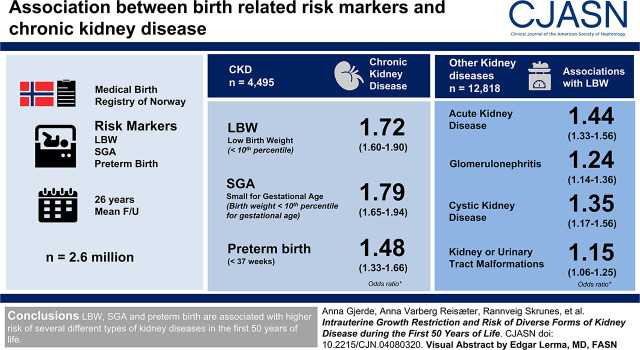
Visual Abstract
Keywords: chronic kidney disease, intrauterine growth, renal development, risk factors
Abstract
Background and objectives
Previous studies have shown that individuals with low birth weight (LBW) or small for gestational age (SGA) have higher risk of kidney failure. This study investigates birth-related exposures and risk of CKD and other kidney diagnoses.
Design, setting, participant, & measurements
The Medical Birth Registry of Norway has registered extensive medical data on all births in Norway since 1967. The Norwegian Patient Registry has registered diagnostic codes for all admissions and outpatient visits to Norwegian hospitals since 2008. Data from these registries were linked, and risk of CKD and other groups of kidney disease were analyzed using logistic regression statistics. LBW (below the tenth percentile), SGA (birth weight below the tenth percentile for gestational age), and preterm birth (<37 weeks) were analyzed as exposures.
Results
A total of 2,663,010 individuals were included. After a mean follow-up of 26 years (maximum 50 years), 4495 had been diagnosed with CKD and 12,818 had been diagnosed with other groups of kidney disease. LBW was associated with an odds ratio (OR) for CKD of 1.72 (95% confidence interval [95% CI], 1.60 to 1.90), SGA with an OR of 1.79 (95% CI, 1.65 to 1.94), and preterm birth with an OR of 1.48 (95% CI, 1.33 to 1.66). Analyses using diagnosis of CKD at stages 3–5 as end point showed similar results. Results were similar for men and women. We analyzed adjusted ORs for other groups of kidney disease and found that LBW was associated with an adjusted OR of 1.44 (95% CI, 1.33 to 1.56) for acute kidney disease, 1.24 (95% CI, 1.14 to 1.36) for GN, 1.35 (95% CI, 1.17 to 1.56) for cystic kidney disease, and 1.15 (95% CI, 1.06 to 1.25) for kidney disease resulting from kidney or urinary tract malformations.
Conclusions
LBW, SGA, and preterm birth are associated with higher risk of CKD in the first 50 years of life. Risk of other groups of kidney disease was less pronounced.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2020_08_17_CJN04080320.mp3
Introduction
In the 1980s, Brenner et al. (1) proposed that intrauterine growth restriction causes a low nephron number, which could predispose to hypertension and kidney disease through mechanisms of increased single-nephron glomerular filtration, compensatory nephron hypertrophy, and decreased functional reserve. Approximately 60% of the nephrons develop during the third trimester of pregnancy, and kidney development ends between 35 and 36 weeks of gestation (2). Thus, preterm birth or impaired intrauterine growth may significantly affect the formation of nephrons and reduce nephron number (3).
Previous cohort studies have linked low birth weight (LBW) and risk of severe kidney failure (4−7), and studies have also linked LBW with lower eGFR or urinary albumin (2,8–10). This is believed to be related to lower numbers of nephrons and glomerular hypertrophy that has been shown in LBW individuals (3,11,12). Recent studies have emphasized the interplay between markers of intrauterine growth such as LBW, small for gestational age (SGA), and prematurity and risk of kidney disease in adult life (2,13–15). An important paper from the Low Birth Weight and Nephron Number Working Group argued that individuals with LBW should undergo screening and follow-up to detect kidney disease or risk factors for kidney disease at an early age (16). Early detection of individuals at risk of kidney disease, as well as early referral to kidney units, may slow disease progression, improve survival in patients with CKD, and reduce total treatment cost (17).
There is a need for better data on the association between different markers of intrauterine growth restriction and risk of clinical kidney disease at an earlier stage than severe kidney failure. In this retrospective, register-based, nationwide cohort study, we linked data from Norwegian registries to explore the association between birth-related variables such as LBW, SGA, preterm birth, and risk of different groups of kidney disease during the first 50 years of life.
Materials and Methods
Data Sources
The study protocol was approved by the regional ethics committee with approval number 2017/627. Since 1967, the Medical Birth Registry of Norway has registered extensive medical data on all births in Norway. The Norwegian Patient Registry (NPR) has registered International Classification of Diseases, Tenth Revision (ICD-10) diagnostic codes for all admissions and outpatient visits to Norwegian hospitals since 2008. In Norway, most specialist care in the field of nephrology is hospital based, and the data are therefore almost complete for specialist care. ICD-10 codes were registered by the treating physicians. For this study, we obtained data from NPR for the period 2008–2016. Date of death was available from the Norwegian Population Registry. We linked these registries using the national identification number.
All individuals born in Norway between 1967 and 2015 were included. We excluded twins, triplets, and quadruplets (n=76,429); individuals who died before age 1 year (n=17,146); and individuals who died before 2008 (n=16,417). Individuals who officially had emigrated from Norway were also excluded (n=80,332).
Birth-Related Variables
LBW was defined as birth weight below the tenth percentile for sex (2940 g for male, 2850 g for female). From 1967 to 1998, gestational age was on the basis of the last menstrual period, and from 1999 onward, it was on the basis of on routine ultrasonographic examination in gestational week 17 to 20. Using birth weight, gestational age, and sex, a z-score of birth weight for gestational age was calculated for all single births. We defined SGA as birth weight below the tenth percentile for gestational age and sex. Preterm birth was defined as birth before 37 weeks of pregnancy. LBW<2500 g was also analyzed as an exposure variable.
Maternal preeclampsia has been diagnosed according to the American College of Obstetricians and Gynecologists criteria (18,19). For this study, pregestational maternal disease was defined as a diagnosis of maternal diabetes mellitus, kidney disease, rheumatic disease, or essential hypertension before pregnancy. Maternal marital status was dichotomized as either single or not single. Congenital malformations in the newborns had been recorded as present if any malformation had been observed before discharge from hospital; in the statistical analyses, a dichotomous variable was used.
Outcome Variables
The data file from NPR included ICD-10 codes for each episode (admission or outpatient visit) with a kidney disease diagnosis (N01–N09, N17–N19, N25–29, or Q60–64). Of the 17,313 individuals with at least one episode with kidney disease, 6494 had one episode, 2377 had two episodes, 1465 had three episodes, 3934 had four to nine episodes, 1751 had ten to 19 episodes, and 1295 had ≥20 episodes (maximum 1370 episodes). Patients were diagnosed with different combinations and sequences of ICD-10 codes. In this study, we analyze whether a diagnosis or group of diagnoses had been recorded at least one time.
The main outcome was defined as having been diagnosed with CKD (ICD-10 code N18) in at least one of the episodes (admissions or outpatient visits). Both main diagnoses and secondary diagnoses were included.
The secondary outcomes were having been diagnosed with different groups of kidney disease: acute kidney disease (N17), glomerular disease (N00–N09), cystic kidney disease (Q61), or kidney or urinary tract malformations (Q60, Q62–Q64). We also analyzed the secondary outcome of having been diagnosed with stage 3–5 of kidney disease (these diagnoses were used in the registry for the time period 2010–2016).
Statistical Analyses
In the statistical analyses, main and secondary outcomes were analyzed as either present or absent. Main exposure variables were LBW, SGA, and preterm birth. LBW<2.5 kg, combinations of the main exposure variables and different cut-offs for birth weight and birth weight for gestational age were also analyzed. For the included participants, 0.1% had missing data on birth weight and 4% for gestational age and z-score. These participants were excluded from the respective analyses. Characteristics of different groups were compared using t tests for continuous variables and Pearson chi-squared test for categorical variables. In the main analyses, logistic regression statistics was used to investigate the associations between exposure variables and the outcome of interest. In adjusted analyses, we adjusted for sex, pregestational maternal disease, maternal marital status, and congenital malformations recorded shortly after birth. In analyses focusing on the associations in adult age, only individuals born before 1990 were included.
In secondary analyses, we used left-truncated Cox regression statistics to complement the logistic regression statistics. Exposure and outcome variables were the same as in the logistic regression analyses. Time until end point was age at first occurrence; time until right censoring was age at death or end of 2016. As we did not have data on outcomes until 2008, analyses were left-truncated for the time period until 2008.
A two-tailed probability value of <0.05 was considered significant. All analyses were performed using STATA version 15.1 (Stata Corp., College Station, TX).
Results
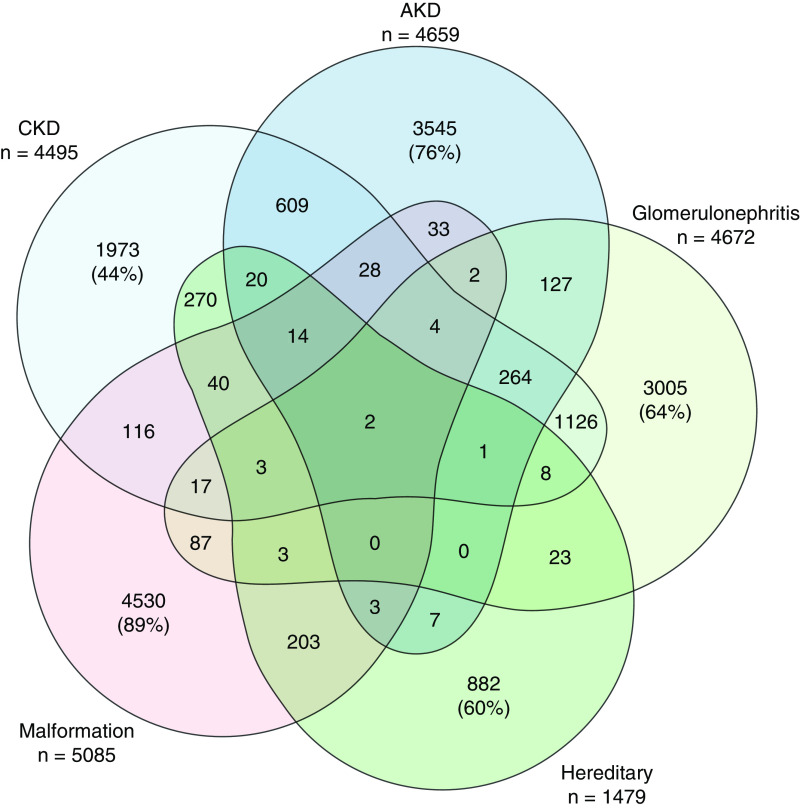
A total of 2,663,010 individuals were included in the study; 51% were male, and the mean birth year was 1991 (range 1967–2015). In the period 2008–2016, 17,313 individuals had been diagnosed with a kidney disease; 4495 had been diagnosed with CKD, 4659 had been diagnosed with acute kidney disease, 4672 had been diagnosed with glomerular disease, 1479 had been diagnosed with cystic kidney disease, and 5085 had been diagnosed with congenital malformations of the kidney or urinary tract. Figure 1 shows how the different groups of kidney disease combined for each patient. Patients with CKD most commonly had other groups of kidney disease. Table 1 shows birth-related characteristics for the total cohort and for the different groups of kidney disease. Mean age at diagnosis differed between groups, with kidney or urinary tract malformations diagnosed in younger patients and CKD diagnosed in older patients. Participants who were diagnosed with CKD had more often LBW and SGA than participants who were diagnosed with other diseases. The same was observed for combinations of diagnoses, i.e., 14% of participants with acute kidney disease but not CKD had LBW, 16% of participants with CKD but not acute kidney disease had LBW, and 17% of participants with CKD and acute kidney disease had LBW.
Figure 1.
Number of individuals diagnosed with combinations of different groups of kidney disease. Patients with CKD most commonly had other groups of kidney disease. In patients with CKD, 14% also had acute kidney disease (AKD), 25% had glomerular disease, 6% had cystic kidney disease, 3% had kidney or urinary tract malformations, 9% had several groups of kidney disease, and 44% were coded with CKD alone.
Table 1.
Characteristics of participants in the Norwegian Patient Registry
| Characteristic | Total | Diagnostic Groups | Any Kidney Disease | ||||
|---|---|---|---|---|---|---|---|
| CKD | Acute Kidney Disease | Glomerular Disease | Cystic Kidney Disease | Congenital Malformations | |||
| N | 2,663,010 | 4495 | 4659 | 4672 | 1479 | 5085 | 17,313 |
| Male | 51% | 61%a | 62%a | 53% | 49% | 57%a | 57%a |
| Birth yr | 1991±14 | 1979±11a | 1980±11a | 1987±14a | 1991±16 | 2003±11a | 1989±15a |
| Duration follow-up,b yr | 26±14 | 33±11 | 33±12 | 25±14 | 21±16 | 9±11 | 23±16 |
| Maternal marital status single | 10% | 12%a | 13%a | 11%c | 10% | 8%a | 11%a |
| LBW (below tenth percentile) | 10% | 16%a | 15%a | 13%a | 15%a | 13%a | 14%a |
| SGA (below tenth percentile) | 10% | 17%a | 15%a | 12%a | 14%a | 11% | 13%a |
| Preterm birth (<37 wk) | 5% | 7%a | 6%a | 5%c | 8%a | 7%a | 6%a |
| Birth weight <2.5 kg | 3% | 6%a | 5%a | 5%a | 5%a | 5%a | 5%a |
| One risk factord | 7% | 10%a | 9%a | 8%c | 9%a | 7% | 8%a |
| Two risk factorsd | 8% | 13%a | 12%a | 10%a | 11%a | 11%a | 11%a |
| Three risk factorsd | 0.6% | 1.5%a | 0.9%a | 1.0%a | 1.5%a | 1.3%a | 1.1%a |
| Congenital malformations | 3.0% | 6.6%a | 4.6%a | 3.9%a | 25%a | 29.4%a | 12.6%a |
| Apgar 5 min<7 | 0.96% | 2.5%a | 3.0%a | 0.96% | 2.6%a | 1.9a | 2.0%a |
| Maternal disease before pregnancye | 2.4% | 3.3%a | 2.8% | 3.1%f | 7.8%a | 4.2%a | 3.6%a |
| Maternal preeclampsia | 2.9% | 3.2%a | 2.6% | 2.9% | 4.5%a | 3.0% | 3.1%a |
LBW, low birth weight; SGA, small for gestational age.
P<0.001 as compared with total.
Duration of follow-up until diagnosis or end of 2016.
P<0.05.
LBW, SGA, or preterm birth.
Maternal diagnosis of diabetes mellitus, CKD, rheumatic disease, or essential hypertension before pregnancy.
P<0.01.
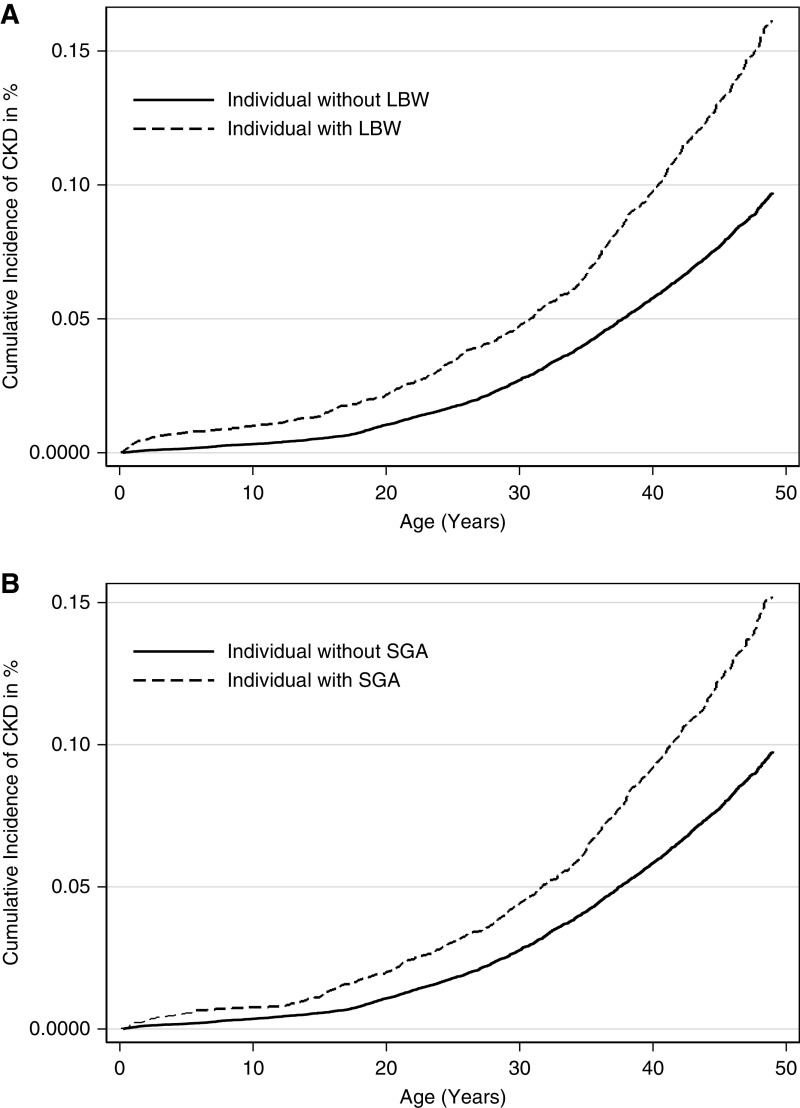
Compared with individuals with birth weight above the tenth percentile, LBW was associated with a higher odds ratio (OR) of 1.72 (95% confidence interval [95% CI], 1.60 to 1.90) for development of CKD (Table 2). Corresponding ORs were 1.80 (95% CI, 1.75 to 1.94) for SGA, 1.50 (95% CI, 1.33 to 1.66) for preterm birth, 1.85 (95% CI, 1.62 to 2.10) for birth weight <2.5 kg, and 1.11 (95% CI, 0.94 to 1.31) for maternal preeclampsia. There were no clear sex differences, except an impression that women seemed to be less affected by preterm birth and more by maternal preeclampsia (Table 2). The adjusted analyses were repeated in the cohort born before 1990 to focus on the adult population, with very similar results. These analyses described in Table 2 were repeated using Cox regression statistics, with virtually identical results. As can be seen from Figure 2, cumulative risk of CKD was higher for both LBW and SGA. We further analyzed the OR of being diagnosed with CKD stage 3–5. In these analyses, LBW was associated with an OR of 1.80 (95% CI, 1.60 to 2.05) for CKD stage 3, 1.84 (95% CI, 1.56 to 2.18) for CKD stage 4, and 1.88 (95% CI, 1.58 to 2.24) for CKD stage 5 (Supplemental Table 1). Corresponding ORs were 1.89 (95% CI, 1.67 to 2.14), 2.04 (95% CI, 1.73 to 2.41), and 1.78 (95% CI, 1.48 to 2.13) for SGA; 1.65 (95% CI, 1.38 to 1.97), 1.59 (95% CI, 1.25 to 2.03), and 1.44 (95% CI, 1.10 to 1.88) for preterm birth; and 2.09 (95% CI, 1.73 to 2.52), 1.82 (95% CI, 1.39 to 2.38), and 1.93 (95% CI, 1.46 to 2.55) for birth weight <2.5 kg for stages 3, 4, and 5, respectively.
Table 2.
Associations of low birth weight, small for gestational age, preterm birth, or maternal preeclampsia with subsequent diagnosis of CKD
| Exposure | Unadjusted | Adjusted | Adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cohort | Men | Women | Cohort Born before 1990 | |||||||||
| N CKD | OR (95% CI) | N CKD | OR (95% CI)a | N CKD | OR (95% CI)a | OR (95% CI)a | ||||||
| Birth weight below tenth percentile, LBW | No | 3759 | 1.0 (ref) | 2304 | 1.0 (ref) | 1455 | 1.0 (ref) | 1.0 (ref) | ||||
| Yes | 724 | 1.72 (1.60 to 1.90) | 427 | 1.56 (1.41 to 1.73) | 297 | 1.72 (1.51 to 2.00) | 1.58 (1.45 to 1.73) | |||||
| z-Score below tenth percentile, SGA | No | 3564 | 1.0 (ref) | 2174 | 1.0 (ref) | 1390 | 1.0 (ref) | 1.0 (ref) | ||||
| Yes | 708 | 1.79 (1.65 to 1.94) | 430 | 1.52 (1.37 to 1.70) | 278 | 1.51 (1.33 to 1.72) | 1.51 (1.40 to 1.63) | |||||
| Preterm birth | No | 4196 | 1.0 (ref) | 2541 | 1.0 (ref) | 1655 | 1.0 (ref) | 1.0 (ref) | ||||
| Yes | 299 | 1.48 (1.33 to 1.66) | 198 | 1.55 (1.34 to 1.80) | 101 | 1.40 (1.14 to 1.71) | 1.35 (1.17 to 1.55) | |||||
| Birth weight <2.5 kg |
No | 4235 | 1.0 (ref) | 2587 | 1.0 (ref) | 1648 | 1.0 (ref) | 1.0 (ref) | ||||
| Yes | 260 | 1.85 (1.62 to 2.10) | 152 | 1.87 (1.58 to 2.20) | 108 | 1.80 (1.49 to 2.2) | 1.79 (1.55 to 2.07) | |||||
| Maternal preeclampsia | No | 4352 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||
| Yes | 143 | 1.11 (0.94 to 1.31) | 1.18 (0.94 to 1.50) | 1.44 (1.12 to 1.9) | 1.26 (1.04 to 1.33) | |||||||
OR, odds ratio; 95% CI, 95% confidence interval; ref, reference; LBW, low birth weight; SGA, small for gestational age.
Adjusted for sex, maternal disease (defined as maternal diabetes mellitus, kidney disease, rheumatic disease, or essential hypertension diagnosed before pregnancy), maternal marital status, and malformations in the newborn.
Figure 2.
Cumulative incidence (%) of CKD according to LBW and SGA. Cumulative risk of CKD was higher for both LBW and SGA, and the graphs separate most strongly in adult age. Cumulative proportion with CKD at 50 years of age was 1% in individuals without LBW and 2% in individuals with LBW (top). Similar association was found in individuals with and without SGA (bottom). LBW, low birth weight; SGA, small for gestational age.
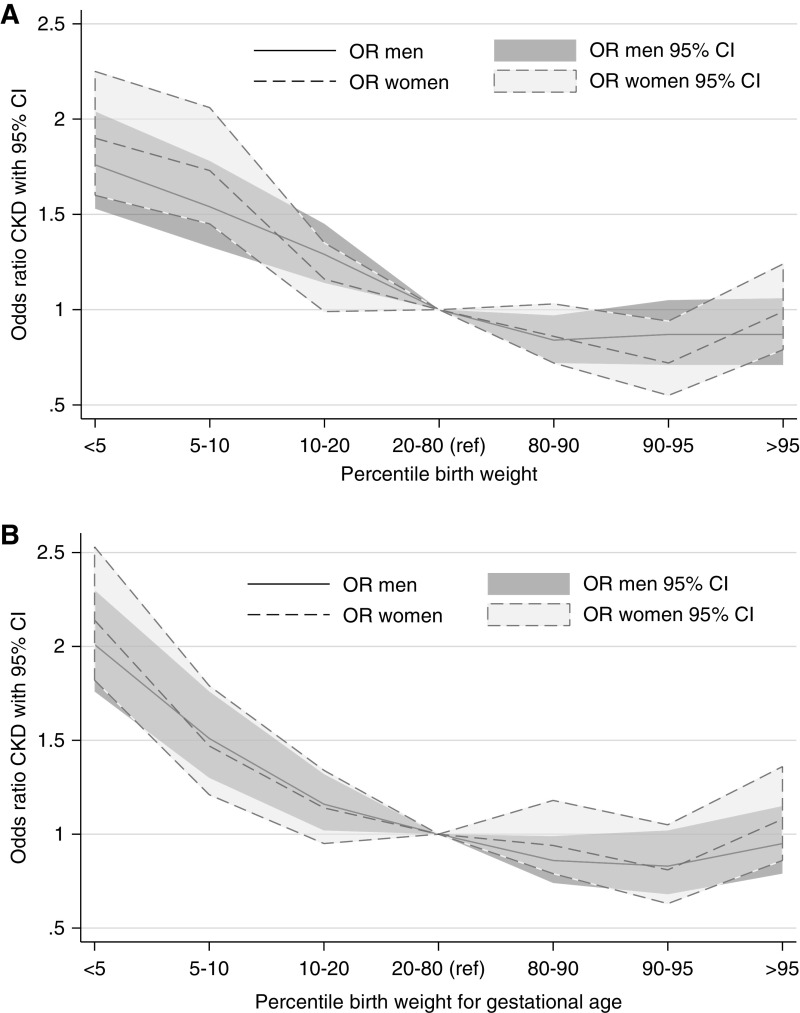
In Figure 3, we investigated possible dose-response relationships for LBW and SGA. In these analyses, we categorized birth weight and birth weight for gestational age according to sex-specific percentiles, and the following groups were analyzed: below the fifth percentile, fifth to tenth percentile, tenth to 20th percentile, 20–80th (reference) percentile, 80–90th percentile, 90–95th percentile, and >95th percentile cut-offs. Dose-response relationships were observed for both LBW and birth weight for gestational age, with higher risks for lower birth weights. Higher risk was seen below the tenth percentile for both LBW and SGA, but significant slightly higher ORs were seen for the tenth to 20th percentile groups for men.
Figure 3.
Odds ratio for CKD according to different percentiles for birth weight (top) and birth weight for gestational age (bottom). Dose-response relationships were observed for both low birth weight and birth weight for gestational age, with higher risks for lower birth weights. Higher risk was seen below the tenth percentile for both LBW and SGA, but significant slightly higher ORs were seen for the tenth to 20th percentile groups for men. 95% CI, 95% confidence interval; OR, odds ratio.
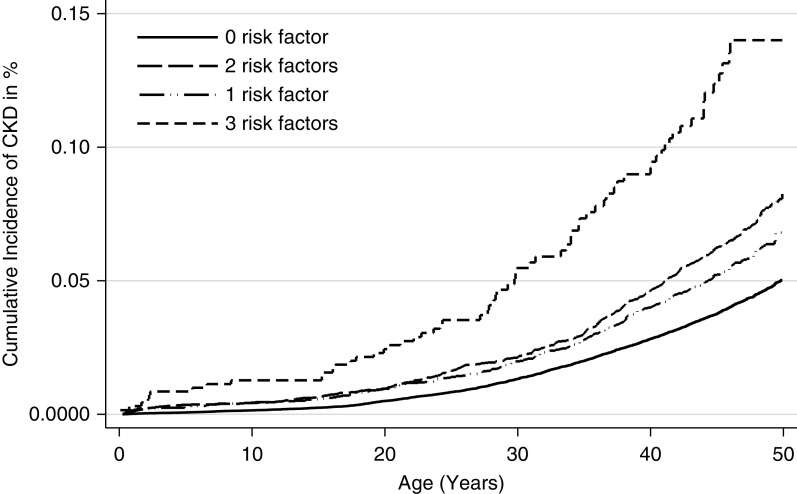
To further analyze the effects of LBW, SGA, and preterm birth, we investigated how combinations of these exposures associated with risk of CKD. As compared with having none of the exposures, individuals with one exposure had a significantly higher risk and the effect seemed similar for the three exposures (Table 3). Individuals with two exposures had a higher risk than individuals with one exposure, especially individuals with LBW and SGA, and individuals with three exposures had an even higher risk (Figure 4). These analyses were also repeated in the cohort born before 1990 so as to focus on the adult population, showing nearly identical results, except that the OR for individuals with only one exposure was attenuated and only significant for LBW or SGA. Analyses described in Table 3 were repeated using Cox regression, with virtually identical results. In order to analyze a possible contribution from preeclampsia, we chose to stratify the analyses in Table 3. The results showed that preeclampsia did not significantly affect the contribution of these other exposures (results not shown).
Table 3.
Associations for subsequent diagnosis of CKD according to whether the individuals had low birth weight, were small for gestational age, or were born preterm
| Exposure | Total Cohort (Born 1967–2015) | Cohort Born before 1990 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total N | N CKD | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | Total N | N CKD | Adjusted ORa (95% CI) | |||
| Term, not LBW or SGA | 2,147,243 | 3248 | 1.0 (ref) | 1.0 (ref) | 955,093 | 2681 | 1.0 (ref) | ||
| Term, not LBW, SGA | 99,862 | 240 | 1.59 (1.39 to 1.81) | 1.57 (1.38 to 1.79) | 56,462 | 210 | 1.32 (1.14 to 1.52) | ||
| Term, LBW, not SGA | 33,877 | 74 | 1.44 (1.15 to 1.82) | 1.41 (1.12 to 1.78) | 13,199 | 53 | 1.41 (1.07 to 1.85) | ||
| Preterm, not LBW or SGA | 40,540 | 89 | 1.45 (1.17 to 1.79) | 1.40 (1.13 to 1.72) | 19,327 | 62 | 1.11 (0.86 to 1.43) | ||
| Preterm, not LBW, SGA | No data | No data | No data | ||||||
| Term, LBW and SGA | 137,891 | 402 | 1.92 (1.74 to 2.14) | 1.88 (1.70 to 2.10) | 72,175 | 345 | 1.70 (1.52 to 1.90) | ||
| Preterm, LBW, not SGA | 65,013 | 141 | 1.43 (1.21 to 1.70) | 1.36 (1.15 to 1.61) | 25,427 | 103 | 1.40 (1.15 to 1.71) | ||
| Preterm, LBW and SGA | 16,299 | 66 | 2.67 (2.10 to 3.42) | 2.50 (2.00 to 3.18) | 6174 | 47 | 2.63 (1.96 to 3.53) | ||
| No. of exposuresb | |||||||||
| 0 | 2,147,243 | 3248 | 1.0 (ref) | 1.0 (ref) | 995,093 | 2681 | 1.0 (ref) | ||
| 1 | 174,279 | 403 | 1. 52 (1.37 to 1.69) | 1.50 (1.35 to 1.66) | 88,988 | 325 | 1.28 (1.14 to 1.44) | ||
| 2 | 202,904 | 543 | 1.76 (1.61 to 1.93) | 1.72 (1.57 to 1.89) | 97,602 | 448 | 1.62 (1.47 to 1.79) | ||
| 3 | 16,299 | 66 | 2.67 (2.09 to 3.41) | 2.49 (1.95 to 3.19) | 6174 | 47 | 2.63 (1.96 to 3.53) | ||
Separate analyses for total cohort and the cohort born before 1990. OR, odds ratio; 95% CI, 95% confidence interval; LBW, low birth weight; SGA, small for gestational age; ref, reference.
Adjusted for sex, maternal disease (defined as maternal diabetes mellitus, kidney disease, rheumatic disease, or essential hypertension diagnosed before pregnancy), maternal marital status, and malformations in the newborn.
Number of exposures LBW (defined as below the tenth percentile), SGA (defined as below the tenth percentile), and preterm birth (<37 weeks).
Figure 4.
Cumulative incidence (%) of CKD according to number of birth-related risk factors (birth weight below the tenth percentile, birth weight for gestational age below the tenth percentile, and preterm birth). Compared with having none of the exposures, individuals with one exposure had a significantly higher risk. Individuals with two exposures had a higher risk than individuals with one exposure, and individuals with three exposures had an even higher risk.
In the above analyses, we have used CKD as the main outcome. In Table 4, we presented results for other groups of kidney disease and showed that birth-related risk factors were most strongly associated with the CKD group. Unadjusted and adjusted analyses showed similar results (only the adjusted results are shown in the table). Supplemental Table 2 shows the results for the adult cohort born before 1990.
Table 4.
Associations of low birth weight, small for gestational age, and preterm birth with diverse forms of kidney disease
| Exposure | CKD | AKD | GN | Hereditary Kidney Disease | Malformations of the Kidney or Urinary Tract | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | aOR (95% CI)a | N | aOR (95% CI)a | N | aOR (95% CI)a | N | aOR (95% CI)a | N | aOR (95% CI)a | ||
| Birth weight below tenth percentile, LBW | No | 3759 | 1.0 (ref) | 3971 | 1.0 (ref) | 4086 | 1.0 (ref) | 1258 | 1.0 (ref) | 4394 | |
| Yes | 724 | 1.62 (1.50 to 1.76) | 678 | 1.44 (1.33 to 1.56) | 581 | 1.24 (1.14 to 1.36) | 219 | 1.35 (1.17 to 1.56) | 679 | 1.15 (1.06 to 1.25) | |
| z-Score below tenth percentile, SGA | No | 3564 | 1.0 (ref) | 3730 | 1.0 (ref) | 3900 | 1.0 (ref) | 1240 | 1.0 (ref) | 4465 | |
| Yes | 708 | 1.52 (1.40 to 1.65) | 675 | 1.60 (1.49 to 1.73) | 543 | 1.18 (1.07 to 1.30) | 194 | 1.31 (1.12 to 1.52) | 531 | 1.13 (1.03 to 1.23) | |
| Preterm birth | No | 4196 | 1.0 (ref) | 4385 | 1.0 (ref) | 4423 | 1.0 (ref) | 1362 | 1.0 (ref) | 4727 | |
| Yes | 299 | 1.49 (1.33 to 1.68) | 274 | 1.30 (1.15 to 1.47) | 249 | 1.17 (1.03 to 1.33) | 117 | 1.43 (1.18 to 1.74) | 358 | 1.08 (0.97 to 1.21) | |
| Birth weight <2.5 kg | No | 4235 | 1.0 (ref) | 4429 | 1.0 (ref) | 4464 | 1.0 (ref) | 1402 | 1.0 (ref) | 4835 | |
| Yes | 260 | 1.84 (1.62 to 2.08) | 230 | 1.56 (1.36 to 1.79) | 208 | 1.40 (1.21 to 1.60) | 77 | 1.30 (1.03 to 1.63) | 250 | 1.07 (0.94 to 1.22) | |
AKD, acute kidney disease; aOR, adjusted odds ratio; 95% CI, 95% confidence interval; LBW, low birth weight; ref, reference; SGA, small for gestational age.
Adjusted for sex, maternal disease (defined as maternal diabetes mellitus, kidney disease, rheumatic disease, or essential hypertension diagnosed before pregnancy), maternal marital status, and malformations in the newborn.
Discussion
This study showed that intrauterine growth restriction was associated with a 60%–70% higher risk of being diagnosed with CKD during the first 50 years of life. The associations were similar for CKD stages 3, 4, and 5 and for men and women, and mostly the same in the adult cohort. Different markers of intrauterine growth restriction were tested, and LBW and SGA yielded similar results that seemed to be stronger than for preterm birth. There was also significantly higher risks for other groups of kidney disease, but not as strongly as for CKD. Given the high population prevalence of CKD (20−23) and the high comorbidity and mortality in CKD (24), our results may have public health importance.
The main finding in our study is that it provides evidence that individuals born with LBW and SGA not only have higher risk for development of kidney failure (4,7), but also for the much more prevalent CKD. Global prevalence of CKD has been shown to be about 12%, with stage 3 prevalence at 8%, stage 4 prevalence at 0.4%, and stage 5 prevalence at 0.1% (20). In this study, individuals born with LBW or SGA had an unadjusted OR of about 1.72 for being diagnosed with CKD. In analyses of birth weight percentiles, we observed higher risks for lower birth weights as well as trends toward lower risks for birth weights above normal. In analyses of combinations of birth-related exposures, having more exposures were associated with higher risks and LBW and SGA seemed more important than preterm birth. Interestingly, we could not find evidence on sex differences, unlike other studies (7,9,13,25).
In this study, cumulative proportion of CKD at 50 years of age was 2% in individuals with LBW compared with 1% in those without LBW. However, the real prevalence of CKD might be much higher because there is a well-known under-reporting of CKD in administrative databases as these rely on the patient having been referred to specialist care for evaluation (26,27). For example, a meta-analysis of CKD prevalence did show that prevalence of stage 3–5 in population screening is about 12% at 50 years of age (20). It could be expected that the higher relative risk would be the same irrespective of the higher prevalence with increasing age; the absolute importance of LBW would thus be likely to be higher (20,28). Review papers have argued for routine follow-up to detect early kidney disease in all individuals with LBW, SGA, or preterm birth (2,16), and our study strengthens these arguments. Studies of the effect of screening in adults and older adults seem warranted to assess cost versus benefit.
Our study confirms previous Norwegian studies that LBW, SGA, and preterm birth are associated with higher risk for kidney disease in adult age (4,7). A recent study showed that combination of these exposures are associated with a further higher risk of CKD, and that individuals with only one exposure did not have a higher risk (29). In contrast, this study found that also individuals with only one exposure had a higher risk, and the contribution from preterm birth seemed to be weaker. The importance of SGA has been documented in this study and several previous studies (4,7,30). A Swedish study showed an important higher risk associated with preterm birth, an effect that was of the same magnitude as for LBW and SGA in our study (31). However, it is difficult to directly compare these studies as there is considerable overlap between the exposures. The Swedish study did not include data on birth weight, and it also showed a much weaker effect for preterm birth after 20 years of age as compared with our study. Our study showed clear associations between LBW and SGA and risk of CKD also in adult age, as well as that the findings of the number of exposures also may be important.
Several studies have described the association between LBW and different indicators of kidney disease, such as albuminuria, low eGFR, or kidney failure (13). LBW has also been linked to moderately higher BP (7), impaired glucose homeostasis (32), microalbuminuria, and endothelial dysfunction. In our study, we found that LBW also was associated with higher risk of acute kidney disease, glomerular disease, cystic kidney disease, and kidney and urinary tract malformations, although these risks were lower than for CKD. As kidney or urinary tract malformations, as well as cystic kidney disease, could cause intrauterine growth restriction, this aspect of our findings could be expected. In a previous paper, we did in fact show that LBW was especially strongly associated with risk of congenital and hereditary kidney disease before 15 years of age (4). However, in this study, we did also show a significantly higher risk in the adult cohort born before 1990 (Supplemental Table 2). Unadjusted analyses, adjusted analyses, and analyses for the adult cohort showed very similar results, and we believe that the potential for residual confounding is of smaller significance. The higher risk of GN could also be expected on the basis of previous studies suggesting that autoimmune disease could be caused by early-life perturbations (33), but the higher risk of being diagnosed with GN has, to our knowledge, not been demonstrated in a population-based study.
The major strengths of our study are the opportunity to use the national registries to include a large number of participants with prospective registration of birth-related variables, the long follow-up period of 50 years, and the stability of the Norwegian population with little or no emigration during follow-up. About 2% of the included population had been officially recorded as emigrated and were excluded from the study, but a crosscheck by Statistics Norway showed that another 2% currently were living abroad. Further strengths are that most kidney disease diagnoses are assessed and treated in hospitals, and that we included both main diagnoses and secondary diagnoses in the data. The study population is mostly White, which is both a strength and a weakness: results might be different in other populations, but as the Norwegian population is quite homogeneous with equal access to specialist health care, this could allow for better internal comparability and reduce potential confounding factors such as low socioeconomic status, educational level, and ethnic origin. We did not have access to these data in our study, but we were able to adjust for single versus nonsingle mother, which is a socioeconomic marker.
The main weakness is that we could not record end points until 2008. Our data thus reflect prevalence of CKD during the years 2008–2015. Given the wide age range of 0–50 years, we believe that our data also could reflect incidence of CKD. On the basis of these reflections, we decided to perform the main statistics as logistic regression statistics, but also performed left-truncated survival statistics to investigate the age-associated risk of CKD. These two approaches showed mainly identical results. As discussed above, there is probably an under-reporting of CKD in administrative databases (26,27,34). An important weakness is also that CKD documented in patient journals by albuminuria or lower eGFR will not be coded in the diagnostic databases if not relevant for the patient care that was given. The treating physicians decide which ICD-10 diagnostic codes to use, and although we believe that these mostly are correct, diagnostic codes of kidney disease have, to our knowledge, not been validated in Norway. Other limitations include lack of data on other important risk factors such as diabetes, hypertension, smoking, dyslipidemia, and other exposures of kidney disease. In conclusion, we have shown that intrauterine growth restriction is associated with a 60%–70% higher risk of being diagnosed with CKD during the first 50 years of age. Findings were similar for men and women and were similar for LBW and SGA. Future studies will need to address whether screening of individuals with intrauterine growth restriction could have a beneficial cost–benefit ratio and also how intrauterine growth restriction modifies the effect of other known kidney disease exposures. Starting now, we suggest that clinicians should ask their kidney patients for information on birth history.
Disclosures
All authors have nothing to disclose.
Funding
This study is supported by grants from Helse Fonna and from the Western Norway Regional Health Authority funds (912186; Principal Investigator: B.E. Vikse). These supporters played no part in development or approval of the manuscript.
Supplementary Material
Acknowledgments
Dr. Anna Gjerde analyzed the data, performed statistical analyses, interpreted results, and drafted and wrote the manuscript. Dr. Anna Varberg Reisæter provided critical review of the manuscript and discussions during drafting of the manuscript, and approved the final version. Dr. Hans-Peter Marti provided intellectual content of critical importance, revised the manuscript, and approved the final version. Dr. Rannveig Skrunes provided intellectual content of critical importance, revised the manuscript, and approved the final version. Dr. Bjørn Egil Vikse provided the research idea and study design, was responsible for data analysis and interpretation of the collected data, was responsible for statistical analysis, provided invaluable supervision and guidance during drafting of the manuscript, and approved the final version.
Data Sharing Statement
Anonymized data for main analyses will be shared on request.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04080320/-/DCSupplemental.
Supplemental Table 1. Associations of low birth weight, small for gestational age, and preterm birth with CKD stages 3–5.
Supplemental Table 2. Associations of low birth weight, small for gestational age, and preterm birth with diverse forms of kidney disease. Analyses for cohort born before 1990.
References
- 1.Brenner BM, Garcia DL, Anderson S: Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Bacchetta J, Harambat J, Dubourg L, Guy B, Liutkus A, Canterino I, Kassaï B, Putet G, Cochat P: Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int 76: 445–452, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D: The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol 99: 296–301, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM: Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 19: 151–157, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan ZJ, Lackland DT, Lipsitz SR, Nicholas JS: The association of low birthweight and chronic renal failure among Medicaid young adults with diabetes and/or hypertension. Public Health Rep 121: 239–244, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ: Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med 160: 1472–1476, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Ruggajo P, Skrunes R, Svarstad E, Skjærven R, Reisæther AV, Vikse BE: Familial factors, low birth weight, and development of ESRD: A nationwide registry study. Am J Kidney Dis 67: 601–608, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Keijzer-Veen MG, Schrevel M, Finken MJ, Dekker FW, Nauta J, Hille ET, Frölich M, van der Heijden BJ; Dutch POPS-19 Collaborative Study Group : Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol 16: 2762–2768, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Chen SC, Shlipak M, Bakris G, McCullough PA, Sowers J, Stevens L, Jurkovitz C, McFarlane S, Norris K, Vassalotti J, Klag MJ, Brown WW, Narva A, Calhoun D, Johnson B, Obialo C, Whaley-Connell A, Becker B, Collins AJ; Kidney Early Evaluation Program Investigators : Low birth weight is associated with chronic kidney disease only in men. Kidney Int 73: 637–642, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Hallan S, Euser AM, Irgens LM, Finken MJ, Holmen J, Dekker FW: Effect of intrauterine growth restriction on kidney function at young adult age: The Nord Trøndelag Health (HUNT 2) study. Am J Kidney Dis 51: 10–20, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF: Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int 63: 2113–2122, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Mañalich R, Reyes L, Herrera M, Melendi C, Fundora I: Relationship between weight at birth and the number and size of renal glomeruli in humans: A histomorphometric study. Kidney Int 58: 770–773, 2000. [DOI] [PubMed] [Google Scholar]
- 13.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, Huxley RR: Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 54: 248–261, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE: Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7: 17–25, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Luyckx VA, Brenner BM: Birth weight, malnutrition and kidney-associated outcomes—A global concern. Nat Rev Nephrol 11: 135–149, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Luyckx VA, Perico N, Somaschini M, Manfellotto D, Valensise H, Cetin I, Simeoni U, Allegaert K, Vikse BE, Steegers EA, Adu D, Montini G, Remuzzi G, Brenner BM; writing group of the Low Birth Weight and Nephron Number Working Group : A developmental approach to the prevention of hypertension and kidney disease: A report from the low birth weight and nephron number working group. Lancet 390: 424–428, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonnemann G, Duttlinger J, Hohmann D, Hickstein L, Reichel H: Timely referral to outpatient nephrology care slows progression and reduces treatment costs of chronic kidney diseases. Kidney Int Rep 2: 142–151, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM: Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 183: S1–S22, 2000. [PubMed] [Google Scholar]
- 20.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD: Global prevalence of chronic kidney disease - A systematic review and meta-analysis. PLoS One 11: e0158765, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A: Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 382: 158–169, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Eriksson JG, Salonen MK, Kajantie E, Osmond C: Prenatal growth and CKD in older adults: Longitudinal findings from the helsinki birth cohort study, 1924-1944. Am J Kidney Dis 71: 20–26, 2018. [DOI] [PubMed] [Google Scholar]
- 26.Gasparini A, Evans M, Coresh J, Grams ME, Norin O, Qureshi AR, Runesson B, Barany P, Ärnlöv J, Jernberg T, Wettermark B, Elinder CG, Carrero JJ: Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 31: 2086–2094, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vlasschaert ME, Bejaimal SA, Hackam DG, Quinn R, Cuerden MS, Oliver MJ, Iansavichus A, Sultan N, Mills A, Garg AX: Validity of administrative database coding for kidney disease: A systematic review. Am J Kidney Dis 57: 29–43, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, Hallan HA, Lydersen S, Holmen J: International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 17: 2275–2284, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Schreuder MF, Wilhelm AJ, Bökenkamp A, Timmermans SM, Delemarre-van de Waal HA, van Wijk JA: Impact of gestational age and birth weight on amikacin clearance on day 1 of life. Clin J Am Soc Nephrol 4: 1774–1778, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gjerde A, Lillås BS, Marti HP, Reisæter AV, Vikse BE: Intrauterine growth restriction, preterm birth and risk of end-stage renal disease during the first 50 years of life [published online ahead of print Feb 10, 2020]. Nephrol Dial Transplant doi:10.1093/ndt/gfaa001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crump C, Sundquist J, Winkleby MA, Sundquist K: Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: National cohort study. BMJ 365: l1346, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson RG, Morgenstern H, Bennett PH: Birth weight and renal disease in Pima Indians with type 2 diabetes mellitus. Am J Epidemiol 148: 650–656, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Ruggajo P, Svarstad E, Leh S, Marti HP, Reisæther AV, Vikse BE: Low birth weight and risk of progression to end stage renal disease in IgA nephropathy--A retrospective registry-based cohort study. PLoS One 11: e0153819, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Middleton RJ, Foley RN, Hegarty J, Cheung CM, McElduff P, Gibson JM, Kalra PA, O’Donoghue DJ, New JP: The unrecognized prevalence of chronic kidney disease in diabetes. Nephrol Dial Transplant 21: 88–92, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.