Abstract
Retinal degenerations present a unique challenge as disease progression is irreversible and the retina has little regenerative potential. No current treatments for inherited retinal disease have the ability to reverse blindness, and current dietary supplement recommendations only delay disease progression with varied results. However, the retina is anatomically accessible and capable of being monitored at high resolution in vivo. This, in addition to the immune-privileged status of the eye, has put ocular disease at the forefront of advances in gene- and cell-based therapies. This review provides an update on gene therapies and randomized control trials for inherited retinal disease, including Leber congenital amaurosis, choroideremia, retinitis pigmentosa, Usher syndrome, X-linked retinoschisis, Leber hereditary optic neuropathy, and achromatopsia. New gene-modifying and cell-based strategies are also discussed.
Keywords: retinal degeneration, gene therapy, stem cells, iPSCs, CRISPR, leber congenital amaurosis, choroideremia, retinitis pigmentosa, Usher syndrome, retinoschisis, leber hereditary optic neuropathy, achromatopsia
INTRODUCTION
Inherited retinal diseases comprise a group of conditions with diverse clinical manifestations and heterogeneous genetic mutations that share a common end-result of progressive photoreceptor death and irreversible blindness. Approximately 1 in 2000 individuals worldwide are affected by inherited retinopathies [Sohocki et al., 2001]. There is currently no proven cure available for patients with inherited retinal degenerations, although management entails specialized genetic counseling, improving the use of residual vision, and treatment of complications that arise [Smith et al., 2015]. However, gene- and cell-based therapeutics hold great promise as next-generation treatment strategies.
The eye is an ideal target for novel therapies due to its accessibility, ease of noninvasive monitoring, significant compartmentalization, immune privileged status, and optical transparency. Additionally, in translational studies and randomized control trials, the contralateral eye serves as a control, providing a more ideal outcome analysis [Lin et al., 2015]. The retina lines the posterior aspect of the inner eye and is made of multiple cellular layers involved in the signal transduction of light stimuli (Fig. 1). Inherited genetic mutations affecting protein function in cells of the retina can lead to pathologic cellular dysfunction and death, causing visual impairment. Within the past decade, researchers have made progress in understanding the underlying pathophysiology contributing to retinal degenerations. This knowledge, along with advances in gene delivery and targeting as well as cell-based techniques, has provided hope for future treatment of inherited retinal disorders.
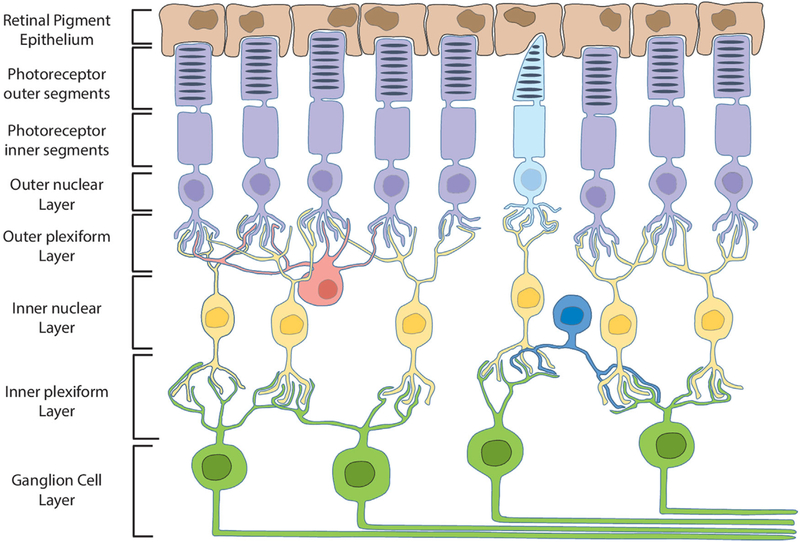
Figure 1. Schematic illustration of retinal cell layers.
RPE cells (brown) maintain close contact with and phagocytose rod (purple) and cone (light blue) photoreceptor outer segments. Bipolar cells (yellow) synapse with and transfer information between photoreceptors and ganglion cells (green). Axons of the ganglion cell layer converge to form the optic nerve. Horizontal (red) and amacrine (dark blue) cells serve multiple functions by integrating and regulating signal transduction throughout the retina.
This review will summarize molecular and biologic strategies currently being tested in clinical trials and those demonstrating potential for clinical application in the future. We include updates regarding gene therapy and randomized control trials for inherited retinal disease, including Leber congenital amaurosis, choroideremia, retinitis pigmentosa, Usher syndrome, X-linked retinoschisis, Leber hereditary optic neuropathy, and achromatopsia. Additionally, the latest advances in genome-engineering techniques, such as clustered regularly interspaced short palindromic repeats (CRISPR) and induced pluripotent stem cell (iPSC) technologies, will be introduced and summarized here.
GENE THERAPY FOR INHERITED RETINAL DISORDERS
Gene therapy uses viral machinery to insert therapeutic genes into native cells. The interventional approach of the therapy is dependent on the inheritance pattern of the condition: recessive conditions, including diseases caused by haploinsufficiency, can be approached through traditional gene addition, meaning a normally functioning gene is provided therapeutically. Patients with dominant or dominant-negative conditions, however, may require gene suppression or repair. In recessive conditions, mutations in both alleles prevent production of a functional gene product, so addition of a wild type copy of the gene can restore the normal phenotype. Adeno-associated virus (AAV) is the safest and most effective viral vector for gene delivery to the retina, and thus far, it is the only viral vector that has shown beneficial results in treating inherited retinal disease [Bainbridge et al., 2008; Cideciyan et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008; Cideciyan et al., 2009; Maguire et al., 2009; Banin et al., 2010; Simonelli et al., 2010; Jacobson et al., 2012; Testa et al., 2013; Boye and Boye, 2015]. Characteristics that make AAV a successful vector include: lack of pathogenicity, low immunogenicity and toxicity, long-term transgene expression observed in animal models, ability to match a variety of serotypes to specific tissue, and relative ease in manipulating genetic elements [Daya and Berns, 2008; Boye and Boye, 2015].
Patients with dominant or dominant-negative conditions, however, may require gene suppression or repair. In recessive conditions, mutations in both alleles prevent production of a functional gene product, so addition of a wild type copy of the gene can restore the normal phenotype.
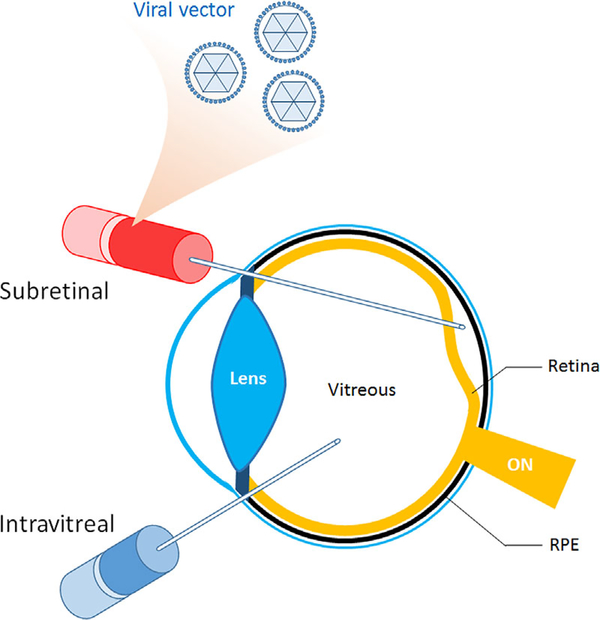
Vector delivery can be achieved by subretinal injection of the viral vector, which involves the formation of a transient retinal detachment that resolves spontaneously (Fig. 2) [Lin et al., 2015]. The vector then infects the cells of the retina, leading to expression of the therapeutic gene, as in the case of a gene addition strategy. Other methods, such as intravitreal injection, are less invasive and produce fewer iatrogenic complications, but may deliver therapeutic genes less efficiently [Yu-Wai-Man, 2016].
Figure 2. Vector delivery.
Schematic illustration of subretinal and intravitreal delivery of therapeutic gene-containing vectors. Vector delivery can be achieved by subretinal injection of the viral vector, which involves the formation of a transient retinal detachment that spontaneously resolves. Intravitreal injection is less invasive and produces fewer iatrogenic complications, but may deliver therapeutic genes less efficiently. ON, optic nerve; RPE, retinal pigment epithelium.
RPE65 Leber Congenital Amaurosis-2 (LCA2)
LCA is an inherited retinal dystrophy first described over 150 years ago by Theodor Leber. Mutations in genes associated with multiple visual pathways have been implicated, making the molecular events that lead to LCA relatively heterogeneous. The original clinical manifestations described by Theodor Leber are still used today: significant vision loss at or soon after birth, wandering nystagmus, amaurotic pupils, and a pigmented retina [Koenekoop, 2004]. Research on a subtype of LCA, retinal pigment epithelium 65 (RPE65)-associated LCA (LCA2), has laid the groundwork for advances in gene therapy of ophthalmic diseases. RPE65 is predominantly expressed in the retinal pigment epithelium (RPE) and encodes a 65 kda protein involved in visual pigment regeneration by isomerizing all-transto 11-cis-retinol (Table I) [Redmond, 2009; Chacon-Camacho and Zenteno, 2015]. Patients with this mutation have marked photoreceptor dysfunction and degeneration along with severely reduced or absent electroretinogram (ERG) signal at birth or first presentation [Redmond, 2009]. Gene therapy for LCA2 is considered to be among the major milestones in precision medicine. An AAV-mediated therapeutic gene delivery was used in a naturally occurring canine model for LCA2 (RPE65−/−) in 2001, which led to the long-term restoration of vision in treated dogs [Acland et al., 2001]. This created a platform for the development of other gene-based therapies in animal models for retinal degenerations and randomized controlled trials for patients suffering from these diseases.
TABLE I.
Expression Patterns for Genes Discussed
| Affected gene | Clinical manifestation | Location of gene expression associated with phenotype |
|---|---|---|
| RPE65 | Leber congenital amaurosis | RPE |
| GUCY2D | Photoreceptor outer segments | |
| CHM | Choroideremia | RPE and photoreceptors later |
| MERTK | Retinitis Pigmentosa | RPE |
| RPGR | Photoreceptor cilia | |
| MYO7a | Usher syndrome | Photoreceptor connecting cilium, RPE, cochlear hair cell bundles |
| RS1 | X-linked retinoschisis | Photoreceptors, bipolar cells |
| ND4 | Leber Hereditary Optic Neuropathy | Retinal ganglion cells |
| CNGB3 | Achromatopsia, or rod monochromatism | Cone photoreceptors |
Following the landmark findings of Acland et al., safety studies for RPE65 delivery by AAV2 were performed in RPE65-mutant canines and normal non-human primates [Jacobson et al., 2006a,b]. Multiple phase I/II gene therapy trials assessed the effects of unilateral, subretinal administration of AAV-RPE65, which demonstrated short-term rescue [Bainbridge et al., 2008; Cideciyan et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008; Cideciyan et al., 2009; Maguire et al., 2009; Banin et al., 2010; Simonelli et al., 2010; Jacobson et al., 2012; Testa et al., 2013]. All trials reported increased light sensitivity and improved vision in some patients, without any significant adverse effects from the vector. The untreated eye of three patients in one study received therapy years later with similar results in a 6-month follow-up period [Bennett et al., 2012].
Long-term follow-up of LCA2 patients in phase I/II trials has continued. Although results initially suggested improvements in photoreceptor function among some individuals, patients continue to show retinal structural loss and degeneration [Cideciyan et al., 2013; Jacobson et al., 2015]. Identifying factors that limit the success of gene therapy is imperative to translating treatments developed in mouse models to human patients. The idea that retinal degenerations reach a “point of no return” has been entertained [Cepko and Vandenberghe, 2013]. Most preclinical mouse models for retinal degenerations were treated prior to disease onset, whereas patients in randomized controlled trials are treated after the onset of photoreceptor degeneration. Fortunately, studies in mouse models show that treatment is likely efficacious at both the early and late stages of disease; this maintains hope that treatment of patients after the onset of retinal degeneration may be practical and capable of preserving vision [Davis et al., 2013; Wert et al., 2014b; Hurley and Chao, 2015; Koch et al., 2015].
Spark Therapeutics (Philadelphia, PA) is sponsoring a phase III clinical trial that launched in 2013 in collaboration with the Children’s Hospital of Philadelphia and the University of Iowa (NCT00999609). Patients aged 3 years or older with retinal dystrophy caused by mutations in RPE65 (LCA2) were administered sub-retinal AAV2-hRPE65v2 (SPK-RPE65) in both eyes. After achieving ‘breakthrough therapy designation’ in late 2014 from the U.S Food and Drug Administration (FDA), Spark announced results of their primary and secondary outcome measures in an October 2015 press release. The trial met its primary endpoint, as SPK-RPE65 gene therapy achieved statistically significant increases in functional vision compared to the control group as measured by bilateral mobility test change score after 1 year. Two of the three secondary end-points were also met: patients receiving SPK-RPE65 had better full-field light sensitivity and favorable mobility test change scores, a measure of performance navigating in environments of various levels of lighting, compared to baseline for the first injected eye; the unmet secondary endpoint was visual acuity, which showed no statistically significant change. The company stated that they will submit a biologics license application with the FDA in 2016. In the meantime, safety of vector administration to the contralateral eye was reported in eleven patients, confirming a previous study with fewer patients [Bennett et al., 2012] and representing the first ocular gene therapy to be successfully delivered to the previously untreated eye [Bennett et al., 2016].
GUCY2D Leber Congenital Amaurosis-1 (LCA1)
LCA1 is caused by homozygous or compound heterozygous mutations in GUCY2D, which encodes for retinal guanylyl cyclase-1 (retGC1). RetGC1 is predominantly expressed in the outer segment of cones but is also present in rods (Table I) [Jacobson et al., 2013; Boye et al., 2015; Chacon-Camacho and Zenteno, 2015]. The normal function of retGC1 in the outer segments is to respond to low intracellular calcium levels and produce cGMP to reopen cGMP-gated channels, thus returning the photoreceptor to its pre-excitation state after a flash of light [Jacobson et al., 2013; Boye et al., 2015]. The mutation causes a severe decrease in visual acuity and produces nystagmus and marked photoreceptor dysfunction on ERG, but interestingly, patients have near-normal laminar structure of the retina, which is unique to this subtype of LCA [Walia et al., 2010; Boye et al., 2015]. Gucy2e is the mouse homolog of GUCY2D, and the Gucy2e−/− model mimics human progression of LCA1 most closely. Multiple proof-of-principle studies have demonstrated restoration of cone function on ERG using AAV-mediated gene delivery [Boye et al., 2010; Boye et al., 2011; Mihelec et al., 2011; Boye et al., 2013]. Because LCA1 predominantly affects foveal cones, and mice do not have a fovea, an all-cone Nrl−/−Gucy2e−/− mouse model was created [Boye et al., 2015]. When AAV5-GRK1-GUCY2D was delivered to this model, retinal function was restored for at least 6 months. While there are currently no clinical trials enrolling patients with LCA1, these results suggest LCA1 patients may benefit from delivery of the GUCY2D gene.
Choroideremia (CHM)
Truncation or deletion of the CHM gene causes choroideremia, an X-linked recessive retinopathy whose genetic cause was described in 1990 [Cremers et al., 1990]. Rab escort protein-1 (REP1) is encoded by CHM and, if deficient, can cause degeneration of the choroid, RPE and neurosensory retina (Table I) [Tolmachova et al., 2006; Zinkernagel and MacLaren, 2015]. Rab proteins are expressed in all cells and, within the eyes, are necessary for transport of proteins used for intracellular signaling in photoreceptors and for phagocytosis and breakdown of outer segment disc membranes in RPE cells [Zinkernagel and MacLaren, 2015]. REP1 facilitates lipid modification on Rab proteins by binding unprenylated Rabs and presenting them to enzymes which catalyze the addition of covalently attached hydrophobic molecules, a process termed prenylation. Prenylation is an important process that mediates the association of cytosolic proteins with biological membranes. The buildup of unprenylated Rab proteins is thought to be a major mechanism of retinal cell death [Pereira-Leal et al., 2001]. The disease shows atrophy of the choroid and thus a pale fundus due to illumination of the sclera behind the degenerating choroid (Fig. 3B). Clinically, the condition starts with night blindness and results in a progressive decrease in peripheral vision [Zinkernagel and MacLaren, 2015]. Because choroideremia progresses slowly and has been characterized in mice [Tolmachova et al., 2006; Tolmachova et al., 2010], it is an attractive candidate for gene therapy.
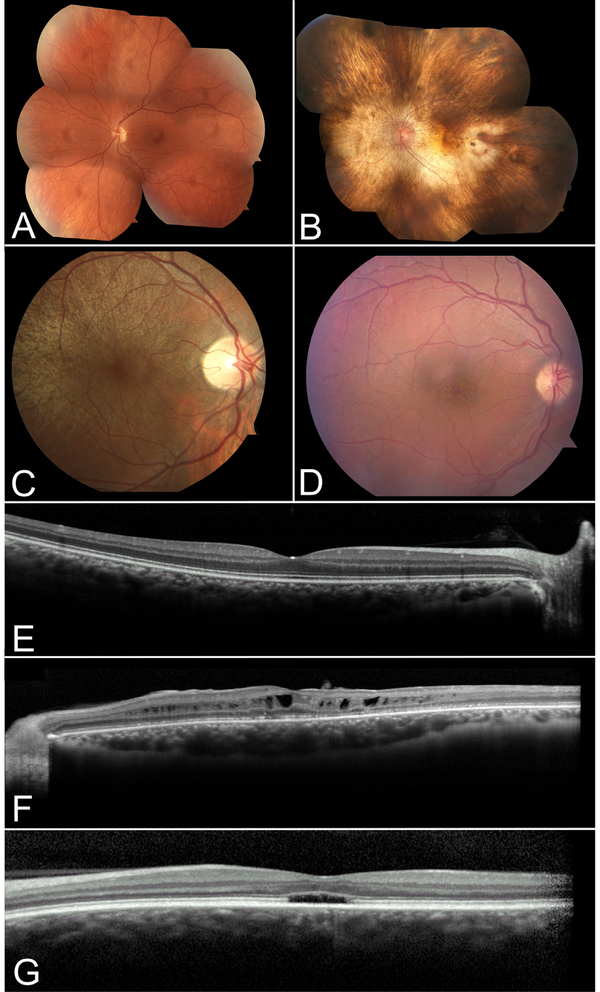
Figure 3. Retinal imaging of patients with inherited retinal disease.
Color fundoscopy of a normal patient (A) compared to patients with choroideremia (B), asymptomatic RPGR-associated RP female carrier (C) and retinoschisis (D). OCT of a normal patient depicting high resolution image of the retinal layers (E) compared to patients with retinoschisis (F) and achromatopsia (G). In B, note the characteristic pale fundus of choroideremia as a result of the sclera transilluminating through a degenerating RPE and choroid. In C, a typical tapetal-like reflex of the retina in an RPGR-associated RP carrier can be seen sparing the macula. In D, the subtle spoke-wheel appearance of retinoschisis is seen on color fundoscopy, and characteristic foveal and perifoveal cysts are seen on OCT in F. Patients with achromatopsia can show a foveal optical gap with loss of the inner and outer segment junction on OCT as depicted in G.
Large animal models for choroideremia do not exist, eliminating the luxury that canine models for LCA2 provided [Boye and Boye, 2015]. However, REP1 replacement therapy proved achievable in Chmnull/WT mice using AAV2 in a dose-dependent fashion and in cells of patients with CHM mutations in vitro, providing support for clinical trials [Anand et al., 2003; Tolmachova et al., 2013]. Tolmachova et al. demonstrated that the vector produced therapeutic REP1 with prenylation in cultured fibroblasts of choroideremia patients. A subsequent phase I/II dose-escalation clinical trial which tested AAV2-REP1 in six patients was recently reported. Two patients experienced two- and four-line improvements in visual acuity, and five patients had increases in mean retinal sensitivity despite detachment of the macula (NCT01461213) [MacLaren et al., 2014]. In light of this trial, [Seitz et al., 2015] performed a retrospective cross-sectional study to define pre-treatment characteristics of choroideremia patients eligible for genetic therapy. This study was important in defining the natural progression of disease as a function of age and determined that there is a high degree of symmetry between eyes, suggesting the untreated eye is an adequate control. Additionally, it was found that the best objective measure of structural disease progression was the use of fundus autofluorescence to quantify the area of remaining RPE. Two additional trials began in 2015 to test the safety and efficacy of the same gene vector evaluated in the previous study (NCT01461213) to also treat CHM patients (NCT02077361 and NCT02341807) [Dimopoulos et al., 2015].
Autosomal Recessive Retinitis Pigmentosa (RP)
RP affects approximately 1 in 4000 individuals worldwide and has a variable inheritance pattern [Berson, 1993]. In RP, progressive atrophy of rods occurs due to an inherited gene mutation, leading to secondary death of cones [Hamel, 2006]. RP is a genetically heterogeneous disease, as over 70 mutated genes have been identified to cause this degeneration of the retina with variable phenotypes [Zhang, 2016]. Patients will typically present with night blindness, which progresses to tunnel vision and ultimately blindness [Fahim et al., 1993; Hartong et al., 2006; Wert et al., 2014a].
A rare form of autosomal recessive RP is caused by mutations in mer receptor tyrosine kinase (MERTK). Homozygous loss-of-function mutations in MERTK result in impaired phagocytosis of the photoreceptor outer segments by RPE cells (Table I) [Gal et al., 2000]. Debris accumulates between the outer segments and RPE over time, causing photoreceptor degeneration and vision loss. Several studies have tested the efficacy of gene therapy in the Royal College of Surgeons (RCS) rat, which was found to carry a Mertk mutation [Vollrath et al., 2001; Smith et al., 2003; Tschernutter et al., 2005; Conlon et al., 2013]. Conlon et al. performed pre-clinical studies with an AAV2 vector carrying MERTK and an RPE-specific promoter (VMD2), which was safe and effective in the RCS model [Conlon et al., 2013]. RCS rats displayed improved ERG response in the eye injected with AAV2-VMD2-hMERTK compared to the contralateral untreated control eye, and the subretinal injection of the vector was well-tolerated. This prompted a clinical trial by collaborators at the King Khaled Eye Specialist Hospital and King Faisal Specialist Hospital & Research Center (NCT01482195) [Conlon et al., 2013]. Safety was confirmed in preclinical studies in monkeys, and a phase I open-label, dose-escalation trial involving subretinal injection of rAAV2-VMD2-hMERTK to six patients with advanced MERTK-associated RP was initiated [Ghazi et al., 2016]. There was transient improvement of visual acuity in half of the patients, although this progress was lost in two of the three patients by the end of the 2-year follow-up.
RPGR X-Linked Retinitis Pigmentosa
X-linked retinitis pigmentosa (XLRP) accounts for approximately 10–20% of all cases of RP, with a mutation in the retinitis pigmentosa GTPase regulator (RPGR) being the most common (Table I) [Hong et al., 2003; Raghupathy et al., 2015]. RPGR has been demonstrated to interact with the delta subunit of cyclic GMP phosphodiesterase, suggesting the defective protein likely causes degeneration due to detrimental effects on the processes that govern protein localization and transport [Linari et al., 1999]. Although no clinical trials for RPGR-XLRP exist at this point, a step toward treatment came with proof-of-concept studies in two canine models in which therapeutic AAV-RPGR preserved photoreceptor function and structural integrity [Beltran et al., 2012]. Other murine models also exist for RPGR-XLRP, but are less representative of the human condition [Hong et al., 2000; Hong et al., 2004; Brunner et al., 2010; Wright et al., 2011; Huang et al., 2012]. Applied Genetic Technologies Corporation (AGTC) (Alachua, FL) is initiating a formal preclinical testing program to provide a clinical evaluation of the gene therapy product used in the canine models.
Although no clinical trials for RPGR-XLRP exist at this point, a step toward treatment came with proof-of-concept studies in two canine models in which therapeutic AAV-RPGR preserved photoreceptor function and structural integrity.
Although RPGR-XLRP almost exclusively affects males, as would be expected due to its X-linked inheritance pattern, females can also be affected [Souied et al., 1997; Churchill et al., 2013]. Heterozygous carrier females exhibit a spectrum of phenotypes, although they typically exhibit no symptoms and have a tapetal-like reflex seen on multiple imaging modalities. In rare cases, they can develop severe phenotypes [Branham et al., 2012]. Recent data suggest that families with no male-to-male transmission who are suspected of having autosomal dominant RP may actually have XLRP instead [Churchill et al., 2013]. For females with XLRP manifesting a mild phenotype, the benefits of gene therapy likely will not outweigh the risks, and enrollment of female patients in clinical trials would require careful consideration.
Usher Syndrome (USH)
Usher syndrome is an autosomal recessive disease characterized by retinitis pigmentosa, progressive hearing loss and possible vestibular dysfunction. Previous studies estimated a prevalence of 1 in 25,000, but recent epidemiological data suggest it may be more common than originally predicted [Kimberling et al., 2010; El-Amraoui and Petit, 2014]. There are three main types that can be distinguished clinically (USH1, USH2, and USH3), but there are over fifteen known loci associated with Usher syndrome affecting both photoreceptors as well as the hair bundle and synapse of the inner ear cells. USH1 patients exhibit congenital profound hearing loss, vestibular dysfunction, and early-onset RP. USH2 patients typically have moderate hearing loss, normal vestibular function, and develop RP in early adulthood. USH3 patients have progressive hearing loss, sporadic vestibular dysfunction, and variable onset of RP [Mathur and Yang, 2015]. USH proteins are part of multiprotein complexes and are involved in actin-based intracellular trafficking, scaffolding, cell adhesion, and signaling [Williams and Lopes, 2011; Mathur and Yang, 2015]. Currently, there is no cure for Usher syndrome, and present management includes administering hearing aids or cochlear implants in those eligible [Jatana et al., 2013].
Retinal degeneration in multiple mouse models for Usher syndrome have been amenable to genetic therapies [Colella et al., 2013; Lopes et al., 2013; Zallocchi et al., 2014]. The shaker1 mouse model of USH1B lacks functional myosin 7a (MYO7A) protein, which has multiple functions, including opsin delivery through the photoreceptor connecting cilium and melanosome transport in RPE (Table I) [Williams and Lopes, 2011]. Subretinal injections of EIAV-CMV-MYO7A (UshStat®) in the shaker1 mouse model for USH1B decreased the amount of photoreceptor loss and restored the α-transducin translocation in photoreceptors. This vector was chosen in part due to its carrying capacity, as the MYO7A gene is large. UshStat proved to be safe and tolerable in macaques in the same study [Zallocchi et al., 2014]. Sanofi (Paris, France) is currently recruiting USH1B patients for a phase I/II trial assessing the safety and tolerability of UshStat in USH1B patients (NCT01505062).
Attempts to restore hearing and vestibular function in an USH1 mouse model have also been undertaken. Lentz et al. restored hearing loss and balance in mice using an anti-sense oligonucleotide (ASO) identified in an in vitro screen. This is the first therapeutic intervention to rescue vestibular and auditory function in a mouse model of human hereditary deafness. The ASO blocks an aberrant splice site in a USH1C mouse model with an Ush1c mutation: c.216G>A. By blocking the aberrant splice site with an ASO to promote correct splicing, functional harmonin protein is synthesized, which restores vestibular and hearing function. This strategy is limited to genetic defects that result in cryptic splice sites [Lentz et al., 2013; Mathur and Yang, 2015].
Attempts to restore hearing and vestibular function in an USH1 mouse model have also been undertaken. Lentz et al. restored hearing loss and balance in mice using an anti-sense oligonucleotide (ASO) identified in an in vitro screen. This is the first therapeutic intervention to rescue vestibular and auditory function in a mouse model of human hereditary deafness.
One challenge for Usher Syndrome is that the carrying capacity of AAV vectors makes larger therapeutic genes like MYO7A more difficult to deliver. To remedy this, large genes can be split in half and delivered in separate AAV vectors. Recombination then occurs in co-infected cells, leading to functional expression of the therapeutic gene [Dyka et al., 2014; Trapani et al., 2014]. The recombination of the two halves of MYO7A results in a sequence that is 100% identical to the predicted sequence in vitro according to one study by Dyka et al. [2014], indicating accurate homologous recombination or splicing for each dual-vector platform. Single AAV vector strategies of delivering MYO7A have been reported, however, which suggests that the gene may undergo spontaneous recombination when fragmentation occurs due to an unfavorable carrying capacity [Allocca et al., 2008; Colella et al., 2013; Lopes et al., 2013]. It remains to be seen which strategy will be the most efficient way to deliver large therapeutic genes in AAV vectors.
X-Linked Retinoschisis (XLRS)
XLRS is a retinal degeneration affecting between 1 in 5,000 to 25,000 individuals worldwide [Byrne et al., 2014]. The condition is characterized by splitting of the retinal layers and the presence of intraretinal fluid-filled cysts, typically in the macula [Schubert and Wissinger, 2015]. A hallmark finding in the functional assessment of XLRS is a diminished ERG b-wave with relative preservation of the a-wave, correlating to decreased synaptic transmission via photoreceptor-bipolar cell synapse [Schubert and Wissinger, 2015]. Mutations in retinoschisin (RS1), a gene encoding a protein thought to be important for retinal cell layer organization and synapse structure, were identified by positional cloning as the cause of XLRS (Table I) [Sauer et al., 1997; Weber et al., 2002]. In a mouse model that lacks the homolog of retinoschisin, the human condition is mimicked, including formation of fluid-filled cavities and progressive photoreceptor death [Weber et al., 2002]. Since the identification of RS1 as the gene associated with XLRS, progress has been made in developing gene therapies to slow degeneration in mice [Zeng et al., 2004; Min et al., 2005; Molday et al., 2006; Kjellstrom et al., 2007; Janssen et al., 2008; Takada et al., 2008; Park et al., 2009; Ou et al., 2015]. Byrne et al. developed methods to deliver RS1 via an AAV vector to specific cell types of the retina, including photoreceptors or Müller glia. It was found that intravitreal delivery of RS1 to photoreceptors at a minimum provided sufficient rescue when compared to gene delivery targeting Müller glia [Byrne et al., 2014]. This study demonstrated the importance of careful optimization for long-lasting gene therapy.
XLRS patients have particularly fragile retinas that may not be suitable for subretinal injections, making other routes of administration favorable [Molday et al., 2006; Park et al., 2009; Byrne et al., 2014]. Two intravitreally-injected vectors, AAV8 and recombinant AAV2 (rAAV2) carrying functional RS1 with different promoters, proved effective in Rs1-deficient mouse models and are currently being studied in clinical trials. The National Eye Institute launched phase I/II clinical trials for AAV8-scRS/IRBPhRS gene transfer in participants affected by XLRS (NCT02317887). AAV8-mediated gene delivery of RS1 induces reorganization of the photoreceptor-depolarizing bipolar cell synapse and restores function in Rs1-KO mice, supporting its use in a clinical trial [Ou et al., 2015]. AGTC is sponsoring a similar phase I/II study for a rAAV2tYF-CB-hRS1 therapy for XLRS patients supported by safety studies in RS1-deficient mice and normal cynomolgus macaques (NCT02416622) [Ye et al., 2015a,b]. Normal macaques showed a dose-dependent inflammatory response in the anterior and posterior segment which improved over time, indicating the need for frequent observation of XLRS patients receiving the recombinant vector in clinical trials [Ye et al., 2015a].
Leber Hereditary Optic Neuropathy (LHON)
LHON is an inherited mitochondrial disorder characterized by optic atrophy, specifically of the retinal ganglion cells (RGCs) [Cwerman-Thibault et al., 2014]. The majority of LHON is caused by mutations in mitochondrial genes ND1, ND4, or ND6 (G3460A, G11778A, and T14484C, respectively); these genes encode for complex I of the electron transport chain, a key enzyme for cellular respiration (Table I) [Cwerman-Thibault et al., 2014]. Irreversible vision loss occurs as the RGCs degenerate, leading to a central visual field defect, loss of color vision and decreased contrast sensitivity [Cwerman-Thibault et al., 2014]. There are features of LHON that make it a desirable target for gene therapy: AAV or other therapeutic agents can be administered to RGCs by injection into the vitreous cavity, and vision loss occurs bilaterally and sequentially, that is, the second eye becomes involved after the first, creating a distinctive therapeutic window [Koilkonda and Guy, 2011; Cwerman-Thibault et al., 2014].
A major limitation to studying mitochondrial diseases is the lack of animal models, partly due to the difficulty of delivering genes directly to the mitochondria [Guy et al., 2002]. Mitochondrial-encoded genes are not directly amenable to gene therapy given their intra-organelle location. Qi et al. [2007] overcame this by using “allotopic expression” to create a rodent model of LHON, which was accomplished by directing expressed mutant ND4 to the mitochondria with a targeting sequence. Using a similar genetic strategy, therapeutic ND4 delivery was shown to effectively rescue an LHON rat model, and intravitreal delivery of ND4 via AAV was safe in normal mice which displayed specific expression in the mitochondria of RGCs [Ellouze et al., 2008; Guy et al., 2009]. These results supported initiation of phase I/II clinical trials.
The University of Miami is leading a clinical trial attempting the ‘allotopic expression’ method to deliver ND4 into patient RGCs (NCT02161380). Initial results published in November 2015 reported no serious safety issues in the first five patients enrolled and increased visual acuity in two of five patients following the delivery of the therapeutic gene [Feuer et al., 2016]. Improved visual acuity in safety studies with low sample size may be due to other factors. In this particular trial, the natural history of LHON may explain the spontaneous improvement in visual acuity, which has been reported in the m.11778G>A patient population that was studied, albeit at much lower rates [Finsterer and Zarrouk-Mahjoub, 2016]. Continued enrollment with an adequate sample size should determine if changes in visual acuity are due to therapeutic gene delivery [Lam et al., 2014]. Similar clinical trials for LHON include those led by GenSight Biologics (Paris, France) (NCT02064569) and Huazhong University of Science and Technology (Wuhan, China) (NCT01267422). Results for the latter study showed intravitreal injection of an AAV vector delivering an allotopically expressed ND4 to be safe during the 9-month follow-up, with six of the nine patients showing increased visual acuity, enlarged visual field, and no change in the retinal nerve fiber layer cross-section on optical coherence tomography (OCT) [Wan et al., 2016].
Achromatopsia
Achromatopsia, also referred to as “rod monochromatism,” is an autosomal recessive retinal condition with a prevalence of approximately 1 in 30,000 [Zobor et al., 2015]. This condition presents early, in infancy or at birth, with pendular nystagmus and photophobia and subsequently reduced visual acuity accompanying poor color vision [Zobor et al., 2015]. There is currently no treatment, and management relies upon low vision aids such as tinted contact lenses or glasses with cutoff filters to reduce symptoms of photophobia [Kohl and Hamel, 2013]. Approximately 50% of cases are associated with altered or truncated cyclic nucleotide-gated ion channel B3 (CNGB3) (Table I) [Kohl et al., 2005]. Other cases are associated with mutant CNGA3, guanine nucleotide-binding protein G subunit alpha-2 (GNAT2), and phosphodiesterase 6C and 6H (PDE6C, PDE6H) [Kohl and Hamel, 2013]. These genes are all crucial for proper cone phototransduction. CNG channels open in the dark and close upon light stimulation due to a decreased concentration of cGMP, which allows for further downstream photoreceptor signal transduction. Mouse models (GNAT2, CNGA3, and CNGB3 deficiency), canine models of CNGB3 deficiency, and a sheep model of CNGA3 deficiency have been treated with gene replacement, yielding increased visual function [Alexander et al., 2007; Komaromy et al., 2010; Michalakis et al., 2010; Carvalho et al., 2011; Pang et al., 2012; Banin et al., 2015]. A phase I/II clinical trial sponsored by AGTC in collaboration with the National Eye Institute (NEI) is currently recruiting patients for a safety and efficacy study for AAV-mediated delivery of CNGB3 to patients with congenital achromatopsia caused by mutations in CNGB3 (NCT02599922).
CHALLENGES FOR GENE THERAPY
Just 15 years after the landmark study by Acland et al. providing gene therapy for dogs with LCA2, the amount of progress in precision medicine research has continued to accelerate. However, several challenges still face researchers. Follow-up studies on the LCA2 trials showed that photoreceptor degeneration continued despite variable improvements in vision among participants [Cideciyan et al., 2013]. Treatment failure could be due to inefficient vector transduction or the timing of rescue in relation to disease onset [Cepko and Vandenberghe, 2013; Wert et al., 2014b]. Recent research approached this question using a genome-engineered mouse model of retinal degeneration, where correction of the deficiency could be controlled pharmacologically in a temporal manner via a tamoxifen inducible Cre-loxP, rescue allele Pde6bStop strategy. This study indicated that treatment is likely efficacious regardless of whether the intervention occurs preor post-onset, as long as there is a sufficient number of rods [Davis et al., 2013]. However, very advanced stages of retinal degeneration are not optimal for gene therapy interventions, which thus far are only able to slow disease progression. Instead, patients at advanced disease stages may benefit more from cell-based transplantation methods, which theoretically could restore visual function.
An alternative hypothesis is that rescued cells may be dying in a non-cell autonomous manner. That is, gene rescue in an individual cell can occur, but extrinsic toxic factors may still cause cell death. Studies discerning the potential factors contributing to non-cell-autonomous death are needed. If these effects prove significant, then for gene therapy to be successful, another mechanism of neuroprotective rescue must be administered in conjunction with therapeutic gene supplementation. This theory is supported by the recent use of a rod-derived cone viability factor (RdCVF). RdCVF is a truncated version of its full-length isoform, which is normally secreted by rods to promote cone survival. It was identified in 2004 via an assay assessing genes that affect cone survival. RdCVF contains a domain which prevents damage from hyperoxemia and was reported to prolong cone survival in rd10 and P23H mouse models of rod-cone dystrophy [Byrne et al., 2015]. rd10 mice model autosomal recessive RP associated with Pde6b dysfunction, and P23H mice model dominantly inherited RP associated with a mutation in rod opsin. In patients, a neuroprotective treatment, ciliary neurotrophic factor (CNTF) delivered as an intraocular encapsulated cell implant, tested alone in early- and late-stage RP patients improved cone survival but had no effect on functional vision [Birch et al., 2013].
Further studies to assess gene therapy in patients are needed, but inherited retinal degenerations are relatively rare, and achieving an adequate sample size to accurately assess the efficacy of these treatments remains difficult. A database created by the Foundation Fighting Blindness (FFB) may remedy the difficulty of low sample numbers in trials. The FFB has recently funded a registry, My Retina Tracker™, to serve as a genetic database for patients suffering from inherited retinal degenerations and their related, unaffected family members (NCT02435940). This database is critical for successful clinical trials, as it collates longitudinal data from participants and provides researchers with institutional review board-approved projects the ability to screen or recruit registrants for clinical studies [Fisher et al., 2016].
Another problem facing clinical trials is surgical complications that hinder effective ocular gene therapy. Subretinal injection to deliver the therapeutic gene provides restricted delivery to a small area and a risk of retinal thinning and visual acuity loss [Boye and Boye, 2015]. Furthermore, diseased retinas are increasingly at risk of damage from subretinal injection. Novel ways to deliver gene therapy to the outer retina via the vitreous are in development and may prove more useful (Fig. 2) since transduction theoretically occurs uniformly across the retina [Lebherz et al., 2008; Natkunarajah et al., 2008; Dalkara et al., 2013; Kay et al., 2013].
ZFN, TALEN, AND CRISPR: THE FUTURE OF GENE THERAPY?
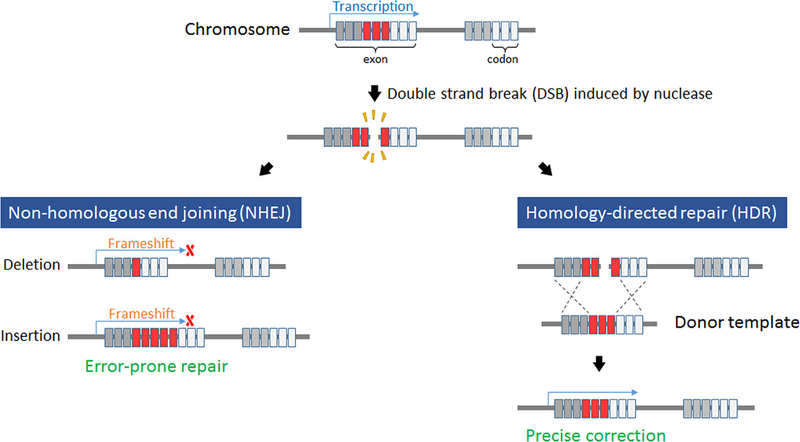
Multiple DNA repair and modification strategies occur naturally and have been a source of inspiration for novel gene therapy approaches. Double-strand breaks (DSBs) are considered a potentially fatal event by the cell and quickly induce two repair mechanisms [Carroll, 2014; Gilles and Averof, 2014]. Non-homologous end joining (NHEJ) results in direct reannealing of the broken strands without concern for sequence fidelity. In this case, preserving the cell and avoiding apoptosis, which can be initiated by compromised DNA, are prioritized over accuracy of the sequence itself [Cong et al., 2013; Gilles and Averof, 2014]. While this strategy is the default method of repair, it often induces insertions or deletions (indels) that code for nonfunctional gene products. The alternative repair mechanism is homology-directed repair (HDR), wherein unbroken sister chromatids from a homologous chromosome are used as a template to ensure the correct sequence is achieved prior to ligation (Fig. 4). While scientists have been eager to implement these strategies in the laboratory, inducing DSBs at loci of interest has been challenging. Only recently has this ability been made efficient by way of protein nucleases, with zinc finger nucleases providing the first generation of gene targeting strategies.
Figure 4. Non-homologous end joining (NHEJ) versus homology-directed repair (HDR).
Illustration depicting differences between NHEJ (left) and HDR (right). A double strand break (DSB) may undergo subsequent error-prone NHEJ via ligation of the ends of the DNA without regard for the sequence accuracy, leading to indels. HDR occurs using a wild type donor template that is homologous to the target site and serves as a template for precise correction.
Zinc Finger Nucleases (ZFNs)
Zinc finger binding domains were fused with the catalytic subunit of the Type IIS restriction enzyme, FokI, to create zinc finger nucleases [Carroll, 2014; Gilles and Averof, 2014]. Zinc fingers are composed of thirty amino acids that can be designed to target specific sequences. Each finger can bind to three nucleotides, and at least three consecutive fingers are required for sufficient binding to DNA. Because FokI must dimerize to cleave the DNA, two sets of three zinc fingers must bind on either side of the cleavage site. These recombinant proteins composed of zinc fingers and the FokI nuclease were designed to identify a DNA sequence and induce a DSB, leading to homologous recombination or re-ligation. Zinc fingers identify the target sequence and the nuclease causes a DSB. When administered with a donor DNA, this strategy theoretically has the potential to treat inherited disease by correcting the gene sequence through HDR [Greenwald et al., 2010]. ZFN technology was among the first of its kind to induce directed gene editing in eukaryotic cells, and it has since gone on to be successfully applied in many tissues, including the retina. Administration of ZFNs targeted to the rhodopsin gene in embryonic retinoblast cells increased the rate of homologous recombination compared with endogenous recombination, with rates reaching as high as 17% [Greenwald et al., 2010]. In a similar study, researchers were able to achieve site-specific gene correction of the p.R31X mutation in the Ush1c gene following ZFN-induced DSB [Overlack et al., 2012]. These results were among the first to demonstrate the feasibility of gene targeting for retinal dystrophies with ZFN technology.
While ZFNs revolutionized the field of genetic medicine and engineering, there are several limitations that detracted from the applicability of this technology. Designing zinc fingers to bind to every combination of three base pairs has not yet been achieved, so there remain many sites that cannot be targeted. Neighboring domains within each finger may lead to interactions that affect binding specificity or affinity [Gilles and Averof, 2014]. Thus, newer technologies were explored to build upon the foundation laid by ZFNs.
Transcription Activator-Like Effector Nucleases (TALEN)
The Xanthomonas plant bacteria species integrates its DNA into its host genome by way of transcription activator-like endonucleases (TALs) [Carroll, 2014; Gilles and Averof, 2014]. Researchers realized that these proteins could be exploited for gene targeting in a parallel manner as zinc finger binding domains. Fusion of TALs with the FokI restriction enzyme led to the creation of TALEN (TAL effector nuclease). TALEN distinguishes itself from ZFN in that the individual TAL proteins, made of tandem repeats of thirty-four amino acids, can bind to individual nucleotides, allowing for a 1:1 ratio of TAL protein to nucleotide. The identity of the nucleotide that the TAL binds to is determined by the amino acids at sites twelve and thirteen. Simplification of the recognition code makes TALEN much easier to design and enhances binding efficiency compared to ZFN. However, at least twelve TALs are required for sufficient binding, and this long array is cumbersome in some assays.
TALEN has been applied to the retina. In mouse embryos expressing the Crb1rd8 mutation, TALEN was used to successfully induce HDR, leading to correction of ocular abnormalities. Single-stranded, complimentary nucleotides were co-injected with TALEN to correct the mutation, and the offspring showed normal retinal phenotype [Low et al., 2014]. However, the CRISPR/Cas9 revolution has recently overtaken TALEN as the primary gene editing strategy.
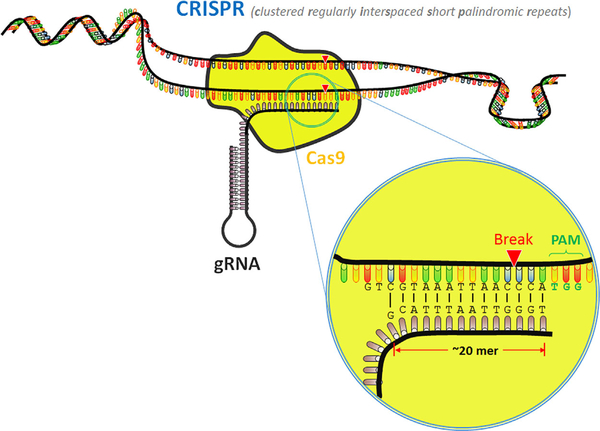
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)
In some bacteria and archaea, immunity against viruses is achieved by breaking the viral genome into pieces that are integrated into the bacterial DNA in regions containing many clustered regularly interspaced short palindromic repeats (CRISPR) [Sander and Joung, 2014]. The viral genes are transcribed into RNA, also called CRISPR RNA (crRNA), which complexes with a CRISPR-associated (Cas) nuclease. When new viruses infect the bacteria, this crRNA/Cas complex can bind to specific sequences on the invading strand, allowing the nuclease to subsequently induce DSBs and prevent infection.
The crRNA/Cas complex also requires a trans-activating crRNA (tracrRNA) to function properly, but researchers developed a chimeric fusion of the crRNA and tracrRNA called guide RNA (gRNA) that is equally as effective in binding to target DNA (Fig. 5) [Cong et al., 2013; Senis et al., 2014]. The gRNA has twenty amino acids at its 5′ end that can be customized to target specific sequences [Jinek et al., 2012]. The 3′ end contains a region that complexes with the Cas protein, and directly downstream of the cleavage site is a protospacer adjacent motif (PAM) that is required for cleavage [Doudna and Charpentier, 2014]. Thus, it is the combination of a gRNA and PAM that enables the site targeting and cleavage to occur [Ran et al., 2013a]. Previous methods for genome engineering, such as gene knockout and knock-in, have also used HDR as part of its mechanism. However, CRISPR does not rely on the low incidence of DNA damage to complete repairs; instead, it actively creates DSBs to allow for gene editing [Dow, 2015].
Figure 5. CRISPR/Cas9.
Schematic illustration of CRISPR/Cas9 associated with a target sequence. gRNA complexes with the Cas9 endonuclease and directs it to the site of interest, where it creates a DSB. gRNA, guide RNA; Cas9, CRISPR-associated protein 9; PAM, protospacer adjacent motif.
CRISPR/Cas-mediated NHEJ is now being applied to inherited retinal disease (Fig. 6). Recently, researchers at Cedars-Sinai Medical Center demonstrated that the mutant gene for an autosomal dominant rodent model of retinitis pigmentosa, S334ter-3 rats, could be ablated to prevent photoreceptor degeneration [Bakondi et al., 2016]. This study paves the way for using gene-modifying techniques such as CRISPR for autosomal dominant diseases. Other strategies have also emerged, including a study by Bassuk et al. demonstrating that a point mutation in induced pluripotent stem cells (iPSCs) from a patient with X-linked retinitis pigmentosa could be corrected with CRISPR/Cas gene repair [Bassuk et al., 2016]. This may prove to be an efficient way of generating healthy iPSCs for future transplantation studies.
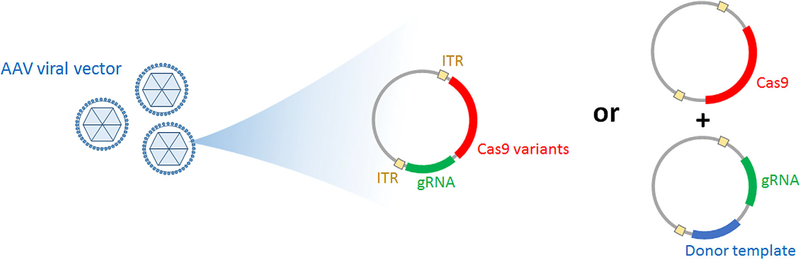
Figure 6. CRISPR/Cas9 delivery.
Illustration depicting how CRISPR/Cas9 can be packaged for gene-modifying therapy. Cas9 and gRNA are delivered via a vector without donor template (left) or with donor template (right). When given without donor template, gene ablation can be achieved at a target sequence specified by gRNA. Cas9 variants can be used to create a shorter sequenced, single construct. Precise homology-directed repair is achieved when the vector includes a donor template. ITR sequences allow identification and encapsulation of the construct by the viral vector. ITR, inverted terminal repeat; gRNA, guide RNA; Cas9, CRISPR-associated protein 9.
CRISPR Interference (CRISPRi): A Substitute for RNAi?
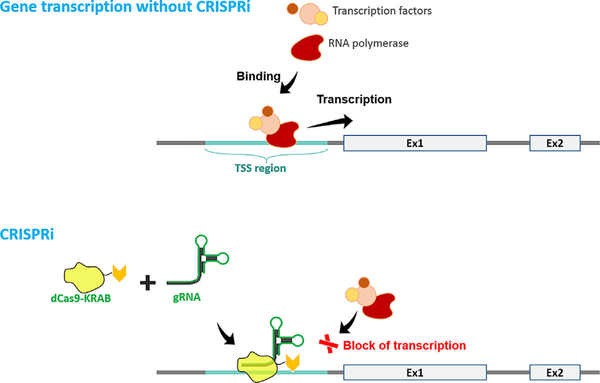
CRISPR tools are evolving quickly and many applications of this technology have been well reviewed [Dow, 2015]. Now, a nuclease-inactive “dead” Cas (dCas) developed by a team at the University of California, San Francisco is being used to decrease gene transcription through a strategy termed ‘CRISPRi’ (Fig. 7) [Qi et al., 2013]. dCas lacks endonuclease activity, so targeted blockage of transcriptional machinery occurs when dCas9 is coupled with a sequence-specific guide RNA, thus preventing RNA polymerase and transcription factors from transcribing genes. This strategy was shown to be applicable to eukaryotes, including human cells, when fused to transcription modulators [Gilbert et al., 2013; Maeder et al., 2013; Gilbert et al., 2014]. CRISPRi is precise and affects gene expression at the DNA level with minimal off-target effects, which is an improvement upon the previous strategy involving RNAi, which decreases expression at the mRNA level [Gilbert et al., 2014]. This may provide yet another strategy for achieving gene ablation for dominantly inherited retinal degenerations.
Figure 7. CRISPRi strategy.
In the absence of CRISPRi, transcription factors and RNA polymerase bind to the transcription start site (TSS), which leads to gene expression. Using CRISPRi, a “dead” Cas9 (dCas9) fused to a Krüppel associated box (KRAB) domain complexes with a gRNA that is complementary to a promoter or exonic sequence. This complex does not create a DSB and instead sterically blocks gene transcription.
CRISPR/Cas is becoming the preferred gene editing strategy due to its higher targeting efficiency and specificity compared with ZFN and TALEN. Unlike TALEN, CRISPR/Cas has been shown to be unaffected by DNA methylation status [Hsu et al., 2013; Ran et al., 2013b; Yaung et al., 2014]. Another recent advancement is the creation of Cas nickases, which provide single strand breaks (SSBs) instead of DSBs. This modification greatly enhances the specificity of this technique and reduces off-targeting effects, as gene correction only occurs if both strands undergo SSBs from Cas [Ran et al., 2013a; Shen et al., 2014]. Progress such as this will continue to expand the applicability of this technology to gene editing.
CELL-BASED THERAPIES FOR INHERITED RETINAL DISEASE
The retina arises from the neuroectoderm and thus, like other central nervous system tissue, has little regenerative potential. Inherited retinal diseases that cause irreversible photoreceptor death and subsequent blindness stand to benefit from cell-based therapies that can, in theory, regenerate functional retina. In contrast to gene therapy which corrects the mutated gene in existing viable cells, cell-based therapies can replace dead cells. Research is currently focused on producing functional retinal graft tissue or generating in vitro disease models to discern disease mechanisms [Yvon et al., 2015; Wang et al., 2016].
In contrast to gene therapy which corrects the mutated gene in existing viable cells, cell-based therapies can replace dead cells. Research is currently focused on producing functional retinal graft tissue or generating in vitro disease models to discern disease mechanisms.
In the early 1990s, adult and fetal RPE tissue was first transplanted in patients with end-stage age-related macular degeneration (AMD) to replace diseased RPE [Peyman et al., 1991; Algvere et al., 1994]. More recently, two phase I/II studies sponsored by Ocata Therapeutics (Marlborough, MA) finished testing the use of embryonic stem (ES) cell-derived RPE cells to treat advanced dry-AMD and Stargardt disease (NCT01344993 and NCT01345006, respectively). A total of 18 patients were enrolled, including nine patients with dry-AMD and nine patients with Stargardt disease. The trial reported adverse effects associated with vitreoretinal surgery and immunosuppression, including cataract progression in four patients and one case each of vitreous inflammation and culture-confirmed postoperative endophthalmitis. Importantly, no systemic or ocular effects occurred as a result of the transplanted tissue. Of the eighteen patients, best-corrected visual acuity improved in ten eyes and decreased more than ten letters in one eye compared to control eyes. Furthermore, subretinal pigmentary changes consistent with transplantation were observed in thirteen patients. OCT imaging of areas with increased pigmentation were suggestive of a layer of cells associated with parts of Bruch’s membrane [Schwartz et al., 2015].
Due to the controversy and ethical considerations surrounding the use of harvested fetal tissue, emphasis was placed on use of cultured cells for transplantation. iPSCs from a patient’s own fibroblasts can circumvent this issue [Lin et al., 2015]. Additionally, using autologous iPSCs decreases the risk of graft rejection. Although the eye is a relatively immune privileged organ, rejection is more likely when using cells from a non-autologous donor. iPSC-derived RPE cells were transplanted successfully in the RPE65rd12/rd12 mouse model [Li et al., 2012]. Li et al. demonstrated that the transplanted cells co-localized with native RPE. This study supports future initiatives to provide autologous iPSC transplantation for retinal degenerations with an etiology that is driven by RPE loss. The first iPSC transplantation in a human was performed in Japan under the direction of a team at the Riken Institute and the Institute of Biomedical Research and Innovation Hospital [Aoi, 2016]. The study was temporarily on hold when a mutation was believed to be found in a known oncogene during a genomic validation step. The study has since resumed, and the results will be important in determining the feasibility of iPSC transplantation in humans.
Meanwhile, the University of California, Davis is attempting other strategies for stem cell delivery. They are sponsoring a phase I trial to assess safety of autologous intravitreal bone-marrow CD34þ stem cells for various retinopathies, which is estimated to be completed in 2017 (NCT01736059). Preliminary findings showed that eyes tolerated the transplant, and cellular adaptive optics OCT imaging suggested incorporation of the transplanted cells into the macula [Park et al., 2015].
Cell-based therapies for disease characterized by an initial photoreceptor insult face larger challenges, because photoreceptors, unlike the RPE, need to correctly integrate with the neuronal circuitry [Lin et al., 2015]. Thus, the future of cell-based strategies in retinal disease with a photoreceptor-predominant pathophysiology will likely depend most on the ability of the cell-based therapy to not only become a viable photoreceptor, but correctly synapse in the complex visual processing pathway. Despite this challenge, progress has been made. Pearson et al. was able to use photoreceptor precursor cells in mice to integrate into host retina and improve vision [Pearson et al., 2012]. Another group confirmed the ability of human embryonic stem cells to form photoreceptor layers, structured graft maturation, and possible synaptic contacts between transplanted and host cells in primate models for photoreceptor degeneration [Shirai et al., 2016].
CONCLUSIONS: GENE AND CELL-BASED THERAPIES ARE PROMISING
The past decade has shown marked improvements in gene therapy and cell-based approaches as well as newer, large-impact techniques such as CRISPR. Ophthalmic conditions continue to be at the forefront of these developments, as ocular disease is more accessible to treatment strategies compared to other pathologies. Continued translational and randomized control studies are important for assessing various treatment modalities and stand to benefit many patients suffering from irreversible blindness.
ACKNOWLEDGMENTS
Jonas Children’s Vision Care, and Bernard & Shirlee Brown Glaucoma Laboratory are supported by the National Institute of Health [5P30EY019007, R01EY018213, R01EY024698, R01EY 026682, R21AG050437], National Cancer Institute Core [5P30CA013696], the Research to Prevent Blindness (RPB) Physician-Scientist Award, unrestricted funds from RPB, New York, NY, USA. J.D.S. is supported by the RPB medical student fellowship. T.C. is supported by the International Council of Ophthalmology—Retina Research Foundation Helmerich Fellowship, honoring Mr. W.H. Helmerich III. S.H.T. is a member of the RD-CURE Consortium and is supported by the Tistou and Charlotte Kerstan Foundation, the Schneeweiss Stem Cell Fund, New York State [C029572], the Foundation Fighting Blindness New York Regional Research Center Grant [C-NY05-0705-0312], the Joel Hoffman Fund, the Professor Gertrude Rothschild Stem Cell Foundation, and the Gebroe Family Foundation.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Contributor Information
JESSE D. SENGILLO, Columbia University Medical Center.
SALLY JUSTUS, Columbia University Medical Center.
YI-TING TSAI, Institute of Human Nutrition at Columbia University.
THIAGO CABRAL, Columbia University.
STEPHEN H. TSANG, New York-Presbyterian Hospital.
REFERENCES
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. 2001. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 28:92–95. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Umino Y, Everhart D, Chang B, Min SH, Li Q, Timmers AM, Hawes NL, Pang JJ, Barlow RB, Hauswirth WW. 2007. Restoration of cone vision in a mouse model of achromatopsia. Nat Med 13:685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algvere PV, Berglin L, Gouras P, Sheng Y. 1994. Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefes Arch Clin Exp Ophthalmol 232:707–716. [DOI] [PubMed] [Google Scholar]
- Allocca M, Doria M, Petrillo M, Colella P, GarciaHoyos M, Gibbs D, Kim SR, Maguire A, Rex TS, Di Vicino U, Cutillo L, Sparrow JR, Williams DS, Bennett J, Auricchio A. 2008. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest 118:1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand V, Barral DC, Zeng Y, Brunsmann F, Maguire AM, Seabra MC, Bennett J. 2003. Gene therapy for choroideremia: In vitro rescue mediated by recombinant adenovirus. Vision Res 43:919–926. [DOI] [PubMed] [Google Scholar]
- Aoi T 2016. 10th anniversary of iPS Cells: The challenges that lie ahead. J Biochem 160:121–129. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. 2008. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 358:2231–2239. [DOI] [PubMed] [Google Scholar]
- Bakondi B, Lv W, Lu B, Jones MK, Tsai Y, Kim KJ, Levy R, Akhtar AA, Breunig JJ, Svendsen CN, Wang S. 2016. In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Mol Ther 24:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin E, Bandah-Rozenfeld D, Obolensky A, Cideciyan AV, Aleman TS, Marks-Ohana D, Sela M, Boye S, Sumaroka A, Roman AJ, Schwartz SB, Hauswirth WW, Jacobson SG, Hemo I, Sharon D. 2010. Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: Human gene therapy initiated in Israel. Hum Gene Ther 21:1749–1757. [DOI] [PubMed] [Google Scholar]
- Banin E, Gootwine E, Obolensky A, Ezra-Elia R, Ejzenberg A, Zelinger L, Honig H, Rosov A, Yamin E, Sharon D, Averbukh E, Hauswirth WW, Ofri R. 2015. Gene augmentation therapy restores retinal function and visual behavior in a sheep model of CNGA3 achromatopsia. Mol Ther 23:1423–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk AG, Zheng A, Li Y, Tsang SH, Mahajan VB. 2016. Precision medicine: Genetic repair of retinitis pigmentosa in patient-derived stem cells. Sci Rep 6:19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A, Chiodo VA, Fajardo DS, Roman AJ, Deng WT, Swider M, Aleman TS, Boye SL, Genini S, Swaroop A, Hauswirth WW, Jacobson SG, Aguirre GD. 2012. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci USA 109:2132–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, McCague S, Pierce EA, Chen Y, Bennicelli JL, Zhu X, Ying GS, Sun J, Wright JF, Auricchio A, Simonelli F, Shindler KS, Mingozzi F, High KA, Maguire AM. 2012. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med 4:120ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Wellman J, Marshall KA, McCague S, Ashtari M, DiStefano-Pappas J, Elci OU, Chung DC, Sun J, Wright JF, Cross DR, Aravand P, Cyckowski LL, Bennicelli JL, Mingozzi F, Auricchio A, Pierce EA, Ruggiero J, Leroy BP, Simonelli F, High KA, Maguire AM. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: A follow-on phase 1 trial. Lancet 388:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson EL. 1993. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 34:1659–1676. [PubMed] [Google Scholar]
- Birch DG, Weleber RG, Duncan JL, Jaffe GJ, Tao W, Ciliary neurotrophic factor retinitis pigmentosa study G. 2013. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am J Ophthalmol 156:283–292e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye S, Boye S. 2015. Genetic therapies for inheritied retinal disease: Adeno-associated viruses have shown promise. Retinal Physician 12:19–24. [Google Scholar]
- Boye SE, Boye SL, Pang J, Ryals R, Everhart D, Umino Y, Neeley AW, Besharse J, Barlow R, Hauswirth WW. 2010. Functional and behavioral restoration of vision by gene therapy in the guanylate cyclase-1 (GC1) knockout mouse. PLoS ONE 5:e11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SL, Conlon T, Erger K, Ryals R, Neeley A, Cossette T, Pang J, Dyka FM, Hauswirth WW, Boye SE. 2011. Long-term preservation of cone photoreceptors and restoration of cone function by gene therapy in the guanylate cyclase-1 knockout (GC1KO) mouse. Invest Ophthalmol Vis Sci 52: 7098–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SL, Peshenko IV, Huang WC, Min SH, McDoom I, Kay CN, Liu X, Dyka FM, Foster TC, Umino Y, Karan S, Jacobson SG, Baehr W, Dizhoor A, Hauswirth WW, Boye SE. 2013. AAV-mediated gene therapy in the guanylate cyclase (RetGC1/RetGC2) double knockout mouse model of Leber congenital amaurosis. Hum Gene Ther 24:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SL, Peterson JJ, Choudhury S, Min SH, Ruan Q, McCullough KT, Zhang Z, Olshevskaya EV, Peshenko IV, Hauswirth WW, Ding XQ, Dizhoor AM, Boye SE. 2015. Gene therapy fully restores vision to the all-Cone nrl(−/−) gucy2e(−/−) mouse model of leber congenital amaurosis-1. Hum Gene Ther 26:575–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branham K, Othman M, Brumm M, Karoukis AJ, Atmaca-Sonmez P, Yashar BM, Schwartz SB, Stover NB, Trzupek K, Wheaton D, Jennings B, Ciccarelli ML, Jayasundera KT, Lewis RA, Birch D, Bennett J, Sieving PA, Andreasson S, Duncan JL, Fishman GA, Iannaccone A, Weleber RG, Jacobson SG, Heckenlively JR, Swaroop A. 2012. Mutations in RPGR and RP2 account for 15% of males with simplex retinal degenerative disease. Invest Ophthalmol Vis Sci 53:8232–8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner S, Skosyrski S, Kirschner-Schwabe R, Knobeloch KP, Neidhardt J, Feil S, Glaus E, Luhmann UF, Ruther K, Berger W. 2010. Cone versus rod disease in a mutant Rpgr mouse caused by different genetic backgrounds. Invest Ophthalmol Vis Sci 51:1106–1115. [DOI] [PubMed] [Google Scholar]
- Byrne LC, Dalkara D, Luna G, Fisher SK, Clerin E, Sahel JA, Leveillard T, Flannery JG. 2015. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J Clin Invest 125:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne LC, Ozturk BE, Lee T, Fortuny C, Visel M, Dalkara D, Schaffer DV, Flannery JG. 2014. Retinoschisin gene therapy in photoreceptors, Muller glia or all retinal cells in the Rs1h−/− mouse. Gene Ther 21: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D 2014. Genome engineering with targetable nucleases. Annu Rev Biochem 83:409–439. [DOI] [PubMed] [Google Scholar]
- Carvalho LS, Xu J, Pearson RA, Smith AJ, Bainbridge JW, Morris LM, Fliesler SJ, Ding XQ, Ali RR. 2011. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet 20:3161–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL, Vandenberghe LH. 2013. Retinal gene therapy coming of age. Hum Gene Ther 24:242–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Camacho OF, Zenteno JC. 2015. Review and update on the molecular basis of Leber congenital amaurosis. World J Clin Cases 3:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill JD, Bowne SJ, Sullivan LS, Lewis RA, Wheaton DK, Birch DG, Branham KE, Heckenlively JR, Daiger SP. 2013. Mutations in the X-linked retinitis pigmentosa genes RPGR and RP2 found in 8.5% of families with a provisional diagnosis of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 54:1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, Flotte TR, Fishman GA, Heon E, Stone EM, Byrne BJ, Jacobson SG, Hauswirth WW. 2008. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA 105:15112–15117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Pang JJ, Roman AJ, Byrne BJ, Jacobson SG. 2009. Human RPE65 gene therapy for Leber congenital amaurosis: Persistence of early visual improvements and safety at 1 year. Hum Gene Ther 20:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komaromy AM, Hauswirth WW, Aguirre GD. 2013. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA 110:E517–E525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Sommella A, Marrocco E, Di Vicino U, Polishchuk E, Garcia Garrido M, Seeliger MW, Polishchuk R, Auricchio A. 2013. Myosin7a deficiency results in reduced retinal activity which is improved by gene therapy. PLoS ONE 8:e72027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon TJ, Deng WT, Erger K, Cossette T, Pang JJ, Ryals R, Clement N, Cleaver B, McDoom I, Boye SE, Peden MC, Sherwood MB, Abernathy CR, Alkuraya FS, Boye SL, Hauswirth WW. 2013. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Dev 24:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers FP, Sankila EM, Brunsmann F, Jay M, Jay B, Wright A, Pinckers AJ, Schwartz M, van de Pol DJ, Wieringa B, et al. 1990. Deletions in patients with classical choroideremia vary in size from 45 kb to several megabases. Am J Hum Genet 47:622–628. [PMC free article] [PubMed] [Google Scholar]
- Cwerman-Thibault H, Augustin S, Ellouze S, Sahel JA, Corral-Debrinski M. 2014. Gene therapy for mitochondrial diseases: Leber Hereditary Optic Neuropathy as the first candidate for a clinical trial. C R Biol 337:193–206. [DOI] [PubMed] [Google Scholar]
- Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. 2013. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 5:189ra176. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Hsu CW, Tsai YT, Wert KJ, Sancho-Pelluz J, Lin CS, Tsang SH. 2013. Therapeutic margins in a novel preclinical model of retinitis pigmentosa. J Neurosci 33: 13475–13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya S, Berns KI. 2008. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev 21:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos IS, Chan S, MacLaren RE, Mac-Donald IM. 2015. Pathogenic mechanisms and the prospect of gene therapy for choroideremia. Expert Opin Orphan Drugs 3:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E. 2014. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346:1258096. [DOI] [PubMed] [Google Scholar]
- Dow LE. 2015. Modeling disease In vivo with CRISPR/Cas9. Trends Mol Med 21:609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyka FM, Boye SL, Chiodo VA, Hauswirth WW, Boye SE. 2014. Dual adeno-associated virus vectors result in efficient in vitro and in vivo expression of an oversized gene, MYO7A. Hum Gene Ther Methods 25:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amraoui A, Petit C. 2014. The retinal phenotype of Usher syndrome: Pathophysiological insights from animal models. C R Biol 337:167–177. [DOI] [PubMed] [Google Scholar]
- Ellouze S, Augustin S, Bouaita A, Bonnet C, Simonutti M, Forster V, Picaud S, Sahel JA, Corral-Debrinski M. 2008. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am J Hum Genet 83:373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim AT, Daiger SP, Weleber RG. 1993. Retinitis pigmentosa overview. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K, editors. Seattle (WA): GeneReviews(R). [Google Scholar]
- Feuer WJ, Schiffman JC, Davis JL, Porciatti V, Gonzalez P, Koilkonda RD, Yuan H, Lalwani A, Lam BL, Guy J. 2016. Gene therapy for leber hereditary optic neuropathy: Initial results. Ophthalmology 123: 558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J, Zarrouk-Mahjoub S. 2016. Re: Feuer et al. : Gene therapy for Leber hereditary optic neuropathy: Initial results (Ophthalmology 2016;123:558–70). Ophthalmology 123:e44–e45. [DOI] [PubMed] [Google Scholar]
- Fisher JK, Bromley RL, Mansfield BC. 2016. My retina tracker: An on-line international registry for people affected with inherited orphan retinal degenerative diseases and their genetic relatives—a new resource. Adv Exp Med Biol 854:245–251. [DOI] [PubMed] [Google Scholar]
- Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. 2000. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet 26:270–271. [DOI] [PubMed] [Google Scholar]
- Ghazi NG, Abboud EB, Nowilaty SR, Alkuraya H, Alhommadi A, Cai H, Hou R, Deng WT, Boye SL, Almaghamsi A, Al Saikhan F, Al-Dhibi H, Birch D, Chung C, Colak D, LaVail MM, Vollrath D, Erger K, Wang W, Conlon T, Zhang K, Hauswirth W, Alkuraya FS. 2016. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: Results of a phase I trial. Hum Genet 135:327–343. [DOI] [PubMed] [Google Scholar]
- Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, Qi LS, Kampmann M, Weissman JS. 2014. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159:647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS. 2013. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles AF, Averof M. 2014. Functional genetics for all: Engineered nucleases, CRISPR and the gene editing revolution. Evodevo 5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald DL, Cashman SM, Kumar-Singh R. 2010. Engineered zinc finger nuclease-mediated homologous recombination of the human rhodopsin gene. Invest Ophthalmol Vis Sci 51:6374–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Qi X, Koilkonda RD, Arguello T, Chou TH, Ruggeri M, Porciatti V, Lewin AS, Hauswirth WW. 2009. Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system. Invest Ophthalmol Vis Sci 50:4205–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Qi X, Pallotti F, Schon EA, Manfredi G, Carelli V, Martinuzzi A, Hauswirth WW, Lewin AS. 2002. Rescue of a mitochondrial deficiency causing leber hereditary optic neuropathy. Ann Neurol 52:534–542. [DOI] [PubMed] [Google Scholar]
- Hamel C 2006. Retinitis pigmentosa. Orphanet J Rare Dis 1:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. 2006. Retinitis pigmentosa. Lancet 368:1795–1809. [DOI] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. 2008. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum Gene Ther 19:979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DH, Pawlyk B, Sokolov M, Strissel KJ, Yang J, Tulloch B, Wright AF, Arshavsky VY, Li T. 2003. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci 44:2413–2421. [DOI] [PubMed] [Google Scholar]
- Hong DH, Pawlyk BS, Adamian M, Li T. 2004. Dominant, gain-of-function mutant produced by truncation of RPGR. Invest Ophthalmol Vis Sci 45:36–41. [DOI] [PubMed] [Google Scholar]
- Hong DH, Pawlyk BS, Shang J, Sandberg MA, Berson EL, Li T. 2000. A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3). Proc Natl Acad Sci USA 97:3649–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. 2013. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31:827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Wright AF, Roman AJ, Cideciyan AV, Manson FD, Gewaily DY, Schwartz SB, Sadigh S, Limberis MP, Bell P, Wilson JM, Swaroop A, Jacobson SG. 2012. RPGR-associated retinal degeneration in human X-linked RP and a murine model. Invest Ophthalmol Vis Sci 53:5594–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JB, Chao JR. 2015. It’s never too late to save a photoreceptor. J Clin Invest 125:3424–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Acland GM, Aguirre GD, Aleman TS, Schwartz SB, Cideciyan AV, Zeiss CJ, Komaromy AM, Kaushal S, Roman AJ, Windsor EA, Sumaroka A, Pearce-Kelling SE, Conlon TJ, Chiodo VA, Boye SL, Flotte TR, Maguire AM, Bennett J, Hauswirth WW. 2006a. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther 13:1074–1084. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Boye SL, Aleman TS, Conlon TJ, Zeiss CJ, Roman AJ, Cideciyan AV, Schwartz SB, Komaromy AM, Doobrajh M, Cheung AY, Sumaroka A, Pearce-Kelling SE, Aguirre GD, Kaushal S, Maguire AM, Flotte TR, Hauswirth WW. 2006b. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther 17:845–858. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Peshenko IV, Sumaroka A, Olshevskaya EV, Cao L, Schwartz SB, Roman AJ, Olivares MB, Sadigh S, Yau KW, Heon E, Stone EM, Dizhoor AM. 2013. Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human Leber congenital amaurosis en route to therapy: Residual cone-photoreceptor vision correlates with biochemical properties of the mutants. Hum Mol Genet 22:168–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, Peden MC, Aleman TS, Boye SL, Sumaroka A, Conlon TJ, Calcedo R, Pang JJ, Erger KE, Olivares MB, Mullins CL, Swider M, Kaushal S, Feuer WJ, Iannaccone A, Fishman GA, Stone EM, Byrne BJ, Hauswirth WW. 2012. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: Safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol 130:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Roman AJ, Sumaroka A, Schwartz SB, Heon E, Hauswirth WW. 2015. Improvement and decline in vision with gene therapy in childhood blindness. N Engl J Med 372:1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen A, Min SH, Molday LL, Tanimoto N, Seeliger MW, Hauswirth WW, Molday RS, Weber BH. 2008. Effect of late-stage therapy on disease progression in AAV-mediated rescue of photoreceptor cells in the retinoschisin-deficient mouse. Mol Ther 16:1010–1017. [DOI] [PubMed] [Google Scholar]
- Jatana KR, Thomas D, Weber L, Mets MB, Silverman JB, Young NM. 2013. Usher syndrome: Characteristics and outcomes of pediatric cochlear implant recipients. Otol Neurotol 34:484–489. [DOI] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J, Dyka FM, Kasuga D, Ayala AE, Van Vliet K, Agbandje-McKenna M, Hauswirth WW, Boye SL, Boye SE. 2013. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS ONE 8:e62097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberling WJ, Hildebrand MS, Shearer AE, Jensen ML, Halder JA, Trzupek K, Cohn ES, Weleber RG, Stone EM, Smith RJ. 2010. Frequency of Usher syndrome in two pediatric populations: Implications for genetic screening of deaf and hard of hearing children. Genet Med 12:512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellstrom S, Bush RA, Zeng Y, Takada Y, Sieving PA. 2007. Retinoschisin gene therapy and natural history in the Rs1hKO mouse: Long-term rescue from retinal degeneration. Invest Ophthalmol Vis Sci 48:3837–3845. [DOI] [PubMed] [Google Scholar]
- Koch SF, Tsai YT, Duong JK, Wu WH, Hsu CW, Wu WP, Bonet-Ponce L, Lin CS, Tsang SH. 2015. Halting progressive neurodegeneration in advanced retinitis pigmentosa. J Clin Invest 125:3704–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenekoop RK. 2004. An overview of Leber congenital amaurosis: A model to understand human retinal development. Surv Ophthalmol 49:379–398. [DOI] [PubMed] [Google Scholar]
- Kohl S, Hamel C. 2013. Clinical utility gene card for: Achromatopsia—update 2013. Eur J Hum Genet 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S, Varsanyi B, Antunes GA, Baumann B, Hoyng CB, Jagle H, Rosenberg T, Kellner U, Lorenz B, Salati R, Jurklies B, Farkas A, Andreasson S, Weleber RG, Jacobson SG, Rudolph G, Castellan C, Dollfus H, Legius E, Anastasi M, Bitoun P, Lev D, Sieving PA, Munier FL, Zrenner E, Sharpe LT, Cremers FP, Wissinger B. 2005. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet 13:302–308. [DOI] [PubMed] [Google Scholar]
- Koilkonda RD, Guy J. 2011. Leber’s hereditary optic neuropathy-gene therapy: From benchtop to bedside. J Ophthalmol 2011: 179412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A, Tanaka JC, Acland GM, Hauswirth WW, Aguirre GD. 2010. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet 19:2581–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BL, Feuer WJ, Schiffman JC, Porciatti V, Vandenbroucke R, Rosa PR, Gregori G, Guy J. 2014. Trial end points and natural history in patients with G11778A Leber hereditary optic neuropathy: Preparation for gene therapy clinical trial. JAMA Ophthalmol 132:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. 2008. Novel AAV serotypes for improved ocular gene transfer. J Gene Med 10:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz JJ, Jodelka FM, Hinrich AJ, McCaffrey KE, Farris HE, Spalitta MJ, Bazan NG, Duelli DM, Rigo F, Hastings ML. 2013. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat Med 19:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tsai YT, Hsu CW, Erol D, Yang J, Wu WH, Davis RJ, Egli D, Tsang SH. 2012. Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa. Mol Med 18:1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MK, Tsai YT, Tsang SH. 2015. Emerging treatments for retinitis pigmentosa: Genes and stem cells, as well as new electronic and medical therapies, are gaining ground. Retin Physician 12:52–70. [PMC free article] [PubMed] [Google Scholar]
- Linari M, Ueffing M, Manson F, Wright A, Meitinger T, Becker J. 1999. The retinitis pigmentosa GTPase regulator, RPGR, interacts with the delta subunit of rod cyclic GMP phosphodiesterase. Proc Natl Acad Sci USA 96:1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes VS, Boye SE, Louie CM, Boye S, Dyka F, Chiodo V, Fofo H, Hauswirth WW, Williams DS. 2013. Retinal gene therapy with a large MYO7A cDNA using adeno-associated virus. Gene Ther 20:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low BE, Krebs MP, Joung JK, Tsai SQ, Nishina PM, Wiles MV. 2014. Correction of the Crb1rd8 allele and retinal phenotype in C57BL/6N mice via TALEN-mediated homology-directed repair. Invest Ophthalmol Vis Sci 55:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, Downes SM, Webster AR, Seabra MC. 2014. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 383:1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, Joung JK. 2013. CRISPR RNA-guided activation of endogenous human genes. Nat Methods 10:977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JI, Hauck B, Zelenaia O, Zhu X, Raffini L, Coppieters F, De Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun J, McDonnell JW, Leroy BP, Simonelli F, Bennett J. 2009. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet 374:1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr., Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. 2008. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 358:2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Yang J. 2015. Usher syndrome: Hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta 1852:406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Muhlfriedel R, Tanimoto N, Krishnamoorthy V, Koch S, Fischer MD, Becirovic E, Bai L, Huber G, Beck SC, Fahl E, Buning H, Paquet-Durand F, Zong X, Gollisch T, Biel M, Seeliger MW. 2010. Restoration of cone vision in the CNGA3−/− mouse model of congenital complete lack of cone photoreceptor function. Mol Ther 18:2057–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihelec M, Pearson RA, Robbie SJ, Buch PK, Azam SA, Bainbridge JW, Smith AJ, Ali RR. 2011. Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of leber congenital amaurosis caused by guanylate cyclase-1 deficiency. Hum Gene Ther 22:1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SH, Molday LL, Seeliger MW, Dinculescu A, Timmers AM, Janssen A, Tonagel F, Tanimoto N, Weber BH, Molday RS, Hauswirth WW. 2005. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of x-linked juvenile retinoschisis. Mol Ther 12:644–651. [DOI] [PubMed] [Google Scholar]
- Molday LL, Min SH, Seeliger MW, Wu WW, Dinculescu A, Timmers AM, Janssen A, Tonagel F, Hudl K, Weber BH, Hauswirth WW, Molday RS. 2006. Disease mechanisms and gene therapy in a mouse model for X-linked retinoschisis. Adv Exp Med Biol 572:283–289. [DOI] [PubMed] [Google Scholar]
- Natkunarajah M, Trittibach P, McIntosh J, Duran Y, Barker SE, Smith AJ, Nathwani AC, Ali RR. 2008. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8. Gene Ther 15:463–467. [DOI] [PubMed] [Google Scholar]
- Ou J, Vijayasarathy C, Ziccardi L, Chen S, Zeng Y, Marangoni D, Pope JG, Bush RA, Wu Z, Li W, Sieving PA. 2015. Synaptic pathology and therapeutic repair in adult retinoschisis mouse by AAV-RS1 transfer. J Clin Invest 125:2891–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overlack N, Goldmann T, Wolfrum U, Nagel-Wolfrum K. 2012. Gene repair of an Usher syndrome causing mutation by zincfinger nuclease mediated homologous recombination. Invest Ophthalmol Vis Sci 53:4140–4146. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Deng WT, Dai X, Lei B, Everhart D, Umino Y, Li J, Zhang K, Mao S, Boye SL, Liu L, Chiodo VA, Liu X, Shi W, Tao Y, Chang B, Hauswirth WW. 2012. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PLoS ONE 7:e35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SS, Bauer G, Abedi M, Pontow S, Panorgias A, Jonnal R, Zawadzki RJ, Werner JS, Nolta J. 2015. Intravitreal autologous bone marrow CD34þ cell therapy for ischemic and degenerative retinal disorders: Preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci 56:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TK, Wu Z, Kjellstrom S, Zeng Y, Bush RA, Sieving PA, Colosi P. 2009. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther 16:916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, Duran Y, Smith AJ, Chuang JZ, Azam SA, Luhmann UF, Benucci A, Sung CH, Bainbridge JW, Carandini M, Yau KW, Sowden JC, Ali RR. 2012. Restoration of vision after transplantation of photoreceptors. Nature 485:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Leal JB, Hume AN, Seabra MC. 2001. Prenylation of Rab GTPases: Molecular mechanisms and involvement in genetic disease. FEBS Lett 498:197–200. [DOI] [PubMed] [Google Scholar]
- Peyman GA, Blinder KJ, Paris CL, Alturki W, Nelson NC Jr., Desai U. 1991. A technique for retinal pigment epithelium transplantation for age-related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surg 22:102–108. [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Sun L, Lewin AS, Hauswirth WW, Guy J. 2007. The mutant human ND4 subunit of complex I induces optic neuropathy in the mouse. Invest Ophthalmol Vis Sci 48:1–10. [DOI] [PubMed] [Google Scholar]
- Raghupathy RK, Gautier P, Soares DC, Wright AF, Shu X. 2015. Evolutionary characterization of the retinitis pigmentosa GTPase regulator gene. Invest Ophthalmol Vis Sci 56:6255–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. 2013a. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154:1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. 2013b. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM. 2009. Focus on Molecules: RPE65, the visual cycle retinol isomerase. Exp Eye Res 88:846–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander JD, Joung JK. 2014. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer CG, Gehrig A, Warneke-Wittstock R, Marquardt A, Ewing CC, Gibson A, Lorenz B, Jurklies B, Weber BH. 1997. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet 17:164–170. [DOI] [PubMed] [Google Scholar]
- Schubert T, Wissinger B. 2015. Restoration of synaptic function in sight for degenerative retinal disease. J Clin Invest 125:2572–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. 2015. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 385:509–516. [DOI] [PubMed] [Google Scholar]
- Seitz IP, Zhour A, Kohl S, Llavona P, Peter T, Wilhelm B, Zrenner E, Ueffing M, Bartz-Schmidt KU, Fischer MD. 2015. Multimodal assessment of choroideremia patients defines pre-treatment characteristics. Graefes Arch Clin Exp Ophthalmol 253:2143–2150. [DOI] [PubMed] [Google Scholar]
- Senis E, Fatouros C, Grosse S, Wiedtke E, Niopek D, Mueller AK, Borner K, Grimm D. 2014. CRISPR/Cas9-mediated genome engineering: An adeno-associated viral (AAV) vector toolbox. Biotechnol J 9:1402–1412. [DOI] [PubMed] [Google Scholar]
- Shen B, Zhang W, Zhang J, Zhou J, Wang J, Chen L, Wang L, Hodgkins A, Iyer V, Huang X, Skarnes WC. 2014. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat Methods 11:399–402. [DOI] [PubMed] [Google Scholar]
- Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, Assawachananont J, Kimura T, Saito K, Terasaki H, Eiraku M, Sasai Y, Takahashi M. 2016. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci USA 113:E81–E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. 2010. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 18:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Schlichtenbrede FC, Tschernutter M, Bainbridge JW, Thrasher AJ, Ali RR. 2003. AAV-Mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa. Mol Ther 8:188–195. [DOI] [PubMed] [Google Scholar]
- Smith J, Ward D, Michaelides M, Moore AT, Simpson S. 2015. New and emerging technologies for the treatment of inherited retinal diseases: A horizon scanning review. Eye (Lond) 29:1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohocki MM, Daiger SP, Bowne SJ, Rodriquez JA, Northrup H, Heckenlively JR, Birch DG, Mintz-Hittner H, Ruiz RS, Lewis RA, Saperstein DA, Sullivan LS. 2001. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat 17:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souied E, Segues B, Ghazi I, Rozet JM, Chatelin S, Gerber S, Perrault I, Michel-Awad A, Briard ML, Plessis G, Dufier JL, Munnich A, Kaplan J. 1997. Severe manifestations in carrier females in X linked retinitis pigmentosa. J Med Genet 34:793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Vijayasarathy C, Zeng Y, Kjellstrom S, Bush RA, Sieving PA. 2008. Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery. Invest Ophthalmol Vis Sci 49:3677–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K, Banfi S, Surace EM, Sun J, Acerra C, Wright JF, Wellman J, High KA, Auricchio A, Bennett J, Simonelli F. 2013. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology 120:1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmachova T, Anders R, Abrink M, Bugeon L, Dallman MJ, Futter CE, Ramalho JS, Tonagel F, Tanimoto N, Seeliger MW, Huxley C, Seabra MC. 2006. Independent degeneration of photoreceptors and retinal pigment epithelium in conditional knockout mouse models of choroideremia. J Clin Invest 116:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmachova T, Tolmachov OE, Barnard AR, de Silva SR, Lipinski DM, Walker NJ, Maclaren RE, Seabra MC. 2013. Functional expression of Rab escort protein 1 following AAV2-mediated gene delivery in the retina of choroideremia mice and human cells ex vivo. J Mol Med (Berl) 91:825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmachova T, Wavre-Shapton ST, Barnard AR, MacLaren RE, Futter CE, Seabra MC. 2010. Retinal pigment epithelium defects accelerate photoreceptor degeneration in cell type-specific knockout mouse models of choroideremia. Invest Ophthalmol Vis Sci 51:4913–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani I, Colella P, Sommella A, Iodice C, Cesi G, de Simone S, Marrocco E, Rossi S, Giunti M, Palfi A, Farrar GJ, Polishchuk R, Auricchio A. 2014. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med 6:194–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschernutter M, Schlichtenbrede FC, Howe S, Balaggan KS, Munro PM, Bainbridge JW, Thrasher AJ, Smith AJ, Ali RR. 2005. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther 12:694–701. [DOI] [PubMed] [Google Scholar]
- Vollrath D, Feng W, Duncan JL, Yasumura D, D’Cruz PM, Chappelow A, Matthes MT, Kay MA, LaVail MM. 2001. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci USA 98:12584–12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia S, Fishman GA, Jacobson SG, Aleman TS, Koenekoop RK, Traboulsi EI, Weleber RG, Pennesi ME, Heon E, Drack A, Lam BL, Allikmets R, Stone EM. 2010. Visual acuity in patients with Leber’s congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology 117:1190–1198. [DOI] [PubMed] [Google Scholar]
- Wan X, Pei H, Zhao MJ, Yang S, Hu WK, He H, Ma SQ, Zhang G, Dong XY, Chen C, Wang DW, Li B. 2016. Efficacy and safety of rAAV2-ND4 treatment for leber’s hereditary optic neuropathy. Sci Rep 6:21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Stern JH, Temple S. 2016. Regenerative medicine: Solution in sight. Adv Exp Med Biol 854:543–548. [DOI] [PubMed] [Google Scholar]
- Weber BH, Schrewe H, Molday LL, Gehrig A, White KL, Seeliger MW, Jaissle GB, Friedburg C, Tamm E, Molday RS. 2002. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proc Natl Acad Sci USA 99:6222–6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wert KJ, Lin JH, Tsang SH. 2014a. General pathophysiology in retinal degeneration. Dev Ophthalmol 53:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wert KJ, Sancho-Pelluz J, Tsang SH. 2014b. Midstage intervention achieves similar efficacy as conventional early-stage treatment using gene therapy in a pre-clinical model of retinitis pigmentosa. Hum Mol Genet 23:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Lopes VS. 2011. The many different cellular functions of MYO7A in the retina. Biochem Soc Trans 39:1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RN, Hong DH, Perkins B. 2011. Misexpression of the constitutive Rpgr(ex1–19) variant leads to severe photoreceptor degeneration. Invest Ophthalmol Vis Sci 52:5189–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaung SJ, Esvelt KM, Church GM. 2014. CRISPR/Cas9-mediated phage resistance is not impeded by the DNA modifications of phage T4. PLoS ONE 9:e98811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye GJ, Budzynski E, Sonnentag P, Miller PE, Sharma AK, Ver Hoeve JN, Howard K, Knop DR, Neuringer M, McGill T, Stoddard J, Chulay JD. 2015a. Safety and biodistribution evaluation in cynomolgus macaques of rAAV2tYF-CB-hRS1, a recombinant adeno-Associated virus vector expressing retinoschisin. Hum Gene Ther Clin Dev 26:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye GJ, Conlon T, Erger K, Sonnentag P, Sharma AK, Howard K, Knop DR, Chulay JD. 2015b. Safety and biodistribution evaluation of rAAV2tYF-CB-hRS1, a recombinant adeno-Associated virus vector expressing retinoschisin, in RS1-Deficient mice. Hum Gene Ther Clin Dev 26:177–184. [DOI] [PubMed] [Google Scholar]
- Yu-Wai-Man P 2016. Genetic manipulation for inherited neurodegenerative diseases: Myth or reality? Br J Ophthalmol 100:1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon C, Ramsden CM, Lane A, Powner MB, da Cruz L, Coffey PJ, Carr AJ. 2015. Using stem cells to model diseases of the outer retina. Comput Struct Biotechnol J 13:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallocchi M, Binley K, Lad Y, Ellis S, Widdowson P, Iqball S, Scripps V, Kelleher M, Loader J, Miskin J, Peng YW, Wang WM, Cheung L, Delimont D, Mitrophanous KA, Cosgrove D. 2014. EIAV-based retinal gene therapy in the shaker1 mouse model for usher syndrome type 1B: Development of UshStat. PLoS ONE 9:e94272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Takada Y, Kjellstrom S, Hiriyanna K, Tanikawa A, Wawrousek E, Smaoui N, Caruso R, Bush RA, Sieving PA. 2004. RS1 gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-Linked retinoschisis. Invest Ophthalmol Vis Sci 45:3279–3285. [DOI] [PubMed] [Google Scholar]
- Zhang Q Retinitis pigmentosa: Progress and perspective. Asia Pac J Ophthalmol (Phila) 5:265–271. [DOI] [PubMed] [Google Scholar]
- Zinkernagel MS, MacLaren RE. 2015. Recent advances and future prospects in choroideremia. Clin Ophthalmol 9:2195–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobor D, Zobor G, Kohl S. 2015. Achromatopsia: On the doorstep of a possible therapy. Ophthalmic Res 54:103–108. [DOI] [PubMed] [Google Scholar]