Genetic approaches identify specific branches of the jasmonate synthesis pathway that are involved in wound-response growth restriction.
Abstract
Wound-response plant growth restriction requires the synthesis of potent mediators called jasmonates (JAs). Four 13-lipoxygenases (13-LOXs) produce JA precursors in Arabidopsis (Arabidopsis thaliana) leaves, but the 13-LOXs responsible for growth restriction have not yet been identified. Through loss-of-function genetic analyses, we identified LOX3 and LOX4 as the principal 13-LOXs responsible for vegetative growth restriction after repetitive wounding. Additional genetic studies were carried out in the gain-of-function fatty acid oxygenation 2 (fou2) mutant that, even when undamaged, shows JA-dependent leaf growth restriction. The fou2 lox3 lox4 triple mutant suppressed the fou2 JA-dependent growth phenotype, confirming that LOX3 and LOX4 function in leaf growth restriction. The fou2 mutation affects the TWO PORE CHANNEL1 (TPC1) ion channel. Additional genetic approaches based on this gene were used to further investigate LOX3 function in relation to leaf growth. To activate LOX3-dependent JA production in unwounded plants, we employed hyperactive TPC1 variants. Expression of the TPC1ΔCai variant in phloem companion cells caused strongly reduced rosette growth in the absence of wounding. Summarizing, in parallel to their established roles in male reproductive development in Arabidopsis, LOX3 and LOX4 control leaf growth rates after wounding. The process of wound-response growth restriction can be recapitulated in unwounded plants when the LOX3 pathway is activated genetically using a hyperactive vacuolar cation channel.
Herbivore damage can lead to spectacular changes in the growth of long-lived plants. Similarly, long-term plant growth can be modified strikingly as a result of repetitive leaf wounding inflicted by skilled bonsai gardeners. These effects are characterized by altered vegetative growth often leading to stunted plants. In the laboratory, and over briefer timescales, comparable effects on plant growth can be observed in short-lived species including Arabidopsis (Arabidopsis thaliana). For example, when the rosettes of this species are subject to repetitive wounds over a period of ∼3 weeks, the newly formed leaves they produce are smaller with shortened petioles compared to those on unwounded plants (Yan et al., 2007). These effects on growth were found to depend on the production of the lipidic regulator jasmonate (JA). Specifically, leaf and petiole growth restriction was strongly attenuated in the JA-synthesis mutant allene oxide synthase (aos; Yan et al., 2007) and in a JA-signaling mutant (Zhang and Turner, 2008). Therefore, a key function of JA pathway-activating signals produced in damaged organs is to travel to apical tissues to reprogram future growth, allowing plants to optimize their defense strategies (Huot et al., 2014; Guo et al., 2018; Ballaré and Austin, 2019; Fernández-Milmanda et al., 2020). Importantly, the activation of JA signaling after wounding requires the de novo synthesis of JA (Browse, 2009; Chini et al., 2016; Howe et al., 2018). Specifically, JA biosynthesis in the aerial tissues of Arabidopsis depends on four distinct 13-lipoxygenases (13-LOXs), namely LOX2, LOX3, LOX4, and LOX6. Each of these 13-LOXs can produce JA precursors in leaves and each LOX appears to contribute in a different way to defense gene expression (Chauvin et al., 2013, 2016; Grebner et al., 2013). However, unlike for reproductive development, the contributions of individual 13-LOXs to vegetative growth modulation in Arabidopsis remains poorly understood.
Like wound-response leaf growth restriction (Zhang and Turner, 2008), Arabidopsis reproductive development requires the CORONATINE-INSENSITIVE1 gene (Xie et al., 1998). CORONATINE-INSENSITIVE1 encodes the receptor for jasmonoyl-Ile (JA-Ile; Sheard et al., 2010; Howe et al., 2018) and 12-hydroxy-JA-Ile (Jimenez-Aleman et al., 2019; Poudel et al., 2019), a compound implicated, together with JA-Ile, in the control of wound-induced growth restriction (Poudel et al., 2019). In terms of JA biosynthetic enzymes, a LOX was found to be essential for male flower formation in maize (Acosta et al., 2009), Subsequently, LOX3 and LOX4 were identified as the 13-LOXs that produce the JA precursors necessary for normal reproductive development in Arabidopsis (Caldelari et al., 2011). That is, although all four Arabidopsis 13-LOXs produce precursors for JA synthesis, LOX2 and LOX6 cannot replace LOX3 and LOX4 to maintain male fertility. The study of the roles of JA in reproductive development has been extended to the cellular level, and the cells in floral organs that are necessary for JA signaling leading to fertility have been identified (Jewell and Browse, 2016). By contrast, the roles of 13-LOXs in vegetative development in Arabidopsis remain poorly understood. To our knowledge, no specific 13-LOXs have an attributed role in any aspect of leaf growth. We therefore set out to address this gap in our knowledge. We used a collection of reduced-function lox single and multiple mutants. In each case, two alleles for each LOX were previously characterized biochemically for their ability to make JAs in wounded leaves (Caldelari et al., 2011; Chauvin et al., 2013). One of each allele was used in this study, allowing the individual inputs of each LOX on rosette growth to be assessed genetically.
To complement loss-of-function studies and to further investigate the activity of 13-LOXs in rosette growth restriction, we also took an opposite, gain-of-function approach. Here, we attempted to activate growth-regulating 13-LOXs in Arabidopsis in the absence of wounding. Our strategy was based on the fatty acid oxygenation upregulated2 (fou2) mutant (Bonaventure et al., 2007a). At the molecular level, the fou2 mutation affects TWO-PORE CHANNEL1 (Peiter et al., 2005; Hedrich et al., 2018; Pottosin and Dobrovinskaya, 2018), increasing the open-probability of this vacuolar cation channel (Bonaventure et al., 2007a; Beyhl et al., 2009; Lenglet et al., 2017). In the undamaged state, adult-phase fou2 leaves have elevated levels of JA compared to the wild type, and the fou2 transcriptome indicates high JA pathway activity (Bonaventure et al., 2007b). Relevant to that study is the fact that fou2 has small, epinastic leaves with short petioles. Generation of fou2 aos double mutants that cannot produce JA is known to suppress much of the effect of fou2 on leaf growth, although this double mutant does not completely rescue the leaf epinasty that is typical of fou2 (Bonaventure et al., 2007b). We investigated whether fou2 complementary DNA (cDNA; TPC1D454N) could be used to activate JA signaling leading to rosette growth restriction. The results reveal roles for specific 13-LOXs in leaf growth and also identify a cell population likely to play critical roles in wound-response vegetative growth plasticity.
RESULTS
Damaged Plants Are Smaller, with Greater Defense Capabilities than Their Undamaged Counterparts
Wild-type plants that had been fed on by Spodoptera littoralis larvae for 11 d under controlled conditions showed obvious signs of damage and their rosettes appeared to be smaller than the undamaged wild type (Fig. 1A). However, each leaf showed variable signs of damage, and a more controlled wounding protocol was therefore employed. We used serial mechanical wounding to better control for the effects of leaf damage and to test whether plants that had been damaged were more resistant to herbivores. Starting with 2-week-old plants, developing leaves were wounded at 3-d intervals in such a way that leaf 1 was damaged first by crushing 50% of the lamina, then leaf 2, and so forth. In total, seven leaves were wounded. As a control, plants were handled identically but not wounded. At the end of the treatment, and 3 d after the last of seven wounds (or handling, as was the case of the control unwounded plants), wounded wild-type rosettes were smaller than those of unwounded plants (Fig. 1B) and showed reduced petiole extension (Fig. 1C). The effects of wounding on petiole growth were reduced in the aos mutant relative to the wild type (Fig. 1, B and C). Reduced growth due to JA pathway activity has been linked to increased levels of defense (Huot et al., 2014; Guo et al., 2018). We compared herbivore performance on unwounded plants and plants that had been mechanically wounded in bioassays using the lepidopteran herbivore S. littoralis and the cabbage aphid Brevicoryne brassicae. Weight gain by S. littoralis was reduced on the prewounded plants compared to undamaged plants (Fig. 1D). Similarly, aphid fecundity, as judged by the production of adult aphids, was reduced on the prewounded plants relative to the undamaged plants (Fig. 1E).
Figure 1.
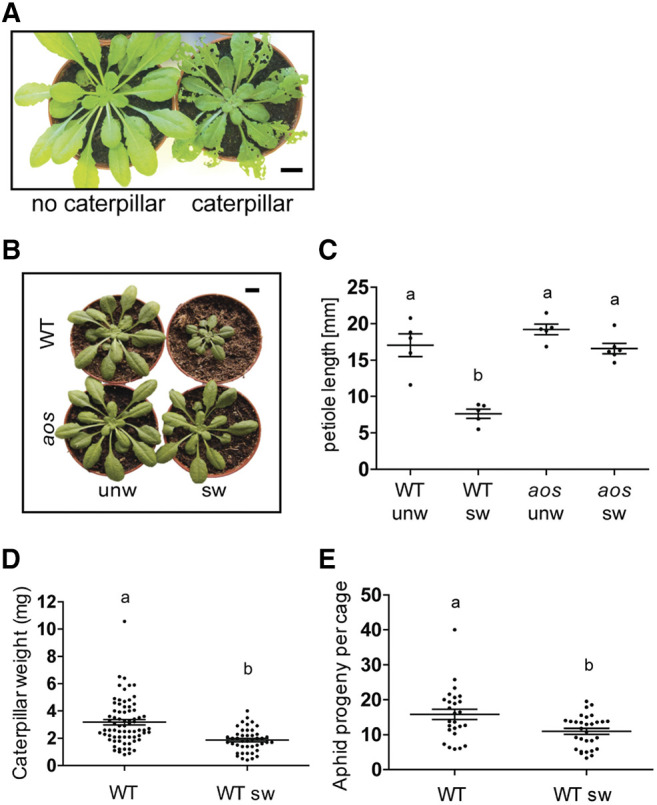
Enhanced defense in serially wounded plants. A, Rosette morphology of undamaged wild-type (WT) plant and plant after 11 d of S. littoralis feeding. Scale bar = 1 cm. B, Rosette morphology of wild type and aos after serial wounding. The background was digitally rendered in white in the image. unw, Unwounded; sw, serially wounded. Scale bar = 1 cm. C, Petiole length after serial wounding in wild type and aos mutant. The mean petiole length of leaves number 6, 7, and 8 in each plant is counted as one biological replicate. Bars represent the means (±sem), n = 5 to 6 D, Biomass of S. littoralis after 11 d feeding on unwounded wild type or serially wounded wild type (WT sw). Bars are means (±sem) of three combined experiments. Wild type, n = 73; wild type sw, n = 44. E, Reproductive success of cabbage aphids (B. brassicae) on unwounded or serially wounded plants. Aphid success was monitored 14 d after placing nymphs on plants. Bars are means (±sem) of three combined experiments; wild type, n = 26; wild type sw, n = 30. Lowercase letters indicate significant difference as determined by Tukey’s HSD test (P < 0.001).
Identification of 13-LOXs that Operate in Wound-Activated Rosette Growth Responses
Because wound-response growth restriction is JA-dependent and is associated with decreased defense against herbivores, we sought to identify the branch of the JA synthesis pathway necessary for this process. Four 13-LOXs contribute to JA production in wounded leaves (Chauvin et al., 2013; 2016). The effects of serial wounding on growth restriction in single lox mutants for each of these genes was tested. Figure 2, A and B, shows that the wild type and all single lox mutants displayed wound-response growth restriction. Next, lox triple mutants (Chauvin et al., 2013) were wounded repetitively. Each of these lines retains a single functional JA precursor-producing LOX. When subjected to serial wounding, the lox2 lox4 lox6 mutant displayed growth restriction similar to the wild type (Fig. 2, C and D), whereas the other triple mutants showed less wound-response growth restriction relative to the wild type. In the triple mutants, mass reduction in response to wounding was lowest in lox2 lox3 lox4, followed by lox3 lox4 lox6, then lox2 lox3 lox6. The lox quadruple mutant that cannot produce JA (Chauvin et al., 2013) showed relatively little wound-response growth restriction. A final series of experiments compared the growth of the lox2 lox4 and lox3 lox4 double mutants in response to serial wounding. In these assays, the lox3 lox4 double mutant behaved like the quadruple lox mutant. The lox2 lox4 double mutant showed slightly stronger growth restriction than lox3 lox4 (Fig. 2, E and F). Comparison of fresh weights of unwounded plants from the same dataset (Supplemental Fig. S1) showed that the fresh masses of the quadruple and triple lox mutants and the lox3 lox4 double mutant were greater than the wild type. In summary, these results based on biochemically and genetically verified loss-of-function lox alleles show that LOX3, and, to a lesser extent, LOX4, produce the majority of JA precursors necessary for wound-response rosette growth restriction.
Figure 2.
Effect of serial leaf wounding on rosette growth of lox mutants. Rosette morphology of unwounded and serially wounded wild type (WT) and lox mutants. unw, Unwounded; sw, serially wounded. In each case, two representative plants are shown. The ratios (percent) represent fresh weight reduction resulted by wounding. A, Rosettes of wild type and lox single mutants. B, Fresh weights of unwounded and serially wounded wild type and lox single mutants. n = 5 to 6. C, Rosette morphology of wild type, lox triple mutants, and the lox quadruple mutant. D, Fresh weights of unwounded and serially wounded plants. n ≥ 10. E, Rosette morphology of wild type, lox double mutants, and the lox quadruple mutant. F, Fresh weights of unwounded and serially wounded plants. n ≥ 7. Arrows in C and E indicate genotypes showing strong growth restriction after wounding. Bars represent the mean of fresh weight (±sd). Asterisks indicate significant difference as determined by Student t test (**P < 0.01 and ***P < 0.001).
The fou2 Phenotype Is Suppressed When LOX3 and LOX4 Are Mutated
A mutant that affects petiole length is fou2, a plant gene that overproduces JA in the adult phase (Bonaventure et al., 2007a). JA production in fou2 is necessary for the shorter-than-wild-type petioles typical of this mutant, and petiole length was restored in fou2 aos double mutants that cannot produce JA (Bonaventure et al., 2007b). Based on the results of the serial wounding assays shown in Figure 2, we therefore generated fou2 lox3 lox4 triple mutants. We found that the strong JA-dependent phenotype of fou2 was suppressed in the fou2 lox3 lox4 triple mutant (Fig. 3). The level of suppression of the fou2 phenotype was similar to that reported for the fou2 aos double mutant (Bonaventure et al., 2007a), which was used as a control in these experiments. These results further confirm the roles for LOX3 and LOX4 in JA-dependent growth control and raise the intriguing possibility that stunted, bonsai-like wound phenotypes could be recapitulated through the genetic activation of 13-LOXs in the absence of wounding. To do this, we chose LOX3 because it had the strongest effect among the four 13-LOXs on wound-response growth restriction. The xylem and phloem were chosen as target tissues because (1) the LOX3 promoter activity domain spans both these regions (Chauvin et al., 2016), and (2) both the phloem and xylem are known to play critical roles in leaf-to-leaf wound signaling (Nguyen et al., 2018). Methods to activate JA production via LOX3, and therefore mimic wound-response growth restriction, were developed taking advantage of the gain-of-function nature of the fou2 mutation.
Figure 3.
Mutations in LOX3 and LOX4 genes largely suppress the fou2 phenotype. Plants were grown for 5 weeks in short-day conditions. A, Rosette morphology of wild type (WT), fou2, fou2 lox3 lox4, and fou2 aos. Scale bar = 1 cm. The phenotype of the fou2 aos mutant was as reported in Bonaventure et al. (2007b). B, Fresh weights of wild type, fou2, fou2 lox3 lox4, and fou2 aos. Data are means (±sd), n = 11 to 12. Lowercase letters indicate statistically significant differences as determined by Tukey’s HSD test (P < 0.05).
Hyperactive TPC1 Stimulates LOX3-Dependent Growth Restriction
In fou2, the Asp 454 to Asn (D454N) mutation in TWO-PORE CHANNEL1 (TPC1D454N) increases the open probability of this channel (Bonaventure et al., 2007a; Beyhl et al., 2009). This is because Asp 454, along with other acidic residues in TPC1, binds inhibitory Ca2+ in the vacuolar lumen, changing the voltage sensitivity of this voltage-gated cation channel (Guo et al., 2017). However, the fou2 mutation is semidominant, and wild-type TPC1 alleles inhibit the effects of TPC1D454N (Bonaventure et al., 2007a). In preliminary experiments to activate JA-dependent growth phenotypes with TPC1D454N, and to overcome its semidominance, TPC1D454N was expressed in the tpc1-2 null background (Peiter et al., 2005), with expression in the phloem driven by the SUC TRANSPORTER2 (SUC2) promoter (Truernit and Sauer, 1995), with expression in xylem contact cells driven by the LIPOXYGENASE6 (LOX6) promoter (Chauvin et al., 2013), or with expression in the bundle sheath driven by the SCARECROW (SCR) promoter (Wysocka-Diller et al., 2000). The 5-week-old SCRpro::TPC1D454N plants (Supplemental Fig. S2A) did not display a fou2-like phenotype, and the LOX6pro::TPC1D454N plants (Supplemental Fig. S2B) displayed only a slight fou2-like phenotype at this stage. By contrast, SUC2pro::TPC1D454N expressed in tpc1-2 phenocopied fou2 (Supplemental Fig. S2C), including causing elevated expression of the JA-signaling marker JAZ10 (Supplemental Fig. S2D). As expected, when the wild-type TPC1 sequence was expressed under the SUC2 promoter in the fou2 background, the TPC1 cDNA partially reverted the fou2 phenotype toward that of the wild type (Supplemental Fig. S2E) and suppressed the elevated levels of JAZ10 transcript detected in fou2 (Supplemental Fig. S2F).
These initial findings raised the possibility that JA precursor production by LOX3 could be activated through the expression of hyperactive TPC1 variants, provided that these variants had sufficient genetic penetrance to overcome the presence of wild-type TPC1 alleles. The single D454N mutation in TPC1D454N reduces Ca2+ binding to a vacuole lumen-located inhibitory sensor (Beyhl et al., 2009). More recently, and together with Asp 454, the residues Asp 240 and Glu 528 were also found to participate in channel-inhibiting Ca2+ coordination. The TPC1 D240A,D454A,E528A variant, termed “TPC1ΔCai,” largely removes luminal calcium inhibition without affecting the cation permeation specificity observed in the wild-type channel (Guo et al., 2017). We reasoned that hyperactive TPC1ΔCai might display sufficient genetic penetrance to be employed in backgrounds harboring wild-type TPC1 alleles to activate growth restriction through LOX3. Experiments were thus designed to test this.
Based on preliminary results (Supplemental Fig. S2), TPC1ΔCai was expressed in the phloem. For this, SUC2pro::TPC1ΔCai was transformed into the wild type and into the lox2 lox4 lox6 triple mutant that retains LOX3 as the only functional 13-LOX gene. Both the wild type and the lox2 lox4 lox6 lines expressing SUC2pro::TPC1ΔCai displayed fou2-like phenotypes (Fig. 4A; Supplemental Fig. S3). As a control, SUC2pro::TPC1ΔCai was transformed into the lox2 lox3 lox4 lox6 quadruple mutant that lacks the ability to make JA. The transformed quadruple mutant displayed a weak phenotype at the rosette center (Fig. 4B; Supplemental Fig. S3), consistent with the fact that part of the effect of fou2 on leaf growth is JA-independent (Bonaventure et al., 2007a, 2007b). Further analyses of the transformants revealed that TPC1ΔCai expression in the wild type and in lox2 lox 4 lox6 reduced rosette fresh weight, but this was not significantly affected in the lox quadruple mutant that does not produce JA (Fig. 4C). JAZ10 levels were found to be elevated in wild type and in lox2 lox4 lox6 triple mutant plants expressing SUC2pro::TPC1ΔCai (Fig. 4D). The relative levels of the LOX3 mRNA were also measured in the wild type, the wild type expressing TPC1ΔCai, and in the lox2 lox4 lox6 triple mutant without and with the TPC1ΔCai transgene. Whereas expression of TPC1ΔCai in the wild type increased LOX3 mRNA levels ∼3-fold, there was no corresponding increase in LOX3 mRNA level in the triple-mutant background (Fig. 4E).
Figure 4.
Expressing TPC1∆Cai in phloem companion cells stimulates LOX3-dependent growth restriction. All plants were 5 weeks old. A, Rosette morphology of wild type (WT), the wild type expressing SUC2pro::TPC1∆Cai, lox2/4/6, and lox2/4/6-expressing SUC2pro::TPC1∆Cai. B, Rosette morphologies of the lox quadruple mutant and the same plant transformed with SUC2pro::TPC1∆Cai. Scale bars = 1 cm. C, Fresh weights of wild type, the wild type expressing SUC2pro::TPC1∆Cai, lox2/4/6, lox2/4/6 expressing SUC2pro::TPC1∆Cai, lox quadruple mutant, and lox quadruple mutant expressing SUC2pro::TPC1∆Cai. D, JAZ10 transcript levels relative to reference gene UBC21. E, LOX3 transcript levels relative to UBC21. F, JA levels in the leaves of the wild type, the wild type expressing SUC2pro::TPC1∆Cai, lox2/4/6, and lox2/4/6 expressing SUC2pro::TPC1∆Cai. The dashed horizontal line shows the limit of quantification. G, JA-Ile levels in the leaves of the wild type, the wild type expressing SUC2pro::TPC1∆Cai, lox2/4/6, and lox2/4/6 expressing SUC2pro::TPC1∆Cai The dashed horizontal line shows the limit of quantification. Data are means (±sd); n = 10 to 11 (C), n = 4 (D and E), and n = 4 to 5 (F and G). Lowercase letters indicate statistically significant differences as determined by Tukey’s HSD test (P < 0.05).
Finally, to investigate whether expression of SUC2pro::TPC1ΔCai in the wild type and in the lox2 lox4 lox6 triple mutant altered the levels of jasmonic acid and JA-Ile in leaves, the levels of these molecules were measured. Increases in both jasmonic acid (Fig. 4F) and JA-Ile (Fig. 4G) were detected in both genetic backgrounds expressing TPC1ΔCai relative to the control genetic backgrounds.
DISCUSSION
JA-controlled leaf growth restriction is likely to be widespread in nature and can be activated by repetitive wounding (Yan et al., 2007) or by repeated mechanostimulation (Chehab et al., 2012). This process is, at least in part, a consequence of repression of cell division (Noir et al., 2013) and is controlled by MYC transcription factors (Major et al., 2017). Although growth and defense can be at least partially uncoupled (Campos et al., 2016), the ability of plants to channel resources from growth to defense is likely to have adaptive value (Huot et al., 2014; Guo et al., 2018). Consistent with this, we found that repetitively wounded plants slowed weight gain in a lepidopteran, and also limited reproduction in a hemipteran, herbivore. The fact that repetitive wounding affected plant growth and that this correlated with resistance to insects prompted us to investigate which branch of the JA biosynthesis pathway operates to control post-wounding leaf growth.
A Role for LOX3 and LOX4 in Wound-Response Growth
Here, we were able to identify LOX3 and LOX4 as the key 13-LOXs that act, through the production of JA precursors, in wound-response leaf growth restriction. We also noted that multiple lox mutations generally caused an increase in the fresh mass of unwounded plants. This was expected because aos mutants in JA biosynthesis have higher dry masses than the wild type (Yan et al., 2007). LOX3 and LOX4 are known to contribute to JA production in response to osmotic stress (Grebner et al., 2013) and after leaf wounding (Chauvin et al., 2013). This same 13-LOX pair in Arabidopsis is essential for male reproductive development (Caldelari et al., 2011) and the two 13-LOXs also play roles in root defense against nematodes (Ozalvo et al., 2014).
With this work, all four JA precursor-producing 13-LOXs in Arabidopsis now have attributed roles in wounded rosettes (Fig. 5). In addition to the functions of LOX3 and LOX4 described herein, LOX2 produces JA precursors as well as defense-related metabolites called “arabidopsides,” which accumulate rapidly in and near wounds (Glauser et al., 2009). Concerning LOX6, corresponding loss-of-function mutants are compromised in rapid JA synthesis in leaves distal to damage sites (Chauvin et al., 2013; Grebner et al., 2013; Gasperini et al., 2015). LOX2 and LOX6 also function together to upregulate LOX3 and LOX4 (Chauvin et al., 2016). In this work, expression of TPC1ΔCai in the wild-type background caused increases in LOX3 mRNA levels. However, and consistent with the model for LOX3/LOX4 regulation by LOX2 and LOX6 proposed in Chauvin et al. (2016), no increases in LOX3 mRNA were detectable in the lox2 lox4 lox6 triple mutant. The result suggests that hyperactive TPC1 variants exert their activating effect on LOX3-dependent JA precursor synthesis at the post-transcriptional level.
Figure 5.
Specific 13-LOX functions in wounded Arabidopsis rosettes. All four 13-LOXs produce JA precursors in wounded leaves and all four play roles in defense against lepidopteran herbivores. LOX2 is expressed throughout soft tissues (including the mesophyll) but not (or at very low levels) in the mature leaf vasculature. LOX3, LOX4, and LOX6 are primarily expressed in phloem- and xylem-associated cells in both developing and mature leaf veins. Only the functions that are largely specific to distinct LOXs in Arabidopsis leaves are emphasized. From Chauvin et al. (2013, 2016), Glauser et al. (2009), Grebner et al. (2013), and this work.
A question emerging from this study is raised by the fact that all four 13-LOXs produce JAs after leaf wounding (Chauvin et al., 2013). LOX3, LOX4, and LOX6 are expressed principally in phloem- and xylem-associated cells in adult-phase primary leaf veins (Chauvin et al., 2013). Moreover, JA precursors made by LOX6 in xylem contact cells can move out of these cell populations (Gasperini et al., 2015). Why, then, do LOX3 and LOX4 and not LOX2 and/or LOX6 operate in the control of wound-response leaf growth restriction? It is possible that JA precursors or JAs produced by LOX6 and LOX2 in response to wounding do not reach the stem cell populations that determine the rate of leaf primordia development. In a potentially interesting parallel, we note that transcripts for all four Arabidopsis 13-LOXs are found in developing flowers (Klepikova et al., 2016), and yet only LOX3 and LOX4 produce JAs essential for reproductive development in this plant (Caldelari et al., 2011).
Phloem-Derived Signals Can Activate the JA Pathway
Which cell types produce the signals that lead to wound-response rosette growth restriction? To investigate this, we sought to control this process genetically in the absence of wounding. The LOX3 promoter activity domain in primary leaf veins spans both the regions of xylem and phloem (Chauvin et al., 2016). Cells in one or both of these tissues were candidates that might produce the signals that stimulate environmentally linked growth restriction. In this respect, the phloem was of particular interest because this tissue plays central roles in growth plasticity (López-Salmerón et al., 2019). Additionally, key roles of the phloem in the induction of antiherbivore defense mechanisms in leaves have been discovered (Nguyen et al., 2018). Remarkably, expressing TPC1D454N in the null background tpc1-2 under the phloem-companion-cell–specific SUC2 promoter phenocopied fou2. To increase the dominance of fou2, we exploited TPC1ΔCai (TPC1D240A,D454A,E528A), which strongly increases the open probability of the channel (Guo et al., 2017). The first key test with the TPC1ΔCai cDNA was to express it in a restricted cellular domain under the SUC2 promoter in the lox2 lox3 lox4 lox6 quadruple mutant background that does not produce biologically active JAs in response to wounding (Chauvin et al., 2013). This test (Fig. 4B; Supplemental Fig. S3) revealed that the rosette diameters and leaf shapes of the lox quadruple mutants with or without TPC1ΔCai were similar. In 5-week-old plants, only a few of the younger leaves in the rosette of the quadruple mutant transformed with TPC1ΔCai, displayed some epinasty. This experiment served as the basis for transforming the lox2 lox4 lox6 triple mutant with TPC1ΔCai to try to activate LOX3, and thereby control rosette growth. A further control was expression of TPC1ΔCai in the wild-type background. The wild-type plants expressing SUC2::TPC1ΔCai displayed a fou2-like phenotype.
By extending this approach to a lox2 lox4 lox6 background that carries the functional LOX3 gene, we found that TPC1ΔCai-dependent ion fluxes generated in the phloem could activate the LOX3 branch of the JA synthesis pathway. The mechanism underlying this phenomenon is unknown, but membrane depolarization after wounding appears to play a role in JA pathway activation in Arabidopsis leaves (e.g. Lenglet et al., 2017; Nguyen et al., 2018). Phloem cells are highly excitable (Sibaoka, 1962; Hafke and van Bel, 2013; Hedrich et al., 2016), and wounding may therefore activate JA synthesis by altering membrane potentials and Ca2+ fluxes in these cells. Such a scenario may be widespread in plants subjected to repetitive wounding. The art of bonsai may rest on related mechanisms.
CONCLUSION
Loss-of-function genetic studies show that LOX3 and LOX4 together control wound-response leaf growth in Arabidopsis. These two 13-LOXs operate in both vegetative growth and reproductive development, highlighting that their functions are distinct from those of LOX2 and LOX6. Further demonstrating a role of LOX3 in controlling leaf growth, we were also able to activate LOX3-dependent JA production in unwounded leaves. This was accomplished using a novel approach based on gain-of-function mutations in a vacuolar ion channel. In terms of future work, hyperactive ion channel variants could find broader use in investigating mechanisms of cell-to-cell signaling within the vasculature, a phenomenon that underlies much of plant physiology.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Transfer-DNA insertion lines were obtained from the Nottingham Arabidopsis Stock Center (http://arabidopsis.info/). Arabidopsis (Arabidopsis thaliana) Columbia (Col-0) was the wild type and the background for lox mutants. The lox alleles used were lox2-1 (Glauser et al., 2009), lox3b (SALK_147830), lox4a (SALK_ 071732), and lox6a (SALK_138907). Other mutants used were tpc1-2 (SALK_145413), fou2 (At4g03560, TPC1D454N; Bonaventure et al., 2007a), and aos (At5g42650; Park et al., 2002), which were all in the wild-type background. Plants were grown individually in 7-cm–diameter pots on soil at 70% humidity and 100 μE m−2 s−1 photosynthetically available radiation under short-day conditions (10-h light at 22°C).
Serial Wounding
For serial wounding, a series of wounds was inflicted from d 14 of growth when the first two leaves were slightly bigger than the cotyledons. Fifty percent of the tip of leaf 1 was wounded with forceps. Control plants were handled identically but not wounded. Plants were then incubated for 3 d before 50% wounding of leaf 2. The same schema was repeated with 3 d of resting between each wound event and the series of wounds was stopped 3 d after leaf 7 was wounded. Five to six plants of each genotype (wild type or aos) were used for petiole measurements. For each plant, petiole lengths of the leaves 6, 7, and 8 were measured from photographs using the software ImageJ (https://imagej.nih.gov/ij/index.html) and their combined lengths were averaged.
Insect Bioassays
No-choice bioassays were performed with neonate Spodoptera littoralis placed on 5-week-old plants grown under short-day conditions identical to those described above. Six larvae were placed on each individual plant (11 plants per genotype) and allowed to feed for 11 d. Larvae were then weighted on a precision balance XP205 DeltaRang (Mettler-Toledo). Aphid bioassays performed with Brevicoryne brassicae. Unwounded or serially wounded plants were placed in cages (11 plants per cage) and five adult aphids were deposed on each plant. After 48 h (day0), the adults were removed. After 72 h, nymphs were removed, leaving only five nymphs on each plant. Nymphs were counted at day 11 and the nymphs and adult aphids were counted at day 14. The aphid performance was calculated as follows: ([number of nymphs on day 11 + number of nymphs on day 14]/ number of adults on day 14).
Gene Expression Analysis
At the time of tissue collection, the leaves (unwounded or wounded) were collected and immediately frozen in liquid N2. RNA isolation and reverse transcription quantitative PCR was as described by Gfeller et al. (2011) using the following primer pairs: UBC21 (At5g25760) transcripts were quantified using the primers 5ʹ-CAGTCTGTGTGTAGAGCTATCATAGCAT-3ʹ (ubc21F) and 5ʹ-AGAAGATTCCCTGAGTCGCAGTT-3ʹ (ubc21R). JAZ10 (At5g13220) transcripts were quantified using primers 5ʹ-ATCCCGATTTCTCCGGTCCA-3ʹ (jaz10F) and 5ʹ-ACTTTCTCCTTGCGATGGGAAGA-3ʹ (jaz10R). LOX3 (At1g17420) transcripts were quantified using primers 5ʹ-AACACAACCACATGGTCTTAAACTC-3ʹ (lox3F) and 5ʹ-GGAGCTCAGAGTCTGTTTTGATAAG-3ʹ (lox3R).
Plasmid Construction and Transformation
Vectors were based on MultiSite Gateway Technology (www.invitrogen.com). Promoters were amplified from wild-type genomic DNA with indicated oligonucleotides for: the 2-kb upstream region directly preceding the first ATG of SCR (At3g54220; Malamy and Benfey, 1997), LOX6 (At1g67560; 5ʹ-CGGGGTACCGGTTGTTGAAATTTCTGATGCT-3ʹ and 5ʹ-TTCCCCCCCGGGTTTTGTTTGGAGTTTGGCAGT-3ʹ), and the 4-kb upstream region directly preceding the first ATG of SUC2 (At1g22710; 5ʹ-CGGGGTACCCTGCTAAAACTATTCCATTTCAAAATG-3ʹ and 5ʹ-TTCCCCCCCGGGATTTGACAAACCAAGAAAGTAAG-3ʹ). After amplification, these sequences were verified and cloned via restriction digestion with XmaI and KpnI into a modified pUC57 (Chauvin et al., 2013) to create pEN-L4-promoter-R1 clones. The open reading frame of TPC1 (At4g03560) and TPC1D454N was amplified from cDNA from wild-type and fou2 plants (5ʹ-ACAAAAAGCAGGCTTAATGGAAGACCC-3ʹ and 5ʹ-AGAAAGCTGGGTTGTGTCAGAAGTGGAACACT-3ʹ). Amplification products were recombined into pDONR221 (Invitrogen) to produce pEN-L1-gene-L2 clones. To generate promoter fusion with proteins under the control of endogenous promoters, pEN-L4-promoter-R1 plasmids were recombined with pEN-L1-CDS-L2 into pEDO097pFR7m24GW by double Gateway Technology to obtain SUC2pro-TPC1D454N, SCRpro-TPC1D454N, LOX6pro-TPC1D454N, and SUC2pro-TPC1 clones. All constructs were introduced into Arabidopsis backgrounds by floral dip Agrobacterium-mediated transformation. For promoter fusions, transformed seeds expressing red fluorescence protein in T1, T2, and T3 lines were selected by fluorescence microscopy. TPC1ΔCai (Guo et al., 2017) was synthesized by GenScript. The following mutations were introduced into the TPC1 coding sequence: Asp (GAC) 240-Ala (GCC), Asp (GAT) 454-Ala (GCT), and Glu (GAA) 528-Ala (GCA). The synthetic gene was cloned into the Entry vector pDONR/Zeo and recombined with pUC57 carrying the SUC2 promoter for plant transformation. From multiple transformants with similar phenotypes, a minimum of two independent transgenic lines for individual each construct was used in experiments.
JA and JA-Ile Analyses
JA and JA-Ile measurements were performed as described by Glauser et al. (2014). Plants were 5 weeks old and had not been wounded. The internal standards used were JA-d5 for JA and JA-Ile-13C6 for JA-Ile. Limits of quantifications for JA and JA-Ile were 0.4 and 0.15 pmol g−1 fresh weight, respectively.
Statistical Analysis
Statistical significance in pairwise comparisons was evaluated by Student’s t test. Multiple comparisons using ANOVA followed by Tukey’s honestly significant difference (HSD) test were performed in the software R 3.2.2 (www.r-project.org).
Accession Numbers
Sequence data TPC1 can be found in the GenBank/EMBL data libraries under accession numbers 825655/ NP_567258.1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Effect of serial leaf wounding on rosette growth of lox mutants.
Supplemental Figure S2. Expressing TPC1D454N in phloem companion cells of tpc1-2 phenocopies fou2.
Supplemental Figure S3. Independent transformants show similar phenotypes and fresh weights to those used for Figure 4 in the main text.
Acknowledgments
Archana Kumari (University of Lausanne) gave us critical advice and Marion Ribault (University of Lausanne) provided valuable technical help.
Footnotes
This work was supported by the Swiss National Science Foundation (grants nos. 31003A–155960 and 31003A–175566/1 to E.E.F.).
Articles can be viewed without a subscription.
References
- Acosta IF, Laparra H, Romero SP, Schmelz E, Hamberg M, Mottinger JP, Moreno MA, Dellaporta SL(2009) Tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science 323: 262–265 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Austin AT(2019) Recalculating growth and defense strategies under competition: Key roles of photoreceptors and jasmonates. J Exp Bot 70: 3425–3434 [DOI] [PubMed] [Google Scholar]
- Beyhl D, Hörtensteiner S, Martinoia E, Farmer EE, Fromm J, Marten I, Hedrich R(2009) The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J 58: 715–723 [DOI] [PubMed] [Google Scholar]
- Bonaventure G, Gfeller A, Proebsting WM, Hörtensteiner S, Chételat A, Martinoia E, Farmer EE(2007a) A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J 49: 889–898 [DOI] [PubMed] [Google Scholar]
- Bonaventure G, Gfeller A, Rodríguez VM, Armand F, Farmer EE(2007b) The fou2 gain-of-function allele and the wild-type allele of Two Pore Channel 1 contribute to different extents or by different mechanisms to defense gene expression in Arabidopsis. Plant Cell Physiol 48: 1775–1789 [DOI] [PubMed] [Google Scholar]
- Browse J.(2009) Jasmonate passes muster: A receptor and targets for the defense hormone. Annu Rev Plant Biol 60: 183–205 [DOI] [PubMed] [Google Scholar]
- Caldelari D, Wang G, Farmer EE, Dong X(2011) Arabidopsis lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Mol Biol 75: 25–33 [DOI] [PubMed] [Google Scholar]
- Campos ML, Yoshida Y, Major IT, de Oliveira Ferreira D, Weraduwage SM, Froehlich JE, Johnson BF, Kramer DM, Jander G, Sharkey TD, et al. (2016) Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat Commun 7: 12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvin A, Caldelari D, Wolfender JL, Farmer EE(2013) Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: A role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol 197: 566–575 [DOI] [PubMed] [Google Scholar]
- Chauvin A, Lenglet A, Wolfender JL, Farmer EE(2016) Paired hierarchical organization of 13-lipoxygenases in Arabidopsis. Plants (Basel) 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab EW, Yao C, Henderson Z, Kim S, Braam J(2012) Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Curr Biol 22: 701–706 [DOI] [PubMed] [Google Scholar]
- Chini A, Gimenez-Ibanez S, Goossens A, Solano R(2016) Redundancy and specificity in jasmonate signalling. Curr Opin Plant Biol 33: 147–156 [DOI] [PubMed] [Google Scholar]
- Fernández-Milmanda GL, Crocco CD, Reichelt M, Mazza CA, Köllner TG, Zhang T, Cargnel MD, Lichy MZ, Fiorucci AS, Fankhauser C, et al. (2020) A light-dependent molecular link between competition cues and defence responses in plants. Nat Plants 6: 223–230 [DOI] [PubMed] [Google Scholar]
- Gasperini D, Chauvin A, Acosta IF, Kurenda A, Stolz S, Chételat A, Wolfender JL, Farmer EE(2015) Axial and radial oxylipin transport. Plant Physiol 169: 2244–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller A, Baerenfaller K, Loscos J, Chételat A, Baginsky S, Farmer EE(2011) Jasmonate controls polypeptide patterning in undamaged tissue in wounded Arabidopsis leaves. Plant Physiol 156: 1797–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G, Dubugnon L, Mousavi SAR, Rudaz S, Wolfender JL, Farmer EE(2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284: 34506–34513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G, Vallat A, Balmer D(2014) Hormone profiling In Sanchez-Serrano JJ, and Salinas J, eds, Arabidopsis Protocols. Humana Press, Totowa, NJ, pp 597–608 [Google Scholar]
- Grebner W, Stingl NE, Oenel A, Mueller MJ, Berger S(2013) Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol 161: 2159–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Major IT, Howe GA(2018) Resolution of growth-defense conflict: Mechanistic insights from jasmonate signaling. Curr Opin Plant Biol 44: 72–81 [DOI] [PubMed] [Google Scholar]
- Guo J, Zeng W, Jiang Y(2017) Tuning the ion selectivity of two-pore channels. Proc Natl Acad Sci USA 114: 1009–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafke JB, van Bel AJE(2013) Cellular basis of electrical potential waves along the phloem and impact of coincident Ca2+ fluxes In Thompson GA, and van Bel AJE, eds, Phloem: Molecular Cell Biology, Systemic Communication, Biotic Interactions. Wiley, New York, pp 122–140 [Google Scholar]
- Hedrich R, Mueller TD, Becker D, Marten I(2018) Structure and function of TPC1 vacuole SV channel gains shape. Mol Plant 11: 764–775 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Salvador-Recatalà V, Dreyer I(2016) Electrical wiring and long-distance plant communication. Trends Plant Sci 21: 376–387 [DOI] [PubMed] [Google Scholar]
- Howe GA, Major IT, Koo AJ(2018) Modularity in jasmonate signaling for multistress resilience. Annu Rev Plant Biol 69: 387–415 [DOI] [PubMed] [Google Scholar]
- Huot B, Yao J, Montgomery BL, He SY(2014) Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol Plant 7: 1267–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JB, Browse J(2016) Epidermal jasmonate perception is sufficient for all aspects of jasmonate-mediated male fertility in Arabidopsis. Plant J 85: 634–647 [DOI] [PubMed] [Google Scholar]
- Jimenez-Aleman GH, Almeida-Trapp M, Fernández-Barbero G, Gimenez-Ibanez S, Reichelt M, Vadassery J, Mithöfer A, Caballero J, Boland W, Solano R(2019) Omega hydroxylated JA-Ile is an endogenous bioactive jasmonate that signals through the canonical jasmonate signaling pathway. Biochim Biophys Acta Mol Cell Biol Lipids 1864: 158520. [DOI] [PubMed] [Google Scholar]
- Klepikova AV, Kasianov AS, Gerasimov ES, Logacheva MD, Penin AA(2016) A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J 88: 1058–1070 [DOI] [PubMed] [Google Scholar]
- Lenglet A, Jaślan D, Toyota M, Mueller M, Müller T, Schönknecht G, Marten I, Gilroy S, Hedrich R, Farmer EE(2017) Control of basal jasmonate signalling and defence through modulation of intracellular cation flux capacity. New Phytol 216: 1161–1169 [DOI] [PubMed] [Google Scholar]
- López-Salmerón V, Cho H, Tonn N, Greb T(2019) The phloem as a mediator of plant growth plasticity. Curr Biol 29: R173–R181 [DOI] [PubMed] [Google Scholar]
- Major IT, Yoshida Y, Campos ML, Kapali G, Xin XF, Sugimoto K, de Oliveira Ferreira D, He SY, Howe GA(2017) Regulation of growth-defense balance by the JASMONATE ZIM-DOMAIN (JAZ)-MYC transcriptional module. New Phytol 215: 1533–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN(1997) Analysis of SCARECROW expression using a rapid system for assessing transgene expression in Arabidopsis roots. Plant J 12: 957–963 [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Kurenda A, Stolz S, Chételat A, Farmer EE(2018) Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc Natl Acad Sci USA 115: 10178–10183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noir S, Bömer M, Takahashi N, Ishida T, Tsui TL, Balbi V, Shanahan H, Sugimoto K, Devoto A(2013) Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol 161: 1930–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozalvo R, Cabrera J, Escobar C, Christensen SA, Borrego EJ, Kolomiets MV, Castresana C, Iberkleid I, Brown Horowitz S(2014) Two closely related members of Arabidopsis 13-lipoxygenases (13-LOXs), LOX3 and LOX4, reveal distinct functions in response to plant-parasitic nematode infection. Mol Plant Pathol 15: 319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R(2002) A knock‐out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31: 1–12 [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D(2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434: 404–408 [DOI] [PubMed] [Google Scholar]
- Pottosin I, Dobrovinskaya O(2018) Two-pore cation (TPC) channel: Not a shorthanded one. Funct Plant Biol 45: 83–92 [DOI] [PubMed] [Google Scholar]
- Poudel AN, Holtsclaw RE, Kimberlin A, Sen S, Zeng S, Joshi T, Lei Z, Sumner LW, Singh K, Matsuura H, et al. (2019) 12-Hydroxy-jasmonoyl-L-isoleucine is an active jasmonate that signals through CORONATINE INSENSITIVE 1 and contributes to the wound response in Arabidopsis. Plant Cell Physiol 60: 2152–2166 [DOI] [PubMed] [Google Scholar]
- Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, et al. (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibaoka T.(1962) Excitable cells in Mimosa. Science 137: 226. [DOI] [PubMed] [Google Scholar]
- Truernit E, Sauer N(1995) The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of beta-glucuronidase to the phloem: Evidence for phloem loading and unloading by SUC2. Planta 196: 564–570 [DOI] [PubMed] [Google Scholar]
- Wysocka-Diller JW, Helariutta Y, Fukaki H, Malamy JE, Benfey PN(2000) Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development 127: 595–60310631180 [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG(1998) COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, Farmer EE(2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Turner JG(2008) Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS One 3: e3699. [DOI] [PMC free article] [PubMed] [Google Scholar]






