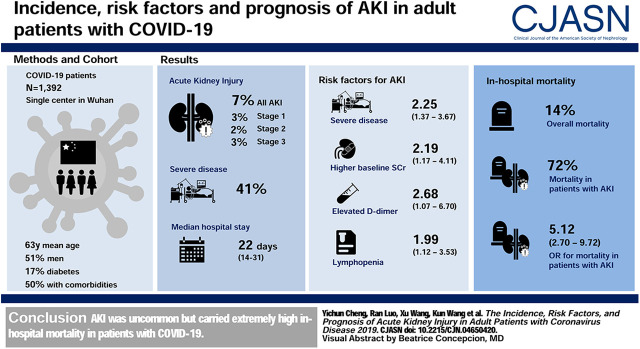
Visual Abstract
Keywords: acute kidney injury, COVID-19, mortality, prognosis, risk factors
Abstract
Background and objectives
Since December 2019, coronavirus disease 2019 (COVID-19) outbreak occurred and has rapidly spread worldwide. However, little information is available about the AKI in COVID-19. We aimed to evaluate the incidence, risk factors, and prognosis of AKI in adult patients with COVID-19.
Design, setting, participants, & measurements
This was a retrospective cohort study of 1392 patients with COVID-19 admitted to a tertiary teaching hospital. Clinical characteristics and laboratory data were extracted from electronic hospitalization and laboratory databases. AKI was defined and staged according to the 2012 Kidney Disease: Improving Global Outcomes criteria. Risk factors for AKI and the association of AKI with in-hospital mortality were assessed.
Results
A total of 7% (99 of 1392) of patients developed AKI during hospitalization, 40% (40 of 99) of which occurred within 1 week of admission. Factors associated with a higher risk of AKI include severe disease (odds ratio [OR], 2.25; 95% confidence interval [CI], 1.37 to 3.67), higher baseline serum creatinine (OR, 2.19; 95% CI, 1.17 to 4.11), lymphopenia (OR, 1.99; 95% CI, 1.12 to 3.53), and elevated D-dimer level (OR, 2.68; 95% CI, 1.07 to 6.70). The in-hospital mortality in patients with AKI stage 1, stage 2, and stage 3 was 62%, 77%, and 80%, respectively. AKI was associated with in-hospital mortality even after adjustment for confounders (OR, 5.12; 95% CI, 2.70 to 9.72).
Conclusions
AKI is uncommon but carries high in-hospital mortality in patients with COVID-19.
Introduction
In December 2019, a new strain of coronavirus was identified and officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously known as 2019-nCoV) (1,2). Because >3 million cases of coronavirus disease 2019 (COVID-19) have been reported in >100 countries, it was officially classified as a pandemic by the World Health Organization on March 11, 2020.
Although acute respiratory distress syndrome due to diffuse alveolar damage was associated with the highest mortality, lungs were not the only organ involved (3,4). It was reported that SARS-CoV-2 interacts with the human angiotensin-converting enzyme II molecule, which is highly expressed in lung tissues and in human kidneys (5,6). Furthermore, immunostaining showed that SARS-CoV-2 nucleoprotein antibody was positive in tubules, and electron microscopic examination showed clusters of coronavirus-like particles, with distinctive spikes in the tubular epithelium and podocytes (7), indicating that the human kidney is a potential target of SARS-CoV-2. A previous study also reported the high prevalence of proteinuria, hematuria, and elevated serum creatinine in patients suffering from COVID-19 (8).
AKI is a common, serious complication in critically ill patients and is associated with higher mortality, longer hospital stay, and higher medical costs (9). The detection rate of AKI in patients with COVID-19 varies from 0.5% to 29% and is dependent on the number of enrolled patients, proportion of severely sick patients, and the duration of follow-up (3,10–13). However, there is limited information concerning epidemiologic characteristics and outcomes of AKI in patients with COVID-19.
The aim of this study is to evaluate the incidence, risk factors, and prognosis of AKI in adult patients with COVID-19 in a tertiary teaching hospital, which was designated for serious COVID-19 cases by local government.
Materials and Methods
Participants
This was a retrospective study conducted in Tongji Hospital (Wuhan, China), a tertiary teaching hospital designated for the treatment of patients with severe COVID-19 by local government. The diagnosis criteria of COVID-19 were as follows: fever or respiratory symptoms, leukopenia or lymphopenia, and computed tomography scan showing radiographic abnormalities in the lungs. Patients fulfilling at least two clinical diagnosis criteria and with positive findings from high-throughput sequencing real-time RT-PCR assay of nasal and pharyngeal swab specimens or a positive antibodies test to SARS-CoV-2 were confirmed as COVID-19 positive. We screened patients who were diagnosed with COVID-19 and admitted to the Sino-French New City branch of Tongji Hospital from January 18 to February 28, 2020 (n=1449). We excluded patients aged <18 years (n=13), patients with a history of maintenance dialysis or kidney transplantation (n=19), and patients who were missing serum creatinine on admission (n=25). A total of 1392 patients were included in the main analysis (Figure 1). It should be noted that 333 of the patients included in the study by Pei et al. (14) and 701 of the patients (8) included in our previous study were also included in this study. The study protocol and waiving of written informed consent was approved by the Medical Ethics Committee of Tongji Hospital (approval number TJ-C20200132).
Figure 1.
Flow chart of the study population selection. COVID-19, coronavirus disease 2019.
The criteria for discharge were absence of fever for at least 3 days; substantial improvement in both lungs on a chest computed tomography scan; clinical remission of respiratory symptoms; and two nasal and pharyngeal swab samples that were negative for SARS-CoV-2 RNA, obtained at least 24 hours apart. By April 22, 2020, all patients in our study had either died or were discharged from the hospital.
Data Collection
The demographic characteristics, clinical symptoms, laboratory data, and outcomes were extracted from electronic medical records. All comorbidities were reported by patients or family members. Laboratory data consisted of kidney function tests, lymphocyte count, and examination of D-dimer, high-sensitivity C-reactive protein, and lactate dehydrogenase levels. eGFR was calculated with the CKD Epidemiology Collaboration equation (CKD-EPI) (15). The baseline clinical characteristics and laboratory data were ascertained at the time of admission. The data were reviewed by a trained team of physicians.
Definition
Fever was defined as an axillary temperature of at least 37.3°C. Severe cases were defined as either (1) a respiratory rate >30/min, (2) oxygen saturation ≤93%, or (3) partial pressure of oxygen/fraction of inspired oxygen ratio ≤300 mm Hg. Lymphopenia was defined as a lymphocyte count of <1.0×109/L (16). Anemia was defined as a hemoglobin level of <13 g/dl in men and <12 g/dl in women.
AKI was defined as an increase in serum creatinine by 0.3 mg/dl within 48 hours or a 50% increase in serum creatinine from baseline within 7 days, according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria (17). Baseline serum creatinine was defined as the lowest serum creatinine value within first 7 days of hospitalization. The date of AKI onset was defined as the earliest day of a serum creatinine change meeting the KDIGO criteria. The maximum stage of AKI was determined using the peak serum creatinine level after AKI detection, with increases of 1.5–1.9, 2.0–2.9, and ≥3 times the baseline being defined as AKI stage 1, 2, and 3, respectively. Recovery from AKI was defined as serum creatinine decreasing below the level determined in the definition of AKI stage 1 (18). We also expanded the AKI criteria and analyzed the association of AKI defined by expanded criteria and in-hospital death. The expanded AKI criteria were defined as a relative slow increasing of, or a small increase in, serum creatinine. In addition to an increase in serum creatinine by 0.3 mg/dl within 48 hours, expanded criteria 1 and 2 were defined as an increase in serum creatinine to 1.5 times the baseline within 14 days and during hospitalization, respectively; Expanded criteria 3, 4, and 5 were defined as an increase in serum creatinine to 1.4, 1.3, and 1.2 times the baseline within 7 days, respectively.
Statistical Analyses
Categorical variables were summarized as percentages, and continuous variables were expressed as median with interquartile range (IQR). Differences in the in-hospital mortality between AKI stages were visualized using a Kaplan–Meier curve and compared using a log-rank test. To explore the risk factors associated with AKI, univariable and multivariable logistic regression models were used. We excluded variables from the univariable analysis if the number of events was too small to estimate odds ratios (ORs; CKD, chronic lung disease, and tumor). The significant variables (P<0.05) in univariable analysis were included in multivariable analysis. The association of AKI and in-hospital mortality was also assessed with logistic regression analysis. Adjusted variables were chosen on the basis of previous findings. Age, lymphopenia, and D-dimer level were reported to be associated with in-hospital mortality (19,20); in addition, previous studies have shown that in-hospital mortality was higher in male patients, as well as in patients with severe disease or those admitted to an intensive care unit (3,21). There is no collinearity between variables in multivariable logistic regression analysis. The missing data of covariates were recorded as “missing,” the level of missingness is presented in Table 1. No imputation was made in logistic regression analysis. Statistical analyses were performed using R software, version 3.6.1, with statistical significance set at a two-sided P<0.05.
Table 1.
Demographic characteristics, laboratory data, and outcomes of patients with coronavirus disease 2019
| Characteristics | All Patients, N=1392 | Non-AKI, n=1293 | AKI Stage 1, n=42 | AKI Stage 2, n=22 | AKI Stage 3, n=35 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Summary | n | Summary | n | Summary | n | Summary | n | Summary | |
| Clinical characteristics | ||||||||||
| Age, yr | 1392 | 63 (50–71) | 1293 | 63 (49–70) | 42 | 65 (56–74) | 22 | 70 (60–79) | 35 | 67 (62–75) |
| Male patients, n (%) | 1392 | 711 (51) | 1293 | 644 (50) | 42 | 29 (69) | 22 | 14 (64) | 35 | 24 (69) |
| Fever on admission, n (%) | 1310 | 334 (25) | 1229 | 311 (25) | 36 | 10 (28) | 17 | 4 (24) | 28 | 9 (32) |
| Systolic BP, mm Hg | 1338 | 130 (118–143) | 1245 | 129 (118–142) | 40 | 134 (117–146) | 20 | 128 (117–143) | 33 | 143 (130–159) |
| Diastolic BP, mm Hg | 1338 | 80 (73–88) | 1245 | 80 (73–88) | 40 | 80 (74–87) | 20 | 79 (71–90) | 33 | 85 (76–96) |
| Severe disease, n (%) | 1392 | 570 (41) | 1293 | 501 (39) | 42 | 27 (64) | 22 | 15 (68) | 35 | 27 (77) |
| Smoking, n (%) | 1392 | 83 (6) | 1293 | 71 (5) | 42 | 6 (14) | 22 | 2 (9) | 35 | 4 (11) |
| Any comorbidity, n (%) | 1392 | 694 (50) | 1293 | 638 (49) | 42 | 24 (57) | 22 | 12 (55) | 35 | 20 (57) |
| CKD | 1392 | 21 (2) | 1293 | 17 (1) | 42 | 3 (7) | 22 | 1 (5) | 35 | 0 (0) |
| Chronic lung disease | 1392 | 77 (6) | 1293 | 66 (5) | 42 | 9 (21) | 22 | 0 (0) | 35 | 2 (6) |
| Diabetes | 1392 | 241 (17) | 1293 | 218 (17) | 42 | 6 (14) | 22 | 6 (27) | 35 | 11 (31) |
| Hypertension | 1392 | 499 (36) | 1293 | 459 (35) | 42 | 14 (33) | 22 | 9 (41) | 35 | 17 (49) |
| Tumor | 1392 | 62 (5) | 1293 | 57 (4) | 42 | 3 (7) | 22 | 2 (9) | 35 | 0 (0) |
| Concomitant other respiratory viral infection, n (%) | 1392 | 351 (25) | 1293 | 330 (26) | 42 | 5 (12) | 22 | 6 (27) | 35 | 10 (29) |
| Laboratory data | ||||||||||
| BUN, mg/dl | 1392 | 13 (9–17) | 1293 | 12 (9–16) | 42 | 19 (11–33) | 22 | 25 (17–38) | 35 | 24 (16–29) |
| Serum creatinine, mg/dl | 1392 | 0.8 (0.6–1.0) | 1293 | 0.8 (0.6–1.0) | 42 | 1.0 (0.7–1.2) | 22 | 1.0 (0.7–1.6) | 35 | 1.0 (0.8–1.2) |
| eGFR, ml/min per 1.73 m2 | 1392 | 92 (77–104) | 1293 | 92 (78–104) | 42 | 80 (58–103) | 22 | 72 (42–83) | 35 | 78 (61–91) |
| eGFR<60 ml/min per 1.73 m2, n (%) | 1392 | 159 (11) | 1293 | 133 (10) | 42 | 10 (24) | 22 | 8 (36) | 35 | 8 (23) |
| Hematuria | 877 | 354 (40) | 834 | 327 (39) | 22 | 12 (55) | 8 | 5 (63) | 13 | 10 (77) |
| 1+ | 877 | 270 (31) | 834 | 256 (31) | 22 | 6 (27) | 8 | 2 (25) | 13 | 6 (46) |
| 2+∼3+ | 877 | 84 (10) | 834 | 71 (9) | 22 | 6 (27) | 8 | 3 (38) | 13 | 4 (31) |
| Proteinuria | 877 | 494 (56) | 834 | 457 (55) | 22 | 18 (82) | 8 | 7 (88) | 13 | 12 (92) |
| 1+ | 877 | 433 (49) | 834 | 405 (49) | 22 | 15 (68) | 8 | 5 (63) | 13 | 8 (62) |
| 2+∼3+ | 877 | 61 (7) | 834 | 52 (6) | 22 | 3 (14) | 8 | 2 (25) | 13 | 4 (31) |
| Lymphopenia, n (%) | 1392 | 772 (55) | 1293 | 691 (53) | 42 | 35 (83) | 22 | 18 (82) | 35 | 28 (80) |
| Anemia, n (%) | 1392 | 599 (43) | 1293 | 552 (43) | 42 | 28 (67) | 22 | 9 (41) | 35 | 10 (29) |
| D-dimer >0.5 mg/L, n (%) | 1320 | 939 (72) | 1224 | 849 (69) | 41 | 36 (88) | 21 | 21 (100) | 34 | 33 (97) |
| hs-CRP≥10 mg/L, n (%) | 1335 | 987 (74) | 1239 | 897 (72) | 42 | 36 (86) | 21 | 21 (100) | 33 | 33 (100) |
| LDH>245 U/L, n (%) | 1391 | 931 (67) | 1292 | 846 (65) | 42 | 33 (79) | 22 | 19 (86) | 35 | 33 (94) |
| Outcomes, n (%) | ||||||||||
| Intensive care unit | 1392 | 140 (10) | 1293 | 66 (5) | 42 | 26 (62) | 22 | 19 (86) | 35 | 29 (83) |
| Mechanical ventilation | 1392 | 284 (20) | 1293 | 204 (16) | 42 | 31 (74) | 22 | 19 (86) | 35 | 30 (86) |
| Noninvasive | 1392 | 259 (19) | 1293 | 191 (15) | 42 | 26 (62) | 22 | 18 (82) | 35 | 24 (69) |
| Invasive | 1392 | 109 (8) | 1293 | 48 (4) | 42 | 24 (57) | 22 | 13 (59) | 35 | 24 (69) |
| Vasopressor | 1392 | 194 (14) | 1293 | 119 (9) | 42 | 27 (64) | 22 | 18 (82) | 35 | 30 (86) |
| ECMO | 1392 | 11 (1) | 1293 | 5 (0) | 42 | 3 (1) | 22 | 1 (5) | 35 | 2 (6) |
| Hospital length of stay, d | 1392 | 22 (14–31) | 1293 | 22 (15–31) | 42 | 23 (12–33) | 22 | 24 (14–33) | 35 | 10 (7–18) |
| In-hospital mortality, n (%) | 1392 | 199 (14) | 1293 | 128 (10) | 42 | 26 (62) | 22 | 17 (77) | 35 | 28 (80) |
Clinical characteristics and laboratory data were assessed on admission. Values for categorical variables are given as count (percentage); values for continuous variables are given as median (interquartile range). Other respiratory viral infection includes influenza virus, parainfluenza virus, adenovirus, and respiratory syncytial virus. Vasopressors include norepinephrine, epinephrine, and dopamine. Severe disease was defined as either (1) respiratory rate >30/min, (2) oxygen saturation ≤93%, or (3) partial pressure of oxygen/fraction of inspired oxygen ratio ≤300 mm Hg. Lymphopenia is defined as lymphocyte count <1.0×109/L. Anemia is defined as a hemoglobin level of <13 g/dl in men and <12 g/dl in women. hs-CRP, high-sensitivity C-reactive protein; LDH, lactose dehydrogenase; ECMO, extracorporeal membrane oxygenation.
Results
Patient Characteristics
A total of 1392 patients with COVID-19 were included in our study. The median age was 63 (IQR, 50–71) years, and 51% patients were men. On admission, 25% of patients had a fever and 41% were classed as severe. A total of 694 (50%) patients had comorbidities, including CKD (2%), chronic lung disease (6%), diabetes (17%), hypertension (36%), and tumor (5%). During hospitalization, 140 (10%) patients were admitted to the intensive care unit, 284 (20%) patients received mechanical ventilation, and 199 (14%) patients died. The median hospital stay was 22 (IQR, 14–31) days (Table 1).
Incidence and Characteristics of AKI
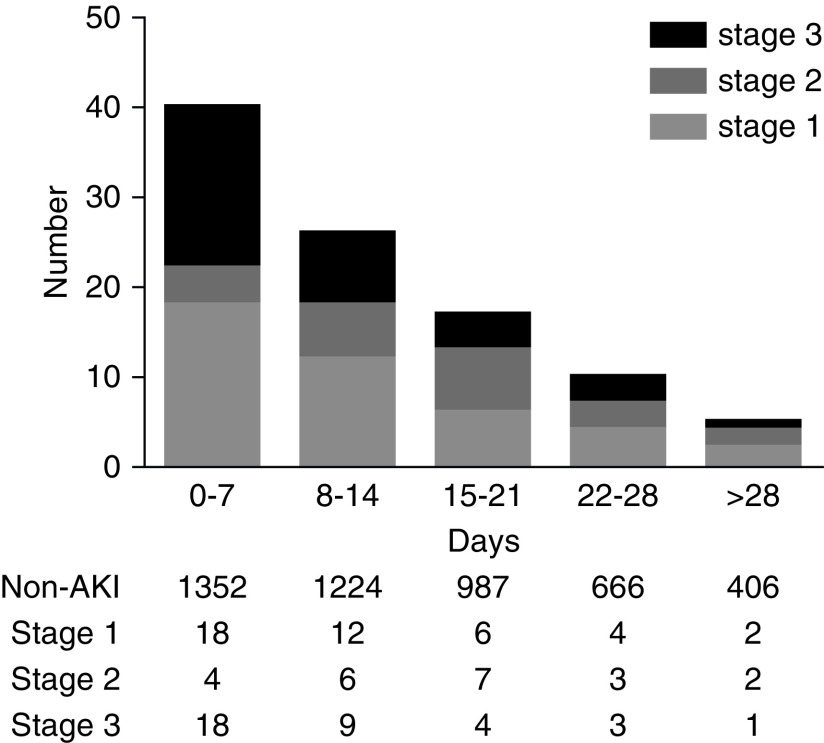
Of the 1392 patients with COVID-19 in our study, 99 (7%) developed AKI during hospitalization, including stage 1 in 42 (3%) patients, stage 2 in 22 (2%) patients, and stage 3 in 35 (3%) patients (Table 1). Of the 99 patients who developed AKI, 40% (40 of 99) developed AKI within 1 week of admission (Figure 2). The incidence of AKI in severe patients was 12% (69 of 570), which was higher than nonsevere patients (4%, 30 of 822). A total of 56% (61 of 109) patients who received invasive mechanical ventilation developed AKI. In addition, when baseline serum creatinine was defined as the serum creatinine on admission, the incidence of AKI was 6% (86 of 1392), and the peak stages of AKI were stage 1 in 39 (3%) patients, stage 2 in 15 (1%) patients, and stage 3 in 32 (2%) patients.
Figure 2.
Most AKI occured early in patients with coronavirus disease 19.
Patients with AKI tended to have more severe disease, had worse kidney function, and were more likely to have hematuria and proteinuria on admission than patients without AKI. In addition, lymphopenia and elevated D-dimer, high-sensitivity C-reactive protein, and lactose dehydrogenase levels were more common in patients with AKI (Table 1). Compared with those who developed AKI >1 week after admission, patients who developed AKI within 1 week tended to have more severe disease and had worse kidney function on admission (Supplemental Table 1).
Risk Factors for AKI
We assessed the potential risk factors of AKI development in patients with COVID-19. Unadjusted analysis showed that older age, male gender, severe disease, diabetes, higher serum creatinine, lymphopenia, and elevated D-dimer, high-sensitivity C-reactive protein, and lactose dehydrogenase levels were associated with AKI. Multivariable analysis showed that factors associated with a higher risk of AKI include severe disease (OR, 2.25; 95% confidence interval [CI], 1.37 to 3.67), higher baseline serum creatinine (OR, 2.19; 95% CI, 1.17 to 4.11), lymphopenia (OR, 1.99; 95% CI, 1.12 to 3.53), and elevated D-dimer level (OR, 2.68; 95% CI, 1.07 to 6.70) (Table 2).
Table 2.
Risk factors for AKI in patients with coronavirus disease 2019
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age, yra | 1.03 | 1.02 to 1.05 | 1.02 | 1.00 to 1.03 |
| Sex (male versus female) | 2.11 | 1.37 to 3.26 | 1.45 | 0.87 to 2.40 |
| Disease severity (severe versus general) | 3.64 | 2.33 to 5.66 | 2.25 | 1.37 to 3.67 |
| Diabetes (yes versus no) | 1.49 | 0.92 to 2.43 | ||
| Hypertension (yes versus no) | 1.23 | 0.81 to 1.87 | ||
| Baseline serum creatinine, mg/dla | 3.34 | 1.95 to 5.70 | 2.19 | 1.17 to 4.11 |
| Lymphopenia (yes versus no) | 3.92 | 2.33 to 6.61 | 1.99 | 1.12 to 3.53 |
| Anemia (yes versus no) | 1.21 | 0.81 to 1.83 | ||
| D-dimer, mg/L (>0.5 versus ≤0.5) | 6.63 | 2.87 to 15.27 | 2.68 | 1.07 to 6.70 |
| hs-CRP, mg/L (≥10 versus <10) | 5.72 | 2.48 to 13.19 | 2.24 | 0.89 to 5.62 |
| LDH, U/L (>245 versus ≤245) | 3.20 | 1.80 to 5.70 | 0.96 | 0.48 to 1.91 |
Baseline serum creatinine was defined as the lowest serum creatinine value within the first 7 days of hospitalization. Clinical characteristics and laboratory data were assessed on admission. Severe disease was defined as either (1) respiratory rate >30/min, (2) oxygen saturation ≤93%, or (3) partial pressure of oxygen/fraction of inspired oxygen ratio ≤300 mm Hg. Lymphopenia is defined as lymphocyte count <1.0×109/L. Anemia is defined as a hemoglobin level of <13 g/dl in men and <12 g/dl in women. OR, odds ratio; CI, confidence interval; hs-CRP, high-sensitivity C-reactive protein; LDH, lactose dehydrogenase.
Per one-unit increase.
We summarized the medications administered during hospitalization in Table 3. Antibiotics (75%), antivirals (89%), and glucocorticoids (47%) were the three most common medications among patients with COVID-19. The percentage of treatment with antibiotics, antidiabetics, diuretics, and glucocorticoids was higher in patients with AKI than those without AKI.
Table 3.
Medications used during hospitalization
| Medications | All Patients, N=1392 | Non-AKI, n=1293 | AKI Stage 1, n=42 | AKI Stage 2, n=22 | AKI Stage 3, n=35 |
|---|---|---|---|---|---|
| RAAS inhibitors, n (%) | 88 (6) | 83 (6) | 4 (10) | 1 (5) | 0 (0) |
| Antibiotics, n (%) | 1044 (75) | 949 (73) | 39 (93) | 22 (100) | 34 (97) |
| Antivirals, n (%) | 1232 (89) | 1157 (89) | 32 (76) | 18 (82) | 25 (71) |
| Antidiabetics, n (%) | 281 (20) | 252 (19) | 11 (26) | 6 (27) | 12 (34) |
| Diuretics, n (%) | 175 (13) | 109 (8) | 22 (52) | 19 (86) | 25 (71) |
| Glucocorticoids, n (%) | 657 (47) | 587 (45) | 29 (69) | 14 (64) | 27 (77) |
RAAS inhibitors, renin-angiotensin-aldosterone system inhibitors, including angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
In-Hospital Outcomes
The overall in-hospital mortality was 14% (199 of 1392) in our study. A stepwise increase in the stage of AKI conferred an incremental risk of in-hospital death (Figure 3). A total of 71 (72%) patients with AKI died in hospital, and the mortality in AKI stage 1, stage 2, and stage 3 was 62%, 77%, and 80%, respectively (Table 1). A total of 21 (21%) patients died within 1 day after AKI occurred. By contrast, the in-hospital mortality of patients who developed AKI stage 1 and did not receive invasive mechanical ventilation or admittance to an intensive care unit was only 23% (three of 13). In addition, compared with those who developed AKI >1 week after admission, patients who developed AKI within 1 week had higher in-hospital mortality (83% versus 64%) (Supplemental Table 1).
Figure 3.
AKI is associated with higher in-hospital mortality in patients with coronavirus disease 19.
Compared with patients without AKI, patients with AKI were more likely to be admitted to an intensive care unit (75% versus 5%), receive vasopressors (76% versus 9%), and receive extracorporeal membrane oxygenation during hospitalization (6% versus 0.4%). Of the 99 patients with AKI, 15 (15%) received KRT during hospitalization. Of the 28 patients who survived to discharge, 19 (68%) patients recovered from AKI.
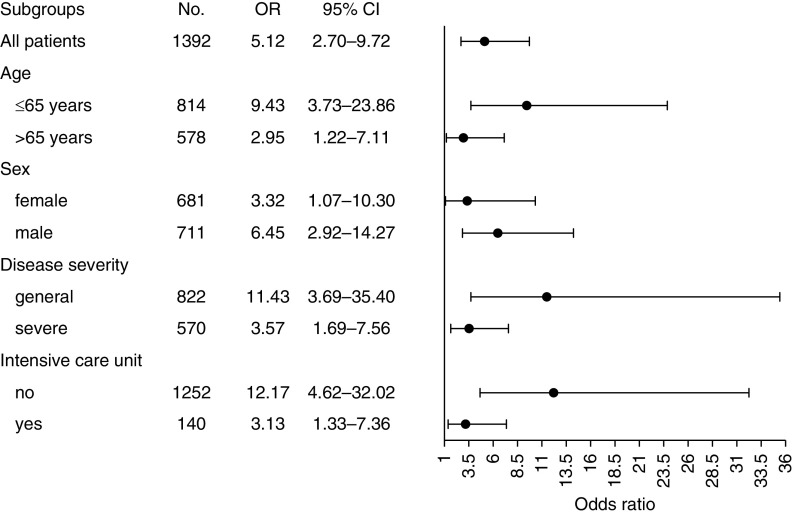
Multivariable logistic regression showed that AKI was associated with high risk of in-hospital death (OR, 5.12; 95% CI, 2.70 to 9.72) after adjustment for age, sex, disease severity, lymphopenia, D-dimer level, and intensive care unit admission. When different specific subgroups of patients were considered, including stratifying by age, sex, disease severity, and admission to intensive care unit, AKI was still a risk factor for in-hospital mortality (Figure 4).
Figure 4.
Association of AKI with in-hospital death in patients with coronavirus disease 2019, by participant characteristics. Odds ratios (ORs) and 95% confidence intervals (CIs) were obtained in multivariable logistic regression models after adjustment for age, sex, disease severity, intensive care unit admission, lymphopenia, and D-dimer level. In subgroup analysis, the grouping variables were not included in the model.
We expanded the AKI criteria, which were defined as a relative slow increasing of, or a small increase in, serum creatinine. When the AKI criteria were expanded to 50% serum creatinine increase within 14 days or the entire hospital stay, AKI was still a risk factor for in-hospital death. Also, the association of AKI and in-hospital death remained significant when the AKI criteria were expanded to 40% or 30% serum creatinine increase within 7 days; however, the association was lost when the AKI criteria were expanded to 20% serum creatinine increase within 7 days (Table 4).
Table 4.
Incidence of AKI defined by different criteria and its association of in-hospital death in patients with coronavirus disease 2019
| Criteria | Incidence, n (%) | OR (95% CI) |
|---|---|---|
| KDIGO criteria | 99 (7) | 5.12 (2.70 to 9.72) |
| Criteria 1: increase in Scr by 50% within 14 d | 101 (7) | 4.89 (2.60 to 9.21) |
| Criteria 2: increase in Scr by 50% within hospital stay | 110 (8) | 5.39 (2.92 to 9.94) |
| Criteria 3: increase in Scr by 40% within 7 d | 140 (10) | 3.92 (2.27 to 6.76) |
| Criteria 4: increase in Scr by 30% within 7 d | 187 (13) | 2.33 (1.41 to 3.85) |
| Criteria 5: increase in Scr by 20% within 7 d | 286 (21) | 1.48 (0.94 to 2.32) |
KDIGO criteria are an increase in Scr by 0.3 mg/dl within 48 hours or a 50% increase in SCr from the baseline within 7 days; in addition to an increase in Scr by 0.3 mg/dl within 48 hours, the expanded AKI criteria were defined as a relative slow increasing of, or a small increase in, serum creatinine. ORs were obtained in multivariable logistic regression models after adjustment for age, sex, disease severity, intensive care unit admission, lymphocyte count, and D-dimer level. OR, odds ratio; CI, confidence interval; KIDGO, Kidney Disease: Improving Global Outcomes; Scr, serum creatinine.
Discussion
In this large, retrospective cohort study conducted in a tertiary teaching hospital in Wuhan, China, we found that the incidence of AKI was 7% in patients with COVID-19. High baseline serum creatinine, elevated D-dimer level, severe disease, and lymphopenia were associated with a high risk for AKI during hospitalization. Strikingly, 72% of patients with COVID-19 with AKI died during hospitalization, and AKI was an independent risk factor of in-hospital mortality.
The detection rate of AKI varied in studies of patients with COVID-19 with different levels of disease severity. The 7% in our study is in line with the 3%–7% found in a previous observational study with a small sample size and a higher proportion of severe patients (10–12); much higher than the 0.5% found in a study of 1099 patients from 30 provinces, with relatively lower proportion of severely sick patients (3); and lower than the 29% found in a study of 52 critically ill adult patients admitted to an intensive care unit in Wuhan, China (22). In our cohort, the large number of >1000 patients in a tertiary hospital for the treatment of patients with severe COVID-19 may represent a more generalizable AKI rate of the hospitalized population, with an average follow-up period of 22 days. However, Hirsch et al. conducted a large, observational study in New York (23) and found that the incidence of AKI was up to 37%, which was much higher than that reported in our study. There are several possible reasons for this. First, the patients in the study by Hirsch et al. showed higher admission serum creatinine (1.0 versus 0.8 mg/dl), indicating that our cohort may have had less underlying CKD or AKI on admission. Second, higher rates of diabetes and hypertension were reported in their study, which may contribute to the higher incidence of AKI. Third, Hirsch et al. defined baseline serum creatinine as the median serum creatinine value from the entirety of the hospitalization period, which may improve AKI detection rate.
In our study, about half of the patients with AKI developed it in the early period (within the first 7 days) during hospitalization, which was also observed in patients with influenza A virus subtype H1N1 in previous studies (24,25). AKI in the early period may be associated with SARS-CoV-2 infection, and AKI in the latter stage of hospitalization may be because of the aggravation of respiratory failure, and, consequently, sepsis, cytokine storm, shock, and acidosis. Of note, we found that AKI developed in the early period tended to be more severe and have high in-hospital mortality. Thus, to improve the diagnostic time of AKI and prevent poor outcome in patients with COVID-19, frequent monitoring of serum creatinine should be encouraged, especially in the early period of hospitalization.
We found that the disease severity and lymphopenia were risk factors for AKI development. Likewise, it is reported that the incidence of AKI was significantly higher in patients with severe COVID-19, as well as patients admitted to the intensive care unit (3,12). A possible explanation is that COVID-19 can be complicated by acute respiratory disease syndrome and septic shock in severe cases, and subsequent hypotension and vasoconstriction plausibly contributed to the development of acute tubular necrosis, as evidenced by histologic findings from kidney tissues of patients with COVID-19 (7). We also found that high baseline serum creatinine was associated with high risk of AKI during hospitalization. Previous studies demonstrated that elevated serum creatinine was common in patients with COVID-19 (8). High serum creatinine on admission may indicate the initial stages of kidney damage and easier progression to AKI. Early intervention in patients with elevated serum creatinine on admission, which may appear before clinical manifestations of kidney failure, is likely to result in better outcomes than treating only established AKI.
It is noteworthy that patients with COVID-19 with AKI demonstrated an extremely poor prognosis. The in-hospital death rate of 72% in our study was comparable with those in patients with severe acute respiratory syndrome in 2003 (26) and influenza A virus subtype H1N1 in 2009 (25), and even higher than those who developed acute respiratory distress syndrome requiring mechanical ventilation in patients without COVID-19 (27), indicating that multiple organ failure was a significant risk factor for patients with COVID-19, especially in this cohort with an average age of 63 years. Indeed, older age and higher Sequential Organ Failure Assessment score were associated with increasing odds of in-hospital death in a small study of 191 patients with COVID-19 (19). However, the in-hospital death of 5449 (35%) patients with COVID-19 in New York in the study by Hirsch et al. (23) was much lower than our study. It is noted that all of the patients in our study were discharged or died, and the ascertainment of in-hospital death was more complete than earlier reports from Wuhan and the Hirsch et al. study. In our study, the likelihood of receiving mechanical ventilation was much higher in patients with AKI. Previous studies showed that AKI worsens lung injury via several mechanisms including impaired fluid excretion, direct capillary endothelial injury, and exacerbating inflammatory response (28), which may greatly increase mortality. We also found that about three quarters of the patients with AKI received vasopressors during hospitalization, indicating a high incidence of septic shock. Cytokine storm and acute cardiac injury could cause hypotension and diminished perfusion of the kidneys, leading to AKI and high mortality in patients with COVID-19. Consistently, histopathological analysis of kidneys in patients with COVID-19 showed that less red blood cell aggregation was present in the peritubular capillaries of patients with predominant glomerular loop occlusion, which is often associated with a relatively long duration of hypotension (7). In addition, some patients were unable to be hospitalized at the time because of limited beds in the early stage of the outbreak, which may also contribute to the high proportion of severe disease and the high mortality in our study.
In light of the high mortality in patients with AKI and the importance of early detection of AKI, we expanded the criteria by extending the time limit or reducing degree of increase in serum creatinine change, and assessed their association with in-hospital mortality. We found that 50% increase in serum creatinine within 14 days or during the entire hospitalization period remained a risk factor of in-hospital death in COVID-19. Moreover, in keeping with previous studies in critically ill patients (29,30), our results showed that even small rises in serum creatinine within 7 days are associated with mortality. However, because of the rapidly increasing number of COVID-19 cases, two serum creatinine tests within 7 days cannot be guaranteed in clinical practice and, therefore, AKI would likely be missed. Further, small increments in creatinine are often attributed to laboratory variations and do not receive much consideration. Therefore, it is essential to pay more attention to patients with mild or slow increased serum creatinine in the treatment of COVID-19.
Our study has several limitations. First, this was an observational study in adult hospitalized patients. Therefore, the results may not be fully suitable to all patients with COVID-19, especially for those with mild symptoms who quarantine themselves at home. Second, an accurate baseline serum creatinine and urine output was not available, which may have led to an underestimation of AKI or erroneous associations. Third, the frequency of serum creatinine tests may also affect the AKI detection rate.
In conclusion, we observed a 7% incidence of AKI in adult inpatients with COVID-19. A significant number of patients developed AKI within 7 days after admission. The in-hospital mortality of patients with COVID-19 with AKI was extremely high, and AKI was strong associated with mortality in the entire cohort. Therefore, careful monitoring of AKI is necessary from the early period. We recommend that physicians carefully monitor kidney function and administer effective multimodality therapy when treating patients with COVID-19.
Disclosures
All authors have nothing to disclose.
Funding
This work was financially supported by National Natural Science Foundation of China and the Deutsche Forschungsgemeinschaft international (regional) cooperation and exchange projects (grant 81761138041); National Natural Science Foundation of China grants 81570667, 81470948, and 81670633; Major Research Plan of the National Natural Science Foundation of China grant 91742204; National Key Research and Development Program of China grants 2018YFC1314003-1 and 2015BAI12B07; and National Key Research and Development Program grant 2016YFC0906103.
Supplementary Material
Acknowledgments
The authors greatly appreciate all the hospital staff for their efforts in recruiting and treating patients, and thank all patients involved in this study.
Prof. Gang Xu and Prof. Shuwang Ge designed the study. Dr. Yichun Cheng, Dr. Ran Luo, Dr. Xu Wang, Dr. Kun Wang, Dr. Nanhui Zhang, Dr. Meng Zhang, Dr. Zhixiang Wang, Dr. Lei Dong, Prof. Junhua Li, Prof. Rui Zeng, and Prof. Ying Yao collected the data and prepared the figures and tables. Dr. Yichun Cheng and Prof. Shuwang Ge contributed analytical tools. Dr. Yichun Cheng and Prof. Shuwang Ge wrote the paper. Prof. Shuwang Ge and Prof. Gang Xu conceived the project and supervised and coordinated all the work.
Data Sharing Statement
The data used and analyzed during this study are available from the corresponding author upon reasonable request.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “COVID-19–Associated Acute Kidney Injury: An Evolving Picture,” on pages 1383–1385.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04650420/-/DCSupplemental.
Supplemental Table 1. Characteristics of patients with AKI, according to the time of AKI occurrence.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao FG, Tan W; China Novel Coronavirus Investigating and Research Team : A novel coronavirus from patients with pneumonia in China 2019. N Engl J Med 382: 727–733, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W: Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 395: 565–574, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19 : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS: Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420–422, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou X, Chen K, Zou J, Han P, Hao J, Han Z: Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14: 185–192, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronco C, Bellomo R, Kellum JA: Acute kidney injury. Lancet 394: 1949–1964, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L: Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395: 507–513, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B: Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z: Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y: Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 8: 475–481, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y, Zeng R, Xu G: Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2009]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Commission of the People’s Republic of China: Chinese Management Guideline for COVID-19 (Version 7.0), 2020. Available at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf. Accessed March 27, 2020
- 17.Kidney Disease Improving Global Outcomes; Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012. Available at: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf [Google Scholar]
- 18.Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettilä V, Prowle JR, Schetz M, Joannidis M: Renal recovery after acute kidney injury. Intensive Care Med 43: 855–866, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: 1054–1062, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y: Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180: 1–11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q: Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study [published correction appears in BMJ 368: m1295, 2020]. BMJ 368: m1091, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, He Q, Chen J, Chen M, Liu X, Zhu Z, Yang L, Lian X, Ding F, Li Y, Wang H, Wang J, Wang R, Mei C, Xu J, Li R, Cao J, Zhang L, Wang Y, Xu J, Bao B, Liu B, Chen H, Li S, Zha Y, Luo Q, Chen D, Shen Y, Liao Y, Zhang Z, Wang X, Zhang K, Liu L, Mao P, Guo C, Li J, Wang Z, Bai S, Shi S, Wang Y, Wang J, Liu Z, Wang F, Huang D, Wang S, Ge S, Shen Q, Zhang P, Wu L, Pan M, Zou X, Zhu P, Zhao J, Zhou M, Yang L, Hu W, Wang J, Liu B, Zhang T, Han J, Wen T, Zhao M, Wang H; ISN AKF 0by25 China Consortiums : Acute kidney injury in China: A cross-sectional survey. Lancet 386: 1465–1471, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell Nephrology COVID-19 Research Consortium : Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joannidis M, Forni LG: Severe viral infection and the kidney: Lessons learned from the H1N1 pandemic. Intensive Care Med 37: 729–731, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung JY, Park BH, Hong SB, Koh Y, Suh GY, Jeon K, Koh SO, Kim JY, Cho JH, Choi HS, Park YB, Kim HC, Kim YS, Lim CY, Park MS: Acute kidney injury in critically ill patients with pandemic influenza A pneumonia 2009 in Korea: A multicenter study. J Crit Care 26: 577–585, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, Fung KS, Tang HL, Yan WW, Chan HW, Lai TS, Tong KL, Lai KN: Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 67: 698–705, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darmon M, Clec’h C, Adrie C, Argaud L, Allaouchiche B, Azoulay E, Bouadma L, Garrouste-Orgeas M, Haouache H, Schwebel C, Goldgran-Toledano D, Khallel H, Dumenil AS, Jamali S, Souweine B, Zeni F, Cohen Y, Timsit JF: Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol 9: 1347–1353, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faubel S, Edelstein CL: Mechanisms and mediators of lung injury after acute kidney injury. Nat Rev Nephrol 12: 48–60, 2016. [DOI] [PubMed] [Google Scholar]
- 29.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Samuels J, Ng CS, Nates J, Price K, Finkel K, Salahudeen A, Shaw A: Small increases in serum creatinine are associated with prolonged ICU stay and increased hospital mortality in critically ill patients with cancer. Support Care Cancer 19: 1527–1532, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.