At the end of the plant reproductive phase, the FRUITFULL-APETALA2 pathway modulates responses to both endogenous and exogenous factors to coordinate inflorescence meristem arrest.
Abstract
The end of the reproductive phase in monocarpic plants is determined by a coordinated arrest of all active meristems, a process known as global proliferative arrest (GPA). GPA is linked to the correlative control exerted by developing seeds and, possibly, the establishment of strong source-sink relationships. It has been proposed that the meristems that undergo arrest at the end of the reproductive phase behave at the transcriptomic level as dormant meristems, with low mitotic activity and high expression of abscisic acid response genes. Meristem arrest is also controlled genetically. In Arabidopsis (Arabidopsis thaliana), the MADS-box transcription factor FRUITFULL induces GPA by directly repressing genes of the APETALA2 (AP2) clade. The AP2 genes maintain shoot apical meristem (SAM) activity in part by keeping WUSCHEL expression active, but the mechanisms downstream of this pathway remain elusive. To identify target genes, we performed a transcriptomic analysis, inducing AP2 activity in meristems close to arrest. Our results suggest that AP2 controls meristem arrest by repressing genes related to axillary bud dormancy in the SAM and negative regulators of cytokinin signaling. In addition, our analysis indicates that genes involved in the response to environmental signals also respond to AP2, suggesting that it could modulate the end of flowering by controlling responses to both endogenous and exogenous signals. Our results support the previous observation that at the end of the reproductive phase the arrested SAM behaves as a dormant meristem, and they strongly support AP2 as a master regulator of this process.
Flower, fruit, and seed production are basic events in plant reproduction. In Arabidopsis (Arabidopsis thaliana) and most of the species with indeterminate inflorescences where the shoot apical meristem (SAM) never differentiates, the number of flowers that an inflorescence is able to produce, and therefore the number of fruits and seeds, depends on the activity of the SAM and the length of the flowering period. Thus, the flowering period lasts from the floral transition until the meristem arrest at the end of flowering. However, while we have a clear understanding of the mechanisms acting at the floral transition, knowledge about the processes that govern the end of flowering at the meristem is still scarce.
In monocarpic plants, the end of flowering is associated with a senescence process named global proliferative arrest (GPA). GPA has been well characterized in Arabidopsis (Hensel et al., 1994; Walker and Bennett, 2018), starting with the arrest of SAM in the main inflorescence after the production of a certain number of flowers. At this point, all the active meristems of the plant also arrest more or less coordinately, responding to both local and systemic cues (Ware et al., 2020). After meristem arrest, the plant completes the filling of the last seeds produced and enters a global senescence program that ends with the death of the plant. The initiation of GPA is clearly affected by seed production and development (correlative control; Hensel et al., 1994; Wuest et al., 2016; Walker and Bennett, 2018). Sterile mutants or plants where flowers are removed manually show a delayed GPA, which leads to an increase in the flowers produced by the SAM (Hensel et al., 1994; Balanzà et al., 2018, 2019). To explain the effect of fertility on GPA, different hypotheses have been proposed. For example, GPA could respond to strong source-sink relations between developing seeds and the SAM (Sinclair and de Wit, 1975; Kelly et al., 1988). The possible existence of a mobile seed-derived signal that could mediate the meristem arrest was also proposed (Leopold et al., 1959; Engvild, 1989; Noodén et al., 2004). Recently, it has been shown that auxin supplied from the last developing fruits proximal to the shoot apex could modify the auxin fluxes in the stem, inducing SAM arrest (Ware et al., 2020). Despite all the information available, little is known about the molecular control of this process.
To assess the molecular responses of the SAM to seed production, Wuest et al. (2016) performed a transcriptomic study providing new evidence regarding the nature of this process. The authors showed that the arrested meristem at the end of flowering retains its identity and functionality, but it has reduced mitotic capacity. They also proposed that arrested meristems resemble dormant meristems, as they present high expression of abscisic acid (ABA) response genes. In agreement with this, the arrested meristems at the moment of GPA are able to recover activity after fruit removal, further supporting the role of seed contribution to meristem arrest (Hensel et al., 1994; Wuest et al., 2016). Although our knowledge of this process is increasing, the molecular events that control the trigger of meristem arrest are still unknown. Recently, the first genetic pathway involved in the control of the length of the flowering phase was described. In Arabidopsis, the end of flowering is controlled by the negative regulation of APETALA2-like (AP2-like) genes by FRUITFULL (FUL) and microRNA172 (miR172) in the SAM (Balanzà et al., 2018). Both the ful loss-of-function mutants and the ap2-170 mutant, an allele of AP2 that is partially resistant to miR172 action, are able to delay GPA, keeping meristems active longer. In addition, both mutants develop more flowers in the main shoot than wild-type plants when fruit and seed production is completely prevented, pointing to a direct effect on SAM activity independent of signals coming from fruits. AP2 has been implicated in the regulation of meristem identity and maintenance through the control of WUSCHEL (WUS) expression (Würschum et al., 2006; Zhao et al., 2007), although the underlying mechanism is not well understood.
The aim of this work was to identify genes working downstream of the FUL-AP2 pathway to control inflorescence meristem arrest. Our results, combined with previously reported data by Wuest et al. (2016), indicate that AP2 controls meristem activity and GPA via two main hormonal responses, ABA and cytokinins (CK), as well as by regulating genes that respond to different environmental signals, such as light quality and temperature. Our results confirm previous observations that arrested meristems at GPA behave as dormant meristems and uncover a direct role for AP2 in the induction of this dormant state.
RESULTS
To understand how AP2 controls SAM activity and represses untimely meristem arrest, we analyzed the transcriptomic response to elevated levels of AP2 in the inflorescence. AP2 has a complex and fine-tuned regulation at the transcriptional, posttranscriptional, and translational levels (Aukerman and Sakai, 2003; Chen, 2004). To simplify this regulation, we decided to take advantage of an inducible system to overexpress an allele of AP2 completely resistant to miR172, AP2m3 (35S::LhGR»AP2m3; Fig. 1A; Chen, 2004). We previously showed that ectopic expression of AP2 miR172-resistant alleles in the SAM is able to restore SAM activity and the production of new flowers when they are induced in an arrested SAM (GPA stage; Fig. 1B; Balanzà et al., 2018). As we were interested in how AP2 modulates the end of flowering and not the process of reversion of arrested meristems, we designed our experiment on meristems that were still active but close to GPA. Two weeks after bolting, inflorescences were treated with Dex or mock solution, and SAM were collected for RNA extraction 6 to 8 h posttreatment by manual dissection to remove floral buds and, as much as possible, floral primordia (Supplemental Fig. 1).
Figure 1.
Inducible expression of the AP2 miR172-resistant allele. A, A two-component inducible system was developed (based on the pOpON vector; Moore et al., 2006) to ectopically express the miR172-resistant allele, AP2m3, where a 35S promoter (black) directed the expression of the artificial transcription factor LhG4 fused to the glucocorticoid receptor (LhGR; green). Once dexamethasone (Dex) is applied (blue circles), the LhG4 can bind the regulatory region (pOp6; red) that controls the expression of the AP2m3 allele (purple) and the GUS reporter gene (blue), inducing its expression. Transformed plants are selected by kanamycin resistance (KANR; white). Triangles represent poly(A) signals. Omegas represent translational enhancer from the tobacco mosaic virus (TMV-Ω). Arrows represent the minimal 35S promoter. B, The system was assayed on arrested meristems at GPA by applying a drop of Dex (or mock) in the center of the SAM. The Dex-treated plants were able to rescue the meristem activity and started to produce new flowers (right).
Most of the Genes Differentially Expressed after AP2 Induction Are Associated with SAM Arrest at GPA under Physiological Conditions
RNA sequencing (RNA-seq) was performed with three independent biological replicates. We selected transcripts with a log2 fold change of greater than 0.75 or less than −0.75 and a corrected false discovery rate P < 0.05. With those thresholds, we identified 1,164 differentially expressed genes (DEG) between mock- (control) and Dex-treated plants (Supplemental Table S1). As expected, AP2 was one of the most up-regulated genes. In addition, we performed reverse transcription quantitative PCR (RT-qPCR) of 17 DEG in equivalent RNA samples. The expression pattern was confirmed for all genes tested (up- and down-regulated), validating the results obtained by RNA-seq (Supplemental Fig. 2).
To discard genes not expressed in the inflorescence meristem that could be regulated specifically by AP2 in very young flower primordia that could not be dissected away, we decided to cross our results with the data published by Wuest et al. (2016) obtained using laser-microdissected inflorescence meristems. We discarded the 62 transcripts that were not present in their data sets (GSE74386), which therefore were considered as not expressed in the SAM (Supplemental Table S2). The resulting list of DEG upon AP2 induction in the inflorescence included 1,102 genes, with 435 genes upregulated and 667 genes downregulated (Supplemental Table S3).
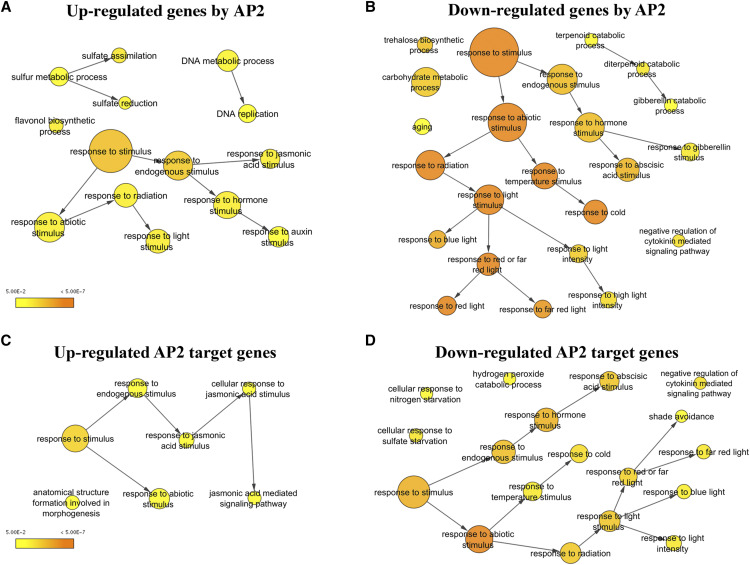
With the final list of 1,102 genes, we conducted a Gene Ontology (GO) analysis with the BiNGO tool (Maere et al., 2005) implemented for Cytoscape (Shannon et al., 2003). We found 49 and 115 categories overrepresented in the up and downregulated genes after AP2 induction, respectively (Supplemental Table S4). Among the categories enriched in the upregulated genes, we found response to auxins, response to jasmonic acid stimulus, DNA replication, and sulfur metabolic process (reduction and assimilation; Fig. 2A; Supplemental Table S4). Among the categories enriched in the down-regulated genes, we found response to stimulus, with the response to ABA, gibberellin, and, interestingly, response to multiple abiotic factors as temperature or light as being highly represented (Fig. 2B; Supplemental Table S4). It is worth noting that the categories of gibberellin catabolic process and negative regulation of cytokinin-mediated signaling pathway were also enriched in the downregulated DEG list (Fig. 2B; Supplemental Table S4). We also observed an enrichment in the categories of aging, trehalose biosynthesis, and carbohydrate catabolic processes (Fig. 2B; Supplemental Table S4).
Figure 2.
Functional enrichment analysis with overrepresented GO biological process categories. The analysis was performed for total DEG, upregulated (A) and downregulated (B) by AP2 induction. The same analysis was later performed for the putative direct targets of AP2 in the upregulated (C) and downregulated (D) groups. Circle size is proportional to gene numbers, and the color of each circle represents the enrichment P value (hypergeometric test) for the GO term label on that circle (scales are shown at bottom left of A and C), with orange representing the highest enrichment and yellow the lowest enrichment above the cutoff (Benjamini and Hochberg false discovery rate-corrected 0.05). Some categories were removed and the distance between nodes was arranged manually to optimize readability (Supplemental Tables S4 and S5). The figure and statistical analysis were generated using BiNGO software.
As AP2 has been described as a repressor of GPA that maintains SAM activity (Balanzà et al., 2018), we hypothesized that genes repressed by AP2 could mediate the entrance into GPA and accumulate at the end of flowering (up in arrested meristems) and also that the genes activated by AP2 could work in the opposite direction, repressing the entrance into GPA (down in arrested meristem). In accordance with this hypothesis, when we compared our results with the expression data produced by Wuest et al. (2016), comparing growing meristems versus arrested meristems (active versus GPA) or reactivated meristems after fruit removal versus arrested meristems (reactivated versus GPA), we observed a high correspondence of the results. Approximately 60% of DEG upon AP2 induction showed a similar up- or down-regulated expression in the equivalent conditions in the two experiments by Wuest et al. (2016), while 75% of D.E.G by AP2 induction behaved similarly in at least one of the experiments by Wuest et al. (2016; Fig. 3). As expected, the observed changes in gene expression upon AP2 induction associate with the changes observed for genes that are important not only for physiological meristem arrest (mostly dependent on seed production) but also for meristem reactivation after fruit removal, suggesting that AP2 could be a key factor integrating age-related signals (Balanzà et al., 2018) as well as the fertility input (Wuest et al., 2016).
Figure 3.
Genes differentially expressed by AP2 induction. A, The heat map shows that most of the changes observed in the genes differentially expressed after AP2 induction associate with the reported changes at the moment of meristem arrest (top) according to Wuest et al. (2016), when comparing growing versus arrested meristems in untreated plants (bottom) or reactivated meristems versus arrested meristems upon fruit removal (middle). B, The Venn diagrams show that 60% of the genes differentially expressed after AP2 induction behaved as expected in the two experiments performed by Wuest et al. (2016) and that 75% of DEG after AP2 induction behaved similarly in at least one of the experiments by Wuest et al. (2016).
We decided to perform a new GO analysis with the reduced list of the common DEG that behaved similarly upon AP2 induction and in at least one of the experiments by Wuest et al. (2016). In the group of genes downregulated by AP2 and genes upregulated at GPA (557 out of the 667 genes downregulated by AP2), 114 categories were identified, and all of them were included in the original GO analysis (115 categories; Supplemental Tables S4 and S5). In the group of genes upregulated by AP2 and downregulated at GPA (277 out of 435 genes), we observed a substantial reduction in the number of enriched categories, obtaining only nine of the original 49 categories found in AP2-activated genes (Supplemental Tables S4 and S5). This analysis indicates a clear association between the repressed responses by AP2 induction and the pathways that become activated at the end of the reproductive phase. However, this association is not clear for genes positively regulated by AP2 induction, as mentioned above.
To understand the differences between the transcriptomic changes described by Wuest et al. (2016) under physiological conditions and those that responded to AP2 induction, we performed an additional GO analysis for the genes that behaved differently after AP2 induction with respect to the described changes under physiological conditions (25% of DEG by AP2 induction). Among the genes downregulated by AP2 induction (and also downregulated in physiological conditions at GPA: 110 genes), we found only six categories related to shoot development, maintenance of floral meristem identity, organ development, and shade avoidance (Supplemental Tables S4 and S5), which could possibly be associated with other AP2 functions related to flower development. In the group of genes upregulated by AP2 induction and also upregulated under physiological conditions at GPA (158 genes), we found 25 categories related to response to stimulus, pigment, and flavonoid biosynthetic process, in addition to other categories, such as response to jasmonic acid stimulus and response to auxin stimulus, all of them previously identified in the GO analysis done with the complete list of DEG (Supplemental Table S5). This analysis suggests that many of the genes included in the categories obtained from the upregulated genes in the initial GO analysis (Supplemental Table S3) are also upregulated when GPA occurs under physiological conditions and therefore may not have a direct function in the control of meristem arrest. Altogether, this analysis suggested that AP2 function in the control of SAM arrest is mainly associated with the repression of genes that tend to accumulate at the end of flowering, which are likely to be responsible for meristem arrest.
Direct Targets of AP2 in the Inflorescence Meristem
Our RNA-seq analysis was done with samples collected at a relatively short time after Dex treatment (6–8 h), given that the two-component system for induction that we used implies transcription and translation of AP2. We observed that AP2 induction elicits a response contrary to that observed during meristem arrest under physiological conditions (Wuest et al., 2016). Thus, we wondered if AP2 could directly regulate part of this response and, therefore, be a key control point. To assess this, we determined how many of the genes differentially expressed in response to AP2 induction (Supplemental Table S3) had been described as putative targets of AP2 in the chromatin immunoprecipitation sequencing data experiments reported by Yant et al. (2010). A total of 26.5% of the DEG (292 genes) were putative targets of AP2 (Supplemental Table S3), 102 and 186 genes in the up and downregulated gene categories, respectively. To determine if these target genes associated preferentially with any of the previously identified GO categories, we performed a new GO analysis using the list of differentially expressed target genes. Within this subgroup, GO analyses rendered only nine and 35 categories overrepresented in the genes up and downregulated by AP2 induction, respectively (Supplemental Table S6). As expected, we obtained a clear reduction in the number of categories obtained in the first analysis (Supplemental Table S4), recovering those with a high number of putative direct targets. Among the nine categories enriched in the up-regulated target genes, we again found the response to stimulus (response to chemical, abiotic, and endogenous stimulus and to organic substance) as well as anatomical structure formation involved in morphogenesis (Fig. 2C; Supplemental Table S6) as well as the response to jasmonic acid stimulus previously highlighted in the initial GO analysis with the DEG (five out of 10 genes [50%]; Supplemental Table S7). Among the direct targets included in the jasmonic acid stimulus category were ARGININE DECARBOXYLASE2 and DWARF4, genes that are rate-limiting enzymes in the biosynthesis of polyamine and brassinosteroids, respectively (Choe et al., 1998; Soyka and Heyer, 1999). The DELLA gene GIBBERELLIC ACID INSENSITIVE was also included in this category, together with the repressors of jasmonic acid response, JASMONATE-ZIM-DOMAIN PROTEIN1 (JAZ1) and JAZ5 (Peng et al., 1997; Chini et al., 2007; Thines et al., 2007).
Among the 35 categories enriched in the down-regulated target genes, we again found the response to stimulus. More specifically, the response to ABA, the response to light stimulus, and the response to temperature as well as the negative regulation of cytokinin-mediated signaling pathway category were highly represented (Fig. 2D; Supplemental Table S6).
In the response to ABA category, we recovered 10 out of 22 DEG (45%) obtained in the first GO analysis performed with all the DEG (Supplemental Table S8). We found genes such as SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE2-3 (SNRK2-3) and ABA INSENSITIVE2 (ABI2; Leung et al., 1997; Boudsocq et al., 2007), which are core members of ABA signaling, and genes such as RESPONSIVE TO DESICCATION20 (RD20), RD22, RD29A, BEL1-LIKE HOMEODOMAIN (BLH1), and GLYCINE-RICH PROTEIN3, which are known ABA response genes (de Oliveira et al., 1990; Matsui et al., 2008; Kim et al., 2013a). Among the down-regulated target genes in the response to light category, we recovered 14 out of 42 DEG (33%; Supplemental Table S9). In this group, the presence of genes such as PHYTOCHROME A (PHYA), involved in the perception of red/far-red light (Dehesh et al., 1993), and PHYTOCHROME RAPIDLY REGULATED1 (PAR1), PAR2, and LONG HYPOCOTYL IN FAR-RED (HFR1), which participate in the far-red light response repressing growth, are noteworthy (Fairchild et al., 2000; Yang et al., 2005; Roig-Villanova et al., 2007; Supplemental Table S9). In the response to temperature category, we recovered 10 out of 30 DEG (33%; Supplemental Table S10). Interestingly, all of the genes in the response to temperature category corresponded to genes also present in the response to cold category (nine out of 10), with the exception of ABI2 (Supplemental Table S10). This same tendency was also observed in the group of genes belonging to this category in the initial GO analysis, with 25 out of 30 also present in the response to cold category (Supplemental Table S10). The negative regulation of cytokinin-mediated signaling pathway category contained two out of three genes of the initial GO analysis (66%), KISS ME DEADLY2 (KMD2) and KMD4, missing only KMD1 (Kim et al., 2013b).
Our RNA-seq data indicated that many direct targets of AP2 fall into specific categories identified in the initial GO analysis, suggesting that AP2 could be a hub or master regulator controlling meristem activity at the end of the flowering phase. In addition, this direct activity is mainly associated with the repression of factors that respond to or modulate both endogenous (ABA and CK) and exogenous (light and temperature) stimuli that have been associated with growth inhibition.
AP2 Modulates Meristem Arrest by Repressing the ABA Response and the Same Regulatory Network That Controls Axillary Meristem Dormancy
It has been suggested that meristems in GPA are similar to dormant meristems (Wuest et al., 2016). ABA has been described to play a crucial role in the maintenance of meristems in the dormant state (Yao and Finlayson, 2015; González-Grandío et al., 2017), and, accordingly, ABA signaling is one of the enriched categories in the GO analysis of DEG (Supplemental Tables S4, S5, and S7). There are 36 DEG related to ABA and dormancy among the genes downregulated upon AP2 induction (Supplemental Table S11). Most of them are also upregulated in arrested meristems at GPA (Wuest et al., 2016; Supplemental Table S11). Interestingly, most of the genes present in this group were previously described by González-Grandío et al. (2017) in the genetic regulatory network controlling Arabidopsis lateral bud dormancy downstream of BRANCHED1 (BRC1; González-Grandío et al., 2013, 2017). More specifically, the common genes include HOMEOBOX PROTEIN21 (HB21) and HB53, which are direct targets of BRC1, NINE-CIS-EPOXYCAROTENOID DIOXYGENASE3 (NCED3), which acts downstream of HB21 and HB53 to direct ABA synthesis (Iuchi et al., 2001), and G-BOX BINDING FACTOR, NAC-LIKE, ACTIVATED BY AP3/PI, ABSCISIC ACID RESPONSIVE ELEMENT-BINDING FACTOR3, and HISTONE H1 to HISTONE H3, which are ABA-responsive genes (Ascenzi and Gantt, 1997; Sablowski and Meyerowitz, 1998; Uno et al., 2000; Garcia et al., 2008; Yang et al., 2014). From the genetic regulatory network described by González-Grandío et al. (2017) downstream of BRC1, we also identified ABNORMAL SHOOT2/NGATHA-LIKE1, AT1G79520, and AT1G11210 as being differentially regulated upon AP2 induction, although a priori these genes are not related to ABA. We checked HB21 expression in the data of Wuest et al., 2016) and observed that the HB21 transcript accumulates in arrested meristems.
HB21 is an upstream regulator of the bud dormancy regulatory network (González-Grandío et al., 2017) and is a putative direct target of AP2 (Yant et al., 2010). Moreover, in our experiment, it was repressed by AP2 induction. Therefore, we decided to explore its possible contribution to GPA. First, we analyzed its expression in the SAM during inflorescence development of wild-type plants. We observed that HB21 expression increased dramatically around 3 weeks after bolting, when meristem arrest occurs (Fig. 4A). Interestingly, in a ful mutant background, where AP2 gene expression is maintained in the SAM, delaying meristem arrest (Balanzà et al., 2018), we did not observe HB21 transcript accumulation in the shoot apex (Fig. 4A).
Figure 4.
Characterization of HB21 and BLH1 genes. A and B, Time-course expression analysis for HB21 (A) and BLH1 (B) during inflorescence development, showing that both transcripts accumulate in the wild type (Columbia [Col.]) at the end of inflorescence development (GPA). This accumulation is not observed in the ful mutant, where meristem arrest is delayed due to a higher activity of AP2. C and D, hb21 and blh1 mutants show delayed meristem arrest, producing plants that grow for longer than wild-type controls, resulting in longer inflorescences (C) and producing more fruits than the wild type (D). Error bars in A, B, and D represent sd, and asterisks indicate significant differences (*P < 0.05) from the wild-type control according to Student’s t test. The plants were digitally extracted for comparison in C. Bar in C = 10 cm.
The response to ABA category also included BLH1. This protein has been related to ABA-mediated seed dormancy (Kim et al., 2013a) and interacts physically with KNOX proteins (Hackbusch et al., 2005; Smaczniak et al., 2012), which are important for meristem activity. Interestingly, BLH1 was also present in the response to light category. In fact, BLH1 accumulates under far-red light and regulates part of the response mediated by PHYA (Staneloni et al., 2009). As mentioned before, other direct targets of AP2 in the response to light category were PHYA, PAR1, PAR2, and HFR1, all of them involved in far-red light perception and response, suggesting a role for red/far-red light signaling in the control of meristem arrest. As BLH1 responds to both ABA and light, we decided to analyze its expression in the SAM during inflorescence development of wild-type and ful mutant plants. As with HB21, we observed a clear accumulation of the transcript 3 weeks after bolting in the wild type, while no transcript accumulation was detected in the ful mutant (Fig. 4B). These results suggest that the accumulation of both genes in the meristem could participate in establishing the meristem arrest observed in wild-type plants as well as reinforcing our hypothesis that AP2 could control meristem arrest, in part, by the direct repression of genes in the SAM that modulate ABA and light responses.
If HB21 and BLH1 play a role in promoting meristem arrest, the corresponding mutants may be expected to present altered GPA timing. blh1-2 and hb21-2 mutants, obtained from the Nottingham Arabidopsis Stock Centre, did not develop any obvious alterations in vegetative or inflorescence development when compared with wild-type plants, but both showed a delayed GPA (Fig. 4C). When we scored the number of fruits produced in the main shoot by the mutants, both of them presented an increased number (blh1, 60.8 ± 4.9; hb21, 63.1 ± 7.8) with respect to the wild type (54.8 ± 4.5; Fig. 4D), indicating that these genes play a role in the meristem arrest at the end of flowering.
AP2 Regulates CK Responsiveness at the GPA
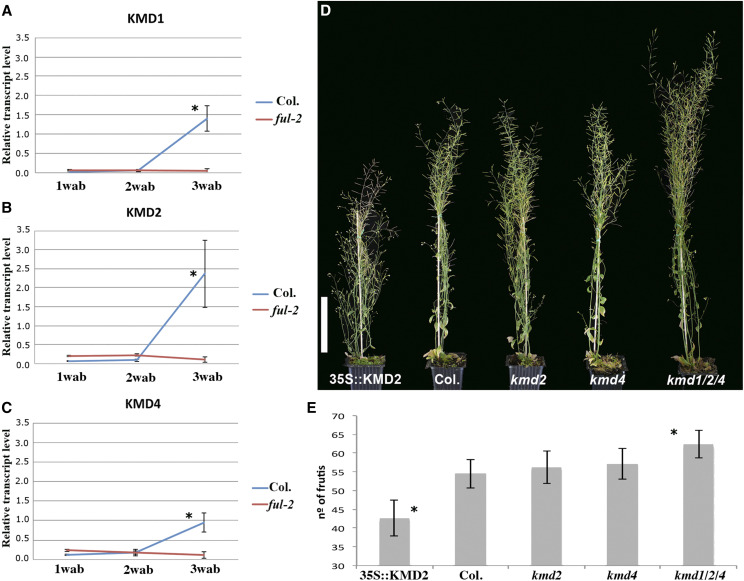
CK have been described to promote SAM activity and dormancy break in most plant species. Our GO analysis indicates that the negative regulation of CK signaling was repressed by AP2 induction. In this category, we recovered three out of four genes from a small F-box protein family, KMD1, KMD2, and KMD4, involved in the proteasomal degradation of the B-type ARR transcription factors that mediate CK signaling (Kim et al., 2013b). Two of the identified genes, KMD2 and KMD4, are putative direct targets of AP2 and have a direct role in the control of meristem activity (Kim et al., 2013b). For this reason, we decided to analyze their expression in the shoot apex meristem during inflorescence development. As for BLH1 and HB21, we observed a clear accumulation of KMD transcripts at the end of flowering in wild-type plants, while in ful mutants the expression of these genes remained low (Fig. 5, A–C). We also checked if KMD2 and KMD4 (putative direct targets of AP2) had a role in meristem arrest by characterizing GPA timing in the corresponding mutants. The single kmd2 and kmd4 mutants were similar to the wild-type plants and produced 56.3 ± 4.4 and 57.1 ± 4 fruits, respectively, showing no significant differences with respect to the wild type (54.5 ± 3.8; Fig. 5, D and E). As redundant roles for these genes have been reported (Kim et al., 2013b), we also tested the triple mutant kmd1/2/4, where all the KMD genes detected in our RNA-seq analysis are inactive. Triple mutants presented a clear delay of meristem arrest (Fig. 5D). Compared with the wild type and the single mutants analyzed, the triple mutant produced more flowers in the main shoot (62.4 ± 3.7), indicating that these genes were also redundant in GPA control (Fig. 5E). Conversely, 35S::KMD2 lines produced a lower number of flowers than wild-type plants (42.7 ± 4.7 versus 54.5 ± 3.8, respectively; Fig. 5, D and E), indicating that high levels of this protein are able to accelerate meristem arrest. Our results confirmed that KMD genes participate in the control of meristem activity and that they accumulate at the end of the reproductive phase. Thus, AP2 could maintain meristem activity, at least in part, by repressing the KMD genes.
Figure 5.
Characterization of KMD genes. A to C, Time-course expression analysis for KMD1 (A), KMD2 (B), and KMD4 (C) during inflorescence development, showing that transcripts for the three genes accumulate in the wild type (Columbia [Col.]) at the end of inflorescence development. This accumulation is not observed in the ful mutant, where meristem arrest is delayed due to the presence of AP2. D and E, kmd2 and kmd4 single mutants have no phenotypes related to meristem arrest, producing a similar number of fruits as wild-type plants. In agreement with the reported redundancy for these genes, we observed clear phenotypes when overexpressing one of them (KMD2) and in analyzing the triple kmd1/2/4 mutant. While the overexpression line shows an early meristem arrest, producing shorter plants and fewer fruits than wild-type plants, the triple mutant presents delayed meristem arrest, developing taller plants that produce more fruits than wild-type controls. Error bars in A, B, C, and E represent sd, and asterisks indicate significant differences (P < 0.05) from the wild-type control according to Student’s t test. The plants were digitally extracted for comparison in D. Bar in D = 10 cm.
DISCUSSION
The genetic and molecular control of the end of flowering and the establishment of GPA has been a forgotten topic in plant science. However, in the last years, detailed transcriptomic landscapes of arrested meristems at this developmental stage, and the first evidence of the involvement of a genetic pathway likely controlling age-dependent cues, the FUL-AP2 pathway, have been published (Wuest et al., 2016; Balanzà et al., 2018, 2019). These publications also show that the proliferative arrest of meristems is reversible and that flower production can resume either by removing mature fruits and seeds or by inducing AP2 expression in the meristems. In this study, our aim was to better understand how these two cues, those coming from seed production under normal physiological conditions and those provided by the regulation of AP2 levels in the meristem, interact to control the end of the reproductive phase.
The combination of previously published data on AP2-binding sites (Yant et al., 2010) with our transcriptomic analysis in response to AP2 induction in meristems close to arrest has allowed us to identify putative molecular mechanisms that function downstream of the FUL-AP2 pathway at the shoot apex, linking AP2 action with the control of hormonal regulation in the SAM and of several genes that respond to environmental factors. In this scenario, AP2 (together, probably, with other AP2-like genes) represses the ABA response and promotes CK responsiveness to stimulate SAM activity. In parallel, AP2 likely mediates, at least to some extent, the effect of environmental signals, such as temperature and light, that also likely impact the regulation of the end of the reproductive phase.
AP2 Promotes SAM Activity, Allowing a High CK Responsiveness
Previous reports have implicated AP2 in the maintenance of WUS expression (Würschum et al., 2006; Zhao et al., 2007), but the mechanism of this regulation has not yet been fully elucidated. In addition, we have previously shown that meristem arrest associates with the absence of WUS expression in the SAM (Balanzà et al., 2018). The results of this study indicate that AP2 could control SAM activity through the regulation of the CK responsiveness in the meristem by repressing the KMD genes, which are negative regulators of CK responses. CK are key factors for SAM formation and maintenance (Werner et al., 2003; Gordon et al., 2009; Bartrina et al., 2011, 2017), activating the expression of the B-type ARR genes that are positive direct regulators of WUS (Meng et al., 2017; Wang et al., 2017). Three out of four genes in the KMD clade were negatively regulated by AP2, and two of them, KMD2 and KMD4, are putative direct targets of AP2. All three transcripts accumulate at the end of the flowering phase, when meristem arrest occurs, and mutant analysis reveals a role for these proteins in the control of meristem arrest. As KMD proteins are involved in the proteasomal degradation of the B-type ARR factors that mediate CK responses (Kim et al., 2013b), our results suggest a clear link between AP2 action and WUS regulation. In this scenario, low levels of AP2 at the end of flowering could allow for the accumulation of the KMD proteins, avoiding CK signaling and turning off the expression of WUS in the SAM, which associates with meristem arrest (Fig. 6).
Figure 6.
Proposed model for the events occurring downstream of the FUL-AP2 pathway in the control of meristem arrest at the end of the reproductive phase. We previously reported that meristem arrest at the end of flowering in Arabidopsis associates with the indirect repression of WUS expression in the SAM by AP2 (Balanzà et al., 2018). AP2 could mediate the meristem arrest through the repression of HB21 and KMD genes as well as genes involved in the perception and response to light (PHYA, PAR1, PAR2, and HFR1) and cold (CBF3). During inflorescence development, AP2 allows high CK response and low ABA levels that promote meristem activity, while at the end of flowering, the low levels of AP2 allow the rise of KMD genes and HB21, which reduce the CK response and increase that of ABA, respectively, promoting meristem arrest. In a second level, AP2 could also repress the response to light (far-red) and cold. The reduction of AP2 levels at the end of flowering would allow the SAM to respond to changes in key environmental signals, such as daylength or low temperatures, to induce arrest. Gray gene names indicate low expression levels.
The Arrested SAM at the End of Flowering Resembles a Dormant Meristem
Wuest et al. (2016) showed that the arrested meristems at the end of flowering behave as dormant meristems. Corroborating this, if seeds, which are key factors to inducing meristem arrest (Hensel et al., 1994; Balanzà et al., 2018, 2019), are removed by fruit pruning after GPA takes place, reactivation of the arrested meristem is forced and flowers are produced again. This response clearly indicates that the arrested SAM is still alive and able to respond to signals. Under physiological conditions, Wuest et al. (2016) showed that ABA-responsive genes accumulated in the SAM at the moment of the arrest, but the levels of those same genes were reduced after meristem reactivation. As we previously showed, AP2 induction after meristem arrest is also able to reactivate flower production (Fig. 1B; Balanzà et al., 2018). Interestingly, here we show that the same groups of genes that are up-regulated at GPA are repressed after AP2 induction in the SAM, indicating that AP2 could delay meristem arrest, at least partially, by repressing their expression.
Our GO analysis showed that several putative direct targets of AP2 are included in the response to ABA category, suggesting a direct relationship between the ABA response and AP2 action (Supplemental Table S11). We observed that AP2 induction represses the expression of ABA biosynthesis (ABA1 and NCED3), perception (PYL7), signaling (SNRK2.3 and ABI2), and response (RD20, RD22, and RD29A) genes. In addition, several genes included in the genetic regulatory network described for the control of axillary bud dormancy (González-Grandío et al., 2017), including the AP2 direct target HB21 as well as HB53, are also found in DEG in response to AP2 induction in the inflorescence meristem. These HB proteins have been shown to act downstream of BRC1 to direct ABA accumulation and response in axillary dormant buds, but no information about their role in the SAM was previously reported. Interestingly, BRC1, or even its close relative BRC2, is not expressed in the SAM, not even at the moment of proliferative arrest (Wuest et al., 2016; this work), suggesting that the acquisition of the dormant stage of the SAM at the end of the reproductive phase is not dependent on BRC1. Since AP2 appears to act by repressing dormancy, we can speculate that AP2 would be either repressing the factor(s) activating the HB pathway or maintaining HB21/53 repression directly in the active SAM, and this repression would disappear at the end of flowering to trigger dormancy and therefore induce meristem arrest. It has been described that AP2 decreases its activity in the SAM progressively through the direct repression by FUL (Balanzà et al., 2018). The decrease in AP2, which down-regulates HB21, would explain the HB21 accumulation at the end of flowering, which will trigger the accumulation of ABA and ABA responses (Fig. 6). Taken together, our results suggest that the combination of high ABA response and low CK responsiveness could mediate meristem arrest at the end of the reproductive phase, inducing the dormant stage, as has been proposed for many species for the establishment and maintenance of bud dormancy (Corot et al., 2017; Tylewicz et al., 2018; Liu and Sherif, 2019; Vayssières et al., 2020).
Possible Role of Environmental Factors in the Control of Meristem Arrest
The different developmental phases that take place in plants are processes that are highly controlled and coordinated by both genetic and environmental factors in order to ensure the reproductive success and fitness of the species. Accordingly, the formation of dormant buds in many perennial plants occurs before the start of winter in order to endure the ensuing adverse conditions. Thus, the environmental conditions (daylength, temperature, etc.) are key factors to determine the precise moment to initiate the dormant stage (Heide and Prestrud, 2005; Maurya and Bhalerao, 2017). Our analysis has revealed a putative function for AP2 as a regulator of genes involved in the responses to temperature and light. Interestingly, most of the genes identified in the response to temperature are related to the response to cold. All the identified genes are repressed by AP2 and, at the same time, accumulate in arrested SAM (Wuest et al., 2016), suggesting that the expression of genes that respond to low temperatures could induce meristem arrest. In fact, among the DEGs we found upon AP2 induction that are putatively direct AP2 targets is the C-REPEAT BINDING FACTOR3 (CBF3) gene that is a key regulator of the cold response, repressing plant growth (Liu et al., 1998; Gilmour et al., 2000, 2004; Chinnusamy et al., 2007; Zhou et al., 2017). Interestingly, one of the few categories recovered from the up-regulated target genes of AP2 was jasmonic acid response, including the response repressors JAZ1 and JAZ5 (Chini et al., 2007). JAZ proteins act as negative regulators of CBFs (Hu et al., 2013; Zhou et al., 2017). While more evidence is required, our results suggest that AP2 could regulate the same group of genes that are controlled in the cold response.
Another important factor controlling dormancy in many plants is light. Shortening of the photoperiod in autumn is an important signal for bud dormancy initiation and growth cessation in trees (Eriksson and Moritz, 2002; Mølmann et al., 2006; Olsen, 2010). The daylength is usually measured by plants as peaks of far-red light at the beginning and end of the day (Johnson et al., 1994; Olsen et al., 1997; Yanovsky and Kay, 2003; Imaizumi, 2010). As observed for the temperature response, AP2 also could regulate many genes involved in light signaling. Among the DEGs identified after AP2 induction that are putatively its direct targets, we have identified core elements in the genetic routes involved in the perception of light quality, such as PHOT1 (blue photoreceptor) and PHYA (red/far-red photoreceptor), as well as in the red/far-red light response. Interestingly, HFR1, PAR1, and PAR2 are activated by low red/far-red light ratios to negatively regulate the shade-avoidance elongation response in a process mediated by PHYA (Johnson et al., 1994; Yanovsky et al., 1995; Devlin et al., 2003. These genes also accumulate in arrested meristems (Wuest et al., 2016) and are negatively regulated by AP2. Thus, AP2 could regulate a subset of genes in the SAM that are involved in the response to light, suggesting that the red/far-red light ratio could modulate SAM activity. We have analyzed the expression of BLH1, one of the far-red light-responsive genes. Similar to other genes in this category, BLH1 accumulates in arrested meristems and is repressed by AP2, and blh1 mutants show delayed meristem arrest, suggesting a role for BLH1 in the control of the end of flowering. BLH1 regulates part of the PHYA response and accumulates under far-red light (Staneloni et al., 2009). In addition, BLH1 is also involved in ABA-mediated seed dormancy and, together with KNAT3, modulates ABA response genes, such as ABI5 (which accumulates in arrested meristems and is downregulated by AP2 induction; Kim et al., 2013a). Clear examples of cross talk between light signaling and the ABA response in the control of seed dormancy have been previously proposed. For example, in the establishment of primary seed dormancy, far-red light has been reported to promote dormancy by upregulating the levels of PIF1 (which also accumulates in arrested meristem and is down-regulated by AP2 induction). This, in turn, promotes ABA accumulation, leading to an increase in ABI5 transcripts (Oh et al., 2004, 2006; Kim et al., 2013a; Vaistij et al., 2018). Taken together, these data suggest that BLH1 could work as a link between ABA and light responses in addition to being regulated by AP2 in the SAM.
We have observed that AP2 regulates many genes involved in the response to light and temperature. It remains to be established if AP2 could mediate the response to these environmental factors by regulating these genes or if the same subset of genes has been recruited to control growth and meristem activity by AP2 independently. However, it is tempting to speculate that environmental factors (temperature and light) and endogenous factors (ABA, CK, age, and even seed-derived signals) could converge in the regulation of meristem activity and that AP2 would be a hub in the regulation of these different pathways. It would be very interesting to carry out systematic studies in order to determine how environmental conditions affect meristem arrest at the end of the reproductive phase and how seasonal variations affect the process.
Moreover, our results reinforce the idea, previously proposed by Wuest et al. (2016), that at the end of flowering, the arrested meristem behaves as a dormant meristem. Our work indicates that genetic pathways that operate in other dormancy processes, such as axillary bud dormancy or seed dormancy, could control the arrest of the SAM. We also propose that environmental factors could be important in the control of meristem arrest, identifying genes involved in both cold and red/far-red light responses as important players in this process (Fig. 6). Recently, auxin transport from fruit to the stem has been proposed as a determinant for meristem arrest when the plant has acquired the competence to stop (Ware et al., 2020). In the initial GO analysis of genes up-regulated upon AP2 induction, we identified the auxin response category (Supplemental Table S4), but those genes were also upregulated under physiological conditions at the moment of meristem arrest (Supplemental Table S5), suggesting that this set of genes is unlikely to mediate AP2 function in the control of SAM activity. Thus, we can speculate that AP2-mediated control of meristem arrest could work in parallel to auxins, as no DEG related to auxins were identified in the list of direct AP2 targets. On the other hand, our data fit perfectly with the acquisition of the SAM competence proposed by Ware et al. (2020): the progressive repression of AP2 genes in the SAM would allow for the activation of ABA, light, and cold response genes that could provide the competence to stop, rendering the SAM able to respond to the change in auxin fluxes that have been proposed by Ware et al. (2020) to be a cue provided from fruits proximal to the inflorescence meristem. Interestingly, the similar expression changes observed between AP2 induction and the changes observed under physiological conditions in fertile plants and, more importantly, with reactivated meristems after fruit (seed) removal point to AP2 as a key factor integrating not only age-related signals (Balanzà et al., 2018) but also the fertility input in the control of SAM activity (Hensel et al., 1994; Wuest et al., 2016). As changes in auxin fluxes have been identified as part of the fertility input that controls meristem arrest, future studies of the interaction between auxin fluxes and AP2 function could contribute to a better understanding of this developmental process.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
For all the analyses described, Arabidopsis (Arabidopsis thaliana) seeds were stratified for 2 d at 4°C after sowing. Plants were grown at 21°C under long-day conditions (16 h of light/8 h of dark) in a 2:1:1 (v/v/v) mixture of peat:perlite:vermiculite. All mutant plants and marker lines used in this study were in the Columbia background. All mutants and overexpression lines used have been described previously: ful-2 (Gu et al., 1998), blh1-2 (Pagnussat et al., 2007), kmd2-1, kmd4-1, kmd1-1 kmd2-1 kmd4-1, and 35S::KMD2 (Kim et al., 2013b), except hb21-2, which is a new allele obtained from the Nottingham Arabidopsis Stock Centre (N835325).
The 35S::LhG4:GR»AP2m3 construct was generated by cloning the AP2m3 allele coding sequence (Chen, 2004) via a Gateway LR clonase II recombination reaction downstream of the artificial pOp6 promoter into the pOpOn2.1 binary vector derived from the pOpOff2 vector (Moore et al., 2006). Primer sequences used are detailed in Supplemental Table S12. Arabidopsis was transformed with Agrobacterium tumefaciens strain C58 pM090 using the floral dip protocol (Clough and Bent, 1998), and transgenic lines carrying a single transgene insertion were selected. The homozygous lines selected were classified by their GUS expression level. For this study, a T3 homozygous line for the transgene with high and consistent GUS expression was used.
Dex Treatment
Plants were grown in soil until 2 weeks after bolting. The induction of 35S::LhG4:GR»AP2m3 in the shoot apex of transgenic plants was carried out by dipping the apices in a Dex solution (5 µm Dex and 0.015% [v/v] Silwet L-77) or a control solution with an equivalent concentration of Silwet L-77 (mock). At 6 h after induction, inflorescence apices were harvested and dissected to eliminate older buds. Three biological replicates were sampled, each containing about 20 inflorescence meristems.
RNA-Seq
RNA for RNA-seq was obtained with the NucleoSpin RNA Plant Kit (Macherey-Nagel), adding a complementary DNase treated with Turbo DNase (Ambion AM1907). RNA concentration and purity were verified using a NanoDrop Spectrophotometer ND-1000 (Thermo Scientific), and RNA integrity was determined according to RNA Integrity Number values using a Bioanalyzer Chip RNA 7500 series II (Agilent). The cDNA libraries were built using the TruSeq Stranded mRNA LT Sample Preparation Kit (Illumina). The size distribution of the libraries was evaluated using the 2100 Bioanalyzer and the High Sensitivity DNA Kit (Agilent). The accurate quantification of the libraries was obtained using the 7500 Fast Real-Time PCR (Applied Biosystems) and the KAPA Library Quantification Kit (Kapa Biosystems). Paired-end sequencing (2 × 300 bp) was performed on a MiSeq (Illumina). For the bioinformatic analysis, reads were aligned to the reference genome of Arabidopsis available at the TAIR database (Lamesch et al., 2012) using TopHat (Trapnell et al., 2009) and Bowtie (Langmead et al., 2009) software. The abundance estimation of the transcripts was performed using the RSEM package (Li and Dewey, 2011), and the differentially expressed transcripts (fragments per kilobase million value) were estimated using Cufflinks (Trapnell et al., 2010). The sequences from DEG were annotated through BLAST search against the TAIR database.
Heat Map and Venn Diagrams
The heat map was generated with the heatmapper tool (http://www.heatmapper.ca/expression/; Babicki et al., 2016). Fold change values for each experiment were normalized with respect to their own maximum and minimum values for comparison. Venn diagrams were generated using the web tool available at Ghent University (http://bioinformatics.psb.ugent.be/webtools/Venn). For expression data from Wuest et al. (2016), we assumed differences in the expression tendency with differences in log2 fold change of > 0.4 or < −0.4.
RT-qPCR Analyses
RNA-Seq Validation
cDNAs were synthesized from 1.5 μg of total RNA using Oligo (dT24V) primers and SuperScript III (Invitrogen). Gene-specific primers were designed using Primer 3 software (Rozen and Skaletsky, 2000). PCR was performed in the 7500 Fast Real-Time PCR detection system (Applied Biosystems) and used SYBRGreen to monitor double-stranded DNA synthesis. The presence of spurious amplification products caused by genomic DNA contamination was verified by the qPCR dissociation profiles. The PCR efficiency and the optimal quantification cycle threshold value were obtained by the online program Real Time PCR Miner (Zhao and Fernald, 2005). The relative expression ratio between control and treated groups was calculated based on the mean of the quantification cycle threshold values and a subsequent statistical test using a pairwise fixed reallocation randomization test (Pfaffl et al., 2002). The significance of the performed randomization was tested by the P value (se < 0.005 at P = 0.05). Three independent biological replicates of each experimental condition (control or treated samples) were evaluated using triplet technical replicates. The reference genes used to normalize the qPCR data have been discussed previously (Czechowski et al., 2005). Primer sequences used are detailed in Supplemental Table S12.
Expression Levels
Inflorescence apices were harvested and trimmed to eliminate older buds. RNA was extracted using the E.Z.N.A. Plant RNA Kit (Omega Bio-tek) and DNase treated with Turbo DNase (Ambion AM1907). RNA concentration and purity were verified using a NanoDrop Spectrophotometer ND-1000 (Thermo Scientific). cDNAs were synthesized from 1 μg of total RNA using random hexamers and SuperScript IV (Invitrogen). The RT-qPCR was performed in the 7500 Fast Real-Time PCR detection system (Applied Biosystems) and used EVAGreen to monitor double-stranded DNA synthesis. The Ct value was obtained from an automatic threshold. Results were normalized to the expression of the ACT reference gene. The 2−ΔCt was shown as relative expression level. Three biological replicates were performed for each sample. Two-tailed Student’s t test was performed to determine statistical significance (P < 0.05). Primer sequences used are detailed in Supplemental Table S12.
Fruit Number Quantification
Plants were grown as described above. Elongated fruits were quantified in the main inflorescence after meristem arrest for at least 12 plants of each genotype. Unhealthy or phenotypically altered plants were discarded. Experiments were replicated independently twice, obtaining comparable results, although only one experiment is represented in each figure.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AT4G36920 (AP2), AT5G60910 (FUL), AT2G18550 (HB21), AT2G35940 (BLH1), AT1G80440 (KMD1), AT1G15670 (KMD2), and AT3G59940 (KMD4). Accession numbers for all the the genes introduced in the work are included in Supplemental Tables S1 to S11. The RNA-seq data discussed in this article have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (Edgar et al., 2002) and are accessible through GEO Series accession number GSE151082 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE151082).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Samples used for transcriptome analyses.
Supplemental Figure S2. Validation of RNA-seq results by RT-qPCR.
Supplemental Table S1. The 1,164 DEG by AP2 induction.
Supplemental Table S2. The 62 DEG by AP2 induction that are not SAM specific.
Supplemental Table S3. Final list of DEG by AP2 induction.
Supplemental Table S4. GO analysis of DEG by AP2 induction.
Supplemental Table S5. GO analysis of genes grouped in the Venn analysis.
Supplemental Table S6. GO analysis of differentially expressed targets by AP2 induction.
Supplemental Table S7. List of genes included in the category response to jasmonic acid.
Supplemental Table S8. List of genes included in the category response to ABA.
Supplemental Table S9. List of genes included in the category response to light.
Supplemental Table S10. List of genes included in the category response to temperature.
Supplemental Table S11. List of ABA- and dormancy-related genes repressed by AP2 induction.
Supplemental Table S12. Primers used in this work.
Acknowledgments
We thank Dr. Eric Schaller for providing the triple mutant kmd1/2/4 seeds and the 35S::KMD2 line; Dr. Fabiano Thompsom and Dr. Louisi Sousa de Oliveira for technical support with the RNA-seq experiment; Javier Forment for help with the RNA-seq analysis; and Dr. Concha Gomez-Mena, Dr. Lynne Yenush, and all members of the Ferrándiz lab for revising the article and for critical discussion.
Footnotes
This work was supported by the Spanish Ministerio de Economía, Industria y Competitividad/Fondo Europeo de Desarrollo Regional, European Union (grant no. BIO2015–64531–R to C.F.), the Spanish Ministerio de Ciencias, Investigación y Universisdades/Agencia estatal de Investigación/Fondo Europeo de Desarrollo Regional, European Union (grant no. RTI2018–099239–B–I00 to C.F.), Generalitat Valenciana (grant no. PROMETEU/2019/004 to C.F.), the National Council for Scientific and Technological Development (grant no. CNPq, 308832/2017–5 to M.A.F.), the National Institute of Science and Technology (grant no. 465480/2014–4 to M.A.F.), the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (grant no. E–26/202.631/2019 to M.A.F.), and the European Union (grant no. FP7–PEOPLE–PIRSES–2009–247589 to C.F. and M.A.F.).
Articles can be viewed without a subscription.
References
- Ascenzi R, Gantt JS(1997) A drought-stress-inducible histone gene in Arabidopsis thaliana is a member of a distinct class of plant linker histone variants. Plant Mol Biol 34: 629–641 [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Sakai H(2003) Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15: 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, Wishart DS(2016) Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res 44: W147–W153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanzà V, Martínez-Fernández I, Sato S, Yanofsky MF, Ferrándiz C(2019) Inflorescence meristem fate is dependent on seed development and FRUITFULL in Arabidopsis thaliana. Front Plant Sci 10: 1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanzà V, Martínez-Fernández I, Sato S, Yanofsky MF, Kaufmann K, Angenent GC, Bemer M, Ferrándiz C(2018) Genetic control of meristem arrest and life span in Arabidopsis by a FRUITFULL-APETALA2 pathway. Nat Commun 9: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartrina I, Jensen H, Novák O, Strnad M, Werner T, Schmülling T(2017) Gain-of-function mutants of the cytokinin receptors AHK2 and AHK3 regulate plant organ size, flowering time and plant longevity. Plant Physiol 173: 1783–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmülling T(2011) Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23: 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Droillard MJ, Barbier-Brygoo H, Laurière C(2007) Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol Biol 63: 491–503 [DOI] [PubMed] [Google Scholar]
- Chen X.(2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303: 2022–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J, Zhu JK(2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12: 444–451 [DOI] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA(1998) The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell 10: 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF(1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Corot A, Roman H, Douillet O, Autret H, Perez-Garcia MD, Citerne S, Bertheloot J, Sakr S, Leduc N, Demotes-Mainard S(2017) Cytokinins and abscisic acid act antagonistically in the regulation of the bud outgrowth pattern by light intensity. Front Plant Sci 8: 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR(2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehesh K, Franci C, Parks BM, Seeley KA, Short TW, Tepperman JM, Quail PH(1993) Arabidopsis HY8 locus encodes phytochrome A. Plant Cell 5: 1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira DE, Seurinck J, Inzé D, Van Montagu M, Botterman J(1990) Differential expression of five Arabidopsis genes encoding glycine-rich proteins. Plant Cell 2: 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Yanovsky MJ, Kay SA(2003) A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol 133: 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE(2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvild KC.(1989) The death hormone hypothesis. Physiol Plant 77: 282–285 [Google Scholar]
- Eriksson ME, Moritz T(2002) Daylength and spatial expression of a gibberellin 20-oxidase isolated from hybrid aspen (Populus tremula L. × P. tremuloides Michx.). Planta 214: 920–930 [DOI] [PubMed] [Google Scholar]
- Fairchild CD, Schumaker MA, Quail PH(2000) HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev 14: 2377–2391 [PMC free article] [PubMed] [Google Scholar]
- Garcia ME, Lynch T, Peeters J, Snowden C, Finkelstein R(2008) A small plant-specific protein family of ABI five binding proteins (AFPs) regulates stress response in germinating Arabidopsis seeds and seedlings. Plant Mol Biol 67: 643–658 [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Fowler SG, Thomashow MF(2004) Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol Biol 54: 767–781 [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF(2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124: 1854–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Grandío E, Pajoro A, Franco-Zorrilla JM, Tarancón C, Immink RG, Cubas P(2017) Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc Natl Acad Sci USA 114: E245–E254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Grandío E, Poza-Carrión C, Sorzano CO, Cubas P(2013) BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell 25: 834–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM(2009) Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci USA 106: 16529–16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Ferrándiz C, Yanofsky MF, Martienssen R(1998) The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 125: 1509–1517 [DOI] [PubMed] [Google Scholar]
- Hackbusch J, Richter K, Müller J, Salamini F, Uhrig JF(2005) A central role of Arabidopsis thaliana ovate family proteins in networking and subcellular localization of 3-aa loop extension homeodomain proteins. Proc Natl Acad Sci USA 102: 4908–4912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide OM, Prestrud AK(2005) Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol 25: 109–114 [DOI] [PubMed] [Google Scholar]
- Hensel LL, Nelson MA, Richmond TA, Bleecker AB(1994) The fate of inflorescence meristems is controlled by developing fruits in Arabidopsis. Plant Physiol 106: 863–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Jiang L, Wang F, Yu D(2013) Jasmonate regulates the inducer of cbf expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 25: 2907–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T.(2010) Arabidopsis circadian clock and photoperiodism: Time to think about location. Curr Opin Plant Biol 13: 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K(2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27: 325–333 [DOI] [PubMed] [Google Scholar]
- Johnson E, Bradley M, Harberd NP, Whitelam GC(1994) Photoresponses of light-grown phyA mutants of Arabidopsis (phytochrome A is required for the perception of daylength extensions). Plant Physiol 105: 141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MO, Davies PJ, Woolhouse HW(1988) The control of whole plant senescence. Crit Rev Plant Sci 7: 139–173 [Google Scholar]
- Kim D, Cho YH, Ryu H, Kim Y, Kim TH, Hwang I(2013a) BLH1 and KNAT3 modulate ABA responses during germination and early seedling development in Arabidopsis. Plant J 75: 755–766 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Chiang YH, Kieber JJ, Schaller GE(2013b) SCF(KMD) controls cytokinin signaling by regulating the degradation of type-B response regulators. Proc Natl Acad Sci USA 110: 10028–10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia-Hernandez M, et al. (2012) The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res 40: 1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL(2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold AC, Niedergang-Kamien E, Janick J(1959) Experimental modification of plant senescence. Plant Physiol 34: 570–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J(1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9: 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Dewey CN(2011) RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sherif SM(2019) Hormonal orchestration of bud dormancy cycle in deciduous woody perennials. Front Plant Sci 10: 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K(1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maere S, Heymans K, Kuiper M(2005) BiNGO: A Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21: 3448–3449 [DOI] [PubMed] [Google Scholar]
- Matsui A, Ishida J, Morosawa T, Mochizuki Y, Kaminuma E, Endo TA, Okamoto M, Nambara E, Nakajima M, Kawashima M, et al. (2008) Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tiling array. Plant Cell Physiol 49: 1135–1149 [DOI] [PubMed] [Google Scholar]
- Maurya JP, Bhalerao RP(2017) Photoperiod- and temperature-mediated control of growth cessation and dormancy in trees: A molecular perspective. Ann Bot 120: 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng WJ, Cheng ZJ, Sang YL, Zhang MM, Rong XF, Wang ZW, Tang YY, Zhang XS(2017) Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell 29: 1357–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mølmann JA, Junttila O, Johnsen O, Olsen JE(2006) Effects of red, far-red and blue light in maintaining growth in latitudinal populations of Norway spruce (Picea abies). Plant Cell Environ 29: 166–172 [DOI] [PubMed] [Google Scholar]
- Moore I, Samalova M, Kurup S(2006) Transactivated and chemically inducible gene expression in plants. Plant J 45: 651–683 [DOI] [PubMed] [Google Scholar]
- Noodén LD, Guiamét JJ, John I(2004) Whole plant senescence In Noodén LD, ed, Plant Cell Death Processes. Academic Press, San Diego, CA, pp 227–244 [Google Scholar]
- Oh E, Kim J, Park E, Kim JI, Kang C, Choi G(2004) PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16: 3045–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Kamiya Y, Bae G, Chung WI, Choi G(2006) Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J 47: 124–139 [DOI] [PubMed] [Google Scholar]
- Olsen JE.(2010) Light and temperature sensing and signaling in induction of bud dormancy in woody plants. Plant Mol Biol 73: 37–47 [DOI] [PubMed] [Google Scholar]
- Olsen JE, Junttila O, Nilsen J, Eriksson ME, Martinussen I, Olsson O, Sandberg G, Moritz T(1997) Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J 12: 1339–1350 [Google Scholar]
- Pagnussat GC, Yu HJ, Sundaresan V(2007) Cell-fate switch of synergid to egg cell in Arabidopsis eostre mutant embryo sacs arises from misexpression of the BEL1-like homeodomain gene BLH1. Plant Cell 19: 3578–3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP(1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11: 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L(2002) Relative Expression Software Tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig-Villanova I, Bou-Torrent J, Galstyan A, Carretero-Paulet L, Portolés S, Rodríguez-Concepción M, Martínez-García JF(2007) Interaction of shade avoidance and auxin responses: A role for two novel atypical bHLH proteins. EMBO J 26: 4756–4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H(2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386 [DOI] [PubMed] [Google Scholar]
- Sablowski RW, Meyerowitz EM(1998) A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell 92: 93–103 [DOI] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T(2003) Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, de Wit CT(1975) Photosynthate and nitrogen requirements for seed production by various crops. Science 189: 565–567 [DOI] [PubMed] [Google Scholar]
- Smaczniak C, Immink RG, Muiño JM, Blanvillain R, Busscher M, Busscher-Lange J, Dinh QD, Liu S, Westphal AH, Boeren S, et al. (2012) Characterization of MADS-domain transcription factor complexes in Arabidopsis flower development. Proc Natl Acad Sci USA 109: 1560–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka S, Heyer AG(1999) Arabidopsis knockout mutation of ADC2 gene reveals inducibility by osmotic stress. FEBS Lett 458: 219–223 [DOI] [PubMed] [Google Scholar]
- Staneloni RJ, Rodriguez-Batiller MJ, Legisa D, Scarpin MR, Agalou A, Cerdán PD, Meijer AH, Ouwerkerk PB, Casal JJ(2009) Bell-like homeodomain selectively regulates the high-irradiance response of phytochrome A. Proc Natl Acad Sci USA 106: 13624–13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J(2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL(2009) TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L(2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28: 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylewicz S, Petterle A, Marttila S, Miskolczi P, Azeez A, Singh RK, Immanen J, Mähler N, Hvidsten TR, Eklund DM, et al. (2018) Photoperiodic control of seasonal growth is mediated by ABA acting on cell-cell communication. Science 360: 212–215 [DOI] [PubMed] [Google Scholar]
- Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K(2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97: 11632–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaistij FE, Barros-Galvão T, Cole AF, Gilday AD, He Z, Li Y, Harvey D, Larson TR, Graham IA(2018) MOTHER-OF-FT-AND-TFL1 represses seed germination under far-red light by modulating phytohormone responses in Arabidopsis thaliana. Proc Natl Acad Sci USA 115: 8442–8447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayssières A, Mishra P, Roggen A, Neumann U, Ljung K, Albani MC(2020) Vernalization shapes shoot architecture and ensures the maintenance of dormant buds in the perennial Arabis alpina. New Phytol 227: 99–115 [DOI] [PubMed] [Google Scholar]
- Walker CH, Bennett T(2018) Forbidden fruit: Dominance relationships and the control of shoot architecture In Roberts JA, ed, Annual Plant Reviews online. John Wiley and Sons, Ltd, New Jersey, pp 1–38 [Google Scholar]
- Wang J, Tian C, Zhang C, Shi B, Cao X, Zhang TQ, Zhao Z, Wang JW, Jiao Y(2017) Cytokinin signaling activates WUSCHEL expression during axillary meristem initiation. Plant Cell 29: 1373–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware A, Walker CH, Šimura J, González-Suárez P, Ljung K, Bishopp A, Wilson ZA, Bennett T(2020) Auxin export from proximal fruits drives arrest in temporally competent inflorescences. Nat Plants 6: 699–707 [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T(2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest SE, Philipp MA, Guthörl D, Schmid B, Grossniklaus U(2016) Seed production affects maternal growth and senescence in Arabidopsis. Plant Physiol 171: 392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würschum T, Gross-Hardt R, Laux T(2006) APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell 18: 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lin R, Sullivan J, Hoecker U, Liu B, Xu L, Deng XW, Wang H(2005) Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in Arabidopsis. Plant Cell 17: 804–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Worley E, Udvardi M(2014) A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis leaves. Plant Cell 26: 4862–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Whitelam GC(1995) Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: Weak de‐etiolation of the phyA mutant under dense canopies. Plant Cell Environ 18: 788–794 [Google Scholar]
- Yanovsky MJ, Kay SA(2003) Living by the calendar: How plants know when to flower. Nat Rev Mol Cell Biol 4: 265–275 [DOI] [PubMed] [Google Scholar]
- Yant L, Mathieu J, Dinh TT, Ott F, Lanz C, Wollmann H, Chen X, Schmid M(2010) Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22: 2156–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Finlayson SA(2015) Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiol 169: 611–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Kim Y, Dinh TT, Chen X(2007) miR172 regulates stem cell fate and defines the inner boundary of APETALA3 and PISTILLATA expression domain in Arabidopsis floral meristems. Plant J 51: 840–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Fernald RD(2005) Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol 12: 1047–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Chen H, Wei D, Ma H, Lin J(2017) Arabidopsis CBF3 and DELLAs positively regulate each other in response to low temperature. Sci Rep 7: 39819. [DOI] [PMC free article] [PubMed] [Google Scholar]