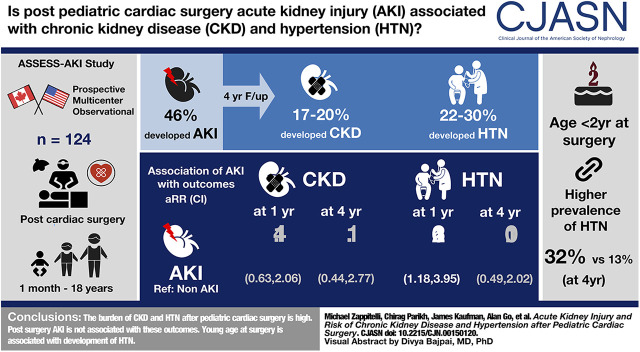
Visual Abstract
Keywords: acute renal failure, chronic kidney disease, clinical hypertension, pediatric nephrology, pediatric intensive care medicine, epidemiology and outcomes, acute kidney injury
Abstract
Background and objectives
The association of AKI after pediatric cardiac surgery with long-term CKD and hypertension development is unclear. The study objectives were to determine whether AKI after pediatric cardiac surgery is associated with incident CKD and hypertension.
Design, setting, participants, & measurements
This was a prospective cohort study of children of 1 month to 18 years old who were undergoing cardiac surgery at two tertiary care centers (Canada, United States). Participants were recruited before cardiac surgery and were followed during hospitalization and at 3, 12, 24, 36, and 48 months after discharge. Exposures were postoperative AKI, based on the Kidney Disease Improving Global Outcomes (KDIGO) definition, and age <2 years old at surgery. Outcomes and measures were CKD (low eGFR or albuminuria for age) and hypertension (per the 2017 American Academy of Pediatrics guidelines) at follow-up, with the composite outcome of CKD or hypertension.
Results
Among 124 participants, 57 (46%) developed AKI. AKI versus non-AKI participants had a median (interquartile range) age of 8 (4.8–40.8) versus 46 (6.0–158.4) months, respectively, and higher preoperative eGFR. From the 3- to 48-month follow-up, the cohort prevalence of CKD was high (17%–20%); hypertension prevalence was also high (22%–30%). AKI was not significantly associated with the development of CKD throughout follow-up. AKI was associated with hypertension development at 12 months after discharge (adjusted relative risk, 2.16; 95% confidence interval, 1.18 to 3.95), but not at subsequent visits. Children aged <2 years old at surgery had a significantly higher prevalence of hypertension during follow-up than older children (40% versus 21% at 3-month follow-up; 32% versus 13% at 48-month follow-up).
Conclusions
CKD and hypertension burden in the 4 years after pediatric cardiac surgery is high. Young age at surgery, but not AKI, is associated with their development.
Introduction
Postoperative AKI occurs in 35%–65% of children after cardiac surgery and is associated with hospital mortality, prolonged length of stay, and prolonged ventilation (1–7). In children, younger age is associated with development of AKI after cardiac surgery (1,6,8). In adults, AKI is a risk factor for long-term incident and progressive CKD, kidney failure, and cardiovascular morbidity (9–14). Three studies in children undergoing cardiac surgery showed discordant results regarding the association of postoperative AKI and development of long-term CKD (15–17). In these studies, the long-term prevalence of CKD and hypertension after cardiac surgery was as high as 18%. The knowledge gap regarding the natural history or progression of CKD and hypertension, over time, after cardiac surgery discharge limits the ability to develop evidence-based recommendations on when and how these children should be screened for development of CKD and hypertension. Uncertainty regarding the association of post–cardiac surgery AKI with long-term CKD and hypertension development limits the ability to conclude whether resources should be used to systematically target children with AKI for post–cardiac surgery discharge kidney outcomes ascertainment.
The ASsessment, Serial Evaluation, and Subsequent Sequelae in AKI (ASSESS-AKI) study included children undergoing cardiac surgery in a multicenter, prospective, observational study to document subsequent kidney outcomes. The primary hypothesis was that postoperative AKI is a risk factor for developing CKD and hypertension, with a secondary goal of documenting the natural history of CKD and hypertension over time after cardiac surgery discharge. A secondary hypothesis for this analysis was that age <2 years old (when kidney maturation is complete) at surgery is associated with worse long-term CKD and hypertension outcomes.
Materials and Methods
Study Design, Setting, and Participant Selection
The ASSESS-AKI study included children undergoing cardiac surgery, in a multicenter, prospective, observational study. Children were enrolled preoperatively from two tertiary care centers (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Montreal Children’s Hospital, Montreal, QC, Canada) between January 14, 2010 and October 18, 2012. Inclusion criteria were those aged 1 month to 18 years old who were undergoing cardiac surgery. Participants who completed a 3-month postdischarge study visit were included. Exclusion criteria included preoperative AKI or severe CKD (Supplemental Table 1) (18).
Research ethics board (Canada) and institutional review board (United States) approvals were obtained before initiation of the study. Parental informed consent and child assent, when appropriate, were obtained before initiating participant activities.
Study Protocol
The protocol included in-person and phone study visits over 48 months (Supplemental Figure 1). Preoperative blood and urine and baseline clinical data were collected. Postoperatively, clinical data (including complications, vasopressor and diuretic use, nephrotoxic medications, hospital outcomes) and serum creatinine concentration, measured at study sites, were recorded daily. Before discharge or shortly thereafter, eligible subjects were invited to participate in this longitudinal study. At 3 months (±4 weeks) and 12, 24, 36, and 48 months (±6 weeks) after discharge, in-person visits were performed at the study center or participant homes. Within 48 hours of study visits, approximately 2.5 ml blood was drawn and urine (≥5 ml in non-diaper wearers; >1.5 ml in diaper wearers) was collected. Height and weight were measured three times, and automated BP was measured three times (seated; right arm, or left arm if right arm not available; size-appropriate cuffs; average of the two lowest measures). Measurement equipment was similar across sites. Questionnaires were completed (including medications and health status since the last visit). Phone visits were performed at 6, 18, 30, and 42 months to ascertain survival and obtain health updates.
Specimen Handling and Laboratory Measures
Blood and urine were spun (1000 × g, 10 minutes, room temperature), aliquoted, and frozen at −80°C within 6 hours of collection. Samples not processed within 30 minutes were placed on ice until processing. Specimen aliquots were bar coded, entered into an online database, and shipped on dry ice to the central biorepository for laboratory measurements. Serum creatinine (creatinine, isotope dilution mass spectrometry–traceable method), urine albumin concentration (nephelometry), and urine creatinine concentration (modified Jaffe assay) were measured. The individuals performing laboratory measurements were blinded to clinical data.
Primary Exposure: Postoperative AKI
AKI was defined based on the Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria (19). KDIGO urine output criteria were not collected because of variability in postoperative urinary catheterization practice across sites (established in our previous studies) (3,20). Postoperative AKI was defined as an increase in creatinine of ≥0.3 mg/dl (to convert to µmol/L, multiply by 88.4) within 48 hours, or to ≥1.5 times the preoperative value within the first 7 postoperative days, or being treated with dialysis for AKI. AKI was staged by severity (stages 1–3) according to the KDIGO guideline (19).
A secondary exposure was age <2 years old at surgery. This age threshold was selected because kidney maturation and function reach adulthood levels by this age, and our previous studies showed that children <2 years old are at higher risk for postoperative AKI (1,6,20,21).
Outcomes: CKD and Hypertension
CKD was defined using the KDIGO criteria, based on abnormal eGFR or elevated urinary albumin-creatinine ratio (UACR) (22). GFR was estimated using the bedside, creatinine-based CKD in Children study equation (23). For patients >18 years old, GFR was estimated by the average of the eGFR obtained from the CKD in Children study equation and the eGFR from the adult CKD–Epidemiology Collaboration creatinine equation, as has been suggested for young adults (24).
Defining abnormal eGFR for young children in longitudinal studies is challenging because GFR increases physiologically until the age of 2 years. We defined low eGFR using published normative data adjusted for age as follows (25,26): ≥2 years old, <90 ml/min per 1.73 m2; ≥18 to <24 months old, <76 ml/min per 1.73 m2; ≥12 to <18 months old, <74 ml/min per 1.73 m2; ≥8 to <12 months old, <65 ml/min per 1.73 m2; <8 months old, <58 ml/min per 1.73 m2.
UACR was assessed from spot urine collections. Because UACR is physiologically higher in children <2 years old, we defined albuminuria as UACR ≥30 mg/g in children ≥2 years old, and UACR ≥75 mg/g in children <2 years old (22).
Hypertension was defined as systolic or diastolic BP ≥95th percentile for age, sex, and height, or above adolescent and adult BP thresholds as appropriate (2017 American Academy of Pediatrics BP guidelines [27]; software available at https://apps.cpeg-gcep.net/BPz_cpeg/). For infants <1 year old, each BP was evaluated manually by two independent raters, using published normative infant curves for hypertension (28). Discrepancies were discussed and finalized (authors V.C. and M.Z.). A total of 11 patients with systolic but no diastolic BP measure were staged using only systolic BP. One patient with a missing height measure was staged using the average heights from the flanking visits. At each study visit, only one assessment was made for CKD and for hypertension. Guidelines state that repeated measures should be made to diagnose CKD and hypertension (22,27); this was not feasible in the context of this study.
A secondary outcome was the composite of CKD or hypertension. For composite outcomes, if one variable was missing, but the nonmissing component was abnormal (e.g., eGFR low but missing UACR), the participant was defined as having the outcome (<1% of total visits). If the nonmissing variable was normal, then the outcome was “missing” (<5% of study visits). Missing data were analyzed by listwise deletion.
Statistical Analyses
When this study was designed, there were no reliable data regarding expected CKD or hypertension event rates. Sample size was determined based on study site feasibility evaluation and funding. Recruitment target was initially 100 children, which was surpassed to increase the likelihood of having approximately 100 participants at all study visits.
Prevalence of CKD, hypertension, and composite outcomes were calculated at each study visit in the whole cohort, by postoperative AKI status, presence versus absence of stage 2 or greater AKI, and by age <2 versus ≥2 years old at surgery. Baseline and postoperative characteristics were compared between AKI groups using univariable analyses appropriate for variable distribution. CKD and hypertension prevalence at study visits were compared between AKI groups and age groups (chi-squared or Fisher exact test, as appropriate). Log-binomial regression (estimation of adjusted relative risk; 95% confidence interval [95% CI]) was performed to evaluate adjusted associations between AKI with CKD and with hypertension at each study visit. In all regression models, age <2 versus ≥2 years old was included a priori. Other covariates were included if they were associated with AKI and the 12-month outcome (e.g., 12-month CKD) in univariable analyses (P<0.05). In secondary analyses, we performed univariable analyses to evaluate associations between CKD at the 3-month visit with CKD at subsequent visits (12, 24, 36, and 48 months), in the whole cohort and by AKI status. Sensitivity analyses were performed to evaluate whether associations between AKI and hypertension at follow-up visits were similar when use of antihypertensive medication was included as a hypertension criterion. Analyses were performed using SAS, version 9.4, and Stata, version 15.1.
Results
Study Population Characteristics
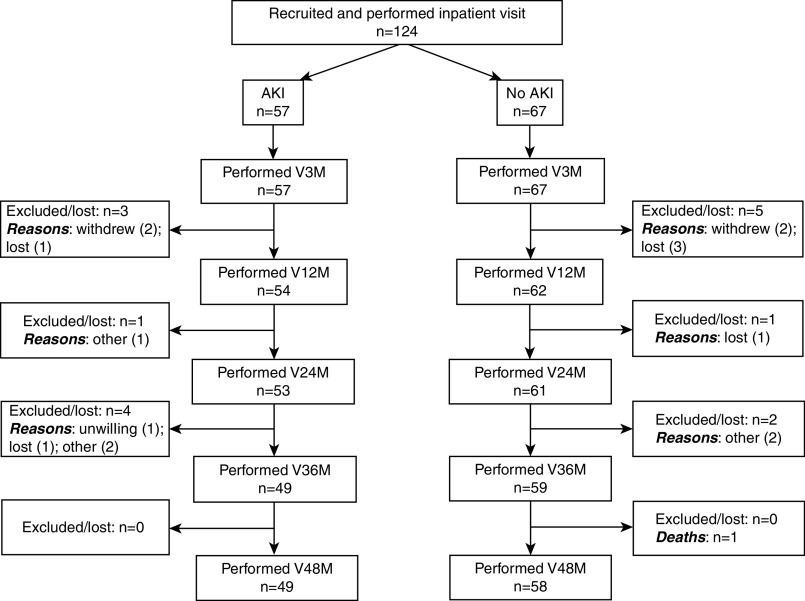
A total of 124 eligible children were enrolled preoperatively. Figure 1 shows the number of patients at study visits. Of these patients, 57 (46%) developed postoperative AKI (29 [51%] with stage 1, 19 [33%] with stage 2, and nine [16%] with stage 3). Patients with AKI were significantly younger and had higher preoperative eGFR than patients without AKI (Table 1). One patient with AKI was briefly treated with dialysis postoperatively. Supplemental Table 2 displays patient characteristics and outcomes by center.
Figure 1.
Flow diagram depicting number of AKI and non-AKI patients between each study visit. The flow diagram displays eligible patients with and without AKI who completed follow-up, in-person study visits. Reasons for exclusions from study visits are shown (“other” refers to inability to perform the visit or invalid visit). The following data are unavailable: (1) total number of potentially eligible patients in preoperative clinics that we were unable to approach; (2) characteristics and number of patients who were not approached for eligibility in preoperative clinics; and (3) number of patients found to be ineligible after screening in preoperative clinics. V3M, 3-month visit; V12M, 12-month visit; V24M, 24-month visit; V36M, 36-month visit; V48M, 48-month visit.
Table 1.
Characteristics of participants with age <18 yr in the ASsessment, Serial Evaluation, and Subsequent Sequelae in AKI (ASSESS-AKI) study
| Characteristicsa | AKI (n=57) | No AKI (n=67) | All (n=124) |
|---|---|---|---|
| Demographic | |||
| Age at surgery (mo), median (IQR) | 8.4 (4.8–40.8) | 45.6 (6–158.4)c | 18 (4.8–78.6) |
| Male sex, n (%) | 31 (54) | 33 (49) | 64 (52) |
| Non-White, n (%)b | 6 (11) | 10 (15) | 16 (13) |
| Preoperative cardiac/health characteristics and medication, n (%) | |||
| Cyanotic heart disease | 17 (30) | 14 (21) | 31 (25) |
| Previous heart surgery | 22 (39) | 30 (45) | 52 (42) |
| Prematurity, n/total with data (%)a | 8/55 (15) | 14/66 (21) | 22/121 (18) |
| Any nonkidney comorbidityd | 32 (56) | 40 (60) | 72 (58) |
| ACE inhibitors | 5 (9) | 6 (9) | 11 (9) |
| Diuretics | 20 (35) | 15 (22) | 35 (28) |
| Aspirin or NSAIDS | 9 (16) | 8 (12) | 17 (14) |
| Preoperative kidney characteristics | |||
| eGFR (ml/min per 1.73 m2), median (IQR)e | 134 (107–155) | 111 (92–125)c | 118 (97–139) |
| Identifiable kidney comorbidity, n (%)a,f | 3 (5) | 5/66 (8) | 8/123 (7) |
| Postoperative characteristics, medication, hospital outcomes | |||
| RACHS-1 surgical severity score ≥3, n (%) | 22 (39) | 38 (57)g | 60 (48) |
| Aminoglycosides, n (%) | 10 (18) | 5 (7) | 15 (12) |
| NSAIDs, n (%) | 22 (39) | 35 (52) | 57 (46) |
| Aspirin, n (%) | 9 (16) | 13 (19) | 22 (18) |
| ACE inhibitors or ARBs, n (%) | 6 (11) | 9 (13) | 15 (12) |
| Diuretics, n (%) | 55 (96) | 61 (91) | 116 (94) |
| Vasopressors, n (%) | 36 (63) | 42 (63) | 78 (63) |
| Complications, n (%)h | 41 (72) | 46 (69) | 87 (70) |
| Invasive mechanical ventilation ≥2 d, n (%) | 15 (26) | 8 (12)g | 23 (19) |
| KRT, n (%) | 1 (2) | 0 (0) | 1 (1) |
| ICU length of stay (d), median (IQR) | 4.0 (2.0–6.0) | 4.0 (2.0–5.0) | 4.0 (2.0–6.0) |
| Hospital length of stay (d), median (IQR) | 8.0 (6.0–12.0) | 7.0 (6.0–11.0) | 8.0 (6.0–11.0) |
Continuous variables expressed as median (IQR); categoric variables expressed as N (column %). IQR, interquartile range; ACE, angiotensin-converting enzyme; NSAIDs, nonsteroidal anti-inflammatory drugs; RACHS-1, Risk Adjustment for Congenital Heart Surgery surgical severity/mortality score; ARBs, angiotensin receptor blockers; ICU, intensive care unit.
There were no missing values, unless otherwise indicated (data not imputed for the variables prematurity and identifiable kidney comorbidity).
Specific self-reported race designations for AKI versus non-AKI groups were White (90% versus 85%), Black (5% versus 0%), and other (5% versus 15%).
P<0.005.
Preoperative nonkidney comorbidity includes diabetes, chronic lung disease, genetic syndrome, neurologic disorder, poor growth, nasogastric feeding, and other. There were no significant differences in any of these disorders between AKI groups (not shown).
Eight of 124 participants had low eGFR for age preoperatively (eight AKI and one non-AKI). eGFR was calculated as per the CKD in Children study equation.
Identifiable kidney comorbidity includes congenital abnormality of the kidney or urinary tract, CKD, or hypertension, ascertained by chart review.
P<0.05, for between-group difference.
Includes sepsis/shock, return to operating room for major surgical procedures, arrhythmias.
Postdischarge Prevalence of CKD and Hypertension over Time in the Whole Cohort
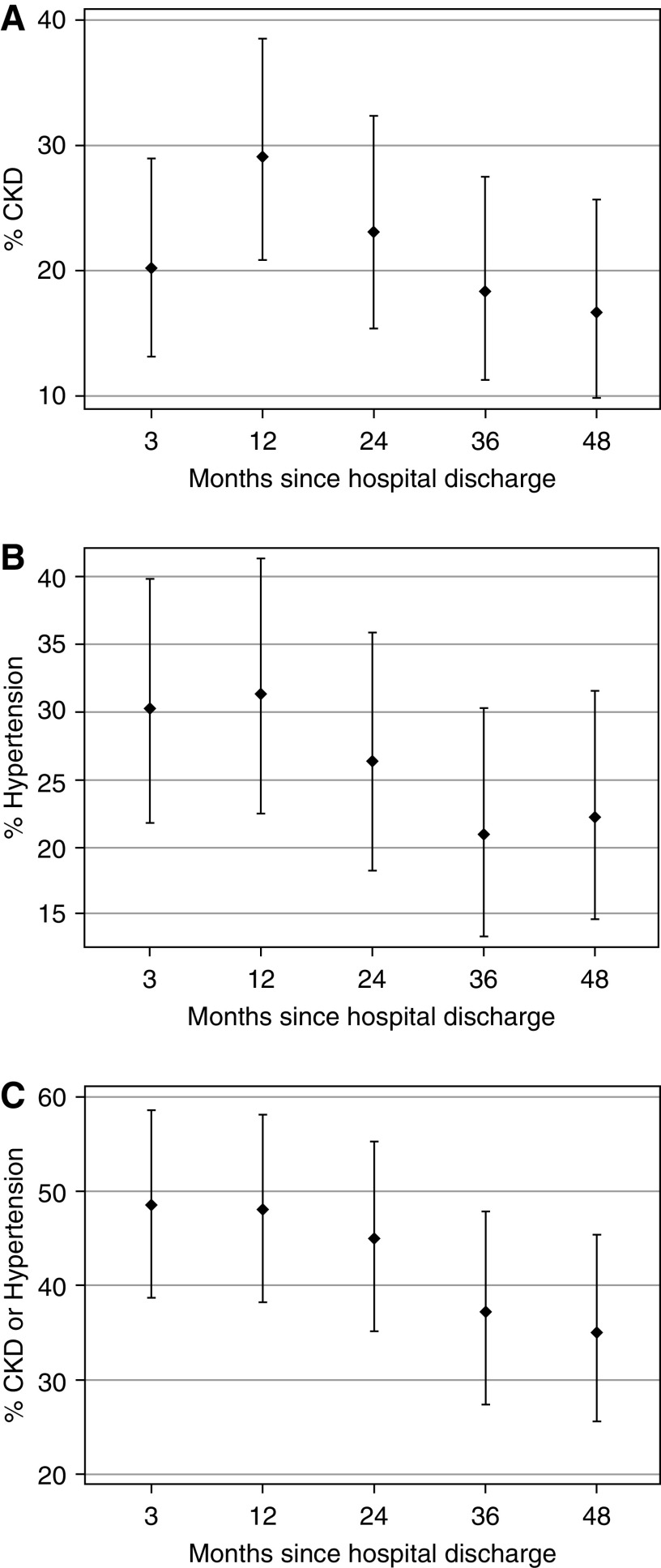
CKD and hypertension data were nonmissing for 88%–96% of participants, depending on study visit. From 3 to 48 months after discharge, the prevalence of CKD (low eGFR or high UACR) increased from 20% to 29% between 3 and 12 months, and then decreased to 17% by 48 months (Figure 2A). From 3 to 48 months after discharge, hypertension prevalence decreased from 30% to 22% (Figure 2B). At the 3-, 12-, 24-, 36-, and 48-month follow-up visits, 13 of 124 (10%), 12 of 116 (10%), 12 of 114 (11%), 11 of 108 (10%) and ten of 107 (9%) patients, respectively, reported treatment with antihypertensive agents (≥90% were angiotensin-converting enzyme inhibitors at all visits). The prevalence of CKD and/or hypertension decreased from 49% to 35% from the 3- to 48-month follow-up (Figure 2C). Supplemental Figure 2 shows that changing outcome status across study visits (e.g., CKD to non-CKD, vice versa) was common.
Figure 2.
Decrease in outcome prevalence over time after cardiac surgery. Prevalence of CKD and hypertension in the whole cohort, from 3 to 48 months after cardiac surgery discharge. In each graph, dots are the point estimates of prevalence (proportions of participants) of the outcome (y axis). Bars represent the upper and lower 95% confidence interval estimates. (A) CKD (low eGFR or high albumin-creatinine ratio) at 3–48 months postdischarge (x axis). (B) Hypertension (systolic or diastolic BP ≥95th percentile for height, age, and sex) at 3–48 months. (C) CKD or hypertension at all study visits.
Prevalence of CKD and Hypertension by AKI Status
At all study visits, CKD prevalence was not significantly different between AKI versus non-AKI groups or between stage 2 or greater AKI versus non-AKI/stage 1 AKI groups (Table 2). In general, most of the patients, regardless of AKI status, were categorized as having CKD due to high UACR (Table 2); Supplemental Table 3 displays the distribution of eGFR and UACR values by CKD status at each study visit. In multivariable analyses (adjusting for age <2 years old at surgery), the association between AKI and CKD at each study visit remained nonsignificant (Supplemental Table 4). Supplemental Table 5 shows that only in the AKI group was there a statistically significant association between 3-month postdischarge CKD with CKD at the three subsequent visits (AKI participants with versus without 3-month CKD: seven of 12 [58%] versus five of 33 [15%] with 12-month CKD; five of ten [50%] versus four of 32 [13%] with 24-month CKD; five of 11 [45%] versus one of 29 [3%] with 36-month CKD) (Supplemental Table 5).
Table 2.
Prevalence of CKD, low eGFR, high urinary albumin-creatinine ratio, and hypertension by postoperative AKI status, from 3 mo to 4 yr after cardiac surgery discharge
| Outcomes | Outcome Prevalence by Presence of Postoperative AKI Stage 1 or Worse versus No AKI, n/total (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 Mo | 12 Mo | 24 Mo | 36 Mo | 48 Mo | ||||||
| AKI | No AKI | AKI | No AKI | AKI | No AKI | AKI | No AKI | AKI | No AKI | |
| CKD | 12/49 (24) | 10/60 (17) | 15/51 (29) | 17/59 (29) | 10/48 (21) | 14/56 (25) | 8/43 (19) | 10/55 (18) | 7/42 (17) | 9/54 (17) |
| Low eGFRa | 2/51 (4) | 4/61 (7) | 2/53 (4) | 5/58 (9) | 1/49 (2) | 3/56 (5) | 1/45 (2) | 3/54 (6) | 0/43 (0) | 5/53 (9) |
| High UACRa | 11/54 (20) | 7/65 (11) | 13/52 (25) | 12/62 (19) | 9/52 (17) | 12/58 (21) | 8/47 (17) | 7/57 (12) | 7/45 (16) | 4/56 (7) |
| Systolic and/or diastolic hypertension | 19/45 (42)b | 14/64 (22) | 22/45 (49)c | 10/57 (18) | 15/47 (32) | 13/59 (22) | 9/44 (20) | 12/56 (21) | 11/46 (24) | 12/57 (21) |
| Hypertension and/or CKDa | 29/46 (63)b | 22/59 (37) | 27/46 (59) | 23/58 (40) | 21/44 (48) | 24/56 (43) | 15/40 (38) | 20/54 (37) | 16/42 (38) | 18/55 (33) |
| Outcomes | Outcome Prevalence by Presence of Postoperative AKI Stage 2 or Worse versus No AKI or Stage 1 AKI, n/total (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 Mo | 12 Mo | 24 Mo | 36 Mo | 48 Mo | ||||||
| ≥Stage 2 AKI | No AKI or Stage 1 | ≥Stage 2 AKI | No AKI or Stage 1 | ≥Stage 2 AKI | No AKI or Stage 1 | ≥Stage 2 AKI | No AKI or Stage 1 | ≥Stage 2 AKI | No AKI or Stage 1 | |
| CKD | 5/23 (22) | 17/86 (20) | 8/24 (33) | 24/86 (28) | 2/23 (9) | 22/81 (27) | 5/17 (29) | 13/81 (16) | 3/18 (17) | 13/78 (17) |
| Low eGFRa | 2/23 (9) | 4/89 (5) | 1/26 (4) | 6/85 (7) | 0/24 (0) | 4/81 (5) | 1/19 (5) | 3/80 (4) | 0/19 (0) | 5/77 (6) |
| High UACRa | 4/27 (15) | 14/92 (15) | 7/25 (28) | 18/89 (20) | 2/25 (8) | 19/85 (22) | 5/20 (25) | 10/84 (12) | 3/20 (15) | 8/81 (10) |
| Systolic and/or diastolic hypertension | 11/22b (50) | 22/87 (25) | 14/22c (64) | 18/80 (23) | 8/22 (36) | 20/84 (24) | 5/20 (25) | 16/80 (20) | 6/21 (29) | 17/82 (21) |
| Hypertension and/or CKDa | 14/21 (67) | 37/84 (44) | 15/22b (68) | 35/82 (43) | 8/20 (40) | 37/80 (46) | 9/17 (53) | 26/77 (34) | 8/18 (44) | 26/79 (33) |
CKD defined as low GFR, estimated using serum creatinine or high UACR. UACR, urinary albumin-creatinine ratio.
Note that composite outcome participant numbers will differ from the numbers for the components of the composite outcomes (e.g., CKD total, versus totals for low eGFR and high UACR).
P<0.05
P<0.005, for difference in proportion with outcome between AKI and non-AKI groups or between stage 2 or worse AKI versus no AKI/stage 1 groups.
The prevalence of hypertension was significantly higher in the AKI group in the first year of follow-up (Table 2), but this association was attenuated at later study visits (e.g., Table 2, the AKI versus non-AKI 48-month hypertension prevalence was 24% versus 21%, respectively). Similar results were found for stage 2 or higher AKI (Table 2). In multivariable analyses (adjusted for age <2 years old and mechanical ventilation duration of ≥2 days), AKI was only associated with hypertension at the 12-month visit (adjusted relative risk, 2.16; 95% CI, 1.18 to 3.95) (Supplemental Table 4). For the composite outcome of CKD and/or hypertension, there was only a statistically significant higher prevalence in the AKI group at the 3-month visit (Table 2).
Prevalence of CKD and Hypertension by Age <2 versus ≥2 Years
Table 3 shows there was no significant association between age groups and CKD prevalence at any study visit. Hypertension prevalence was higher at all study visits in patients <2 years old at surgery (statistically significant at 3-, 12- and 48-month visits, with the younger age group having approximately 2, 3.5, and 2.5 times higher hypertension prevalence, respectively; Table 3).
Table 3.
Prevalence of CKD and hypertension by age group (<2 versus ≥2 yr old at surgery), from 3 mo to 4 yr after cardiac surgery discharge (n/total %)
| Outcomes | 3 Mo | 12 Mo | 24 Mo | 36 Mo | 48 Mo | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| <2 yr | ≥2 yr | <2 yr | ≥2 yr | <2 yr | ≥2 yr | <2 yr | ≥2 yr | <2 yr | ≥2 yr | |
| CKD | 7/56 (13)a | 15/53 (28) | 13/57 (23) | 19/53 (36) | 13/55 (24) | 11/49 (22) | 7/49 (14) | 11/49 (22) | 6/46 (13) | 10/50 (20) |
| Systolic and/or diastolic hypertension | 21/53 (40) | 12/56 (21)a | 24/47 (51) | 8/55 (15)b | 17/53 (32) | 11/53 (21) | 13/48 (27) | 8/52 (15) | 16/50 (32) | 7/53 (13)a |
| Hypertension and/or CKD | 28/50 (56) | 23/55 (42) | 27/49 (55) | 23/55 (42) | 26/50 (52) | 19/50 (38) | 19/45 (42) | 16/49 (33) | 20/46 (43) | 14/51 (27) |
CKD defined as low GFR, estimated using serum creatinine or high UACR. UACR, urinary albumin-creatinine ratio.
P<0.05.
P<0.005, for difference in proportion with outcome between AKI and non-AKI groups.
Sensitivity Analyses
When antihypertensive medication treatment was included as a criterion for hypertension, the directions of associations between AKI with hypertension at each study visit were similar (Supplemental Table 6).
Discussion
This study confirms that children undergoing cardiac surgery are at high risk for development of CKD and hypertension, but CKD and hypertension prevalence decrease over time. AKI was not associated with CKD from 3 months to 4 years after cardiac surgery discharge. Hypertension prevalence was higher in participants with AKI for only the first year after discharge. Age <2 years at surgery was associated with higher prevalence of post–cardiac surgery hypertension, but not CKD.
Four years after hospital discharge, 17% of participants fulfilled CKD criteria, similar to our previous cross-sectional cohort studied 5 years after cardiac surgery (17). Adult CKD prevalence is <15%; childhood CKD is rare (29). Thus, children undergoing cardiac surgery are at high risk for CKD relative to the general pediatric population. Most CKD was ascertained due to elevated UACR. Despite controversy regarding prognostic significance of albuminuria in children (30), microalbuminuria has been shown to be associated with other cardiometabolic risk factors (31,32), suggesting that detecting albuminuria and targeting such patients for cardiovascular risk reduction may be beneficial (30). Although hypertension prevalence decreased from 28% to 20% throughout the 4-year follow-up, this prevalence is much higher than the 1%–3% reported in North American children (33,34). Reasons for decreasing prevalence of CKD and hypertension over time remain unclear. It is possible that kidney (glomerular filtration) reserve from less-injured nephrons increases after initial injury, preserving GFR, or that initial changes to the microvascular kidney environment improve over time. It is also possible that, with improved cardiac function after future surgeries or with age, kidney blood flow, and thus function, improves. Future longer-term prospective studies should address whether this apparent improvement in kidney function or hypertension is permanent. There are no post–pediatric cardiac surgery kidney or hypertension follow-up guidelines. Cardiologists, who follow these patients long term, would be ideal to target for knowledge translation regarding monitoring of CKD or hypertension and interventions to decrease kidney and cardiovascular risk. CKD and hypertension are potent and treatable cardiovascular and morbidity risk factors (35–37). Childhood hypertension is associated with hypertension in adulthood (35,38). Children undergoing cardiac surgery are at the beginning of their life span; thus, developing multidisciplinary kidney follow-up guidelines and evaluating whether follow-up interventions improve patient outcomes in this population are warranted.
Many studies in adults have shown that AKI is a risk factor for CKD, cardiovascular events, mortality, and hypertension (9–13); these associations are supported by fundamental science and animal studies (13,39–42). In this study, postoperative AKI was not significantly associated with long-term CKD or hypertension. This was important to confirm in a longitudinal study with multiple assessments because recent studies have been contradictory (15–17). It is unclear why AKI after pediatric cardiac surgery would not be associated with later CKD or hypertension development; other factors like age, cardiac disease (e.g., aortic coarctation or other vascular anomalies for hypertension, cyanotic diseases for CKD and hypertension), or postoperative management may be more potent risk factors. Young age was significantly associated with postdischarge hypertension over time. Children undergoing cardiac surgery as infants, before kidney maturation is complete, may have higher risk for kidney damage, leading to hypertension. Future studies should distinguish the effect of age from cardiac diagnosis and procedures on this association, and should be powered to evaluate multiple CKD and hypertension risk factors, to provide a targeted approach to long-term follow-up guidelines.
Participants with CKD at the 3-month visit were more likely to have CKD at subsequent visits; however, this association was much stronger in children with AKI. Routine creatinine or UACR measurement is not standard of care after cardiac surgery (17). Although these results should be interpreted with caution, given the limited subgroup sizes, the presence of past AKI plus CKD at 3 months after discharge may help decide which patients to monitor for albuminuria or low eGFR over time. Hypertension monitoring after cardiac surgery (as per guidelines, at least yearly and at all health visits in patients at risk) (27) is important. This is relevant because North American data show that BP screening and follow-up by primary care providers is often suboptimal (43,44).
A strength of this study was the prospective follow-up with low attrition. However, efforts to enhance feasibility and retention came with a cost of internal validity for ascertainment of CKD and hypertension. Guidelines state that repeated eGFR, UACR, and BP measures are needed to diagnose CKD and confirm hypertension (22,27). We performed a single assessment at each visit. We did not rule out postural proteinuria, which may have led to increased variability of our measures (favoring AKI associations with CKD toward the null) and may have led to CKD overestimation. We measured casual BP, which is susceptible to white-coat hypertension and masked hypertension, known to be common in children (45). Future research should consider performing measured GFR and reference standard hypertension ascertainment. About 10% of patients received antihypertensive medications at each follow-up visit; however, we did not determine the indication for treatment. Most antihypertensives were angiotensin-converting enzyme inhibitors, which may also be used to treat heart failure. Evaluating CKD prevalence over time in infants is complex because normal GFR and UACR levels change over the first 2 years of life, as glomerular and tubular function improve (22,26). We carefully considered this by using age-specific thresholds for normal GFR and albuminuria. We did not formally assess CKD or hypertension during preoperative recruitment; therefore, some participants may have had CKD or hypertension before study entry. The sample size was relatively low; this limited the ability to adjust for covariates in multivariable analyses and the study’s negative results should, therefore, be interpreted with caution. However, our 48-month AKI versus non-AKI prevalence of outcomes were very similar, suggesting we were not underpowered to detect a clinically significant association of AKI with outcomes. Our use of listwise deletion for handling missing data was a limitation. Our findings may not generalize to all centers performing cardiac surgery.
Larger studies of well-matched patients are needed to provide more definitive insights into the relationship between pediatric AKI and long-term adverse kidney outcomes, on risk and protective factors for development of CKD and hypertension, and on cost-effective monitoring of CKD and hypertension after pediatric cardiac surgery.
Disclosures
S.G. Coca reports receiving grants, personal fees, and equity and stock options from RenalytixAI, during the conduct of the study. He also reports receiving personal fees from Bayer, Boehringer-Ingelheim, CHF Solutions, Relypsa, and Takeda, outside the submitted work. C. Hsu reports receiving royalties from UpToDate; consulting fees from EcoR1 Capital Fund, Ice Miller LLP, and Health Advances; personal fees from Satellite Healthcare; nonfinancial support from Microlife; and grants from Satellite Healthcare, outside the submitted work. T. Alp Ikizler reports receiving personal fees from Abbott Renal Care, Fresenius Kabi, and International Society of Nephrology, during the conduct of the study. J.S. Kaufman reports receiving personal fees from NIDDK, during the conduct of the study. C.R. Parikh reports receiving consulting fees from Akebia Therapeutics, Inc. and Genfit Biopharmaceutical Company; grants from NIDDK and National Heart, Lung and Blood Institute; and other from Renaltix AI, outside the submitted work. P.L. Kimmel is the coeditor of Chronic Renal Disease (Second Edition, Academic Press, San Diego, CA, 2020). He is also employed by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the funder of the study. M.M. Moxey-Mims was employed by the NIDDK during the conduct of the study. All remaining authors have nothing to disclose.
Funding
This project was supported by National Institutes of Health, NIDDK grants UO1 DK082185, P50 DK096418 (to P. Devarajan), R01 DK098233 (to C. Hsu and A.S. Go), and K08 DK110536 (to J.H. Greenberg). M. Zappitelli received a Fonds de Recherche du Québec - Santé research salary award.
Supplementary Material
Acknowledgments
We acknowledge Dr. Yaqi Jia (MD) and Ms. Heather R. Thiessen-Philbrook (MMath) for contributing to the creation of some figures and the approach to the analysis, and Ms. Stella Wang (MSc Biostatistics) for help with revision analyses.
The opinions expressed in this article are the authors’ own and do not necessarily reflect the views of the NIDDK, the National Institutes of Health, the Department of Health and Human Services, or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00150120/-/DCSupplemental.
Supplemental Table 1. Detailed description of exclusion criteria.
Supplemental Figure 1. Study visit outline and procedures.
The figure describes the overall study visit outline and procedures.
Supplemental Table 2. Comparison of characteristics and of outcome prevalence rates by participating center.
Supplemental Figure 2. Dot plots of CKD and hypertension status at each follow-up visit, stratified by patients with versus without the outcome at the 3-month follow-up visit.
Supplemental Table 3. Distribution of eGFR and of UACR in participants without and with CKD at each follow-up visit.
Supplemental Table 4. Adjusted associations between AKI with CKD and with hypertension at 3 months to 48 months follow-up study visits.
Supplemental Table 5. Association of having the CKD outcome at the 3 month visit with having the CKD outcome at future visits in the total sample and by post-operative AKI status.
Supplemental Table 6. Prevalence hypertension (based on BP and/or use of anti-hypertensive medication) by post-operative AKI, post-operative AKI Stage 2 or worse and age < versus ≥2 years old at surgery, from 3 months to 4 years after cardiac surgery discharge.
References
- 1.Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, Phan V, Zappitelli M: Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: A two-center retrospective cohort study. Crit Care 15: R146, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, Hyder A, Alkandari O: A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int 76: 885–892, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Zappitelli M, Krawczeski CD, Devarajan P, Wang Z, Sint K, Thiessen-Philbrook H, Li S, Bennett MR, Ma Q, Shlipak MG, Garg AX, Parikh CR; TRIBE-AKI Consortium : Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int 80: 655–662, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blinder JJ, Asaro LA, Wypij D, Selewski DT, Agus MSD, Gaies M, Ferguson MA: Acute kidney injury after pediatric cardiac surgery: A secondary analysis of the safe pediatric euglycemia after cardiac surgery trial. Pediatr Crit Care Med 18: 638–646, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano D, Ito A, Yamada A, Kakegawa D, Miwa S, Umeda C, Chiba K, Takemasa Y, Tokunaga A, Ida H: Independent risk factors and 2-year outcomes of acute kidney injury after surgery for congenital heart disease. Am J Nephrol 46: 204–209, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Morgan CJ, Zappitelli M, Robertson CM, Alton GY, Sauve RS, Joffe AR, Ross DB, Rebeyka IM; Western Canadian Complex Pediatric Therapies Follow-Up Group : Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr 162: 120–127.e1, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Tanyildiz M, Ekim M, Kendirli T, Tutar E, Eyileten Z, Ozcakar ZB, Kavaz A, Yalcınkaya F, Uysalel A, Atalay S: Acute kidney injury in congenital cardiac surgery: Pediatric risk-injury-failure-loss-end-stage renal disease and Acute Kidney Injury Network. Pediatr Int (Roma) 59: 1252–1260, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Morgan C, Al-Aklabi M, Garcia Guerra G: Chronic kidney disease in congenital heart disease patients: A narrative review of evidence. Can J Kidney Health Dis 2: 27, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heung M, Steffick DE, Zivin K, Gillespie BW, Banerjee T, Hsu CY, Powe NR, Pavkov ME, Williams DE, Saran R, Shahinian VB; Centers for Disease Control and Prevention CKD Surveillance Team : Acute kidney injury recovery pattern and subsequent risk of CKD: An analysis of veterans health administration data. Am J Kidney Dis 67: 742–752, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu CY, Hsu RK, Yang J, Ordonez JD, Zheng S, Go AS: Elevated BP after AKI. J Am Soc Nephrol 27: 914–923, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickering JW, James MT, Palmer SC: Acute kidney injury and prognosis after cardiopulmonary bypass: A meta-analysis of cohort studies. Am J Kidney Dis 65: 283–293, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James MT, Grams ME, Woodward M, Elley CR, Green JA, Wheeler DC, de Jong P, Gansevoort RT, Levey AS, Warnock DG, Sarnak MJ; CKD Prognosis Consortium : A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis 66: 602–612, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madsen NL, Goldstein SL, Frøslev T, Christiansen CF, Olsen M: Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int 92: 751–756, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Cooper DS, Claes D, Goldstein SL, Bennett MR, Ma Q, Devarajan P, Krawczeski CD: Follow-Up Renal Assessment of Injury Long-Term After Acute Kidney Injury (FRAIL-AKI). Clin J Am Soc Nephrol 11: 21–29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, Garg AX, Coca S, Parikh CR; TRIBE-AKI Consortium : Kidney outcomes 5 years after pediatric cardiac surgery: The TRIBE-AKI Study. JAMA Pediatr 170: 1071–1078, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Go AS, Parikh CR, Ikizler TA, Coca S, Siew ED, Chinchilli VM, Hsu CY, Garg AX, Zappitelli M, Liu KD, Reeves WB, Ghahramani N, Devarajan P, Faulkner GB, Tan TC, Kimmel PL, Eggers P, Stokes JB; Assessment Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury Study Investigators : The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: Design and methods. BMC Nephrol 11: 22, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease Improving Global Outcomes : KDIGO Clinical Practice Guideline for Acute Kidney Injury. Available at: https://kdigo.org/guidelines/acute-kidney-injury/. Accessed August 13, 2020
- 20.Zappitelli M, Coca SG, Garg AX, Krawczeski CD, Thiessen Heather P, Sint K, Li S, Parikh CR, Devarajan P; TRIBE-AKI Consortium : The association of albumin/creatinine ratio with postoperative AKI in children undergoing cardiac surgery. Clin J Am Soc Nephrol 7: 1761–1769, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zappitelli M, Ambalavanan N, Askenazi DJ, Moxey-Mims MM, Kimmel PL, Star RA, Abitbol CL, Brophy PD, Hidalgo G, Hanna M, Morgan CM, Raju TNK, Ray P, Reyes-Bou Z, Roushdi A, Goldstein SL: Developing a neonatal acute kidney injury research definition: A report from the NIDDK neonatal AKI workshop. Pediatr Res 82: 569–573, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidney Disease Improving Global Outcomes : KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Available at: https://kdigo.org/guidelines/acute-kidney-injury/. Accessed August 24, 2020 [DOI] [PubMed]
- 23.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng DK, Schwartz GJ, Schneider MF, Furth SL, Warady BA: Combination of pediatric and adult formulas yield valid glomerular filtration rate estimates in young adults with a history of pediatric chronic kidney disease. Kidney Int 94: 170–177, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piepsz A, Tondeur M, Ham H: Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging 33: 1477–1482, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Work DF: Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4: 1832–1843, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM; Subcommittee on Screening and Management of High Blood Pressure in Children : Clinical practice guideline for screening and management of high blood pressure in children and adolescents [published onlime ahead of print August 21, 2017]. Pediatrics 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 28.Task Force on Blood Pressure Control in Children: Report of the second task force on blood pressure control in children--1987. Task force on blood pressure control in children. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics 79: 1–25, 1987. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention : Age-adjusted prevalence of CKD stages 1-4 by gender 1999-2012. Chronic Kidney Disease (CKD) Surveillance Project website. Available at https://nccd.cdc.gov.
- 30.Tsioufis C, Mazaraki A, Dimitriadis K, Stefanidis CJ, Stefanadis C: Microalbuminuria in the paediatric age: Current knowledge and emerging questions. Acta Paediatr 100: 1180–1184, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Cho H, Kim JH: Prevalence of microalbuminuria and its associated cardiometabolic risk factors in Korean youth: Data from the Korea national health and nutrition examination survey. PLoS One 12: e0178716, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lurbe E, Torro MI, Alvarez J, Aguilar F, Fernandez-Formoso JA, Redon J: Prevalence and factors related to urinary albumin excretion in obese youths. J Hypertens 31: 2230–2236; discussion 2236, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Paradis G, Tremblay MS, Janssen I, Chiolero A, Bushnik T: Blood pressure in Canadian children and adolescents. Health Rep 21: 15–22, 2010. [PubMed] [Google Scholar]
- 34.Xi B, Zhang T, Zhang M, Liu F, Zong X, Zhao M, Wang Y: Trends in elevated blood pressure among US children and adolescents: 1999-2012. Am J Hypertens 29: 217–225, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J; American Heart Association Expert Panel on Population and Prevention Science; Council on Cardiovascular Disease in the Young; Council on Epidemiology and Prevention; Council on Nutrition; Council on Physical Activity and Metabolism; Council on High Blood Pressure Research; Council on Cardiovascular Nursing; Council on the Kidney in Heart Disease; Interdisciplinary Working Group on Quality of Care and Outcomes Research : Cardiovascular risk reduction in high-risk pediatric patients: A scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs 22: 218–253, 2007. [DOI] [PubMed] [Google Scholar]
- 36.James MT, Hemmelgarn BR, Tonelli M: Early recognition and prevention of chronic kidney disease. Lancet 375: 1296–1309, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Carmody JB, Charlton JR: Short-term gestation, long-term risk: Prematurity and chronic kidney disease. Pediatrics 131: 1168–1179, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM: Cardiac and vascular consequences of pre-hypertension in youth. J Clin Hypertens (Greenwich) 13: 332–342, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devarajan P, Jefferies JL: Progression of chronic kidney disease after acute kidney injury. Prog Pediatr Cardiol 41: 33–40, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferenbach DA, Bonventre JV: Acute kidney injury and chronic kidney disease: From the laboratory to the clinic. Nephrol Ther 12[Suppl 1]: S41–S48, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldstein SL, Devarajan P: Progression from acute kidney injury to chronic kidney disease: A pediatric perspective. Adv Chronic Kidney Dis 15: 278–283, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Humphreys BD, Bonventre JV: Pathophysiology of acute kidney injury to chronic kidney disease: Maladaptive repair. Contrib Nephrol 174: 149–155, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Aliarzadeh B, Meaney C, Moineddin R, White D, Birken C, Parkin P, Greiver M: Hypertension screening and follow-up in children and adolescents in a Canadian primary care population sample: A retrospective cohort study. CMAJ Open 4: E230–E235, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen ML, Gunn PW, Kaelber DC: Underdiagnosis of hypertension in children and adolescents. JAMA 298: 874–879, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Acosta AA, McNiece KL: Ambulatory blood pressure monitoring: A versatile tool for evaluating and managing hypertension in children. Pediatr Nephrol 23: 1399–1408, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.