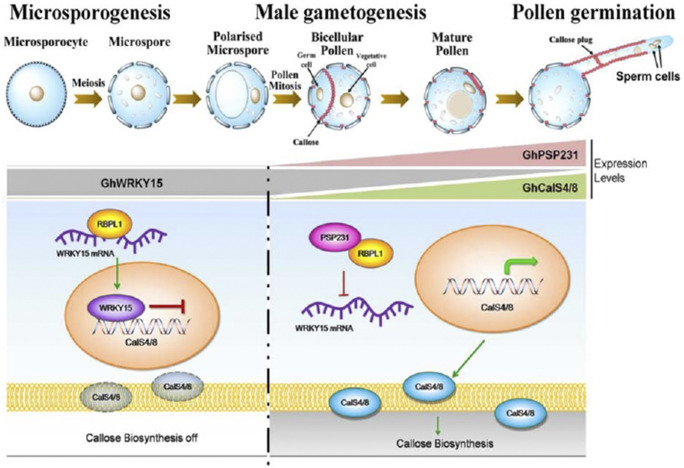
Callose is a cell wall component that is dynamically deposited and degraded during pollen development. Thanks to a new article investigating pollen formation in cotton (Gossypium hirsutum), we now know that a pollen-specific protein regulates callose deposition by inhibiting the action of a transcription factor, WRKY15 (Li et al., 2020).
Callose is a polymer formed primarily of Glc units connected by β1-3-linkages. It is transiently deposited at sites of wounding, plasmodesmata, and pollen cell walls (Chen and Kim, 2009). Callose is also frequently present as a transient wall component in newly formed cell plates during cytokinesis of mitotically dividing plant cells (Scherp et al., 2001).
In the anthers of many angiosperms, a transient callose-rich cell wall is laid down, surrounding and separating each microsporocyte and callose is also deposited on the outer pollen wall (Blackmore et al., 2007; De Storme and Geelen, 2013). Callose is thought to isolate microspores from one another and is considered an impermeable barrier. Additionally, in many species that form these callose-rich walls, mutants with defective callose production do not create viable pollen (De Storme and Geelen, 2013). Nevertheless, the essential role of callose is somewhat contested, as there is some evidence that large molecules can still cross the callose wall and some species do not deposit callose at this early meiotic stage (Scott et al., 2004). Despite this, callose deposition during meiosis has been observed across land plants including in mosses, liverworts, hornworts, and lycophytes (Flowers, 2018).
Li et al. (2020) have identified a gene expressed in cotton pollen, POLLEN SPECIFIC PROTEIN 231 (PSP231). They uncovered a sequence of regulatory interactions leading from PSP231 through to callose deposition phenotypes. PSP231 is part of the SKU5-SIMILAR (SKS) family of genes, which are frequently glycosylated but otherwise have unknown biochemical functions. Some SKS genes are involved in root development, and others have been shown to be expressed in pollen (Albani et al., 1992; Wittink et al., 2000; Zhou, 2019b).
Studying gametogenesis is notoriously difficult as mutants are frequently sterile and it is even more difficult in species such as Gossypium spp., in which established tools for pollen germination research are not yet available. Despite this, the authors persevered by using in vitro methods as well as RNA interference (RNAi) and heterologous expression.
Li et al. (2020) found that PSP231-RNAi lines were defective in male gametogenesis, forming pollen grains that were frequently withered and misshapen. Overexpressing PSP231 in tobacco (Nicotiana tabacum) pollen also resulted in reduced pollen germination. These findings illustrated an essential role for PSP231 and suggested that its expression had to be carefully balanced to achieve its correct effects.
To understand what PSP231 might be doing in cotton, Li et al. (2020) analyzed the transcriptome of wild-type and PSP231-RNAi pollen. They found that two callose synthase genes were downregulated in the knockdown line compared to wild type. Furthermore, in the pollen of the tobacco PSP231 overexpressors, aniline-blue staining highlighted increased callose deposition both in the newly formed wall dividing the vegetative and germ cells and in mature pollen.
In the absence of PSP231, GhWRKY15 showed increased expression. The GhWRKY15 protein is a transcriptional repressor and is able to bind to the promoters of the two callose synthase genes, consistent with the decreased expression levels of these genes.
Li et al. (2020) also used a variety of in vitro and in vivo methods to establish that PSP231 forms a protein–protein complex with an RNA-binding protein, GhRBPL1. To complete the links in the chain, Li et al. (2020) then demonstrated that GhRBPL1 is able to specifically bind to the sense strand of GhWRKY15 mRNA. Taking the data together, they suggested a model in which PSP231 binds to and inhibits the actions of GhRBPL1. When de-repressed, GhRBPL1 is thought to bind to and stabilize GhWRKY15 mRNA. In turn, GhWRKY15 represses transcription of callose synthase genes. When PSP231 is present, GhWRKY15 is therefore downregulated and callose synthesis can take place (Fig. 1). As PSP231 is largely expressed at later stages of pollen development, it is likely that additional regulators also exist that influence the early-stage deposition of callose.
Figure 1.
Schematic illustrating features of male gametogenesis and genetic interactions. Adapted from figure 8 in Li et al. (2020).
Remarkably, altered callose deposition was not only seen in plants, but also in yeast (Schizosaccharomyces pombe) cells overexpressing PSP231. In fission yeast, dividing cells have callose in their new cell plates. When S. pombe overexpressed PSP231, cells were more often seen at late stages of cell division compared to wild type, exhibiting callose-rich cell plates. These cells also showed growth retardation and decreased viability, likely due to the inability to degrade the excessively callosic septa.
While SKS-like genes have been known for some time, their roles have not been extensively studied. In Arabidopsis (Arabidopsis thaliana), SKU5 regulates root growth and mutants show twisted roots (Sedbrook et al., 2002). Similarly to PSP231, Arabidopsis sks mutants show modified cell geometry phenotypes with increased cell wall thickness in seedling roots (Zhou, 2019b). Previous research has demonstrated that SKS genes are frequently glycosylphosphatidylinositol (GPI)-anchored. Supporting this, Li et al. (2020) found that PSP231 is glycosylated. It is therefore likely that the PSP231 is anchored to lipid membranes within the cell, as has been demonstrated for other SKS proteins (Sedbrook et al., 2002; Wittink et al., 2000; Zhou, 2019a). In Arabidopsis, disruption of GPI reduces the prevalence of GPI-anchored proteins including SKU5, and results in nonviable pollen and general disruption to cell wall synthesis (Gillmor et al., 2005). It will be interesting to see whether this type of post-translational modification is also essential for PSP231 function.
This is the one of the first instances in which a regulatory network with an SKS-like gene has been established, and it may open up further avenues of research for the roles of SKS genes in other species and tissues. The ability of PSP231 to substantially impact not only plant pollen development but also yeast cell division suggests that there may be common regulatory processes for callose synthesis across species and kingdoms. This has already been hinted at by the observation that both the plant and fungal genes that synthesize callose share significant sequence similarity (Latgé, 2007). Li et al. (2020) have established an important set of regulatory interactions governing the development and cellular structure of pollen, which will lead to a greater understanding of male reproductive development in plants.
References
- Albani D, Sardana R, Robert LS, Altosaar I, Arnison PG, Fabijanski SF(1992) A Brassica napus gene family which shows sequence similarity to ascorbate oxidase is expressed in developing pollen: Molecular characterization and analysis of promoter activity in transgenic tobacco plants. Plant J 2: 331–342 [PubMed] [Google Scholar]
- Blackmore S, Wortley AH, Skvarla JJ, Rowley JR(2007) Pollen wall development in flowering plants. New Phytol 174: 483–498 [DOI] [PubMed] [Google Scholar]
- Chen X-Y, Kim J-Y(2009) Callose synthesis in higher plants. Plant Signal Behav 4: 489–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Geelen D(2013) Cytokinesis in plant male meiosis. Plant Signal Behav 8: e23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers N. (2018) Sporogenesis and callose localization in Anthocerotophyta. PhD Thesis. Illinois Univerysity, Carbondale, IL
- Gillmor CS, Lukowitz W, Brininstool G, Sedbrook JC, Hamann T, Poindexter P, Somerville C(2005) Glycosylphosphatidylinositol-anchored proteins are required for cell wall synthesis and morphogenesis in Arabidopsis. Plant Cell 17: 1128–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé JP.(2007) The cell wall: A carbohydrate armour for the fungal cell. Mol Microbiol 66: 279–290 [DOI] [PubMed] [Google Scholar]
- Li Y, Li L, Wang Y, Wang Y-C, Wang NN, Lu R, Wu YW, Li XB(2020) Pollen-specific protein PSP231 activates callose synthesis to govern male gametogenesis and pollen germination. Plant Physiol 184: 1024–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherp P, Grotha R, Kutschera U(2001) Occurrence and phylogenetic significance of cytokinesis-related callose in green algae, bryophytes, ferns and seed plants. Plant Cell Rep 20: 143–149 [DOI] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Dickinson HG(2004) Stamen structure and function. Plant Cell 16: S46 LP-S60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook JC, Carroll KL, Hung KF, Masson PH, Somerville CR(2002) The Arabidopsis SKU5 gene encodes an extracellular glycosyl phosphatidylinositol-anchored glycoprotein involved in directional root growth. Plant Cell 14: 1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittink FRA, Knuiman B, Derksen J, Čapková V, Twell D, Schrauwen JAM, Wullems GJ(2000) The pollen-specific gene Ntp303 encodes a 69-kDa glycoprotein associated with the vegetative membranes and the cell wall. Sex Plant Reprod 12: 276–284 [Google Scholar]
- Zhou K.(2019a) Glycosylphosphatidylinositol-anchored proteins in Arabidopsis and one of their common roles in signaling transduction. Front Plant Sci 10: 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K.(2019b) GPI-anchored SKS proteins regulate root development through controlling cell polar expansion and cell wall synthesis. Biochem Biophys Res Commun 509: 119–124 [DOI] [PubMed] [Google Scholar]