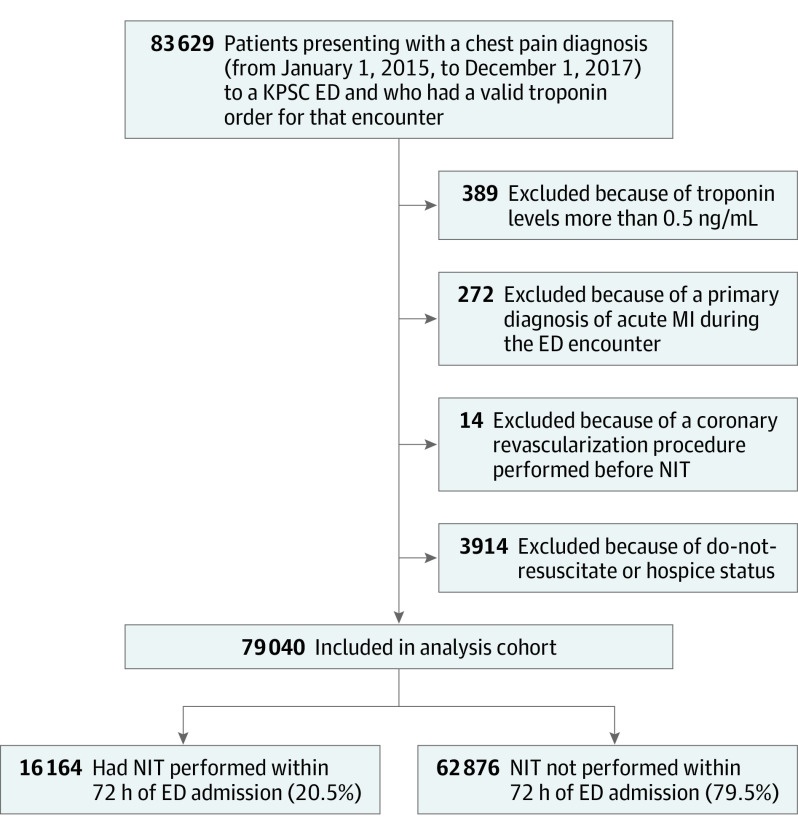
The total cohort included 79 040 adults (Figure), of whom 16 164 (20.5%) completed a NIT within 72 hours of admission (Table 1). Among the 16 164 tested, 2796 (17.3%) completed the test as an outpatient while 13 368 (82.7%) completed it either prior to or on the day of discharge. The distribution of the type of NIT included 47.5% stress ECG, 17.2% stress echocardiogram, 35.2% stress myocardial perfusion, and 0.1% coronary computed tomography angiogram. The mean (SD) age of the cohort was 57 (16) years and the majority were female (57.7%) and White (51.6%). The combined risk of death or MI was 0.5% in the control cohort, while in the NIT cohort it was 0.3% (Table 1). The independent risk of death was 0.2% in the control cohort vs 0.1% in the early NIT cohort; of MI was 0.3% in the control cohort vs 0.2% in the early NIT cohort; of coronary revascularization was 0.2% in the control cohort vs 0.4% in the early NIT cohort; and of MACE was 0.5% in the control cohort vs 0.3% in the early NIT cohort (Table 1).
Figure. Flowchart of Patients Presenting With Chest Pain to an Emergency Department (ED) and Patients Included in the Analysis.
KPSC indicates Kaiser Permanente Southern California; MI, myocardial infarction; NIT, noninvasive cardiac testing.
Specification testing of the IV models suggested that day of the week and medical center’s NIT practice pattern were strong instruments. Independently, a 1% increase in a medical center’s past practice pattern for NIT was associated with an odds ratio of 6.4% (95% CI, 6.0%-6.9%) higher of ordering early NIT. Similarly, as compared with an ED encounter during any weekday (Monday through Friday), the odds ratio of ordering early NIT was lower by 18% (95% CI, 14%-21%) during a weekend (eTable 1 in the Supplement). All assumptions necessary for consistent parameter estimates from IV analysis were satisfied (eTable 2 in the Supplement).
The average adjusted risk reduction for death or MI was 0.4% (95% CI, 0.3%-0.6%) while that for death was 0.18% (95% CI, 0.1%-0.2%) (Table 2). Similarly, the adjusted risk reduction for secondary aims of MI was 0.3% (95% CI, 0.1%-0.5%) and MACE was 0.5% (95% CI, 0.3%-0.7%) (Table 4). The difference in coronary revascularization rate was not statistically significant. The NNT was 250 for the death or MI composite outcome, while for death the NNT was 500, for MI the NNT was 333, and for MACE the NNT was 200 (Table 2). Sensitivity analysis using inverse probability weighted models showed similar results with slightly smaller treatment effect (hence higher NNT), but the MI outcome was not found to be significantly different (Table 3).
In the traditional subgroups associated with high cardiac risk, the absolute risk of death and MI composite outcome ranged between 0.3% (BMI ≥30) and a maximum of 7% (elevated troponin) in the controls (Table 4). Early NIT reduced the absolute risk of death or MI by 0.3% to 7%. Consequently, the NNT ranged between a low of 14 and a high of 333.
Discussion
This study evaluated the association of early NIT, in a large cohort of patients admitted to the ED presenting with suspected ACS, with the risk of death, MI, coronary revascularization, and MACE within 30 days of discharge. To our knowledge, few prior studies have evaluated the association of NIT with cardiovascular outcomes and only 1 study has evaluated the association of NIT, performed within 30 days, with cardiovascular death.2,23,24,25,35 This study focuses on evaluating outcomes in the 30-day follow-up period as it allows disentangling the immediate association of early NIT with outcomes as opposed to that observed from the cascade of events leading to improved downstream processes of care that ultimately may have led to the lower outcomes that have been reported in prior studies. The 30-day follow-up is also more closely related to ED decision-making and any benefit of early NIT should be identified within this timeframe. By combining the comprehensive EMRs with California State–level death data and national-level death data obtained from the Social Security Administration, we believe that this is one of the first studies to report on the association of early (within 72 hours) NIT and the risk of death at the population level.
In this cohort, the absolute risks of death or MI, death, MI, revascularization, and MACE within 30 days of ED discharge were low (<1%) and early NIT had a minor benefit in reducing these risks. We find that to benefit from American Heart Association/American College of Cardiology NIT guidelines, the NNT to avoid 1 death or MI was 250, while 500 patients with suspected ACS need to be tested to avoid 1 death. Although we do not find a benefit of NIT in reducing coronary revascularization, it is interesting to note that revascularization procedures were not increased with early NIT. The lack of increased revascularization rates among patients who receive NIT suggests that other factors are likely driving the reduced event rate. For instance, better medical optimization may play a role, as we noted that the early NIT arm had higher rates of use of antihyperlipidemics (16.1% vs 9.7%; P < .001); antihypertensives (13.8% vs 10.2%; P < .001); anticoagulants (4.7% vs 3.6%; P < .001); and antidiabetic medication (4.4% vs 3.3%; P < .001) compared with the no early NIT arm in the 90-day postdischarge period. Thus, NIT may identify patients who could benefit from additional contact with outpatient health care professionals where lifestyle interventions and medication adherence may be emphasized. Hence, if used appropriately, NIT could serve a role in downstream risk stratification to identify CAD and may improve outcomes beyond 30 days.
The absolute risk of death or MI was highest in patients with elevated troponin who also experienced the most risk reduction (7%) related to early NIT. With an NNT of 14 observed in traditional troponin assays, there appears promise in adoption of high-sensitivity troponin assays for future ACS evaluation. High-sensitivity cardiac troponin assays increase diagnostic accuracy for MI at the time of presentation and allow for a more rapid assessment of the probability of MI.15 In most other subgroups with high cardiac risk, the NNT was above 100. The findings of this study suggest a need for implementation of risk stratification models in the ED to better identify those more likely to benefit from NIT and avoid unhelpful tests. For example, in addition to biomarkers and ECG, a low HEART (History, Electrocardiogram, Age, Risk Factors, Troponin) score has been associated with low 30-day MACE outcome.36,37 Along with increased adoption of HEART score for ED evaluation of ACS, refinement of existing HEART score with high-sensitivity cardiac troponin assays could significantly reduce unhelpful NIT.
The results of this study regarding MI and coronary revascularization are similar to those of other published reports.2,23,25,35 Using IV analysis in a retrospective cohort of privately insured patients, Sandhu et al23 reported that cardiac testing was associated with increased revascularization without a significant change in MI. Foy et al2 reported that patients admitted to the ED with chest pain who do not have an MI are at very low risk of experiencing an MI during short-term and longer-term follow-up and this low risk does not appear to be affected by the initial testing strategy. These 2 studies do not include patients older than 65 years, do not include Medicare/Medicaid enrollees, and have not adjusted for race-related or ethnicity-related differences. Roifman et al25 have reported on the effect of NIT performed within 30 days of chest pain visit on composite MI or death in 90-day and 1-year follow-up in a population in Ontario, Canada. Their propensity score–matched analysis estimated an NNT of 974 to prevent 1 event of MI or cardiovascular death in a 1-year follow-up. In the short-term 90-day follow-up, the NIT arm had marginally higher composite outcome, which could be due to unmeasured confounding that was not addressed in their analysis.38 The majority of these prior studies have lacked information on clinically important variables such as initial troponin value and hence may not have identified type 2 MI, which is based on the level of troponin. Reinhardt et al35 performed a secondary analysis of the ROMICAT-II trial and reported that stress testing leads to longer length of stay, more downstream testing, more radiation exposure, and greater cost, without an improvement in clinical outcomes.
Cumulatively, these consistent results observed across geographically diverse populations call into question the current American Heart Association/American College of Cardiology recommendations of early NIT in patients in the ED with suspected ACS.39 The PROMISE40 and SCOT-HEART41 trials, as well as several population-based studies,25 including this study, have found low rates of MI and death. It is difficult to further reduce what are already low rates. Hence, future guideline revisions on NIT could recommend increased role for risk stratification to identify patients at high risk and soften NIT recommendation for those at low risk. Additionally, once ACS is ruled out for patients at low risk, they could be treated according to guidance for the management of suspected CAD, which is aimed at primary care physicians and/or cardiologists.
We also foresee benefits of developing new risk stratification models using high-sensitivity troponin assays or modifying existing models to incorporate high-sensitivity troponin assays instead of traditional troponin assays. Additionally, increased adoption and documentation of shared decision–based treatment where patients understand their options and the tradeoffs involved with NIT may reduce overuse of NIT and allow patients to protect themselves financially from the inevitable gaming involved in the complex US health care reimbursement system.42
Limitations
There are several potential limitations to our study. This study provides data on the short-term safety of early NIT in a low-risk population, which is typical of most suspected ACS ED encounters. These findings may not apply beyond the 30-day post-ED discharge period. However, other studies have failed to show significant benefit of NIT for longer-term outcomes.2,23 Additionally, results do not apply to patients with MI who present without chest pain, which can be seen in older patients, women, patients with diabetes, and those with heart failure. Also, the patient population is geographically limited to Southern California and belongs to a single integrated health care system, which may limit practice pattern variation observed across the US and in fee-for-service systems. The lack of high-sensitivity cardiac troponin assay is a limitation that influences the generalizability of the results of this study. High-sensitivity cardiac troponin assay is theoretically better for risk stratification of patients on presentation and hence adoption of high-sensitivity assays will likely further drive down rates of NIT from the ED. We also do not have patient-level social risk data, which may contribute to the receipt of early NIT because those who lack transportation, do not speak English well, or have lower education levels may not be able to navigate the health system as well.
Conclusions
In patients with suspected ACS for whom MI has been ruled out, early NIT results in minor reductions (0.4%) in death or MI outcome, but the large NNT required to benefit 1 patient calls into question this clinical strategy for most patients. The findings of this study support selective use of NIT by avoiding such testing for most patients evaluated in the ED and reserving NIT for patients at substantial risk of 30-day adverse cardiovascular outcomes.
eTable 1. Logistic Regression of First Stage IV Model Predicting Early NIT
eTable 2. Overall Diagnostic Test for the IV Model Assumptions
References
- 1.Vedanthan R, Seligman B, Fuster V. Global perspective on acute coronary syndrome: a burden on the young and poor. Circ Res. 2014;114(12):1959-1975. doi: 10.1161/CIRCRESAHA.114.302782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foy AJ, Liu G, Davidson WR Jr, Sciamanna C, Leslie DL. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern Med. 2015;175(3):428-436. doi: 10.1001/jamainternmed.2014.7657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Centers for Disease Control . National Hospital Ambulatory Medical Care Survey: 2010 emergency department summary tables. 2010. Accessed September 8, 2020. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf
- 4.US Centers for Disease Control/National Center for Health Statistics. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. 2010. Accessed September 16, 2014. https://www.cdc.gov/nchs/data/databriefs/db43.pdf
- 5.Sharp AL, Baecker AS, Shen E, et al. Effect of a HEART care pathway on chest pain management within an integrated health system. Ann Emerg Med. 2019;74(2):171-180. doi: 10.1016/j.annemergmed.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee TH, Rouan GW, Weisberg MC, et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60(4):219-224. doi: 10.1016/0002-9149(87)90217-7 [DOI] [PubMed] [Google Scholar]
- 7.McCarthy BD, Beshansky JR, D’Agostino RB, Selker HP. Missed diagnoses of acute myocardial infarction in the emergency department: results from a multicenter study. Ann Emerg Med. 1993;22(3):579-582. doi: 10.1016/S0196-0644(05)81945-6 [DOI] [PubMed] [Google Scholar]
- 8.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163-1170. doi: 10.1056/NEJM200004203421603 [DOI] [PubMed] [Google Scholar]
- 9.Schull MJ, Vermeulen MJ, Stukel TA. The risk of missed diagnosis of acute myocardial infarction associated with emergency department volume. Ann Emerg Med. 2006;48(6):647-655. doi: 10.1016/j.annemergmed.2006.03.025 [DOI] [PubMed] [Google Scholar]
- 10.Brooker JA, Hastings JW, Major-Monfried H, et al. The association between medicolegal and professional concerns and chest pain admission rates. Acad Emerg Med. 2015;22(7):883-886. doi: 10.1111/acem.12708 [DOI] [PubMed] [Google Scholar]
- 11.Brown TW, McCarthy ML, Kelen GD, Levy F. An epidemiologic study of closed emergency department malpractice claims in a national database of physician malpractice insurers. Acad Emerg Med. 2010;17(5):553-560. doi: 10.1111/j.1553-2712.2010.00729.x [DOI] [PubMed] [Google Scholar]
- 12.Amsterdam EA, Kirk JD, Bluemke DA, et al. ; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research . Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122(17):1756-1776. doi: 10.1161/CIR.0b013e3181ec61df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amsterdam EA, Wenger NK, Brindis RG, et al. ; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):e344-e426. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(23):e179-e347. doi: 10.1016/j.jacc.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 15.Roffi M, Patrono C, Collet JP, et al. ; ESC Scientific Document Group . 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267-315. doi: 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence . Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. March 24, 2010. Updated November 30, 2016. Accessed September 8, 2020. https://www.nice.org.uk/guidance/CG95 [PubMed]
- 17.Venkatesh AK, Geisler BP, Gibson Chambers JJ, Baugh CW, Bohan JS, Schuur JD. Use of observation care in US emergency departments, 2001 to 2008. PLoS One. 2011;6(9):e24326. doi: 10.1371/journal.pone.0024326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services. Office of Inspector General. Memorandum report: hospitals' use of observation stays and short inpatient stays for Medicare beneficiaries, OEI-02-12-00040. July 29, 2013. Accessed Sep 16, 2014. https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf
- 19.Sabbatini AK, Nallamothu BK, Kocher KE. Reducing variation in hospital admissions from the emergency department for low-mortality conditions may produce savings. Health Aff (Millwood). 2014;33(9):1655-1663. doi: 10.1377/hlthaff.2013.1318 [DOI] [PubMed] [Google Scholar]
- 20.Goldstein JA, Chinnaiyan KM, Abidov A, et al. ; CT-STAT Investigators . The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-1422. doi: 10.1016/j.jacc.2011.03.068 [DOI] [PubMed] [Google Scholar]
- 21.Prasad V, Cheung M, Cifu A. Chest pain in the emergency department: the case against our current practice of routine noninvasive testing. Arch Intern Med. 2012;172(19):1506-1509. doi: 10.1001/archinternmed.2012.4037 [DOI] [PubMed] [Google Scholar]
- 22.Redberg RF. Stress testing in the emergency department: not which test but whether any test should be done. JAMA Intern Med. 2015;175(3):436. doi: 10.1001/jamainternmed.2014.7698 [DOI] [PubMed] [Google Scholar]
- 23.Sandhu AT, Heidenreich PA, Bhattacharya J, Bundorf MK. Cardiovascular testing and clinical outcomes in emergency department patients with chest pain. JAMA Intern Med. 2017;177(8):1175-1182. doi: 10.1001/jamainternmed.2017.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safavi KC, Li SX, Dharmarajan K, et al. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174(4):546-553. doi: 10.1001/jamainternmed.2013.14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roifman I, Han L, Koh M, et al. Clinical effectiveness of cardiac noninvasive diagnostic testing in patients discharged from the emergency department for chest pain. J Am Heart Assoc. 2019;8(21):e013824. doi: 10.1161/JAHA.119.013824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollander JE, Blomkalns AL, Brogan GX, et al. ; Multidisciplinary Standardized Reporting Criteria Task Force; Standardized Reporting Criteria Working Group of Emergency Medicine Cardiac Research and Education Group-International . Standardized reporting guidelines for studies evaluating risk stratification of emergency department patients with potential acute coronary syndromes. Ann Emerg Med. 2004;44(6):589-598. doi: 10.1016/j.annemergmed.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 27.Natsui S, Sun BC, Shen E, et al. Evaluation of outpatient cardiac stress testing after emergency department encounters for suspected acute coronary syndrome. Ann Emerg Med. 2019;74(2):216-223. doi: 10.1016/j.annemergmed.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharp AL, Wu YL, Shen E, et al. The HEART score for suspected acute coronary syndrome in U.S. emergency departments. J Am Coll Cardiol. 2018;72(15):1875-1877. doi: 10.1016/j.jacc.2018.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heckman J, Robb R. Alternative methods for evaluating the impact of interventions: an overview. J Econom. 1985;30(1-2):239-267. doi: 10.1016/0304-4076(85)90139-3 [DOI] [Google Scholar]
- 30.Holland P. Statistics and causal inference. J Am Stat Assoc. 1986;81(396): 945-960. doi: 10.1080/01621459.1986.10478354 [DOI] [Google Scholar]
- 31.Heckman J, Navarro-Lozano S. Using matching, instrumental variables, and control functions to estimate economic choice models. Rev Econ Stat. 2004;86(1):30–57. doi: 10.1162/003465304323023660 [DOI] [Google Scholar]
- 32.Angrist J, Imbens G, Rubin D.. Identification of causal effects using instrumental variables. JAm Stat Assoc. 1996;91(434):444-455. doi: 10.1080/01621459.1996.10476902 [DOI] [Google Scholar]
- 33.Angrist JD, Pischke S Jr. Mostly Harmless Econometrics: An Empiricist's Companion. Princeton University Press; 2009. doi: 10.1515/9781400829828 [DOI] [Google Scholar]
- 34.Wooldridge JM. Econometric Analysis of Cross Section And Panel Data. 2nd ed. MIT Press; 2010. [Google Scholar]
- 35.Reinhardt SW, Lin CJ, Novak E, Brown DL. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178(2):212-219. doi: 10.1001/jamainternmed.2017.7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255 [DOI] [PubMed] [Google Scholar]
- 37.Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9(3):164-169. doi: 10.1097/HPC.0b013e3181ec36d8 [DOI] [PubMed] [Google Scholar]
- 38.Sun BC, Redberg RF. Cardiac testing after emergency department evaluation for chest pain: time for a paradigm shift? JAMA Intern Med. 2017;177(8):1183-1184. doi: 10.1001/jamainternmed.2017.2439 [DOI] [PubMed] [Google Scholar]
- 39.Booth J, Thomas JJ. Provocative testing for low-risk chest pain patients, must we continue? J Nucl Cardiol. 2019;26(5):1647-1649. doi: 10.1007/s12350-018-1202-2 [DOI] [PubMed] [Google Scholar]
- 40.Douglas PS, Hoffmann U, Patel MR, et al. ; PROMISE Investigators . Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-1300. doi: 10.1056/NEJMoa1415516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newby DE, Adamson PD, Berry C, et al. ; SCOT-HEART Investigators . Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi: 10.1056/NEJMoa1805971 [DOI] [PubMed] [Google Scholar]
- 42.Figueroa JF, Joynt Maddox KE. The case of noninvasive cardiac testing—for every action there is a reaction. JAMA Intern Med. 2019;179(12):1706-1707. doi: 10.1001/jamainternmed.2019.4265 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Logistic Regression of First Stage IV Model Predicting Early NIT
eTable 2. Overall Diagnostic Test for the IV Model Assumptions