A multilayered regulatory cascade controls ethylene biosynthesis during the climacteric ripening in banana.
Abstract
Ethylene plays a critical regulatory role in climacteric fruit ripening, and its biosynthesis is fine-tuned at the transcriptional and posttranslational levels. Nevertheless, the mechanistic link between transcriptional and posttranslational regulation of ethylene biosynthesis during fruit ripening is largely unknown. This study uncovers a coordinated transcriptional and posttranslational mechanism of controlling ethylene biosynthesis during banana (Musa acuminata) fruit ripening. NAC (NAM, ATAF, and CUC) proteins MaNAC1 and MaNAC2 repress the expression of MaERF11, a protein previously known to negatively regulate ethylene biosynthesis genes MaACS1 and MaACO1. A RING E3 ligase MaXB3 interacts with MaNAC2 to promote its ubiquitination and degradation, leading to the inhibition of MaNAC2-mediated transcriptional repression. In addition, MaXB3 also targets MaACS1 and MaACO1 for proteasome degradation. Further evidence supporting the role of MaXB3 is provided by its transient and ectopic overexpression in banana fruit and tomato (Solanum lycopersicum), respectively, which delays fruit ripening via repressing ethylene biosynthesis and thus ethylene response. Strikingly, MaNAC1 and MaNAC2 directly repress MaXB3 expression, suggesting a feedback regulatory mechanism that maintains a balance of MaNAC2, MaACS1, and MaACO1 levels. Collectively, our findings establish a multilayered regulatory cascade involving MaXB3, MaNACs, MaERF11, and MaACS1/MaACO1 that controls ethylene biosynthesis during climacteric ripening.
The plant hormone ethylene is involved in a wide range of physiological responses, including seed germination, flowering, fruit ripening, senescence, abscission, and response to abiotic and biotic stresses (Dubois et al., 2018). In climacteric fruits, such as tomato (Solanum lycopersicum), banana (Musa acuminata), apple (Malus domestica), mango (Mangifera indica), and kiwifruit (Actinidia deliciosa), ethylene plays an important role in triggering the onset of ripening and is an essential regulatory molecule of the ripening process (Bapat et al., 2010; Liu et al., 2015). Most of our knowledge of the processes underlying ethylene biosynthesis, perception and signal transduction, and ripening-related genes in climacteric fruits has been achieved from the model species tomato and partly from apple (Klee and Giovannoni, 2011; Seymour et al., 2013a; Li et al., 2016, 2017), but little is known about other climacteric fruit types. Genes encoding ethylene biosynthesis ACC synthase (ACS) and ACC oxidase (ACO), perception (ETRs), signal transduction (CTR, EIN2, EIN3, EBF, etc.) and ethylene response factors (ERFs) are now well described in a number of plant species (Gapper et al., 2013; Seymour et al., 2013b; Liu et al., 2015; Giovannoni et al., 2017; Dubois et al., 2018). Other components that affect ethylene biology, such as signaling, including REVERSION TO ETHYLENE SENSITIVITY1 (RTE1) and RTE1-HOMOLOG (RTH), GREEN RIPE (GR), and GREEN-RIPE LIKE (GRL) have also been reported (Giovannoni, 2007; Lin et al., 2009; Gapper et al., 2013). Nevertheless, a full understanding of the diversity and complexity of the mechanisms by which ethylene mediates fruit ripening in different fleshy fruit species still awaits the discovery of upstream regulators of ethylene biosynthesis and the signaling pathway.
The vital regulatory roles played by ethylene in triggering and coordinating fruit ripening relies on the precise control of the responses to this hormone in a timely and tissue-specific manner. Two modes (system 1 and system 2) of ethylene production have been proposed. System 1 ethylene is generated during normal vegetative growth at a basal level and is autoinhibitory, while the system 2 ethylene is produced at the onset of ripening and is autocatalytic (Alexander and Grierson, 2002). Transition from system 1 to system 2 ethylene synthesis during the onset of ripening mainly depends on the induced expression of ACSs and ACOs (Fujisawa et al., 2013; Gao et al., 2018). To date, most of the transcription factors (TFs) reported to regulate fruit ripening act through modulating system 2 ethylene biosynthesis by directly targeting ACSs and ACOs. For example, tomato MADS-box protein RIPENING INHIBITOR (RIN) and Homeodomain-Leu zipper (HD-Zip) homeobox protein LeHB-1 promote fruit ripening through transcriptionally activating the expression of ACS2 and ACO1, respectively (Ito et al., 2008; Lin et al., 2008; Fujisawa et al., 2013). Apple MdACS1 and MdACO1 are directly activated by MdMADS8/MdMADS9 and MdbHLH3, leading to the increase in ethylene production (Ireland et al., 2013; Hu et al., 2019). Likewise, MdERF3 and MdERF2 promote or repress MdACS1 transcription, respectively (Li et al., 2016, 2017). In kiwifruit, the activation of AdACO1 by AdEIL2 and AdEIL3 leads to the stimulation of ethylene production during fruit ripening (Yin et al., 2010). From these examples, it is clear that ethylene biosynthesis during fruit ripening is tightly controlled at the transcriptional level.
Posttranslational modifications are other important regulatory factors involved in the modulation of ethylene biosynthesis and signaling (Guo and Ecker, 2003; Potuschak et al., 2003; Qiao et al., 2009; Deng et al., 2018). Protein ubiquitination, a common posttranslational modification, has been well documented in the regulation of ethylene production in the model plant Arabidopsis (Arabidopsis thaliana; Yoon, 2015; Lee et al., 2017). For instance, the Arabidopsis BTB-subtype E3 ubiquitin ligases ETHYLENE OVERPRODUCER1 (ETO1), ETO1-like (EOL1), and EOL2 target type-2 ACS5 and ACS9 enzymes for degradation via the ubiquitin 26S proteasome system (UPS) and inhibits their activities (Christians et al., 2009). In Arabidopsis, a member of the RING E3 ligase XBAT32 negatively modulates ethylene production via the proteasomal degradation of type-2 ACS4 and type-3 ACS7 proteins (Prasad et al., 2010; Lyzenga et al., 2012). In addition, the key components of ethylene signaling, including ETHYLENE-INSENSITIVE3 (EIN2) and the master TFs EIN3/EIN3-like (EIL), are strictly controlled by the UPS. In Arabidopsis, ethylene blocks the ubiquitin-mediated proteasomal degradation of EIN2 and EIN3 by SCF-type CRL E3 ligases containing EIN2-TARGETING PROTEIN1/2 (ETP1/2) and EIN3-BINDING F-BOX1/2 (EBF1/2) substrate recruiting F-box proteins, respectively (Guo and Ecker, 2003; Potuschak et al., 2003; Binder et al., 2007; Qiao et al., 2009). Interestingly, in tomato, silencing of SlEBF1 and SlEBF2 genes results in constitutive ethylene response phenotypes, accelerated plant senescence, and fruit ripening (Yang et al., 2010). Recently, a tomato F-box protein, SlEBF3, has been shown to directly interact with EIL proteins to induce their degradation, thus impairing ethylene-dependent fruit ripening (Deng et al., 2018). However, the findings that the mechanisms of protein ubiquitination are involved in ethylene biosynthesis, and the signaling pathway has been limited so far to the model plant species, and it remains largely unknown whether the same mechanisms are operating in a similar way in economically important fruit species.
Banana is one of the important fruits globally by economic value and one of the top 10 crops by production (Paul et al., 2017). Bananas are part of a balanced human diet and represent staple foods for more than 400 million people in the tropics (Hölscher et al., 2014). Banana is a typical climacteric fruit, characterized by a peak of ethylene production that orchestrates ripening-associated processes, resulting in a short postharvest life of 10 to 15 d when stored at ambient temperature (Bapat et al., 2010; Shan et al., 2012; Xiao et al., 2013; Han et al., 2016). A better understanding of the mechanism underlying banana fruit ripening is important toward designing new strategies for maintaining quality and extending fruit shelf life. Genes encoding the two main enzymes of the ethylene biosynthesis pathway ACS and ACO have been identified in bananas (Liu et al., 1999; Inaba et al., 2007). In addition, three ERS genes (Yan et al., 2011), one CTR1 gene (Hu et al., 2012), five EIL genes (Mbéguié-A-Mbéguié et al., 2008), and two EBF genes (Kuang et al., 2013) have been isolated. All members of the 10 banana gene families related to ethylene biosynthesis and signaling pathways have been recently identified using genome-scale approaches (Jourda et al., 2014). Together, these results expand our knowledge of genes involved in ethylene biosynthesis and signaling in banana and provide the basis for further studies on the regulatory network of banana fruit ripening. More importantly, several ripening-related TFs, including MADS-box, NAC, and ERF, have been identified in banana fruit (Shan et al., 2012; Xiao et al., 2013; Elitzur et al., 2016). Six members of the NAC family involved in banana fruit ripening have been previously characterized, among which MaNAC1/MaNAC2 are induced by ethylene (Shan et al., 2012). By contrast, the EAR-containing MaERF11 is down-regulated during banana fruit ripening or upon ethylene treatment, and this ERF directly suppresses the expression of MaACS1 and MaACO1 (Xiao et al., 2013), two important genes previously identified to be responsible for system 2 ethylene biosynthesis (Inaba et al., 2007; Jourda et al., 2014). However, whether there is any link between MaNAC1/MaNAC2 and MaERF11 and whether these factors operate within the same mechanism remain completely unknown. This study showed that MaNAC1 and MaNAC2 directly targeted MaERF11 to suppress its expression. Moreover, we identified a C3HC4-type RING E3 ligase named MaXB3, which physically interacted with MaNAC2, MaACS1, and MaACO1 and specifically mediated their ubiquitination and subsequent proteasome degradation. The transient and ectopic expression of MaXB3 in banana fruit and tomato delayed fruit ripening, accompanied by the repression of ethylene biosynthesis and response. Intriguingly, MaNAC1 and MaNAC2 were also capable of direct binding to the MaXB3 promoter and repressed its expression. Overall, our findings establish a multilayered regulatory cascade involving MaXB3, MaNACs, MaERF11, MaACS1, and MaACO1 that modulates ethylene biosynthesis during banana fruit ripening.
RESULTS
MaNAC1 and MaNAC2 Directly Target and Repress MaERF11
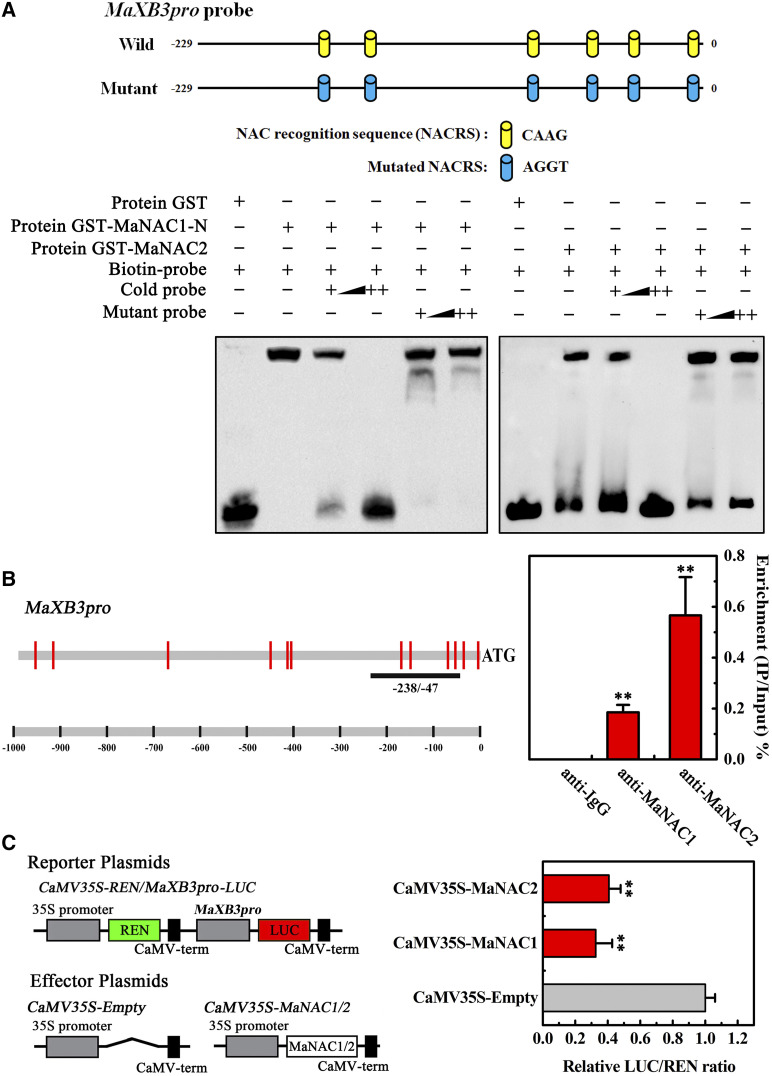
A dual-luciferase transient expression system in BY2 protoplasts clearly indicated that both MaNAC1 and MaNAC2 have transcriptional repression activity (Supplemental Fig. S1). MaNAC1 and MaNAC2 are ethylene inducible and positively associated with fruit ripening (Shan et al., 2012), which raises the possibility that negative regulators of ethylene response and fruit ripening are the candidate targets of these NAC proteins. Because MaERF11 negatively affects ethylene biosynthesis by suppressing MaACS1 and MaACO1 transcription (Xiao et al., 2013), it therefore represents a potential target of MaNAC1 and MaNAC2. In support to this hypothesis, a number of cis-elements that represent the putative NAC recognition sequence (NACRS; Puranik et al., 2012) were identified in the MaERF11 promoter (Supplemental Text S1). First, electrophoretic mobility shift assay (EMSA) experiments were performed using purified recombinant proteins of MaNAC1-N and MaNAC2 (Supplemental Fig. S2). While no mobility shift was observed in the presence of GST alone, both MaNAC1-N and MaNAC2 proteins were able to bind to the labeled probes containing NACRS derived from the MaERF11 promoter and caused mobility shifts. In addition, the shifted bands could be effectively competed by the addition of increasing amounts of unlabeled cold probes with the same sequence but not by the mutated probes (Fig. 1A). Furthermore, the interaction in vivo between MaNAC1/MaNAC2 and the MaERF11 promoter was revealed by chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) analysis using MaNAC1 and MaNAC2 antibodies. As shown in Figure 1B, the MaERF11 promoter region was specifically enriched by anti-MaNAC1 and -MaNAC2 antibodies, but not by nonspecific antibody (preimmune rabbit IgG). These results support a direct binding of MaNAC1 and MaNAC2 to the MaERF11 promoter in banana fruit pulp.
Figure 1.
MaNAC1 and MaNAC2 directly repress MaERF11 expression through binding its promoter. A, EMSA showing the GST-MaNAC1-N terminus or -MaNAC2 fusion protein binds to DNA probe from the MaERF11 promoter in vitro. NACRS and mutated NACRS in the probe are shown in the top. Biotin-labeled probe containing NACRS was incubated with GST-MaNAC1-N or MaNAC2 protein, and the DNA-protein complexes were separated on 6% native polyacrylamide gels. The probe with the mutated NACRS was used to test binding specificity. Triangles indicate increasing amounts (100× and 500×) of unlabeled cold or mutated probes for competition. B, ChIP-qPCR assay for direct binding of MaNAC1 or MaNAC2 protein to the promoter of MaERF11. The promoter structure of MaERF11 is shown in the left and red lines indicate cis elements of NAC. The underlined fragment was used for ChIP-qPCR. Values are the percentage of DNA fragments that coimmunoprecipitated with anti-MaNAC1 or -MaNAC2 antibodies or nonspecific antibody (anti-IgG) relative to the input DNA. Error bars represent the se of three independent experiments. C, MaNAC1 and MaNAC2 repress the activity of MaERF11 promoter in a dual luciferase assay. The reporter and effector vectors, as indicated, were cotransformed into Nicotana tabacum ‘Bright Yellow 2’ (BY2) protoplasts. Data show ratios of LUC to REN. Each value represents the mean ± se of six biological replicates. In B and C, asterisks indicate statistically significant differences as determined by Student’s t test (**P < 0.01).
To further investigate the ability of MaNAC1 or MaNAC2 to act as a repressor on the native MaERF11 promoter, we carried out a transient dual luciferase assay (Fig. 1C). The data indicate that the firefly luciferase (LUC)/renilla luciferase (REN) ratio significantly decreased in the presence of MaNAC1 or MaNAC2 (Fig. 1C). Taken together, these data clearly support the notion that MaNAC1 and MaNAC2 directly repress MaERF11 through targeting its promoter.
MaXB3 Physically Interacts with MaNAC2 In Vitro and In Vivo
To gain further insight on the factors and mechanisms by which MaNAC1/2 regulate MaERF11 expression and hence ethylene biosynthesis, we carried out yeast two-hybrid screens to search for putative MaNAC1/2 interaction proteins in a cDNA library corresponding to a ripening banana fruit. Among the eight positive clones, a RING finger domain-containing protein (GSMUA_Achr7G18180_001) showed the strongest interaction with MaNAC2. Sequence analysis of this isolated banana clone, named MaXB3, revealed high similarity to the rice (Oryza sativa) XA21 binding protein3 (XB3; Wang et al., 2006), characterized by the presence of multiple copies of ankyrin repeats and a C3HC4-type RING finger motif (Supplemental Fig. S3). MaXB3 shares 70% identity with the rice XB3 and with four o’clock flower (Mirabilis jalapa) MjXB3, which are involved in pathogen-induced programmed cell death and flower senescence, respectively (Wang et al., 2006; Xu et al., 2007; Supplemental Fig. S4). Expression studies conducted by reverse transcription (RT)-qPCR revealed a dramatic decrease in MaXB3 transcript levels at the ripening stages of banana fruit, and transactivation assay in N. tabacum ‘BY2’ cells indicated a negative regulation by ethylene (Supplemental Fig. S5). During banana fruit development, transcription levels of MaNAC1 and MaNAC2, as well as their protein levels, increased from early developmental stage (immature to late mature green) to later developmental stage (turn yellow and ripen), while transcription and protein levels of MaXB3 decreased (Supplemental Fig. S6). Similarly, MaNAC1 and MaNAC2 proteins accumulated during natural and ethylene-induced banana fruit ripening, whereas protein levels of MaXB3 decreased (Supplemental Fig. S7). These inverted accumulation profiles raise the hypothesis of a possible interaction between MaXB3 and MaNAC1/2 proteins.
Yeast two-hybrid assay revealed positive interaction between MaXB3 and MaNAC2, whereas no interaction was detected with MaNAC1 (Fig. 2A). To substantiate the interaction between MaXB3 and MaNAC2 in plant cells, an in vivo interaction assay was carried out using bimolecular fluorescence complementation (BiFC) in N. tabacum ‘BY2’ protoplasts. Coexpression of MaXB3-YC and MaNAC2-YN, or MaXB3-YN and MaNAC2-YC, reconstituted a functional yellow florescent protein (YFP) in the nucleus, whereas coexpression of MaXB3-YC and MaNAC1-YN, or MaXB3-YN and MaNAC1-YC, as well as the control combinations, failed to generate YFP signal (Fig. 2B). In addition, although the presence of MaXB3 protein was detected in nucleus, cytoplasm, and plasma membrane, the colocalization of MaXB3 and MaNAC2 proteins was restricted to the nuclear compartment (Fig. 2C). The MaXB3-MaNAC2 interaction was further verified by coimmunoprecipitation (Co-IP) assay, which showed that MaXB3-His fusion proteins were detected following immunoprecipitation of MaNAC2-GFP using anti-GFP antibody microbeads (Fig. 2D). Collectively, these data clearly demonstrate that MaXB3 physically interacts with MaNAC2 in plant.
Figure 2.
MaXB3 interacts with MaNAC2 in vitro and in vivo. A, Yeast two-hybrid assay for the interaction between MaXB3 and MaNAC2. The coding regions of MaXB3 and MaNACs were fused with DNA-binding domain (DBD) and activation domain (AD) vectors, respectively, as indicated, and cotransformed into the yeast (Saccbaromyces cerevisiae) strain Y2H Gold. The ability of yeast cells to grow on synthetic medium lacking Trp, Leu, His, and adenine and to turn blue in the presence of the chromogenic substrate α-galactoside, was scored as a positive interaction. B, BiFC in N. tabacum ‘BY2’ protoplasts showing the interaction between MaXB3 and MaNAC2 in living cells. MaXB3 and MaNACs were fused with the C terminus of YFP (YC) and the N terminus of YFP (YN) respectively, as indicated, and cotransfected into protoplasts. Expressions of MaXB3 or MaNACs alone were used as negative controls. The VirD2NLS-mCherry was included in each transfection to serve as a control for successful transfection as well as for nuclear localization (NLS-mCherry). Merge is digital merge of bright field and fluorescent images. The length of the bar indicated in the photographs is 25 μm. C, Nuclear colocalization of MaXB3 and MaNAC2 in N. tabacum ‘BY2’ protoplasts. The indicated constructs were cotransfected into protoplasts. Bars = 25 μm. D, Co-IP assay showing the interaction between MaNAC2 with MaXB3. Nicotiana benthamiana leaves coexpressing MaNACs-GFP and MaXB3-His, or empty-GFP and MaXB3-His, was used to immunoprecipitate with the anti-GFP antibody, and the immunoblot was performed with the anti-GFP and anti-His antibodies, respectively. The molecular weight (kilodalton) is indicated on the right side of the gel.
MaXB3 Destabilizes MaNAC2 Protein, Resulting in Suppression of the Transcriptional Repression Activity of MaNAC2 on MaERF11
The rice protein XB3 and its Arabidopsis ortholog XBAT32 function as E3 ubiquitin ligases that target their substrates for ubiquitylation and subsequent degradation (Wang et al., 2006; Prasad et al., 2010; Lyzenga et al., 2012). The impact of MaXB3-MaNAC2 interaction on the transcriptional repression activity of MaNAC2 was assessed in vivo through a dual-luciferase reporter approach with the MaERF11 promoter. Coexpression of MaNAC2 with MaERF11 Pro-LUC resulted in decreased a LUC/REN ratio that was abolished when MaXB3 was coexpressed (Fig. 3), consistent with the hypothesis that MaXB3-MaNAC2 interaction prevents the transcriptional repression of MaERF11 mediated by MaNAC2. Importantly, MG132, a known selective inhibitor of proteasomal activity (Yang et al., 2004), maintained the MaNAC2-dependent transcriptional repression of MaERF11 (Fig. 3). These data are consistent with a mechanism where MaXB3 possibly mediates the degradation of MaNAC2 protein via the proteasome pathway, then linking MaXB3, MaNAC2, and MaERF11 in a common regulatory pathway.
Figure 3.
MaXB3 destabilizes MaNAC2 protein, suppressing the transcriptional repression activity of MaNAC2 on the MaERF11 promoter. The reporter and effector vectors used in the transient assay, as indicated at the top, were cotransformed into N. tabacum ‘BY2’ protoplasts. MG132 was included as a proteasome inhibitor. Data show ratios of LUC to REN. Each value represents the mean ± se of six biological replicates. Lowercase letters above bars indicate a statistical difference at P < 0.05 compared with the empty vector.
MaNAC2 Protein Is Ubiquitinated by MaXB3 and Degraded via the Ubiquitin-Proteasome System
To test whether MaNAC2 protein is marked for degradation by the 26S proteasome through polyubiquitination mediated by MaXB3, we carried out in vitro and in vivo ubiquitination assays. Following the incubation of recombinant glutathione S-transferases (GST)-MaNAC2 and maltose-binding protein (MBP)-MaXB3 proteins (Supplemental Fig. S2) in the presence or absence of Ub, ATP, E1, and E2 at 30°C for 2 h, the reaction mixture was analyzed by immunoblotting using anti-GST antibody. Coincubation of GST-MaNAC2 and MBP-MaXB3 in the presence of Ub, E1, and E2 leads to the appearance of high-molecular-mass bands likely corresponding to ubiquitinated MaNAC2 as the exclusion of Ub, E1, or E2 from the reaction mixture abolished these bands (Fig. 4A). Since MaXB3 did not interact with MaNAC1, we also found that MaXB3 could not ubiquitinate MaNAC1 in vitro (Supplemental Fig. S8A). The ubiquitination of MaNAC2 was further confirmed in planta via coexpression of MaNAC2-GFP and MaXB3-His constructs in N. benthamiana leaves. As shown in Figure 4B, immunoblotting analysis using anti-GFP antibody and anti-Ub antibody, respectively, showed smear bandings representing the polyubiquitinated MaNAC2 protein in the anti-GFP-immunoprecipitated complex when the coexpression is performed with MaXB3-His, but not with the empty-His control vector (Fig. 4B). These data clearly reveal that MaXB3 is able to ubiquitinate MaNAC2 both in vitro and in vivo.
Figure 4.
MaXB3 mediates MaNAC2 protein degradation via the ubiquitin-proteasome system. A, In vitro ubiquitination of MaNAC2 by MaXB3. Recombinant MBP-MaXB3 protein was coincubated with GST-MaNAC2 protein in the presence or absence of Ub, ATP, human E1, and human E2 at 30°C for 2 h. The reaction mixture was analyzed by immunoblotting with an anti-GST antibody. Ubiquitination results in a heterogeneous collection of higher-molecular mass proteins that were detected using an anti-GST antibody. The molecular weight (kilodarcy) is indicated on the left of the gel. B, In vivo ubiquitination of MaNAC2 by MaXB3. Ubiquitylated proteins in total protein extracts from N. benthamiana leaves transiently expressing MaNAC2-GFP, MaXB3-His, and empty-His with different combinations were captured with the anti-GFP antibody. The ubiquitylated MaNAC2 protein was detected using anti-GFP and anti-ubiquitin antibodies, respectively. Actin was used as the loading control. The molecular weight (kilodarcy) is indicated on the right of the gel. Relative mRNA abundance of MaNAC2 in each combination is shown at the bottom of the image. C, Proteasome-mediated degradation assay of MaNAC2 in plant cells. As indicated, MaNAC2 fused with GFP and LUC was expressed alone or coexpressed with MaXB3 in N. benthamiana leaves in the presence or absence of MG132. The resulting protein extracts were analyzed using an anti-GFP antibody. Actin was used as the loading control. The molecular weight (kilodarcy) is indicated on the right side of the gel. LUC activity and relative mRNA abundance of MaNAC2 in each sample is shown at the bottom of the image. Each value represents the mean ± se of six biological replicates. Asterisks indicate statistically significant differences as determined by Student’s t test (**P < 0.01). NS, No significant difference.
To investigate whether MaXB3 mediates the degradation of MaNAC2 protein, the stability of the MaNAC2 protein in plant cells was examined using a cell-free proteasome degradation assay. As shown in Figure 4C, MaNAC2 amounts declined substantially in the presence of MaXB3, whereas in the absence of MaXB3, MaNAC2 remained relatively stable. The fact that this MaXB3-mediated destabilization of MaNAC2 is inhibited by MG132 (Fig. 4C) strongly suggests that the MaNAC2 degradation process occurs via the proteasome pathway. Quantification of LUC activity also showed that expression of MaXB3 protein resulted in the degradation of MaNAC2 protein, while in the presence of MG132, the degradation of MaNAC2 was abolished (Fig. 4C). As expected, the stability of the MaNAC1 protein was not affected by MaXB3 (Supplemental Fig. S8B), since MaXB3 could not ubiquitinate MaNAC1. These results clearly reveal that MaXB3 protein mediates the proteasome degradation of MaNAC2 protein, thus preventing the transcriptional repression of its target genes such as MaERF11.
MaXB3 Interacts with and Ubiquitinates MaACS1 and MaACO1 for the Proteasome Degradation
XBAT32 interacts with and ubiquitinates type 2 ACS4 and type 3 ACS7, suggesting that XBAT32 negatively regulates ethylene biosynthesis by modulating the abundance of ACS proteins (Prasad et al., 2010; Lyzenga et al., 2012). Banana fruit MaACS1 belongs to the type 1 ACS family (Supplemental Fig. S9), and MaACS1 and MaACO1 are the most important members that are responsible for system 2 ethylene biosynthesis during fruit ripening (Liu et al., 1999; Inaba et al., 2007; Xiao et al., 2013; Jourda et al., 2014). To test the interaction between MaXB3 and both MaACS1 and MaACO1, we first used yeast two-hybrid system. As shown in Figure 5A, yeast cells cotransformed with MaXB3 and either MaACS1 or MaACO1 turn blue in the presence of the chromogenic substrate α-Gal, indicating that MaXB3 interacts with both MaACS1 and MaACO1. To further confirm the observed interaction between MaXB3 and MaACS1 or MaACO1 in the yeast two-hybrid assay, BiFC assay in N. tabacum ‘BY2’ protoplasts was performed. As shown in Figure 5B, strong YFP fluorescent signal was detected in the entire cells of cv BY2 protoplasts expressing MaXB3-YN with MaACS1-YC or MaACO1-YC, and MaXB3-YC with MaACS1-YN or MaACO1-YN, whereas coexpression with the control combinations failed to generate YFP fluorescence (Fig. 5B). Moreover, Co-IP assay showed that MaACS1-GFP or MaACO1-GFP coimmunoprecipitate with MaXB3-His in plants (Fig. 5C). These results suggest that MaACS1 and MaACO1 are putative substrates for the MaXB3 protein.
Figure 5.
MaXB3 interacts with MaACS1 or MaACO1 in vitro and in vivo. A, Yeast two-hybrid assay for the interactions between MaXB3, MaACS1, and MaACO1. The coding regions of MaXB3, MaACS1, and MaACO1 were fused with DNA-binding domain (DBD) and activation domain (AD) vectors, respectively, as indicated, and cotransformed into the yeast strain Y2H Gold. The ability of yeast cells to grow on synthetic medium lacking Trp, Leu, His, and adenine and to turn blue in the presence of the chromogenic substrate X-α-Gal, was scored as a positive interaction. B, BiFC in N. tabacum ‘BY2’ protoplasts showing the interactions between MaXB3, MaACS1, and MaACO1 in living cells. MaXB3, MaACS1, and MaACO1 were fused with the YC and YN as indicated and cotransfected into protoplasts. Expressions of MaXB3, MaACS1, or MaACO1 alone were used as negative controls. Merge is digital merge of bright field and fluorescent images. Bar = 25 μm. C, Co-IP assay showing the interaction between MaXB3 and MaACS1 or MaACO1. N. benthamiana leaves coexpressing MaACS1-GFP and MaXB3-His, MaACO1-GFP, and MaXB3-His, or empty-GFP and MaXB3-His were used to immunoprecipitate with the anti-GFP antibody, and the immunoblot was performed with the anti-GFP and anti-His antibodies, respectively. The molecular weight (kilodarcy) is indicated on the right side of the gel.
To determine whether MaXB3 can mediate MaACS1 and MaACO1 protein ubiquitination, in vitro and in vivo ubiquitination assays were performed. As shown in Figure 6A, in the presence of Ub, E1, E2, and MBP-MaXB3, polyubiquitinated GST-MaACS1 or GST-MaACO1 protein was detected by anti-GST immunoblot analysis, while lack of Ub, E1, E2, MBP-MaXB3, or substrate from the reaction mixture abolished the ubiquitinated bands. Similarly, polyubiquitination of MaACS1 or MaACO1 protein was detected in vivo by immunoblotting using anti-ubiquitin antibody and anti-GFP antibody, respectively, after immunoprecipitation of MaACS1- or MaACO1-GFP protein (Fig. 6B). Furthermore, cell-free degradation assay showed that MaACS1 or MaACO1 was degraded by MaXB3 and that this degradation was inhibited by MG132 (Fig. 6C). These data suggest that MaXB3 negatively regulates ethylene biosynthesis via ubiquitin-dependent degradation of ACS and ACO proteins.
Figure 6.
MaXB3 mediates MaACS1 and MaACO1 protein degradation via the ubiquitin-proteasome system. A, In vitro ubiquitination of MaACS1 and MaACO1 by MaXB3. Recombinant MBP-MaXB3 protein was coincubated with GST-MaACS1 or -MaACO1 protein in the presence or absence of Ub, ATP, human E1, and human E2 UbcH5b at 30°C for 2 h. The reaction mixture was analyzed by immunoblotting with an anti-GST antibody. Ubiquitination results in a heterogeneous collection of higher-molecular mass proteins that were detected using an anti-GST antibody. The molecular weight (kilodarcy) is indicated on the left side of the gel. B, In vivo ubiquitination of MaACS1 and MaACO1 by MaXB3. Ubiquitylated proteins in total protein extracts from N. benthamiana leaves coexpressing MaACS1-GFP, MaACO1-GFP, MaXB3-His, and empty-His with indicated combinations were used to immunoprecipitate with the anti-GFP antibody. The ubiquitylated MaACS1 or MaACO1 protein was detected using anti-GFP and anti-ubiquitin antibodies, respectively. Actin was used as the loading control. The molecular weight (kilodarcy) is indicated on the right side of the gel. Relative mRNA abundance of MaACS1 and MaACO1 in each combination is shown at the bottom of the image. C, Proteasome-mediated degradation assay of MaACS1 and MaACO1 in plant cells. As indicated, MaACS1 and MaACO1 fused with GFP and LUC was expressed alone or coexpressed with MaXB3 in N. benthamiana leaves in the presence or absence of MG132. The resulting protein extracts were analyzed using an anti-GFP antibody. Actin was used as the loading control. The molecular weight (kDa) is indicated on the right side of the gel. LUC activity and relative mRNA abundance of MaACS1 and MaACO1 in each sample are shown at the bottom of the image. Each value represents the mean ± se of six biological replicates. Asterisks indicate statistically significant differences as determined by Student’s t test (**P < 0.01). NS, No significant difference.
Transient Overexpression of MaXB3 in Banana Fruit Delays Ripening by Repressing Ethylene Biosynthesis
The function of MaXB3 in regulating fruit ripening by affecting ethylene biosynthesis was investigated through its transient overexpression in banana fruit (Fig. 7, A–C). The MaXB3-overexpressing fruits showed a distinct delay in fruit ripening, as the peel of fruits transiently overexpressing MaXB3 exhibited less yellowing than the fruits expressing the empty vector (control; Fig. 7B). Concomitantly, lower color index was found in MaXB3-injected fruits (Fig. 7D). In parallel, the increase in ethylene production and the decrease in fruit firmness during ripening were inhibited in MaXB3-overexpressing fruits (Fig. 7, E and F). Because MaXB3 mediates the degradation of the MaNAC2, MaACS1, and MaACO1 proteins, accumulations of these proteins, as well as MaNAC1, were detected in MaXB3-overexpressing and control fruits. The results showed that the levels of MaNAC2, MaACS1, and MaACO1 proteins in control fruits increased following ripening, while overexpression of MaXB3 in fruits reduced their accumulations but did not affect MaNAC1 protein accumulation (Fig. 7G). Additionally, the expression of MaACS1, MaACO1, and two cell-wall-modifying genes (MaEXP2 and MaEXP8) was down-regulated, whereas MaERF11 was up-regulated in MaXB3-overexpressing fruits during ripening (Fig. 7H). Taken together, MaXB3 delays banana fruit ripening by negatively impacting ethylene biosynthesis through the degradation of MaNAC2, MaACS1, and MaACO1.
Figure 7.
Effects of transient overexpression of MaXB3 in banana fruits on ripening and ethylene biosynthesis. A, Schematic diagram showing the inoculation of Agrobacterium tumefaciens solution into banana pulp through the distal end of fruits. B, Appearance of banana fruits transiently overexpressing MaXB3 and empty vector (control) during ripening. C, Immunoblotting analysis of bananas inoculated with empty vector and MaXB3-His. Fruit tissues of injection region were used for the protein detection. Total proteins were extracted and probed with an anti-His monoclonal antibody. Actin was used as the loading control. D to F, Color index, fruit firmness, and ethylene production in MaXB3-overexpressing and control banana fruits during ripening. Data represent the means of six biological replicates, and vertical bars represent the se. G, MaNAC1, MaNAC2, MaACS1, and MaACO1 protein accumulation in MaXB3-overexpressing and control banana fruits during ripening. Total proteins in tissue samples from each time point were extracted and immunodetected using the anti-MaNAC1, -MaNAC2, -MaACS1, and -MaACO1 specific polyclonal antibodies, respectively. Actin was used as the loading control. E, Empty vector. M, MaXB3-His. H, Relative mRNA abundance of MaERF11, MaACO1, MaEXP2, MaACS1, and MaEXP8 in MaXB3-overexpressing and control banana fruits during ripening. Data represent the means of three biological replicates, and vertical bars represent the se. Asterisks indicate statistically significant differences as determined by Student’s t test (**P < 0.01).
MaXB3 Delays Fruit Ripening and Down-Regulates Ethylene Biosynthetic and Responsive Gene Expression in Transgenic Tomato
The role of MaXB3 in fruit ripening and ethylene biosynthesis was further confirmed by its stable overexpression in tomato, which is considered the optimal model system for the study of climacteric ripening. Three independent transgenic lines (OE-1, OE-2, and OE-3) that accumulated high levels of MaXB3 transcript and protein were chosen for further investigation (Fig. 8, A and B). As shown in Figure 8A, the differences in fruit ripening between the MaXB3-overexpressing lines and wild type became apparent at 51 d post-anthesis (dpa). A visible color change could be observed at this stage in the wild-type fruit, whereas MaXB3-overexpressing tomatoes remained green. At 54 and 57 dpa, the wild-type fruit had a homogenous orange color and red color, respectively, while fruit from the MaXB3-overexpressing lines were only just beginning to change color. Consistently, ethylene production in MaXB3-overexpressing fruits was obviously reduced compared with that in wild-type tomatoes during ripening (Fig. 8C). Furthermore, the expression at the transcript level of ethylene biosynthetic genes ACS2, ACS4, and ACO1 important for fruit ripening (Qin et al., 2012; Deng et al., 2018) and two known ethylene-responsive genes E4 and E8 (Deng et al., 2018) in MaXB3-overexpressing fruits was significantly down-regulated from 47 to 54 dpa compared to the wild-type tomatoes (Fig. 8D). These data further validate the function of MaXB3 in modulating fruit ripening by repressing ethylene biosynthesis or ethylene responses.
Figure 8.
Overexpression of MaXB3 in tomato delays fruit ripening and impacts ethylene biosynthesis and response. A, Fruit ripening process of wild-type (WT) and MaXB3-overexpressing lines. Fruit at 47, 51, 54, and 57 dpa from wild-type and three MaXB3-overexpressing lines (OE-1, OE-2, and OE-3) are shown. B, RT-PCR and immunoblotting analysis of MaXB3 expression. Pericarp tissues of MaXB3-overexpressing lines and wild type at 47 dpa were used for detection. For immunoblotting analysis, total proteins were extracted and probed with an anti-HA monoclonal antibody. Actin was used as internal reference. C, Ethylene production in fruit of wild-type and MaXB3-overexpressing lines during ripening. D, Relative mRNA abundance of three ethylene biosynthetic genes (ACS2, ACS4, and ACO1) and two genes known to be ethylene responsive (E4 and E8) in fruit of wild type and MaXB3-overexpressing lines during ripening. The gene transcript levels were determined by RT-qPCR using Actin as the reference gene, followed by normalization against the wild type at 47 DPA. Data in C and D represent the means of three biological replicates, and vertical bars represent the se. Asterisks indicate statistically significant differences as determined by Student’s t test (**P < 0.01).
MaNAC1 and MaNAC2 Repress MaXB3 by Directly Binding to Its Promoter
MaXB3 is ethylene- and ripening-inhibited (Supplemental Fig. S5), which raises the possibility that MaXB3, like MaERF11, might also be a target of MaNAC1 and MaNAC2. Further supporting this hypothesis, several cis-elements corresponding to putative NAC binding sites were present in the MaXB3 promoter (Supplemental Text S1). EMSA showed that the GST-MaNAC1-N and -MaNAC2 recombinant proteins were able to bind to the MaXB3 promoter fragment with specificity, and the addition of unlabeled MaXB3 promoter fragment competed for the binding in a dose-dependent manner, whereas unlabeled mutant probes failed to do so (Fig. 9A). Moreover, ChIP-qPCR assay revealed that MaNAC1 and MaNAC2 directly bind to the promoter of MaXB3 in banana fruit pulp (Fig. 9B). Further in vivo analysis by dual-luciferase showed that coexpression of MaNAC1 or MaNAC2 effectively inhibited the expression of the MaXB3 pro-LUC reporter gene (Fig. 9C). All together, the data support the conclusion that MaXB3 transcription is repressed by MaNAC1 and MaNAC2, uncovering a feedback regulatory mechanism in which MaNAC1 and MaNAC2 antagonize MaXB3 to prevent the degradation of MaNAC2, MaACS1, and MaACO1 proteins and to maintain the transcriptional repression of MaERF11 mediated by MaNAC2.
Figure 9.
MaNAC1 and MaNAC2 bind to the promoter of MaXB3 and repress its transcription. A, Binding of MaNAC1 and MaNAC2 to the promoter of MaXB3 in EMSA assays. NACRS and mutated NACRS in the probe are shown at the top. Biotin-labeled DNA probe containing NACRS from the MaXB3 promoter was incubated with GST-MaNAC1-N or -MaNAC2 protein, and the DNA-protein complexes were separated on 6% native polyacrylamide gels. The probe with the mutated NACRS was used to test binding specificity. Triangles indicate increasing amounts (100× and 500×) of unlabeled cold or mutated probes for competition. B, ChIP-qPCR assay for direct binding of MaNAC1 or MaNAC2 protein to the promoter of MaXB3. The promoter structure of MaXB3 is shown in the left and red lines indicate cis-elements of NAC. The underlined fragment was used for ChIP-qPCR. Values are the percentage of DNA fragments that coimmunoprecipitated with anti-MaNAC1 or -MaNAC2 antibodies or nonspecific antibody (anti-IgG) relative to the input DNA. Error bars represent the se of three independent experiments. C, MaNAC1 and MaNAC2 repress the activity of the MaXB3 promoter in a dual luciferase assay. The reporter and effector vectors, as indicated, were cotransformed into N. tabacum ‘BY2’ protoplasts. Data show ratios of LUC to REN. Each value represents the mean ± se of six biological replicates. Asterisks indicate significant differences in values by Student’s t test (**P < 0.01).
DISCUSSION
As a vital fruit-ripening hormone, the biosynthesis and signaling of ethylene must be precisely regulated at both the transcriptional and posttranscriptional levels (Lin et al., 2009; Klee and Giovannoni, 2011; Gapper et al., 2013; Seymour et al., 2013a; Yoon 2015; Lee et al., 2017). So far, ethylene biosynthesis controlled by coordinated transcriptional and posttranslational regulators such as UPS during fruit ripening remains poorly documented. In this work, we provide several lines of evidence to unravel a multilayered regulatory cascade involving MaXB3, MaNACs, MaERF11, and MaACS1/MaACO1, among which MaXB3 mediates the ubiquitination of MaNAC2, MaACS1, and MaACO1 for proteasome degradations, thereby inhibiting ethylene biosynthesis during banana fruit ripening.
Substantial progress has highlighted that ripening-related TFs, such as MADS-box/RIN, NAC/NOR, CNR, and ERF, especially in tomato fruit, act upstream of ethylene-dependent and ethylene-independent pathways (Giovannoni, 2007; Lin et al., 2009; Klee and Giovannoni, 2011; Gapper et al., 2013; Seymour et al., 2013a, 2013b; Giovannoni et al., 2017). The repression of tomato TF SlNAC4 results in decreased ethylene synthesis with reduced fruit ripening, carotenoid accumulation, chlorophyll breakdown, and down-regulation of a variety of ethylene- and ripening-associated genes (Zhu et al., 2014). Recently, a tomato NAC TF, NOR-like1, has been identified as a new positive regulator of fruit ripening, which targets genes involved in ethylene biosynthesis (SlACS2, SlACS4), color formation (SlGgpps2, SlSGR1), and cell wall metabolism (SlPG2a, SlPL, SlCEL2, and SlEXP1; Gao et al., 2018). In our previous work, banana MaERF11 was negatively associated with ethylene biosynthesis via suppressing MaACS1 and MaACO1 transcriptions (Xiao et al., 2013). In this study, we demonstrate that MaNAC1 and MaNAC2 act as transcriptional repressors and directly bind to the MaERF11 promoter to repress its expression (Fig. 1). The Arabidopsis NAC TF SHYG activates ACO5, leading to elevated ethylene formation and waterlogging-induced hyponastic leaf growth (Rauf et al., 2013). Tomato SlNAC1 and NOR-like1 control ethylene biosynthesis and fruit ripening by binding to the promoter of SlACO1, SlACS2, and SlACS4, respectively (Ma et al., 2014; Gao et al., 2018). Thus, present results suggest that MaNAC1 and MaNAC2 might impact ethylene biosynthesis through directly repressing MaERF11. Interestingly, a recent fruitENCODE project proposed that there is an additional loop between the NAC and MADS that enables banana fruit to synthesize ethylene in the presence of ethylene inhibitor 1-MCP after ripening initiation, indicating a transition from autocatalytic to ethylene-independent ripening, which is a unique ripening feature in banana (Lü et al., 2018; Gao et al., 2019). These findings indicate that the control of ethylene biosynthesis during climacteric fruit ripening is more robust and complex than previously thought.
ETO1/EOL2 and XBAT32-mediated UPS in Arabidopsis regulates ethylene production by modulating the stability of type-2 and type-3 ACS proteins (Christians et al., 2009; Prasad et al., 2010; Lyzenga et al., 2012). Joo et al. (2008) showed that MG132 can greatly enhance the stability of ACS6 protein, suggesting the possible involvement of UPS in regulating the stability of type I ACS. However, no E3 ligase has been identified that targets type I ACS and ACO proteins. In this study, we showed that the banana fruit RING E3 ligase MaXB3 could not only interact with type I MaACS1, but also with MaACO1, and regulated MaACS1 and MaACO1 protein stability via UPS (Figs. 5 and 6). Furthermore, transient overexpression of MaXB3 in bananas delayed fruit ripening, which was accompanied by a reduction in the level of MaACS1 and MaACO1 proteins (Fig. 7). Meanwhile stable overexpression of MaXB3 in tomatoes down-regulated three ethylene biosynthetic and two ethylene-responsive genes, thus repressing ethylene biosynthesis (Fig. 8). Together, these observations provide the supporting evidence that MaXB3 is a possible negative regulator of fruit ripening by inhibiting ethylene biosynthesis through the modulation of MaACS1 and MaACO1 protein stability. Besides ubiquitin-proteasome degradation, ACSs are also phosphorylated by MAPKs and CPKs and play a key role in antagonizing ubiquitination and stabilizing ACS proteins (Joo et al., 2008; Yoon, 2015). Contrarily, Tan and Xue (2014) reported that a casein kinase1 (CK1) phosphorylated ACS5 and promoted its ubiquitin-proteasome degradation, thereby negatively regulating ethylene biosynthesis in Arabidopsis. Banana MaACS1 is reported to be phosphorylated by a Ser/Thr protein kinase during fruit ripening (Choudhury et al., 2012); thus, it is interesting to investigate whether phosphorylation of MaACS1 is also antagonistic to its proteasomal degradation.
Several NAC TFs undergo posttranslational modification (Puranik et al., 2012). Arabidopsis RING-type ubiquitin E3 ligase SINAT5 ubiquitinates AtNAC1 for proteasomal degradation, thereby weakening auxin signaling (Xie et al., 2002). The rice NAC TF RIM1 involved in jasmonic acid signaling degrades through the UPS upon jasmonic acid treatment (Yoshii et al., 2010). A tomato ubiquitin ligase termed SEVEN IN ABSENTIA3 (SINA3), a negative regulator of defense signaling, ubiquitinates a defense-related NAC TF SlNAC1 for the UPS-mediated degradation (Huang et al., 2013; Miao et al., 2016). These data indicate that the transcriptional activities of NAC proteins are regulated by UPS-mediated degradation. In this work, we provided evidence showing that the ripening- and ethylene-induced MaNAC2 is degraded by MaXB3 via the UPS (Fig. 4) and consequently suppresses the transcriptional repression of MaERF11 by MaNAC2 (Fig. 3). This further confirms that MaXB3 acts as a negative regulator of ethylene biosynthesis during banana fruit ripening. Similarly, the high Glc-repressed U-box-type E3 ubiquitin ligase MdPUB29 in apple influences ethylene biosynthesis by direct UPS degradation of MdbHLH3 protein during fruit quality formation, establishing the cross talk between ethylene and fruit quality in plants (Hu et al., 2019). Additionally, it is interesting to observe that MaNAC1 and MaNAC2 targeted the MaXB3 promoter and repressed its expression (Fig. 9), demonstrating a feedback regulatory mechanism of MaNAC2 preventing its or ACS and ACO degradations.
Based on our present and previous findings, we propose a working model (Fig. 10) for MaXB3 controlling ethylene biosynthesis during banana fruit ripening. MaXB3targets both MaACS1 and MaACO1 for ubiquitination and degradation to negatively regulate ethylene biosynthesis. MaXB3 also modulates the abundance of MaNAC2, resulting in preventing the MaNAC2-mediated transcriptional repression of MaERF11, thereby leading to the inhibition of MaACS1 and MaACO1 expression. Meanwhile, MaNAC1 and MaNAC2 also directly repress MaXB3 expression to antagonize the degradations of MaNAC2, MaACS1, and MaACO1. Taken together, our findings present new insights on climacteric fruit ripening and establish a multilayered regulatory cascade involving MaXB3, MaNACs, MaERF11, and MaACS1/MaACO1 that modulates ethylene biosynthesis during the climacteric ripening.
Figure 10.
A proposed working model demonstrating MaXB3 represses ethylene biosynthesis during banana fruit ripening through the degradation of MaNAC2, MaACS1, and MaACO1. MaNAC1 and MaNAC2 repress the activity of MaERF11, which binds to the promoters of both MaACS1 and MaACO1 and inhibit their action. MaXB3 targets both MaACS1 and MaACO1 for ubiquitination and degradation to negatively regulate ethylene biosynthesis. MaXB3 also modulates the abundance of MaNAC2, resulting in suppressing the MaNAC2-mediated transcriptional repression of MaERF11, also leading to the inhibition of MaACS1 and MaACO1 transcription. Meanwhile, MaNAC1 and MaNAC2 also directly repress MaXB3 transcription to antagonize the degradation of MaNAC2, MaACS1, and MaACO1. Our findings illustrate a multilayered regulatory cascade involving MaXB3, MaNACs, MaERF11, and MaACS1/MaACO1 that modulates ethylene biosynthesis during the climacteric ripening. Arrows, Positive effect; bar, negative regulation.
MATERIALS AND METHODS
Plant Materials, Treatments, and Growth Conditions
Preclimacteric banana (Musa acuminata, AAA group, ‘Cavendish’) fruit at 75% to 80% maturation obtained from a local commercial plantation near Guangzhou, China, was used for this study. Four postharvest treatments, including a control (natural ripening), ethylene-induced ripening (100 µL L−1 ethylene, 18 h), 1-methylcyclopropene (1-MCP)-delayed ripening (0.5 µL L−1 1-MCP, 18 h), and a combination of 1-MCP with ethylene treatment (1-MCP + ethylene), were performed to create four different ripening conditions, as described previously (Shan et al., 2012). At each sampling time, ethylene production and fruit firmness were recorded, as described by Shan et al. (2012).
Nicotiana benthamiana plants were planted in a growth chamber of 22°C under long-day conditions (16-h light/8-h dark), and 4- to 6-week-old plants were selected for Agrobacterium tumefaciens-mediated transient expression assays.
Tomato (Solanum lycopersicum) ‘Ailsa Craig’ was used for transformation, and seedlings were grown in a standard glasshouse with a 12-h light (28°C)/12-h dark (22°C) cycle.
Gene Expression Analysis
Total RNA was extracted from banana and tomato fruit samples using the hot borate method and the RNeasy plant mini kit (Qiagen), respectively. RT-qPCR was performed using the step one plus a CFX96 touch real-time PCR detection system (Bio-Rad) and GoTaq qPCR master mix kit (Promega). Banana MaRPS2 (ribosomal protein 2; Han et al., 2016) and tomato SlActin (Qin et al., 2012) were adopted as the reference genes.
Purification of Recombinant Proteins and MaNAC1, MaNAC2, MaACS1, MaACO1, or MaXB3-Specific Antibody Production
The N-terminal of MaNAC1 (1–199 amino acids) consisting of the DNA-binding domain and the full-length cDNA fragments of MaNAC2, MaACS1, or MaACO1 were amplified and inserted into pGEX-4T-1 (Amersham Biosciences) to fuse in frame with GST, while MaXB3 was cloned into the pMAL-c2X expression vector (New England Biolabs) to fuse in frame with MBP. These recombinant proteins were expressed in the Escherichia coli strain BM Rosetta (DE3) and induced by isopropyl β-d-1-thiogalactopyranoside. The recombinant proteins were purified using a Pierce GST (Thermo Scientific) or MBP (New England Biolabs) spin purification kit.
To produce anti-MaNAC1, -MaNAC2, -MaACS1, or -MaACO1 antibody, the purified recombinant MaNAC1-N, -MaNAC2, -MaACS1, or -MaACO1 protein was used to immunize rabbits, while anti-MaXB3 antibody was raised in rabbits using polypeptide VADSWGFARF as antigen. Polyclonal antibody was affinity purified from rabbit antisera by Hangzhou HuaAn Biotechnology. In brief, 0.5 mg of the purified fusion protein or polypeptide was injected (subcutaneous) into a rabbit after being emulsified with Freund’s complete adjuvant. Four booster injections were given at a 10-d interval, and the antiserum was collected 10 d after the last injection. The polyclonal antibodies were purified by affinity chromatography (Affi-Gel protein A sepharose) and affinity column using antigens coupled to cyanogen bromide-activated sepharose 4B (GE Healthcare Lifesciences; Alarcon et al., 2019). The specificity of the anti-MaNAC1, -MaNAC2, or -MaXB3 antibody was confirmed by immunoblotting analysis using in vitro-translated recombinant protein and total protein extracts from banana fruit pulp (Supplemental Fig. S10).
Dual-Luciferase Transient Expression Assay
For the analysis of transrepression of MaERF11 and MaXB3 promoter activity by MaNAC1 and MaNAC2, two pGreenII vectors, pGreenII 0800-LUC reporter vector and pGreenII 62-SK effector vector, were used as described by Hellens et al. (2005). The MaERF11 or MaXB3 promoter was cloned into pGreen 0800-LUC reporter vector as described above. MaNAC1 or MaNAC2 was cloned into the pGreenII 62-SK vector to generate the 35S pro-MaNAC1 or -MaNAC2 effector, respectively.
The constructed effector and reporter plasmids were cotransfected into BY2 protoplasts by the polyethylene glycol method as described previously (Shan et al., 2012). The transformed protoplasts were incubated at 23°C for 16 h in dark and then harvested to determine the LUC and REN activity on a Luminoskan Ascent Microplate Luminometer (Thermo Scientific) using the dual luciferase assay kit (Promega).
EMSA
The MaERF11 and MaXB3 promoter fragments used as the probes were amplified with primers and labeled with biotin at the 5′ end. EMSA was performed using a LightShift chemiluminescent EMSA kit (Thermo Scientific), as previously described (Shan et al., 2014). In brief, GST-MaNAC1-N or GST-MaNAC2 protein and biotin-labeled probes were incubated together, while unlabeled cold and mutated probes and the GST protein alone were used as competitors and negative controls, respectively. Biotin-labeled DNA was detected by the chemiluminescence method according to the manufacture’s protocol on a ChemiDoc MP imaging system (Bio-Rad).
ChIP-qPCR Analysis
ChIP-qPCR analysis was performed as described by Fan et al. (2018). In brief, the banana fruit pulp was submerged in 1% (v/v) formaldehyde to cross link genomic DNA and protein. The chromatin was sheared to an average length of 500 bp by sonication. Immunoprecipitation of MaNAC1 or MaNAC2 cross linked DNA was performed using affinity-purified polyclonal antibody of MaNAC1 or MaNAC2 for 12 h at 4°C with rotation. The reaction with preimmune serum IgG or without antibody was used as a mock/negative control. The DNA-protein-antibody complex was captured on Protein A/agarose beads by incubating for 1 h at 4°C. The beads were pelleted and washed sequentially for 10 min at 4°C with low-salt wash buffer, high-salt wash buffer, lithium chloride wash buffer, and tris-ethylenediaminetetraacetic acid buffer, and the immunoprecipitated material was eluted by gently rotating for 15 min at 65°C. Cross linking of immunoprecipitated DNA was reversed by incubation in 0.2 m NaCl at 65°C overnight. After proteinase K treatment, the immunoprecipitated DNA was purified and eluted. The amount of each precipitated DNA fragment was determined by RT-qPCR as described above.
Yeast Two-Hybrid Assay
The yeast two-hybrid assay was performed using the Matchmaker GoldYeast two-hybrid systems (Clontech). The coding sequences of MaNAC1, MaNAC2, MaACS1, MaACO1, and MaXB3 were subcloned into pGBKT7 or pGADT7 vector to fuse with the DNA-binding domain and activation domain, to create the bait and prey, respectively. The bait and prey constructs were cotransformed into yeast (Saccbaromyces cerevisiae) strain Y2H Gold by the lithium acetate method and grown on double dropout supplements medium (minimal media double dropouts, synthetic dropout medium with -Leu/-Trp) for 3 d according to the manufacturer’s protocol. Transformed colonies were plated onto quadruple dropout supplements medium (minimal media quadruple dropouts, synthetic dropout medium with -Leu/-Trp/-Ade/-His), and the possible interaction among MaNAC1, MaNAC2, MaACS1, MaACO1, and MaXB3 was evaluated according to their growth status and the activity of α-galactosidase.
BiFC
For BiFC assay, full-length coding sequences of MaNAC1, MaNAC2, MaACS1, MaACO1, and MaXB3 (without their stop codons) were subcloned into pUC-pSPYNE or pUC-pSPYCE vectors (Walter et al., 2004). Expressions of target genes alone were used as negative controls. The resulting constructs were cotransfected into cv BY2 protoplasts as described above. YFP fluorescence was observed using a florescence microscope (Zeiss Axioskop 2 plus).
Subcellular Localization and Colocalization Assay
To construct vectors for subcellular localization and colocalization assays, the coding regions of MaXB3 and MaNAC2 were amplified and subcloned into the pBI221 vector containing a GFP gene, resulting in MaXB3-GFP and MaNAC2-GFP, respectively. NLS-mCherry was used as the nuclear marker. The coding sequence of mCherry was amplified from NLS-mCherry as template, and the PCR product was inserted into the pUC18 vector to replace the GFP, resulting in the vector pUC18-mCherry. The coding region of MaXB3 was inserted into the pUC18-mCherry to generate MaXB3-mCherry. The fusion constructs and the control GFP and mCherry vectors were cotransformed into Nicotiana tabacum ‘BY2’ suspension culture cell protoplasts. GFP and mCherry fluorescence signals were observed with a fluorescence microscope (Zeiss Axioskop 2 Plus). The excitation wavelengths for GFP and mCherry detection were 488 and 561 nm, respectively.
In Vivo Co-IP and Ubiquitination Assay
The high-level simultaneous expression pEAQ binary vectors (Sainsbury et al., 2009) were used in the assay. To create MaXB3-His and MaNAC1-, MaNAC2-, MaACS1-, or MaACO1-GFP constructs, full-length MaXB3, MaNAC1, MaNAC2, MaACS1, or MaACO1 were obtained by PCR amplification and cloned into pEAQ-HT-His and pEAQ-HT-GFP vectors, respectively. Then the resulting constructs were introduced into A. tumefaciens strain GV3101 and infiltrated into the abaxial side of 4- to 6-week-old N. benthamiana leaves using a 1-mL needleless syringe as described previously (Sainsbury et al., 2009).
In vivo Co-IP and ubiquitination assays were performed as described by Han et al. (2016), Liu et al. (2010), and Hu et al. (2019), respectively. For proteasome inhibition, leaves were infiltrated with 10 μm MG132 (Merck) solution for 12 h before sample collection. Forty-eight hours after infiltration, N. benthamiana leaves were ground in liquid nitrogen. Proteins were extracted using an extraction buffer including protease inhibitor cocktail. N. benthamiana cell debris was pelleted, and the supernatant was incubated with 10 μL of anti-GFP antibody (Abcam) at 4°C overnight to capture the epitope-tagged protein. The second day, 50 μL of protein A agarose beads (Roche) was added. After 4 h of incubation at 4°C, the beads were centrifuged and washed three times using a washing buffer. The immunoprecipitated protein complex was eluted with 40 μL of 2.5× SDS sample buffer, separated by SDS-PAGE, and then subjected to immunoblotting analysis using anti-His antibody (Abcam) and anti-GFP antibody (Abcam) for Co-IP assay, while anti-GFP antibody and anti-ubiquitin antibody (Sigma) were used for examining the ubiquitination of MaNAC2, MaACS1, or MaACO1. Detection was carried out using the chemiluminescent substrate SuperSignalWest Pico (Thermo Scientific) for horseradish peroxidase and imaged on a ChemiDoc MP imaging system (Bio-Rad Laboratories).
In Vitro Ubiquitination Assay
The ubiquitination assay was generally performed as described by Prasad et al. (2010). In brief, reactions (30 μL) containing 50 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 0.05 mm ZnCl2, 1 mm ATP (Sigma-Aldrich), 0.2 mm dithiothreitol, 10 mm phosphocreatine, 0.1 unit of creatine kinase (Sigma-Aldrich), 50 ng of human E1 (Boston Biochem), 250 ng of human E2 (Boston Biochem), 2 mg of ubiquitin (Boston Biochem), 500 ng of MBP-MaXB3, and 500 ng of GST-MaNAC2, -MaACS1, or -MaACO1 were incubated at 30°C for 2 h. Reactions were stopped by adding sample buffer and analyzed by SDS-PAGE followed by immunoblot analysis using anti-GST antibody (Sigma-Aldrich).
In Vivo Proteasomal Degradation Assay
To construct pEAQ-MaNAC2-, MaACS1-, and MaACO1-GFP-LUC vectors, first the coding sequence of the MaNAC2-, MaACS1-, or MaACO1-GFP was cut from the pBI221 containing MaNAC2-, MaACS1-, or MaACO1-GFP, and the corresponding fragments were ligated into pEAQ to generate pEAQ-MaNAC2-, MaACS1-, or MaACO1-GFP. Then the coding region of firefly LUC was amplified from pGreenII 0800-LUC as template and inserted into pEAQ-MaNAC2-, MaACS1-, or MaACO1-GFP, generating pEAQ-MaNAC2-, MaACS1-, or MaACO1-GFP-LUC. The full-length cDNA of MaXB3 without the stop codon was cloned into pEAQ vector, generating pEAQ-MaXB3. Equal volumes of different combinations of the A. tumefaciens strain GV3101 harboring each construct were mixed and coinfiltrated into N. benthamiana leaves.
Leaves were harvested and ground into fine powder in liquid nitrogen. For MG132 treatment, leaves were infiltrated with 10 μm MG132 or distilled water, respectively, and incubated for 12 h before harvesting. The cell-free proteasomal degradation assay was performed as described previously (Zaltsman et al., 2013; García-Cano et al., 2014). In brief, total protein extracts from 12.5 mg of fresh leaf weight, prepared by bead-beating the tissue in 25 μL of degradation/DNase digestion buffer, were incubated at room temperature for 30 min in a final reaction volume of 120 μL. The protein concentration was determined using the Bradford reagent (Bio-Rad Laboratories). Reactions were terminated by boiling in SDS sample buffer and then subjected to SDS-PAGE followed by immunoblot analysis using anti-GFP antibody (Abcam). LUC activity was also measured using the dual luciferase assay kit (Promega).
Transient Overexpression Analysis in Banana Fruit
The coding region of MaXB3 fused with the 6× His tag (GTGATGGTGATGGTGATG) was amplified and subcloned into pCXUN vector under the control of a maize (Zea mays) Ubiquitin promoter (Chen et al., 2009). The recombinant MaXB3-His construct and the vector control pCXUN were transferred to A. tumefaciens strain EHA105. The A. tumefaciens suspension containing MaXB3-His and the control pCXUN was then separately introduced into the mature green banana fruits by injecting it through the distal end of the fruits, as previously described by Xiao et al. (2018). Transformed fruits were treated with 100 µL L−1 ethylene on day 1 after introducing the A. tumefaciens and kept at 22°C and 90% relative humidity for 7 d. Samples were collected on day 0, 1, 3, 5, and 7 following A. tumefaciens introduction for the measurement of color index, fruit firmness, ethylene production, gene expression, and protein accumulation.
Tomato Genetic Transformation
To overexpress MaXB3, its full-length cDNA was inserted into the plant transformation vector pFGC1008-HA containing the Cauliflower mosaic virus 35S promoter. The constructed plasmid was then introduced into the A. tumefaciens strain EHA105 by electroporation. Tomato ‘Ailsa Craig’ plants were used for transformation, as described by Fillatti et al. (1987). Transgenic plants overexpressing the MaXB3 transgene were identified by the transcript and protein levels of MaXB3. Three independent homozygous lines (T1) were used for experiments. Tomato fruits of wild type and MaXB3-overexpression at 47, 51, 54, and 57 dpa were sampled for analysis.
Statistical Analysis
All experiments were performed at least in triplicate. Data were recorded as mean ± ses of three or six independent biological replicates. Statistical differences between samples were analyzed by Student’s t test (P < 0.05 or 0.01).
Primers
All primers designed and used in this study are listed in Supplemental Table S1.
Accession Numbers
Sequence data from this article can be found in the GenBank data libraries (https://www.ncbi.nlm.nih.gov) under the following accession numbers: MaXB3 (XP_009410017), MaNAC1 (XP_00940629), MaNAC2 (XP_009406749), MaACS1 (XP_009397633), MaACO1 (AAR00930), MaERF11 (XP_009412068), MaEXP2 (XP_009384678), MaEXP8 (XP_009388613), SlACS2 (X59139), SlACS4 (M88487), SlACO1 (X58273), SlE4 (S44898), and SlE8 (X13437).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. MaNAC1 and MaNAC2 have transcriptional repression activity.
Supplemental Figure S2. SDS-PAGE gel stained with Coomassie blue demonstrating affinity purification of the recombinant proteins.
Supplemental Figure S3. Structure of MaXB3 and amino acid alignment of MaXB3 and other plant XB3/XB3-like proteins.
Supplemental Figure S4. Phylogenetic analysis of the XB3/XB3-like proteins.
Supplemental Figure S5. Molecular characterization of MaXB3.
Supplemental Figure S6. Transcription and protein levels of MaNAC1, MaNAC2, and MaXB3 during banana fruit development.
Supplemental Figure S7. MaNAC1, MaNAC2, and MaXB3 protein accumulation in banana fruit pulp during natural and ethylene-induced ripening.
Supplemental Figure S8. MaXB3 does not ubiquitinate and mediate MaNAC1 protein degradation.
Supplemental Figure S9. Phylogenetic analysis of ACS proteins.
Supplemental Figure S10. Immunoblot analysis of the specificity of polyclonal antibodies.
Supplemental Table S1. Summary of primers used in this study.
Supplemental Text S1. The cis-elements of NAC TFs in the nucleotide sequences of MaERF11 and MaXB3 promoters.
Acknowledgments
The authors thank Jörg Kudla (Universität Münster), Seiichiro Hasezawa (The University of Tokyo), Shouyi Chen (Chinese Academy of Sciences), Junping Gao (China Agricultural University), and Dr. George P. Lomonossoff (John Innes Centre, Norwich Research Park) for the generous gift of BiFC vectors, N. tabacum BY2 suspension cells, transient expression vectors, and pEAQ vectors, respectively.
Footnotes
This work was supported by the National Key R&D Program of China (grant no. 2016YFD0400100), the National Natural Science Foundation of China (grant nos. 31830071, 31701652, and 31372111), the National Natural Science Foundation of Guangdong Province (grant no. 2017A030310353) and the China Agriculture Research System (grant no. CARS–31–11).
References
- Alexander L, Grierson D(2002) Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J Exp Bot 53: 2039–2055 [DOI] [PubMed] [Google Scholar]
- Alarcon M, Fuentes E, Maldonado X, Mardones C, Palomo I(2019) Methodology of generation and purification of anti-beta 2 glycoprotein I antibodies. MethodsX 6: 986–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapat VA, Trivedi PK, Ghosh A, Sane VA, Ganapathi TR, Nath P(2010) Ripening of fleshy fruit: Molecular insight and the role of ethylene. Biotechnol Adv 28: 94–107 [DOI] [PubMed] [Google Scholar]
- Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, Vierstra RD(2007) The Arabidopsis EIN3 binding F-box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell 19: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Songkumarn P, Liu J, Wang GL(2009) A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol 150: 1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury SR, Roy S, Sengupta DN(2012) A Ser/Thr protein kinase phosphorylates MA-ACS1 (Musa acuminata 1-aminocyclopropane-1-carboxylic acid synthase 1) during banana fruit ripening. Planta 236: 491–511 [DOI] [PubMed] [Google Scholar]
- Christians MJ, Gingerich DJ, Hansen M, Binder BM, Kieber JJ, Vierstra RD(2009) The BTB ubiquitin ligases ETO1, EOL1 and EOL2 act collectively to regulate ethylene biosynthesis in Arabidopsis by controlling type-2 ACC synthase levels. Plant J 57: 332–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Pirrello J, Chen Y, Li N, Zhu S, Chirinos X, Bouzayen M, Liu Y, Liu M(2018) A novel tomato F-box protein, SlEBF3, is involved in tuning ethylene signaling during plant development and climacteric fruit ripening. Plant J 95: 648–658 [DOI] [PubMed] [Google Scholar]
- Dubois M, Van den Broeck L, Inzé D(2018) The pivotal role of ethylene in plant growth. Trends Plant Sci 23: 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elitzur T, Yakir E, Quansah L, Zhangjun F, Vrebalov J, Khayat E, Giovannoni JJ, Friedman H(2016) Banana MaMADS transcription factors are necessary for fruit ripening and molecular tools to promote shelf-life and food security. Plant Physiol 171: 380–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan ZQ, Ba LJ, Shan W, Xiao YY, Lu WJ, Kuang JF, Chen JY(2018) A banana R2R3-MYB transcription factor MaMYB3 is involved in fruit ripening through modulation of starch degradation by repressing starch degradation-related genes and MabHLH6. Plant J 96: 1191–1205 [DOI] [PubMed] [Google Scholar]
- Fillatti JJ, Kiser J, Rose R, Comai L(1987) Efficient transfer of a glyphosate tolerance gene into tomato using a binary Agrobacterium tumefaciens vector. Nat Biotechnol 5: 726–730 [Google Scholar]
- Fujisawa M, Nakano T, Shima Y, Ito Y(2013) A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 25: 371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Wei W, Zhao X, Tan X, Fan Z, Zhang Y, Jing Y, Meng L, Zhu B, Zhu H, et al. (2018) A NAC transcription factor, NOR-like1, is a new positive regulator of tomato fruit ripening. Hortic Res 5: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhu N, Zhu X, Wu M, Jiang CZ, Grierson D, Luo Y, Shen W, Zhong S, Fu DQ, et al. (2019) Diversity and redundancy of the ripening regulatory networks revealed by the fruitENCODE and the new CRISPR/Cas9 CNR and NOR mutants. Hortic Res 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapper NE, McQuinn RP, Giovannoni JJ(2013) Molecular and genetic regulation of fruit ripening. Plant Mol Biol 82: 575–591 [DOI] [PubMed] [Google Scholar]
- García-Cano E, Zaltsman A, Citovsky V(2014) Assaying proteasomal degradation in a cell-free system in plants. J Vis Exp 85: 51293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni J, Nguyen C, Ampofo B, Zhong S, Fei Z(2017) The epigenome and transcriptional dynamics of fruit ripening. Annu Rev Plant Biol 68: 61–84 [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ.(2007) Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol 10: 283–289 [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR(2003) Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Han YC, Kuang JF, Chen JY, Liu XC, Xiao YY, Fu CC, Wang JN, Wu KQ, Lu WJ(2016) Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol 171: 1070–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA(2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher D, Dhakshinamoorthy S, Alexandrov T, Becker M, Bretschneider T, Buerkert A, Crecelius AC, De Waele D, Elsen A, Heckel DG, et al. (2014) Phenalenone-type phytoalexins mediate resistance of banana plants (Musa spp.) to the burrowing nematode Radopholus similis. Proc Natl Acad Sci USA 111: 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DG, Yu JQ, Han PL, Xie XB, Sun CH, Zhang QY, Wang JH, Hao YJ(2019) The regulatory module MdPUB29-MdbHLH3 connects ethylene biosynthesis with fruit quality in apple. New Phytol 221: 1966–1982 [DOI] [PubMed] [Google Scholar]
- Hu HL, Do YY, Huang PL(2012) Expression profiles of a MhCTR1 gene in relation to banana fruit ripening. Plant Physiol Biochem 56: 47–55 [DOI] [PubMed] [Google Scholar]
- Huang W, Miao M, Kud J, Niu X, Ouyang B, Zhang J, Ye Z, Kuhl JC, Liu Y, Xiao F(2013) SlNAC1, a stress-related transcription factor, is fine-tuned on both the transcriptional and the post-translational level. New Phytol 197: 1214–1224 [DOI] [PubMed] [Google Scholar]
- Inaba A, Liu X, Yokotani N, Yamane M, Lu WJ, Nakano R, Kubo Y(2007) Differential feedback regulation of ethylene biosynthesis in pulp and peel tissues of banana fruit. J Exp Bot 58: 1047–1057 [DOI] [PubMed] [Google Scholar]
- Ireland HS, Yao JL, Tomes S, Sutherland PW, Nieuwenhuizen N, Gunaseelan K, Winz RA, David KM, Schaffer RJ(2013) Apple SEPALLATA1/2-like genes control fruit flesh development and ripening. Plant J 73: 1044–1056 [DOI] [PubMed] [Google Scholar]
- Ito Y, Kitagawa M, Ihashi N, Yabe K, Kimbara J, Yasuda J, Ito H, Inakuma T, Hiroi S, Kasumi T(2008) DNA-binding specificity, transcriptional activation potential, and the rin mutation effect for the tomato fruit-ripening regulator RIN. Plant J 55: 212–223 [DOI] [PubMed] [Google Scholar]
- Joo S, Liu Y, Lueth A, Zhang S(2008) MAPK phosphorylation-induced stabilization of ACS6 protein is mediated by the non-catalytic C-terminal domain, which also contains the cis-determinant for rapid degradation by the 26S proteasome pathway. Plant J 54: 129–140 [DOI] [PubMed] [Google Scholar]
- Jourda C, Cardi C, Mbéguié-A-Mbéguié D, Bocs S, Garsmeur O, D’Hont A, Yahiaoui N(2014) Expansion of banana (Musa acuminata) gene families involved in ethylene biosynthesis and signalling after lineage-specific whole-genome duplications. New Phytol 202: 986–1000 [DOI] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ(2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45: 41–59 [DOI] [PubMed] [Google Scholar]
- Kuang J-F, Chen L, Shan W, Yang S, Lu W-J, Chen J-Y(2013) Molecular characterization of two banana ethylene signaling component MaEBFs during fruit ripening. Postharvest Biol Technol 85: 94–101 [Google Scholar]
- Lee HY, Chen YC, Kieber JJ, Yoon GM(2017) Regulation of the turnover of ACC synthases by phytohormones and heterodimerization in Arabidopsis. Plant J 91: 491–504 [DOI] [PubMed] [Google Scholar]
- Li T, Jiang Z, Zhang L, Tan D, Wei Y, Yuan H, Li T, Wang A(2016) Apple (Malus domestica) MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J 88: 735–748 [DOI] [PubMed] [Google Scholar]
- Li T, Xu Y, Zhang L, Ji Y, Tan D, Yuan H, Wang A(2017) The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 29: 1316–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Hong Y, Yin M, Li C, Zhang K, Grierson D(2008) A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J 55: 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Zhong S, Grierson D(2009) Recent advances in ethylene research. J Exp Bot 60: 3311–3336 [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang Y, Tang S, Zhao Q, Zhang Z, Zhang H, Dong L, Guo H, Xie Q(2010) An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana. Plant J 61: 893–903 [DOI] [PubMed] [Google Scholar]
- Liu M, Pirrello J, Chervin C, Roustan JP, Bouzayen M(2015) Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation. Plant Physiol 169: 2380–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shiomi S, Nakatsuka A, Kubo Y, Nakamura R, Inaba A(1999) Characterization of ethylene biosynthesis associated with ripening in banana fruit. Plant Physiol 121: 1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü P, Yu S, Zhu N, Chen YR, Zhou B, Pan Y, Tzeng D, Fabi JP, Argyris J, Garcia-Mas J, et al. (2018) Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat Plants 4: 784–791 [DOI] [PubMed] [Google Scholar]
- Lyzenga WJ, Booth JK, Stone SL(2012) The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1-carboxylate synthase 7. Plant J 71: 23–34 [DOI] [PubMed] [Google Scholar]
- Ma N, Feng H, Meng X, Li D, Yang D, Wu C, Meng Q(2014) Overexpression of tomato SlNAC1 transcription factor alters fruit pigmentation and softening. BMC Plant Biol 14: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbéguié-A-Mbéguié D, Hubert O, Fils-Lycaon B, Chillet M, Baurens FC(2008) EIN3-like gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Grande naine). Physiol Plant 133: 435–448 [DOI] [PubMed] [Google Scholar]
- Miao M, Niu X, Kud J, Du X, Avila J, Devarenne TP, Kuhl JC, Liu Y, Xiao F(2016) The ubiquitin ligase SEVEN IN ABSENTIA (SINA) ubiquitinates a defense-related NAC transcription factor and is involved in defense signaling. New Phytol 211: 138–148 [DOI] [PubMed] [Google Scholar]
- Paul JY, Khanna H, Kleidon J, Hoang P, Geijskes J, Daniells J, Zaplin E, Rosenberg Y, James A, Mlalazi B, et al. (2017) Golden bananas in the field: elevated fruit pro-vitamin A from the expression of a single banana transgene. Plant Biotechnol J 15: 520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P(2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Prasad ME, Schofield A, Lyzenga W, Liu H, Stone SL(2010) Arabidopsis RING E3 ligase XBAT32 regulates lateral root production through its role in ethylene biosynthesis. Plant Physiol 153: 1587–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranik S, Sahu PP, Srivastava PS, Prasad M(2012) NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci 17: 369–381 [DOI] [PubMed] [Google Scholar]
- Qiao H, Chang KN, Yazaki J, Ecker JR(2009) Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev 23: 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Wang Y, Cao B, Wang W, Tian S(2012) Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening. Plant J 70: 243–255 [DOI] [PubMed] [Google Scholar]
- Rauf M, Arif M, Fisahn J, Xue GP, Balazadeh S, Mueller-Roeber B(2013) NAC transcription factor speedy hyponastic growth regulates flooding-induced leaf movement in Arabidopsis. Plant Cell 25: 4941–4955 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sainsbury F, Thuenemann EC, Lomonossoff GP(2009) pEAQ: Versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol J 7: 682–693 [DOI] [PubMed] [Google Scholar]
- Seymour GB, Østergaard L, Chapman NH, Knapp S, Martin C (2013a) Fruit development and ripening. Annu Rev Plant Biol 64: 219–241journal [DOI] [PubMed] [Google Scholar]
- Seymour GB, Chapman NH, Chew BL, Rose JKC(2013b) Regulation of ripening and opportunities for control in tomato and other fruits. Plant Biotechnol J 11: 269–278 [DOI] [PubMed] [Google Scholar]
- Shan W, Kuang JF, Chen L, Xie H, Peng HH, Xiao YY, Li XP, Chen WX, He QG, Chen JY, et al. (2012) Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. J Exp Bot 63: 5171–5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W, Kuang J-F, Lu W-J, Chen J-Y(2014) Banana fruit NAC transcription factor MaNAC1 is a direct target of MaICE1 and involved in cold stress through interacting with MaCBF1. Plant Cell Environ 37: 2116–2127 [DOI] [PubMed] [Google Scholar]
- Tan ST, Xue HW(2014) Casein kinase 1 regulates ethylene synthesis by phosphorylating and promoting the turnover of ACS5. Cell Rep 9: 1692–1702 [DOI] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking C, et al. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang YS, Pi LY, Chen X, Chakrabarty PK, Jiang J, De Leon AL, Liu GZ, Li L, Benny U, Oard J, et al. (2006) Rice XA21 binding protein 3 is a ubiquitin ligase required for full Xa21-mediated disease resistance. Plant Cell 18: 3635–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y-Y, Kuang J-F, Qi X-N, Ye Y-J, Wu Z-X, Chen J-Y, Lu W-J(2018) A comprehensive investigation of starch degradation process and identification of a transcriptional activator MabHLH6 during banana fruit ripening. Plant Biotechnol J 16: 151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YY, Chen JY, Kuang JF, Shan W, Xie H, Jiang YM, Lu WJ(2013) Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J Exp Bot 64: 2499–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Guo HS, Dallman G, Fang S, Weissman AM, Chua NH(2002) SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature 419: 167–170 [DOI] [PubMed] [Google Scholar]
- Xu X, Jiang CZ, Donnelly L, Reid MS(2007) Functional analysis of a RING domain ankyrin repeat protein that is highly expressed during flower senescence. J Exp Bot 58: 3623–3630 [DOI] [PubMed] [Google Scholar]
- Yan SC, Chen JY, Yu WM, Kuang JF, Chen WX, Li XP, Lu WJ(2011) Expression of genes associated with ethylene-signalling pathway in harvested banana fruit in response to temperature and 1-MCP treatment. J Sci Food Agric 91: 650–657 [DOI] [PubMed] [Google Scholar]
- Yang P, Fu H, Walker J, Papa CM, Smalle J, Ju YM, Vierstra RD(2004) Purification of the Arabidopsis 26 S proteasome: Biochemical and molecular analyses revealed the presence of multiple isoforms. J Biol Chem 279: 6401–6413 [DOI] [PubMed] [Google Scholar]
- Yang Y, Wu Y, Pirrello J, Regad F, Bouzayen M, Deng W, Li Z(2010) Silencing Sl-EBF1 and Sl-EBF2 expression causes constitutive ethylene response phenotype, accelerated plant senescence, and fruit ripening in tomato. J Exp Bot 61: 697–708 [DOI] [PubMed] [Google Scholar]
- Yin XR, Allan AC, Chen KS, Ferguson IB(2010) Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol 153: 1280–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon GM.(2015) New insights into the protein turnover regulation in ethylene biosynthesis. Mol Cells 38: 597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii M, Yamazaki M, Rakwal R, Kishi-Kaboshi M, Miyao A, Hirochika H(2010) The NAC transcription factor RIM1 of rice is a new regulator of jasmonate signaling. Plant J 61: 804–815 [DOI] [PubMed] [Google Scholar]
- Zaltsman A, Lacroix B, Gafni Y, Citovsky V(2013) Disassembly of synthetic Agrobacterium T-DNA-protein complexes via the host SCF(VBF) ubiquitin-ligase complex pathway. Proc Natl Acad Sci USA 110: 169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Chen G, Zhou S, Tu Y, Wang Y, Dong T, Hu Z(2014) A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol 55: 119–135 [DOI] [PubMed] [Google Scholar]