Slow development is a general mechanism for photoperiod-sensitive genic male sterility.
Abstract
Photoperiod- and thermosensitive genic male sterility (P/TGMS) lines are widely used in crop breeding. The fertility conversion of Arabidopsis (Arabidopsis thaliana) TGMS lines including cals5-2, which is defective in callose wall formation, relies on slow development under low temperatures. In this study, we discovered that cals5-2 also exhibits PGMS. Fertility of cals5-2 was restored when pollen development was slowed under short-day photoperiods or low light intensity, suggesting that slow development restores the fertility of cals5-2 under these conditions. We found that several other TGMS lines with defects in pollen wall formation also exhibited PGMS characteristics. This similarity indicates that slow development is a general mechanism of PGMS fertility restoration. Notably, slow development also underlies the fertility recovery of TGMS lines. Further analysis revealed the pollen wall features during the formation of functional pollens of these P/TGMS lines under permissive conditions. We conclude that slow development is a general mechanism for fertility restoration of P/TGMS lines and allows these plants to take different strategies to overcome pollen formation defects.
Hybrid breeding, using three-line and two-line systems, is applied in increasing crop yields to meet the rising needs for food worldwide (Li et al., 2007). The three-line system involves a cytoplasmic male-sterile line, a restorer line, and a maintainer line. The two-line system only requires a male-sterile line and a restorer line, which simplifies the breeding procedures and has been widely used in numerous crops, including rice (Oryza sativa), maize (Zea mays), cotton (Gossypium hirsutum), and rape (Brassica napus; Virmani and Ilyas-Ahmed, 2001). The most extensively used male-sterile lines in the two-line system exhibit thermosensitive genic male sterility (TGMS) and/or photoperiod-sensitive genic male sterility (PGMS). TGMS lines are sterile under normal temperature range but turn fertile under low temperatures, while PGMS lines are sterile under long-day (LD) photoperiod but are fertile under short-day (SD) photoperiod (Chen and Liu, 2014).
Much progress about the TGMS mechanism has been made in recent years. A number of TGMS loci have been identified in rice. In AnnongS-1, a premature stop codon is introduced in the RNase ZS1 gene (Zhou et al., 2014). The genome of HengnongS-1 contains a mutation in the homolog of Arabidopsis (Arabidopsis thaliana) MALE STERILITY1 (MS1; Qi et al., 2014). An artificial TGMS rice line was created by silencing a UGPase (Chen et al., 2007). In addition, TGMS in indica rice Peiai64S is determined by the mutation of the genetic locus for a long noncoding RNA (PMS1T and PMS3; Zhou et al., 2012). Similar to rice, several TGMS loci have been identified in Arabidopsis, including the PLANT U-BOX 4 (PUB4), MYB33, and MYB65 (Millar and Gubler, 2005). Recently, a new TGMS locus, REVERSIBLE MALE STERILE1 (RVMS), was identified. RVMS encodes a GDSL-motif lipase that hydrolyzes triglycerides into glycerol and hexadecanoic acid, which are the components of plasma membrane (Zhu et al., 2020). The male-sterile mutants acyl-coa synthetase5-2 (acos5-2), cyp703a2, callose synthase5-2 (cals5-2), and ruptured pollen grain (rpg1) have defective pollen walls (Dong et al., 2005; Morant et al., 2007; Guan et al., 2008; de Azevedo Souza et al., 2009). These lines also show the TGMS phenotype (Zhu et al., 2020). Analysis of the underlying mechanism revealed that slow development induced by low temperature is involved in the fertility conversion in these TGMS lines (Zhu et al., 2020).
The first PGMS line was discovered in 1973 in the japonica rice var NongKen 58S (Shi, 1985). Then, a number of PGMS lines derived from NongKen 58S with significant hybrid vigor, were created for application in agriculture (Fan et al., 2016). Photoperiod was considered to be the only environmental regulator of fertility conversion of the sterile lines until the discovery of TGMS in the late 1980s (Sun et al., 1989; Chen, 2001). In the summer of 1989 in China, low temperatures unusually led to unexpected fertility restoration in several rice sterile lines, including W6541S, AnnongS-1, and HengnongS-1 (Chen, 2001). In 1991, the unusual high autumn temperatures caused unintentional sterility in the 7001S rice line (Chen, 2001). Later on, it was found that both photoperiod and temperature may contribute to the sterility or fertility recovery (He et al., 1987). In addition, researchers found that in rice, japonica varieties are mainly affected by photoperiod, while indica varieties are mainly affected by temperature (Sun et al., 1991). Scientists also discovered that mutation of a noncoding RNA shows different environmental genic male sterility (EGMS) traits: PGMS in japonica rice var NongKen 58S and TGMS in indica rice var Peiai64S (Ding et al., 2012). Nevertheless, the mechanism of the fertility conversion in PGMS lines and how it is coordinated by photoperiod and temperature are still unclear.
In Arabidopsis, the callose synthase CalS5 is required for the callose wall formation during early pollen wall development (Dong et al., 2005). The cals5-2 knockout line exhibits the TGMS phenotype, and its fertility can be restored by low temperature (Zhu et al., 2020). In this study, we found that cals5-2 is also a PGMS line. Slow development under SD photoperiod or low light intensity restores its fertility. We found several other TGMS lines also showed the PGMS phenotype. Further analysis revealed that slow development was responsible for fertility restoration in these PGMS lines, revealing a shared mechanism for PGMS and TGMS.
RESULTS
cals5-2 Exhibits PGMS
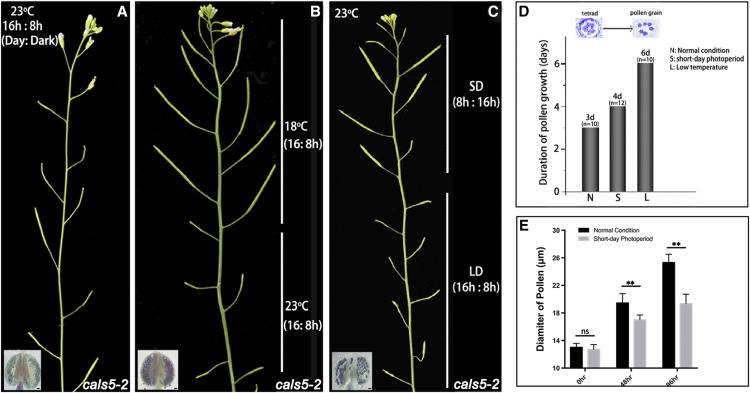
It was shown that the fertility and pollen production of cals5-2 line can be restored under low temperature (Zhu et al., 2020). In this study, we investigated whether its fertility was also photoperiod sensitive. A typical photoperiod for growing Arabidopsis is 16 h of light and 8 h of darkness at 23°C, the LD condition. To test its sensitivity to photoperiod, we grew the cals5-2 line under SD photoperiod with 8 h of light and 16 h of darkness at 23°C. Under LD, cals5-2 plants were severely sterile with few pollen grains (Fig. 1A). However, under the SD photoperiod, the seed set of cals5-2 was significantly restored (Fig. 1C), similar to that observed under low temperature (Fig. 1B). In addition, results from Alexander staining showed the formation of mature pollen grains (purple) in cals5-2 under SD conditions (Fig. 1C). Thus, SD photoperiod restores pollen formation and fertility of cals5-2.
Figure 1.
cals5-2 is a PGMS line. A, cals5-2 exhibited severely reduced male fertility under normal conditions. Few pollen grains were produced in cals5-2 anther. B, The fertility of cals5-2 was restored under low temperature. C, The fertility of cals5-2 was restored under SD conditions, and pollen maturation was recovered. The images of plants shown in A, B, and C were digitally extracted for comparison. Bars = 20 μm. D, Pollen maturation was delayed by 1 d under SD conditions compared with LD conditions. E, Diameters of microspores at different developmental stages. Twenty microspores or pollen grains were used to measure the diameter at each developmental stage, respectively. One-way ANOVA was performed to compare the diameter of microspores (*P < 0.05 and **P < 0.001; ns, not significant [P > 0.05]). Error bars represent the sd of pollen diameters. The diameter of microspores at 48 and 96 h under a SD photoperiod was significantly reduced compared to a LD photoperiod.
The pollen development process, microgametogenesis, depends on timely coordination of meiosis, mitosis, cell growth, and expansion (Sanders et al., 1999). Our previous work showed that the pollen development is slowed under low temperature (Zhu et al., 2020). To analyze whether the development is also slowed under SD conditions, we compared the pollen growth under LD and SD conditions using two different approaches. First, we analyzed the growth rate of cals5-2 from tetrad to mature pollen grain under LD and SD conditions. One tetrad encloses four microspores in callose wall, which can be easily observed using light microscopy. In Arabidopsis, mature pollen grains contain three nuclei, which are easy to identify. Under the LD condition, a few fertile pollen grains can be occasionally observed in cals5-2 anthers (Fig. 1A). It took about 3 d for tetrads to develop into mature pollen grains under LD photoperiod (n = 10; Fig. 1D), compared to about 4 d under the SD photoperiod (n = 12; Fig. 1D). The second approach was to measure the size of developing microspores during microgametogenesis, as previously described (Zhu et al., 2020). Under the LD condition, the microspores released from the tetrad were about 13.0 μm in diameter. The microspore size was 19.4 and 25.3 μm after 2 and 4 d growth, respectively (Fig. 1E). Under the SD condition, the released microspores were about 12.7 μm in diameter, and their sizes were 17.0 to 19.3 μm after 2 and 4 d growth, respectively (Fig. 1E). These results demonstrated that SD photoperiod significantly slowed microspore development. A similar delay in microspore development was observed under the low-temperature conditions, which can restore the fertility of cals5-2 and other TGMS lines (Zhu et al., 2020). Therefore, both low temperatures and SD photoperiod restore the fertility of cals5-2 plants through slowing microspore development.
Low Light Intensity Restores the Fertility of cals5-2
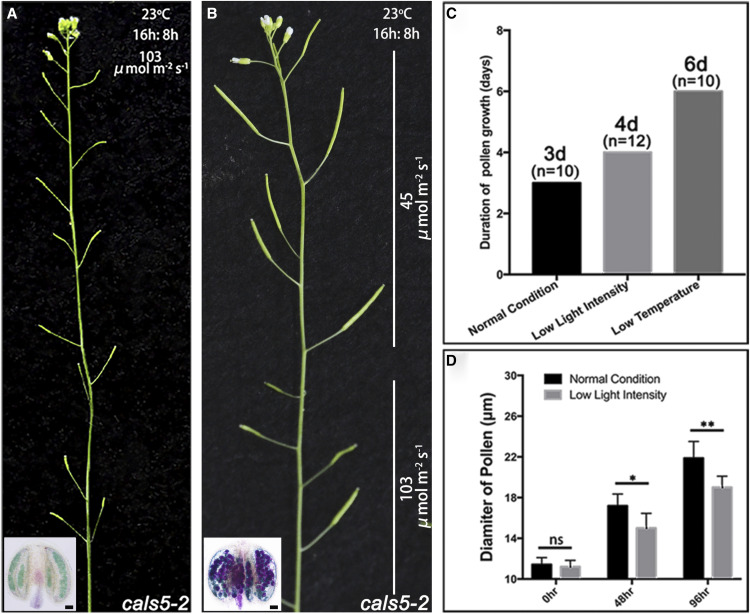
In addition to photoperiod, light intensity is another important factor that affects light signaling and general energy production. Thus, we further investigated whether the fertility of cals5-2 can be restored by low light intensity. We placed cals5-2 in the growth chamber with low light intensity (45 μmol m−2 s−1) for 10 d. The control cals5-2 plants were grown under the normal conditions with light intensity of 103 μmol m−2 s−1. Under low light intensity, seeds were produced normally (Fig. 2A; Supplemental Fig. S1), and fertile pollen grains were observed in cals5-2 (Fig. 2B). We further analyzed the pollen development process under different light intensity. Pollen maturation under normal conditions and under low light intensity took about 3 and 4 d, respectively (Fig. 2C). By measuring microspore diameters at 2 and 4 d after release from tetrad, we found the microspores were significantly smaller at both time points under the low light intensity condition (Fig. 2D). Therefore, low intensity of light also slowed microspore development. Taken together, low light intensity restores cals5-2 fertility through slowing its microspore growth, which is the same as the low temperature and SD photoperiod.
Figure 2.
Fertility of cals5-2 is restored under low light intensity. A, cals5-2 showed severe male sterility under normal conditions. Pollen production was abnormal in cals5-2 anther. B, The fertility of cals5-2 was restored under low light intensity. Pollen grains in cals5-2 were produced under low light intensity. Bars = 20 μm. C, Pollen growth, from tetrad to mature pollen, was delayed by 1 d under low light intensity (n = 12). D, Diameter of microspores at different developmental stages. Twenty microspores or pollen grains were used to measure the diameter at each developmental stage, respectively. One-way ANOVA was performed to compare the diameter of microspores (*P < 0.05 and **P < 0.001; ns, not significant [P > 0.05]). Error bars represent the sd of pollen diameters. The diameter of microspores at 48 and 96 h was significantly smaller under low light intensity compared to normal conditions.
Slow Development Overcomes Sexine Defects and Restores the Formation of Functional Pollen in cals5-2
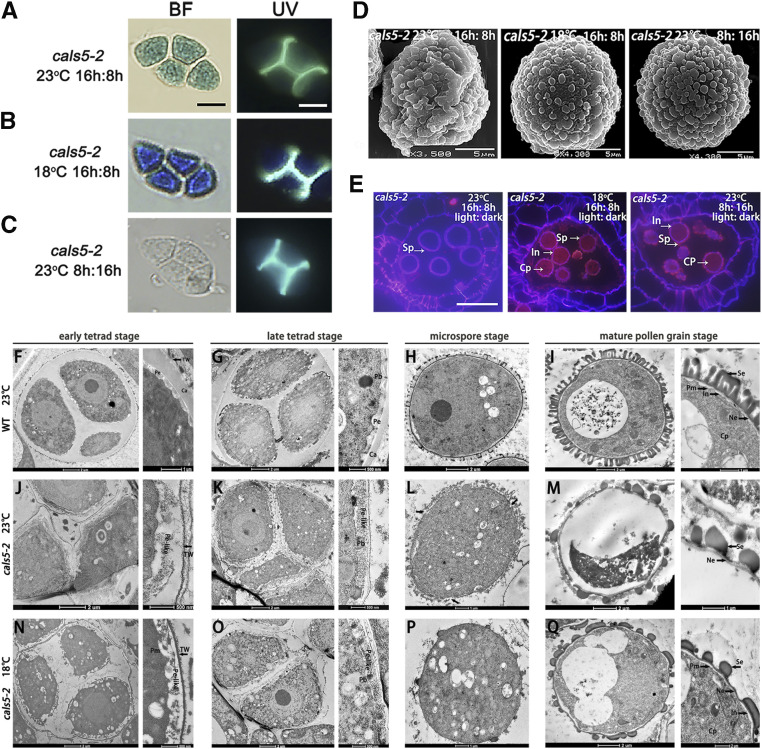
After meiosis, all four microspores are enclosed in callose wall. Primexine, formed between the callose wall and microspore plasma membrane, is responsible for pollen wall pattern establishment (Xu et al., 2016). CalS5 encodes a callose synthase involved in callose wall formation. Peripheral callose wall is absent in cals5-2, which leads to defective exine pattern formation and male sterility under normal conditions (Dong et al., 2005). We used the callose stain aniline blue to reveal the wall features of tetrads in fertility-restored plants. The peripheral callose wall of the fertile cals5-2 tetrad was found to be absent, similar to what was observed in normal (sterile) conditions (Fig. 3, A–C). We further observed microspore development of fertile and sterile cals5-2 plants using a scanning electron microscope (SEM). The pollen surface of the microspore was rough and abnormal in the fertility-restored plants of cals5-2, again similar to cals5-2 male-sterile plants under normal conditions (Fig. 3D). Thus, even though the pollen grains became fertile in the restored plants under SD photoperiod and low temperatures, their callose wall and pollen wall remained defective. Furthermore, we stained the pollen wall with Tinopal and DiOC2. The intine can be stained purple by Tinopal, and the lipid contents and sexine of the mature pollen grain can be stained red by DIOC2 (Gu et al., 2014). The intine layer and lipid contents were not visible in pollen grains of cals5-2 plants under normal (sterile) conditions (Fig. 3E). In contrast, in the fertile cals5-2 plants, the intine layer and lipid contents were clearly visible in the fertile cals5-2 pollen grains under SD photoperiod or low temperature (Fig. 3E). This indicates that cals5-2 microspore could overcome pollen wall pattern defect to further develop into mature functional pollen under these permissive conditions.
Figure 3.
Fertility-restored cals5-2 line exhibits pollen wall defects but recovered pollen wall integrity. A to C, Results from aniline blue staining of the callose wall of tetrads. Callose deposition was not recovered in cals5-2 under low temperature or SD conditions. Bars = 10 μm. The scale bar in A also represents ones in B and C. D, SEM observation of the mature pollen of cals5-2 plants. The pollen surface remained rough and irregular under low temperature or SD conditions. E, Tinopal and DIOC2 staining of cals5-2 anther and pollen grains. The intine of cals5-2 pollen was intact under low temperature or SD conditions. Cp, Cytoplasm; Sp, sporopollenin; In, intine. Bars = 20 μm. F to I, TEM images of wild-type pollen from the early tetrad stage to mature pollen stage at 23°C. J to M, TEM images of cals5-2 pollen from the early tetrad stage to mature pollen stage under 23°C. The callose wall and primexine were not observed around microspores in tetrads. The nexine was discontinuous, and intine formation was disrupted at 23°C. N to Q, TEM images of cals5-2 pollen from the early tetrad stage to mature pollen stage at 18°C. The callose wall and primexine were not restored, but the integrity of nexine and intine was much better maintained than those under normal temperature (23°C). Ca, Callose wall; TW, tetrad wall; Pb, probacular; Pe, primexine; Pm, plasma membrane; Pe-like, primexine-like materials; In, intine; Se, sexine; Ne, nexine; Cp, cytoplasm.
During anther development, the pollen wall materials from the tapetum deposit outside of the microspore to form a wall with a reticular pattern. Both callose wall and primexine are important for pollen wall establishment (Xu et al., 2016). Transmission electron microscope (TEM) observation revealed that the primexine remained absent in cals5-2 tetrads under low temperature (Fig. 3N), but some electron-dense (pe-like) materials were visible between the plasma membrane and the thin wall (Fig. 3, N and O). Globular sporopollenin was randomly deposited on the plasma membrane at the late tetrad stage under both low-temperature and normal conditions (Fig. 3, J to M, P, and Q). Thus, callose wall is essential for primexine formation. The defective callose wall and primexine are in agreement with defective pollen wall pattern in the restored cals5-2 plant under low temperature. Under normal conditions, the cals5-2 nexine layer was discontinuous (Fig. 3L). However, under low temperature, the nexine layer was continuous and intact at the microspore stage (Fig. 3P), and the intine layer also exhibited good integrity at the mature pollen stage (Fig. 3Q). These results show that the formation of functional mature pollen in the fertility restored cals5-2 is associated with restoration of nexine and intine layers, while the outer sexine wall remained defective. Slowed development may therefore overcome certain pollen wall pattern defects to support its further development into functional pollen.
Fertility of cals5-5 Is Reduced under High Temperature
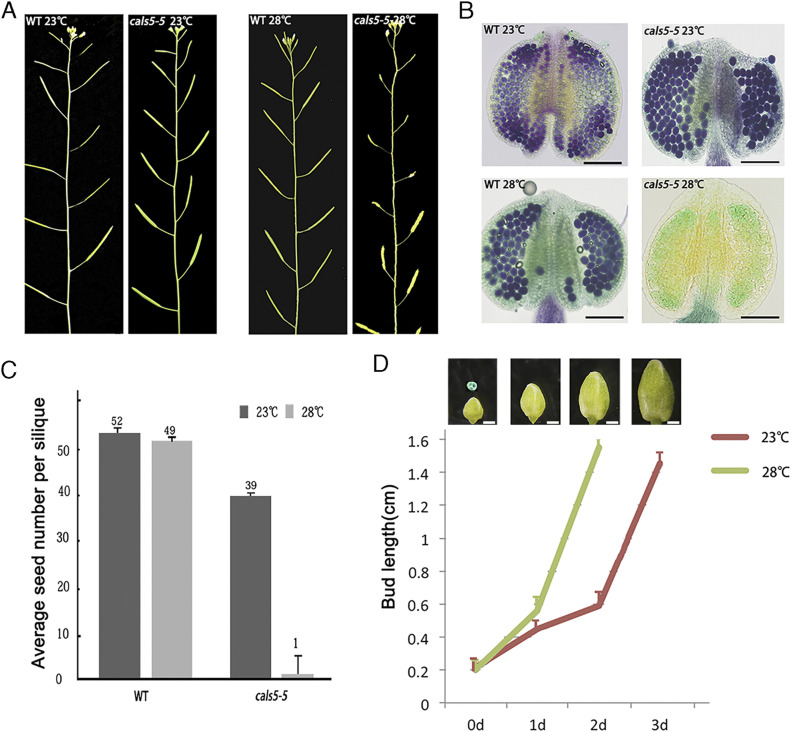
Our results suggest that slowed development led to the fertility restoration of P/TGMS lines under permissive conditions (Figs. 1 and 2). To further test this hypothesis, we investigated whether faster pollen development could affect the fertility of P/TGMS lines. cals5 has several alleles, and cals5-5 is a weak allele with a transfer DNA insertion in the third intron (Supplemental Fig. S2A; Nishikawa et al., 2005). The expression level of CalS5 in cals5-5 was found to be about 54.9% of that of wild type (Supplemental Fig. S2B). cals5-5 was fully fertile under normal conditions (Fig. 4A). In cals5-5, pollen production and callose wall formation were similar to those of wild type (Fig. 4B; Supplemental Fig. S2D). To find out whether high temperature could cause male sterility in the mutant, cals5-5 was placed under high temperature (28°C). The wild-type control was fully fertile at both 23°C and 28°C (Fig. 4A). However, the fertility of cals5-5 was severely compromised at 28°C (Fig. 4A). The average seed set of the cals5-5 was significantly decreased (Fig. 4C), and few pollen grains were produced in cals5-5 anthers at 28°C (Fig. 4B). It was shown that high temperature may impact the mRNA splicing in the rice ugp1-RNAi plants (Chen et al., 2007). The intron of CalS5 contains a transfer DNA insertion in cals5-5 plants. Thus, we further investigated whether the transcription and splicing of CalS5 in cals5-5 had the similar effect to the ugp1-RNAi line. We analyzed the expression level of the full-length mature CalS5 transcript in wild type and cals5-5 under normal and high temperature by RT-PCR. Results showed that the expression of CalS5 was reduced in cals5-5 compared with in wild type (Supplemental Fig. S2C). However, the CalS5 transcripts under 28°C was slightly higher than the ones under 23°C either in wild type or in cals5-5, respectively (Supplemental Fig. S2C). This result indicates that the full-length CalS5 transcripts in cals5-5 under high temperature was not reduced compared with that under low temperature. Thus, the sterility in cals5-5 under high temperature was not due to the temperature-sensitive mRNA splicing. As there were no pollen grains in cals5-5 at 28°C, we analyzed the growth rate of the buds from the tetrad stage to mature pollen grain. It took 2 d for the buds to grow from 0.2 (tetrad stage) to 1.58 cm (mature pollen stage) at 28°C, while it took 3 d to complete the same process at 23°C (Fig. 4D). These results indicate that high temperature accelerates the growth rate of plants, which is associated with male sterility in cals5-5.
Figure 4.
Fertility of cals5-5 is significantly reduced under high temperature. A, Fertility of cals5-5 was reduced at 28°C, while the fertility of the wild-type plants was normal. The images of plants shown in A were digitally extracted for comparison. B, Results from Alexander staining of the pollen grains. A number of pollen grains were observed in the wild-type anther but not in the cals5-5 anther under high temperature. Bars = 50 μm. C, The seed number per pod in the wild-type and cals5-5 plants growing at 23°C and 28°C (n = 12). Error bars represent the sd of seed setting per silique. There was almost no seed in the cals5-5 siliques under high temperature. D, Growth rate of the pollen from the tetrad to mature stage in wild-type plants. The tetrad over the first flower bud represents that it was at the tetrad stage. Error bars represent the sd of bud length (n = 10). The buds developed much quicker at 28°C than at 23°C. Bars = 100 μm.
SD Photoperiod Restores Fertility of Several Other TGMS Lines
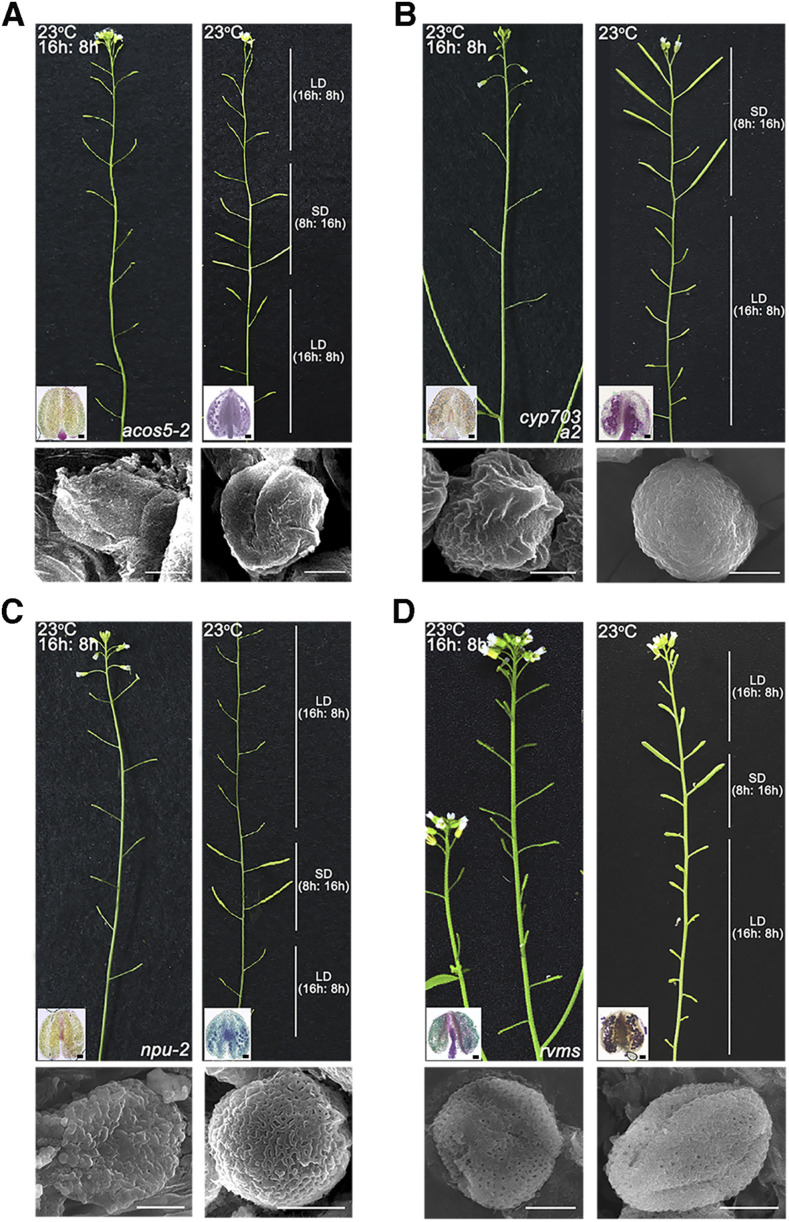
The pollen wall is composed of outer exine (the outer sexine and inner nexine) and inner intine. The development of the exine requires the synthesis of wall materials for sexine and nexine and the assembly of these materials to form a particular pollen wall pattern (sexine pattern; Xu et al., 2016). ACoS5 and CYP703A2 are responsible for the synthesis of sexine material. The acos5-2 and cyp703a2 single mutants are male sterile and have defective pollen wall formation (Morant et al., 2007; de Azevedo Souza et al., 2009). NPU is responsible for the primexine formation. npu-2 is also male sterile. In npu-2, primexine cannot be formed, and pollen wall pattern is defective (Chang et al., 2012). RVMS is responsible for the synthesis of membrane material of microspores. The rvms line also showed male sterility and defective pollen development (Zhu et al., 2020). Previous investigation shows that acos5-2, cyp703a2, and rvms are all TGMS lines (Zhu et al., 2020). We found npu-2 is also a TGMS line, as its fertility was restored under low temperature (Supplemental Fig. S3). Here, we further investigated their fertility under SD photoperiod. As shown in Figure 5, they exhibited full siliques of seeds under the tested conditions. Results from Alexander staining assay further indicated the production of fertile pollen (Fig. 5). Thus, these TGMS lines also showed PGMS features that their fertility is restored under SD photoperiod. This indicates that slow development is a general mechanism for fertility restoration of PGMS lines, and both PGMS and TGMS may commonly function through slowed growth to rescue the fertility. To further understand the pollen wall development of these lines under SD conditions, SEM observations were then performed. We found that the pollen grains of these lines mostly turned round and plump, indicating morphological restoration (Fig. 5). The pollen surface of the functional npu-2 pollen grains exhibited regular reticulated pattern under the permissive conditions (Fig. 5C). The formation of the primexine was restored in npu-2 under the permissive condition (Supplemental Fig. S4), indicating the full recovery of the pollen wall pattern. However, the cell wall surface of the pollen grains of acos5-2 and cyp703a2 remained rough and irregular, similar to what was observed for cals5-5 (Fig. 5, A and B). Thus, despite the pollen wall defects in these lines under low temperature, SD photoperiod, and low light intensity, the pollen fertility can be restored through slow development (Figs. 1 and 2).
Figure 5.
Fertility restoration of TGMS lines under SD photoperiod. A, acos5-2 is male sterile under normal conditions, but its fertility was restored under short photoperiod or low light intensity. SEM observations showed that the defective pollen surface of acos5-2 remained defective under SD photoperiod or low light intensity by SEM observations. B, cyp703a2 is male sterile under normal conditions, but its fertility was largely restored under SD photoperiod or low light intensity. C, npu-2 showed male sterility under normal conditions, but its fertility was significantly restored under SD photoperiod or low light intensity. SEM observations revealed that the pollen surface was restored to a normal reticulated pattern under SD photoperiod or low light intensity. D, rvms exhibited male sterility under normal conditions but was fertile under SD photoperiod or low light intensity. The pollen wall of rvms remained rough and irregular under SD photoperiod or low light intensity. Bars = 20 μm (pollen staining) and = 5 μm (SEM).
DISCUSSION
The discovery of a PGMS line in the japonica rice var NongKen 58S in 1973 initiated the research and breeding of two-line hybrid rice in China and led to the discovery and development of TGMS germplasm (Fan and Zhang, 2018). The mechanisms underlying the EGMS fertility recovery remain of great interests to plant scientists and agronomists. Our previous study shows that Arabidopsis cals5-2 is a TGMS line and that slowed development is a general mechanism for fertility restoration of all investigated TGMS lines (Zhu et al., 2020). In this study, we found that cals5-2 is also a PGMS line, and its fertility restoration under SD photoperiod is associated with slowed development (Fig. 1). We show that other TGMS lines, cyp703a2, npu-2, and rvms, also feature PGMS characteristics (Fig. 5). Furthermore, low light intensity, another environmental factor that results in slowed pollen development, also restored pollen fertility of the P/TGMS line (Fig. 2). Thus, we propose that slowed development is the mechanism for the fertility restoration of PGMS, and both TGMS and PGMS lines share the common mechanism for fertility restoration. For most plants, suboptimal conditions including low temperature, low light intensity, or SD photoperiod are known to result in slowed growth and development. For instance, we previously established that pollen development was significantly slowed in rice and Brassica spp. under low-temperature conditions (Zhu et al., 2020). Reproductive processes like anther development and pollen formation are quite conserved in plants (Wilson and Zhang, 2009; Gómez et al., 2015). It is likely that slowed development is also involved in fertility restoration of rice P/TGMS lines. In rice, PGMS and TGMS are two types of male-sterile lines widely used for hybrid breeding. However, results from agriculture practice already indicate the possible interactions between PGMS and TGMS (Fan and Zhang, 2018). For example, photoperiod and temperature were found to act synergistically in PGMS and TGMS lines (He et al., 1987). In addition, the same genetic locus for a noncoding RNA that determines PGMS in japonica rice var NongKen 58S but TGMS in indica rice var Peiai64S (Ding et al., 2012; Zhou et al., 2012). All these information support a common mechanism for the fertility restoration shared by both PGMS and TGMS lines.
The restoration of fertility in TGMS and PGMS plants depends on the successful formation of functional pollen. The genes determining P/TGMS such as CalS5, NPU, CYP703A2, ACoS5, and RVMS play different roles in pollen formation. This is reflected by our findings, which reveal that multiple mechanisms are involved in the recovery of functional pollen (Fig. 6). CalS5 is responsible for callose wall formation and ACoS5 and CYP703A2 for synthesis of sexine materials. Under normal conditions, loss of any of these genes results in a defective sexine layer, pollen rupture, and male sterility (Dong et al., 2005; Morant et al., 2007; de Azevedo Souza et al., 2009). However, our results suggest that in these P/TGMS lines, permissive conditions relieve the requirement for an intact sexine, thereby restoring fertility (Figs. 3 and 5). It indicates that slow development overcomes sexine defect during the formation of fertile pollen, which leads to the fertility restoration. Every plant species has a unique and reticular pollen wall pattern. Callose wall and primexine are critical for pollen wall pattern establishment (Xu et al., 2016). In the npu-2 line, primexine is absent and pollen wall pattern is defective (Chang et al., 2012). However, in fertility-restored npu-2 plant, primexine was observed, and its pollen wall pattern was established (Supplemental Fig. S4). Several other genes were reported to be involved in primexine formation (Paxson-Sowders et al., 2001; Ariizumi et al., 2004; Guan et al., 2008; Hu et al., 2014; Li et al., 2017; Suzuki et al., 2017). The slower pace of development may overcome the loss of NPU’s function in coordination of these genes to allow primexine formation. Nevertheless, in fertility-restored cals5-2 plants, primexine remained absent, and the pollen wall pattern was still defective (Fig. 3). Therefore, primexine is critical for pollen wall pattern establishment. RVMS encodes a member of the GDSL-motif lipase family that may provide materials for plasma-membrane formation during microspore enlargement. In Arabidopsis, this gene family contains about 108 members (Zhu et al., 2020). Under permissive conditions, the remaining RVMS lipase activity in the rvms mutant or other members in this family may provide sufficient plasma membrane for slow microspore development. In conclusion, slow development, as a general mechanism, may allow different P/TGMS lines to adopt different strategies to overcome pollen formation defects and to restore fertility under permissive condition.
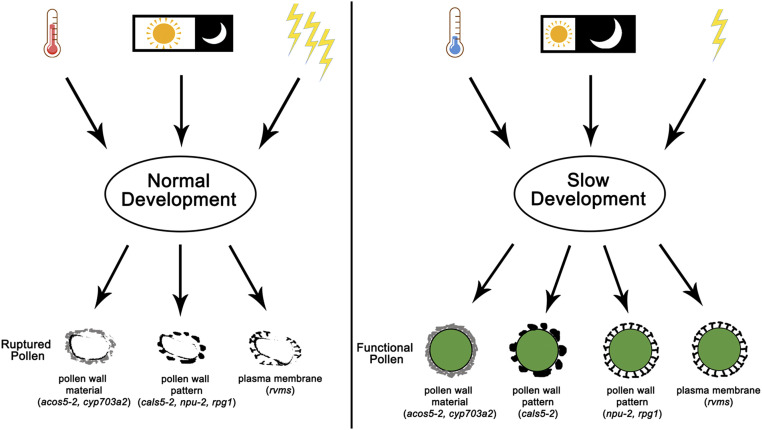
Figure 6.
A schematic model for slow development functions in the fertility restoration of different TGMS/PGMS lines. Temperature, photoperiod, and light intensity are all crucial for the fertility restoration of TGMS/PGMS lines. Under restrictive conditions, pollen development is rapid, leading to the pollen rupture due to various pollen defects in different T/PGMS lines (left). However, under permissive conditions, pollen development is slowed down, which promotes the functional pollen formation (right). The restored pollen remains morphological defects in some lines or shows restored cell wall features in others under all tested conditions, suggesting that multiple molecular mechanisms are involved.
Normal plant growth and reproduction rely on suitable environmental conditions such as certain temperature, photoperiod, and light intensity. Based on the responses to environmental factors, EGMS has been divided into four types: PGMS, TGMS, reverse PGMS (rPGMS) and rTGMS (Chen and Liu, 2014; Fan and Zhang, 2018). rPGMS lines show male sterility under SD conditions and normal fertility under LD conditions. rTGMS lines show male sterility under normal temperature and fertility under high temperature. In this work, we showed that light intensity also affected fertility of male-sterile lines (Fig. 2). Together with previous work (Zhu et al., 2020), we show slow development is the common mechanism for the fertility restoration of TGMS and PGMS lines. The responses of rPGMS/TGMS lines to environmental change are opposite to that of PGMS/TGMS lines. rPGMS/TGMS may have different mechanisms to restore their fertility.
MATERIALS AND METHODS
Plant Growth Conditions
Arabidopsis (Arabidopsis thaliana) plants, cals5-2 (Columbia, SALK_026354) and cals5-5 (Columbia, SALK_072226), were obtained from the Arabidopsis Biological Resource Center. Plants were grown on soil under normal conditions (16 h light/8 h dark, 103 μmol m−2 s−1, 23°C) in a greenhouse. For low-temperature treatments, the plants were grown for 5 weeks in the same conditions as described above and then transferred into a low-temperature greenhouse (16 h light/ 8 h dark, 103 μmol m−2 s−1, 18°C). Similarly, for the SD photoperiod treatment, the plants were first grown for 5 weeks under normal conditions and then were transferred to a SD photoperiod growth chamber (8 h light/16 h dark, 103 μmol m−2 s−1, 23°C) for 2 weeks. For the low light intensity treatment, after 5 weeks growth under normal conditions, the plants were transferred into low light intensity conditions (16 h light/8 h dark, 43 μmol m−2 s−1, 23°C) for 2 weeks. For the high-temperature treatment, the plants were grown for 5 weeks under normal temperature and then transferred to a growth chamber with 16 h light/8 h dark, 103 μmol m−2 s−1, at 28°C for 7 d.
Genetic Analysis and Expression Analysis
To obtain the cals5-2 and cals5-5 homozygous mutants, the primers of genetic analysis for locus identification were designed using SIGnALiSect Tools (http://signal.salk.edu/isects.html). PCR primers used to amplify the specific genomic fragments for each allele were as follows: cals5-2 (CalS5-2-F, 5′-TGCTTCTGTGGTGGTCACAGG-3′; CalS5-2-R, 5′-GCATACCAAATTTGAGTGTCCAT-3′) and cals5-5 (CalS5-5-F, 5′-CCAGATTTCCGGTTTTCTTTC-3′; CalS5-5-R, 5′-TGTGGATTTCTCCATCGGTAG-3′). Total RNA was isolated from Arabidopsis Col-0 inflorescences using Trizol reagent (Invitrogen). cDNA was reverse-transcribed from RNA using the SuperScript first-strand synthesis system (Invitrogen). The relative expression levels of CalS5 in the two mutant alleles were assayed by RT-PCR/RT-qPCR using gene-specific primers: CalS5-RT-F, 5′-TTCAGGGAAGGGGATCTGAAAG-3′; CalS5-RT-R, 5′-TCAGAGAAAGTTAGTCTGAGACTT-3′; CalS5qRT3F, 5′-GGGGATGCTATTCCACTTCTTGA-3′; CalS5qRT3R, 5′-CAACTCCCATGATGTACTCATACCC-3′. The RT-PCR program for CalS5 used was as follows: 94°C for 5 min, (94°C for 15 s, 55°C for 15 s, 72°C for 3 min) × 36 cycles, 72°C for 5 min. The qPCR was performed as described (Peng et al., 2020). All reactions were repeated three times with β-TUBULIN as the normalizing gene.
Pollen Viability Assay and Callose Wall and Intine Staining
Pollen grains were collected on a slide. They were stained according to the Alexander staining method (Alexander, 1969), and the photos of the stained pollen grains were taken using an Olympus optical microscope with an Olympus digital camera. Aniline blue staining of callose was performed as described in a previous report (Zhang et al., 2007). The anthers were fixed in Carnoy’s fixative for 2 h, and then the tetrads were separated to a glass slide. The tetrads were stained with 0.1% (m/v) aniline blue. The pictures of callose staining were taken with an Olympus BX-51 microscope (Olympus). T&D staining assay was performed as described in a previous report (Lou et al., 2014). The inflorescence of wild type and mutant were embedded into spur resin, and the sections of pollen were put on the surface of the 50°C dryer. Then the desiccation of sections was performed, and the sections were stained with toluidine blue for 5 min (10 mg mL−1), Tinopal for 15 min (10 mg mL−1; Sigma) and DiOC2 for 5 min (5 mg mL−1; Sigma).
SEM and TEM Sample Preparation and Observation
For SEM observation, pollen grains were applied by flicking the anthers of mature flowers over mounting tape on a stub. The pollen grains were allowed to air dry for about 30 min, sputter coated with gold, and observed with a JSM-840 microscope (JEOL, Japan). For TEM observation, the same-stage inflorescences of wild type and mutant were fixed in 0.1 m phosphate buffer (pH 7.2) with 2.5% (v/v) glutaraldehyde. After 7 d of fixation, the material was washed thoroughly with phosphate buffer (pH 7.2). After that, the material was gradient dehydrated to 100% (v/v) ethyl alcohol and the inflorescences were then embedded and polymerization was performed (65°C, 24 h). Finally, ultra-thin sections (70–100 nm thick) were made and observed using a TEM microscope (JEOL).
The Measure of Pollen Growth Rate
The tetrad-stage buds of wild type were marked and retained at 8:00 am under the different conditions. The anthers were carefully separated and dissected. The anthers of different stages were respectively fixed in Carnoy’s fixative for 2 h, and the pollen grains were separated on a glass slide using a needle. The pollen grains were stained in toluidine blue solution and then observed and measured using an Olympus optical microscope. To determine the microspore or pollen diameters, images were captured using a BX51 Olympus microscope and measured using ImageJ software (NIH). The F-test was performed to compare the diameters between normal condition and low light intensity conditions.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: CalS5 (AT2G13680); NPU (AT3G51610); CYP703A2 (AT1G01280); AcoS5 (AT1G62940); and RVMS1 (AT4G10950).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Seed production analysis of cals5-2 under different conditions.
Supplemental Figure S2. Characterization of two alleles of cals5.
Supplemental Figure S3. Fertility of npu-2 is restored under low temperature.
Supplemental Figure S4. Primexine formation and pollen wall pattern are restored in npu-2 at low temperature.
Acknowledgments
We thank Jeremy Murray (Shanghai Institute of Plant Physiology and Ecology) for critical reading and revising of this manuscript.
Footnotes
This work was supported by the grants from the National Natural Science Foundation of China (grant nos. 31930009 and 31970335), the National Key Research and Development Program of China (grant no. 2016YFD0100902), the Shanghai Municipal Education Commission (grant no. 2019–01–07–00–02–E00006), and the Science and Technology Commission of Shanghai Municipality (grant nos. 18DZ2260500 and 17DZ2252700).
Articles can be viewed without a subscription.
References
- Alexander MP.(1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K(2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39: 170–181 [DOI] [PubMed] [Google Scholar]
- Chang HS, Zhang C, Chang YH, Zhu J, Xu XF, Shi ZH, Zhang XL, Xu L, Huang H, Zhang S, et al. (2012) No primexine and plasma membrane undulation is essential for primexine deposition and plasma membrane undulation during microsporogenesis in Arabidopsis. Plant Physiol 158: 264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu YG(2014) Male sterility and fertility restoration in crops. Annu Rev Plant Biol 65: 579–606 [DOI] [PubMed] [Google Scholar]
- Chen LY.(2001) The Principles and Techniques of Two-Line Hybrid Rice. Shanghai Scientific & Technical Publishers, Shanghai [Google Scholar]
- Chen R, Zhao X, Shao Z, Wei Z, Wang Y, Zhu L, Zhao J, Sun M, He R, He G(2007) Rice UDP-glucose pyrophosphorylase1 is essential for pollen callose deposition and its cosuppression results in a new type of thermosensitive genic male sterility. Plant Cell 19: 847–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azevedo Souza C, Kim SS, Koch S, Kienow L, Schneider K, McKim SM, Haughn GW, Kombrink E, Douglas CJ(2009) A novel fatty Acyl-CoA Synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell 21: 507–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Lu Q, Ouyang Y, Mao H, Zhang P, Yao J, Xu C, Li X, Xiao J, Zhang Q(2012) A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc Natl Acad Sci USA 109: 2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP(2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42: 315–328 [DOI] [PubMed] [Google Scholar]
- Fan Y, Zhang Q(2018) Genetic and molecular characterization of photoperiod and thermo-sensitive male sterility in rice. Plant Reprod 31: 3–14 [DOI] [PubMed] [Google Scholar]
- Fan YR, Cao XF, Zhang QF(2016) The progress on photoperiod thermo-sensitive genic male sterile rice. Chin Sci Bull 61: 3822–3832 [Google Scholar]
- Gómez JF, Talle B, Wilson ZA(2015) Anther and pollen development: A conserved developmental pathway. J Integr Plant Biol 57: 876–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JN, Zhu J, Yu Y, Teng XD, Lou Y, Xu XF, Liu JL, Yang ZN(2014) DYT1 directly regulates the expression of TDF1 for tapetum development and pollen wall formation in Arabidopsis. Plant J 80: 1005–1013 [DOI] [PubMed] [Google Scholar]
- Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN(2008) RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol 147: 852–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HH, Zhang ZG, Yuan SC(1987) A preliminary study on the effects of temperature on the development and fertility conversion of light-sensitive genic male sterile rice. J Wuhan Univ 7: 87–93 [Google Scholar]
- Hu J, Wang Z, Zhang L, Sun MX(2014) The Arabidopsis exine formation defect (EFD) gene is required for primexine patterning and is critical for pollen fertility. New Phytol 203: 140–154 [DOI] [PubMed] [Google Scholar]
- Li S, Yang D, Zhu Y(2007) Characterization and use of male sterility in hybrid rice breeding. J Integr Plant Biol 49: 791–804 [Google Scholar]
- Li WL, Liu Y, Douglas CJ(2017) Role of glycosyltransferases in pollen wall primexine formation and exine patterning. Plant Physiol 173: 167–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Xu XF, Zhu J, Gu JN, Blackmore S, Yang ZN(2014) The tapetal AHL family protein TEK determines nexine formation in the pollen wall. Nat Commun 5: 3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AA, Gubler F(2005) The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 17: 705–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morant M, Jørgensen K, Schaller H, Pinot F, Møller BL, Werck-Reichhart D, Bak S(2007) CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell 19: 1473–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Zinkl GM, Swanson RJ, Maruyama D, Preuss D(2005) Callose (beta-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA(2001) DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol 127: 1739–1749 [PMC free article] [PubMed] [Google Scholar]
- Peng X, Wang M, Li Y, Yan W, Chang Z, Chen Z, Xu C, Yang C, Wang Deng X, Wu J, et al. (2020) Lectin receptor kinase OsLecRK-S.7 is required for pollen development and male fertility. J Integr Plant Biol 62: 1227–1245 [DOI] [PubMed] [Google Scholar]
- Qi Y, Liu Q, Zhang L, Mao B, Yan D, Jin Q, He Z(2014) Fine mapping and candidate gene analysis of the novel thermo-sensitive genic male sterility tms9-1 gene in rice. Theor Appl Genet 127: 1173–1182 [DOI] [PubMed] [Google Scholar]
- Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu YC, Lee PY, Truong MT, Beals TP, Goldberg RB(1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11: 297–322 [Google Scholar]
- Shi MS.(1985) The discovery and preliminary studies of the photoperiod-sensitive recessive male sterile rice (Oryza sativa L. subsp. japonica). Scientia Agricultura Sinica 2: 44–48 [Google Scholar]
- Sun ZX, Cheng SH, Si HM, Yang RC, Liang KJ, Wang NY(1991) Fertility of photoperiod-sensitive genie male-sterile lines of early indica rice under photo-and thermo-period controlled conditions. Ada Agriculturae Zhejiangensis 3: 101–105 [Google Scholar]
- Sun ZX, Xiong ZM, Min SK, Si HM(1989) Identification of the temperature sensitive male sterile rice. Zhongguo Shuidao Kexue 3: 49–55 [Google Scholar]
- Suzuki T, Narciso JO, Zeng W, van de Meene A, Yasutomi M, Takemura S, Lampugnani ER, Doblin MS, Bacic A, Ishiguro S(2017) KNS4/UPEX1: A type II arabinogalactan beta-(1,3)-galactosyltransferase required for pollen exine development. Plant Physiol 173: 183–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani SS, Ilyas-Ahmed M(2001) Environment-sensitive genic male sterility (EGMS) in crops. Adv Agron 72: 139–195 [Google Scholar]
- Wilson ZA, Zhang DB(2009) From Arabidopsis to rice: Pathways in pollen development. J Exp Bot 60: 1479–1492 [DOI] [PubMed] [Google Scholar]
- Xu T, Zhang C, Zhou Q, Yang ZN(2016) Pollen wall pattern in Arabidopsis. Sci Bull (Beijing) 61: 832–837 [Google Scholar]
- Zhang ZB, Zhu J, Gao JF, Wang C, Li H, Li H, Zhang HQ, Zhang S, Wang DM, Wang QX, et al. (2007) Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J 52: 528–538 [DOI] [PubMed] [Google Scholar]
- Zhou H, Liu Q, Li J, Jiang D, Zhou L, Wu P, Lu S, Li F, Zhu L, Liu Z, et al. (2012) Photoperiod- and thermo-sensitive genic male sterility in rice are caused by a point mutation in a novel noncoding RNA that produces a small RNA. Cell Res 22: 649–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zhou M, Yang Y, Li J, Zhu L, Jiang D, Dong J, Liu Q, Gu L, Zhou L, et al. (2014) RNase Z(S1) processes UbL40 mRNAs and controls thermosensitive genic male sterility in rice. Nat Commun 5: 4884. [DOI] [PubMed] [Google Scholar]
- Zhu J, Lou Y, Shi QS, Zhang S, Zhou WT, Yang J, Zhang C, Yao XZ, Xu T, Liu JL, et al. (2020) Slowing development restores the fertility of thermo-sensitive male-sterile plant lines. Nat Plants 6: 360–367 [DOI] [PubMed] [Google Scholar]