A mutation that causes multiple aleurone layers disrupts the scaffolding subunit of a regulatory complex and dysregulates genes involved in cell division, signaling, differentiation, and metabolism.
Abstract
Maize (Zea mays) thick aleurone1 (thk1-R) mutants form multiple aleurone layers in the endosperm and have arrested embryogenesis. Prior studies suggest that thk1 functions downstream of defective kernel1 (dek1) in a regulatory pathway that controls aleurone cell fate and other endosperm traits. The original thk1-R mutant contained an ∼2-Mb multigene deletion, which precluded identification of the causal gene. Here, ethyl methanesulfonate mutagenesis produced additional alleles, and RNA sequencing from developing endosperm was used to identify a candidate gene based on differential expression compared with the wild-type progenitor. Gene editing confirmed the gene identity by producing mutant alleles that failed to complement existing thk1 mutants and that produced multiple-aleurone homozygous phenotypes. Thk1 encodes a homolog of NEGATIVE ON TATA-LESS1, a protein that acts as a scaffold for the CARBON CATABOLITE REPRESSION4-NEGATIVE ON TATA-LESS complex. This complex is highly conserved and essential in all eukaryotes for regulating a wide array of gene expression and cellular activities. Maize also harbors a duplicate locus, thick aleurone-like1, which likely accounts for the ability of thk1 mutants to form viable cells. Transcriptomic analysis indicated that THK1 regulates activities involving cell division, signaling, differentiation, and metabolism. Identification of thk1 provides an important new component of the DEK1 regulatory system that patterns cell fate in endosperm.
Cereal endosperm is a nutrient reserve that fuels seedling growth and development during germination until phototrophy can be established. It is also a critically important source of human food and livestock feed as well as an industrial feedstock. Starchy endosperm cells form the central mass of the endosperm and are the primary storage cell type where starch and storage proteins accumulate. Starchy endosperm cells undergo programmed cell death and are not viable in a mature grain (Young and Gallie, 2000). An epidermal-like layer called aleurone forms from the outer cell layer(s) of the endosperm. The aleurone cells remain viable in mature grains and secrete amylases and proteases into the starchy endosperm during germination to remobilize storage products and support seedling growth. The aleurone is also a key site for mineral storage and an abundant source of fiber, antioxidants, and other dietary benefits.
In most cereals, including most lines of maize (Zea mays), the aleurone is a single cell layer. Barley (Hordeum vulgare) is an exception, with multiple layers of aleurone cells. During development, unknown positional cues specify cells in the surface layer as aleurone (Becraft and Asuncion-Crabb, 2000). Cell identity remains plastic, and when a periclinal division occurs in the aleurone, the inner daughter cell will transdifferentiate to starchy endosperm according to its new position. The defective kernel1 (dek1) gene is required for aleurone cell fate specification in maize (Lid et al., 2002). Kernels mutant for dek1 lack aleurone cells, and the outermost cell layer of the endosperm has starchy endosperm identity instead. Analysis of unstable dek1 mutant alleles demonstrated that gene function is required throughout development for aleurone identity. Loss of dek1 function late in development caused aleurone cells to redifferentiate into starchy endosperm, while restoration of dek1 function in mutant kernels allowed cells on the surface of the endosperm to transdifferentiate from starchy endosperm to aleurone (Becraft and Asuncion-Crabb, 2000).
The dek1 gene encodes a plasma membrane-localized mechanosensitive calcium channel with a cytoplasmic calpain-like protease domain (Lid et al., 2002; Wang et al., 2003; Tran et al., 2017). A hypothesis is that mechanical stress in the surface cells opens the Ca2+ channel, leading to activation of the calpain-like protease, which in turn cleaves regulatory substrates that control cellular processes (Tran et al., 2017), in this case aleurone cell identity. However, to date, no substrates or other downstream factors have been reported. DEK1 proteins are conserved in plants but do not exist in other kingdoms (Zhao et al., 2012).
The thick aleurone1 (thk1) gene is also involved in patterning maize endosperm cell fate. Kernels mutant for thk1 have multiple layers of aleurone cells, indicating that the thk1 gene is required to block aleurone fate or promote starchy endosperm fate in cells internal from the surface (Yi et al., 2011). The original thk1-R mutant allele was caused by an ∼2-Mb multigene deletion, which precluded identifying the causal gene. Interestingly, thk1 mutants are epistatic to dek1; double mutants had multiple layers of aleurone cells. This suggests that the thk1 gene product likely functions downstream of DEK1.
The CARBON CATABOLITE REPRESSION4-NEGATIVE ON TATA-LESS (CCR4-NOT) complex is a multifunctional protein complex that is essential for cell viability in eukaryotes. It regulates many aspects of gene expression, including chromatin modification, transcription initiation and elongation, mRNA quality control and export, mRNA turnover, protein translation, and microRNA-mediated mRNA decay (Collart, 2016; Collart and Panasenko, 2017). The composition of this complex varies among species, but there are seven highly conserved subunits considered core subunits (Collart and Panasenko, 2017). The CCR4 and CCR4-ASSOCIATED FACTOR (CAF1) core subunits are each deadenylases that remove poly(A) tails to initiate mRNA degradation (Tucker et al., 2001). The NOT4 subunit is a RING domain E3 ubiquitin ligase that polyubiquitinates protein substrates, targeting them for degradation (Albert et al., 2002). The NOT1 subunit serves as a scaffold protein to which all the other subunits of the complex bind, either directly or indirectly (Collart, 2016). Other core subunits are believed to function largely as docking proteins that recruit various cellular proteins to the CCR4-NOT complex (Collart and Panasenko, 2017). There are also variable numbers of species-specific subunits that have been described primarily in yeast (Saccharomyces cerevisiae), Drosophila melanogaster, and humans (Homo sapiens; Collart, 2016).
Biologically, the CCR4-NOT complex, or specific subunits thereof, regulates many diverse processes, including energy metabolism and cellular homeostasis (Shirai et al., 2014), autophagy (Yamaguchi et al., 2018), embryonic patterning (Fujino et al., 2018), stem cell maintenance and fate (Yamaji et al., 2017), tumor suppression (Vicente et al., 2018), and fungal pathogenicity (Dai et al., 2016). Despite the ubiquitous importance and wide-ranging functions of the CCR4-NOT complex, there have been few reports in plants.
CCR4-NOT complexes were analyzed in Arabidopsis (Arabidopsis thaliana) using epitope-tagged AtCCR4b to pull down complexes that were analyzed by mass spectrometry. All but one of the ubiquitous core subunits were identified, including CAF1, CCR4, NOT1, NOT2, NOT3, and NOT9 (CAF40). The NOT4 subunit was not reported. Additionally, NOT10 and NOT11 were identified. Some of the identified subunits are encoded by multigene families, suggesting that different isoforms may have unique functions and that the CCR4-NOT complex may be composed of different subunits in different contexts within an individual organism. Eleven genes encode CAF1 proteins (AtCAF1a to AtCAF1k), and seven genes encode CCR4 subunits (AtCCR4a to AtCCR4g; Walley et al., 2010; Arae et al., 2019).
The AtCAF1a and AtCAF1b genes are inducible by mechanical wounding, biotic stress, and stress hormones, regulate gene expression in response to pathogen infection and wounding, and promote resistance to pathogen and abiotic stresses (Walley et al., 2007; Liang et al., 2009). The wound-inducible gene sets regulated by AtCAF1a and AtCAF1b were largely distinct, and the genes showed differential effects on conferring salt tolerance. Furthermore, the 11 CAF1 genes encoded by the Arabidopsis genome showed differential responses to wounding (Walley et al., 2010). Rice (Oryza sativa) contains 18 OsCAF1 genes (Arae et al., 2019), which show tissue-specific expression patterns, differential responses to abiotic stresses, and differential subcellular localization (Chou et al., 2014). These results support the hypothesis that different subunit isoforms have different functions.
In Arabidopsis, AtNOT1 is required for the normal spatial organization of male gametophytes. Mutant pollen failed to germinate, and mutant alleles were not transmitted through the male (Motomura et al., 2020). Mutant alleles also showed reduced transmission through the female, and a portion of female gametophytes showed defects in cell type specification and compromised ability to attract pollen tubes. Fertilized ovules often aborted with arrested embryos. Transcriptome analysis suggested that AtNOT1 is important for pollen maturation.
Here, we report that maize thk1, located on chromosome 1, encodes a NOT1 homolog. A duplicated region of chromosome 9 contains a second locus, thick aleurone-like1 (thk-like1), that appears to have partially subfunctionalized. Transcriptomic analysis supports the phenotypic interpretation that Thk1+ is a negative regulator of aleurone cell fate and suggests that cell division and differentiation processes may be key targets of regulation.
RESULTS
Identification of the thk1 Gene
The original thk1-R mutation was an ∼2-Mb deletion that encompassed dozens of genes on chromosome 1 (Yi et al., 2011). This precluded identifying the causal gene with standard mapping methods and left open the possibility that the mutant phenotype was due to a combination of multiple genes lost in the deletion. To address these issues, an ethyl methanesulfonate (EMS) mutagenesis was undertaken. A total of 8,000 M1 plants were screened as described in “Materials and Methods,” and three independent new alleles were generated. PCR marker assays indicated they all contained genomic DNA in the region that was missing in the original allele. Each of the new alleles showed phenotypes indistinguishable from that described for the thk1-R mutant, which, combined with additional alleles described below, indicates that the thick aleurone and arrested embryo phenotypes are most likely due to pleiotropy caused by loss of function of a single gene.
To identify a candidate gene(s), RNA sequencing (RNA-seq) was performed to identify genes that mapped within the chromosomal region of the thk1-R deletion and that showed altered expression and/or sequence polymorphisms in each of two new EMS alleles, thk1-iso15 and thk1-iso17, compared with the wild-type progenitor. RNA was isolated from endosperms dissected at 19 d after pollination (DAP), and RNA-seq was performed on three biological replicates of each genotype. A total of 434,256,513 reads were mapped to the B73v4 reference genome (Jiao et al., 2017), with an average of 48,250,724 reads per replicate. Fifty-six gene models were identified within the 2-Mb thk1-R region (Supplemental Data Set S1). Differential gene expression analysis and single-nucleotide polymorphism (SNP) calling were performed as described in “Materials and Methods.” Four genes contained SNPs in either thk1-iso15 or thk1-iso17, but not both, and none of these genes showed any expression differences. Five genes showed differential expression in both thk1-iso15 and thk1-iso17 (Supplemental Table S1). One, Zm00001d027278, showed a particularly interesting pattern of differential expression in both alleles, with a strikingly greater fold difference in reads mapping to 5′ exons than to 3′ exons (Fig. 1, A and B). These 5′ exons showed the greatest fold change of any differentially expressed gene (DEG) within the 2-Mb region.
Figure 1.

Identification of the thk1 gene. A, RNA-seq reads show differential expression between EMS mutant alleles and the wild-type progenitor at Zm00001d27278. B, 5′ exons show greater differential expression than 3′ exons. Tukey’s honestly significant difference test was used in the analysis, and the asterisks indicate that read counts of B73 are significantly greater than those of iso15 and iso17 (*P < 0.05). Error bars represent se. C, DNA sequences and encoded amino acids at the guide RNA (gRNA) target site in the wild type (WT) and two edited thk1 alleles. D and E, Histological sections of wild-type and edited thk1 kernels, respectively, segregating on a self-pollinated ear from a heterozygous plant. The arrow in D indicates the normal single layer of aleurone cells; the bracket in E denotes the multiple layers in the edited thk1 mutant. Bars = 200 μm.
Public transposon collections were searched for available insertion mutants, and UFMu-00684:mu1065075 was identified near the Zm00001d027278 locus in the UniformMu collection (Settles et al., 2007). This insertion was annotated as located on chromosome 8, but PCR genotyping (Supplemental Table S2) and sequencing confirmed the presence of a Mu13 insertion in the 5′ untranslated region, 97 bp upstream of the ATG start codon of Zm00001d027278_T001. When heterozygotes were crossed with the thk1-R mutant, the resultant ears contained segregated kernels with typical thk1 mutant phenotypes. Thus, the thk1-Mu allele failed to complement the thk1-R mutant, indicating that they are likely allelic. However, no mutant phenotype was observed when plants harboring this Mu13 insertion allele were self-pollinated, possibly because thk1-Mu is a hypomorphic mutant.
To further test the identity of the thk1 gene, a clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein9 (Cas9) gene-editing construct was designed to target exon 3 of the Zm00001d027278_T001 gene model and transformed into Hi-II. Of 13 independent transformation events, three produced edits. Events 1 and 8 each produced only a single plantlet containing an edit (out of eight or 10, respectively), whereas for event 13, all seven regenerated plantlets contained edits. Interestingly, each plantlet contained different edits and some leaf samples contained as many as four different alleles, suggesting that the CRISPR/Cas9 system was active during callus growth and plantlet regeneration. Two lines containing independent editing events, thk1-B4 and thk1-CH4, were selected for further analysis. These contained a 1-bp insertion and a 4-bp deletion, respectively, each of which caused a frame shift and premature stop codon in exon 3. The predicted proteins encoded by each edited allele are 181 amino acids for thk1-B4 and 179 amino acids for thk1-CH4, whereas the predicted full-length protein is 2,454 amino acids (Fig. 1C).
Heterozygous plants harboring each edit were crossed with wild-type inbred line A636, and F1 grains showed normal phenotypes. Upon self-pollination, the resultant ears contained segregated homozygous kernels that displayed characteristic thk1 mutant phenotypes (Fig. 1, D and E; Supplemental Fig. S1), whereas self-pollinating F1 plants that did not inherit the edits showed only normal kernels. To test allelism, plants containing each of these edits were crossed to thk1-iso17/+ plants. F1 ears segregated kernels showing the thk1 mutant phenotype (Supplemental Fig. S1), demonstrating that the new gene edits fail to complement, and are therefore allelic to, known thk1 mutant alleles. This confirms that the thk1 gene indeed corresponds to gene model Zm00001d027278. The RESPONSIVE TO ABSCISIC ACID17-YELLOW FLUORESCENT PROTEIN (RAB17–YFP) aleurone marker (Gontarek et al., 2016) was introduced into a thk1-B4 line and mutant kernels showed multiple layers of YFP-fluorescing cells (Supplemental Fig. S1), demonstrating the identity of the multiple cell layers as aleurone.
The thk1 Gene Encodes a NOT1 Homolog with a Syntelogous Duplication
As annotated at EnsemblePlants (https://plants.ensembl.org/Zea_mays/Info/Index), Zm00001d027278, the thk1 locus, encompasses 40,719 bp on chromosome 1, from 1,492,439 to 1,533,158 (Fig. 2A). It is a complex locus with 68 alternative transcripts annotated. Transcript _T001 contains 51 exons, produces an mRNA of 7,741 nucleotides (coding region of 7,365), and encodes a predicted protein of 2,454 amino acids. This protein contains highly conserved domains throughout its length (Fig. 2B; Table 1); thus, we conclude that transcript _T001 is likely to be functional. InterPro (https://www.ebi.ac.uk/interpro/) analysis identified this protein as a member of the IPR040398 protein family, CCR4-NOT complex subunit 1 (NOT1). As described, NOT1 serves as a scaffold for other subunits of the CCR4-NOT complex. NOT1 functional domains, as defined by UniProt (A0A1D6JJP2), are shown in Figure 2B, Table 1, and Supplemental Figure S2.
Figure 2.
Maize contains two loci encoding NOT1 subunits. A, Structures of the thk1 and thkl1 loci. Gene models are from EnsemblePlants (https://plants.ensembl.org/Zea_mays/Info/Index). Black boxes represent coding exons, white boxes are noncoding exons, and lines between boxes are introns. B, NOT1 signature domains in THK1 and THKL1. C, Molecular phylogeny of NOT1 proteins. The evolutionary tree was inferred using the maximum likelihood method and is drawn to scale, with branch lengths measured in the number of substitutions per site (Jones et al., 1992). Species and accessions are listed in Supplemental Table S3.
Table 1. NOT1 signature domains in THK1 and THKL1 proteins.
| InterPro Domain | Motif Name | Function | THK1 Residues | THKL1 Residues |
|---|---|---|---|---|
| IPR032194 | HEAT repeat | NOT10 binding (Basquin et al., 2012; Bawankar et al., 2013) | 473–617 | 470–615 |
| IPR032193 | TTP-binding domain | CAF1 binding (Fabian et al., 2013) | 653–820 | 652–826 |
| IPR032191 | MIF4G | CAF1 binding (Petit et al., 2012) | 975–1,194 | 982–1,201 |
| IPR024557 | DUF3819 | CAF40 binding (Mathys et al., 2014) | 1,325–1,465 | 1,332–1,453 |
| IPR007196 | NOT1 C terminus | NOT2/NOT5 binding (Bhaskar et al., 2013) | 2,062–2,435 | 2,149–2,522 |
| NOT4 binding (Bhaskar et al., 2015) |
The thk1 locus is in a region of chromosome 1 with a syntenic duplication on chromosome 9 (Schnable et al., 2009), containing a second NOT1-homologous locus, Zm00001d048546 (Fig. 2A). This locus, hereafter called thkl1, occupies 59,560 bases, 158,405,008 to 158,464,567, on the reverse strand and is similarly complex with 63 annotated transcripts. The longest, _T008, is 8,156 nucleotides, has 52 exons, and encodes a predicted protein of 2,541 amino acids that also contains the signature NOT1 motifs throughout its length (Fig. 2B; Table 1; Supplemental Fig. S2).
The two loci share several structural characteristics (Fig. 2A). They both have large numbers of introns and exons with complex splicing patterns. Each has a large intron just over 15 kb and one or more additional introns of 6 to 8 kb. Beyond that, there is little obvious similarity, and the thkl1 locus is nearly 50% larger than thk1.
Despite the variations in gene structures, the THK1 and THKL1 protein sequences were collinear over most of their lengths and show a high level of conservation with the exception of several insertions/deletions in one relative to the other (Supplemental Fig. S3). The N-terminal half is most highly conserved, with 88% identity up to residue 1,445 on THK1 (1,452 on THKL1). Here, there is an insertion of 166 amino acids in THKL1. From THK1 residue 1,446 (1,619 of THKL1) to the C termini there is 79% identity and several insertions in the THK1 protein relative to THKL1. These include 42 amino acids at 1,616 to 1,657 and 35 amino acids at 1,980 to 2,014. Given the transcript complexity, it is unclear whether the amino acids apparently inserted into the predicted THK1 and THKL1 proteins are functional or are artifacts of the gene model predictions.
Gene expression atlases indicate that each of these genes is widely expressed in nearly all tissues examined (Stelpflug et al., 2016; Hoopes et al., 2019). The qTeller tool was used to compile publicly available expression data, and on average, thk1 transcript levels were 2.2 times as high as thkl1, ranging from 0.4 in mature pollen to 10.9 in leaf primordia (Supplemental Fig. S4; Supplemental Data Set S2).
NOT1 protein sequences representing a range of eukaryotic diversity (Supplemental Table S3) were subjected to multiple sequence alignment (Supplemental Fig. S2). Each maize protein contains all the highly conserved signature domains (Table 1; Supplemental Fig. S2). Each also contains unique sequences. THKL1 contains insertions of 166 amino acids (1,453–1,618) near the C-terminal end of the DUF3819 domain and 14 amino acids (694–707) in the TTP-binding domain. THK1 has an insertion of 35 residues (1,979–2,013) in the center of the NOT1 domain. THKL1 also harbors a gap of 49 residues following Lys-1947, which includes about half moderately conserved residues and half variable positions.
The aligned proteins were subjected to phylogenetic analysis, and the maximum likelihood tree is shown in Figure 2C. The duplicated thk1 and thkl1 genes arose recently in the maize lineage, due to the recent whole-genome duplication about 5 million years ago (Swigoňová et al., 2004). The relationship to NOT1 proteins of other species largely follows phylogenetic relationships among taxa, particularly within the Plantae. The most closely related protein is from sorghum (Sorghum bicolor), which diverged from maize about 12 million years ago (Swigoňová et al., 2004), followed by other grasses, then dicots, and Selaginella spp. and Physcomitrella sp. Interestingly, sorghum and rice each contains a second locus encoding predicted NOT1 proteins. In each case, the syntenic loci (SORBI_3001G542400 and rice LOC4331296; Buell et al., 2005) were more similar to maize than the nonsyntenic loci (SORBI_3001G299700 and Os10g0556700).
Thk1 Regulates Genes for Cell Division, Signaling, Differentiation, and Metabolism
To explore the effect of THK1 on gene expression in developing endosperm, RNA-seq data were analyzed from two independent thk1 mutant alleles, thk1-iso15 and thk1-iso17. Principal component analysis showed separation between the B73 progenitor and the two thk1 mutant transcriptomes, whereas the two thk1 mutants showed a similar position along the PC1 axis (Supplemental Fig. S5A). DEGs were defined as log2 fold change > 1 and adjusted P < 0.05. Of 31,952 expressed genes, 1,599 (5%) were differentially expressed between B73 and both thk1 mutants. Another 1,794 were differentially expressed with one allele but not the other: 973 for thk1-iso15 and 821 for thk1-iso17 (Fig. 3A). The overlapping DEGs showed high correlation (R2 = 0.9513, P < 0.0001) between the two mutant alleles, and a strong correlation extended to the entire set of all DEGs (Supplemental Fig. S5, B and C), consistent with MA plots that showed consistent distributions of DEGs between both alleles (Supplemental Fig. S5D). This indicated that the two alleles caused similar effects on gene expression, and subsequent analyses focused on the DEGs that were significant in both thk1 alleles.
Figure 3.
Transcriptomic profiling of thk1. A, DEG comparisons among the three data sets, thk1-iso15, thk1-iso17, and the wild-type progenitor. B, Overlap among thk1 DEGs and endosperm tissue-specific genes detected by a hypergeometric test (Zhan et al., 2015). There was overrepresentation of thk1 up-regulated genes in the aleurone-specific gene coexpression module. The top number in each cell represents the number of overlapping genes, and the bottom number in parentheses is the corresponding P value. The color key represents the level of significance, where red and blue represent high and low levels, respectively. AL, Aleurone; BETL, basal endosperm transfer layer; CSE, central starchy endosperm; CZ, conducting zone; ESR, embryo-surrounding region.
The relationship between thk1 DEGs and tissue-specific genes previously identified from 8-DAP endosperm (Zhan et al., 2015) was examined with a hypergeometric test. As shown in Figure 3B, there was significant overrepresentation of aleurone-specific genes among the thk1 up-regulated DEGs, with 95 genes contained in the set (Supplemental Data Set S3). This is consistent with the mutant phenotype and the interpretation of Thk1+ wild-type gene function as a negative regulator of aleurone development (Yi et al., 2011). Gene Ontology (GO) enrichment analysis of the 95 thk1 up-regulated aleurone-specific genes identified two terms: (1) transcription factor (TF) activity (GO:0003700; false discovery rate [FDR] 0.018), with 14 TF genes (Supplemental Table S4), and (2) cytokinesis by cell plate formation (GO:0000911; FDR 0.0061), with seven cytokinesis-related genes (Supplemental Table S5). There were also lesser, albeit significant, overlaps among basal endosperm transfer layer- and embryo-surrounding region-specific and thk1 up-regulated genes. The meaning of these is not clear because there did not appear to be an obvious phenotypic effect of thk1 mutants on the basal endosperm transfer layer.
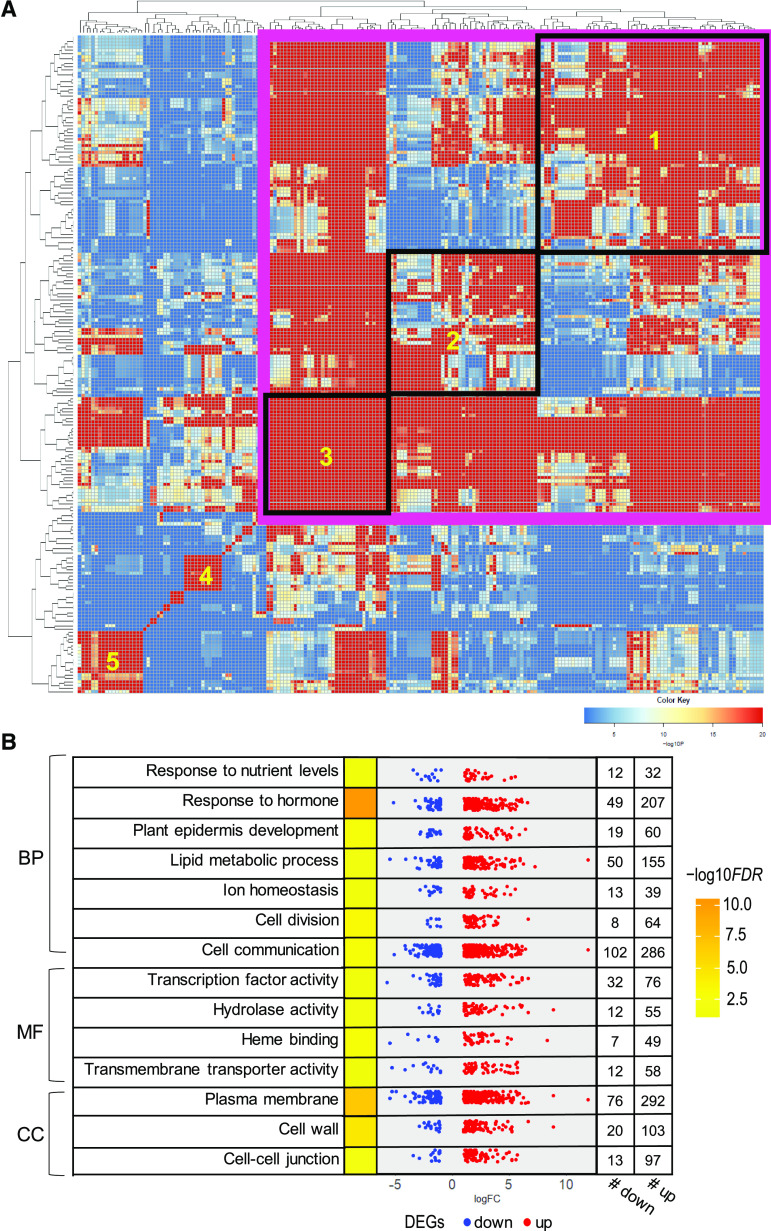
Considering the full complement of DEGs common to both alleles, 234 GO terms were significantly enriched (FDR < 0.05), with 200 terms of biological process, 13 of molecular function, and 21 of cellular component (Supplemental Data Set S4). A hypergeometric test (Zhan et al., 2015) collapsed the terms into several major clusters of related biological processes (Fig. 4A). Group 1 contained terms related to cell cycle, cell division, cell differentiation, and tissue development. Group 2 consisted of responses to hormones and nutrients. Group 3 contained cell signaling, cell communication, and response to stress. Together, these can be considered as a super group related to cell signaling, growth, division, and differentiation. Additional smaller clusters of interest included ion homeostasis as well as hormone and lipid biosynthesis (groups 4 and 5, respectively). Enriched molecular functions sorted into five small groups (fatty acid elongase activity, hydrolase activity, active transmembrane transporter activity, TF activity, and heme binding), and there was one large cluster for cellular component (cell wall, cell membrane, cell-cell junction, and extracellular region; Supplemental Fig. S6).
Figure 4.
GO enrichment analysis of thk1 function. A, Correlation in gene content among GO terms reveals related biological processes. Group 1, Cell cycle, cell division, cell differentiation, and tissue development; group 2, hormone and nutrient response; group 3, signaling, cell communication, and response to stress; group 4, ion homeostasis; group 5, hormone and lipid biosynthesis. The pink box including groups 1 to 3 represents a supergroup related to cellular development, including cell signaling, growth, division, and differentiation. B, Higher proportion of up-regulated genes versus down-regulated genes in selected GO terms. BP, Biological process; CC, cellular component; MF, molecular function.
Of the 1,599 DEGs common to both thk1 alleles, 1,194 showed increased expression in the thk1 mutants while 405 showed decreased expression. The trend of more genes showing increased expression extended to the full complement of all DEGs in either mutant. Within GO terms, there was also a strong propensity for greater proportions of elevated DEGs than decreased expression (Fig. 4B). None of the enriched GO terms contained more down-regulated genes than up-regulated genes. Overall, the function of THK1 appears to be primarily a negative regulator of specific transcript levels as well as biological processes.
Given the strict pattern of cell division in normal aleurone (Becraft and Asuncion-Crabb, 2000) and the nature of the thk1 mutant phenotype, the cell division GO term was of particular interest. Seventy-two DEGs were identified, including cyclins and other cell cycle genes (Supplemental Data Set S3). These include up-regulated genes encoding a D-type cyclin associated with G1-to-S transition (Oakenfull et al., 2002), three A-type cyclins associated with S-to-G2 and G2-to-M transitions (Renaudin et al., 1996; Sabelli et al., 2005a; Wang and Ruan, 2013), five B-type cyclins associated with G2-to-M transition (Renaudin et al., 1994, 1996; Criqui et al., 2000), and CELL DIVISION CYCLE201 (CDC201), a cofactor for the anaphase-promoting complex (Niu et al., 2015). There was also down-regulation of the gene for RETINOBLASTOMA-RELATED PROTEIN3 (RBR3), which inhibits entry into S phase. Taken together, these results suggest that THK1 acts as a negative regulator of the cell cycle (Supplemental Fig. S7).
To study the effect of thk1 mutants on metabolism, DEGs were mapped onto metabolic pathways using CornCyc9.0. Multiple metabolic pathways were predicted to be altered in the mutants, including fatty acids, amino acids, carbohydrates, and hormones (Table 2; Supplemental Fig. S7; Supplemental Data Set S3). In particular, very-long-chain fatty acid biosynthesis and starch degradation are up-regulated, whereas starch biosynthesis is down-regulated, in thk1 mutants (Table 2). Several key genes involved in cytokinin biosynthesis were up-regulated (cis-zeatin O-glucosyltransferase1 [czog1], czog2, and lonely guy7), while the auxin biosynthetic gene defective endosperm18, encoding an endosperm-specific YUCCA1 protein (Bernardi et al., 2012), was down-regulated, suggesting that THK1 might regulate hormone balance.
Table 2. Metabolic pathways associated with DEGs.
Dashes indicate no data.
| Category | Up-Regulated Pathways | Down-Regulated Pathways |
|---|---|---|
| Fatty acid and lipid biosynthesis | (1) Superpathway of fatty acid biosynthesis II (plant) | Superpathway of phosphatidylcholine biosynthesis |
| (2) Very-long-chain fatty acid biosynthesis I and II | ||
| (3) Phospholipid remodeling | ||
| Fatty acid and lipid degradation | (1) Triglyceride degradation | (1) Phosphatidylcholine degradation |
| (2) Fatty acid α-oxidation I | (2) Ceramide degradation | |
| (3) Phosphatidylcholine degradation | ||
| Amino acid biosynthesis | Leu, Asn, Gln, Pro, | Ile, Arg, Gly, Ala |
| Amino acid degradation | Val, Leu, Pro, Glu, Gln, Lys | Ala |
| Sugar transport | Suc, hexose | – |
| Carbohydrate biosynthesis | Stachyose, xyloglucan, Suc, trehalose | Suc, starch, trehalose, |
| Carbohydrate degradation | Starch, Suc, chitin, stachyose | Xylopyranose, Suc |
| Hormone biosynthesis | Jasmonic acid, gibberellin12, ethylene, cytokinin | Indole-3-acetate |
| Hormone degradation | Cytokinin, brassinosteroid inactivation, abscisic acid, gibberellin inactivation | – |
DISCUSSION
Here, we report that maize thk1 encodes a homolog of NOT1, an essential scaffolding protein for the CCR4-NOT complex. In spite of the many diverse aspects of gene expression and cellular activity regulated by the CCR4-NOT complex, it has gone undetected in prior forward genetic analyses in plants. Redundancy among gene family members for various subunits may provide a partial explanation. For example, mutants of individual not2 genes in Arabidopsis did not show obvious phenotypes but not2a;not2b double mutants disrupted both male and female gametogenesis and microRNA biogenesis (Wang et al., 2013). The essential nature of the CCR4-NOT complex might also contribute to the lack of mutants identified in forward genetic screens. The NOT1 protein is essential for cell viability in most eukaryotes (Collart, 2016), and not1 transfer DNA alleles could not be made homozygous in Arabidopsis because they failed to transmit through the pollen (Motomura et al., 2020). Interestingly, the mutant alleles could transmit through the female gametophyte, albeit at reduced frequency. Several possible explanations for this surprising result include that residual maternal NOT1 function might be sufficient to support embryo sac development, that essential NOT1 functions might be compensated by alternative gene products, or that cellular processes in developing embryo sacs might not be dependent on NOT1 functions.
The ability of thk1 mutants, including deletion alleles, to transmit through the haploid gametophytes, and of homozygous mutants to produce viable endosperm cells, is likely due to the duplicate thkl1 gene providing essential functions. The viability of endosperm and gametophyte cells in the thk1-R deletion mutant suggests that the two genes are partially redundant, while the mutant phenotype indicates that they have subfunctionalized distinct developmental roles in endosperm, embryogenesis, and adult plant organs (Yi et al., 2011). An interesting future direction will be to study the subunit composition and functions of each complex. None of the known CCR4 or NOT subunit genes were differentially expressed in the thk1 mutants, indicating that THK1 does not regulate the expression of its binding partners.
The maize CCR4-NOT regulatory system has tremendous potential for complexity and functional diversity. Having duplicate NOT1 genes is unusual (Fig. 2) and provides potential for functional diversification. The thk1 and thkl1 loci show similarly complex structures with many exons and several large introns, and each locus is annotated with over 60 potential splice isoforms. While it remains to be determined how many are biologically relevant, the human CNOT1 gene is similarly complex and produces at least 24 different mRNA isoforms involving alternative transcription start sites and alternative splicing (Thierry-Mieg and Thierry-Mieg, 2006). Multigene families for several CCR4-NOT subunits (Arae et al., 2019) further indicate that multiple forms of the complex exist, which multiplies the potential complexity.
Loss-of-function thk1 mutants produce extra aleurone layers, indicating that the normal gene functions in cell patterning by restricting aleurone differentiation to the outermost cell layer (Yi et al., 2011). Arabidopsis mutants showed defects in cell patterning and cell fate specification in pollen and embryo sacs (Motomura et al., 2020), suggesting that CCR4-NOT functions might be required for these processes throughout angiosperms. Transcriptomic data showed an abundance of aleurone-associated transcripts in the thk1 mutant samples, including aleurone-specific TFs, genes involved in cytokinesis, and genes involved in lipid metabolism. In contrast, the starch biosynthetic pathway, prototypical of starchy endosperm, was down-regulated. This is consistent with the interpretation that normal Thk1+ negatively regulates aleurone formation. Also, genes associated with the plant epidermis were enriched, which supports a relationship between aleurone and the shoot epidermis that was proposed based on the propensity for mutants that disrupt the aleurone to also affect the shoot epidermis (Becraft and Yi, 2011).
Genes associated with cell differentiation, cell cycle or cell division, and cell communication were enriched, as might be expected for a mutant that alters cell patterning. Cell cycle genes misregulated in the mutant suggest that THK1 normally functions as a negative regulator of cell division. This would fit with the normal pattern of endosperm development, where frequent cell divisions in the aleurone maintain a large surface of small cells while cells in the starchy endosperm transition to growth mode and begin accumulating storage products. In addition, RBR3 is associated with the onset of endoreduplication, which would also be consistent with the transition to starchy endosperm cell identity (Sabelli et al., 2005b). Overexpression of the pepper (Capsicum annuum) CAF1 subunit in tomato (Solanum lycopersicum) induced the expression of cell cycle genes and enhanced cell enlargement (Sarowar et al., 2007). In yeast, CCR4-NOT directly regulates a cell cycle-regulated protein kinase, DUMBELL FORMATION2, through binding to the complex (Liu et al., 1997), while in human cells, it can inhibit cell division and arrest the cell cycle at G1 (Yi et al., 2012). In plants, it is unknown whether CCR4-NOT directly or indirectly regulates the cell cycle.
Factors known to regulate aleurone development include DEK1 (Lid et al., 2002), CRINKLY4 (CR4; Becraft et al., 1996), SUPERNUMERARY ALEURONE LAYERS1 (Shen et al., 2003), NAKED ENDOSPERM1 (NKD1) and NKD2 (Yi et al., 2015; Gontarek et al., 2016), and DNA-BINDING ONE ZINC FINGER3 (Qi et al., 2017) in maize as well as RICESEED BASIC-ZIPPER1, RICE PROLAMINE BINDING FACTOR (Kawakatsu et al., 2009), and REPRESSOR OF SILENCING1 (Liu et al., 2018) in rice. Mutants of dek1 or cr4 are associated with loss of aleurone cell fate, whereas the rest are associated with increased numbers of aleurone cell layers. None of these genes were differentially expressed in the thk1 mutant transcriptomes, indicating that THK1 does not regulate them at the level of transcript abundance. The thk1 mutants produce multiple layers of apparently fully differentiated aleurone cells, whereas nkd mutants produce multiple layers of cells with compromised aleurone characteristics. Comparison of these mutants may provide a means of identifying genes involved in the specification of layer number versus differentiation of aleurone cell characteristics.
Double mutants between dek1 and thk1 display the multiple aleurone phenotype of the thk1 mutant even though dek1 single mutants cannot produce aleurone (Yi et al., 2011). This striking epistatic interaction is deeply informative and suggested a model where THK1 inhibits aleurone fate and DEK1 inhibits THK1 to permit aleurone specification in the outermost cell layer (Becraft and Yi, 2011; Yi et al., 2011). Molecularly, the regulatory relationship need not involve THK1 per se but could involve any component specific to the THK1 (versus THKL1) CCR4-NOT complex. Given that DEK1 is a protease (Wang et al., 2003; Lid et al., 2005), this might involve a regulatory cleavage or degradation of one or more subunits; however, it remains unclear whether this interaction is direct or indirect.
Aleurone cells are particularly rich in nutrition and dietarily beneficial compounds (Brouns et al., 2012). Increasing the number of aleurone layers in cereal grains is one way to improve their dietary value, as was recently shown for the rice thick aleurone2 mutant (Liu et al., 2018). Identification of THK1 and the CCR4-NOT complex as part of the pathways that regulate aleurone cell layers adds to our understanding of endosperm development and may help achieve this goal.
MATERIALS AND METHODS
Mutagenesis Screen for New thk1 Alleles
Pollen was collected from a wild-type, purple-kernel strain of the inbred B73 maize (Zea mays), treated with EMS (Neuffer, 1994), and then used to pollinate B73 ears. Successful creation of a new mutant allele in the pollen would thus produce a heterozygous thk1-*/+ M1 embryo. The resultant M1 grains were planted in an isolation plot and the plants were detasseled. Half the ovules on the rare heterozygous plant would carry the new thk1-* allele. For the pollen donor, a line was planted that segregated 1:2:1 for the original thk1-R allele, and the field was allowed to open pollinate. Because thk1-R homozygotes are inviable, two-thirds of pollen plants were heterozygous thk1-R/+ and one-third were homozygous wild type. As such, one-third of the pollen available to fertilize the M1 ears carried the thk1-R allele. Taken together, an ear on a rare M1 plant heterozygous for a new mutant allele was expected to produce one-sixth mutant kernels. Viable heterozygous kernels from such an ear were then planted to recover the new thk1-* mutant allele.
RNA-Seq
RNA-seq was performed on endosperm tissue of two independent EMS mutant alleles, thk1-iso15 and thk1-iso17, and the wild-type B73 progenitor. Field grown plants were self-pollinated, and endosperms were dissected at 19 DAP. Mutant kernels from segregating ears were identified by microscopy. Three replicates were conducted per genotype by collecting endosperms from ears of three independent plants. For each replicate, endosperms were dissected from five kernels and pooled. Hence, a total of nine samples were collected. Dissected samples were immediately frozen in liquid N2 and stored at −80°C. RNA was isolated as previously described (Wang et al., 2012), and RNA quality was examined by an Agilent 2100 Bioanalyzer (Agilent). Samples were submitted to the Iowa State University DNA Facility for library preparation with a TruSeq RNA Library Prep Kit (Illumina) and 100-bp end sequencing using an Illumina HiSeq 2500 sequencer. Three samples, one replicate of each genotype, were multiplex sequenced in each of three different flow cell lanes.
RNA-Seq Data Analysis and Identification of the thk1 Candidate Gene
Quality control of raw reads was performed by FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The raw reads were mapped to the maize reference genome (B73 RefGen_v4, AGPv4) by HISAT2 with default parameters specific for paired-end reads (v2.1.0; Kim et al., 2015; Jiao et al., 2017), and 88.42% to 97.55% of reads were successfully mapped. The output SAM files were converted to BAM files and were sorted by SAMtools (v1.9; Li et al., 2009). For SNP calling, the sorted BAM files of each genotype were merged for better calling power. The Mpileup-BCFtools pipeline (by SAMtools v1.9) and freebayes pipeline (v1.1.0; Li et al., 2009; Li, 2011) were used to identify SNPs in transcripts from thk1-iso15 or thk1-iso17 mutant samples compared with the B73 reference within the 2-Mb thk1-R region. Candidate SNPs were discarded if they were also found in reads from the lab-specific B73 progenitor line.
For differential expression analysis, mapped transcripts in sorted BAM files were assembled, and normalized reads per kilobase of transcript per million mapped reads and transcripts per million values were calculated with StringTie (v1.3.6; Pertea et al., 2016) using the maize genome annotation file Zea_mays.B73_RefGen_v4.43.gtf downloaded from Gramene (http://www.gramene.org/). Read counts were calculated by HTSeq (v0.11.1; Anders et al., 2015) and were normalized and analyzed by DEseq2 (v1.26.0; Love et al., 2014). DEGs were called using an empirical Bayes approach with the criteria log2 fold change (log2FC) > 1 or < −1 and FDR < 0.05 between each pair of samples, thk1-iso15, thk1-iso17, or B73 (Love et al., 2014). Genes within the 2-Mb region deleted in thk1-R (Yi et al., 2011) were evaluated as candidates for thk1.
GO term enrichment analysis was performed at AgriGOv2 (http://systemsbiology.cau.edu.cn/agriGOv2/) using the Singular Enrichment Analysis tool with FDR < 0.05 (Tian et al., 2017). Metabolic pathway analysis was performed using CornCyc v9.0 (https://www.plantcyc.org/databases/corncyc/9.0; Schläpfer et al., 2017). GO terms are hierarchically clustered from general to specific, and closely related GO terms in a certain cluster contain overlapping genes. A hypergeometric test (Zhan et al., 2015) was conducted to statistically evaluate if two GO terms are significantly related by calculating the number of overlapping genes relative to the total number of protein-coding genes (39,324) in the B73 genome (Jiao et al., 2017). Highly related GO terms (−log10p > 20) form a cluster on a heat map. A hypergeometric test (Zhan et al., 2015) was also used to analyze if thk1 DEGs are significantly enriched in genes specific to endosperm tissues using the same reference gene list as above.
CRISPR/Cas9 Editing of the thk1 Gene
Potential gRNA targets were evaluated using the CRISPR Genome Analysis Tool supplied by Iowa State University’s Crop Bioengineering Center (Brazelton et al., 2015). Based on the criteria of specificity (to avoid targeting thkl1 on chromosome 9) and targeting an exon likely to disrupt protein function, the sequence 5′-GTGTCCAGATGCTTCAAC-3′, corresponding to bases 1,493,925 to 1,493,942 on chromosome 1, was chosen as a gRNA target. The corresponding oligonucleotide was ligated into a pENTR vector and subsequently recombined into the pGW-Cas9 vector (Char et al., 2017). The construct was submitted to the Iowa State University Plant Transformation Facility and introduced into Hi-II immature embryos by biolistic transformation. Target site DNA from transgenic plantlets was PCR amplified with the primer pair NotI-CRTF/R (Supplemental Table S2), and edits were identified using a T7 endonuclease I assay (New England Biolabs). PCR amplicons from edited plants were cloned into the pGEMT-easy vector and sequenced at the Iowa State University DNA sequencing facility. For subsequent inheritance and genetic crosses, plants were screened for the presence of the edits using the primer pair NotI-CRTF/R as well as selected for the absence of the Cas9 cassette using primer pair Cas9F/R (Supplemental Table S2).
Histological Analysis of Kernel Phenotypes
Kernels were harvested at 19 DAP, cut longitudinally, and fixed in 5% (v/v) formaldehyde 10% (v/v) glacial acetic acid, and 50% (v/v) ethanol. Kernels were embedded in Paraplast+, sectioned longitudinally to 10 μm, and mounted on glass microscope slides. Samples were stained with periodic acid-Schiff and Toluidine Blue and then mounted in Permount. Sections were examined with an Olympus BX-60 microscope, and images were captured with a Jenoptik C5 camera.
thk1 and thkl1 Annotation, Structure, Expression, and Protein Prediction
Information about the maize thk1 and thkl1 loci, transcripts, and encoded proteins was retrieved from EnsemblePlants Zea mays version 97.7 (B74_RefGen_v4; https://plants.ensembl.org/Zea_mays/Info/Index). Public gene expression data were queried using the qTeller tool at MaizeGDB (https://qteller.maizegdb.org/).
Protein Phylogenetic Analysis
The yeast (Saccharomyces cerevisiae) CDC39 protein was used to search the National Center for Biotechnology Information Reference Protein database. Protein sequences listed in Supplemental Table S3 were downloaded, and multiple sequence alignment was performed using ClustalO (Madeira et al., 2019). The alignments were imported into MEGA7 (Kumar et al., 2016), and molecular phylogeny was analyzed using the maximum likelihood method (Jones et al., 1992). The tree with the highest log likelihood (−26,987.2808) is shown in Figure 2. Initial trees for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model and then selecting the topology with a superior log likelihood value. All positions containing gaps were eliminated.
Statistical Analysis
Count per million (CPM) reads of specific chromosome regions were calculated by SAMtools (v1.9; Li et al., 2009). Exon groups 1 to 5 were located within the positions 1,492,285 to 1,497,210; 1,498,801 to 1,502,872; 1,504,102 to 1,507,314; 1,522,039 to 1,525,010; and 1,530,793 to 1,533,161 of chromosome 1, respectively. Means and se values of log2(CPM+1) of the three replicates from B73, thk1-iso15, and thk1-iso17 were calculated and analyzed by Tukey’s honestly significant difference test in JMP Pro 14 (SAS Institute) with the level of significance P < 0.05. Read-count processing and DEG analysis were performed using algorithms incorporated in DEseq2 (v1.26.0; Love et al., 2014). In brief, read counts were normalized to fit a generalized linear model. Principal component analysis used a regularized log transformation algorithm (DEseq2 function rlog) of the normalized read counts. Empirical Bayes shrinkage estimation was used for calculating dispersions and fold changes. Genes with log2FC > 1 or < −1 and FDR < 0.05 between each pair of samples are considered to be significantly differentially expressed. Log2FC correlation coefficients between DEGs of B73 versus thk1-iso15 and B73 versus thk1-iso17 were tested by JMP Pro 14 (SAS Institute) with the level of significance P < 0.05.
A hypergeometric test of overlap was performed using a previously reported method (Zhan et al., 2015) to test if two groups of genes (GO-related genes, DEGs, or tissue-specific genes) are significantly related by calculating the number of overlapping genes relative to the total number of protein-coding genes in the B73 genome (Jiao et al., 2017). The P values were transformed by −log10p, and the greater the value, the higher the level of significance.
Accession Numbers
Accession numbers of genes, proteins, and RNA-seq data are listed in Supplemental Table S6
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Noncomplementation between the CRISPR/Cas9-edited thk1 allele and thk1-iso17, and expression of the Rab17-YFP aleurone marker.
Supplemental Figure S2. Protein multiple sequence alignment used for phylogenetic analysis.
Supplemental Figure S3. ClustalW alignment of maize THK1 and THKL1.
Supplemental Figure S4. Expression atlas data for thk1 and thkl1 genes.
Supplemental Figure S5. Comparison of RNA-seq data among the wild type, thk1-iso15, and thk1-iso17 shows highly similar effects on gene expression between the two mutant alleles.
Supplemental Figure S6. Correlations in gene content among GO terms for molecular function and cellular component.
Supplemental Figure S7. Select pathways identified by mapping DEGs with CornCyc.
Supplemental Table S1. Differentially expressed genes within the 2-Mb region.
Supplemental Table S2. Primers and oligonucleotides used in this study.
Supplemental Table S3. Species and accessions used in protein phylogenetic analysis.
Supplemental Table S4. Aleurone-specific TF genes up-regulated in the thk1 mutant.
Supplemental Table S5. Aleurone-specific cytokinesis-related genes up-regulated in thk1 mutant endosperm.
Supplemental Table S6. Accession numbers of genes, proteins, and RNA-seq data.
Supplemental Data Set S1. Gene models contained within the 2.1-Mb region deleted in the thk1-R mutant.
Supplemental Data Set S2. qTeller compilation of public expression data for the thk1 and thkl1 genes.
Supplemental Data Set S3. Summary of all DEGs among thk1-iso15, thk1-iso17, and B73 wild-type progenitor plants.
Supplemental Data Set S4. GO terms enriched among DEGs.
Acknowledgments
The Maize Genetics Cooperation Stock Center was instrumental in providing critical genetic materials. The Iowa State University Roy J. Carver High Resolution Microscopy Facility provided technical training for histology.
Footnotes
This work was supported by the National Science Foundation (grant no. 1444568).
Articles can be viewed without a subscription.
References
- Albert TK, Hanzawa H, Legtenberg YI, de Ruwe MJ, van den Heuvel FA, Collart MA, Boelens R, Timmers HT(2002) Identification of a ubiquitin-protein ligase subunit within the CCR4-NOT transcription repressor complex. EMBO J 21: 355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W(2015) HTSeq: A Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arae T, Morita K, Imahori R, Suzuki Y, Yasuda S, Sato T, Yamaguchi J, Chiba Y(2019) Identification of Arabidopsis CCR4-NOT complexes with Pumilio RNA binding proteins, APUM5 and APUM2. Plant Cell Physiol 60: 2015–2025 [DOI] [PubMed] [Google Scholar]
- Basquin J, Roudko VV, Rode M, Basquin C, Séraphin B, Conti E(2012) Architecture of the nuclease module of the yeast Ccr4-Not complex: The Not1-Caf1-Ccr4 interaction. Mol Cell 48: 207–218 [DOI] [PubMed] [Google Scholar]
- Bawankar P, Loh B, Wohlbold L, Schmidt S, Izaurralde E(2013) NOT10 and C2orf29/NOT11 form a conserved module of the CCR4-NOT complex that docks onto the NOT1 N-terminal domain. RNA Biol 10: 228–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft PW, Asuncion-Crabb Y(2000) Positional cues specify and maintain aleurone cell fate in maize endosperm development. Development 127: 4039–4048 [DOI] [PubMed] [Google Scholar]
- Becraft PW, Stinard PS, McCarty DR(1996) CRINKLY4: A TNFR-like receptor kinase involved in maize epidermal differentiation. Science 273: 1406–1409 [DOI] [PubMed] [Google Scholar]
- Becraft PW, Yi G(2011) Regulation of aleurone development in cereal grains. J Exp Bot 62: 1669–1675 [DOI] [PubMed] [Google Scholar]
- Bernardi J, Lanubile A, Li QB, Kumar D, Kladnik A, Cook SD, Ross JJ, Marocco A, Chourey PS(2012) Impaired auxin biosynthesis in the defective endosperm18 mutant is due to mutational loss of expression in the ZmYuc1 gene encoding endosperm-specific YUCCA1 protein in maize. Plant Physiol 160: 1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar V, Basquin J, Conti E(2015) Architecture of the ubiquitylation module of the yeast Ccr4-Not complex. Structure 23: 921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar V, Roudko V, Basquin J, Sharma K, Urlaub H, Séraphin B, Conti E(2013) Structure and RNA-binding properties of the Not1-Not2-Not5 module of the yeast Ccr4-Not complex. Nat Struct Mol Biol 20: 1281–1288 [DOI] [PubMed] [Google Scholar]
- Brazelton VA Jr., Zarecor S, Wright DA, Wang Y, Liu J, Chen K, Yang B, Lawrence-Dill CJ(2015) A quick guide to CRISPR sgRNA design tools. GM Crops Food 6: 266–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns F, Hemery Y, Price R, Anson NM(2012) Wheat aleurone: Separation, composition, health aspects, and potential food use. Crit Rev Food Sci Nutr 52: 553–568 [DOI] [PubMed] [Google Scholar]
- Buell CR, Yuan Q, Ouyang S, Liu J, Zhu W, Wang A, Maiti R, Haas B, Wortman J, Pertea M, et al. (2005) Sequence, annotation, and analysis of synteny between rice chromosome 3 and diverged grass species. Genome Res 15: 1284–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Char SN, Neelakandan AK, Nahampun H, Frame B, Main M, Spalding MH, Becraft PW, Meyers BC, Walbot V, Wang K, et al. (2017) An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotechnol J 15: 257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WL, Huang LF, Fang JC, Yeh CH, Hong CY, Wu SJ, Lu CA(2014) Divergence of the expression and subcellular localization of CCR4-associated factor 1 (CAF1) deadenylase proteins in Oryza sativa. Plant Mol Biol 85: 443–458 [DOI] [PubMed] [Google Scholar]
- Collart MA.(2016) The Ccr4-Not complex is a key regulator of eukaryotic gene expression. Wiley Interdiscip Rev RNA 7: 438–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart MA, Panasenko OO(2017) The Ccr4-Not complex: Architecture and structural insights. Subcell Biochem 83: 349–379 [DOI] [PubMed] [Google Scholar]
- Criqui MC, Parmentier Y, Derevier A, Shen WH, Dong A, Genschik P(2000) Cell cycle-dependent proteolysis and ectopic overexpression of cyclin B1 in tobacco BY2 cells. Plant J 24: 763–773 [DOI] [PubMed] [Google Scholar]
- Dai Y, Cao Z, Huang L, Liu S, Shen Z, Wang Y, Wang H, Zhang H, Li D, Song F(2016) CCR4-Not complex subunit Not2 plays critical roles in vegetative growth, conidiation and virulence in watermelon fusarium wilt pathogen Fusarium oxysporum f. sp. niveum. Front Microbiol 7: 1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MR, Frank F, Rouya C, Siddiqui N, Lai WS, Karetnikov A, Blackshear PJ, Nagar B, Sonenberg N(2013) Structural basis for the recruitment of the human CCR4-NOT deadenylase complex by tristetraprolin. Nat Struct Mol Biol 20: 735–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino Y, Yamada K, Sugaya C, Ooka Y, Ovara H, Ban H, Akama K, Otosaka S, Kinoshita H, Yamasu K, et al. (2018) Deadenylation by the CCR4-NOT complex contributes to the turnover of hairy-related mRNAs in the zebrafish segmentation clock. FEBS Lett 592: 3388–3398 [DOI] [PubMed] [Google Scholar]
- Gontarek BC, Neelakandan AK, Wu H, Becraft PW(2016) NKD transcription factors are central regulators of maize endosperm development. Plant Cell 28: 2916–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes GM, Hamilton JP, Wood JC, Esteban E, Pasha A, Vaillancourt B, Provart NJ, Buell CR(2019) An updated gene atlas for maize reveals organ-specific and stress-induced genes. Plant J 97: 1154–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Peluso P, Shi J, Liang T, Stitzer MC, Wang B, Campbell MS, Stein JC, Wei X, Chin CS, et al. (2017) Improved maize reference genome with single-molecule technologies. Nature 546: 524–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM(1992) The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8: 275–282 [DOI] [PubMed] [Google Scholar]
- Kawakatsu T, Yamamoto MP, Touno SM, Yasuda H, Takaiwa F(2009) Compensation and interaction between RISBZ1 and RPBF during grain filling in rice. Plant J 59: 908–920 [DOI] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL(2015) HISAT: A fast spliced aligner with low memory requirements. Nat Methods 12: 357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K(2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.(2011) A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27: 2987–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R(2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Li C, Liu F, Jiang H, Li S, Sun J, Wu X, Li C(2009) The Arabidopsis homologs of CCR4-associated factor 1 show mRNA deadenylation activity and play a role in plant defence responses. Cell Res 19: 307–316 [DOI] [PubMed] [Google Scholar]
- Lid SE, Gruis D, Jung R, Lorentzen JA, Ananiev E, Chamberlin M, Niu X, Meeley R, Nichols S, Olsen OA(2002) The defective kernel 1 (dek1) gene required for aleurone cell development in the endosperm of maize grains encodes a membrane protein of the calpain gene superfamily. Proc Natl Acad Sci USA 99: 5460–5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lid SE, Olsen L, Nestestog R, Aukerman M, Brown RC, Lemmon B, Mucha M, Opsahl-Sorteberg HG, Olsen OA(2005) Mutation in the Arabidopsis thaliana DEK1 calpain gene perturbs endosperm and embryo development while over-expression affects organ development globally. Planta 221: 339–351 [DOI] [PubMed] [Google Scholar]
- Liu HY, Toyn JH, Chiang YC, Draper MP, Johnston LH, Denis CL(1997) DBF2, a cell cycle-regulated protein kinase, is physically and functionally associated with the CCR4 transcriptional regulatory complex. EMBO J 16: 5289–5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wu X, Yao X, Yu R, Larkin PJ, Liu CM(2018) Mutations in the DNA demethylase OsROS1 result in a thickened aleurone and improved nutritional value in rice grains. Proc Natl Acad Sci USA 115: 11327–11332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S(2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, et al. (2019) The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47: W636–W641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Basquin J, Ozgur S, Czarnocki-Cieciura M, Bonneau F, Aartse A, Dziembowski A, Nowotny M, Conti E, Filipowicz W(2014) Structural and biochemical insights to the role of the CCR4-NOT complex and DDX6 ATPase in microRNA repression. Mol Cell 54: 751–765 [DOI] [PubMed] [Google Scholar]
- Motomura K, Arae T, Araki-Uramoto H, Suzuki Y, Takeuchi H, Suzuki T, Ichihashi Y, Shibata A, Shirasu K, Takeda A, et al. (2020) AtNOT1 is a novel regulator of gene expression during pollen development. Plant Cell Physiol 61: 712–721 [DOI] [PubMed] [Google Scholar]
- Neuffer MG.(1994) Mutagenesis In Freeling M, and Walbot V, eds, The Maize Handbook. Springer-Verlag, New York, pp 212–219 [Google Scholar]
- Niu B, Wang L, Zhang L, Ren D, Ren R, Copenhaver GP, Ma H, Wang Y(2015) Arabidopsis cell division cycle 20.1 is required for normal meiotic spindle assembly and chromosome segregation. Plant Cell 27: 3367–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakenfull EA, Riou-Khamlichi C, Murray JAH(2002) Plant D-type cyclins and the control of G1 progression. Philos Trans R Soc Lond B Biol Sci 357: 749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL(2016) Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 11: 1650–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit AP, Wohlbold L, Bawankar P, Huntzinger E, Schmidt S, Izaurralde E, Weichenrieder O(2012) The structural basis for the interaction between the CAF1 nuclease and the NOT1 scaffold of the human CCR4-NOT deadenylase complex. Nucleic Acids Res 40: 11058–11072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Li S, Zhu Y, Zhao Q, Zhu D, Yu J(2017) ZmDof3, a maize endosperm-specific Dof protein gene, regulates starch accumulation and aleurone development in maize endosperm. Plant Mol Biol 93: 7–20 [DOI] [PubMed] [Google Scholar]
- Renaudin JP, Colasanti J, Rime H, Yuan Z, Sundaresan V(1994) Cloning of four cyclins from maize indicates that higher plants have three structurally distinct groups of mitotic cyclins. Proc Natl Acad Sci USA 91: 7375–7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudin JP, Doonan JH, Freeman D, Hashimoto J, Hirt H, Inzé D, Jacobs T, Kouchi H, Rouzé P, Sauter M, et al. (1996) Plant cyclins: A unified nomenclature for plant A-, B- and D-type cyclins based on sequence organization. Plant Mol Biol 32: 1003–1018 [DOI] [PubMed] [Google Scholar]
- Sabelli PA, Dante RA, Leiva-Neto JT, Jung R, Gordon-Kamm WJ, Larkins BA(2005a) RBR3, a member of the retinoblastoma-related family from maize, is regulated by the RBR1/E2F pathway. Proc Natl Acad Sci USA 102: 13005–13012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelli PA, Leiva-Neto JT, Dante RA, Nguyen H, Larkins BA(2005b) Cell cycle regulation during maize endosperm development. Maydica 50: 485–496 [Google Scholar]
- Sarowar S, Oh HW, Cho HS, Baek KH, Seong ES, Joung YH, Choi GJ, Lee S, Choi D(2007) Capsicum annuum CCR4-associated factor CaCAF1 is necessary for plant development and defence response. Plant J 51: 792–802 [DOI] [PubMed] [Google Scholar]
- Schläpfer P, Zhang P, Wang C, Kim T, Banf M, Chae L, Dreher K, Chavali AK, Nilo-Poyanco R, Bernard T, et al. (2017) Genome-wide prediction of metabolic enzymes, pathways, and gene clusters in plants. Plant Physiol 173: 2041–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Liang C, Zhang J, Fulton L, Graves TA, et al. (2009) The B73 maize genome: Complexity, diversity, and dynamics. Science 326: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Settles AM, Holding DR, Tan BC, Latshaw SP, Liu J, Suzuki M, Li L, O’Brien BA, Fajardo DS, Wroclawska E, et al. (2007) Sequence-indexed mutations in maize using the UniformMu transposon-tagging population. BMC Genomics 8: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Li C, Min Z, Meeley RB, Tarczynski MC, Olsen OA(2003) sal1 determines the number of aleurone cell layers in maize endosperm and encodes a class E vacuolar sorting protein. Proc Natl Acad Sci USA 100: 6552–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai YT, Suzuki T, Morita M, Takahashi A, Yamamoto T(2014) Multifunctional roles of the mammalian CCR4-NOT complex in physiological phenomena. Front Genet 5: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelpflug SC, Sekhon RS, Vaillancourt B, Hirsch CN, Buell CR, de Leon N, Kaeppler SM(2016) An expanded maize gene expression atlas based on RNA sequencing and its use to explore root development. Plant Genome 9: 1–16 [DOI] [PubMed] [Google Scholar]
- Swigoňová Z, Lai J, Ma J, Ramakrishna W, Llaca V, Bennetzen JL, Messing J(2004) Close split of sorghum and maize genome progenitors. Genome Res 14: 1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry-Mieg D, Thierry-Mieg J(2006) AceView: A comprehensive cDNA-supported gene and transcripts annotation. Genome Biology 7: S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Liu Y, Yan H, You Q, Yi X, Du Z, Xu W, Su Z(2017) agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res 45: W122–W129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D, Galletti R, Neumann ED, Dubois A, Sharif-Naeini R, Geitmann A, Frachisse JM, Hamant O, Ingram GC(2017) A mechanosensitive Ca2+ channel activity is dependent on the developmental regulator DEK1. Nat Commun 8: 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, Valencia-Sanchez MA, Staples RR, Chen J, Denis CL, Parker R(2001) The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell 104: 377–386 [DOI] [PubMed] [Google Scholar]
- Vicente C, Stirparo R, Demeyer S, de Bock CE, Gielen O, Atkins M, Yan J, Halder G, Hassan BA, Cools J(2018) The CCR4-NOT complex is a tumor suppressor in Drosophila melanogaster eye cancer models. J Hematol Oncol 11: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley JW, Coughlan S, Hudson ME, Covington MF, Kaspi R, Banu G, Harmer SL, Dehesh K(2007) Mechanical stress induces biotic and abiotic stress responses via a novel cis-element. PLoS Genet 3: 1800–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley JW, Kelley DR, Nestorova G, Hirschberg DL, Dehesh K(2010) Arabidopsis deadenylases AtCAF1a and AtCAF1b play overlapping and distinct roles in mediating environmental stress responses. Plant Physiol 152: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Barry JK, Min Z, Tordsen G, Rao AG, Olsen OA(2003) The calpain domain of the maize DEK1 protein contains the conserved catalytic triad and functions as a cysteine proteinase. J Biol Chem 278: 34467–34474 [DOI] [PubMed] [Google Scholar]
- Wang G, Wang G, Zhang X, Wang F, Song R(2012) Isolation of high quality RNA from cereal seeds containing high levels of starch. Phytochem Anal 23: 159–163 [DOI] [PubMed] [Google Scholar]
- Wang L, Ruan YL(2013) Regulation of cell division and expansion by sugar and auxin signaling. Front Plant Sci 4: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Song X, Gu L, Li X, Cao S, Chu C, Cui X, Chen X, Cao X(2013) NOT2 proteins promote polymerase II-dependent transcription and interact with multiple microRNA biogenesis factors in Arabidopsis. Plant Cell 25: 715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Suzuki T, Sato T, Takahashi A, Watanabe H, Kadowaki A, Natsui M, Inagaki H, Arakawa S, Nakaoka S, et al. (2018) The CCR4-NOT deadenylase complex controls Atg7-dependent cell death and heart function. Sci Signal 11: eaan3638. [DOI] [PubMed] [Google Scholar]
- Yamaji M, Jishage M, Meyer C, Suryawanshi H, Der E, Yamaji M, Garzia A, Morozov P, Manickavel S, McFarland HL, et al. (2017) DND1 maintains germline stem cells via recruitment of the CCR4-NOT complex to target mRNAs. Nature 543: 568–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G, Lauter AM, Scott MP, Becraft PW(2011) The thick aleurone1 mutant defines a negative regulation of maize aleurone cell fate that functions downstream of defective kernel1. Plant Physiol 156: 1826–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G, Neelakandan AK, Gontarek BC, Vollbrecht E, Becraft PW(2015) The naked endosperm genes encode duplicate INDETERMINATE domain transcription factors required for maize endosperm cell patterning and differentiation. Plant Physiol 167: 443–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, Hong M, Gui B, Chen Z, Li L, Xie G, Liang J, Wang X, Shang Y(2012) RNA processing and modification protein, carbon catabolite repression 4 (Ccr4), arrests the cell cycle through p21-dependent and p53-independent pathway. J Biol Chem 287: 21045–21057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TE, Gallie DR(2000) Programmed cell death during endosperm development. Plant Mol Biol 44: 283–301 [DOI] [PubMed] [Google Scholar]
- Zhan J, Thakare D, Ma C, Lloyd A, Nixon NM, Arakaki AM, Burnett WJ, Logan KO, Wang D, Wang X, et al. (2015) RNA sequencing of laser-capture microdissected compartments of the maize kernel identifies regulatory modules associated with endosperm cell differentiation. Plant Cell 27: 513–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Liang Z, Demko V, Wilson R, Johansen W, Olsen OA, Shalchian-Tabrizi K(2012) Massive expansion of the calpain gene family in unicellular eukaryotes. BMC Evol Biol 12: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]