Key Points
Question
Does prehospital administration of tranexamic acid compared with placebo result in lower 30-day mortality in patients at risk for hemorrhage after trauma?
Findings
In this multicenter randomized clinical trial of 927 patients, patients who received tranexamic acid compared with placebo in the prehospital setting did not have a significantly lower rate of 30-day mortality (8.1% vs 9.9%). There were no differences in the incidence of pulmonary embolism, deep vein thrombosis, seizures, or adverse events, including thrombotic complications, across arms.
Meaning
Prehospital administration of tranexamic acid is safe but does not significantly reduce mortality in patients at risk for hemorrhage after injury.
Abstract
Importance
In-hospital administration of tranexamic acid after injury improves outcomes in patients at risk for hemorrhage. Data demonstrating the benefit and safety of the pragmatic use of tranexamic acid in the prehospital phase of care are lacking for these patients.
Objective
To assess the effectiveness and safety of tranexamic acid administered before hospitalization compared with placebo in injured patients at risk for hemorrhage.
Design, Setting, and Participants
This pragmatic, phase 3, multicenter, double-blind, placebo-controlled, superiority randomized clinical trial included injured patients with prehospital hypotension (systolic blood pressure ≤90 mm Hg) or tachycardia (heart rate ≥110/min) before arrival at 1 of 4 US level 1 trauma centers, within an estimated 2 hours of injury, from May 1, 2015, through October 31, 2019.
Interventions
Patients received 1 g of tranexamic acid before hospitalization (447 patients) or placebo (456 patients) infused for 10 minutes in 100 mL of saline. The randomization scheme used prehospital and in-hospital phase assignments, and patients administered tranexamic acid were allocated to abbreviated, standard, and repeat bolus dosing regimens on trauma center arrival.
Main Outcomes and Measures
The primary outcome was 30-day all-cause mortality.
Results
In all, 927 patients (mean [SD] age, 42 [18] years; 686 [74.0%] male) were eligible for prehospital enrollment (460 randomized to tranexamic acid intervention; 467 to placebo intervention). After exclusions, the intention-to-treat study cohort comprised 903 patients: 447 in the tranexamic acid arm and 456 in the placebo arm. Mortality at 30 days was 8.1% in patients receiving tranexamic acid compared with 9.9% in patients receiving placebo (difference, –1.8%; 95% CI, –5.6% to 1.9%; P = .17). Results of Cox proportional hazards regression analysis, accounting for site, verified that randomization to tranexamic acid was not associated with a significant reduction in 30-day mortality (hazard ratio, 0.81; 95% CI, 0.59-1.11, P = .18). Prespecified dosing regimens and post-hoc subgroup analyses found that prehospital tranexamic acid were associated with significantly lower 30-day mortality. When comparing tranexamic acid effect stratified by time to treatment and qualifying shock severity in a post hoc comparison, 30-day mortality was lower when tranexamic acid was administered within 1 hour of injury (4.6% vs 7.6%; difference, −3.0%; 95% CI, −5.7% to −0.3%; P < .002). Patients with severe shock (systolic blood pressure ≤70 mm Hg) who received tranexamic acid demonstrated lower 30-day mortality compared with placebo (18.5% vs 35.5%; difference, −17%; 95% CI, −25.8% to −8.1%; P < .003).
Conclusions and Relevance
In injured patients at risk for hemorrhage, tranexamic acid administered before hospitalization did not result in significantly lower 30-day mortality. The prehospital administration of tranexamic acid after injury did not result in a higher incidence of thrombotic complications or adverse events. Tranexamic acid given to injured patients at risk for hemorrhage in the prehospital setting is safe and associated with survival benefit in specific subgroups of patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT02086500
This randomized clinical trial assesses the effectiveness and safety of tranexamic acid administered before hospitalization compared with placebo in injured patients at risk for hemorrhage.
Introduction
Trauma remains a leading cause of death worldwide, and the management of injured patients at risk for hemorrhage has evolved over time.1,2,3,4,5,6 Changes in management, including prevention of coagulopathy by early ratio-based blood component resuscitation and early antifibrinolytic therapy with tranexamic acid after arrival to definitive care, contribute to improved outcomes.7,8,9,10,11,12
Interventions provided to injured patients during prehospital care, close to the time of injury, result in improved outcomes and survival.13,14 On the basis of extrapolation from hospital-based data, guidelines now recommend prehospital tranexamic acid administration.15,16,17 However, the risks and benefits associated with tranexamic acid initiated in the prehospital environment, before trauma center evaluation, are unknown.18,19 No high-level evidence demonstrates the efficacy and safety of administering tranexamic acid in the prehospital setting.15
The Study of Tranexamic Acid During Air Medical and Ground Prehospital Transport (STAAMP) trial tests the clinical impact and safety of administering tranexamic acid during the prehospital phase of care.20 We enrolled patients at risk for hemorrhage with a broad range of injury and shock severity and allocated patients to 3 different tranexamic acid dosing regimens or placebo. We hypothesized that early administration of tranexamic acid in the prehospital environment would improve 30-day mortality.
Methods
Trial Design
The STAAMP study was a phase 3, multicenter, double-blind, placebo-controlled, randomized clinical trial that compared outcomes in patients at risk for hemorrhage receiving tranexamic acid (single dose) before hospitalization administered during air medical or ground transport. During the in-hospital phase of care, 3 dosing schemes of tranexamic acid were compared as a prespecified subgroup analysis. A total of 6559 patients who were transported via participating emergency medical services to 4 participating trauma centers were screened. We enrolled 927 patients at these 4 US level 1 trauma centers from May 1, 2015, through October 31, 2019. The treatment arms received a 1-g bolus of tranexamic acid (for 10 minutes) en route to the hospital. After patients arrived at the trauma center, tranexamic acid administration followed the prehospital phase assignment. Intervention arm patients received no additional tranexamic acid, 1 g of tranexamic acid infused during 8 hours, or a bolus of 1 g of tranexamic acid followed by 1 g of tranexamic acid infused during 8 hours. We did not alter other aspects of prehospital or in-hospital care besides administration of tranexamic acid. Prehospital tranexamic acid administration was not usual care for participating sites during the trial. The trial protocol can be found in Supplement 1. The US Food and Drug Administration (Investigational New Drug 121102), the Human Research Protection Offices of the US Department of Defense, and all site institutional review boards approved the trial. An external data and safety monitoring board oversaw the trial. Institutional review boards at each site approved an exception from informed consent to enroll participants. This approval included community consultation and notification. We notified enrolled participants or their legally authorized representatives as soon as feasible and obtained consent for continued participation.21 This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Patient Population
We selected inclusion criteria to include a broad range of shock and injury severity and prior trial results.8 Prehospital personnel enrolled patients before hospitalization based on all information available during the prehospital phase of care. Injured patients at risk for hemorrhage transported from the scene or transferred from an outside emergency department to a participating site within an estimated 2 hours of the time of injury were eligible for enrollment if they experienced at least 1 episode of hypotension (systolic blood pressure ≤90 mm Hg) or tachycardia (heart rate ≥110 beats per minute) before arrival at a participating center. Exclusion criteria included age older than 90 years or younger than 18 years, lack of intravenous or intraosseous access, isolated fall from standing, documented cervical cord injury, known prisoner or pregnancy, traumatic arrest of more than 5 minutes, penetrating brain injury, isolated drowning or hanging, objection to study voiced at scene, or wearing a STAAMP study opt-out bracelet.
Randomization and Masking
We generated a 1:1:1:1 ratio random allocation sequence with a block size of 12 using a computer random-number generator. We placed sealed drug kits on each participating ambulance or aircraft according to the allocation sequence. Each kit contained the group allocation and appropriate drug or placebo, blinded for the prehospital and in-hospital phase interventions. Randomization occurred with kit opening. The primary randomization procedure assigned a prehospital phase assignment (tranexamic acid vs placebo) and a corresponding in-hospital assignment. On arrival, personnel communicated the treatment kit number to research staff, allowing in-hospital random allocation. Patients randomized to the prehospital placebo group were allocated to receive placebo during the in-hospital phase. Patients randomized to the prehospital tranexamic acid group were randomly allocated to 3 in-hospital phase tranexamic acid dosing regimens.20 The Investigational Drug Services at the University of Pittsburgh monitored the intervention for the trial, unblinded to the prehospital and in-hospital phase treatment assignment.
Intervention and Comparison Arms
The Investigational Drug Services at the University of Pittsburgh created numerically labeled intervention kits divided into 3 separate components. Each kit A was a prehospital-phase intervention stored at the ambulance or air medical unit. Kits B and C were in-hospital phase interventions stored at the receiving hospital pharmacy (eFigure in Supplement 2). The A kits contained 1 g of tranexamic acid in 10 mL of solution or 10 mL of sterile water placebo. Paramedics added these vials to a 100-mL bag of 0.9% saline and infused the drug or placebo for 10 minutes. If the infusion was not completed by trauma center arrival, the infusion continued in the trauma bay. On patient arrival at a participating center, research staff verified inclusion and exclusion criteria, and a pharmacist mixed the B and C interventions with numbers matching the A intervention. The B phase intervention was 1 g of tranexamic acid in 10 mL of solution or 10 mL of placebo (sterile water) added to a 100-mL bag of 0.9% saline and infused for 10 minutes. In the C phase intervention, 1 g of tranexamic acid in 10 mL of solution or 10 mL of placebo was added to a 100-mL bag of 0.9% saline and infused for 8 hours.
Enrolled patients received 1 of 4 treatments. The control regimen was placebo bolus (phase A), placebo bolus (phase B), and placebo infusion (phase C). The abbreviated dosing regimen was 1 g of tranexamic acid bolus (phase A), placebo bolus (phase B), and placebo infusion (phase C). The standard dosing regimen was 1 g of tranexamic acid bolus (phase A), placebo bolus (phase B), and 1 g of tranexamic acid infusion (phase C). The repeat bolus dosing regimen was 1 g of tranexamic acid bolus (phase A), 1 g of tranexamic acid bolus (phase B), and 1 g of tranexamic acid infusion (phase C).
Outcome
The primary outcome for the trial was 30-day mortality. Prespecified secondary outcomes included (1) 24-hour and in-hospital mortality; (2) blood component resuscitation volumes at 6 and 24 hours from admission; (3) incidence of multiple organ failure; (4) acute respiratory distress syndrome; (5) nosocomial infection; (6) early seizures (initial 24 hours); (7) pulmonary embolism and deep vein thrombosis; (8) crystalloid resuscitation over 24 hours from admission; and (9) incidence of coagulopathy and hyperfibrinolysis as measured and defined by international normalized ratio and thromboelastography. Prespecified subgroup analyses for 30-day mortality included (1) patients who did or did not require blood transfusion; (2) significant traumatic brain injury (head Abbreviated Injury Scale score >2) vs those without; (3) patients enrolled from the scene of injury vs a referral hospital; (4) patients who required early operative intervention (initial 24 hours); (5) history of vitamin K antagonist medication; (6) history of antiplatelet medication; and (7) patients who required massive transfusion (≥10 units of blood in first 24 hours) vs those did not.
Statistical Analysis
The primary intention-to-treat analysis compared 30-day mortality across the prehospital tranexamic acid and placebo groups using a 2-sided Mantel-Haenszel test adjusting for site. We estimated that enrollment of 994 individuals with complete data, using a mortality estimate of 16%,8 a 2-sided z test with pooled variance, and a 2-sided α = .05, would provide 90% power to detect a difference of 7 percentage points (16.0% vs 9.0%) in 30-day mortality between the prehospital assigned tranexamic acid and placebo groups. We assumed that a prehospital intervention would provide a robust treatment effect.
For patients missing 30-day mortality outcomes, we performed multiple imputation after confirming outcomes were missing at random (eAppendix 1 in Supplement 2). Prespecified sensitivity analyses excluded patients missing the primary outcome or assumed all missing patients survived. We computed 30-day survival curves using a Cox proportional hazards regression model with the site covariate included as a random effect. We planned to assess the effects of tranexamic acid dose and the rate of 30-day mortality compared with placebo. We expected that the effect of tranexamic acid would be modified by time to treatment and qualifying shock severity and planned secondary analyses to measure these effects via post hoc analysis.14,22 The critical level of significance for the primary analysis (P < .038) was adjusted for 2 interim analyses. All comparisons were conducted using 2-sided tests. All analyses were adjusted for clustering by site. False discovery rate correction was used to account for multiple comparisons across prespecified secondary outcomes (eAppendix 2 in Supplement 2). Analyses were performed using Stata MP software, version 15 (StataCorp).
Results
A total of 927 patients (mean [SD] age, 42 [18] years; 686 [74.0%] male) were deemed eligible for prehospital enrollment, with 460 randomized to the tranexamic acid intervention and 467 randomized to the placebo intervention. Excluding 24 patients found later to be ineligible or who withdrew their consent, the intention-to-treat study cohort comprised a total of 903 patients; there were 447 in the tranexamic acid arm and 456 in the placebo arm (Figure 1). We halted the trial early, at 93% of planned enrollment because of slower than expected enrollment and financial limitations.
Figure 1. Flow of Patients in the Study of Tranexamic Acid During Air and Ground Prehospital Transport (STAAMP) Trial.
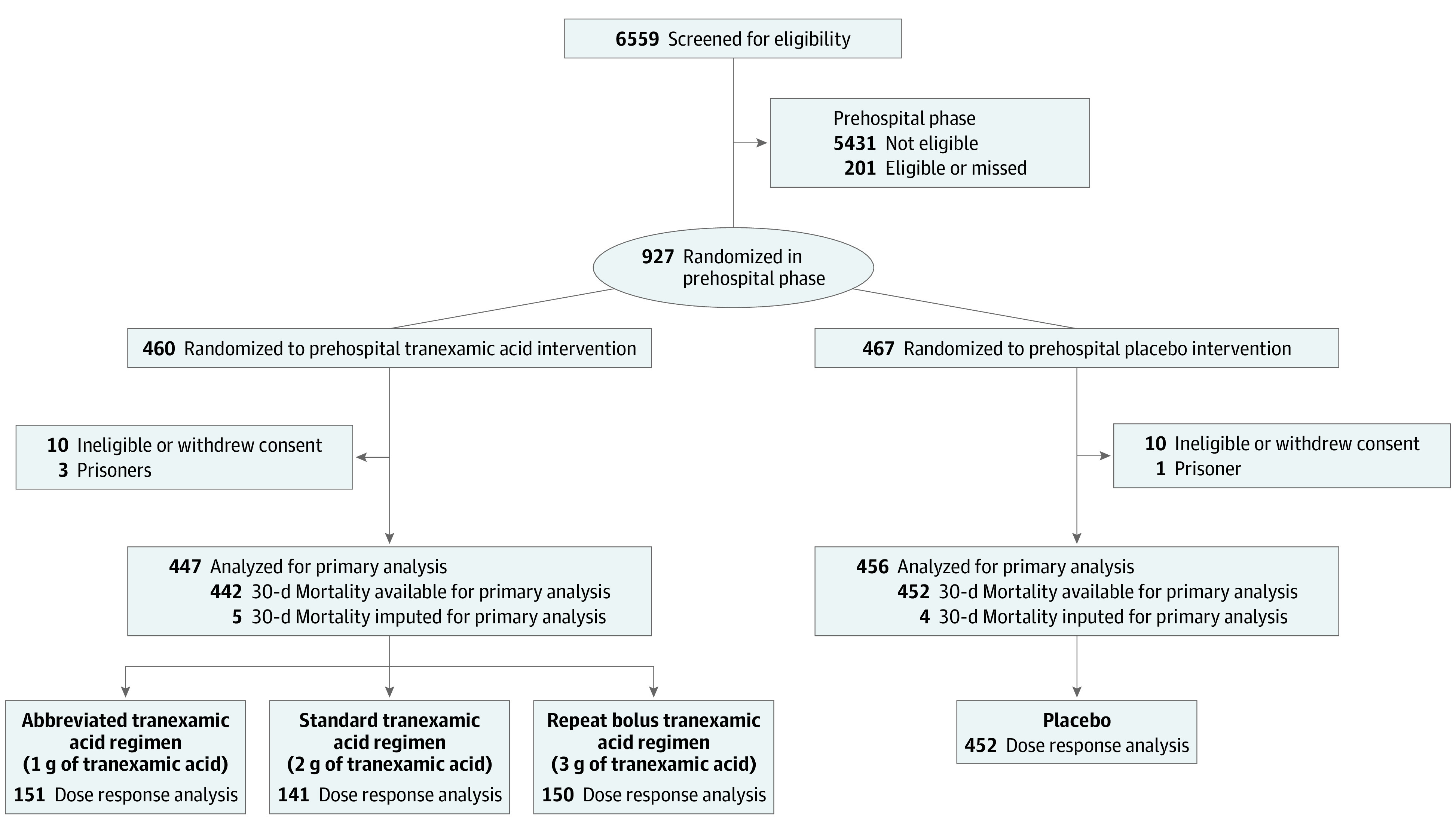
Screening, randomization, and follow-up of the study participants. Per site institutional review board requirements, data from participants who withdrew consent or were excluded based on ineligibility in certain situations could not be included for the intention-to-treat analysis. Multiple imputation was performed for the 9 participants missing the primary outcome for the primary analysis as prespecified in the study protocol. Dose response analyses were performed on those with 30-day mortality data available.
Patients had a median Injury Severity Score (eAppendix 3 in Supplement 2) of 12 (interquartile range [IQR], 5-22) and an all-cause 30-day mortality rate of 9.1% with data available. Tachycardia was the qualifying vital sign for 642 patients (71%), with 203 enrolled patients (22%) having initial prehospital hypotension (systolic blood pressure ≤90 mm Hg). A total of 311 of the 903 enrolled patients (34%) required blood component transfusion in the first 24 hours from randomization. Surgeons performed operative procedures on 406 patients (45%) in the initial 24 hours, with 260 (64%) being taken to the operating theater directly from the trauma bay (eTable 1 in Supplement 2). As expected, 30-day mortality increased with qualifying shock severity, based on initial qualifying prehospital vital signs (tachycardia alone, 6%; systolic blood pressure ≤90 mm Hg, 13%; and systolic blood pressure ≤70 mm Hg, 28%; P < .001).
Prehospital teams delivered the assigned prehospital infusion in 887 of the 903 patients (98%). For the tranexamic acid arm, 440 of the 447 patients (98%) received the full prehospital infusion. For the placebo arm, 447 of the 456 patients (98%) received the full prehospital infusion. For the tranexamic acid arms, 412 of 447 patients (92%) received full or partial B phase bolus, and 412 of 447 (92%) received full or partial C phase bolus. For the placebo arm, 423 of 456 patients (95%) received the full or partial B phase intervention, and 422 of 456 patients (93%) received full or partial C phase bolus. The placebo and tranexamic acid arms were similar in demographics, prehospital characteristics, and injury severity (Table 1).
Table 1. Patient Characteristics by Treatment Groupa.
| Characteristic | Placebo (n = 456) | Tranexamic acid (n = 447) |
|---|---|---|
| Age, mean (SD), yb | 42 (18) | 41 (17) |
| Male sexc | 341 (74.8) | 327 (73.2) |
| Race | ||
| White | 361 (79.2) | 353 (79.0) |
| African American | 40 (8.8) | 49 (11.0) |
| Asian | 3 (0.7) | 2 (0.4) |
| Other | 3 (0.7) | 2 (0.4) |
| Unknown | 49 (10.7) | 41 (9.2) |
| Hispanic ethnicity | 34 (7.5) | 24 (5.4) |
| Any blunt mechanism of injury | 389 (85.3) | 371 (83.0) |
| Fall from height | 60 (15.4) | 41 (11.1) |
| Motor vehicle collision | 203 (52.2) | 205 (55.3) |
| Motorcycle collision | 59 (15.2) | 62 (16.7) |
| Pedestrian or bicycle | 22 (5.7) | 17 (4.6) |
| Assault | 7 (1.8) | 7 (1.9) |
| Other blunt mechanism | 38 (9.8) | 39 (10.5) |
| Any penetrating mechanism of injury | 70 (15.4) | 78 (17.4) |
| Firearm | 34 (48.5) | 42 (53.8) |
| Impalement or stabbing | 26 (37.1) | 28 (35.9) |
| Other penetrating mechanism | 10 (14.3) | 8 (10.3) |
| Transported from referral hospital | 61 (13.4) | 66 (14.8) |
| Prehospital crystalloid volume, median (IQR), mL | 500 (100-1000) | 500 (125-1000) |
| Prehospital red blood cell count, ×106/μL | 52 (11.4) | 48 (10.7) |
| Initial Glasgow Coma Scale score <8 | 107 (23.5) | 89 (19.9) |
| Prehospital systolic blood pressure, median (IQR), mm Hg | 126 (87-148) | 123 (88-143) |
| Prehospital heart rate, median (IQR), min | 117 (112-124) | 118 (112-127) |
| Prehospital intubation | 120 (26.3) | 110 (24.6) |
| Prehospital CPR | 9 (2.0) | 11 (2.5) |
| Prehospital transport time, median (IQR) | 39 (30.5-49) | 39 (30-50) |
| Injury Severity Score, median (IQR)d | 11 (4-22) | 13 (5-22) |
| Head AIS score | ||
| Median (IQR) | 0 (0-2) | 0 (0-3) |
| >2 | 107 (23.5) | 116 (26.0) |
| Preinjury vitamin K antagonist | 11 (2.4) | 5 (1.1) |
| Preinjury antiplatelet medication | 49 (10.7) | 45 (10.1) |
Abbreviations: AIS, Abbreviated Injury Scale; CPR, cardiopulmonary resuscitation; IQR, interquartile range.
SI conversion factor: to convert red blood cells to ×1012 per liter, multiply by 1.
Data are presented as number (percentage) of patients unless otherwise indicated. No statistically significant differences were observed between baseline characteristics.
Continuous variables were compared with the Wilcoxon rank-sum test.
Categorical variables were compared with the Fisher exact test.
The score range was 0 to 75, with a score greater than 15 indicating major trauma.
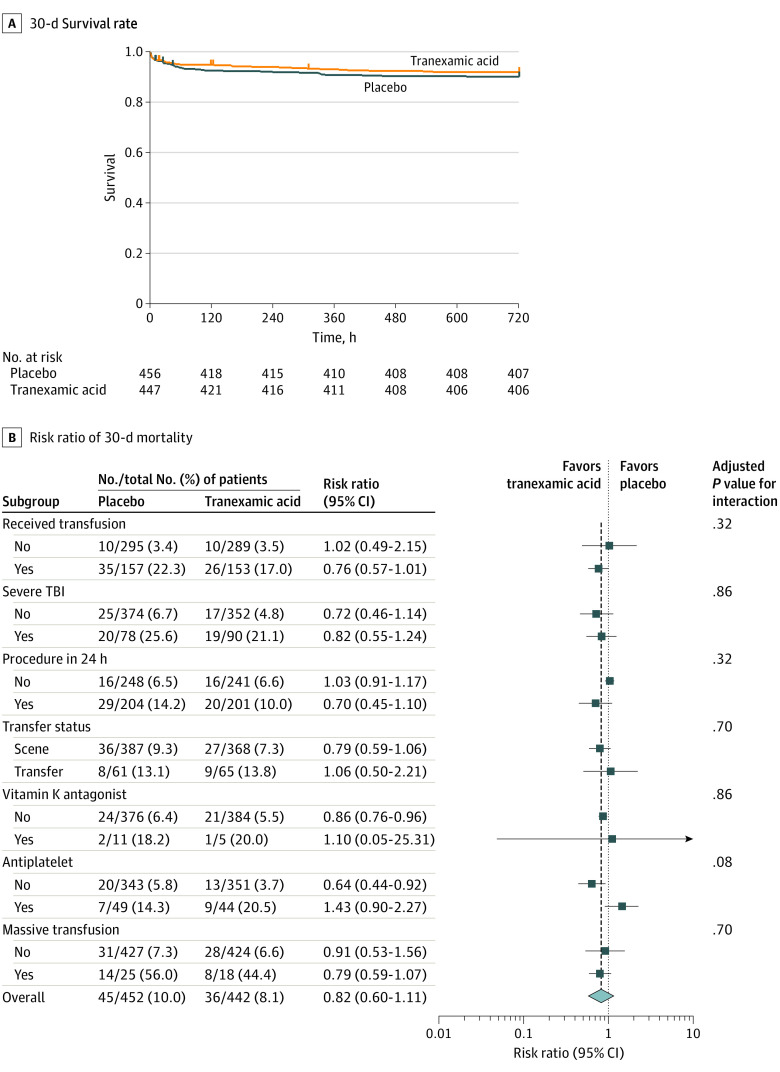
The primary outcome was available in 894 patients (99%). At 30 days after randomization, 36 deaths (8%) had occurred in the tranexamic acid intervention arm and 45 deaths (10%) in the placebo arm. After multiple imputation for the 9 patients missing the primary outcome (5 in the tranexamic acid arm and 4 in the placebo arm), patients who received tranexamic acid compared with placebo did not differ in 30-day mortality (8.1% vs 9.9%; difference, −1.8; 95% CI, −5.6% to 1.9%; P = .17). Sensitivity analysis assuming all patients with missing 30-day mortality outcomes were alive demonstrated similar results. Kaplan-Meier survival curves are shown in Figure 2A (log-rank χ2 = 0.91, P = .34). In a Cox proportional hazards regression analysis, accounting for site, assignment to the prehospital tranexamic acid group did not change the hazards of 30-day mortality (hazard ratio, 0.81; 95% CI, 0.59-1.11; P = .18).
Figure 2. Survival and Subgroup Analysis for 30-Day Mortality.
A, Kaplan-Meier estimates of 30-day survival rate among patients randomized to the prehospital tranexamic acid or placebo intervention. The time when qualifying vital signs occurred in the prehospital environment represents time zero. B, Risk ratio of 30-day mortality in the 7 prespecified subgroups. The diamond represents the point estimate of the risk ratio, and horizontal bars represent the 95% CI. The dotted vertical line represents a risk ratio of 1.0, indicating no difference in mortality between standard care and plasma groups. The dashed vertical line represents the overall treatment risk ratio in the intention-to-treat cohort for patients not missing the primary outcome of 30-day mortality. Adjusted P values are for the interaction term between each subgroup and treatment group in a logistic regression model with 30-day mortality as the outcome to determine whether there was a significantly different effect of treatment group across the levels of each subgroup on the outcome, adjusted for multiple comparisons using false discovery rate correction. TBI indicates traumatic brain injury.
Mortality in the 7 prespecified subgroups is depicted in Figure 2B. No heterogeneity of treatment effect across the subgroups (heterogeneity χ2 = 18.31, P = .15) was found.
No group differences were found in 24-hour mortality (difference, 0.15; 95% CI, −2.3 to 2.6; adjusted P = .98) or in-hospital mortality (difference, 1.1; 95% CI, −2.7 to 4.9; P = .94) (Table 2). The tranexamic acid and placebo groups had similar 6- and 24-hour blood and blood component transfusion requirements (6-hour total blood component transfusion, 0; IQR, 0-2; adjusted P = .97 for both groups). No differences were found in the incidence of pulmonary embolism (−1.3; 95% CI, −3.3 to 0.5; adjusted P = .78), deep vein thrombosis (−1.2; 95% CI, −3.3 to 0.5; adjusted P = .83), or seizures (0.4; 95% CI, −1.0 to 1.9; P = .94) across groups. After adjustment for multiple comparisons, no differences were found in the incidence of multiple organ failure (1.2; 95% CI, −2.4 to 4.7; adjusted P = .94), nosocomial infection (−5.2; 95% CI, −10.1 to −0.3; adjusted P = .75), or any other secondary outcome.
Table 2. Secondary Trial Outcomes by Treatment Groupa.
| Outcome | Tranexamic Acid (n = 447) | Placebo (n = 453) | Difference, % (95% CI) | P value | |
|---|---|---|---|---|---|
| Observedb | Adjustedc | ||||
| 24-h Mortality | 16 (3.6) | 17 (3.7) | 0.15 (−2.3 to 2.6) | .90 | .98 |
| In-hospital mortality | 37 (8.6) | 43 (9.7) | 1.1 (−2.7 to 4.9) | .58 | .94 |
| 6-h Outcomes | |||||
| Laboratory values, median (IQR) | |||||
| Total blood component transfusion, U | 0 (0 to 2) | 0 (0 to 2) | NA | .75 | .97 |
| PRBC transfusion, U | 0 (0 to 1) | 0 (0 to 1) | NA | .54 | .94 |
| Plasma transfusion, U | 0 (0 to 0) | 0 (0 to 0) | NA | .16 | .78 |
| Platelet transfusion, U | 0 (0 to 1) | 0 (0 to 1) | NA | .54 | .94 |
| Crystalloid infusion volume, mL | 1600 (600 to 3300) | 1600 (600 to 3200) | NA | .94 | .98 |
| 24-h Outcomes | |||||
| Laboratory values, median (IQR) | |||||
| Total blood component transfusion, U | 0 (0 to 2) | 0 (0 to 2) | NA | .69 | .97 |
| PRBC transfusion, U | 0 (0 to 1) | 0 (0 to 1) | NA | .47 | .94 |
| Plasma transfusion | 0 (0 to 0) | 0 (0 to 0) | NA | .11 | .78 |
| Platelet transfusion | 0 (0 to 0) | 0 (0 to 0) | NA | .98 | .98 |
| Crystalloid infusion volume, mL | 3100 (1235 to 5600) | 2750 (1282.5 to 5525) | NA | .39 | .94 |
| Lactate, median (IQR), mmol/Ld | 2.9 (1.9 to 3.9) | 2.6 (1.8 to 4.2) | NA | .74 | .97 |
| Initial presenting international normalized ratioe | |||||
| Median (IQR) | 1.1 (1 to 1.2) | 1.1 (1 to 1.2) | NA | .95 | .98 |
| >1.4 | 43 (9.6) | 49 (10.7) | 1.1 (−2.8 to 5.1) | .58 | .94 |
| Initial presenting rapid thromboelastography measurements, median (IQR) | |||||
| Activated clotting time, sf | 113 (105 to 121) | 113 (105 to 128) | NA | .48 | .94 |
| K-time, ming | 1.5 (1.2 to 2) | 1.5 (1.1 to 2) | NA | .29 | .94 |
| α-Angleh | 72.4 (68.4 to 75.7) | 73.35 (68.2 to 76.5) | NA | .19 | .78 |
| Maximal amplitudei | 61.7 (57 to 66.1) | 62.8 (57 to 67.5) | NA | .07 | .75 |
| LY30, %j | 5.45 (1.7 to 50) | 4.35 (1.7 to 50) | NA | .80 | .97 |
| Hyperfibrinolysisj | 145 (32.4) | 144 (31.6) | −0.9 (−7.0 to 5.2) | .78 | .97 |
| Multiple organ failure | 33 (7.4) | 39 (8.6) | 1.2 (−2.4 to 4.7) | .52 | .94 |
| Acute respiratory distress syndrome | 42 (9.4) | 39 (8.6) | −0.8 (−4.6 to 2.9) | .66 | .97 |
| Nosocomial infection | 88 (19.7) | 66 (14.5) | −5.2 (−10.1 to −0.3) | .04 | .75 |
| Seizure in first 24 h | 5 (1.1) | 7 (1.5) | 0.4 (−1.0 to 1.9) | .58 | .94 |
| Pulmonary embolism | 13 (2.9) | 7 (1.5) | −1.3 (−3.3 to 0.5) | .16 | .78 |
| Deep vein thrombosis | 12 (2.7) | 7 (1.5) | −1.2 (−3.3 to 0.5) | .23 | .83 |
Abbreviations: IQR, interquartile range; LY30, 30-minute fibrinolysis; NA, not applicable; PRBC, packed red blood cells.
Data are presented as number (percentage) of patients unless otherwise indicated. For all transfusion volume outcomes, the 24-hour period began at the time of enrollment or randomization in the prehospital setting. Thromboelastography measurements provide viscoelastic properties of a blood sample. Activated clotting time is the time in seconds between initiation of the test and the initial fibrin formation and is increased with factor deficiency or severe hemodilution. The α-angle is the slope of the tracing that represents the rate of clot formation, decreasing with hypofibrinogenemia or platelet deficiency. K-time is the time in minutes needed to reach 20-mm clot strength and is generally increased with hypofibrinogenemia or platelet deficiency. The maximal amplitude is the greatest amplitude of the tracing and reflects platelet contribution to clot strength. LY30 is the percent amplitude reduction at 30 minutes after the maximal amplitude and when elevated reflects a state of hyperfibrinolysis (estimated percent lysis >7.5%).
Continuous variables were compared with the Wilcoxon rank-sum test. Categorical variables were compared with the Fisher exact test.
Adjusted using Benjamini-Hochberg procedure to account for the false discovery rate with multiple comparisons. False discovery rate correction is a powerful method to ensure the probability of a type I error remains at the prespecified level across all hypotheses tested (35 tests including secondary outcomes and prespecified subgroup interactions).
Unavailable in 140 patients in the tranexamic acid group (n = 307) and 149 patients in the placebo group (n = 304).
Unavailable in 25 patients in the tranexamic acid group and 23 patients in the placebo group (n = 422; n = 433 respectively).
Unavailable in 74 patients in the tranexamic acid group (n = 373) and 58 patients in the placebo group (n = 398).
Unavailable in 45 patients in the tranexamic acid group (n = 402) and 33 patients in the placebo group (n = 423).
Unavailable in 42 patients in the tranexamic acid group (n = 405) and 26 patients in the placebo group (n = 430).
Unavailable in 44 patients in the tranexamic acid group (n = 403) and 29 patients in the placebo group (n = 427).
Unavailable in 153 patients in the tranexamic acid group (n = 294) and 156 patients in the placebo group (n = 300) and defined by an estimated percent lysis greater than 7.5%.
The number of adverse events was similar between the arms of the trial (eTable 2 in Supplement 2). No group differences were found in the incidence of arterial thrombotic complications (stroke [1 (0.2%) in the tranexamic acid group and 4 (0.9%) in the placebo group] or myocardial infarction or ischemia [1 (0.9% in the tranexamic acid group and 0 in the placebo group]) across groups. We observed 8 adverse events (4 serious) in the tranexamic acid groups and 21 adverse events (7 serious) in the placebo group.
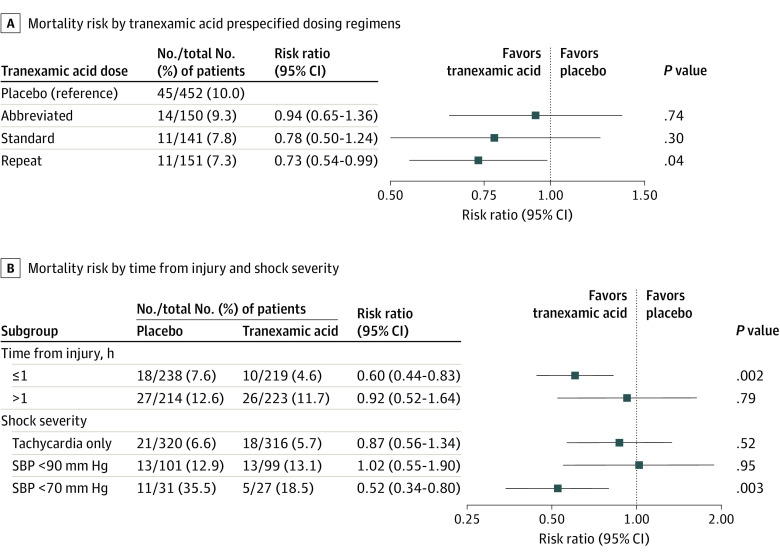
When comparing tranexamic acid dosing regimens in patients with 30-day mortality outcomes available, mortality rates were 10.0% for placebo, 9.3% for abbreviated, 7.8% for standard, and 7.3% for repeat bolus tranexamic acid groups. Among the prespecified comparisons of each tranexamic acid regimen to placebo, the repeat bolus regimen had lower 30-day mortality after adjusting for site (7.3% vs 10.0%; difference, −2.7%; 95% CI, −5.0% to −0.4%; P = .04) (Figure 3A).
Figure 3. Prespecified Tranexamic Acid Dose Response Analysis, Time to Intervention, and Shock Severity Post Hoc Subgroup Analysis for 30-Day Mortality.
A, Risk of 30-day mortality across tranexamic acid prespecified dosing regimens, accounting for site clustering. All risk ratios are in reference to the placebo group. The abbreviated dose represents a single 1-g bolus dose. The standard dose represents a 2-g dose administered as a 1-g bolus dose followed by a 1-g infusion during 8 hours. The repeat dose represents a 3-g dose administered as 2 separate 1-g boluses followed by a 1-g infusion during 8 hours. The repeat dose had lower risk of 30-day mortality than placebo group. B, Risk of 30-day mortality of the tranexamic acid group compared with placebo accounting for site clustering across post hoc subgroups for time of tranexamic acid administration from injury and shock severity based on qualifying inclusion vital signs. The dotted vertical line represents a risk ratio of 1.0 (no difference between groups). The squares represent the point estimate of the risk ratio, with the horizontal solid lines representing the 95% CIs. Time of tranexamic acid administration from injury was stratified by 1 hour or less and greater than 1 hour. The risk of 30-day mortality was lower in the tranexamic acid group when the drug was administered within 1 hour of injury. The risk of 30-day mortality was lower in the tranexamic acid group among patients in severe shock with systolic blood pressure less than 70 mm Hg based on qualifying inclusion vital signs. SBP indicates systolic blood pressure.
When comparing tranexamic acid effect stratified by time to treatment and qualifying shock severity in a post hoc comparison, a lower 30-day mortality was found when tranexamic acid was administered within 1 hour of injury (4.6% vs 7.6%; difference, −3.0%; 95% CI, −5.7% to −0.3%; P < .002) (Figure 3B). Patients who received tranexamic acid with prehospital severe shock (systolic blood pressure ≤70 mm Hg) had a lower 30-day mortality compared with the placebo group (18.5% vs 35.5%; difference, −17%; 95% CI, −25.8% to −8.1%; P < .003) (Figure 3B).
Discussion
Resuscitation strategies for injured patients at risk for hemorrhage have evolved, with patients benefiting from receiving less crystalloid and early balanced blood component therapy after arriving at definitive care sites.1,2,23 Studies7,8,22 demonstrate the survival benefits that result from administration of tranexamic acid soon after arrival to definitive trauma care sites. Most deaths from traumatic hemorrhage occur in the first hours of arrival at the trauma center, underscoring the importance of the early prehospital interventions that provide benefit.1,2,23,24 As a result, recent guidelines have been developed, which include the prehospital use of tranexamic acid after trauma because of this early time-to-treatment effect.15,16,25 However, the effectiveness and safety of tranexamic acid when provided in a pragmatic fashion in the prehospital environment remain poorly characterized.15,26,27
Among the 903 eligible patients who were enrolled in the prehospital setting, those who received prehospital tranexamic acid administration compared with placebo had a 30-day mortality rate that did not reach statistical significance. Of interest, the mortality difference for the primary analysis was similar to prior randomized trials7,8 that studied tranexamic acid after injury with larger patient populations. Despite the potential concerns that prehospital tranexamic acid administration may be associated with a greater risk of thromboembolic complications, we did not find a higher rate of pulmonary embolism, deep vein thrombosis, or arterial thrombotic complications.
A prespecified dose response analysis demonstrated a survival benefit attributable to tranexamic acid in those patients who received a repeat bolus (3 g of tranexamic acid total; 2 1-g bolus infusions during 10 minutes and 1 g during 8 hours) compared with placebo. This finding is novel in current patients at risk for hemorrhage and suggests that different dosing and administration (bolus vs infusion) regimens alter the effect of tranexamic acid on mortality and warrant further assessment with appropriately powered trials in the future. We assessed, via post hoc analysis, the association between time of intervention and the severity of shock with qualifying vital signs across tranexamic acid and placebo arms for 30-day mortality. The subgroup of patients who received tranexamic acid within 1 hour of injury had a lower 30-day mortality rate compared with the patients who received placebo. This time-to-treatment relationship is well documented for tranexamic acid after injury.7,22 This mortality difference was of a magnitude similar to a prior trial22 with tranexamic acid provided after arrival to definitive care.
An association with decreased mortality was found in the subgroup of patients who had the highest shock severity based on qualifying prehospital vital sign inclusion criteria. Although derived from a smaller subgroup, the mortality difference between the tranexamic acid arm and placebo arm is robust (18.5% vs 35.5%) in the severe shock subgroup. It may be that the effect of tranexamic acid is accentuated when provided early, in the prehospital setting, which comports with a prior study.8 Future appropriately powered trials may be needed for these subgroups.
Strengths and Limitations
This clinical trial has strengths. The design of the trial was pragmatic, with simple vital sign inclusion criteria and limited exclusion criteria. The study was blinded and randomized at the level of the patient. A wide spectrum of injury types, injury severities, and patients with variable shock severity were enrolled. The current results build on prior trials7,11,22 of tranexamic acid after injury in military and civilian settings and in those with brain injury. This analysis validates the safety of prehospital administration of tranexamic acid and potential efficacy in prespecified and post hoc subgroups and provides outcome data on dosage effects of tranexamic acid as does another recently presented prehospital trial.28 Of importance, the current trial results reveal similar rates of arterial and venous thrombotic complications and adverse events and highlight the importance of randomized clinical trial comparisons relative to adjusted observational or secondary analyses.18,29,30
Limitations of the trial include an overall low injury severity and blood transfusion requirement, which resulted in an overall low mortality rate and may be in part attributable to the range of vital sign inclusion criteria used in the prehospital phase of care. Inclusion of a diverse range of injury and shock severities improves the generalizability of the safety analyses. Because the trial was a multicenter, pragmatic study, there may be site differences in prehospital settings and in hospital management, which cannot be controlled for by adjustment. The current trial used trauma centers with robust prehospital trauma systems, and the results may not be applicable to trauma systems with different prehospital capabilities. Subgroup analyses are underpowered, and results do not establish causation. Specific mechanisms of injury, unmeasured injury and patient characteristics, prehospital transport modes, or other unmeasured variables may modify any benefit derived from prehospital tranexamic acid administration. Differences were found in the rate of enrollment across participating sites, which were accounted for in all analyses. Missing data limited the ability to draw conclusions from comparisons of laboratory and thromboelastography measurements. Given the early termination, the overall study is underpowered, and with a larger study cohort, primary outcome differences may be more apparent.
Conclusions
Prehospital administration of tranexamic acid compared with placebo did not result in a lower rate of 30-day mortality in this population. Prespecified dose response analyses demonstrate that receipt of a repeat bolus regimen (3 g of tranexamic acid) results in significantly lower 30-day mortality compared with placebo. In patients who receive prehospital tranexamic acid treatment within 1 hour of injury and in those with evidence of prehospital severe shock, post hoc subgroup analysis suggests that prehospital tranexamic acid is associated with lower 30-day mortality. The administration of prehospital tranexamic acid during air or ground transport is safe and can be provided to patients at risk for hemorrhage.
Trial Protocol
eFigure. STAAMP Randomization and Intervention Schematic
eAppendix 1. Missing Data and Multiple Imputation Procedures.
eAppendix 2. Multiple Comparison Procedures
eAppendix 3. Description of Injury Severity Score
eTable 1. Distribution of Operative Procedures Performed in Initial 24 Hours
eTable 2. Adverse Events
eReferences
Data Sharing Statement
References
- 1.Holcomb JB, Jenkins D, Rhee P, et al. . Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307-310. doi: 10.1097/TA.0b013e3180324124 [DOI] [PubMed] [Google Scholar]
- 2.Holcomb JB, Tilley BC, Baraniuk S, et al. ; PROPPR Study Group . Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi: 10.1001/jama.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disease GBD, Injury I, Prevalence C; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34(1):158-163. doi: 10.1007/s00268-009-0266-1 [DOI] [PubMed] [Google Scholar]
- 5.Rhee P, Joseph B, Pandit V, et al. . Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13-21. doi: 10.1097/SLA.0000000000000600 [DOI] [PubMed] [Google Scholar]
- 6.Murphy SL, Xu J, Kochanek KD, Arias E. Mortality in the United States, 2017. NCHS Data Brief. 2018;(328):1-8. [PubMed] [Google Scholar]
- 7.CRASH-3 Trial Collaborators Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394(10210):1713-1723. doi: 10.1016/S0140-6736(19)32233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CRASH-2 Trial Collaborators, Shakur H, Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23-32. [DOI] [PubMed] [Google Scholar]
- 9.Howard JT, Stockinger ZT, Cap AP, Bailey JA, Gross KR. Military use of tranexamic acid in combat trauma: does it matter? J Trauma Acute Care Surg. 2017;83(4):579-588. doi: 10.1097/TA.0000000000001613 [DOI] [PubMed] [Google Scholar]
- 10.Rappold JF, Pusateri AE. Tranexamic acid in remote damage control resuscitation. Transfusion. 2013;53(suppl 1):96S-99S. doi: 10.1111/trf.12042 [DOI] [PubMed] [Google Scholar]
- 11.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119. doi: 10.1001/archsurg.2011.287 [DOI] [PubMed] [Google Scholar]
- 12.Harris T, Davenport R, Mak M, Brohi K. The evolving science of trauma resuscitation. Emerg Med Clin North Am. 2018;36(1):85-106. doi: 10.1016/j.emc.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 13.Shackelford SA, Del Junco DJ, Powell-Dunford N, et al. . Association of prehospital blood product transfusion during medical evacuation of combat casualties in Afghanistan with acute and 30-day survival. JAMA. 2017;318(16):1581-1591. doi: 10.1001/jama.2017.15097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperry JL, Guyette FX, Brown JB, et al. ; PAMPer Study Group . Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379(4):315-326. doi: 10.1056/NEJMoa1802345 [DOI] [PubMed] [Google Scholar]
- 15.Napolitano LM. Prehospital tranexamic acid: what is the current evidence? Trauma Surg Acute Care Open. 2017;2(1):e000056. doi: 10.1136/tsaco-2016-000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudreau RM, Deshpande KK, Day GM, et al. . Prehospital tranexamic acid administration during aeromedical transport after injury. J Surg Res. 2019;233:132-138. doi: 10.1016/j.jss.2018.07.074 [DOI] [PubMed] [Google Scholar]
- 17.Fisher AD, Carius BM, April MD, Naylor JF, Maddry JK, Schauer SG. An analysis of adherence to tactical combat casualty care guidelines for the administration of tranexamic acid. J Emerg Med. 2019;57(5):646-652. doi: 10.1016/j.jemermed.2019.08.027 [DOI] [PubMed] [Google Scholar]
- 18.Myers SP, Kutcher ME, Rosengart MR, et al. . Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism. J Trauma Acute Care Surg. 2019;86(1):20-27. doi: 10.1097/TA.0000000000002061 [DOI] [PubMed] [Google Scholar]
- 19.Valle EJ, Allen CJ, Van Haren RM, et al. . Do all trauma patients benefit from tranexamic acid? J Trauma Acute Care Surg. 2014;76(6):1373-1378. doi: 10.1097/TA.0000000000000242 [DOI] [PubMed] [Google Scholar]
- 20.Brown JB, Neal MD, Guyette FX, et al. . Design of the Study of Tranexamic Acid during Air Medical Prehospital Transport (STAAMP) trial: addressing the knowledge gaps. Prehosp Emerg Care. 2015;19(1):79-86. doi: 10.3109/10903127.2014.936635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services ; US Food and Drug Administration. Guidance for institutional review boards clinical investigators and sponsors: exception from informed consent requirements for emergency research. 2013. Accessed December 21, 2017. https://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM249673.pdf
- 22.CRASH-2 Trial collaborators, Roberts I, Shakur H, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011;377(9771):1096-1101, e1091-e1092. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez EA, Moore FA, Holcomb JB, et al. . Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112-119. doi: 10.1097/01.ta.0000250497.08101.8b [DOI] [PubMed] [Google Scholar]
- 24.Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC; PROPPR Study Group . Earlier endpoints are required for hemorrhagic shock trials among severely injured patients. Shock. 2017;47(5):567-573. doi: 10.1097/SHK.0000000000000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huebner BR, Dorlac WC, Cribari C. Tranexamic acid use in prehospital uncontrolled hemorrhage. Wilderness Environ Med. 2017;28(2S):S50-S60. doi: 10.1016/j.wem.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napolitano LM, Cohen MJ, Cotton BA, Schreiber MA, Moore EE. Tranexamic acid in trauma: how should we use it? J Trauma Acute Care Surg. 2013;74(6):1575-1586. doi: 10.1097/TA.0b013e318292cc54 [DOI] [PubMed] [Google Scholar]
- 27.Pusateri AE, Weiskopf RB, Bebarta V, et al. ; US DoD Hemorrhage and Resuscitation Research and Development Steering Committee . Tranexamic acid and trauma: current status and knowledge gaps with recommended research priorities. Shock. 2013;39(2):121-126. doi: 10.1097/SHK.0b013e318280409a [DOI] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov Prehospital Tranexamic Acid Use for Traumatic Brain Injury (TXA). NCT01990768. Accessed February 10, 2020. https://clinicaltrials.gov/ct2/show/results/NCT01990768
- 29.Johnston LR, Rodriguez CJ, Elster EA, Bradley MJ. Evaluation of military use of tranexamic acid and associated thromboembolic events. JAMA Surg. 2018;153(2):169-175. doi: 10.1001/jamasurg.2017.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore HB, Moore EE, Huebner BR, et al. . Tranexamic acid is associated with increased mortality in patients with physiological fibrinolysis. J Surg Res. 2017;220:438-443. doi: 10.1016/j.jss.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. STAAMP Randomization and Intervention Schematic
eAppendix 1. Missing Data and Multiple Imputation Procedures.
eAppendix 2. Multiple Comparison Procedures
eAppendix 3. Description of Injury Severity Score
eTable 1. Distribution of Operative Procedures Performed in Initial 24 Hours
eTable 2. Adverse Events
eReferences
Data Sharing Statement