Abstract
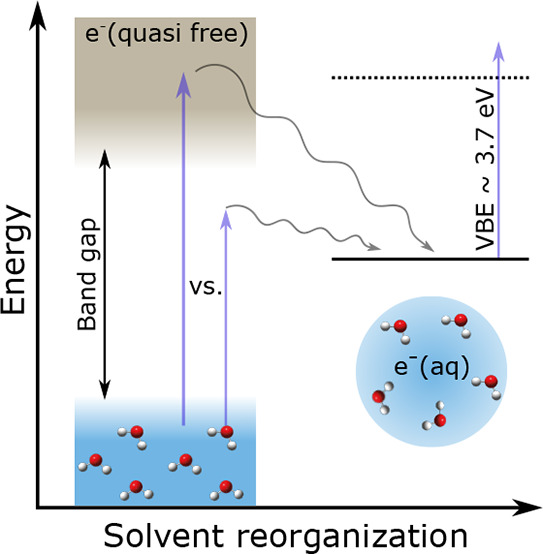
Below band gap formation of solvated electrons in neutral water clusters using pump–probe photoelectron imaging is compared with recent data for liquid water and with above band gap excitation studies in liquid and clusters. Similar relaxation times on the order of 200 fs and 1–2 ps are retrieved for below and above band gap excitation, in both clusters and liquid. The independence of the relaxation times from the generation process indicates that these times are dominated by the solvent response, which is significantly slower than the various solvated electron formation processes. The analysis of the temporal evolution of the vertical electron binding energy and the electron binding energy at half-maximum suggests a dependence of the solvation time on the binding energy.
Introduction
Solvated electrons in molecular liquids, especially water, have sparked broad interest because of their widespread occurrence and their intriguing fundamental properties. Numerous experimental and theoretical studies have provided insights into the electronic properties, the generation mechanism, and the relaxation dynamics of solvated electrons in liquid water, amorphous ice, anionic water clusters, neutral water clusters, and sodium-doped water clusters1−48 (and references therein).
A series of comparable, femtosecond time-resolved photoelectron studies on liquid water and neutral water clusters49−51 has now opened up the possibility to address two aspects in greater detail: (i) similarities and differences of the relaxation dynamics after below versus above band gap generation of the hydrated electron and (ii) similarities and differences of the relaxation dynamics in two different aqueous environments, i.e., liquid water versus neutral water clusters. The formation of hydrated electrons by irradiation with photons has been observed down to the absorption edge of water at ∼6 eV37−39,52 (and references therein), far below the bottom of the conduction band.53−56 Different mechanisms have been suggested for the below band gap formation of solvated electrons, involving very fast (<10 fs) water dissociation and proton and electron transfer processes (“hot H atom mechanism”, “proton-coupled electron transfer”, “consecutive proton transfer, electron transfer process”, “electron transfer to preexisting sites”; see refs (37−39 and 52) and references therein). These ultrafast processes are followed by slower solvent rearrangement (fs to ps time scales) and slow geminate recombination37,40,47,57−59 (and references therein). Irradiation with photon energies above the band gap, by contrast, produces delocalized, quasi-free conduction band electrons by the ionization of water. These conduction band electrons relax rapidly toward the bottom of the conduction band by fast energy dissipation through electron scattering on time scales of several 10 fs.15,31,60−63 Localization from the bottom of the conduction band to interband trapped states likely takes place on time scales faster than ∼100 fs,15,25,30,34 followed by slower solvent rearrangement and geminate recombination.
The present time-resolved photoelectron imaging study addresses the question of whether the formation of solvated electrons by below band gap excitation is also feasible in neutral water clusters, as previously observed in liquid water38,50 (and references therein). The relaxation dynamics after one-photon excitation at 7.8 eV photon energy is probed and compared with a recent pump–probe study for liquid water using an excitation energy of 7.7 eV.50 At these excitation energies, it is assumed that solvated electron formation follows absorption into a localized 11B1 excited state of water. The results for below band gap excitation are compared with previous above band gap excitation photoelectron studies for the liquid and clusters.49−51
Methods
The velocity map imaging (VMI) photoelectron spectrometer used in this work is described in refs (36, 49, 64, and 65) and the Supporting Information (SI). Neutral water clusters consisting of ∼500 water molecules (r ∼ 1.5 nm, see refs (64 and 66) for cluster size determination) are generated in supersonic expansions. Femtosecond (fs) laser pulses of 7.8 eV photon energy (pump) from high harmonic generation (HHG) are used to generate solvated electrons by below band gap excitation in water clusters. The relaxation dynamics are probed with a femtosecond probe pulse of 4.7 eV photon energy by varying the pump–probe time delay t. Time-resolved electron binding energy spectra (eBE) and photoelectron angular distributions (PADs, β-parameters eq S1, SI) are retrieved from the recorded time-dependent photoelectron images (SI). The instrument response function (IRF) determined from (1 + 1’) nonresonant ionization of Xe is 150 ± 35 fs.
Results
Figure 1a shows time-resolved photoelectron spectra (TRPES) recorded with a 4.7 eV probe pulse following below band gap excitation at 7.8 eV pump energy. Within the first picosecond, the eBE spectrum changes rapidly and approaches an asymptote at longer times, accompanied by a decrease in the overall signal intensity. The observed spectral signature is characteristic of hydrated electron relaxation dynamics, where the fully relaxed hydrated electron in its ground state is formed after ∼2 ps,49−51 followed by depopulation due to geminate recombination on a much longer time scale.50 This confirms hydrated electron formation in neutral clusters following below band gap excitation.
Figure 1.

Time-resolved photoelectron spectra (TRPES) of the hydrated electron in neutral water clusters. (a) Experimental TRPES measured with a 7.8 eV pump pulse and a 4.7 eV probe pulse (without subtraction of the impulsive component). (b) Global Lifetime Analysis (GLA) fit to the TRPES in panel a after subtraction of the impulsive component at early pump–probe delays (see also Figure S1).
We expect the short-lived signal observed around zero time delay extending toward maximum eBEs of ∼4.7 eV (toward zero photoelectron kinetic energy, eKE) to originate either from the fast dynamics (much faster than our IRF) of the background water vapor67 or the impulsive (1 + 1’) ionization of water clusters. To subtract these spectral components, we performed a Global Lifetime Analysis (GLA) of our data.18,68,69 GLA assumes a number of time-independent spectra and assigns them a corresponding exponentially decaying population. The spectral profiles and the decay dynamics are then fit to the experimental data. This technique has recently been applied by Hara et al.(69) to model detailed kinetics following conduction-band excitation of methanol. Since the relaxation dynamics are faster in water than in methanol,50,69,70 much better time resolution than that currently available and excellent signal-to-noise levels would be required to extract similarly detailed kinetic information for aqueous systems. We thus use here a simple sequential kinetics model (eq S2, SI) with three exponentially decaying features and a Gaussian distribution for the IRF to fit the TRPES in Figure 1a (SI, section S2), similar to the approaches in refs (50 and 69). The best TRPES fit (Figure 1b and Figure S1, middle panel) corresponds to a Gaussian with a full-width at half-maximum FWHMIRF = 166 ± 5 fs, consistent with the experimentally determined IRF of 150 ± 35 fs, and three exponential decays with lifetimes of τ1GLA = 221 ± 56 fs, τ2 = 447 ± 100 fs, and τ3GLA = 17.74 ± 5.55 ps (SI and Figure 2) with the corresponding Decay Associated Spectra (DAS; Figure 2b). This analysis allows us to subtract the impulsive component from the TRPES (Figure 1b, see also eBE spectra in Figures S3 and S4).
Figure 2.

(a) Experimental (circles) and fitted (full black line) time-dependent relative photoelectron yield with the contributions from the corresponding decay associated spectra (DAS, dashed colored lines). The Gaussian component (cyan dashed-dotted line, FWHMIRF = 166 ± 5 fs) matches well with the measured instrument response function. Contributions from DAS1 (blue dashed line, τ1GLA = 221 ± 56 fs), DAS2 (red dashed, τ2 = 447 ± 100 fs) line, and DAS3 (green dashed line, τ3GLA = 17.74 ± 5.55 ps) follow a sequential kinetics mechanism. (b) The three DAS obtained from the fit (SI, section S2). DAS1, blue line; DAS2, red line; DAS3, green line.
Figure 2a shows the time-dependent, relative photoelectron yield of the hydrated electron (experimental data: circles, GLA fit: full black line) with the contributions from the corresponding decay components (dashed colored lines). The hydrated electron yield decays to ∼45% after 2 ps and to ∼33% after 5 ps of the yield measured at 200 fs. Within our uncertainties (±15%), these values are comparable to the measurements in liquid water of ∼31% and ∼22%, respectively,50 hinting at similar loss mechanisms in clusters and liquid, at least for these relatively short pump–probe delay range. Electron yields recorded at long pump–probe time delays have previously been used to extract geminate recombination rates, survival probabilities, and retrieved electron ejection lengths in the liquid50 (and references therein). In clusters, it is difficult to obtain reliable experimental data for long pump–probe delays because of the generally lower signal-to-noise level.
Experimental time-dependent eBE spectra are shown before subtraction of the impulsive component in Figure S2 and after subtraction of the impulsive component in Figure S3. Fitting these spectra with an exponentially modified Gaussian function (eq S6 and Figures S2 and S3) allows us to retrieve the time evolution of the vertical electron binding energy (VBE; most probable eBE) and the electron binding energy at half-maximum (HBE; see below). At short time delays <60 fs, subtraction of the impulsive component is required to obtain reliable VBEs, while the VBEs at longer time delays are indistinguishable within our experimental uncertainty regardless of whether the impulsive component is subtracted or not (Figures S2 and S3). The experimental VBEs at time delays >60 fs are shown in Figure 3a (circles). The VBE shifts from an initial value of 3.0 to 3.75 eV, which is reached at ∼2 ps and remains constant afterward. The eBE spectra with a VBE of 3.75 eV recorded after ∼2 ps (Figures S2 and S3) coincide with those of the relaxed ground state hydrated electron recorded in clusters and the liquid at a probe energy of 4.7 eV.2,13,31,46,49,71 We have also performed cluster size-dependent studies for average cluster sizes between <n> ∼ 250 and 500 molecules. No cluster size-dependence of the VBE (at ∼2 ps) is observed within the experimental uncertainty (see ref (49) for comparison with water anion and Na-doped water clusters). In agreement with our previous study using 10.9 eV pump photons,49 we retrieve a β-parameter of ∼0.2 for the relaxed ground state hydrated electron (after ∼2 ps). After scattering corrections,31,49,61,64 this corresponds to a genuine β-parameter in the range of 0.51–0.66. For the present pump photons of 7.8 eV, no significant time-dependence is observed for the β-parameter, i.e., the value of β lies around ∼0.2 for all pump–probe delays. This contrasts with our previous results using 10.9 eV pump photons, where the initial (t = 0 fs) β-parameter of ∼0.4 exceeded the ground state value of ∼0.2.49 However, such differences must be viewed with caution given our relatively high experimental uncertainty of ∼ ±0.15.
Figure 3.

(a) Time-dependent vertical binding energy (VBEs, black circles, left abscissa) and binding energy at half-maximum (HBEs, red triangles, right abscissa) for neutral water clusters recorded at a pump energy of 7.8 eV. The data are extracted from the TRPES in Figure 1 (see text and SI). The biexponential fit to the VBEs with time constants τ1VBE = 220 fs and τ2 = 1.6 ps is shown as the black full line. The biexponential fit to the HBEs with time constants τ1HBE = 220 fs and τ2 = 3.3 ps is shown as the red dashed line. Blue diamonds: time-dependent VBEs for liquid water extracted from Figure 2d of ref (50) recorded at a pump energy of 7.7 eV. (b) VBE at a pump–probe delay of ∼0 fs (VBE(t ∼ 0 fs), see Table 1) for the liquid from ref50 (blue triangles) and different cluster studies (black circles: this work and ref.,49 red squares: ref51) as a function of the pump energy hυ. The gray line is to guide the eye. The horizontal dashed line at ∼3.75 eV indicates the VBE of the relaxed ground state hydrated electron (ehyd-) which is reached after ∼2 ps. Note that the different studies use somewhat different analysis methods and different time resolutions.
Discussion
The temporal evolution of the VBE provides data on the relaxation dynamics following below band gap excitation and allows us to compare them with data from previous studies. We find that the time-dependence of the VBEs in Figure 3a (black circles) is well represented by a biexponential function (black full line): VBE (t) = a1 exp(−t/τ1VBE) + a2 exp(−t/τ2) + VBE(t = ∞), with VBE(t = ∞) = 3.8 eV and time constants τ1VBE = 220 fs and τ2 = 1.6 ps. These time constants agree well (see Table 1) with those reported in a recent liquid microjet study of water by Suzuki and co-workers,50 following below band gap excitation using a very similar pump energy of 7.7 eV (blue diamonds in Figure 3a). A similarly good agreement between the relaxation times of clusters and liquid was also observed at a pump energy of 9.3 eV (Table 1). The former time scale (τ1VBE = 220 fs) has been assigned to fast solvent reorganization and electron localization into a cavity with size on the order of a few Å, which is further stabilized by slow solvent reorganization (τ2 = 1.6 ps).8,30,34 Very similar time scales are also retrieved from the sequential kinetics model (Figure 2b). The relaxed hydrated electron (DAS3, green line) is formed in <2 ps, through the transient states represented by DAS1 (blue line) and DAS2 (red line). The shorter time constant (τ1GLA = 221 ± 56 fs) is associated with the DAS1 evolving into DAS2 with a VBE shift of about 0.7 eV, consistent with the fast solvent reorganization (τ1 = 220 fs). The evolution of DAS2 into DAS3 shows only a minor shift in the VBE (slow solvent reorganization) and significant loss of signal due to geminate recombination. However, this comparison is only qualitative since DAS1 and DAS2 only contain information about the average spectrum of all transient species probed within our IRF.
Table 1. Comparison of the Time Constants τ1VBE and τ2 for the Solvation of the Hydrated Electron in Water Clusters and Liquid Water Retrieved from the Time Evolution of the VBEsc.
| Liquid50 | Cluster (this work) | Cluster51 | Liquid50 | Cluster49 | Liquid (two-photon)50 | Cluster51 | |
|---|---|---|---|---|---|---|---|
| hυ [eV] | 7.7 | 7.8 | 9.3 | 9.3 | 10.9 | 11.0 | 15.5 |
| τ1VBE [ps] | 0.2 | 0.2 | 0.21 | 0.3 | 0.2 | 0.18 | |
| τ2VBE [ps] | 1.0 | 1.6 | 1.4 | 0.9 | 2.0 | 1.3 | |
| VBE(t ∼ 0 fs) [eV] | 2.8 | 3.0 | 2.5 | 2.6a | 2.6 | 2.4a | 2.2b |
Value estimated from Figure S6c in ref (51).
hυ is the pump photon energy. Except for the data at 11 eV, all excitations are single-photon excitations. VBE (t ∼ 0 fs) is the vertical binding energy at a pump-probe delay of ∼0 fs. Note that the different studies use somewhat different analysis methods and different time resolutions.
Considering that τ1VBE and τ2 are likely dominated by the response of the solvent (see introduction and ref (15)), the similarity of clusters and liquid does not appear so obvious. Clusters have greater surface area and are thus structurally less restricted than the liquid. One would thus expect solvent rearrangement to be faster in the cluster than in the liquid. The cluster temperatures are likely lower than those of the liquid, which would result in the opposite effect on the relaxation times, i.e., one would expect an increase of the solvent rearrangement times in the cluster compared with the liquid. Furthermore, one would expect both effects to be more pronounced for the slower solvent motions (larger amplitude reorientation), i.e., more pronounced for τ2VBE than for τ1. This appears to be the case for the data at 7.8 and 9.3 eV (see Table 1). The slightly higher values of τ2VBE in the clusters might imply that the cluster temperature is the dominant effect. However, such a conclusion remains speculative with the caveat of the limited comparability of cluster data with the liquid jet results. Apart from the somewhat different analysis methods used, τ2 is generally less well constrained by the cluster data with their lower signal-to-noise and fewer data points at longer time delays.
The observed independence of τ1VBE and τ2 on the pump energy used (see data for the different hυ in Table 1) supports the conclusions that these time scales are dominated by solvent response. The generation process will mainly influence the very fast processes (e.g., electron scattering, localization from the conduction band into the band gap, the different below band gap formation mechanisms) taking place on time scales of a few tens of fs, while the generally slower solvent responses will at most weakly depend on the generation process. The fast component of the solvent relaxation would still be expected to correlate to some extent with electronic deactivation and localization so that it might come as a surprise to find almost identical values for τ1VBE at all different pump energies (Table 1). However, one needs to keep in mind that these values lie close to the time resolution (on the order of ∼100 fs) of the studies. To properly access these fast processes, one would need a significantly better time resolution by about a factor of ∼10. Observable effects of using different excitation energies are mainly limited to the PADs and VBEs at zero pump–probe delay (t ∼ 0 fs). As mentioned above, the initial β-parameter recorded at 10.9 eV appears to be higher (∼0.4) than the one measured at 7.8 eV (∼0.2). Similarly, the initial VBE (VBE(t ∼ 0 fs)) seems to decrease systematically with increasing pump energy by about 0.7 eV (Figure 3b). At 7.8 eV, VBE(t ∼ 0 fs) lies ∼0.7 eV below the values for the relaxed ground state of the hydrated electron (horizontal dashed line at ∼3.75 eV), while this difference increases to ∼1.4 at 15.5 eV pump energy. Together with the above information, this suggests that fast electronic relaxation processes remain largely hidden within the time resolution. As a result, different pump–probe studies in Table 1 probe very similar solvation dynamics probably starting from similar localized initial states in the band gap, independently of the pump energy.
The temporal evolution of the VBE is a simple representation of the complex underlying dynamics because it only probes the dynamics of states that correspond to the maximum of the eBE spectrum (Figure S2). In Figure 3a, we also show the time-dependence of the binding energy at half-maximum (HBE, red triangles) on the rising edge of the eBE spectrum together with a biexponential fit (red dashed line), resulting in time constants of τ1HBE = 220 fs and τ2 = 3.3 ps. τ2HBE exceeds τ2 by a factor of 2. The HBE data represents the temporal evolution of a population with a lower binding energy compared with the population represented by the VBE data. This implies a correlation between the binding energy and the relaxation time. A potential explanation for this correlation could be that states with lower binding energy require more time to relax because more extensive solvent rearrangement is needed to reach the relaxed ground state. To represent this behavior, we therefore suggest using the HBE in addition to the VBE. A comparison with previous studies is currently not possible because the temporal evolution of the HBE is not available from those studies. Finally, we would like to highlight another advantage of the HBE when comparing different studies. Many of the solvated electron photoelectron studies use ultraviolet probe photons in the range below 5.8 eV; i.e., in a range where the energy dependence of electron scattering has a pronounced influence on the shape of the measured binding energy spectra.2,31Figure 3 in ref (31) shows that the position of the HBE only shifts by 50 meV when changing the probe photons from 4.4 to 5.8 eV, while the position of the VBE shifts by more than 200 meV over the same probe energy range. The HBE is less affected by the influence of scattering than the VBE and thus makes comparisons between studies using different probe wavelengths more reliable. We would like to emphasize that for solvated electrons in water, the preferred method to correct for such biases arising from electron scattering are detailed scattering simulations to extract genuine binding energy spectra.31,61−64,72 However, in most studies, this correction has not yet been implemented.
Conclusions
We have investigated the relaxation dynamics of the solvated electron in neutral water clusters (∼500 molecules) after below band gap excitation with photons of 7.8 eV using ultrafast photoelectron imaging. The comparison with a recent liquid water study at an excitation energy of 7.7 eV50 reveals similar solvation times on the order of ∼200 fs and ∼1–2 ps in the clusters and the liquid. It has been suggested that at these excitation energies, hydrated electrons are generated by water dissociation and proton and electron transfer processes38 after one-photon absorption to the predominately localized 11B1 excited state in water. The comparison of the below band gap investigations with previous above band gap photoelectron studies49−51 shows that the observable relaxation dynamics are essentially independent of the excitation energy. This is not surprising considering the time scales accessible in these experiments (∼100 fs range) that mainly probe the solvent response, which is largely independent of the generation process of the solvated electron. A much higher time-resolution (by at least better than a factor of 10) would be required to detect excitation energy dependent differences in the generation process of the solvated electron, arising from ultrafast processes, such as electron scattering, localization into the band gap, and different below band gap electron formation mechanisms. Finally, the different solvation dynamics observed for the binding energy at half-maximum and the vertical electron binding energy suggest that states with a lower electron binding energy correlate with longer solvation times, potentially because more extensive solvent rearrangement is required to relax states with lower binding energy.
Acknowledgments
We thank David Stapfer and Markus Steger for technical support. This project has received funding from the European Union’s Horizon 2020 research and innovation program from the European Research Council under the Grant Agreement No 786636, and the research was supported by the NCCR MUST, funded by the Swiss National Science Foundation (SNSF), through ETH-FAST, and through SNSF project no. 200020_172472. C.W.W. acknowledges funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 801459 - FP-RESOMUS - and the Swiss National Science Foundation through the NCCR MUST. R.S. is a grateful recipient of a Humboldt Research Prize form the Alexander von Humboldt Foundation and a Mildred Dresselhaus Guestprofessorship from the Centre for Ultrafast Imaging in Hamburg.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpca.0c06935.
Details on the experiment and data analysis (PDF)
The authors declare no competing financial interest.
Notes
Data required to reproduce the results presented in the paper can be found in the open access data collection of ETHZ at https://doi.org/10.3929/ethz-b-000438331.
Supplementary Material
References
- Yamamoto Y.; Suzuki Y.-I.; Tomasello G.; Horio T.; Karashima S.; Mitríc R.; Suzuki T. Time- and Angle-Resolved Photoemission Spectroscopy of Hydrated Electrons Near a Liquid Water Surface. Phys. Rev. Lett. 2014, 112, 187603. 10.1103/PhysRevLett.112.187603. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y.-I. I.; Karashima S.; Adachi S.; Suzuki T. Wavelength Dependence of UV Photoemission from Solvated Electrons in Bulk Water, Methanol, and Ethanol. J. Phys. Chem. A 2016, 120, 1153–1159. 10.1021/acs.jpca.5b09601. [DOI] [PubMed] [Google Scholar]
- Elles C. G.; Jailaubekov A. E.; Crowell R. A.; Bradforth S. E. Excitation-Energy Dependence of the Mechanism for Two-Photon Ionization of Liquid H2O and D2O from 8.3 to 12.4eV. J. Chem. Phys. 2006, 125, 044515. 10.1063/1.2217738. [DOI] [PubMed] [Google Scholar]
- Young R. M.; Neumark D. M. Dynamics of Solvated Electrons in Clusters. Chem. Rev. 2012, 112, 5553–5577. 10.1021/cr300042h. [DOI] [PubMed] [Google Scholar]
- Chen X.; Bradforth S. E. The Ultrafast Dynamics of Photodetachment. Annu. Rev. Phys. Chem. 2008, 59, 203–231. 10.1146/annurev.physchem.58.032806.104702. [DOI] [PubMed] [Google Scholar]
- Bragg A. E.; Verlet J. R. R. R.; Kammrath A.; Cheshnovsky O.; Neumark D. M. Electronic Relaxation Dynamics of Water Cluster Anions. J. Am. Chem. Soc. 2005, 127, 15283–15295. 10.1021/ja052811e. [DOI] [PubMed] [Google Scholar]
- Griffin G. B.; Young R. M.; Ehrler O. T.; Neumark D. M. Electronic Relaxation Dynamics in Large Anionic Water Clusters: (H2O) n– and (D2O) n– (N = 25–200). J. Chem. Phys. 2009, 131, 194302. 10.1063/1.3263419. [DOI] [PubMed] [Google Scholar]
- Savolainen J.; Uhlig F.; Ahmed S.; Hamm P.; Jungwirth P. Direct Observation of the Collapse of the Delocalized Excess Electron in Water. Nat. Chem. 2014, 6, 697–701. 10.1038/nchem.1995. [DOI] [PubMed] [Google Scholar]
- Herbert J. M.; Coons M. P. The Hydrated Electron. Annu. Rev. Phys. Chem. 2017, 68, 447. 10.1146/annurev-physchem-052516-050816. [DOI] [PubMed] [Google Scholar]
- Uhlig F.; Marsalek O.; Jungwirth P. Electron at the Surface of Water: Dehydrated or Not?. J. Phys. Chem. Lett. 2013, 4, 338–343. 10.1021/jz3020953. [DOI] [PubMed] [Google Scholar]
- Casey J. R.; Schwartz B. J.; Glover W. J. Free Energies of Cavity and Noncavity Hydrated Electrons Near the Instantaneous Air/Water Interface. J. Phys. Chem. Lett. 2016, 7, 3192–3198. 10.1021/acs.jpclett.6b01150. [DOI] [PubMed] [Google Scholar]
- Borgis D.; Rossky P. J.; Turi L. Electronic Excited State Lifetimes of Anionic Water Clusters: Dependence on Charge Solvation Motif. J. Phys. Chem. Lett. 2017, 8, 2304–2309. 10.1021/acs.jpclett.7b00555. [DOI] [PubMed] [Google Scholar]
- Horio T.; Shen H.; Adachi S.; Suzuki T. Photoelectron Spectra of Solvated Electrons in Bulk Water, Methanol, and Ethanol. Chem. Phys. Lett. 2012, 535, 12–16. 10.1016/j.cplett.2012.03.051. [DOI] [Google Scholar]
- Coons M. P.; You Z. Q.; Herbert J. M. The Hydrated Electron at the Surface of Neat Liquid Water Appears to Be Indistinguishable from the Bulk Species. J. Am. Chem. Soc. 2016, 138, 10879–10886. 10.1021/jacs.6b06715. [DOI] [PubMed] [Google Scholar]
- Stähler J.; Deinert J.-C. C.; Wegkamp D.; Hagen S.; Wolf M. Real-Time Measurement of the Vertical Binding Energy during the Birth of a Solvated Electron. J. Am. Chem. Soc. 2015, 137, 3520–3524. 10.1021/ja511571y. [DOI] [PubMed] [Google Scholar]
- Sagar D. M.; Bain C. D.; Verlet J. R. R. Hydrated Electrons at the Water/Air Interface. J. Am. Chem. Soc. 2010, 132, 6917–6919. 10.1021/ja101176r. [DOI] [PubMed] [Google Scholar]
- Nowakowski P. J.; Woods D. A.; Verlet J. R. R. Charge Transfer to Solvent Dynamics at the Ambient Water/Air Interface. J. Phys. Chem. Lett. 2016, 7, 4079–4085. 10.1021/acs.jpclett.6b01985. [DOI] [PubMed] [Google Scholar]
- Elkins M. H.; Williams H. L.; Neumark D. M. Isotope Effect on Hydrated Electron Relaxation Dynamics Studied with Time-Resolved Liquid Jet Photoelectron Spectroscopy. J. Chem. Phys. 2016, 144, 184503. 10.1063/1.4948546. [DOI] [PubMed] [Google Scholar]
- Riley J. W.; Wang B.; Woodhouse J. L.; Assmann M.; Worth G. A.; Fielding H. H. Unravelling the Role of an Aqueous Environment on the Electronic Structure and Ionization of Phenol Using Photoelectron Spectroscopy. J. Phys. Chem. Lett. 2018, 9, 678–682. 10.1021/acs.jpclett.7b03310. [DOI] [PubMed] [Google Scholar]
- Coe J. V.; Arnold S. T.; Eaton J. G.; Lee G. H.; Bowen K. H. Photoelectron Spectra of Hydrated Electron Clusters: Fitting Line Shapes and Grouping Isomers. J. Chem. Phys. 2006, 125, 14315. 10.1063/1.2212415. [DOI] [PubMed] [Google Scholar]
- Ma L.; Majer K.; Chirot F.; Von Issendorff B. Low Temperature Photoelectron Spectra of Water Cluster Anions. J. Chem. Phys. 2009, 131, 144303. 10.1063/1.3245859. [DOI] [PubMed] [Google Scholar]
- Ehrler O. T.; Neumark D. M. Dynamics of Electron Solvation in Molecular Clusters. Acc. Chem. Res. 2009, 42, 769–777. 10.1021/ar800263z. [DOI] [PubMed] [Google Scholar]
- Lietard A.; Verlet J. R. R. R. Selectivity in Electron Attachment to Water Clusters. J. Phys. Chem. Lett. 2019, 10, 1180–1184. 10.1021/acs.jpclett.9b00275. [DOI] [PubMed] [Google Scholar]
- Faubel M.; Siefermann K. R.; Liu Y.; Abel B. Ultrafast Soft X-Ray Photoelectron Spectroscopy at Liquid Water Microjets. Acc. Chem. Res. 2012, 45, 120–130. 10.1021/ar200154w. [DOI] [PubMed] [Google Scholar]
- Turi L.; Rossky P. J. Theoretical Studies of Spectroscopy and Dynamics of Hydrated Electrons. Chem. Rev. 2012, 112, 5641–5674. 10.1021/cr300144z. [DOI] [PubMed] [Google Scholar]
- Kambhampati P.; Son D. H.; Kee T. W.; Barbara P. F. Solvation Dynamics of the Hydrated Electron Depends on Its Initial Degree of Electron Delocalization. J. Phys. Chem. A 2002, 106, 2374–2378. 10.1021/jp014291p. [DOI] [Google Scholar]
- Lian R.; Oulianov D. A.; Shkrob I. A.; Crowell R. A. Geminate Recombination of Electrons Generated by Above-the-Gap (12.4eV) Photoionization of Liquid Water. Chem. Phys. Lett. 2004, 398, 102–106. 10.1016/j.cplett.2004.09.081. [DOI] [Google Scholar]
- Kratz S.; Torres-Alacan J.; Urbanek J.; Lindner J.; Vöhringer P. Geminate Recombination of Hydrated Electrons in Liquid-to-Supercritical Water Studied by Ultrafast Time-Resolved Spectroscopy. Phys. Chem. Chem. Phys. 2010, 12, 12169. 10.1039/c0cp00762e. [DOI] [PubMed] [Google Scholar]
- Paik D. H.; Lee I. R.; Yang D. S.; Baskin J. S.; Zewail A. H. Electrons in Finite-Sized Water Cavities: Hydration Dynamics Observed in Real Time. Science 2004, 306, 672–675. 10.1126/science.1102827. [DOI] [PubMed] [Google Scholar]
- Wilhelm J.; VandeVondele J.; Rybkin V. V. Dynamics of the Bulk Hydrated Electron from Many-Body Wave-Function Theory. Angew. Chem., Int. Ed. 2019, 58, 3890–3893. 10.1002/anie.201814053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhaus D.; Yamamoto Y.; Suzuki T.; Signorell R. Genuine Binding Energy of the Hydrated Electron. Sci. Adv. 2017, 3, e1603224 10.1126/sciadv.1603224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuch T.; Buck U. Sodium Doped Hydrogen Bonded Clusters: Solvated Electrons and Size Selection. Chem. Phys. Lett. 2013, 579, 1–10. 10.1016/j.cplett.2013.06.011. [DOI] [Google Scholar]
- West A. H. C.; Yoder B. L.; Luckhaus D.; Saak C. M.; Doppelbauer M.; Signorell R. Angle-Resolved Photoemission of Solvated Electrons in Sodium-Doped Clusters. J. Phys. Chem. Lett. 2015, 6, 1487–1492. 10.1021/acs.jpclett.5b00477. [DOI] [PubMed] [Google Scholar]
- Pizzochero M.; Ambrosio F.; Pasquarello A. Picture of the Wet Electron: A Localized Transient State in Liquid Water. Chem. Sci. 2019, 10, 7442–7448. 10.1039/C8SC05101A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefermann K. R.; Liu Y.; Lugovoy E.; Link O.; Faubel M.; Buck U.; Winter B.; Abel B. Binding Energies, Lifetimes and Implications of Bulk and Interface Solvated Electrons in Water. Nat. Chem. 2010, 2, 274–279. 10.1038/nchem.580. [DOI] [PubMed] [Google Scholar]
- Signorell R.; Yoder B. L.; West A. H. C. C.; Ferreiro J. J.; Saak C.-M. M. Angle-Resolved Valence Shell Photoelectron Spectroscopy of Neutral Nanosized Molecular Aggregates. Chem. Sci. 2014, 5, 1283–1295. 10.1039/C3SC53423E. [DOI] [Google Scholar]
- Madsen D.; Thomsen C. L.; Thøgersen J.; Keiding S. R. Temperature Dependent Relaxation and Recombination Dynamics of the Hydrated Electron. J. Chem. Phys. 2000, 113, 1126–1134. 10.1063/1.481891. [DOI] [Google Scholar]
- Bartels D. M.; Crowell R. A. Photoionization Yield vs Energy in H2O and D2O. J. Phys. Chem. A 2000, 104, 3349–3355. 10.1021/jp9941460. [DOI] [Google Scholar]
- Crowell R. A.; Bartels D. M. Multiphoton Ionization of Liquid Water with 3.0–5.0eV Photons. J. Phys. Chem. 1996, 100, 17940–17949. 10.1021/jp9610978. [DOI] [Google Scholar]
- Pépin C.; Goulet T.; Houde D.; Jay-Gerin J. P. Observation of a Continuous Spectral Shift in the Solvation Kinetics of Electrons in Neat Liquid Deuterated Water. J. Phys. Chem. A 1997, 101, 4351–4360. 10.1021/jp970354l. [DOI] [Google Scholar]
- Loh Z. H.; et al. Observation of the Fastest Chemical Processes in the Radiolysis of Water. Science 2020, 367, 179–182. 10.1126/science.aaz4740. [DOI] [PubMed] [Google Scholar]
- Lan J.; Kapil V.; Gasparotto P.; Ceriotti M.; Iannuzzi M.; Rybkin V. V.. Simulating the Ghost: Quantum Dynamics of the Solvated Electron. 2020. 10.26434/chemrxiv.12551234.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabattoni A.; et al. Photoelectron Spectroscopy of Large Water Clusters Ionized by an XUV Comb. J. Phys. Photonics 2020, 2, 035007. 10.1088/2515-7647/ab92b1. [DOI] [Google Scholar]
- Lübcke A.; Buchner F.; Heine N.; Hertel I. V.; Schultz T. Time-Resolved Photoelectron Spectroscopy of Solvated Electrons in Aqueous NaI Solution. Phys. Chem. Chem. Phys. 2010, 12, 14629–14634. 10.1039/c0cp00847h. [DOI] [PubMed] [Google Scholar]
- Buchner F.; Schultz T.; Lübcke A. Solvated Electrons at the Water-Air Interface: Surface versus Bulk Signal in Low Kinetic Energy Photoelectron Spectroscopy. Phys. Chem. Chem. Phys. 2012, 14, 5837. 10.1039/c2cp23305c. [DOI] [PubMed] [Google Scholar]
- Shreve A. T.; Elkins M. H.; Neumark D. M. Photoelectron Spectroscopy of Solvated Electrons in Alcohol and Acetonitrile Microjets. Chem. Sci. 2013, 4, 1633–1639. 10.1039/c3sc22063j. [DOI] [Google Scholar]
- Elkins M. H.; Williams H. L.; Shreve A. T.; Neumark D. M. Relaxation Mechanism of the Hydrated Electron. Science 2013, 342, 1496–1499. 10.1126/science.1246291. [DOI] [PubMed] [Google Scholar]
- Karashima S.; Yamamoto Y. I.; Suzuki T. Resolving Nonadiabatic Dynamics of Hydrated Electrons Using Ultrafast Photoemission Anisotropy. Phys. Rev. Lett. 2016, 116, 137601. 10.1103/PhysRevLett.116.137601. [DOI] [PubMed] [Google Scholar]
- Gartmann T. E.; Ban L.; Yoder B. L.; Hartweg S.; Chasovskikh E.; Signorell R. Relaxation Dynamics and Genuine Properties of the Solvated Electron in Neutral Water Clusters. J. Phys. Chem. Lett. 2019, 10, 4777–4782. 10.1021/acs.jpclett.9b01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y.; Suzuki T. Ultrafast Dynamics of Water Radiolysis: Hydrated Electron Formation, Solvation, Recombination, and Scavenging. J. Phys. Chem. Lett. 2020, 11, 5510–5516. 10.1021/acs.jpclett.0c01468. [DOI] [PubMed] [Google Scholar]
- Svoboda V.; Michiels R.; LaForge A. C.; Med J.; Stienkemeier F.; Slavíček P.; Wörner H. J. Real-Time Observation of Water Radiolysis and Hydrated Electron Formation Induced by Extreme-Ultraviolet Pulses. Sci. Adv. 2020, 6, eaaz0385 10.1126/sciadv.aaz0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C. L.; Madsen D.; Keiding S. R.; Thøgersen J.; Christiansen O. Two-Photon Dissociation and Ionization of Liquid Water Studied by Femtosecond Transient Absorption Spectroscopy. J. Chem. Phys. 1999, 110, 3453–3462. 10.1063/1.478212. [DOI] [Google Scholar]
- Winter B.; Weber R.; Widdra W.; Dittmar M.; Faubel M.; Hertel I. V. Full Valence Band Photoemission from Liquid Water Using EUV Synchrotron Radiation. J. Phys. Chem. A 2004, 108, 2625–2632. 10.1021/jp030263q. [DOI] [Google Scholar]
- Chen W.; Ambrosio F.; Miceli G.; Pasquarello A. Ab Initio Electronic Structure of Liquid Water. Phys. Rev. Lett. 2016, 117, 186401. 10.1103/PhysRevLett.117.186401. [DOI] [PubMed] [Google Scholar]
- Ambrosio F.; Guo Z.; Pasquarello A. Absolute Energy Levels of Liquid Water. J. Phys. Chem. Lett. 2018, 9, 3212–3216. 10.1021/acs.jpclett.8b00891. [DOI] [PubMed] [Google Scholar]
- Gaiduk A. P.; Pham T. A.; Govoni M.; Paesani F.; Galli G. Electron Affinity of Liquid Water. Nat. Commun. 2018, 9, 247. 10.1038/s41467-017-02673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchiz V. H.; Kloepfer J. A.; Germaine A. C.; Lenchenkov V. A.; Bradforth S. E. Map for the Relaxation Dynamics of Hot Photoelectrons Injected into Liquid Water via Anion Threshold Photodetachment and above Threshold Solvent Ionization. J. Phys. Chem. A 2001, 105, 1711–1723. 10.1021/jp003974m. [DOI] [Google Scholar]
- Nishitani J.; Yamamoto Y.; West C. W.; Karashima S.; Suzuki T. Binding Energy of Solvated Electrons and Retrieval of True UV Photoelectron Spectra of Liquids. Sci. Adv. 2019, 5, eaaw6896 10.1126/sciadv.aaw6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertwig A.; Hippler H.; Unterreiner A. N. Transient Spectra, Formation, and Geminate Recombination of Solvated Electrons in Pure Water UV-Photolysis: An Alternative View. Phys. Chem. Chem. Phys. 1999, 1, 5633–5642. 10.1039/a906950j. [DOI] [Google Scholar]
- Mozumder A. Conjecture on Electron Trapping in Liquid Water. Int. J. Radiat. Appl. Instrumentation. Part C. Radiat. Phys. Chem. 1988, 32, 287–291. 10.1016/1359-0197(88)90200-7. [DOI] [Google Scholar]
- Signorell R. Electron Scattering in Liquid Water and Amorphous Ice: A Striking Resemblance. Phys. Rev. Lett. 2020, 124, 205501. 10.1103/PhysRevLett.124.205501. [DOI] [PubMed] [Google Scholar]
- Michaud M.; Wen A.; Sanche L. Cross Sections for Low-Energy (1–100eV) Electron Elastic and Inelastic Scattering in Amorphous Ice. Radiat. Res. 2003, 159, 3–22. 10.1667/0033-7587(2003)159[0003:CSFLEE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Signorell R.; Goldmann M.; Yoder B. L.; Bodi A.; Chasovskikh E.; Lang L.; Luckhaus D. Nanofocusing, Shadowing, and Electron Mean Free Path in the Photoemission from Aerosol Droplets. Chem. Phys. Lett. 2016, 658, 1–6. 10.1016/j.cplett.2016.05.046. [DOI] [Google Scholar]
- Gartmann T. E.; Hartweg S.; Ban L.; Chasovskikh E.; Yoder B. L.; Signorell R. Electron Scattering in Large Water Clusters from Photoelectron Imaging with High Harmonic Radiation. Phys. Chem. Chem. Phys. 2018, 20, 16364–16371. 10.1039/C8CP02148A. [DOI] [PubMed] [Google Scholar]
- Yoder B. L.; West A. H. C. C.; Schläppi B.; Chasovskikh E.; Signorell R. A Velocity Map Imaging Photoelectron Spectrometer for the Study of Ultrafine Aerosols with a Table-Top VUV Laser and Na-Doping for Particle Sizing Applied to Dimethyl Ether Condensation. J. Chem. Phys. 2013, 138, 44202. 10.1063/1.4788620. [DOI] [PubMed] [Google Scholar]
- Yoder B. L.; Litman J. H.; Forysinski P. W.; Corbett J. L.; Signorell R. Sizer for Neutral Weakly Bound Ultrafine Aerosol Particles Based on Sodium Doping and Mass Spectrometric Detection. J. Phys. Chem. Lett. 2011, 2, 2623–2628. 10.1021/jz201086v. [DOI] [Google Scholar]
- Baumann A.; et al. Weak-Field Few-Femtosecond VUV Photodissociation Dynamics of Water Isotopologues. Phys. Rev. A: At., Mol., Opt. Phys. 2017, 96, 013428. 10.1103/PhysRevA.96.013428. [DOI] [Google Scholar]
- van Stokkum I. H. M.; Larsen D. S.; van Grondelle R. Global and Target Analysis of Time-Resolved Spectra. Biochim. Biophys. Acta, Bioenerg. 2004, 1657, 82–104. 10.1016/j.bbabio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Hara A.; Yamamoto Y. I.; Suzuki T. Solvated Electron Formation from the Conduction Band of Liquid Methanol: Transformation from a Shallow to Deep Trap State. J. Chem. Phys. 2019, 151, 114503. 10.1063/1.5116818. [DOI] [PubMed] [Google Scholar]
- Mosyak A. A.; Prezhdo O. V.; Rossky P. J. Solvation Dynamics of an Excess Electron in Methanol and Water. J. Chem. Phys. 1998, 109, 6390–6395. 10.1063/1.477282. [DOI] [Google Scholar]
- Signorell R. Can Current Experimental Data Exclude Non-Gaussian Genuine Band Shapes in Ultraviolet Photoelectron Spectra of the Hydrated Electron?. J. Phys. Chem. Lett. 2020, 11, 1516–1519. 10.1021/acs.jpclett.0c00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartweg S.; Yoder B. L.; Garcia G. A.; Nahon L.; Signorell R. Size-Resolved Photoelectron Anisotropy of Gas Phase Water Clusters and Predictions for Liquid Water. Phys. Rev. Lett. 2017, 118, 103402. 10.1103/PhysRevLett.118.103402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


