Abstract
Background
Cardiac involvement with COVID-19 is increasingly being recognised. Clinical characteristics and outcomes of patients with COVID-19 complicated by secondary Takotsubo cardiomyopathy (TC) is poorly understood.
Methods
This retrospective case series was conducted between March and April 2020 at four hospitals of Steward Health Care Network of Massachusetts, USA. Seven patients out of 169 who had echocardiogram were identified to have features of TC. Demographic, clinical, laboratory, management and outcome were gathered from their electronic medical records. We also reviewed all the published cases of COVID-19 and TC in the literature to recognise their common clinical characteristics, risk factors and outcomes.
Results
In our series of seven patients, three typical, two inverted, one biventricular and one global TC were recognised. Three were females and four were males. The mean age was 71±11 years. In-hospital death was observed in 57% of patients. Patients who belonged to the high-risk group and had high-risk echocardiographic features in our series had a 100% mortality rate.
Conclusions
COVID-19 complicated by TC has a high mortality rate. Early identification of patients with COVID-19 who are at higher risk for developing secondary TC is important for the prevention of complications, and thus improved outcomes.
Keywords: heart failure treatment, left ventricular assist device, echocardiography, stress
Key questions.
What is already known about this subject?
Clinicians globally have reported an increased incidence of Takotsubo cardiomyopathy (TC) with COVID-19 pneumonia. However, very less is known about the characteristics and outcome of secondary TC associated with COVID-19.
What does this study add?
To the best of our knowledge, this is the most extensive series of COVID-19-associated TC cases described in the literature. Our study details the clinical course and outcome of TC associated with COVID-19 pneumonia. It describes typical and atypical variants of TC associated with SARS-CoV-2 infection. Literature review of cases of TC with COVID-19 contributes to a better understanding of TC associated with SARS-CoV-2 infection.
How might this impact on clinical practice?
The development of secondary TC in COVID-19 is multifactorial. The exact role of SARS-CoV-2 in TC development is yet to be determined. Our study serves to enhance the understanding of secondary TC associated with COVID-19 pneumonia. Mortality of secondary TC with COVID-19 is high, and early diagnosis and appropriate management are imperative to improve outcomes.
Introduction
Takotsubo cardiomyopathy (TC) is a clinical syndrome characterised by an acute and transient left ventricular (LV) dysfunction often related to an acute physical or emotionally stressful event.1 COVID-19 is a clinical syndrome caused by SARS-CoV-2. Although respiratory disease is the major clinical presentation of COVID-19 infection, cardiac involvement is now increasingly being recognised. Secondary TC complicating COVID-19 pneumonia is rare. Here we present a series of seven patients hospitalised with COVID-19 pneumonia who subsequently developed secondary TC. Furthermore, we review all the published cases of COVID-19 and TC in the literature to recognise their common clinical characteristics, risk factors and outcomes.
Materials and methods
We included all adult patients (>18 years) with laboratory-confirmed COVID-19 admitted to four hospitals of Steward Health Care Network in Massachusetts between 22 March 2020 and 30 April 2020. A confirmed case of COVID-19 was defined as a positive nasopharyngeal swab for SARS-CoV-2 by reverse transcriptase PCR assay. The study was approved by the institutional review board.
Data collection
All patients with a transthoracic echocardiogram (TTE) performed during their hospital stay were included in the study. One hundred and sixty-nine patients underwent TTE between 22 March 2020 and 30 April 2020, in four hospitals, of which seven (4.14%) patients had features consistent with TC. Their clinical, laboratory and ECG data were obtained from their electronic medical records. These seven patients were followed through their hospital course to determine their outcome.
Statistical methods
The data from this descriptive study are presented as counts, percentages, mean and SD. SPSS V.16 was used for the descriptive analysis. The results of the variables are presented in the tables.
Results
In our series of seven patients, 43% (three) were females and 57% (four) were males. Two of the four males were under the age of 60 years, while the rest of the patients in our series were over 70 years of age. The mean age was 71±11 years. Hypertension (HTN, 85.7%), diabetes mellitus (DM, 71.4%), hyperlipidaemia (HLD, 100%), atrial fibrillation (AF, 57%) and previous embolic strokes (57%) were the most commonly observed comorbidities in our patient series. Except for one patient, who was provided with comfort measures, all others required intubation and pressor support, while none of the patients required continuous venovenous haemofiltration or dialysis for acute kidney injury. In 85.7% (six) patients, TTE was performed in the first week of hospitalisation. 85.7% (six) patients had elevated troponins, 75% (five) had an elevation in NT-pro-BNP and 100% had ECG changes at the time of TTE. Cardiac biomarkers, inflammatory markers, ECG changes and the hospital day of diagnosis of TC are detailed in table 1. 85.7% (six) patients had an ejection fraction (EF) ≤45%, 57% (four) had right ventricular (RV) involvement and 57% (four) had moderate-severe mitral regurgitation (table 2).
Table 1.
Patient characteristics and outcome in our series of seven patients with TC secondary to COVID-19
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Age | 71 | 78 | 70 | 78 | 88 | 58 | 56 |
| Gender | Female | Male | Female | Female | Male | Male | Male |
| Presentation | Cough, myalgia, lethargy | AMS, fever, urinary incontinence | SOB | Fever, cough, SOB | SOB, lethargy, worsening hypoxia | SOB, DOE | SOB and fever |
| History | DM, HLD, HTN | DM, HLD, HTN, CVA, AF | DM, HTN, HLD | DM, HLD, HTN, CVA, AF | DM, HTN, HLD, CVA, AF, CAD, HFrEF, CRF | HLD | Schizophrenia HTN AF, stroke, HLD |
| Intubation | Yes | Yes | Yes | No | Yes | Yes | Yes |
| PaO2/FiO2 | 64.7 | 242 | 82 | 75 | 279 | 138 | 124 |
| Vasopressors | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Peak leucocyte count (x103/μL) (reference 4.5–11.0) |
12.1 | 11.1 | 15.5 | 13.5 | 28.1 | 28 | 10.5 |
| Troponin (ng/mL) (reference <0.03) | 3.77 | 0.3 | <0.01 | 0.03 | 0.2 | 0.22 | 0.12 |
| CPK (U/L) (reference 39–397) |
– | 1128 | 301 | 351 | 43 | 135 | 1959 |
| NT-pro-BNP (pg/mL) (reference 0–900) |
954 | 1674 | 788 | 42 837 | 46 568 | 86 | 1226 |
| CRP (mg/dL) (reference <0.5) |
26.1 | 23.44 | 26.70 | 28.10 | 1.30 | 18.43 | 6 |
| Ferritin (ng/mL) (reference 30–400) |
– | 2787 | 258 | 9445 | 1926 | 684 352 | 4809 |
| D-Dimer quant (μg/mL FEU) (reference <0.5) |
2.95 | 4.06 | 16.28 | >20 | 2 | >20 | 10.27 |
| LDH (U/L) (reference 102–266) |
497 | 945 | 328 | 1224 | 344 | 11 403 | 356 |
| ECG changes | Atrial flutter RVR with diffuse ST elevations (figure 3) | AF with RVR, diffuse deep T-wave inversions (figure 4) | Sinus rhythm with diffuse ST-T changes (figure 5) |
Sinus rhythm with deep T-wave inversions (figure 6) |
AF, with diffuse ST-T changes (figure 7) |
Sinus tachycardia with PACs and T-wave inversions (figure 8) |
Sinus tachycardia with diffuse ST-T changes (figure 9) |
| Hospital day TTE done | 1 | 4 | 3 | 7 | 2 | 20 | 3 |
| Ejection fraction | 15% | 53% | 45% | 20% | 30% | 40% | 45% |
| Variant of TC | Typical | Biventricular | Reverse | Typical | Global with apical cap sparing | Reverse | Typical |
| Hydroxychloroquine/azithromycin use | No | No | Yes | Yes | No | Yes | No |
| Length of hospital stay | 2 | 16 | 25 | 12 | 8 | 44 | 17 |
| ICU days | 2 | 10 | 24 | 0 | 4 | 39 | 15 |
| Complications | AKI, shock liver, AF RVR | AKI recovered | ARDS, chronic respiratory failure | ARDS Shock |
Bilateral pleural effusion s/p thoracentesis, AKI, NSVT | Bilateral pneumothorax s/p chest tube placement, transient transaminitis | AKI, metabolic encephalopathy |
| Outcome | Death | SNF | LTAC | Death | Recovered cardiac function but died secondary to other complications of COVID-19 | Recovered cardiac function LTAC |
Recovered cardiac function Death |
AF, atrial fibrillation; AKI, acute kidney injury; AMS, altered mental status; ARDS, acute respiratory distress syndrome; CAD, coronary artery disease; CPK, creatine phospho kinase; CRF, chronic renal failure; CRP, C reactive protein; CVA, cerebrovascular accident; DM, diabetes mellitus; DOE, dyspnoea on exertion; F, female; FEU, fibrinogen equivalent units; FiO2, fractional inspired oxygen; HFrEF, heart failure with reduced ejection fraction; HLD, hyperlipidaemia; HTN, hypertension; ICU, intensive care unit; LDH, lactate dehydrogenase; LTAC, long-term acute care; M, male; NSVT, non-sustained ventricular tachycardia; PAC, premature atrial contractions; PaO2, arterial oxygen pressure; RVR, rapid ventricular rate; SNF, skilled nursing facility; SOB, shortness of breath; s/p, status post; TC, Takotsubo cardiomyopathy; TTE, transthoracic echocardiogram.
Table 2.
High-risk echocardiographic features in our series of seven patients with COVID-19 and Takotsubo cardiomyopathy
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
| Ejection fraction | 15% | 53% | 45% | 20% | 30% | 40% | 45% |
| Mitral regurgitation >Mild |
Yes | No | No | Yes | Yes | No | Yes |
| Right ventricular dysfunction | Yes | No | No | Yes | Yes | No | Yes |
| Tachycardia >110 bpm at the time of examination | Yes | No | No | Yes | Yes | No | Yes |
| Outcome | Death | Discharge to SNF | Long term acute care hospital | Death | Long term acute care hospital | Death |
bpm, beats per minute; SNF, skilled nursing facility.
Figure 3.
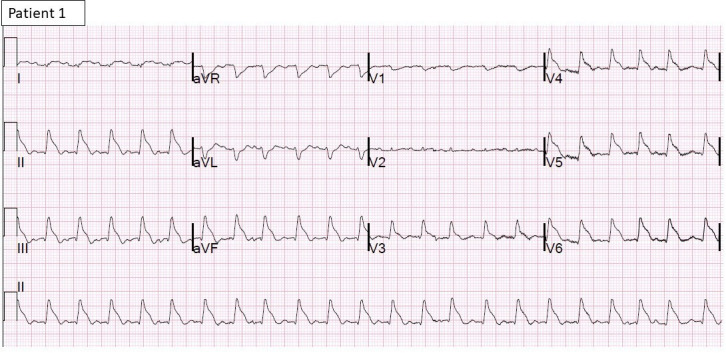
12-lead ECG of patient 1 shows atrial flutter with rapid ventricular rate and diffuse ST-T elevations.
Figure 4.

12-lead ECG of patient 2 shows atrial fibrillation with rapid ventricular rate and diffuse deep T inversions.
Figure 5.
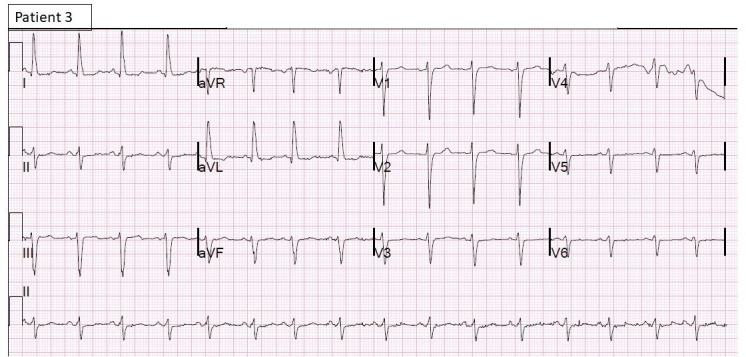
12-lead ECG of patient 3 showing sinus rhythm with diffuse ST-T changes.
Figure 6.
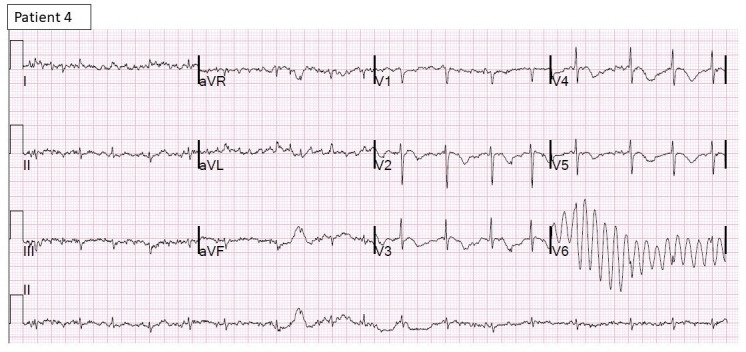
12-lead ECG of patient 4 shows sinus rhythm with deep T inversions.
Figure 7.
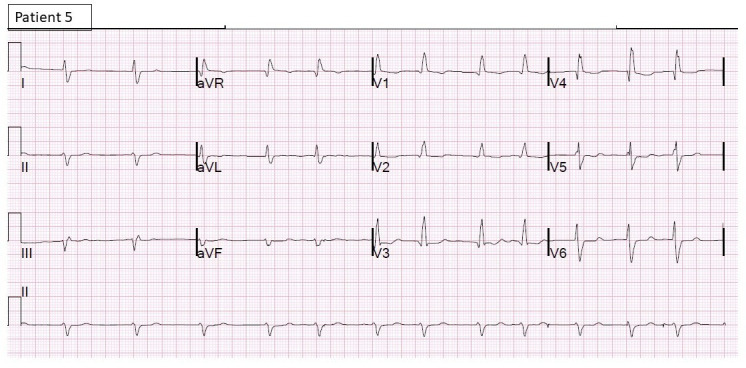
12-lead ECG of patient 5 shows atrial fibrillation with rapid ventricular rate with diffuse ST-T changes.
Figure 8.
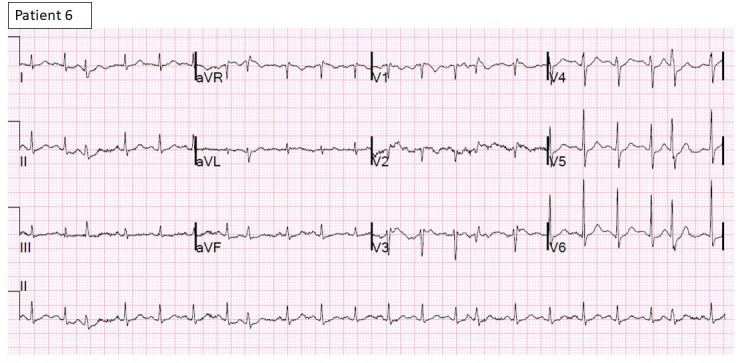
12-lead ECG of patient 6 shows sinus tachycardia with PACs and T inversions.
Figure 9.
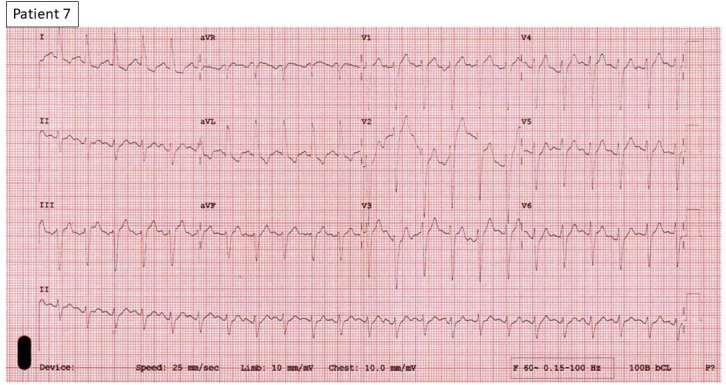
12-Lead ECG of patient 7 shows sinus tachycardia with diffuse ST-T changes.
Out of seven patients in our series, 43% (three) received treatment with hydroxychloroquine and azithromycin for COVID-19. Of the three, two of them died in the hospital. All patients were anticoagulated with heparin drip as per the hospital protocol. None received treatment with steroids. All patients who survived (43%) had a complicated hospital course with elevated inflammatory markers and multiorgan failure. In this series of seven patients with TC and COVID-19, in-hospital mortality was observed in 57% (four) patients, two were discharged to long-term acute care with tracheostomy and percutaneous endoscopic grastrostomy tube placement and one was discharged to short-term rehabilitation facility after successful extubation.
In the review of literature of published cases on COVID-19 and TC, mean age of the population was 75 (±14) years. 62.5% were females, 75% had HTN, 38% were diabetic and 38% (three) had HLD. 100% of patients had troponin elevation. Six out of eight cases reported ECG findings and all had ECG changes with the diagnosis of TC. Mechanical ventilatory support was required in 38% of patients; ionotropic support was used in two patients (one patient died and the other in hospital at the time of publication of case). Out of eight published cases, only six had outcomes reported. The mortality rate was 16.7% (table 3), which refers to one out of six patients.
Table 3.
Literature review of cases of secondary TC in patients with COVID-19
| Case by Minhas et al5 | Case by Meyer et al6 |
Case by Solano-López et al7 | Case by Nguyen et al8 |
Case by Roca et al9 |
Case series by Pasqualetto et al10 | |||
| Patient 1 | Patient 2 | Patient 3 | ||||||
| Age in years | 58 | 83 | 50 | 71 | 87 | 84 | 85 | 81 |
| Gender | Female | Female | Male | Female | Female | Male | Female | Male |
| Presenting symptoms | Cough, fatigue, fever, diarrhoea for 5 days | Chest pain, dry cough and mild dyspnoea | Cough, dyspnoea and fever for 8 days | Fainting | Fever, fatigue, dyspnoea | Fever, cough, dyspnoea and atypical chest pain for ~10 days prior to presentation | ||
| Comorbidities | HTN, DM, HLD | HTN | Benign mediastinal tumour since childhood | HTN HLD NPH s/p VP shunt |
h/o breast cancer | HTN, DM | HTN | HTN, DM |
| ET intubation | Yes | No | No | Yes | No | No | Yes | No |
| PaO2/FiO2 | NA | NA | NA | NA | 226 | >300 | <100 | >300 |
| Troponin | NA | 1142 ng/L (ref <14 ng/L) | 64 ng/mL | 412.7 ng/L (ref <14) | 5318 ng/L (<6) | 70 ng/mL* | 647 ng/mL* | 621 ng/mL* |
| NT-pro-BNP | 11.02 ng/mL | NA | 790 pg/mL | NA | NA | 1381 ng/mL* | 3000 ng/mL* | 12586 ng/mL* |
| CRP | NA | NA | NA | NA | 205.6 (n<5) | 168.8 mg/L* | 170.9 mg/L* | 190.4 mg/L* |
| D-Dimer | NA | NA | NA | NA | NA | 1381 ng/mL | 1227 ng/mL | 3340 ng/mL |
| Procalcitonin | NA | NA | NA | NA | NA | 0.35 ng/mL* | 3.01 ng/mL* | 0.07 ng/mL* |
| ECG | 1 mm upsloping ST elevation in lead 1 and aVL, diffuse PR depression an ST-T changes | Diffuse ST elevation (<1 mm) and T inversions | 2 mm inferolateral ST elevation | Sinus rhythm with prolonged QT | Negative T waves and repolarisation alterations | Deep T-inversions in all leads | NA | NA |
| Variant of TC | Typical | Typical | Reverse | Median | Typical | Typical | Unclear probably typical | Typical |
| EF | 20% | NA | NA | NA | 48% | 53% | 30% | 42% |
| Coronary angiogram | Not performed | Non-significant lesions | Negative | Proximal LAD and D1 significant lesion requiring intervention | Not performed | Negative† | Normal coronary anatomy on autopsy | Negative† |
| In-hospital treatment | Dobutamine | NA | NA | NA | Ceftriaxone, azithromycin, methylprednisone | ASA, fondaparinux subcutaneous, nitroglycerin intravenous, metoprolol intravenous | ASA, clopidogrel, fondaparinux subcutaneous, ionotropic support | ASA, fondaparinux subcutaneous, metoprolol intravenous |
| Outcome | Resolution of TC but worsening ARDS‡ | Near complete recovery of LV function at the time of discharge | Improvement in LV function at the time of discharge | NA | Discharge home | Discharge home | Death | Discharge home |
*Peak levels reported.
†A coronary angiogram done after resolution of initial COVID-19 pneumonia.
‡Patient in-hospital at the time of publication of the case. The final outcome is unknown.
AF, atrial fibrillation; ARDS, acute respiratory distress syndrome; ASA, aspirin; CRP, C reactive protein; D1, first diagonal; DM, diabetes mellitus; EF, ejection fraction; ET, endotracheal; FiO2, fractional inspired oxygen; HLD, hyperlipidaemia; HTN, hypertension; LAD, left anterior descending; LV, left ventricle; NA, not available; NPH s/p VP, normal pressure hydrocephalus status post ventriculoperitoneal shunt; PaO2, arterial oxygen pressure; RVR, rapid ventricular rate; TC, Takotsubo cardiomyopathy; TTE, transthoracic echocardiography.
Discussion
TC, also known as stress cardiomyopathy or broken heart syndrome, was first described in Japan in 1990. It is a clinical syndrome characterised by an acute and transient LV dysfunction often related to an acute physical or emotional stressful event.1 TC has previously been described in patients with other viral infections, including influenza A2 and influenza B3 in its classic form, as well as in its variant forms.4 There are only a few reported cases of TC described in literature among patients with COVID-19.4–10 In this case series, we describe the clinical characteristics of seven patients who initially presented with COVID-19 infection and subsequently developed TC.
Diagnosis of TC is based on clinical setting, ECG abnormalities, significant elevation of N-terminal-pro-Beta natriuretic peptide (NT-pro-BNP) and echocardiogram,11–13 which was the case for all of our patients (table 1). A coronary angiogram is performed under normal circumstances. However, given active COVID-19 illness and the risk of exposure to infection of healthcare workers, we deferred coronary angiography in our patients and based the diagnosis on the above-mentioned well-established parameters.11–13
Non-coronary distribution of LV wall motion abnormities is the hallmark of TC. The most commonly encountered pattern is apical hypokinesia/akinesia/dyskinesia with basal hyperkinesis (typical variant). Less than 5% of patients develop the reverse pattern called inverted or reverse TC characterised by basal hypokinesis/akinesis with apical hyperkinesia; other rare patterns like global TC, global with apical cap sparing, midventricular and focal have also been described.12 13 In the literature review of eight described cases of COVID-19 TC (table 3), five had typical TC, one reverse variant, one median TC and one patient had unclear variant of TC. In our series of seven patients, three were typical TC and two patients had an inverted or reverse variant of TC. One of our patients (patient 2) also had RV TC in addition to typical LV TC, which represents a rare variant of biventricular TC. Ours is the first report on biventricular TC with SARS-CoV-2 infection. In addition, patient 5 in our series had a global TC variant with apical cap sparing, with complete recovery of his LV function to baseline within 2 weeks. This is the first description of global TC with SARS-CoV-2 infection.
The exact mechanism of TC is unclear; however, several hypotheses have been proposed. The most widely accepted hypothesis is related to complex systemic responses to acute, severe stress and the response of the cardiovascular system to sudden surges in endogenous or exogenously administered catecholamines. In primary TC, acute cardiac symptoms resulting from emotional or physical stress are the main reason for seeking medical attention. In contrast, secondary TC develops in patients who are hospitalised for other reasons. The intense sympathetic stimulation in these patients caused by the primary condition or its treatment results in the development of TC.12 13 Some of the commonly identified triggers for the development of secondary TC are respiratory conditions, intubation, medication use, epinephrine use, anxiety and beta-blocker withdrawal.14–17 Many of these triggers are present in patients with severe COVID-19 pneumonia. This could possibly be the explanation for a higher number of reported cases of TC with SARS-CoV-2 compared with other similar respiratory viruses.8 9
Previous observational studies have shown that patients with secondary TC are older, males are more commonly affected than females and they have higher rates of HTN, DM, dyslipidaemia, cerebrovascular disease and cardiac arrhythmia.12 14–17 The same trend was also observed in our series. A similar pattern was also seen in eight cases of TC and COVID-19 described in the literature (table 3). Furthermore, increased risk of life-threatening complications and a 10-fold higher mortality rate as compared with primary TC were also observed in these studies.14 15 Hence, early identification of patients with COVID-19 who are at higher risk for developing secondary TC is important for appropriate management and prevention of complications, and thus improved outcomes.
Certain risk factors can be identified from our series and the study of cases described in the literature that predispose patients with COVID-19 to secondary TC. These are older age, presence of HTN, DM, HLD, prior stroke, AF and psychiatric illness, hypoxia and severe COVID-19 pneumonia requiring mechanical ventilatory support. Patients with the above-mentioned risk factors should be considered for closer monitoring on the telemetry floor, and development of new tachycardia or hypotension should be further investigated with ECG and blood work for cardiac biomarkers (troponin and NT-pro-BNP). Any new ECG changes or abnormalities in cardiac biomarkers should be followed by a TTE (figure 1).
Figure 1.
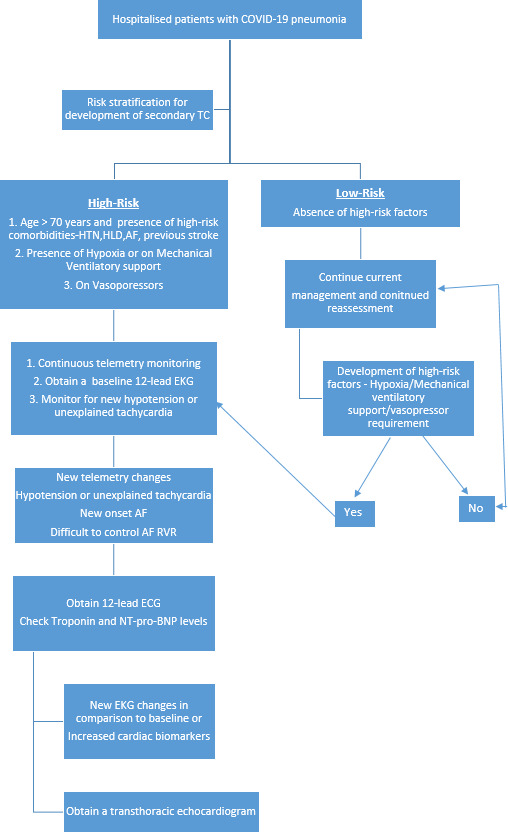
Approach to hospitalised patients with COVID-19 pneumonia who are at risk for secondary TC. AF, atrial fibrillation; HLD, hyperlipidaemia; HTN, hypertension; RVR, rapid ventricular rate; TC, Takotsubo cardiomyopathy.
Patients with secondary TC are more likely to experience cardiogenic shock, respiratory failure requiring mechanical ventilatory support and coagulation disorder.14–18 In our case series, all patients were diagnosed with TC around the time of intubation. It is difficult to ascertain if the deterioration of respiratory status was secondary to TC complicating COVID-19 or, more likely, TC was caused by worsening of COVID-19 pneumonia. Previous studies on secondary TC indicate that males have a higher rate of complications and in-hospital mortality.16 17. However, in our case series of seven patients with COVID-19 complicated by TC, death was observed in two out of four males and two out of three females. Furthermore, complications and mortality rates in secondary TC was found to be higher in older patients (>70 years), lower blood pressure (<110 mm Hg), lower EF (<45%), presence of RV involvement and mitral regurgitation.12 19 20 Three out of four patients who died in our series had EF less than 45% and older (>70 years). All four patients who died in our series had mitral regurgitation, RV involvement and required more than one pressor for the management of their hypotension (table 2). From the literature review of cases, the person who died was also older, required ionotropic support and had EF <40%.
There are no randomised controlled trials or guidelines on the management of patients with secondary TC. Management of these patients is generally supportive. However, after careful review of patients from our series and of published available cases of secondary TC complicating COVID-19, we propose dividing these patients into two groups (figure 2):
Figure 2.
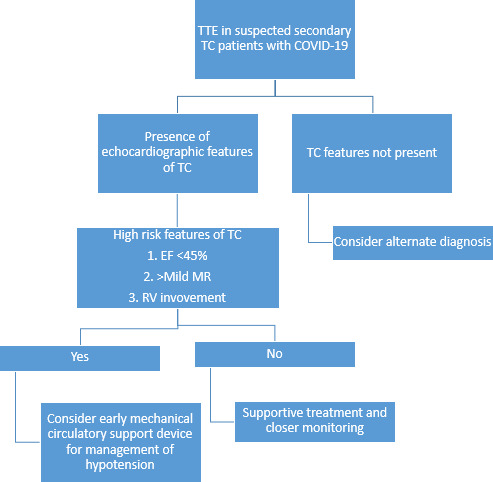
Proposed approach to patients with features of TC on TTE. EF, ejection fraction; MR, magnetic resonance; RV, right ventricle; TC, Takotsubo cardiomyopathy; TTE, transthoracic echocardiogram.
Those who are at high risk for complications, as indicated by their advanced age (>70 years), comorbidities (HTN, DM, HLD, previous stroke and psychiatric illness), severe COVID-19 pneumonia, requirement for mechanical ventilation, hypotension, lower EF (<45%), presence of moderate-severe mitral regurgitation and RV involvement.
Lower risk group: those who do not have above-mentioned high-risk features.
Lower risk groups can be managed with supportive care. However, higher risk groups may require more aggressive treatment with mechanical circulatory support devices for the management of cardiogenic shock,12 rather than traditional approach with inotropes and vasopressors which can worsen TC leading to poor outcome.12 19 21 22 Patients who belonged to high-risk group in our series of seven patients from echocardiographic features had a 100% mortality rate (table 2).
We recognised three patterns of outcome of patients with COVID-19 pneumonia complicated by secondary TC:
In-hospital death secondary to low output state likely due to TC complicating COVID-19. This was observed in four out of seven patients in our series.
Recovery of LV function, with death due to multiorgan failure secondary to COVID-19 disease. This was observed in one patient in our series.
Recovery with or without sequelae: two patients recovered with sequelae and one without.
SARS-CoV-2 is now known to affect the myocardium. Studies from Wuhan, China, indicate that patients who develop cardiac injury manifested by elevation of high-sensitivity troponin I (TnI) levels have higher overall mortality, with rates as high as 59.6%.23 24 However, not all patients who have elevation in their cardiac biomarkers have viral myocarditis, but it can be due to the development of secondary TC. The exact role of SARS-CoV-2 in TC development has yet to be determined. Possible mechanisms could be an interaction of viral spike protein with ACE2 receptors in the heart, procoagulant state created by the virus, direct myocardial damage, endothelial injury and microvascular dysfunction. Further research is required to determine the role of viral interaction with cardiac ACE2 receptors and the development of cardiomyopathy in patients with COVID-19. Nevertheless, the diagnosis of COVID-19 and the state of being in a pandemic itself produces a huge emotional stress in patients, which predisposes them to develop TC.
We believe that the development of TC in patients with COVID-19 is multifactorial in nature and starts from being in a pandemic state, the stress associated with a COVID-19 diagnosis, hypoxia secondary to COVID-19 pneumonia and intense inflammatory state caused by the virus with a release of the cytokine storm. These characteristics along with the presence of predisposing factors, such as DM, intubation and use of vasopressors could trigger TC in patients with COVID-19.
To the best of our knowledge, this is the largest series of COVID-19-associated TC cases described in the literature. A literature review of the available cases suggested a higher rate of recovery of cardiac function and lower mortality rate than in our series. This is mainly attributable to the severity of cases in our cohort. However, more studies are required to understand the role of SARS-CoV-2 in the development of TC.
Limitations
The inability to completely rule out coronary artery disease was a limitation in our case series. However, three of our patients had echocardiograms documenting the recovery of cardiac function, and one had had a recent cardiac catheterisation with no evidence of epicardial coronary artery disease.
The proposed algorithms are based on our literature review of studies on secondary TC and not validated for secondary TC in patients with COVID-19 pneumonia. This is meant to serve just as a guide for clinicians.
Conclusions
The development of secondary TC in COVID-19 is multifactorial. Exact role of SARS-CoV-2 in TC development is yet to be determined. Possible mechanisms could be an interaction of viral spike protein with ACE2 receptors in the heart, procoagulant state created by the virus, direct myocardial damage, endothelial injury and microvascular dysfunction. Mortality of patients with secondary TC with COVID-19 is high, and early diagnosis and appropriate management are imperative to improve outcomes (figures 1 and 2).
We report the first case of biventricular and global TC with apical sparing variant TC with SARS-CoV-2 infection.
openhrt-2020-001360supp001.mp4 (71.7MB, mp4)
openhrt-2020-001360supp002.pdf (17.6KB, pdf)
Acknowledgments
The authors thank the faculty involved in the Division of Cardiovascular Medicine at St. Elizabeth's Medical Center, Tufts University School of Medicine.
Footnotes
Twitter: @shrutihegde16
Contributors: All authors have contributed significantly to the content of the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. All relevant data are included in the article.
References
- 1.Sato H, Tateishi H, Uchida T. Takotsubo-type cardiomyopathy due to multivessel spasm : Kodama K, Haze K, Hon M, Clinical aspect of myocardial injury: from ischemia to heart failure. Tokyo, Japan: Kagakuhyouronsha, 1990: 56–64. [Google Scholar]
- 2.Buzon J, Roignot O, Lemoine S, et al. Takotsubo cardiomyopathy triggered by influenza A virus. Intern Med 2015;54:2017–9. 10.2169/internalmedicine.54.3606 [DOI] [PubMed] [Google Scholar]
- 3.Elikowski W, Małek-Elikowska M, Lisiecka M, et al. Takotsubo cardiomyopathy triggered by influenza B. Pol Merkur Lekarski 2018;45:67–70. [PubMed] [Google Scholar]
- 4.Golfeyz S, Kobayashi T, Aoi S, et al. Possible association of influenza A infection and reverse Takotsubo syndrome. BMJ Case Rep 2018;11:e226289. 10.1136/bcr-2018-226289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minhas AS, Scheel P, Garibaldi B, et al. Takotsubo syndrome in the setting of COVID-19. JACC Case Rep 2020;2:1321–5. 10.1016/j.jaccas.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer P, Degrauwe S, Van Delden C, et al. Typical Takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J 2020;41:41. 10.1093/eurheartj/ehaa306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solano-López J, Sánchez-Recalde A, Zamorano JL. SARS-CoV-2, a novel virus with an unusual cardiac feature: inverted Takotsubo syndrome. Eur Heart J 2020:ehaa390. 10.1093/eurheartj/ehaa390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen D, Nguyen T, De Bels D, et al. A case of Takotsubo cardiomyopathy with COVID 19. Eur Heart J Cardiovasc Imaging 2020;jeaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roca E, Lombardi C, Campana M, et al. Takotsubo syndrome associated with COVID-19. Eur J Case Rep Intern Med 2020;7:1. 10.12890/2020_001665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasqualetto MC, Secco E, Nizzetto M, et al. Stress cardiomyopathy in COVID-19 disease. Eur J Case Rep Intern Med 2020;7:001718. 10.12890/2020_001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghadri J-R, Wittstein IS, Prasad A, et al. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–46. 10.1093/eurheartj/ehy076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a position statement from the Taskforce on Takotsubo syndrome of the Heart Failure Association of the European Society of cardiology. Eur J Heart Fail 2016;18:8–27. 10.1002/ejhf.424 [DOI] [PubMed] [Google Scholar]
- 13.Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1955–71. 10.1016/j.jacc.2018.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hessel EA. Takotsubo cardiomyopathy and its relevance to anesthesiology: a narrative review. Can J Anaesth 2016;63:1059–74. 10.1007/s12630-016-0680-4 [DOI] [PubMed] [Google Scholar]
- 15.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–38. 10.1056/NEJMoa1406761 [DOI] [PubMed] [Google Scholar]
- 16.Singh K, Carson K, Shah R, et al. Meta-analysis of clinical correlates of acute mortality in Takotsubo cardiomyopathy. Am J Cardiol 2014;113:1420–8. 10.1016/j.amjcard.2014.01.419 [DOI] [PubMed] [Google Scholar]
- 17.Khera R, Light-McGroary K, Zahr F, et al. Trends in hospitalization for Takotsubo cardiomyopathy in the United States. Am Heart J 2016;172:53–63. 10.1016/j.ahj.2015.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiermaier T, Eitel C, Desch S, et al. Incidence, determinants and prognostic relevance of cardiogenic shock in patients with Takotsubo cardiomyopathy. Eur Heart J Acute Cardiovasc Care 2016;5:489–96. 10.1177/2048872615612456 [DOI] [PubMed] [Google Scholar]
- 19.Citro R, Rigo F, D'Andrea A, et al. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in tako-tsubo cardiomyopathy. JACC Cardiovasc Imaging 2014;7:119–29. 10.1016/j.jcmg.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 20.Elesber AA, Prasad A, Bybee KA, et al. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol 2006;47:1082–3. 10.1016/j.jacc.2005.12.004 [DOI] [PubMed] [Google Scholar]
- 21.Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation 2012;126:697–706. 10.1161/CIRCULATIONAHA.112.111591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherif K, Sehli S, Jenkins LA. Takotsubo cardiomyopathy after administration of norepinephrine. Proc 2016;29:166–7. 10.1080/08998280.2016.11929402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2020-001360supp001.mp4 (71.7MB, mp4)
openhrt-2020-001360supp002.pdf (17.6KB, pdf)


