Abstract
Malaysia is faced with a high HIV/AIDS burden that poses a public health threat. We constructed and applied a compartmental model to understand the spread and control of HIV/AIDS in Malaysia. A simple model for HIV and AIDS disease that incorporates condom and uncontaminated needle-syringes interventions and addresses the relative impact of given treatment therapy for infected HIV newborns on reducing HIV and AIDS incidence is presented. We demonstrated how treatment therapy for new-born babies and the use of condoms or uncontaminated needle-syringes impact the dynamics of HIV in Malaysia. The model was calibrated to HIV and AIDS incidence data from Malaysia from 1986 to 2011. The epidemiological parameters are estimated using Bayesian inference via Markov chain Monte Carlo simulation method. The reproduction number optimal for control of the HIV/AIDS disease obtained suggests that the disease-free equilibrium was unstable during the 25 years. However, the results indicated that the use of condoms and uncontaminated needle-syringes are pivotal intervention control strategies; a comprehensive adoption of the intervention may help stop the spread of HIV disease. Treatment therapy for newborn babies is also of high value; it reduces the epidemic peak. The combined effect of condom use or uncontaminated needle-syringe is more pronounced in controlling the spread of HIV/AIDS.
Keywords: Mathematical transmission modeling, HIV/AIDS incidence, Interventions, Bayesian inference, Basic reproduction number
1. Introduction
Since the first case of human immunodeficiency virus (HIV) in Malaysia was reported in 1986, HIV, which leads to acquired immunodeficiency syndrome (AIDS), has spread to all parts of Malaysia (MOH, 2015). According to HIV Estimations and Projections under the Ministry of Health, at the end of 2013, Malaysia was estimated to have 86,324 people living with HIV (PLHIV) (MOH, 2012, 2015). However, there is still a need for data collection from the country, indicating that the magnitude and trend of HIV/AIDS are very high (MOH, 2015). The National Strategic Plan, established in 2002 under the auspices of the Ministry of Health, has made significant progress in controlling the spread of HIV/AIDS nation-wide (MOH, 2012). For instance, a significant reduction in numbers was reported from 6978 HIV cases in 2002 to 3393 HIV cases reported in 2013 (MOH, 2012). Some of the problems and challenges identified include how to assess the effectiveness of various preventive measures and control strategies accurately from the public health policy point of view (Apenteng & Ismail, 2015). These objectives are clearly stated in the Malaysia National Strategic Plan on HIV/AIDS prevention, control, and treatment programmes (MOH, 2012, 2015). Malaysia is a country with a concentrated HIV epidemic, with infection rates as high as 5 percent among most-at-risk populations (MARPS). This most at-risk population includes people who inject drugs (PWID), sex workers, transgender, and men who have sex with men (MSM) subpopulation (Huang & Hussein, 2004; Reid et al., 2007; Singh et al., 2013).
Mathematical models are powerful tools for investigating human infectious diseases, such as HIV and AIDS, contributing to the understanding of the dynamics of infections which can provide valuable information for public health policymakers (Bramson et al., 2015; Luboga et al., 2010; Padilla, Reyes, & Connolly, 2012). Many models have been developed to analyze the effectiveness of the control strategies in the past (Abdullah et al., 2002; Levine et al., 2015; Negredo et al., 2015; Nyabadza & Mukandavire, 2011; Sripan et al., 2015). Epidemic models date back to the early twentieth century, to a set of three articles from 1927, 1932, and 1933 by Kermack and McKendrick (Kermack & McKendrick, 1927; 1991a; 1991b). Today, there is a growing need to model the effects of environmental factors, including treatment therapy on newborn babies, condom use, and the supply of uncontaminated needle-syringe on the spread of HIV and AIDS. Such models will provide an understanding of how the spread of HIV/AIDS could be minimized. Cai, Guo, & Wang, 2014 investigated an HIV/AIDS epidemic model with treatment to find out the impact of remedy on HIV (Cai, Guo, & Wang, 2014). Huo and Feng presented global stability for HIV and AIDS epidemic models at different latent levels and treatment in 2013 (Huo & Feng, 2013). Mathematical analyses of a different strain of HIV/AIDS and at population-based levels with antiretroviral therapy have been modeled (Bhunu et al., 2009; Eaton & Hallett, 2014; Falconer et al., 2009; Tamizhmani et al., 2004; Wilson & Zhang, 2011). Greenhalgh, Doyle, & Lewis, 2001 formulated a mathematical treatment of AIDS and the use of condoms in San Francisco, USA. They suggested that the use of condoms has important implications for control of the disease to reduce the spread of HIV (Greenhalgh, Doyle, & Lewis, 2001). In the past, the use of Markov chain Monte Carlo (MCMC) techniques in fitting statistical epidemiological models was conventional (Berzuini et al., 1997; Calderhead, 2008). Recently, MCMC has been put to advantageous use in estimating existing and formulated models (Neal and Terry Huang 2015; Solonen et al., 2013; Sun, Xiao, Peng, & Wang, 2013; Xun et al., 2013). The computational intractability of the model developed in this paper will be addressed using MCMC methods.
To the best of our knowledge, existing mathematical models of HIV dynamics models failed to incorporate the following: firstly, the infected individuals are capable of having children that are either infected or do not have the disease; secondly, by putting the new-born babies who may be exposed to HIV on treatment therapy, and thirdly, incorporating the use of condom as well as a supply of uncontaminated needle-syringes. We used compartmental models to understand the effects of persistent spread and the control of HIV in Malaysia. We assumed homogeneous mixing among the entire high-risk population for simplicity. We then formulated a nonlinear mathematical model to describe the impact of preventive measures on the spread of HIV. We used this formulated model to understand the HIV epidemic in Malaysia comprehensively and to explore policy-related questions, including an investigation of the impact of treatment therapy for new-born babies and the use of condoms or uncontaminated needle syringes on the dynamics of HIV in Malaysia. It is assumed that susceptible become infected via sexual contacts as well as people who inject drugs and that all of the infectives eventually developed AIDS. This approach is different from the ones in most of the papers referenced or cited. It is hoped that the empirical results will improve our understanding of the HIV and AIDS epidemic. Our ultimate goal is to help in formulating a useful model for public-health control strategies. We expect our intermediate results may apply to other countries to control the spread of HIV/AIDS.
2. Materials and methods
2.1. Data
We constructed a deterministic population-based compartmental model of HIV/AIDS transmission with intervention measures. Data for the model were obtained from Malaysia epidemiological data on HIV and AIDS yearly reported cases (MOH, 2012). We chose the year 1986 as a starting point since 1986 was when the first HIV case made its debut, HIV has become one of the country’s most severe health and development challenges. In 1986, the total population in Malaysia was 16,329,400. In 1986, three individuals clinically confirmed HIV patients, and these cases represented the initial infected (compartment), and one individual developed AIDS (the compartment).
2.2. Formulation of the model
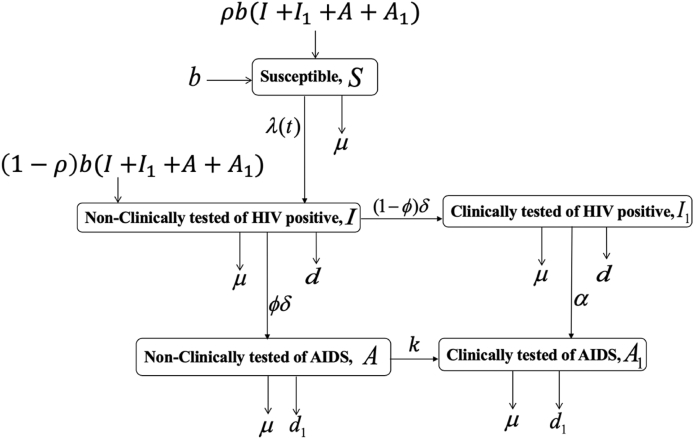
The five compartments model adopted here is specified by adding disease preventive measures: treatment therapy for new cases and condom use or supply of uncontaminated needle-syringes. Let be the number of susceptible individuals in the population, be the number of non-clinically confirmed (early-stage infection) HIV carriers, be the number of clinically confirmed HIV positive individuals, ) be the number of non-clinically tested AIDS cases, and be the number of clinically confirmed AIDS patients at a time . The size of the total population is given by . We assume the disease occurs with equal probability across all sexually active age groups. Susceptible individuals become infected at rates , where is the product of preventive measure (condom use or supply of uncontaminated syringe-needle) and the efficacy of the intervention to prevent the acquisition of infection by susceptible individuals. The parameter is the probabilities of transmission per either sexual action or contaminated syringe-needle use. The assumption here is that the protection (preventability) reduces the risk of infection and transmission, . The endpoint would depicts that preventive measure is useless or meaningless, and implies that preventive measure is comprehensively effective. The parameters , and are modification factors accounting for varying levels of spreading of clinically tested HIV-positive, non-clinically tested AIDS and clinically tested AIDS individuals. Moreover, we incorporate infants whose mothers are HIV/AIDS patients. Krist & Crawford-Faucher, 20022 showed that aggressive maternal antiretroviral treatment therapy and management during pregnancy and labor, followed by after birth treatment of neonatal zidovudine decreases the risk of mother to baby transmission of HIV (Krist & Crawford-Faucher, 2002). Antiretroviral therapy can suppress HIV and stop the progression of the disease (WHO, 2014). Prevention of vertical transmission of HIV has been at the forefront of global prevention efforts. Let represent birth rate; we assume that out of newborns who are exposed to maternal HIV infection, a fraction (where is the product of the treatment rate and diagnosis rate) are identified and successfully undergo treatment therapy. That is, babies move to the susceptible class. The remaining babies are not identified during pregnancy or at birth and enter the non-clinically tested compartment . Let be the rate at which individuals exit the non-clinically tested HIV class, a fraction of where , progress to the non-clinically tested AIDS class ; whereas the remaining of non-clinically tested HIV patients move to the class of individuals who are clinically tested HIV positive. The progression rate of clinically tested HIV individuals to clinically tested AIDS class is represented by . We also assume that natural death is constant across all compartments of the model at rate The parameters anddenote additional death rates due to HIV and AIDS positive, respectively. Finally, represents the progression of non-clinically AIDS to clinically tested AIDS class. See Fig. 1 for the flow diagram of the model.
Fig. 1.
Flow diagram of the model.
With these assumptions, the model is described by the following set of nonlinear differential equations:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
The total population is
| (6) |
To determine whether the disease continues to spread, we must find the stability of the disease-free equilibrium point. The basic reproduction number () is the expected number of secondary cases produced by a single infection in a completely susceptible population(Van Den Driessche & James, 2002; Hethcote, 2000; Huo & Feng, 2013; Jones, 2013). Thus, the disease will not continue to spread if . To determine whether the disease will continue to spread, we must study the dynamics when. If the infection persists (), then either the system will be stable around the interior equilibrium, or there may exist a periodic attractor. Using the notations by Van Den Driessche and James (2002), is the rate of appearance of new infections in compartment , and represents the rate of infections from one compartment to another. Here, and are given by
| (7) |
| (8) |
The corresponding Jacobian matrices at the disease-free equilibrium () are as follows:
where and .
The basic reproduction number () is the spectral radius of. That is,
where
and
3. Model validation
We explore the use of Markov chain Monte Carlo (MCMC) simulation method to estimate uncertainty in all the unknown parameters incorporated in our proposed model. MCMC methods are useful to obtain samples from the posterior distributions(Putter et al., 2002). In choosing an optimal parameterization to fit this model, several considerations play a role. For example, it is essential to note that it is advantageous to transform the unknown parameters to attempt to minimize correlation and to make sure that constraints on parameters are naturally fulfilled. Let denote the epidemiological data, and denote a set of the model parameters, as well as the missing data. Let the joint probability distribution to be overall random quantities. The joint distribution consists of two main parts, that is the prior distribution and likelihood. These two will give a full probability model as. We use the Bayes theorem to determine the distribution of conditional on (for more information see (Eaton & Hallett, 2014)). For a good introduction to MCMC methods, we refer to (Apenteng & Ismail, 2015; Laine, 2008; Soetaert & Petzoldt, 2010). The posterior for the parameters are estimated as:
| (9) |
where is the sum of squares function and is a non-informative prior for indexing the model, where we assume that . To obtain proper results from the MCMC method, a constrained least squares approach is necessary to provide initial estimates of . In addition, the model is not identifiable, and thus constraints on the parameters are warranted. Local sensitivity analysis (Soetaert & Petzoldt, 2010) was used to obtain suitable initial parameter estimates of . For the reciprocal of the error variance, a gamma distribution is used:
| (10) |
The reciprocal of the error variance at each MCMC step is sampled from a gamma distribution (Gelman et al., 2013) as follows:
| (11) |
where and are input arguments to the function and the number of observations, respectively. Observe that the function in solves the system of equations (1), (2), (3), (4), (5), (6). The unknown parameters were estimated using the FME package in R software (Soetaert & Petzoldt, 2010). The estimated parameters obtained were used as an initial guess to ensure good convergence of the MCMC chain (Apenteng & Ismail, 2015). The Metropolis Hasting algorithm (Christen et al., 2005; Haario et al., 2006; Solonen et al., 2013) was applied to our epidemiological dataset. The method samples from the posterior distribution by constructing Markov chains that converge to the target posterior distribution.
4. Results
The estimated epidemiological parameters, together with their 95% credible intervals (CI), are shown in Table 1, and these were used to calculate the basic reproduction number. We observe that estimated parameters have shorter CI widths. Interestingly, the lower limits of parameters and are less than zero. This, of course, is not surprising since the system is underdetermined.
Table 1.
Summary of the estimated parameters.
| Parameter | Estimated Value | 95% CI | Source |
|---|---|---|---|
| 9.9100e-01 | (9.8885e-01, 9.9314e-01) | Estimated | |
| 8.2160e-02 | (8.1993e-02, 8.2327e-02) | Estimated | |
| 8.9020e-01 | (8.8691e-01, 8.9348e-01) | Estimated | |
| 6.3300e-04 | (-6.5920e-05, 1.3319e-03) | Estimated | |
| 2.4950e-01 | (2.4801e-01, 2.5098e-01) | Estimated | |
| 9.9990e-01 | (9.9818e-01, 1.0016e+00) | Estimated | |
| 1.0861e-12 | (-6.2360e-03, 6.236e-03) | Estimated | |
| 4.8050e-02 | (4.4412e-02, 5.1687e-02) | Estimated | |
| 1.0400e-02 | (7.1726e-03, 1.3627e-02) | Estimated | |
| 3.3690e-01 | (3.3443e-01, 3.3937e-01) | Estimated | |
| 2.9560e-09 | (-2.2106e-03, 2.2107e-03) | Estimated | |
| 4.3740e-02 | (4.1194e-02, 4.6286e-02) | Estimated | |
| 4.8390e-03 | (2.8273e-03, 6.8507e-03) | Estimated | |
| 9.0990e-01 | (9.0712e-01, 9.1267e-01) | Estimated |
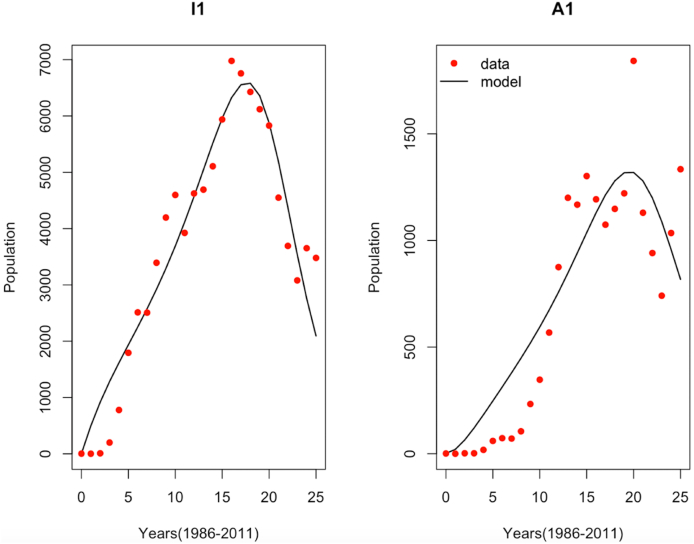
The plot of the data and the best-fit model for yearly reported cases of clinically tested HIV positive and AIDS are shown in Fig. 2. We observe that generally, the reported HIV and AIDS incidence data are calibrated and validated well during the 25 years. However, the calibration and validation during the first five and ten years for the HIV and AIDS incidence cases respectively perform poorly.
Fig. 2.
Fitted model for HIV and AIDS cases.
Table 2 presents a summary of the sensitivity analysis of the estimated epidemiological parameters. We observe that the parameter has both the highest while has the lowest and norms. This suggest that the parameter has the least effect on the output variables and less sensitive, while is the highly sensitivity parameter.
Table 2.
Summary of sensitivity parameters.
| Parameter | Estimated Values | Mean | Min | Max | ||
|---|---|---|---|---|---|---|
| 9.9100e-01 | 5.6000e+00 | 8.8000e-01 | 5.6000e+00 | 0.0000e+00 | 9.7000e+00 | |
| 8.2160e-02 | 4.9000e-01 | 7.6000e-02 | 1.5000e-01 | −5.2000e-01 | 9.2000e-01 | |
| 8.9020e-01 | 1.6000e+00 | 2.7000e-01 | −1.5000e+00 | −3.1000e+00 | 6.5000e-01 | |
| 6.3300e-04 | 2.3000e-03 | 3.8000e-04 | 2.2000e-03 | −5.7000e-04 | 4.6000e-03 | |
| 2.4950e-01 | 1.4000e+00 | 2.2000e-01 | 1.4000e+00 | 0.0000e+00 | 2.5000e+00 | |
| 9.9990e-01 | 3.8000e-01 | 5.6000e-02 | 3.8000e-01 | 0.0000e+00 | 5.2000e-01 | |
| 1.0861e-12 | 8.1000e-13 | 1.4000e-13 | 8.1000e-13 | 0.0000e+00 | 1.6000e-12 | |
| 4.8050e-02 | 2.8000e-01 | 4.4000e-02 | −2.8000e-01 | −4.9000e-01 | 0.0000e+00 | |
| 1.0400e-02 | 4.3000e-02 | 9.6000e-03 | 4.3000e-02 | 0.0000e+00 | 2.1000e-01 | |
| 3.3690e-01 | 4.2000e+00 | 6.8000e-01 | −4.2000e+00 | −8.4000e+00 | 0.0000e+00 | |
| 2.9560e-09 | 2.4000e-08 | 3.7000e-09 | −2.4000e-08 | −3.9000e-08 | 0.0000e+00 | |
| 4.3740e-02 | 6.7000e-02 | 1.2000e-02 | −6.7000e-02 | −1.6000e-01 | 4.3000e-03 | |
| 4.8390e-03 | 4.7000e-03 | 1.3000e-03 | −8.2000e-04 | −4.8000e-03 | 5.6000e-02 | |
| 9.0990e-01 | 1.1000e-01 | 1.7000e-02 | −1.1000e-01 | −1.9000e-01 | 0.0000e+00 |
The pairwise relationships among the parameters are shown in Fig. 3. We observe that most of the relationships are linear. For example, there is a strong positive linear relationship between parameters and . In addition, there is a strong negative linear relationship between and .
Fig. 3.
Pairs plot of the MCMC samples for the fourteen parameters.
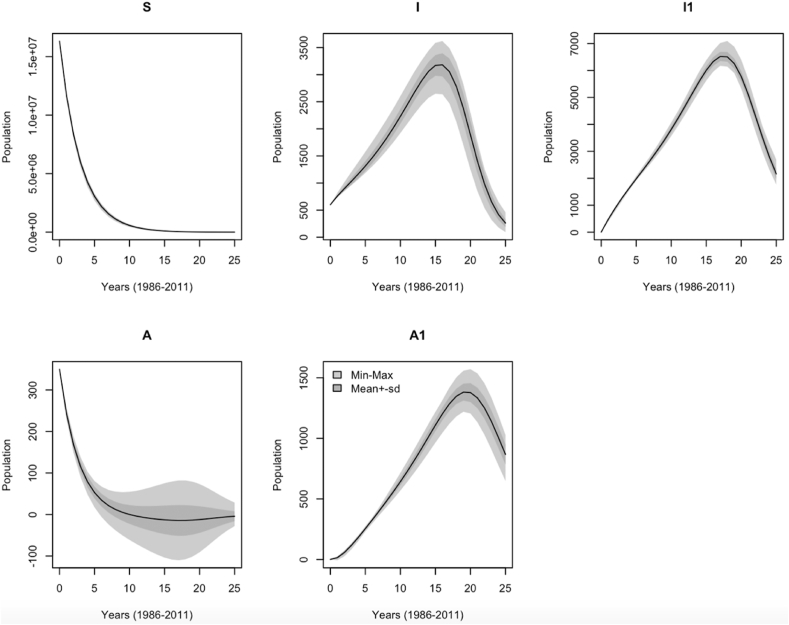
Fig. 4 shows yearly reported HIV positive and AIDS cases based on parameter distribution as generated with the MCMC simulation of the calibration period from 1986 to 2011. The high variances were observed in the following compartment order: (). This shows that there was predictive accuracy of the model, reflected by the variance of the predictive distribution. The large number for the variance is due to either the uncertainties in the model or noise in data collection, and the model fits the noisy data reasonably well.
Fig. 4.
Predictive envelopes of the model showing the sensitivity range of yearly reported HIV and AIDS cases. The light grey shade by Min-Max represents the minimum and maximum model response at each time step, whereas the dark grey shade by Meansd refers to the mean model response plus/minus one standard deviation.
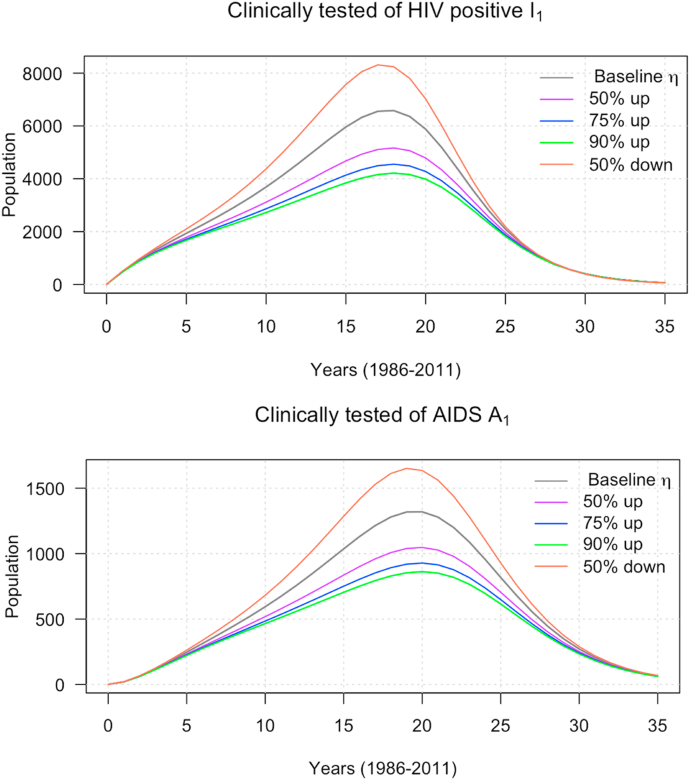
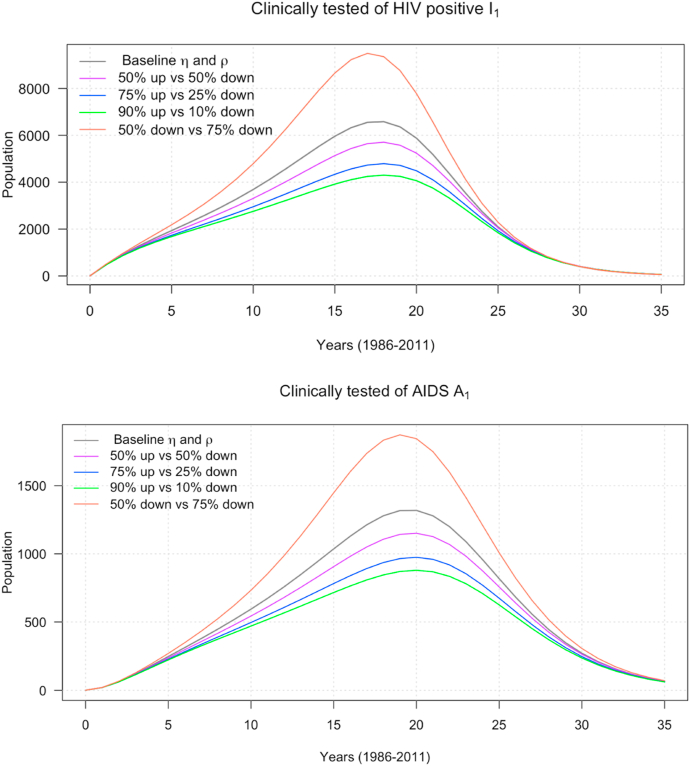
Next, we investigate the impact of the treatment therapy for new-born babies’ control parameter , and the use of condoms or uncontaminated needle syringes on HIV and AIDS incidence. We begin by considering the effect of increasing baseline parameter while is fixed. Fig. 5 depicts the impact as we increase baseline from 50% to 90% and decrease by 50%. We notice that both HIV and AIDS incidence cases have the same effect as we increase or decrease from the baseline. Furthermore, the spread of the HIV and AIDS peak between years 15 and 20. This suggests that increasing the baseline will help to reduce the spread of the disease.
Fig. 5.
The impact of control strategy on HIV and AIDS incidence cases.
Finally, we consider the combined effect of increasing or decreasing and decreasing from their baseline values. The results of the impact on HIV and AIDS incidence cases are shown in Fig. 6. From the figure, we observe that the peaks, both HIV and AIDS incidence drop as we increase and decrease . It is worth noting that the newborn who went through treatment therapy was comprehensively successful with almost equals to one. Thus, can only be decreasing in one direction compare with .
Fig. 6.
The impact of control strategy and on HIV and AIDS incidence cases.
Summary and conclusion
HIV disease is caused by the human immunodeficiency virus that infects humans. Over time, they cause AIDS. Once affected, the immune system is destroyed within a few months. In this paper, we modeled the practical impact of given treatment therapy for new-born babies exposed to HIV, and the use of condoms, or the supply of uncontaminated needle-syringes on reducing HIV and AIDS incidence in Malaysia. We assumed that there were sexual interactions between the susceptible and all the infectious sub-populations, and known exposed new-born babies undergo successful treatment therapy during pregnancy or after birth. The model parameters were estimated using the FME package in R software. We also explored the use of MCMC simulation method to determine the uncertainty in all the unknown parameters incorporated in our proposed model.
It is well known that if (basic reproduction number), then, the spread of disease will die out, and if, then, the infection will persist and continue to spread. Based on the estimated parameters, we computed the reproduction number to be 1.1799, implying that the disease-free equilibrium is unstable. This is not a good indicator from the public health point of view since the aim is to stabilize the infection at the disease-free equilibrium.
Sensitivity analysis was performed to identify the parameters that influence the spread of the disease; this is important to make forecasts and predictions. Sensitivity analysis can also help in public health policy decisions. The results show that the probability transmission per either unprotected sexual action or contaminated needle-syringe use had the highest sensitivity value, and impacts the dynamics of HIV transmission in Malaysia. As stated in the model formulation, the parameter, , decreases the transmission probability. More aggressive adoption of condoms and uncontaminated needle-syringe use is very crucial to control the spread of HIV. We varied and further examined the effects of the parameters and . As exhibited in Fig. 5, a larger reduces the peak of the epidemic curve significantly, and the number of infected individuals (the area under the epidemic curve). For example, in Fig. 5, with a baseline value , the epidemic peak happens at about 6500 clinically tested HIV, and about 1300 clinically confirmed AIDS patients. A 50% increment in the baseline produces less confirmed HIV/AIDS cases (purple curves). The reduction in HIV/AIDS patients is more pronounced with a higher increase in the ; see Fig. 5. We considered the combined effects of the and to demonstrate further, their impact on the dynamics of the proposed model. As shown in Fig. 6, the right combination implementation of these preventive measures is the key to curb the disease in the absence of a vaccine for a cure. We strongly recommend a comprehensive adoption of condoms or uncontaminated needle syringe use and treatment therapy for newborn babies preventive measures to the general public and public health policymakers to contain the disease.
Our results have some limitations, which should be acknowledged. Estimates for the model were obtained based on the yearly reported national cases of HIV/AIDS data. The lack of complete datasets from the onset of the HIV and AIDS disease to date may affect results. Nevertheless, our estimate of the epidemiological parameters, as well as the basic reproduction number of our study, is the first important step in quantifying the magnitude and trend of the HIV/AIDS epidemic in Malaysia. The study provides vital information to both researchers and public health policymakers.
Declaration of competing interest
The authors have no conflict of interest to declare.
Handling editor: Hulin Wu
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Ofosuhene O. Apenteng, Email: ofap@food.dtu.dk.
Prince P. Osei, Email: prinpep@gmail.com.
Bismark Oduro, Email: oduro@calu.edu.
Mavis Pearl Kwabla, Email: mkwabla@uhas.edu.gh.
Noor Azina Ismail, Email: nazina@um.edu.my.
References
- Abdullah A.S.M., Fielding R., Hedley A.J. Sexually Transmitted Diseases; 2002. Risk factors for sexually transmitted diseases and casual sex among Chinese patients attending sexually transmitted disease clinics in Hong Kong. [DOI] [PubMed] [Google Scholar]
- Apenteng O.O., Ismail N.A. A Markov chain Monte Carlo approach to estimate AIDS after HIV infection. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0131950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzuini C., Larizza C., Best N.G., Gilks W.R. Dynamic conditional independence models and Markov chain Monte Carlo methods. Journal of the American Statistical Association. 1997;92(440):1403–1412. [Google Scholar]
- Bhunu C.P., Garira W., Magombedze G. Mathematical analysis of a two strain HIV/AIDS model with antiretroviral treatment. Acta Biotheoretica. 2009;57(3):361–381. doi: 10.1007/s10441-009-9080-2. [DOI] [PubMed] [Google Scholar]
- Bramson H., Des Jarlais, Arasteh K., Nugent A., Guardino V., Feelemyer J., Hodel D. State laws, syringe exchange, and HIV among persons who inject drugs in the United States: History and effectiveness. Original Article Journal of Public Health Policy. 2015;36(2):212–230. doi: 10.1057/jphp.2014.54. [DOI] [PubMed] [Google Scholar]
- Cai L., Guo S., Wang S. Analysis of an extended HIV/AIDS epidemic model with treatment. Applied Mathematics and Computation. 2014;236:621–627. [Google Scholar]
- Calderhead B. 2008. A study of population MCMC for estimating factors over nonlinear ODE models. [Google Scholar]
- Christen J.A., Fox C., Andrés J. Markov chain Monte Carlo using an approximation radiocarbon dating-temporal and spatio-temporal models and inference view project numerical analysis of MCMC view project Markov chain Monte Carlo using an approximation. Article in Journal of Computational and Graphical Statistics. 2005;14(4):795–810. [Google Scholar]
- Eaton J.W., Hallett T.B. Why the proportion of transmission during early-stage HIV infection does not predict the long-term impact of treatment on HIV incidence. National Acad Sciences. 2014;111(45):16202–16207. doi: 10.1073/pnas.1323007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer K., Sandberg J.K., Reichard O., Alaeus A. HCV/HIV Co-infection at a large HIV outpatient clinic in Sweden: Feasibility and results of hepatitis C treatment. Scandinavian Journal of Infectious Diseases. 2009;41(11–12):881–885. doi: 10.3109/00365540903214272. [DOI] [PubMed] [Google Scholar]
- Gelman A., Carlin J.B., Stern H.S., Dunson D.B., Aki V., Rubin D.B. 3rd ed. CRC Press; 2013. Bayesian data analysis. [Google Scholar]
- Greenhalgh D., Doyle M., Lewis F. A mathematical treatment of AIDS and condom use. Mathematical Medicine and Biology: A Journal of the IMA. 2001;18(3):225–262. [PubMed] [Google Scholar]
- Haario H., Laine M., Mira A., Saksman E. Dram: Efficient adaptive MCMC. Statistics and Computing. 2006;16(4):339–354. [Google Scholar]
- Hethcote H.W. Mathematics of infectious diseases. SIAM Review. 2000;42(4):599–653. [Google Scholar]
- Huang M., Hussein H. The HIV/AIDS epidemic country paper: Malaysia. AIDS Education and Prevention. 2004;16(3 SUPPL):100–109. doi: 10.1521/aeap.16.3.5.100.35532. [DOI] [PubMed] [Google Scholar]
- Huo H.F., Feng L.X. Global stability for an HIV/AIDS epidemic model with different latent stages and treatment. Applied Mathematical Modelling. 2013;37(3):1480–1489. [Google Scholar]
- Jones J.H. 2013. Notes on R 0 1 the basic reproduction number in a nutshell. [Google Scholar]
- Kermack W.O., McKendrick A.G. A contribution to the mathematical theory of epidemics. Proceedings of the Royal Society A: Mathematical, Physical & Engineering Sciences. 1927;115(772):700–721. [Google Scholar]
- Kermack W.O., McKendrick A.G. Contributions to the mathematical theory of epidemics-II. The problem of endemicity. Bulletin of Mathematical Biology. 1991;53(1–2):57–87. doi: 10.1007/BF02464424. [DOI] [PubMed] [Google Scholar]
- Kermack W.O., McKendrick A.G. Contributions to the mathematical theory of epidemics-III. Further studies of the problem of endemicity. Bulletin of Mathematical Biology. 1991;53(1–2):89–118. doi: 10.1007/BF02464425. [DOI] [PubMed] [Google Scholar]
- Krist A.H., Crawford-Faucher A. Management of newborns exposed to maternal HIV infection. American Family Physician. 2002;65(10):2049. [PubMed] [Google Scholar]
- Laine M. 2008. Adaptive MCMC methods with applications in environmental and geophysical model. [Google Scholar]
- Levine B., Leskowitz R., Davis M. Personalized gene therapy locks out HIV, paving the way to control virus without antiretroviral drugs. Expert Opinion on Biological Therapy. 2015;15(6):831–843. doi: 10.1517/14712598.2015.1035644. [DOI] [PubMed] [Google Scholar]
- Luboga S., Galukande M., Mabweijano J., Ozgediz D., Jayaraman S. Key aspects of health policy development to improve surgical services in Uganda. World Journal of Surgery. 2010;34(11):2511–2517. doi: 10.1007/s00268-010-0585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOH . 2012. Global AIDS response country progress report. Malaysia 2012. [Google Scholar]
- MOH . 2015. The global AIDS response progress report, 2014. Malaysia. [Google Scholar]
- Neal P., Huang C.L.T. Forward simulation Markov chain Monte Carlo with applications to stochastic epidemic models. Scandinavian Journal of Statistics. 2015;42(2):378–396. [Google Scholar]
- Negredo E., Bonjoch A., Pérez-Álvarez N., Ornelas A., Puig J., Herrero C., Estany C., del Río L., di Gregorio S., Echeverría P., Clotet B. Comparison of two different strategies of treatment with zoledronate in HIV-infected patients with low bone mineral density: Single dose versus two doses in 2 years. HIV Medicine. 2015;16(7):441–448. doi: 10.1111/hiv.12260. [DOI] [PubMed] [Google Scholar]
- Nyabadza F., Mukandavire Z. Modelling HIV/AIDS in the presence of an HIV testing and screening campaign. Journal of Theoretical Biology. 2011;280(1):167–179. doi: 10.1016/j.jtbi.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Padilla M.B., Reyes A.M., Connolly M. Examining the policy climate for HIV prevention in the caribbean tourism sector: A qualitative study of policy makers in the Dominican republic. Health Policy and Planning. 2012;27(3):245–255. doi: 10.1093/heapol/czr021. [DOI] [PubMed] [Google Scholar]
- Putter H., Heisterkamp S.H., Lange J.M.A., de Wolf F. A bayesian approach to parameter estimation in HIV dynamical models. Statistics in Medicine. 2002;21(15):2199–2214. doi: 10.1002/sim.1211. [DOI] [PubMed] [Google Scholar]
- Reid G., Kamarulzaman A., Kaur Sran S. Malaysia and harm reduction: The challenges and responses. International Journal of Drug Policy. 2007;18(2):136–140. doi: 10.1016/j.drugpo.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Singh D., Chawarski M.C., Schottenfeld R., Vicknasingam B. Journal of Food and drug analysis. Vol. 21. Elsevier Taiwan LLC; 2013. Substance abuse and the HIV situation in Malaysia; pp. S46–S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetaert K., Petzoldt T. Inverse modelling, sensitivity and Monte Carlo analysis in R using package FME. Journal of statistical software. 2010;33(3):1–28. [Google Scholar]
- Solonen A., Haario H., Michel Tchuenche J., Rwezaura H. Studying the identifiability of epidemiological models using MCMC. International Journal of Biomathematics. 2013;6(2) [Google Scholar]
- Sripan P., Le Coeur S., Amzal B., Ingsrisawang L., Traisathit P., Ngo-Giang-Huong N., McIntosh K., Cressey T.R., Sangsawang S., Rawangban B., Kanjanavikai P., Tréluyer J.-M., Jourdain G., Lallemant M., Urien S. Modeling of in-utero and intra-partum transmissions to evaluate the efficacy of interventions for the prevention of perinatal HIV. Chung M.H., editor. PloS One. 2015;10(5) doi: 10.1371/journal.pone.0126647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Xiao Y., Peng Z., Wang N. Modelling HIV/AIDS epidemic among men who have sex with men in China. BioMed Research International. 2013;2013 doi: 10.1155/2013/413260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamizhmani K.M., Ramani A., Grammaticos B., Carstea A.S. Modelling AIDS epidemic and treatment with difference equations. Springer 2004. 2004;(3):183–193. [Google Scholar]
- Van Den Driessche P., James W. 2002. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. [DOI] [PubMed] [Google Scholar]
- WHO . WHO; 2014. WHO | care of the HIV-exposed or infected newborn. [Google Scholar]
- Wilson D.P., Zhang L. Characteristics of HIV epidemics driven by men who have sex with men and people who inject drugs. Current Opinion in HIV and AIDS. 2011;6(2):94–101. doi: 10.1097/COH.0b013e328343ad93. [DOI] [PubMed] [Google Scholar]
- Xun X., Cao J., Mallick B., Maity A., Raymond, Carroll J.J. Parameter estimation of partial differential equation models. Journal of the American Statistical Association. 2013;108:1009–1020. doi: 10.1080/01621459.2013.794730. [DOI] [PMC free article] [PubMed] [Google Scholar]