Key Points
Question
What are the current trends in central catheter–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) among critically ill neonates and children?
Findings
This cross-sectional study using 2013-2018 Centers for Disease Control and Prevention surveillance data from 176 hospitals suggests that prior improvements in CLABSI rates have plateaued. However, there has been a statistically significant incremental decrease in population-based CAUTI rates and use of indwelling urinary catheters over time.
Meaning
The findings of this study suggest that, while modest improvements in population-based CAUTI rates may reflect more judicious use of urinary catheters, novel approaches to health care–associated infection surveillance and prevention are likely needed to encourage further improvements.
Abstract
Importance
Central catheter–associated bloodstream infections (CLABSIs) and catheter-associated urinary tract infections (CAUTIs) increase morbidity, mortality, and health care costs in pediatric patients.
Objective
To examine changes over time in CLABSI and CAUTI rates between 2013 and 2018 in neonatal intensive care units (NICUs) and pediatric intensive care units (PICUs) using prospective surveillance data from community hospitals, children’s hospitals, and pediatric units within general hospitals.
Design, Setting, and Participants
This time series study included 176 US hospitals reporting pediatric health care–associated infection surveillance data to the National Healthcare Safety Network from January 1, 2013, to June 30, 2018. Patients aged 18 years or younger admitted to PICUs or level III NICUs were included in the analysis.
Main Outcomes and Measures
The primary outcomes were device-associated rates of CLABSI in NICUs and PICUs and CAUTI in PICUs (infections per 1000 device-days). Secondary outcomes included population-based rates (infections per 10 000 patient-days) and device utilization (device-days per patient-days). Regression models were fit using generalized estimating equations to assess yearly changes in CLABSI and CAUTI rates, adjusted for birth weight (≤1500 vs >1500 g) in neonatal models.
Results
Of the 176 hospitals, 132 hospitals with NICUs and 114 hospitals with PICUs contributed data. Of these, NICUs reported 6 064 172 patient-days and 1 363 700 central line-days and PICUs reported 1 999 979 patient-days, 925 956 central catheter–days, and 327 599 indwelling urinary catheter–days. In NICUs, there were no significant changes in yearly trends in device-associated (incidence rate ratio [IRR] per year, 0.99; 95% CI, 0.95-1.03) and population-based (IRR, 0.96; 95% CI, 0.92-1.00) CLABSI rates or central catheter utilization (odds ratio [OR], 0.97; 95% CI, 0.95-1.00). Results were similar in PICUs, with device-associated (IRR, 1.03; 95% CI, 0.99-1.07) and population-based (IRR, 1.03; 95% CI, 0.99-1.07) CLABSI rates and central catheter utilization (OR, 0.99; 95% CI, 0.97-1.01) remaining stable. While device-associated CAUTI rates in PICUs also remained unchanged over time (IRR, 0.97; 95% CI, 0.91-1.03), population-based CAUTI rates significantly decreased by 8% per year (IRR, 0.92; 95% CI, 0.86-0.98) and indwelling urinary catheter utilization significantly decreased by 6% per year (OR, 0.94; 95% CI, 0.91-0.96).
Conclusions and Relevance
Recent trends in CLABSI rates noted in this study among critically ill neonates and children in a large cohort of US hospitals indicate that past gains have held, without evidence of further improvements, suggesting novel approaches for CLABSI prevention are needed. Modest improvements in population-based CAUTI rates likely reflect more judicious use of urinary catheters.
This cross-sectional study examines changes in the rates of central catheter–associated bloodstream infections and catheter-associated urinary tract infections over time in critically ill pediatric patients.
Introduction
Health care–associated infections are a major and often preventable risk to the safety of hospitalized children, resulting in increased morbidity and mortality, prolonged hospitalization, and higher health care costs.1,2,3,4 Central catheter–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are among the most common health care–associated infections in hospitalized patients, including children.5,6 Critically ill newborns and children cared for in the neonatal intensive care unit (NICU) and pediatric intensive care unit (PICU) are at particularly high risk for these infections.7,8,9,10 Many single and multicenter collaborative efforts focused on neonatal and pediatric patients have reported success with CLABSI and CAUTI reduction.1,11,12,13,14,15,16,17,18,19 National trends in CLABSI rates from 2007 to 2012 reported to the Centers for Disease Control and Prevention’s National Healthcare Safety Network (NHSN) suggest that these improvement efforts have benefited neonatal and pediatric patients; however, similar decreases were not noted for device-associated CAUTI rates during the same period.9 We sought to reexamine recent national trends in CLABSI and CAUTI incidence rates in NICUs and PICUs using prospectively collected surveillance data from community hospitals, pediatric units affiliated with general hospitals, and freestanding pediatric hospitals in the US between January 1, 2013, and June 30, 2018. We hypothesized that CLABSI rates would continue to decrease in NICUs and PICUs and CAUTI rates would remain stable.
Methods
Study Design
We used a time-series design to examine trends in surveillance-based rates of health care–associated infections in critically ill children from January 1, 2013, through June 30, 2018. We included data from acute care hospitals reporting prospective surveillance data from level III NICUs and PICUs to the Center for Disease Control and Prevention’s NHSN. Study hospitals were a subset of those enrolled in the Preventing Avoidable Infectious Complications by Adjusting Payment (PAICAP) study.20 Hospitals were included if they reported CLABSIs from the NICU or CLABSIs or CAUTIs from the PICU.
We obtained data on hospital characteristics from the 2015 American Hospital Association (AHA) annual survey,21 including hospital size, teaching status, type of ownership, location, whether the hospital restricts admissions primarily to children, the number of NICU beds, the number of PICU beds, and the number of full-time equivalent NICU and PICU intensivists. To understand whether NHSN hospitals included in the study are representative of the general population of hospitals caring for critically ill neonates and children, we compared the characteristics of NHSN study hospitals with those of AHA survey hospitals in the US that indicated that the hospital offers neonatal or pediatric intensive care services. The Harvard Pilgrim Health Care Institute Institutional Review Board approved this study with a waiver of informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Outcomes
The primary outcomes examined were device-associated rates of CLABSI in NICUs and PICUs (infections per 1000 central catheter–days) and device-associated rates of CAUTI in PICUs (infections per 1000 indwelling urinary catheter–days). Secondary outcomes examined included population-based health care–associated infection rates (CLABSI or CAUTI per 10 000 patient-days) and device utilization ratios (central catheter–days or indwelling urinary catheter–days per patient-days). We chose device-associated health care–associated infection rates as the primary outcome because these measures are traditionally prioritized for reporting to the NHSN and Solutions for Patient Safety, the national quality improvement network of children’s hospitals that tracks hospital-associated conditions among hospitalized children.19 We did not include any CAUTI measures from NICUs in this study given the infrequent use of indwelling urinary catheters in neonates and the consequent paucity of NICUs reporting CAUTI rates to the NHSN.
We defined all health care–associated infections using NHSN standardized surveillance case definitions. To ensure consistent case definitions, rate analyses included only CAUTI cases associated with urine cultures growing more than 100 000 colony-forming units of bacteria in accordance with a 2015 NHSN CAUTI surveillance case definition revision that excluded urine cultures with lower growth bacteria or nonbacterial organisms.22 While the NHSN did not make any changes directly to the CLABSI case definition during the study period, there were NHSN protocol revisions in 2015 that led to reclassification of certain bloodstream infections as CLABSI rather than bacteremia secondary to another infectious source for patients with a central catheter in situ.23,24 The NHSN revisions leading to this reclassification included addition of more-stringent criteria for when bloodstream infections may be attributed as secondary to other localized site-specific infections (eg, surgical site infections), alterations in which organisms are recognized as causative for particular site-specific infections, and new guidance in how infection preventionists are directed to combine multiple infections in an individual patient as a single reportable event. In combination, these changes led to reported increases in CLABSIs in quarter 1 of 2015.23,24,25 Because we were unable to account directly for the 2015 NHSN revisions in our primary CLABSI analyses, we completed additional sensitivity analyses that included only CLABSIs reported in 2016 onward to mitigate ascertainment bias related to reclassification of other infections as CLABSI in 2015.
We additionally identified the top 5 most frequent causative pathogens for CLABSI in each setting and for CAUTI in the PICU by year for 2015-2018. We included only the primary pathogen reported to the NHSN, although the NHSN surveillance allows hospitals to report up to 3 pathogens growing in a blood culture per CLABSI event and up to 2 pathogens growing in a urine culture per CAUTI event.
Statistical Analysis
In the primary analysis, we fit negative binomial models for quarterly CLABSI and CAUTI counts with device-days in the offset term and logistic regression models for device utilization to describe changes in the trends of these measures over time. For secondary analyses, we included patient-days in the offset term of negative binomial models. We used generalized estimating equations with robust sandwich variance estimators and an independent correlation structure to account for possible clustering at the hospital level.26 We aggregated all health care–associated infection counts and proportions to the quarterly level to increase the stability of estimates in the models and rescaled results to report incident rate ratios (IRRs) or odds ratios (ORs) per year to allow for more clinically meaningful interpretation. All models included time to model secular trends. Birth weight (≤1500 vs >1500 g) was included in neonatal models, as well as an interaction term (birth weight × time) to explore whether there were differences in CLABSI rates over time by birth weight category. Because this interaction term was not significant, we present results from the more parsimonious NICU models. We considered P values <.05, determined with 2-tailed, unpaired testing, to be statistically significant. All analyses were performed in SAS, version 9.4 (SAS Institute Inc).
We conducted sensitivity analyses for device-associated health care–associated infection rates that included only consistently reporting hospital units that contributed data in both the first and last years of the study period, adjusted for children’s hospital status, and adjusted for PICU or NICU bed size. For CLABSI, we additionally conducted sensitivity analyses including only data from 2016-2018 to exclude data from the period during which the 2015 NHSN protocol changes may have affected CLABSI rates.24,25
Results
Study Population
Between January 1, 2013, and June 30, 2018, 176 PAICAP study hospitals from 42 states and the District of Columbia reported CLABSIs and/or CAUTIs in critically ill children to the NHSN. The PAICAP hospital NICUs reported 6 064 172 patient-days and 1 363 700 central catheter–days. PICUs reported 1 999 979 patient-days, 925 956 central catheter–days, and 327 599 indwelling urinary catheter–days. Pediatric CLABSIs were reported by 132 hospitals with NICUs and 114 hospitals with PICUs. Approximately half of hospitals reporting NICU CLABSIs also reported PICU CLABSIs (70/132 [53%]). Most hospitals reporting PICU CLABSIs also reported PICU CAUTIs (112/114 [98%]). Data on causative pathogens were available from 104 of 132 NICUs (79%) and 70 of 114 PICUs (61%) for CLABSIs and 60 of 112 for CAUTIs (54%). The PAICAP hospitals had a median number of 26.0 NICU beds (interquartile range [IQR], 14.0-40.0) and 10.0 PICU beds (IQR, 6.0-16.0) (Table 1). Nine PAICAP hospitals (5%) were children’s hospitals. Compared with AHA survey hospitals, PAICAP hospitals tended to be more frequently located in the northeast US and have more total beds and more NICU beds.
Table 1. Characteristics of PAICAP Study Hospitals vs 2015 AHA Survey Hospitals With PICUs and/or NICUs.
| Characteristic | Hospitals, No. (%) | |||
|---|---|---|---|---|
| With NICU | With PICU | |||
| PAICAP study (n = 132) | AHA survey (n = 1127) | PAICAP study (n = 114) | AHA survey (n = 475) | |
| Consistent reportinga | ||||
| CLABSI | 107 (81) | NA | 91 (80) | NA |
| CAUTI | NA | NA | 87 (76) | NA |
| Region | ||||
| Midwest | 30 (23) | 242 (21) | 20 (18) | 137 (29) |
| Northeast | 43 (33) | 172 (15) | 32 (28) | 79 (17) |
| South | 33 (25) | 440 (39) | 39 (34) | 169 (36) |
| West | 26 (20) | 273 (24) | 23 (20) | 90 (19) |
| Location | ||||
| Metropolitan | 131 (99) | 1061 (94) | 114 (100) | 427 (90) |
| Micropolitan | 1 (1) | 50 (4) | 0 | 29 (6) |
| Rural | 0 | 16 (1) | 0 | 19 (4) |
| Total hospital bed size | ||||
| <100 | 1 (1) | 76 (7) | 3 (3) | 58 (12) |
| 100-399 | 54 (41) | 653 (58) | 41 (36) | 174 (37) |
| ≥400 | 77 (58) | 398 (35) | 70 (61) | 243 (51) |
| NICU beds, median (IQR)b | 26.0 (14.0-40.0) | 15.0 (8.0-30.0) | NA | NA |
| PICU beds, median (IQR)c | NA | NA | 10.0 (6.0-16.0) | 8.0 (1.0-15.0) |
| Full-time equivalent NICU intensivists, median (IQR)b | 6.0 (2.0-11.0) | 4.0 (0.0-7.0) | NA | NA |
| Full-time equivalent PICU intensivists, median (IQR)c | NA | NA | 5.0 (3.0-8.5) | 4.0 (2.0-8.0) |
| Type of ownership | ||||
| Federal | 0 | 4 (<1) | 0 | 4 (<1) |
| For-profit | 26 (20) | 204 (18) | 34 (30) | 57 (12) |
| Not-for-profit | 94 (71) | 772 (69) | 72 (63) | 335 (71) |
| Public | 12 (9) | 147 (13) | 8 (7) | 79 (17) |
| Teaching statusd | ||||
| Graduate | 55 (42) | 532 (47) | 43 (38) | 205 (43) |
| Major | 64 (48) | 222 (20) | 56 (49) | 156 (33) |
| Minor | 4 (3) | 63 (6) | 3 (3) | 14 (3) |
| Nonteaching | 9 (7) | 310 (28) | 12 (11) | 100 (21) |
| Children’s hospitale | 7 (5) | 53 (5) | 9 (8) | 58 (12) |
Abbreviations: AHA, American Hospital Association; CAUTI, catheter-associated urinary tract infection; CLABSI, central catheter–associated bloodstream infection; IQR, interquartile range; NA, not applicable; NICU, neonatal intensive care unit; PAICAP, Preventing Avoidable Infectious Complications by Adjusting Payment; PICU, pediatric intensive care unit.
Consistently reporting hospitals were defined as hospitals contributing data to the study in both the first and last years of the study period. Percentages were calculated based on total hospitals reporting each outcome.
Descriptive statistics for NICU beds and full-time equivalent NICU intensivists were calculated for hospitals reporting NICU CLABSI (n = 132).
Descriptive statistics for PICU beds and full-time equivalent PICU intensivists were calculated for hospitals reporting PICU CLABSI (n = 114).
All hospitals were placed into 1 of 4 categories based on their response to the AHA survey: major teaching hospitals (those that are members of the Council of Teaching Hospitals [COTH]), graduate teaching hospitals (non-COTH members with a residency training program approved by the Accreditation Council for Graduate Medical Education), minor teaching hospitals (non-COTH members with a medical school affiliation reported to the American Medical Association), and nonteaching hospitals (all other institutions).
A children’s hospital was defined as a hospital reporting that it restricts admissions primarily to children in the AHA survey. Three PICU study hospitals and 1 NICU study hospital had missing children’s hospital status.
Outcomes
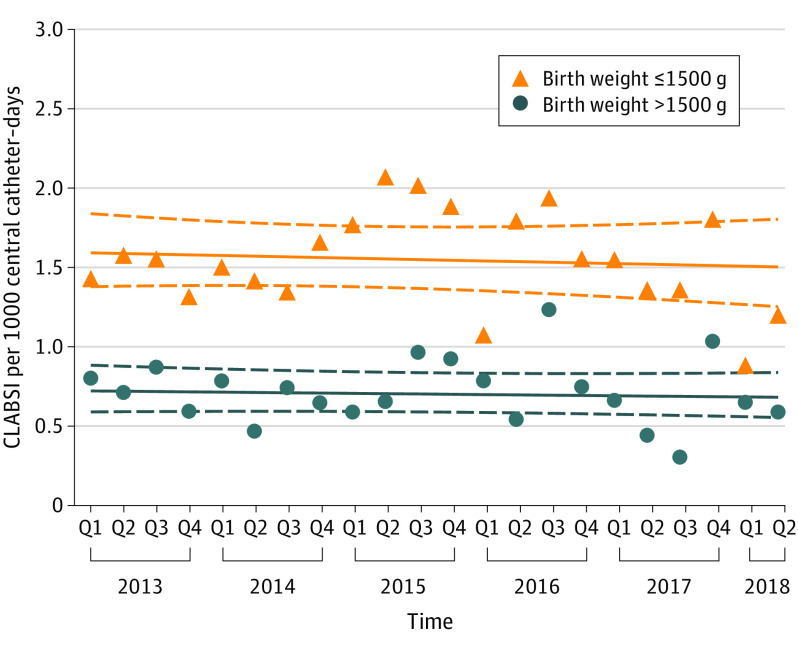
Among hospitalized neonates in NICUs, we did not find evidence to suggest that CLABSI trends over time differed across birth weight categories (P = .99 for interaction) and therefore present results for models that exclude the interaction. Device-associated CLABSI rates remained stable among hospitalized neonates throughout the study period, with no significant time trend (IRR, 0.99 per year; 95% CI, 0.95-1.03) (Figure 1; Table 2). Neonates with birth weights less than or equal to 1500 g were more likely to develop CLABSIs compared with those with birth weights greater than 1500 g (IRR, 2.20; 95% CI, 1.84-2.65). The mean device-associated rates were 1.56 CLABSIs per 1000 central catheter–days for neonates with birth weight less than or equal to 1500 g and 0.72 per 1000 central catheter–days for those with birth weight greater than 1500 g. Similarly, population-based rates of CLABSIs per 10 000 patient-days (IRR, 0.96; 95% CI, 0.92-1.00) and central catheter utilization (OR, 0.97; 95% CI, 0.95-1.00) remained stable over time among hospitalized neonates (Table 2). Sensitivity analyses of device-associated CLABSI rates that included only NICUs contributing data in both the first and last years of the study period, adjusted for number of NICU beds or children’s hospital status, or limited data included to 2016-2018, were also consistent with the main analysis (eTable 1 in the Supplement).
Figure 1. Observed Device-Associated Central Catheter–Associated Bloodstream Infections (CLABSI) Rates by Birth Weight Over Time Among Hospitalized Neonates.
The triangles and circles depict observed quarterly device-associated central catheter–associated bloodstream infection rates (infections per 1000 central catheter–days) aggregated for all study hospitals by birth weight category. Solid lines indicate estimated trend lines and dashed lines represent the corresponding upper and lower limit of the 95% CIs of the estimated trend lines. Q indicates quarter.
Table 2. Regression Models to Evaluate Changes in Rates of NICU CLABSI Over Time by Birth Weight Category.
| Model | Yearly change (95% CI)a |
|---|---|
| CLABSI per 1000 central catheter–days | |
| Time | 0.99 (0.95-1.03) |
| Birth weight ≤1500 gb | 2.20 (1.84-2.65) |
| CLABSI per 10 000 patient-days | |
| Time | 0.96 (0.92-1.00) |
| Birth weight ≤1500 gb | 3.65 (2.82-4.73) |
| Central catheter utilization | |
| Time | 0.97 (0.95-1.00) |
| Birth weight ≤1500 gb | 1.88 (1.62-2.18) |
Abbreviations: CLABSI, central catheter–associated bloodstream infection; NICU, neonatal intensive care unit.
Incidence rate ratios are reported for device-associated and population-based rates. Odds ratios are reported for device utilization.
Birth weight greater than 1500 g is the reference category. Time 0 is quarter 1 of 2013.
The most frequent primary causative pathogens for CLABSIs reported in the NICU were Staphylococcus aureus, which caused 11% to 34% of infections, and coagulase-negative staphylococci, which caused 15% to 25% of infections (eFigure, A in the Supplement). Additional organisms reported within the top 5 most frequent causative pathogens by year included Escherichia coli, Enterococcus species, Klebsiella pneumoniae, and yeast.
Among hospitalized children in PICUs, device-associated CLABSI rates remained stable over the study period (IRR per year, 1.03; 95% CI, 0.99-1.07), with a mean rate of 1.39 CLABSIs per 1000 central catheter–days (Figure 2; Table 3). Results were similar for population-based CLABSI rates (IRR, 1.03; 95% CI, 0.99-1.07) and PICU central catheter utilization (OR, 0.99; 95% CI, 0.97-1.01), which also did not change significantly over time. Sensitivity analyses of device-associated CLABSI rates that included only PICUs contributing data in both the first and last years of the study period, adjusted for number of PICU beds or children’s hospital status, or limited data included to 2016-2018 were also consistent with the main analysis (eTable 2 in the Supplement).
Figure 2. Observed Device-Associated Central Catheter–Associated Bloodstream Infection (CLABSI) and Catheter-Associated Urinary Tract Infection (CAUTI) Rates Over Time Among Hospitalized Children.
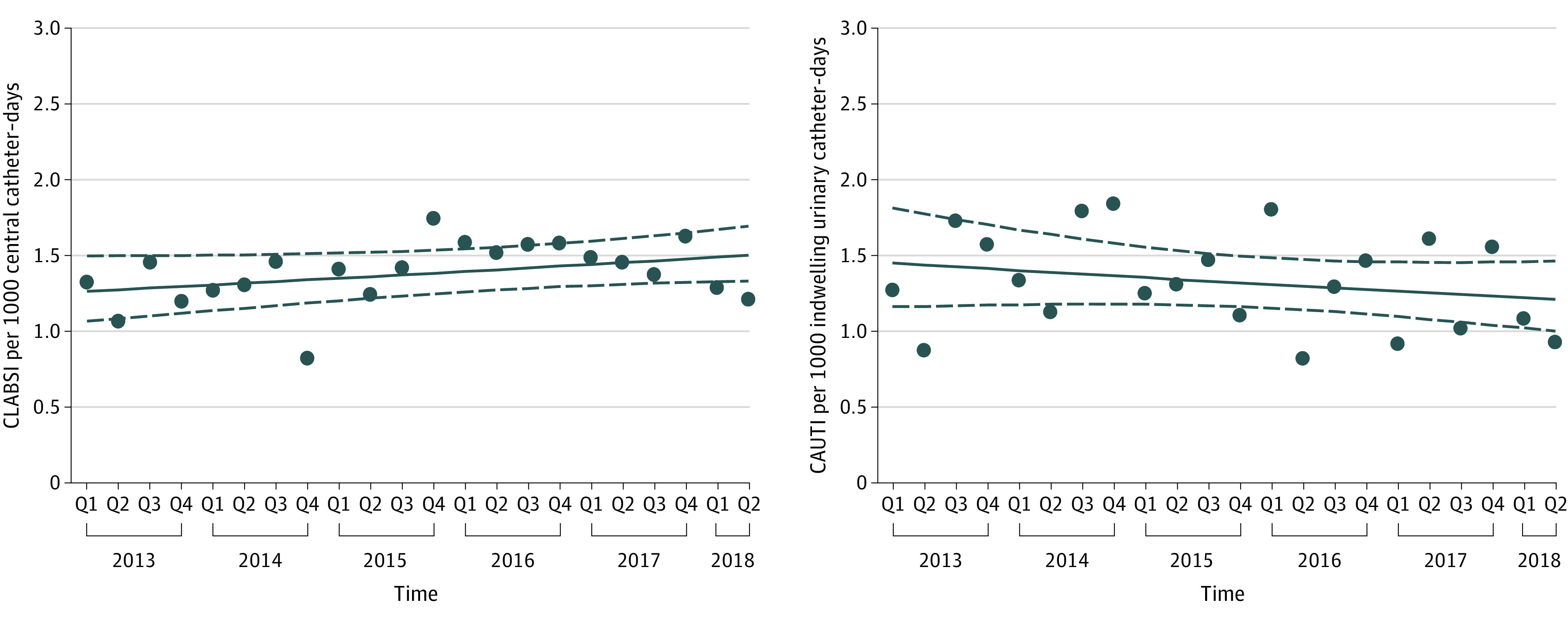
Each series depicts observed quarterly device-associated infection rates aggregated for all study hospitals for CLABSI (A) and CAUTI (B). Solid lines indicate estimated trend lines and dashed lines represent the corresponding upper and lower limit of the 95% CIs of the estimated trend lines. Q indicates quarter.
Table 3. Regression Models to Evaluate Changes in Rates of PICU CLABSI and CAUTI Over Time.
| Model | Yearly change (95% CI)a |
|---|---|
| CLABSI (n = 114 hospitals) | |
| CLABSI per 1000 central catheter–days | |
| Time | 1.03 (0.99-1.07) |
| CLABSI per 10 000 patient-days | |
| Time | 1.03 (0.99-1.07) |
| Central catheter use | |
| Time | 0.99 (0.97-1.01) |
| CAUTI (n = 112 hospitals) | |
| CAUTI per 1000 indwelling urinary catheter–days | |
| Time | 0.97 (0.91-1.03) |
| CAUTI per 10 000 patient-days | |
| Time | 0.92 (0.86-0.98) |
| Indwelling urinary catheter utilization | |
| Time | 0.94 (0.91-0.96) |
Abbreviations: CAUTI, catheter-associated urinary tract infection; CLABSI, central catheter–associated bloodstream infection; PICU, pediatric intensive care unit.
Incidence rate ratios are reported for device-associated and population-based rates. Odds ratios are reported for device utilization.
The most frequent causative pathogen for CLABSIs reported in the PICU in 3 of the 4 years examined was Enterococcus species, which caused 17% to 23% of infections (eFigure, B in the Supplement). Additional organisms reported within the top 5 most frequent causative pathogens by year included coagulase-negative staphylococci, S aureus, Enterobacter cloacae, yeast, K pneumoniae, and Serratia marcescens.
Device-associated CAUTI rates among hospitalized children in PICUs remained unchanged throughout the study period (IRR per year, 0.97; 95% CI, 0.91-1.03), with a mean rate of 1.33 infections per 1000 indwelling urinary catheter–days (Figure 2; Table 3). However, population-based CAUTI rates significantly decreased by 8% per year (IRR, 0.92; 95% CI, 0.86-0.98) and indwelling urinary catheter device utilization significantly decreased by 6% per year (OR, 0.94; 95% CI, 0.91-0.96). Sensitivity analyses of device-associated CAUTI rates that included only PICUs contributing data in both the first and last years of the study period or adjusted for number of PICU beds or children’s hospital status were also consistent with the main analysis (eTable 3 in the Supplement).
The most frequent causative pathogen for CAUTI reported in the PICU was E coli, which caused from 29% to 40% of infections (eFigure, panel C in the Supplement). Additional organisms reported within the top 5 most frequent causative pathogens for CAUTI included Pseudomonas aeruginosa, Enterococcus species, E cloacae, K pneumoniae, Klebsiella oxytoca, and Citrobacter freundii.
Discussion
Device-associated CLABSI and CAUTI rates appeared to remain stable between 2013 and 2018 among a large sample of hospitals in the US caring for critically ill neonates and children, and the pathogen distribution for CLABSI and CAUTI was similar to previously reported US surveillance data.27,28 When expressed as population-based rates, we found no significant change in CLABSI trends over time or central catheter utilization in either the PICU or NICU. In contrast, we observed a statistically significant decrease in the population-based CAUTI rate in conjunction with significant decreases in the use of indwelling urinary catheters.
Our findings for CLABSI differ from those in a similar study examining device-associated CLABSI rates in the NICU and PICU from 2007 to 2012,9 which reported substantial decreases in device-associated CLABSI rates over the 6-year study period. The newly observed plateau in CLABSI rates raises questions for continued quality improvement efforts in infection prevention and control. The first question is whether the persistent focus on improving adherence to central catheter insertion and maintenance bundles has led to the lowest feasible CLABSI rates.29,30,31,32 Central catheter bundles are structured sets of evidence-based practices that, when applied together, have been reported to improve patient outcomes.32 Although the focus of CLABSI improvement work has been on adherence to central catheter bundles, national surveillance data on adherence to all elements of the maintenance bundle in particular are not well described. Second, if adherence to current maintenance bundles used in neonates and children is consistently high, we may need to consider other strategies to enhance these bundles. For example, different strategies for bundle customization to specific patient populations have been studied, including the use of ethanol locks, with reported success in patients with intestinal failure, or inclusion of oral care to reduce the risk of translocation from mucositis in patients with cancer.33,34,35,36 Since the pathophysiologic factors and risk for occurrence of CLABSI vary between patient populations, tailoring bundles accordingly could be a reasonable approach. A third question raised by the observation of plateauing CLABSI rates and central catheter utilization is whether it is time to consider expanding surveillance efforts beyond CLABSI to improve overall patient safety more broadly. For example, hospital-onset bacteremia, defined as a positive blood culture from any cause (including CLABSI) with onset at least 48 hours after hospital admission, is a more inclusive measure that could be tracked. Prior research has reported that approximately two-thirds of hospital-onset bacteremia events are likely preventable,37 and reduction in hospital-onset bacteremia has been associated with reduction in CLABSI.38 By extending the focus beyond CLABSI, this broader approach to surveillance for bloodstream infections may lead to identification of additional opportunities to improve patient outcomes.37,38,39
Efforts for CAUTI prevention have similarly targeted indwelling urinary catheter bundle adherence, with the greatest focus extending beyond aseptic technique and emphasizing appropriate indications for placement and timely removal of indwelling urinary catheters.17,40 These efforts to reduce device utilization are important to consider when assessing whether any progress has been made in CAUTI prevention. Device-associated CAUTI rates, which include patient catheter–days in the denominator, are the most commonly tracked and reported CAUTI outcome measure. Device-associated CAUTI rates, however, may either remain stable or even increase as device utilization (the rate’s denominator) decreases.41 Calculation of population-based rates, which incorporate all hospitalized patient-days from a particular unit in the denominator rather than just the catheter-days, more accurately reflect CAUTI prevention efforts to reduce indwelling urinary catheter use.42,43 For example, in our study, we found no change in device-associated CAUTI rates over time in PICUs. However, there were statistically significant decreases in both population-based CAUTI rates, as well as indwelling urinary catheter use, likely representing the success of efforts to encourage more judicious use of indwelling urinary catheters in critically ill hospitalized children.
Strengths and Limitations
We consider the following factors as strengths of our study. To our knowledge, this is the first report of national-level pediatric health care–associated infection, hospital-based surveillance data since the earlier analysis from 2007-2012.9 Inclusion of hospitalized children cared for in a variety of settings, including nonchildren’s hospitals, community hospitals, and a selection of children’s hospitals, allows for a more diverse and comprehensive capture of national trends than analyses of administrative data from freestanding children’s hospitals alone. Monitoring health care–associated infection trends from nonfreestanding children’s hospitals is particularly important for NICUs given that most critically ill newborns are cared for in a NICU within their birth hospital rather than in a children’s hospital. In addition, to our knowledge, this is the first study to examine population-based rates and device utilization in addition to device-associated rates for CLABSI and CAUTI. Given that duration of catheterization is an important risk factor for infectious and noninfectious complications of both central catheters and urinary catheters, improvement efforts have emphasized limiting unnecessary use of central catheters and indwelling urinary catheters; as such, population-based rates and device utilization, rather than device-associated rates alone, may be the more appropriate measures to track to detect improvements in patient safety.10,44
Our study has several limitations. First, the cohort of hospitals may not be representative of all US hospitals caring for critically ill neonates and children. For example, fewer study hospitals were located in rural or micropolitan areas compared with all US hospitals providing NICU and PICU care. However, the primary modeling approach accounted for hospital-level differences and additional sensitivity analyses, including adjustment for children’s hospital status and NICU or PICU bed size, did not appear to influence the findings. Second, we were unable to precisely account for changes in NHSN protocols that affected CLABSI rates in 2015.19,23,45 This lack of specificity may have limited our ability to detect true changes in CLABSI rates across the full study period. In response, we performed sensitivity analyses using CLABSI data from only 2016-2018 (the post-NHSN protocol revision period). For both the NICU and PICU, these sensitivity analyses demonstrated similar results to the main analysis, although the analyses may have been underpowered to detect changes within this more limited time frame. Third, not all of our study hospitals contributed data for the full study period. However, restricting the analyses to hospitals contributing data consistently did not alter the findings.
Conclusions
The findings of this study suggest that CLABSI rates among critically ill neonates and children in a large cohort of US hospitals have plateaued, indicating that past gains have held with no evidence of further improvement. Future improvement efforts may involve rethinking surveillance approaches and the customization of central catheter bundle components for certain at-risk patient populations. While device-associated CAUTI rates remained similarly stable, incremental improvements in population-based CAUTI rates and indwelling urinary catheter use likely reflect the success of ongoing efforts to encourage appropriate placement and timely removal of indwelling urinary catheters. Including population-based health care–associated infection rates and device utilization in the surveillance approach may enhance the capture of ongoing and meaningful improvements.
eTable 1. Sensitivity Analyses for Regression Models to Evaluate Changes in Rates of Device-Associated Central Line–Associated Bloodstream Infections per 1000 Central Line-Days Among Patients Cared for in Neonatal Intensive Care Units
eTable 2. Sensitivity Analyses for Regression Models to Evaluate Changes in Rates of Device-Associated Central Line–Associated Bloodstream Infections per 1000 Central Line-Days Among Patients Cared for in Pediatric Intensive Care Units
eTable 3. Sensitivity Analyses for Regression Models to Evaluate Changes in Rates of Device-Associated Catheter-Associated Urinary Tract Infections per 1000 Indwelling Urinary Catheter Days Over Time Among Patients Cared for in Pediatric Intensive Care Units
eFigure. Top Five Pathogens Causing Central Line–Associated Bloodstream Infections and Catheter-Associated Urinary Tract Infections Over Time Among Patients Cared for in Pediatric and Neonatal Intensive Care Units
References
- 1.Miller MR, Niedner MF, Huskins WC, et al. ; National Association of Children’s Hospitals and Related Institutions Pediatric Intensive Care Unit Central Line–Associated Bloodstream Infection Quality Transformation Teams . Reducing PICU central line-associated bloodstream infections: 3-year results. Pediatrics. 2011;128(5):e1077-e1083. doi: 10.1542/peds.2010-3675 [DOI] [PubMed] [Google Scholar]
- 2.Polin RA, Denson S, Brady MT; Committee on Fetus and Newborn; Committee on Infectious Diseases . Epidemiology and diagnosis of health care-associated infections in the NICU. Pediatrics. 2012;129(4):e1104-e1109. doi: 10.1542/peds.2012-0147 [DOI] [PubMed] [Google Scholar]
- 3.Septimus EJ, Moody J. Prevention of device-related healthcare-associated infections. F1000Res. Published online January 14, 2016. Accessed September 16, 2019. https://f1000research.com/articles/5-65/v1 [DOI] [PMC free article] [PubMed]
- 4.Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101-114. doi: 10.1086/657912 [DOI] [PubMed] [Google Scholar]
- 5.Wisplinghoff H, Seifert H, Tallent SM, Bischoff T, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in pediatric patients in United States hospitals: epidemiology, clinical features and susceptibilities. Pediatr Infect Dis J. 2003;22(8):686-691. doi: 10.1097/01.inf.0000078159.53132.40 [DOI] [PubMed] [Google Scholar]
- 6.Richards MJ, Edwards JR, Culver DH, Gaynes RP; National Nosocomial Infections Surveillance System . Nosocomial infections in pediatric intensive care units in the United States. Pediatrics. 1999;103(4):e39. doi: 10.1542/peds.103.4.e39 [DOI] [PubMed] [Google Scholar]
- 7.Ahmed SS, McCaskey MS, Bringman S, Eigen H. Catheter-associated bloodstream infection in the pediatric intensive care unit: a multidisciplinary approach. Pediatr Crit Care Med. 2012;13(2):e69-e72. doi: 10.1097/PCC.0b013e31820ac2e1 [DOI] [PubMed] [Google Scholar]
- 8.Mobley RE, Bizzarro MJ. Central line-associated bloodstream infections in the NICU: successes and controversies in the quest for zero. Semin Perinatol. 2017;41(3):166-174. doi: 10.1053/j.semperi.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 9.Patrick SW, Kawai AT, Kleinman K, et al. Health care–associated infections among critically ill children in the US, 2007-2012. Pediatrics. 2014;134(4):705-712. doi: 10.1542/peds.2014-0613 [DOI] [PubMed] [Google Scholar]
- 10.Letica-Kriegel AS, Salmasian H, Vawdrey DK, et al. Identifying the risk factors for catheter-associated urinary tract infections: a large cross-sectional study of six hospitals. BMJ Open. 2019;9(2):e022137. doi: 10.1136/bmjopen-2018-022137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) . Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. [PubMed] [Google Scholar]
- 12.Bundy DG, Gaur AH, Billett AL, He B, Colantuoni EA, Miller MR; Children’s Hospital Association Hematology/Oncology CLABSI Collaborative . Preventing CLABSIs among pediatric hematology/oncology inpatients: national collaborative results. Pediatrics. 2014;134(6):e1678-e1685. doi: 10.1542/peds.2014-0582 [DOI] [PubMed] [Google Scholar]
- 13.Edwards JD, Herzig CT, Liu H, et al. Central line–associated blood stream infections in pediatric intensive care units: longitudinal trends and compliance with bundle strategies. Am J Infect Control. 2015;43(5):489-493. doi: 10.1016/j.ajic.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MR, Griswold M, Harris JM II, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI’s quality transformation efforts. Pediatrics. 2010;125(2):206-213. doi: 10.1542/peds.2009-1382 [DOI] [PubMed] [Google Scholar]
- 15.Piazza AJ, Brozanski B, Provost L, et al. SLUG bug: quality improvement with orchestrated testing leads to NICU CLABSI reduction. Pediatrics. 2016;137(1). doi: 10.1542/peds.2014-3642 [DOI] [PubMed] [Google Scholar]
- 16.Schulman J, Stricof R, Stevens TP, et al. ; New York State Regional Perinatal Care Centers . Statewide NICU central-line–associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436-444. doi: 10.1542/peds.2010-2873 [DOI] [PubMed] [Google Scholar]
- 17.Davis KF, Colebaugh AM, Eithun BL, et al. Reducing catheter-associated urinary tract infections: a quality-improvement initiative. Pediatrics. 2014;134(3):e857-e864. doi: 10.1542/peds.2013-3470 [DOI] [PubMed] [Google Scholar]
- 18.Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. doi: 10.1542/peds.2016-3494 [DOI] [PubMed] [Google Scholar]
- 19.Children's Hospitals' Solutions for Patient Safety . Our results. Accessed September 16, 2019. https://www.solutionsforpatientsafety.org/our-results/
- 20.Calderwood MS, Vaz LE, Tse Kawai A, et al. Impact of hospital operating margin on central line-associated bloodstream infections following Medicare’s hospital-acquired conditions payment policy. Infect Control Hosp Epidemiol. 2016;37(1):100-103. doi: 10.1017/ice.2015.250 [DOI] [PubMed] [Google Scholar]
- 21.American Hospital Association . AHA annual survey database. Accessed August 28, 2020. https://www.aha.org/data-insights/aha-data-products
- 22.Centers for Disease Control and Prevention. NSHN e-News. NHSN HAI surveillance changes for 2015. Published September 2014. Accessed August 8, 2019. https://www.cdc.gov/nhsn/pdfs/newsletters/vol9-3-enl-sept-2014.pdf
- 23.Corley A, Cantara M, Gardner J, Trexler P, Rock C, Maragakis LL. Central line–associated bloodstream infection rate elevation: attributable to National Healthcare Safety Network surveillance definition changes, ongoing opportunities for infection prevention, or both? Am J Infect Control. 2017;45(9):1030-1032. doi: 10.1016/j.ajic.2017.04.282 [DOI] [PubMed] [Google Scholar]
- 24.National Healthcare Safety Network . Bloodstream infection event (central line–associated bloodstream infection and non-central line associated bloodstream infection). Updated January 2020. Accessed June 29, 2019. https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf
- 25.Fakih MG, Groves C, Bufalino A, Sturm LK, Hendrich AL. Definitional change in NHSN CAUTI was associated with an increase in CLABSI events: evaluation of a large health system. Infect Control Hosp Epidemiol. 2017;38(6):685-689. doi: 10.1017/ice.2017.41 [DOI] [PubMed] [Google Scholar]
- 26.Liang KY ZS. Longitudinal data analysis using generalized linear models. Biometrika. 1986; 73(1):13-22. doi: 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 27.Lake JG, Weiner LM, Milstone AM, Saiman L, Magill SS, See I. Pathogen distribution and antimicrobial resistance among pediatric healthcare-associated infections reported to the National Healthcare Safety Network, 2011-2014. Infect Control Hosp Epidemiol. 2018;39(1):1-11. doi: 10.1017/ice.2017.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiner-Lastinger LM, Abner S, Benin AL, et al. Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015-2017. Infect Control Hosp Epidemiol. 2020;41(1):19-30. doi: 10.1017/ice.2019.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. doi: 10.1016/S0140-6736(00)02291-1 [DOI] [PubMed] [Google Scholar]
- 30.Institute for Healthcare Improvement . What is a bundle? Published 2011. Accessed September 17, 2019. http://www.ihi.org/Topics/Bundles/Pages/default.aspx
- 31.Furuya EY, Dick A, Perencevich EN, Pogorzelska M, Goldmann D, Stone PW. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS One. 2011;6(1):e15452. doi: 10.1371/journal.pone.0015452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central line–associated bloodstream infection reduction and bundle compliance in intensive care units: a national study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. doi: 10.1017/ice.2016.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ardura MI, Lewis J, Tansmore JL, Harp PL, Dienhart MC, Balint JP. Central catheter–associated bloodstream infection reduction with ethanol lock prophylaxis in pediatric intestinal failure: broadening quality improvement initiatives from hospital to home. JAMA Pediatr. 2015;169(4):324-331. doi: 10.1001/jamapediatrics.2014.3291 [DOI] [PubMed] [Google Scholar]
- 34.Wolf J, Connell TG, Allison KJ, et al. Treatment and secondary prophylaxis with ethanol lock therapy for central line–associated bloodstream infection in paediatric cancer: a randomised, double-blind, controlled trial. Lancet Infect Dis. 2018;18(8):854-863. doi: 10.1016/S1473-3099(18)30224-X [DOI] [PubMed] [Google Scholar]
- 35.Dandoy CE, Hausfeld J, Flesch L, et al. Rapid cycle development of a multifactorial intervention achieved sustained reductions in central line-associated bloodstream infections in haematology oncology units at a children’s hospital: a time series analysis. BMJ Qual Saf. 2016;25(8):633-643. doi: 10.1136/bmjqs-2015-004450 [DOI] [PubMed] [Google Scholar]
- 36.Ormsby JA, Bukoye B, Lajoie D, et al. Enhanced central venous catheter bundle for pediatric parenteral-dependent intestinal failure. Am J Infect Control. 2018;46(11):1284-1289. doi: 10.1016/j.ajic.2018.04.209 [DOI] [PubMed] [Google Scholar]
- 37.Dantes RB, Abbo LM, Anderson D, et al. Hospital epidemiologists’ and infection preventionists’ opinions regarding hospital-onset bacteremia and fungemia as a potential healthcare-associated infection metric. Infect Control Hosp Epidemiol. 2019;40(5):536-540. doi: 10.1017/ice.2019.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rock C, Thom KA, Harris AD, et al. A multicenter longitudinal study of hospital-onset bacteremia: time for a new quality outcome measure? Infect Control Hosp Epidemiol. 2016;37(2):143-148. doi: 10.1017/ice.2015.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leekha S, Li S, Thom KA, et al. Comparison of total hospital-acquired bloodstream infections to central line–associated bloodstream infections and implications for outcome measures in infection control. Infect Control Hosp Epidemiol. 2013;34(9):984-986. doi: 10.1086/671730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel BI, Figueroa J, Stockwell JA. Impact of a daily PICU rounding checklist on urinary catheter utilization and infection. Pediatr Qual Saf. 2018;3(3):e078. doi: 10.1097/pq9.0000000000000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fakih MG, Gould CV, Trautner BW, et al. Beyond infection: device utilization ratio as a performance measure for urinary catheter harm. Infect Control Hosp Epidemiol. 2016;37(3):327-333. doi: 10.1017/ice.2015.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu HE, Wang R, Jentzsch MS, et al. Association between value-based incentive programs and catheter-associated urinary tract infection rates in the critical care setting. JAMA. 2019;321(5):509-511. doi: 10.1001/jama.2018.18997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fakih MG, Greene MT, Kennedy EH, et al. Introducing a population-based outcome measure to evaluate the effect of interventions to reduce catheter-associated urinary tract infection. Am J Infect Control. 2012;40(4):359-364. doi: 10.1016/j.ajic.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018;178(8):1078-1085. doi: 10.1001/jamainternmed.2018.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Healthcare Safety Network . FAQs: NHSN CLABSI definition & rebaseline. Published 2017. Accessed May 16, 2020. https://www.cdc.gov/nhsn/pdfs/rebaseline/faq-clabsi-rebaseline.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Sensitivity Analyses for Regression Models to Evaluate Changes in Rates of Device-Associated Central Line–Associated Bloodstream Infections per 1000 Central Line-Days Among Patients Cared for in Neonatal Intensive Care Units
eTable 2. Sensitivity Analyses for Regression Models to Evaluate Changes in Rates of Device-Associated Central Line–Associated Bloodstream Infections per 1000 Central Line-Days Among Patients Cared for in Pediatric Intensive Care Units
eTable 3. Sensitivity Analyses for Regression Models to Evaluate Changes in Rates of Device-Associated Catheter-Associated Urinary Tract Infections per 1000 Indwelling Urinary Catheter Days Over Time Among Patients Cared for in Pediatric Intensive Care Units
eFigure. Top Five Pathogens Causing Central Line–Associated Bloodstream Infections and Catheter-Associated Urinary Tract Infections Over Time Among Patients Cared for in Pediatric and Neonatal Intensive Care Units