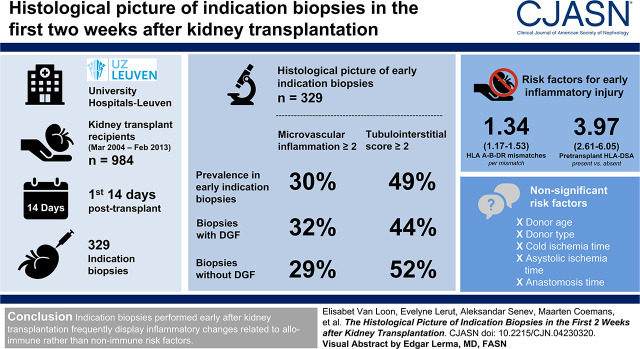
Visual Abstract
Keywords: acute allograft rejection, ischemia-reperfusion injury, delayed graft function, kidney biopsy, kidney transplantation
Abstract
Background and objectives
In preclinical studies, ischemia-reperfusion injury and older donor age are associated with graft inflammation in the early phase after transplantation. In human kidney transplantation, impaired allograft function in the first days after transplantation is often adjudicated to donor- and procedure-related characteristics, such as donor age, donor type, and ischemia times.
Design, setting, participants, & measurements
In a cohort of 984 kidney recipients, 329 indication biopsies were performed within the first 14 days after transplantation. The histologic picture of these biopsies and its relationship with alloimmune risk factors and donor- and procedure-related characteristics were studied, as well as the association with graft failure. Multivariable Cox models were applied to quantify the cause-specific hazard ratios for early rejection and early inflammatory scores, adjusted for potential confounders. For quantification of hazard ratios of early events for death-censored graft failure, landmark analyses starting from day 15 were used.
Results
Early indication biopsy specimens displayed microvascular inflammation score ≥2 in 30% and tubulointerstitial inflammation score ≥2 in 49%. Rejection was diagnosed in 186 of 329 (57%) biopsies and associated with the presence of pretransplant donor-specific HLA antibodies and the number of HLA mismatches, but not nonimmune risk factors in multivariable Cox proportional hazards analysis. In multivariable Cox proportional hazards analysis, delayed graft function, the graft dysfunction that prompted an early indication biopsy, HLA mismatches, and pretransplant donor-specific HLA antibodies were significantly associated with a higher risk for death-censored graft failure, whereas early acute rejection was not.
Conclusions
Indication biopsies performed early after kidney transplantation display inflammatory changes related to alloimmune risk factors. Nonimmune risk factors for ischemia-reperfusion injury, such as cold and warm ischemia time, older donor age, and donor type, were not identified as strong risk factors for early inflammation after human kidney transplantation.
Introduction
To mitigate the deceased donor organ shortage, donor acceptance criteria for kidney transplantation were expanded over the past several decades, leading to increased numbers of transplantation with kidneys from older donors and donation after cardiac death (1,2). These donor characteristics are risk factors for delayed graft function (DGF), graft dysfunction, and graft failure, both in preclinical and in large cohort studies (3−7). Despite the preclinical demonstration of an association between donor characteristics and inflammation (3), the histologic relevance early after human kidney transplantation remains poorly studied.
Ischemia-reperfusion injury was extensively studied as a risk factor for kidney graft failure and rejection. In preclinical studies, prolonged cold ischemia time was associated with a proinflammatory response after reperfusion of the graft because of ischemia-reperfusion injury (8,9). This inflammatory response is thought to increase the risk of allograft rejection (10−13). However, the effect of cold ischemia time on allograft rejection was not consistent between studies (14,15). Further, warm ischemia time during organ procurement and additional warm ischemia during kidney implantation (anastomosis time) have been linked with DGF and graft outcome (6,16–19). Their effect on inflammation is less well studied, but a small study showed a lower rate of DGF and acute rejection and lower inflammatory molecular signals when limiting warm ischemia during anastomosis time (20).
Despite the extensive literature on the effect of these donor- and procedure-related characteristics on graft failure, the relevance for the allograft’s histologic presentation early after kidney transplantation has not been sufficiently studied. In this study, we evaluated the contribution of donor- and procedure-related factors, next to immunologic risk factors, to the observed risk of inflammation in human kidney transplant biopsies performed at the time of graft dysfunction in the first 2 weeks after transplantation, and to the risk of DGF. Finally, we evaluated the relevance of these early events for graft survival.
Materials and Methods
Study Population and Data Collection
All consecutive adult recipients of a kidney transplant at the University Hospitals Leuven between March 2004 and February 2013 were eligible. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. We excluded recipients of combined transplants or kidney transplants after another solid-organ transplant. All transplants were performed with negative complement‐dependent cytotoxicity crossmatches on T and B lymphocytes. The clinical data were prospectively collected during routine clinical follow‐up. This study was approved by the Ethics Committee of the University Hospitals Leuven (approval numbers S53364 and S61971). Decline in initially good kidney function, insufficient kidney functional recovery after transplantation, and DGF are all considered as allograft dysfunction. DGF was defined as the need for dialysis in the first week after transplantation.
Biopsies and Histologic Scoring
In the early biopsy group, we included all post-transplant kidney allograft biopsies performed at time of graft dysfunction in the first 14 days after transplantation. One pathologist (E.L.) reviewed all of these biopsies. The severity of the histologic lesions was semiquantitatively scored according to Banff grades. An immunohistochemical C4d stain (mAb, dilution 1:500; Quidel Corporation, Santa Clara, CA) was performed on a frozen tissue in clinical routine. The diagnosis of the histologic phenotypes was on the basis of the criteria as defined by the Banff 2017 consensus (21).
Borderline changes were defined as foci of tubulitis (t>0) with minor interstitial inflammation (i1) or moderate‐to-severe interstitial inflammation (i2 or i3) with mild (t1) tubulitis. Early inflammatory changes were defined as microvascular inflammation (glomerulitis and peritubular capillaritis) score ≥2, tubulointerstitial inflammation (tubulitis and interstitial inflammation) score ≥2, and intimal arteritis (v) score ≥1 within the first 2 weeks after transplantation.
Detection of Circulating Anti-HLA Antibodies and HLA Genotyping
The follow-up of anti-HLA antibodies was systematically monitored in one histocompatibility laboratory (Histocompatibility and Immunogenetics Laboratory, Belgian Red Cross Flanders); details on this assessment were previously published (22,23). For most patients, anti-HLA donor-specific antibodies (DSA) were assessed retrospectively. For some patients, presence of pretransplant DSA was known before transplantation, but they were accepted because of low-grade DSA with negative complement-dependent cytotoxicity crossmatches. We screened all sera using a LIFECODES LifeScreen Deluxe (LMX) kit (Immucor, Norcross, GA), and in case of positive screening, we assessed the donor-specificity using LIFECODES Single Antigen Bead (LSA) kits (Immucor). To determine true donor specificity, we genotyped the transplant pairs at second field high-resolution level for the HLA-A, -B, -C, -DRB1, -DRB3, -DRB4, -DRB5, -DQA1, -DQB1, -DPA1, and -DPB1 loci. HLA mismatches were calculated at the antigen split level.
Statistical Analyses
From the initial cohort of 1000 patients, 16 patients were excluded because of missing data on asystolic ischemia time (n=6) and anastomosis time (n=10). All other relevant variables (donor age, donor type, cold ischemia time, HLA A-B-DR mismatches, and HLA DSAs) were complete, leaving 984 patients for complete case analysis. Variables with normal distribution are displayed as mean±SD. Median and interquartile range are given for variables without normal distribution. For variance analysis of continuous variables in different groups, the t test was used. Dichotomous variables were compared using the chi-squared test. Multivariable Cox models were applied to quantify the cause-specific hazard ratios (HRs) for early rejection and early inflammatory scores, adjusted for potential confounders. For these analyses, patients without biopsy in the first 14 days were censored at day 15. In patients with more than one biopsy in the first 14 days, only the first biopsy was taken into account for analyses. For quantification of odds ratios of baseline characteristics for DGF, unadjusted and multivariable logistic regression models were used. For quantification of HRs of early events for death-censored graft failure, landmark analyses starting from day 15 were used. In these analyses, recipients were censored at time of death with a functioning graft or at time of last follow-up date. Eight grafts were lost in the first 2 weeks after transplantation (two to recipient death and six to graft failure), leaving 976 patients with 148 events for this analysis. All multivariable models were adjusted for recipient age, recipient and donor sex, recipient body mass index at transplantation, repeat transplantation, pretransplant diabetes mellitus, use of induction therapy, and immunosuppressive regimen at transplantation. For all models, we graphically explored the form of the loess line through the Martingale residuals, supplemented with the Kolmogorov-type supremum test, and supplemented with graphical inspection of Martingale residuals when discordant. The time dependency (nonproportionality) of the relevant variables was checked by including an interaction term with the covariate and time in the Cox model, and supplemented with graphical inspection of Schoenfeld residuals when discordant. There were no major linearity or proportionality assumptions violated for relevant covariates in any of the models. We checked for interaction effects between relevant baseline risk factors in the models. When a significant interaction was found, further elaboration was done by assessing specific estimates for the interaction effect and plotting the log(odds) and probability for visualization. We used SAS software (version 9.4; SAS Institute, Cary, NC) for statistical analysis and GraphPad Prism (version 8; GraphPad Software, San Diego, CA) for data presentation.
Results
Study Population Characteristics
Between 2004 and 2013, 1137 patients were transplanted. Patients with combined transplants or with a history of another organ transplantation before kidney transplantation were excluded (n=137), as well as patients with missing data on the relevant variables (n=16), leaving 984 transplantations in 968 recipients available for this study. Median follow‐up time was 7.4 (interquartile range, 4.9–10.0) years. Baseline characteristics of the study population are described in Table 1. In total, 329 biopsies were performed at time of graft dysfunction in 291 patients (30%) in the first 14 days after transplantation. The mean time till biopsy was 7.4±2.8 days post-transplant. In comparison with grafts without a biopsy in the first 14 days (n=693), grafts that underwent an early biopsy (n=291) were more often from older donors, transplanted with more pretransplant HLA DSAs and HLA antigen mismatches into recipients with higher body mass index, receiving a more standard immunosuppression regimen consisting of tacrolimus, mycophenolate mofetil, and corticosteroids (Table 1).
Table 1.
Demographics for the cohort with and without early indication biopsies, within the first 14 days post-transplant
| Characteristic | Entire Cohort, N=984 | Grafts with Early Indication Biopsy, n=291 | Grafts without Early Indication Biopsy, n=693 |
|---|---|---|---|
| Recipient characteristics at transplantation | |||
| Age, yr, mean±SD | 54±13 | 54±13 | 54±13 |
| Recipient BMI at time of transplantation, kg/m2, mean±SD | 25.4±4.5 | 25.8±4.7 | 25.2±4.3 |
| Sex, male, n (%) | 598 (61%) | 179 (62%) | 419 (61%) |
| White race, n (%) | 968 (98%) | 299 (100%) | 678 (98%) |
| Pretransplant diabetes mellitus, n (%) | 172 (17%) | 61 (21%) | 111 (16%) |
| Repeat transplantation, n (%) | 153 (16%) | 52 (18%) | 101 (15%) |
| Donor characteristics at transplantation | |||
| Age, yr, mean±SD | 48±15 | 50±15 | 47±15 |
| Sex, male, n (%) | 527 (54%) | 146 (50%) | 381 (55%) |
| Type of donor | |||
| Living donor, n (%) | 57 (6%) | 15 (5%) | 42 (6%) |
| Donation after brain death, n (%) | 774 (79%) | 222 (76%) | 552 (80%) |
| Donation after cardiac death, n (%) | 153 (16%) | 54 (19%) | 99 (14%) |
| Transplant characteristics | |||
| Cold ischemia time, h, mean±SD | 14.2±5.6 | 14.5±5.7 | 14.1±5.6 |
| Asystolic ischemia time, min mean±SD (only in donation after cardiac death donors) | 17.1±10.3 | 18.9±9.9 | 16.2±10.5 |
| Anastomosis time, min, mean±SD | 35.1±8.2 | 35.6±8.1 | 34.9±8.2 |
| Total HLA-A, -B, -DR mismatches, mean±SD | 2.7±1.3 | 3.0±1.3 | 2.6±1.3 |
| Immunosuppression regimen TAC‐MPA‐CS, n (%) | 862 (88%) | 266 (91%) | 596 (86%) |
| Induction therapy, n (%)a | 411 (42%) | 121 (42%) | 290 (42%) |
| Pretransplant donor-specific anti-HLA antibodies, n (%) | 108 (11%) | 54 (19%) | 54 (8%) |
| Delayed graft function | 188 (19%) | 101 (35%) | 87 (13%) |
| Overall survival 1 yr | 93% | 87% | 96% |
| Overall survival 3 yr | 87% | 81% | 90% |
| Overall survival 5 yr | 80% | 71% | 84% |
| Death-censored graft survival 1 yr | 95% | 91% | 97% |
| Death-censored graft survival 3 yr | 92% | 87% | 95% |
| Death-censored graft survival 5 yr | 89% | 82% | 92% |
BMI, body mass index; TAC-MPA-CS, tacrolimus-mycophenolic acid-corticosteroids.
Induction agents most often consisted of anti-IL2 receptor blockade (basiliximab, daclizumab) (380 of 411, 93%), other agents included thymoglobulins (6%), alefacept (1%), Igs (0.5%), and rituximab (0.2%).
Biopsy Characteristics and Risk Factors for Early Rejection
Histologic lesions in the 329 early biopsies are shown in Table 2. Microvascular inflammation (g+ptc) score ≥2 was present in 99 of 329 biopsies (30%), tubulointerstitial inflammation (i+t) score ≥2 was present in 162 of 329 biopsies (49%), and intimal arteritis score ≥1 was present in 119 of 329 (36%) biopsies. Biopsy-proven acute rejection was present in 186 of 329 (57%) biopsies, with 14 (4%) antibody-mediated rejection, 95 (29%) T cell–mediated rejection, 31 (9%) borderline changes, and 46 (14%) mixed rejections. In total, 19 samples met the inflammatory lesions criteria but not full Banff criteria for acute rejection. A total of 166 of 186 rejections (89%) were treated with antirejection therapy. Borderline changes were less often treated (59%), whereas antibody-mediated rejection (12 of 14), mixed (43 of 46), and T cell–mediated rejection (92 of 95) were almost all treated. Antirejection therapy most often consisted of corticosteroids (86%).
Table 2.
Histologic picture of early indication biopsies (n=329)
| Histological Characteristic | Prevalence in Early Indication Biopsies (n=329), n (%) | Biopsies with DGF (n=127) | Biopsies without DGF (n=202) |
|---|---|---|---|
| Rejection phenotypes (Banff 2017) | |||
| No rejection | 143 (43%) | 63 (50%) | 80 (40%) |
| Borderline changes | 31 (9%) | 7 (6%) | 24 (12%) |
| Acute T cell–mediated rejection | 95 (29%) | 33 (26%) | 62 (31%) |
| Antibody-mediated rejection | 14 (4%) | 3 (2%) | 11 (5%) |
| Mixed rejection (TCMR and ABMR) | 46 (14%) | 21 (17%) | 25 (12%) |
| Histologic lesion scores | |||
| Glomerulitis >0; >1 | 88 (27%); 57 (17%) | 37 (29%); 27 (21%) | 51 (25%); 30 (15%) |
| Peritubular capillaritis >0; >1 | 118 (36%); 59 (18%) | 50 (39%); 23 (18%) | 68 (34%); 36 (18%) |
| Microvascular inflammation (g+ptc) ≥2 | 99 (30%) | 41 (32%) | 58 (29%) |
| C4d deposition ptc >0; >1 | 94 (29%); 58 (18%) | 37 (29%); 21 (17%) | 57 (28%); 37 (18%) |
| Tubulitis >0; >1 | 185 (56%); 74 (22%) | 67 (53%); 25 (20%) | 118 (58%); 49 (24%) |
| Interstitial inflammation >0; >1 | 157 (48%); 101 (31%) | 54 (43%); 36 (28%) | 103 (51%); 65 (32%) |
| Tubulointerstitial score ≥ 2 | 162 (49%) | 56 (44%) | 105 (52%) |
| Intimal arteritis >0; >1 | 119 (36%); 18 (5%) | 52 (41%); 8 (6%) | 67 (33%); 10 (5%) |
| Vascular intimal thickening >0; >1 | 138 (42%); 53 (16%) | 52 (41%); 23 (18%) | 86 (43%); 30 (15%) |
| Arteriolar hyalinosis >0; >1 | 90 (27%); 32 (10%) | 26 (20%); 8 (6%) | 64 (32%); 24 (12%) |
| Tubular atrophy >0; >1 | 155 (47%); 9 (3%) | 52 (41%); 4 (3%) | 103 (51%); 5 (2%) |
| Intimal fibrosis >0; >1 | 41 (12%); 12 (4%) | 14 (11%); 5 (4%) | 27 (13%); 7 (3%) |
| Thrombi | 18 (5%) | 4 (3%) | 14 (7%) |
| Transplant glomerulopathy >0; | 1 (0.3%) | 0 | 1 (0.5%) |
| Mesangial matrix expansion >0; >1 | 14 (4%); 5 (2%) | 4 (3%) | 10 (5%) |
DGF, delayed graft function; TCMR, T cell-mediated rejection; ABMR, antibody-mediated rejection; g, glomerulitis; ptc, peritubular capillaritis.
We performed cause-specific Cox proportional hazard analyses to determine which baseline characteristics were associated with inflammatory lesions and acute rejection (Table 3). In multivariable analysis, only pretransplant HLA DSAs were retained as a significant risk factor for microvascular inflammation. Risk factors for tubulointerstitial inflammation were the number of HLA mismatches and the presence of pretransplant HLA DSAs (Figures 1 and 2B). For acute rejection (antibody-mediated rejection, T cell–mediated rejection, mixed rejection, and/or borderline changes), risk factors in multivariable analyses consisted of HLA mismatches and pretransplant HLA-DSA. There was no significant interaction effect between donor age and cold ischemia time.
Table 3.
Risk factor assessment for early inflammatory injury
| Covariate | Microvascular Inflammation ≥2 | Tubulointerstitial Inflammation ≥2 | Acute Rejection |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| No. of events | n=86 | n=146 | n=166 |
| Donor age, per yr | 1.01 (0.99 to 1.03) | 1.01 (0.99 to 1.02) | 1.01 (0.99 to 1.02) |
| Living donor versus donation after brain death | 0.51 (0.15 to 1.70) | 0.51 (0.19 to 1.32) | 0.43 (0.17 to 1.10) |
| Donation after circulatory death versus donation after brain death | 1.00 (0.30 to 3.29) | 0.97 (0.43 to 2.16) | 1.16 (0.56 to 2.41) |
| Cold ischemia time, h | 0.98 (0.94 to 1.04) | 0.99 (0.95 to 1.03) | 0.99 (0.96 to 1.03) |
| Asystolic ischemia time, min | 0.99 (0.93 to 1.06) | 1.01 (0.97 to 1.05) | 1.00 (0.97 to 1.04) |
| Anastomosis time, min | 1.01 (0.98 to 1.04) | 1.00 (0.97 to 1.02) | 1.00 (0.98 to 1.02) |
| HLA A-B-DR mismatches, per mismatch | 1.15 (0.96 to 1.38) | 1.38 (1.20 to 1.60) | 1.34 (1.17 to 1.53) |
| Pretransplant HLA DSAs, present versus absent | 6.94 (4.04 to 11.94) | 3.02 (1.87 to 4.89) | 3.97 (2.61 to 6.05) |
Risk factors assessed by multivariable Cox proportional hazard analysis (n=291 biopsies). HR, hazard ratio; 95% CI, 95% confidence interval; DSAs, donor-specific antibodies. Adjusted for recipient age, recipient body mass index at transplantation, recipient and donor sex, pretransplant diabetes mellitus, repeat transplantation, immunosuppressive regimen at time of transplantation, and induction therapy.
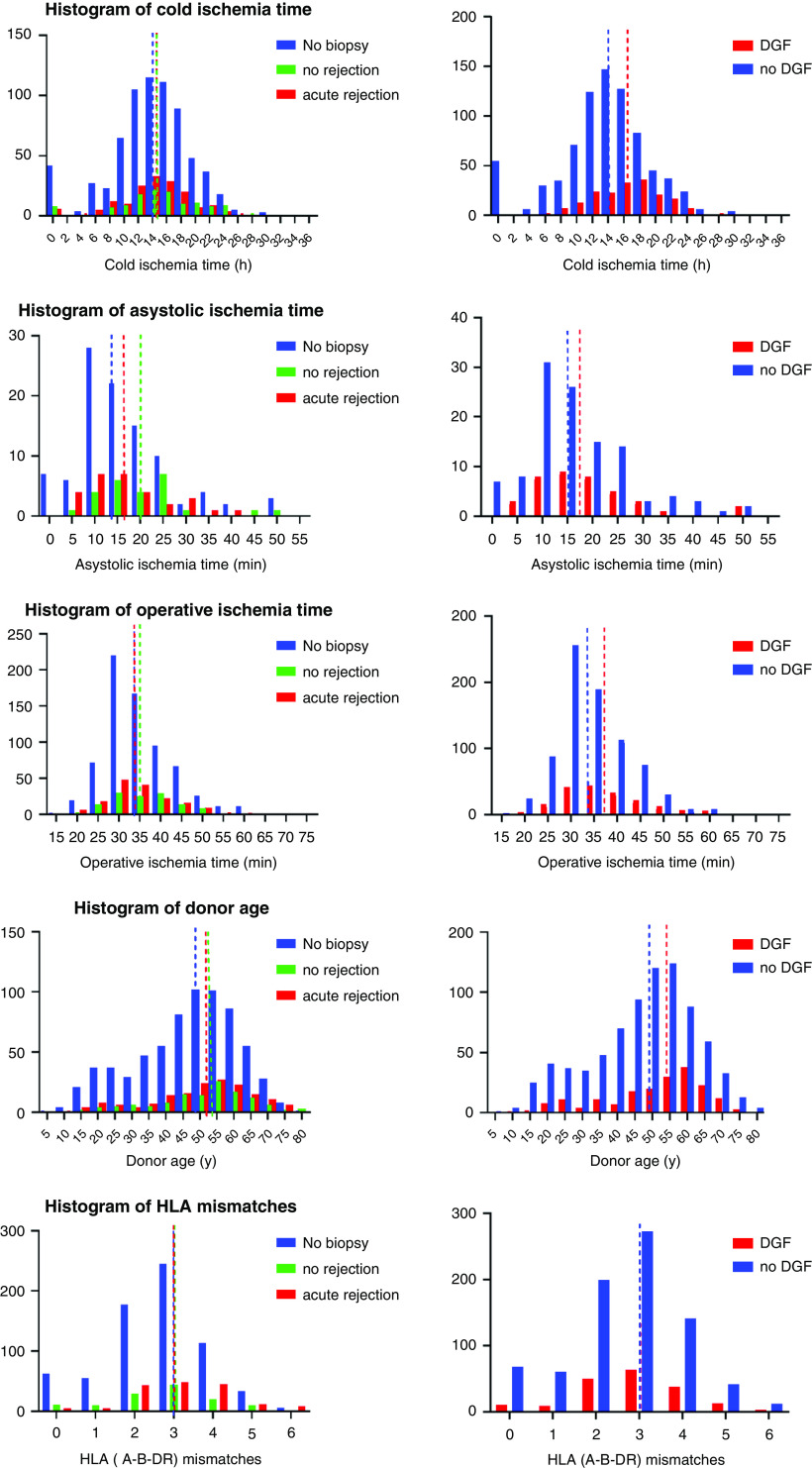
Figure 1.
Histograms of cold ischemia time, donor age, asystolic ischemia time, anastomosis time, and HLA antigen mismatches for delayed graft function (DGF) and acute rejection in the first 2 weeks after transplantation. In comparison with grafts without a biopsy in the first 14 days (n=693), grafts that underwent an early biopsy (n=291) were more often from older donors. No significant differences in baseline variables between acute rejection and no rejection were noted for ischemia times and donor age. Longer ischemia times and older donor age were seen in patients experiencing DGF versus without DGF. For the histogram of asystolic ischemia time, only donation after cardiac death is represented. Dashed lines represent the median.
Figure 2.
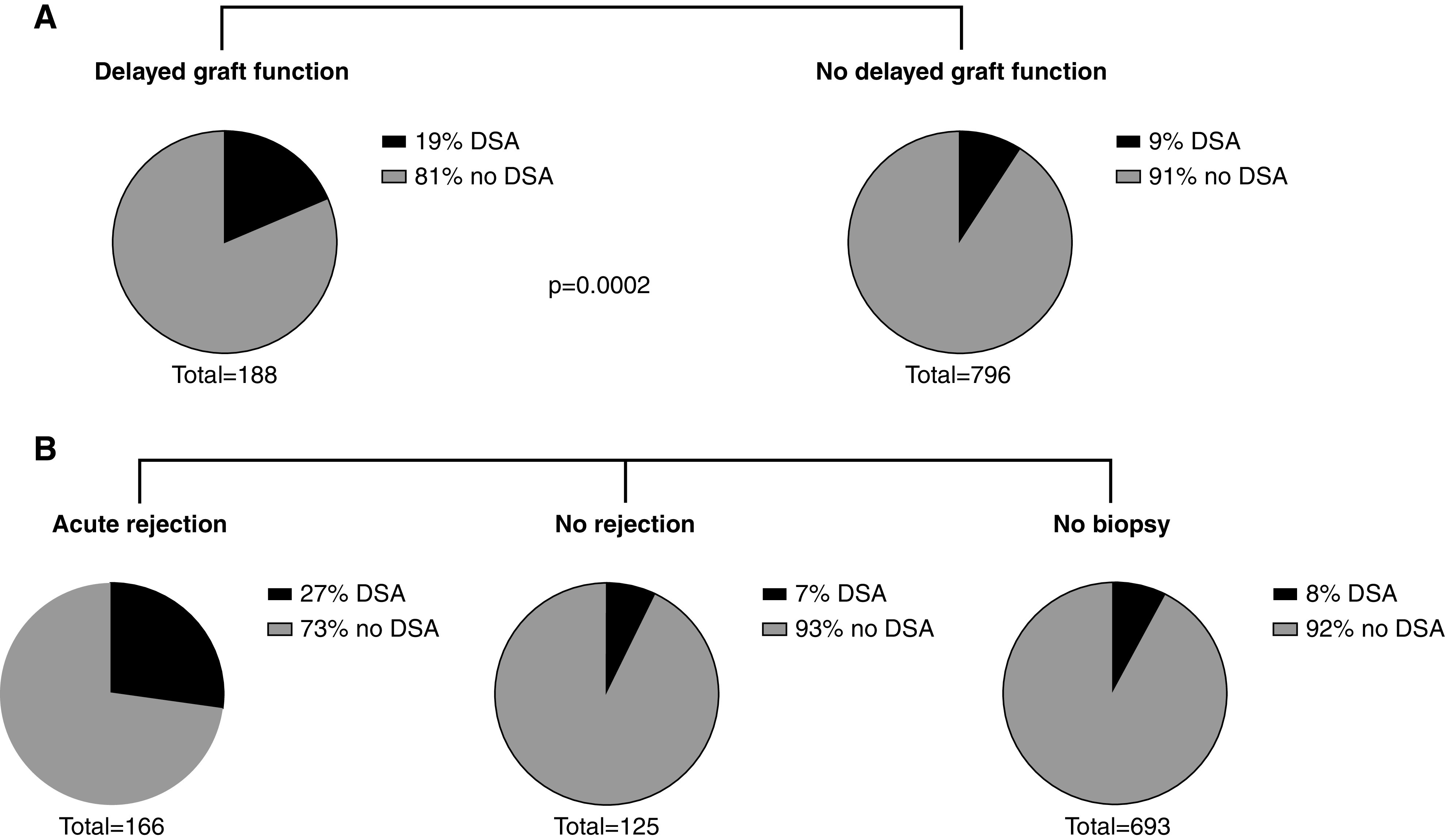
Distribution of pretransplant HLA donor-specific antibodies (DSAs) according to delayed graft function and acute rejection in the first 2 weeks after transplantation. (A) Patients experiencing DGF (n=188) had signficantly more pretransplant HLA-DSA compared with patients who did not experience DGF (n=796). (B) For patients experiencing acute rejection in the first 2 weeks after transplantation, a higher prevalence of pretransplant HLA-DSA was noted compared with patients without rejection/biopsy in the first 2 weeks after transplantation.
Risk Factors for DGF
In total, 188 of 984 grafts (19%) experienced DGF. A biopsy was not performed in all grafts with DGF; in 87 of 188 (46%) grafts, no biopsy was performed, whereas in the remaining 101 grafts (54%), a total of 127 biopsies were performed in the first 2 weeks after transplantation. We found no significant differences in baseline characteristics between grafts experiencing DGF that received a biopsy compared with those that did not, except for more induction therapy and more frequent prescription of a standard immunosuppressive regimen (54% versus 37%, P=0.02 and 96% versus 87%, P=0.03, respectively). Acute rejection was present in 64 of 127 (50%) biopsies performed in grafts experiencing DGF that had received a biopsy, and was mostly T cell–mediated rejection (33 of 64, 52%), followed by mixed rejections (21 of 64, 33%), borderline changes (seven of 64, 11%), and antibody-mediated rejection (three of 64, 5%). This rejection rate was not significantly different compared with early indication biopsies from grafts without DGF (acute rejection in 122 of 202, 60%; P=0.08). When looking at individual histologic lesions in early biopsies from grafts experiencing DGF compared with early biopsies from grafts without DGF, no significant differences were noted (Table 2). Baseline risk factors differing between patients with an early biopsy at time of DGF versus patients with an early biopsy without DGF are described in Supplemental Table 1.
Donor age, deceased donor type, cold ischemia time, asystolic ischemia time and anastomosis time, and pretransplant HLA-DSA were associated with DGF in unadjusted analysis (Figures 1 and 2A, Supplemental Table 2). There was no significant interaction effect between donor age and cold ischemia time, donor age and deceased donor type, or cold ischemia time and deceased donor type. There was a significant interaction effect found between donor age and anastomosis time for the risk of DGF (P=0.04). In multivariable analysis, donor age, cold ischemia time, and anastomosis time were associated with DGF (Supplemental Table 1). We did not include the interaction effect between donor age and anastomosis time in the multivariable analysis because this did not relevantly change the results and would lead to less interpretable results. We elaborate on this interaction effect in Supplemental Figure 1. With higher donor age, the probability of DGF increases to a larger extent, with longer anastomosis times, compared with younger donors.
Early Risk Factors for Graft Failure
During follow-up, 154 of 984 grafts experienced graft failure, with six grafts lost and two recipient deaths with a functioning transplant in the first 14 days, leaving 148 events in 976 grafts for landmark analysis, starting at 15 days post-transplant. Grafts with an indication biopsy in the first 14 days after transplantation had a worse outcome compared with those without early biopsy (HR, 1.91; 95% confidence interval [95% CI], 1.36 to 2.67; P<0.001) independent of the baseline demographics (Figure 3). In multivariable analysis adjusted for all baseline risk factors, presence or absence of acute rejection in this biopsy made no difference in outcome (effect of rejection: HR, 0.99; 95% CI, 0.76 to 1.31; P=0.99) (Figure 3). DGF was associated with graft failure (HR, 2.51; 95% CI, 1.73 to 3.64; P<0.001), after adjusting for all baseline covariates. A final multivariable Cox model, taking into account these early events on top of donor- and procedure-related risk factors, illustrated that DGF, the graft dysfunction that prompted the act of an early indication biopsy, HLA mismatches, and pretransplant HLA DSAs were significantly associated with a higher risk for death-censored graft failure, whereas early acute rejection was not (Table 4).
Figure 3.
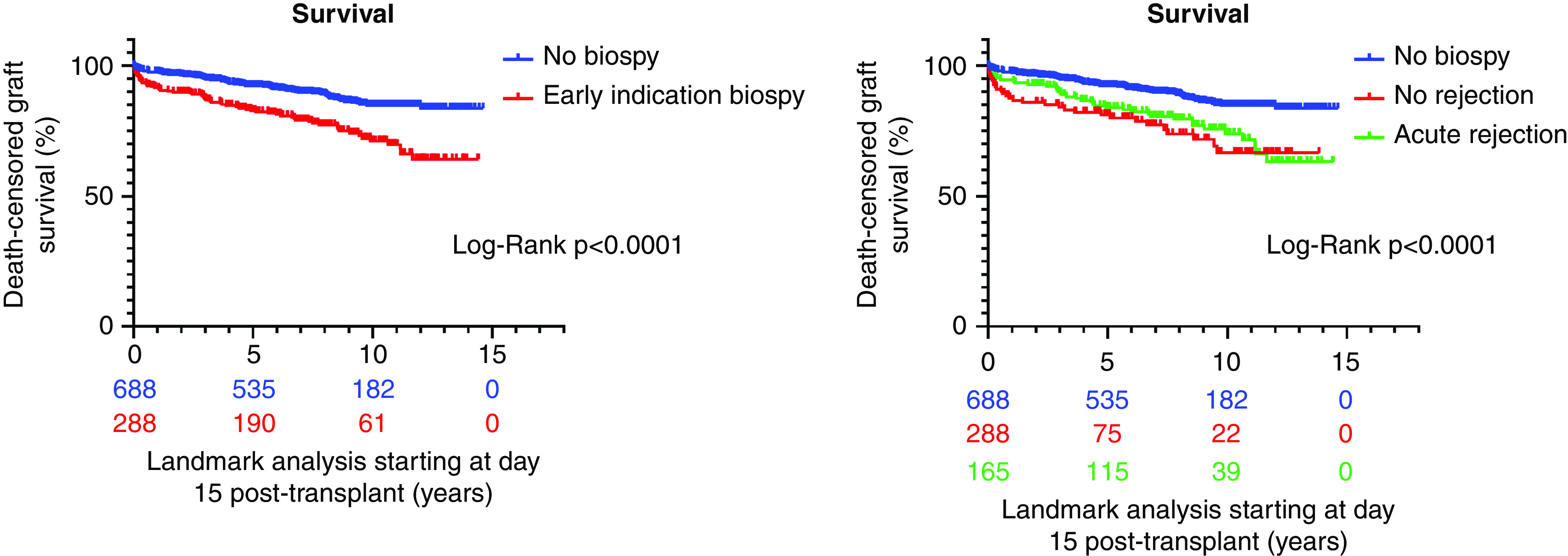
Kaplan–Meier survival curves starting at day 15 after transplantation, stratified according to early indication biopsy in the first 2 weeks after transplantation (left panel), and stratified according to acute rejection in the early biopsy, no rejection in the early biopsy, or no biopsy (right panel). Grafts with an indication biopsy in the first 14 days after transplantation had a worse outcome compared with those without early biopsy; presence or absence of acute rejection in this biopsy made no difference in outcome.
Table 4.
Risk assessment of early events and baseline characteristics on death-censored graft failure
| Covariate | Hazard Ratio (95% CI) |
|---|---|
| No. of events/no. of transplants | 148/976 |
| Donor age, per yr | 1.01 (1.00 to 1.02) |
| Donor type | |
| Living donor versus donation after brain death | 0.36 (0.13 to 1.05) |
| Donation after circulatory death versus donation after brain death | 0.49 (0.19 to 1.27) |
| Cold ischemia time, h | 0.99 (0.96 to 1.03) |
| Asystolic ischemia time, min | 1.02 (0.98 to 1.07) |
| Anastomosis time, min | 1.00 (0.98 to 1.02) |
| HLA A-B-DR mismatches, per mismatch | 1.20 (1.04 to 1.38) |
| Pretransplant HLA DSAs, present versus absent | 1.88 (1.16 to 3.04) |
| Delayed graft function, yes or no | 2.03 (1.38 to 3.01) |
| Any rejection | 0.67 (0.40 to 1.12) |
| Early indication biopsy | 2.12 (1.36 to 3.29) |
Multivariable model of effect of delayed graft function, histologic rejection in early indication biopsies, and baseline characteristics on death-censored graft failure as assessed by multivariable Cox proportional hazard analysis. Landmark analysis starting at 15 days post-transplantation. Six grafts were lost and two patients died within 14 days post-transplantation and were not included in analysis. 95% CI, 95% confidence interval; DSAs, donor-specific antibodies.
Discussion
In this observational, single-center, cohort study, we confirm that inflammatory lesions are often present in early indication biopsies. However, their presence was most importantly related to alloimmune factors, and not to nonimmune factors like ischemia times, donor age, and donor type. Graft dysfunction in the first 14 days that triggered performing a biopsy translated into a worse graft survival beyond 14 days, independent of other baseline risk factors. Whether this biopsy demonstrated inflammatory injury or not did not alter the survival of these grafts. Ischemia times, donor type, and donor age were not associated with worse graft survival after adjustment for DGF.
From preclinical studies, cold ischemia time is thought to induce a proinflammatory response through ischemia-reperfusion injury (8,9), enhancing the risk of allograft rejection (10,14,24), but we could not confirm this in our study. This hypothesis has not been consistently validated in clinical studies, and our study cannot confirm a significant effect of ischemia-reperfusion injury on histologic inflammation in the early phase after transplantation, in contrast to an earlier study (25). We did find an interaction effect between anastomosis time and donor age, and illustrated that older donors are more susceptible to increasing anastomosis times in their risk for DGF. But this did not lead to a significant association between donation after brain death or donation after cardiac death grafts and early inflammatory lesions.
On the other hand, these nonimmune factors (cold ischemia time, anastomosis time, donor age, and donation after cardiac death grafts) are clearly associated with DGF, as illustrated by the multivariable analysis. In grafts experiencing DGF where a biopsy was performed, there was no difference in histologic lesions nor rate of rejection compared with early biopsies with graft dysfunction but no need for dialysis. Thus, although nonimmune factors are strongly associated with DGF, there was no strong effect found on histologic inflammation, nor was there a distinctive histologic correlate for DGF.
Inflammatory lesions like tubulointerstitial inflammation and microvascular inflammation are the hallmarks for well-defined rejection phenotypes like T cell–mediated rejection and antibody-mediated rejection, respectively. Therefore, it is not surprising that alloimmune factors like HLA mismatches and HLA DSAs are identified as strong risk factors in this study. Although the donor–recipient HLA mismatch is associated with T cell–mediated rejection, only specific mismatches leading to HLA DSA are associated with antibody-mediated rejection. HLA DSA–mediated injury is targeted to the graft microvasculature and endothelium (26). In this study, early biopsies for graft dysfunction were hallmarked by a high rate of inflammatory lesions, where much of the microvascular inflammation was accompanied by tubulointerstitial inflammation, leading to a high rate of mixed rejection (antibody-mediated rejection with concomitant T cell–mediated rejection). We could not demonstrate a deleterious effect of these early rejections, taking into account that the graft dysfunction warranting an early biopsy (independent of histologic findings) was already associated with worse graft survival, and that these rejections were treated.
Alloimmune factors like HLA mismatches and HLA DSAs remained significant risk factors for worse graft survival, independent of early events. This illustrates that, even with current powerful immunosuppressive agents for prevention of rejection, rejection is still very common early after transplantation and could best be avoided by further decreasing the immunologic risk (avoid pretransplant DSAs, better HLA matching, adequate induction therapy). This study does not support the hypothesis that early inflammation is a consequence of ischemia-reperfusion injury even in cases considered at high risk for such injury, such as donation after cardiac death, older donor kidneys, or kidneys with longer ischemia times.
There are several limitations to this study. Because only biopsies at time of graft dysfunction are performed in the first 3 months after transplantation, there is considerable selection bias, and subclinical inflammation will be missed. Also, as illustrated in this study, not all patients with DGF underwent a biopsy, which could either be related to fast recovery from the need for dialysis or clinical decision, as the indication for a biopsy is left to each physician’s discretion. Because the large majority of early rejection episodes were treated in this study, no conclusions can be made regarding whether these rejections might have had a more detrimental effect on outcome without treatment. Similarly, all patients received powerful immunosuppressive regimens with almost universal perioperative high-dose steroids, which could have attenuated inflammatory changes related to nonimmune risk factors, and possibly explain the difference with the results from animal studies. Next, the progressive awareness and efforts to minimize cold ischemia time in the past decades are clearly reflected by the low proportion of extended cold ischemia time in this cohort. Furthermore, our study population consisted largely of White patients and most of them were on a tacrolimus-based regimen, with low to intermediate immunologic risk, and with only a proportion of patients receiving induction therapy, limiting the generalizability of these findings to other and higher-risk populations. Finally, from the statistical analyses not showing a significant association between nonimmune factors and inflammation, we cannot conclude that there is complete absence of an effect on inflammation of nonimmune donor and procedure characteristics from this study; larger, multicenter studies with higher heterogeneity in nonimmune risk factors like cold ischemia time are needed to address this. Moreover, no inference can be made on events later after transplantation.
In conclusion, these clinical data illustrate that indication biopsies performed in the earliest days after kidney transplantation often display inflammatory changes and that these changes seem to be related to alloimmune risk factors. In contrast to preclinical observations, nonimmune factors like ischemia times, older donor age, and donor type were not identified as strong risk factors for early inflammation after human kidney transplantation in this patient cohort.
Disclosures
M. Naesens reports a patent EP19152365.3 issued to KU Leuven. All remaining authors have nothing to disclose.
Funding
This study was funded by the Research Foundation–Flanders (Fonds Wetenschappelijk Onderzoek [FWO]) and Flanders Innovation and Entrepreneurship (VLAIO) with a TBM (Toegepast Biomedisch onderzoek met een primair Maatschappelijke finaliteit) project (grant IWT.150199), awarded to M. Naesens. This study was also funded by Onderzoeksraad, KU Leuven grant C32/17/049. E. Van Loon holds an FWO fellowship grant (1143919N). M. Naesens and B. Sprangers are senior clinical investigators of FWO (grants 1844019N and 1842919N).
Supplementary Material
Acknowledgments
The authors thank the centers of the Leuven Collaborative Group for Renal Transplantation, the clinicians and surgeons, nursing staff, and the patients.
Dr. Elisabet Van Loon, Mr. Maarten Coemans, Dr. Aleksandar Senev, Prof., Dr. Evelyne Lerut, Prof., Dr. Dirk Kuypers, Prof., Dr. Marie-Paule Emonds, Prof., Dr. Ben Sprangers, and Prof., Dr. Maarten Naesens collected the data. Dr. Elisabet Van Loon and Prof., Dr. Maarten Naesens designed the study, analyzed the data, and prepared figures. Dr. Elisabet Van Loon, Mr. Maarten Coemans, Dr. Aleksandar Senev, Prof., Dr. Evelyne Lerut, Prof., Dr. Jacques Pirenne, Prof., Dr. Diethard Monbaliu, Prof., Dr. Ina Jochmans, Prof., Dr. Mauricio Sainz Barriga, Prof., Dr. Katrien De Vusser, Dr. Amaryllis H. Van Craenenbroeck, Prof., Dr. Marie-Paule Emonds, Prof., Dr. Ben Sprangers, Prof., Dr. Dirk Kuypers, and Prof., Dr. Maarten Naesens contributed to the report and have read and agreed with the manuscript as written.
Data Sharing Statement
All of the individual, deidentified participant data that underlie the results reported in this article (text, tables, figures, and appendices) can be made available on a collaborative basis after institutional review board approval, immediately after publication, without end date and for any purpose. Proposals should be directed to the corresponding author.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04230320/-/DCSupplemental.
Supplemental Table 1. Demographics for the cohort with an early indication biopsy within the first 14 days post-transplant, with or without delayed graft function (DGF).
Supplemental Table 2. Baseline risk factors for delayed graft function (DGF) in univariate and multivariable analysis.
Supplemental Figure 1. Visualization of interaction effect between donor age and anastomosis time for delayed graft function (DGF). The left panel illustrates the log(odds) of DGF with increasing anastomosis time per predefined donor age category. The right panel illustrates the increase in probability of DGF with increasing anastomosis time, categorized by donor age.
References
- 1.Summers DM, Watson CJ, Pettigrew GJ, Johnson RJ, Collett D, Neuberger JM, Bradley JA: Kidney donation after circulatory death (DCD): State of the art. Kidney Int 88: 241–249, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Mirshekar-Syahkal B, Summers D, Bradbury LL, Aly M, Bardsley V, Berry M, Norris JM, Torpey N, Clatworthy MR, Bradley JA, Pettigrew GJ: Local expansion of donation after circulatory death kidney transplant activity improves waitlisted outcomes and addresses inequities of access to transplantation. Am J Transplant 17: 390–400, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Denecke C, Yuan X, Ge X, Kim IK, Bedi D, Boenisch O, Weiland A, Jurisch A, Kotsch K, Pratschke J, Reutzel-Selke A, Tullius SG: Synergistic effects of prolonged warm ischemia and donor age on the immune response following donation after cardiac death kidney transplantation. Surgery 153: 249–261, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Summers DM, Johnson RJ, Hudson A, Collett D, Watson CJ, Bradley JA: Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: A cohort study. Lancet 381: 727–734, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA: Outcomes of kidneys from donors after cardiac death: Implications for allocation and preservation. Am J Transplant 7: 1797–1807, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Heylen L, Jochmans I, Samuel U, Tieken I, Naesens M, Pirenne J, Sprangers B: The duration of asystolic ischemia determines the risk of graft failure after circulatory-dead donor kidney transplantation: A eurotransplant cohort study. Am J Transplant 18: 881–889, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Schaapherder A, Wijermars LGM, de Vries DK, de Vries APJ, Bemelman FJ, van de Wetering J, van Zuilen AD, Christiaans MHL, Hilbrands LH, Baas MC, Nurmohamed AS, Berger SP, Alwayn IP, Bastiaannet E, Lindeman JHN: Equivalent long-term transplantation outcomes for kidneys donated after brain death and cardiac death: Conclusions from a nationwide evaluation. EClinicalMedicine 4–5: 25–31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uehara M, Solhjou Z, Banouni N, Kasinath V, Xiaqun Y, Dai L, Yilmam O, Yilmaz M, Ichimura T, Fiorina P, Martins PN, Ohori S, Guleria I, Maarouf OH, Tullius SG, McGrath MM, Abdi R: Ischemia augments alloimmune injury through IL-6-driven CD4+ alloreactivity. Sci Rep 8: 2461, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alegre ML, Florquin S, Goldman M: Cellular mechanisms underlying acute graft rejection: Time for reassessment. Curr Opin Immunol 19: 563–568, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Tilney NL, Guttmann RD: Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation 64: 945–947, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Pratschke J, Kofla G, Wilhelm MJ, Vergopoulos A, Laskowski I, Shaw GD, Tullius SG, Volk HD, Neuhaus P, Tilney NL: Improvements in early behavior of rat kidney allografts after treatment of the brain-dead donor. Ann Surg 234: 732–740, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuquay R, Renner B, Kulik L, McCullough JW, Amura C, Strassheim D, Pelanda R, Torres R, Thurman JM: Renal ischemia-reperfusion injury amplifies the humoral immune response. J Am Soc Nephrol 24: 1063–1072, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenig A, Chen CC, Marçais A, Barba T, Mathias V, Sicard A, Rabeyrin M, Racapé M, Duong-Van-Huyen JP, Bruneval P, Loupy A, Dussurgey S, Ducreux S, Meas-Yedid V, Olivo-Marin JC, Paidassi H, Guillemain R, Taupin JL, Callemeyn J, Morelon E, Nicoletti A, Charreau B, Dubois V, Naesens M, Walzer T, Defrance T, Thaunat O: Missing self triggers NK cell-mediated chronic vascular rejection of solid organ transplants. Nat Commun 10: 5350, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postalcioglu M, Kaze AD, Byun BC, Siedlecki A, Tullius SG, Milford EL, Paik JM, Abdi R: Association of cold ischemia time with acute renal transplant rejection. Transplantation 102: 1188–1194, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayler LK, Magliocca J, Zendejas I, Srinivas TR, Schold JD: Impact of cold ischemia time on graft survival among ECD transplant recipients: A paired kidney analysis. Am J Transplant 11: 2647–2656, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Weissenbacher A, Oberhuber R, Cardini B, Weiss S, Ulmer H, Bösmüller C, Schneeberger S, Pratschke J, Öllinger R: The faster the better: Anastomosis time influences patient survival after deceased donor kidney transplantation. Transpl Int 28: 535–543, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Heylen L, Naesens M, Jochmans I, Monbaliu D, Lerut E, Claes K, Heye S, Verhamme P, Coosemans W, Bammens B, Evenepoel P, Meijers B, Kuypers D, Sprangers B, Pirenne J: The effect of anastomosis time on outcome in recipients of kidneys donated after brain death: A cohort study. Am J Transplant 15: 2900–2907, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Heylen L, Pirenne J, Samuel U, Tieken I, Naesens M, Sprangers B, Jochmans I: The impact of anastomosis time during kidney transplantation on graft loss: A eurotransplant cohort study. Am J Transplant 17: 724–732, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Tennankore KK, Kim SJ, Alwayn IP, Kiberd BA: Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int 89: 648–658, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Kamińska D, Kościelska-Kasprzak K, Chudoba P, Hałoń A, Mazanowska O, Gomółkiewicz A, Dzięgiel P, Drulis-Fajdasz D, Myszka M, Lepiesza A, Polak W, Boratyńska M, Klinger M: The influence of warm ischemia elimination on kidney injury during transplantation - Clinical and molecular study. Sci Rep 6: 36118, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E, Alachkar N, Bagnasco S, Bouatou Y, Becker JU, Cornell LD, Duong van Huyen JP, Gibson IW, Kraus ES, Mannon RB, Naesens M, Nickeleit V, Nickerson P, Segev DL, Singh HK, Stegall M, Randhawa P, Racusen L, Solez K, Mengel M: The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senev A, Coemans M, Lerut E, Van Sandt V, Daniëls L, Kuypers D, Sprangers B, Emonds MP, Naesens M: Histological picture of antibody-mediated rejection without donor-specific anti-HLA antibodies: Clinical presentation and implications for outcome. Am J Transplant 19: 763–780, 2019. [DOI] [PubMed] [Google Scholar]
- 23.Senev A, Lerut E, Van Sandt V, Coemans M, Callemeyn J, Sprangers B, Kuypers D, Emonds MP, Naesens M: Specificity, strength, and evolution of pretransplant donor-specific HLA antibodies determine outcome after kidney transplantation. Am J Transplant 19: 3100–3113, 2019. [DOI] [PubMed] [Google Scholar]
- 24.Mikhalski D, Wissing KM, Ghisdal L, Broeders N, Touly M, Hoang AD, Loi P, Mboti F, Donckier V, Vereerstraeten P, Abramowicz D: Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation 85[Suppl]: S3–S9, 2008. [DOI] [PubMed] [Google Scholar]
- 25.de Fijter JW, Mallat MJ, Doxiadis II, Ringers J, Rosendaal FR, Claas FH, Paul LC: Increased immunogenicity and cause of graft loss of old donor kidneys. J Am Soc Nephrol 12: 1538–1546, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Louis K, Hertig A, Taupin JL, Buob D, Jamme M, Brocheriou I, Luque Y, Jouanneau C, Ouali N, Audouin M, Rondeau E, Xu-Dubois YC: Markers of graft microvascular endothelial injury may identify harmful donor-specific anti-HLA antibodies and predict kidney allograft loss. Am J Transplant 19: 2434–2445, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.