Abstract
Background
Sleep disorders have emerged as potential cancer risk factors.
Objective
This review discusses the relationships between sleep, obesity, and breathing disorders with concomitant risks of developing cancer.
Results
Sleep disorders result in abnormal expression of clock genes, decreased immunity, and melatonin release disruption. Therefore, these disorders may contribute to cancer development. Moreover, in sleep breathing disorder, which is frequently experienced by obese persons, the sufferer experiences intermittent hypoxia that may stimulate cancer cell proliferation.
Discussion
During short- or long- duration sleep, sleep-wake rhythm disruption may occur. Insomnia and obstructive sleep apnea increase cancer risks. In short sleepers, an increased risk of stomach cancer, esophageal squamous cell cancer, and breast cancer was observed. Among long sleepers (>9 hours), the risk of some hematologic malignancies is elevated.
Conclusion
Several factors including insomnia, circadian disruption, obesity, and intermittent hypoxia in obstructive sleep apnea are contributing risk factors for increased risk of several types of cancers. However, further studies are needed to determine the more significant of these risk factors and their interactions.
Keywords: Cancer, risk factors, obesity, sleep, sleep apnea, intermittent hypoxia
1. INTRODUCTION
1.1. Obesity as a Cancer Risk Factor
Among the various exogenous and endogenous risk factors for carcinogenesis, obesity plays a major contributing role [1-3]. Meta-analyses reveal that adiposity is correlated with various cancers including colorectal, breast, ovary, endometrium, kidney, gastric cardia, pancreas, biliary tract, esophageal adenocarcinoma, and multiple myeloma [4].Moreover, the International Agency for Research on Cancer listed thyroid gland cancer, liver cancer, and meningioma, among pathologies associated with excess body fat [5]. Additionally, it has been noted that breast cancer along with colon cancer and endometrium cancer in women of postmenopausal age is associated with high body mass index (BMI) [6]. Therefore, various possible mechanisms have been proposed to correlate obesity and cancer.
Previous studies indicate that obesity causes oxidative stress, lowering antioxidant defense [7, 8]. Furthermore, obesity-related oxidative stress and obesity-related chronic low-grade inflammation may induce DNA damage and inhibit DNA reparative processes that may contribute to cancer cell growth [8]. There is evidence linking cancer development with the inappropriate secretion of adipokines, i.e. hormone-like substances produced by adipocytes, in obese individuals [9]. For example, adiponectin secretion is reduced in obesity; and low levels of this molecule have been associated with cancerogenesis [10, 11]. A recent meta-analysis revealed that the concentration of adiponectin in the serum was associated with diminished cancer risk for breast, colorectal, and endometrial cancers (odds ratio (OR) 0.7; 95% confidence interval (CI), 0.6-0.80) [12, 13]. On the other hand, the increased concentration of leptin has been shown to promote the proliferation of cancer cells [14-16]. In this regard, the same meta-analysis confirmed a positive association between leptin serum concentration and cancer risk (OR 1.26; 95% CI 1.05-1.51) of endometrium and kidney cancers [12]. Finally, the term “adiponcosis” was introduced to indicate the potential pathogenic association between obesity and cancerogenesis [17].
Obesity is correlated with sleep problems; however, there appears to be a bidirectional influence: obesity may lead to poor sleep and vice versa [18-21]. As but one example, obstructive sleep apnea (OSA) is the most common breathing disorder during sleep, and in the vast majority of cases, it is correlated with obesity [22]. OSA causes numerous pathophysiological consequences, including sleep fragmentation, sleep deprivation, disturbances of sleep architecture, intermittent hypoxia, autonomic system dysfunction, chronic inflammation, oxidative stress, and cardiovascular disturbances [23-26]. Combined results suggest that there is a relation between obesity, sleep problems, and cancer. These 3 conditions have become global epidemics [27-32]. In the present review, we analyze some recent studies focusing on the associations between sleep disorders, obesity, and cancer risk.
2. Sleep Disorders in Cancer Patients
Sleep disturbances are among the common symptoms experienced by patients with different kinds of cancer [33]. Sleep quality is poor in cancer patients according to the Pittsburgh Sleep Quality Index [34], Questionnaire Core-30 developed by the European Organization for Research and Treatment of Cancer [35], or by polysomnography [36]. The study performed with the use of actimeters and sleep diaries in women with advanced breast cancer obtained a negative correlation with sleep quality [37]. However, the association of cancer and the quality of sleep needs further study [38].
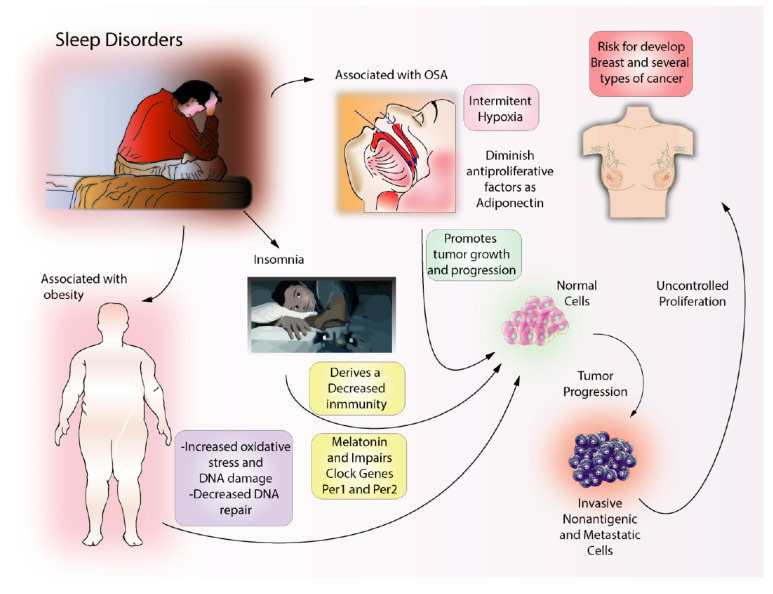
It has been hypothesized that cancer disrupts homeostatic mechanisms in the brain leading to associated sleep problems. The molecules that are associated with cancer may influence sleep through somnogenic or anti-somnogenic actions. These molecules are interleukin-ß, interleukin-6, interleukin-4, interleukin-10, tumor necrosis factor (TNF), transforming growth factor ß, ghrelin and leptin [39]. Importantly, sleep problems in cancer patients may be regarded not only as a consequence of cancer or its treatment but also as one of the main risk factors for its development (Fig. 1).
Fig. (1).
Sleep disorders and associated diseases linked to cancer risk. Obesity is a well-known condition associated with cancer. Obesity increases oxidative stress in the body and induces DNA damage. In turn, DNA damage may promote oncogenic processes. Insomnia is a complex condition that may result in depression of the immune system and is associated with impaired melatonin secretion and regulation of clock genes. Obstructive Sleep Apnea may cause intermittent hypoxia with the resulting alteration of adiponectin, a key antiproliferative factor. It is possible obesity, insomnia, and OSA increase the risk for the development of several types of cancer including, breast, prostate, thyroid, gastric, and lung cancer. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. Sleep Duration and Cancer Risk
Previous studies revealed a correlation between short sleep duration and decreased immunity [40]. Insufficient sleep correlates with increased levels of circulating TNF-α [41]. The up-regulation of TNF-α mRNA [42], as well as the activation of the transcription factors NF-κB in peripheral blood mononuclear cells, indicate a genomic response to inadequate sleep [43]. In humans, sleep deprivation leads to a decrease in the number of natural killers cells (NK) that play an important role in controlling cancer development [44]. It has been reported that sleep-deprived mice develop pulmonary metastasis earlier than normal mice with the same disease [45]. In addition, there is a decrease in NK cells, CD8+T cells, and cytotoxicity during sleep deprivation in the cancer microenvironment [45]. However, either abnormally short or long sleep durations were found to be associated with increased cancer risk.
In short sleepers, i.e. in patients sleeping 5-6 hours as compared with sleeping 7-8 hours, increased risk of stomach cancer was detected in the study encompassing 173,327 men (HR 1.29; 95% CI 1.05-1.59) [46]. The study involving 527 patients revealed significantly increased risk for esophageal squamous cell cancer associated with short sleep duration, i.e. <7 hours (OR 3.18; 95% CI 1.55-6.53) [47]. The risk of breast cancer was more than doubled (HR 2.1; 95% CI 1.12-3.59) in women younger than 60 years and reporting short sleep duration [48]. In a prospective study, the risk of estrogen-negative/progesterone-negative breast cancer was increased in black women sleeping <8 hours and more than doubled in those sleeping 6 hours (OR 2.22; 95% CI 1.19-4.12).
However, in assessing the entire population, the association between sleep duration and breast cancer was not confirmed [49]. Short sleep duration (<6 hours) associated with increased risk of breast cancer was confirmed (OR 1.53; 95% CI 1.10-2.12) [50].
But long sleep duration (>9 hours) was also found to be significantly associated with an increased risk of breast cancer (OR 1.59; 95% CI 1.17-2.17) [50]. Lower risk of hormone-related cancers, including breast cancer (HR 0.79; 95% CI 0.65, 0.97) was previously reported among long sleepers [51]. However, a recent large population-based study involving 5790 breast cancer patients did not provide evidence of a relation between sleep duration and breast cancer. This same study found a clear association with obesity [52]. Another study encompassing 4869 patients with colorectal cancer showed increased cancer mortality associated with short sleep duration (<5 hours) in the pre-diagnosis period [53]. However, a meta-analysis of different studies encompassing 723337 persons revealed that long sleepers were at greater risk of colorectal cancer (HR 1.29; 95% CI 1.09-1.52) [51]. Furthermore, a study encompassing 123858 women in the United States showed an increased risk of non-Hodgkin lymphoma in long sleepers (>9 hours) compared to those sleeping 7-8 hours (HR 1.45; 95% CI 1.00-2.11) [46]. Long sleep duration (>9h) was confirmed to be associated with non-Hodgkin lymphoma (hazard ratio, HR, 2.14; 95% CI 1.14-4.01) and other hematologic neoplasms (HR 1.70; 95% CI 1.03-2.82), in the study of 45984 adult Canadians [54].
In the general population, all-cancer mortality is weakly but significantly associated with abnormal sleep duration, both short sleep (five hours or less) and long sleep (>8 hours). This was reported in a meta-analysis of 14 cohort studies [55]. However, after taking into account 24-hour sleep-wake pattern, the prevalence of cancer in elderly persons (on average 74 years old) in Chinese general population appeared to be lower (OR 0.48; 95% CI 0.26-0.85) in those sleeping long at night with additional daytime naps as compared with persons sleeping shortly at night with additional daytime naps [56].
4. Sleep-Wake Rhythm Abnormalities and Cancer Risk
There is evidence of an association between circadian rhythm abnormalities and cancer development. Clock genes control the process of daily physiological changes associated with circadian rhythm. Abnormal expression of clock genes like per 1, per 2, and different cyclins that regulate cell proliferation as well as cell apoptosis may contribute to the development of cancer [57, 58]. Disturbances in the expression of clock genes are linked to cancer cells’ uncontrolled proliferation [57]. Both over- and under-expression of different clock genes were found in prostate cancer [59], non-small-cell lung cancer, kidney, liver, colon, and pancreatic cancers [60, 61].
The association between sleep-wake disruption and cancer development may be a result of melatonin release disruption. Melatonin, a hormone of the pineal gland, is produced in a cyclic rhythm depending on exposure to darkness and light and in normal conditions achieves its highest levels during nocturnal hours [62]. Melatonin has recently emerged as a strong suppressor in cancer development [63]. Excess light at night may affect the normal physiological nocturnal increase in melatonin concentrations, causing circadian disruption that may be one of the factors associated with carcinogenesis [64].
Melatonin acts through numerous pathways including actions against tumor cells proliferation and metastatic formation as well as against concomitant inflammation [65]. The anti-tumorigenic action of melatonin is related to its antioxidant properties [66]. In this aspect, the antioxidant activity of melatonin leads to destabilization of HIF-1α protein and to angiogenesis inhibition induced by hypoxia, as shown by in vitro studies of colon cancer cell line [67]. Adequate levels of melatonin may have protective effects against the development of cancer through its action as an inducer of apoptosis in cancer cells [68].
Another meta-analysis revealed low concentrations of melatonin in breast cancer patients (n=963) as compared with healthy controls. This finding suggests decreased levels of melatonin may be a risk factor for breast cancer [69]. Additionally, possible synergistic effects of melatonin combined with chemotherapy have been postulated in non-small-cell-lung cancer [65], pancreatic cancer [68], skin cancer [70], ovarian cancer [71], colorectal cancer [72], and other cancers, including breast, hepatic, and prostate cancer [73].
The circadian disruption caused by shift work or transoceanic flights has been classified by the International Agency for Research on Cancer as a probable carcinogenic factor [74]. However, a direct relationship between shift work, chronodisruption, and carcinogenesis in humans remains to be fully elucidated [75, 76]. A meta-analysis of 24 articles revealed that nurses’ night shift work constituted a risk factor for various metabolic and cardiovascular disorders, as well as risk of breast cancer [77]. However, a study performed in the United Kingdom involving 2059 women with breast cancer revealed no risk of this neoplasm associated with night shift work, except a slight, albeit significant (p<0.05) trend associated with the average duration of night work per week [78].
Similarly, the results of a study conducted in Canada encompassing 406 patients with epithelial ovarian cancer did not confirm an increased risk of neoplastic disease associated with night shift work. However, an increased risk of epithelial ovarian cancer was found in the patients with the longest period during which night shift work was performed and women who self-identified themselves as “morning” chronotype (OR 1.64; 95% CI 1.01-2.65) [79]. These studies suggest additional research is needed to better understand the relationship between sleep disorders and cancer incidence.
5. Insomnia and Cancer Risk
The risk of breast cancer was found to be increased in patients with insomnia (HR 1.73; 95% CI 1.57-1.90) [80]. Patients with breast cancer suffered from sleep disturbances including 8 percent insomnia syndrome prior to diagnosis [81]. Another study with 33332 women, who were followed for a mean 14.7 years, indicated that insomnia was a risk factor of breast cancer in patients suffering from all three symptoms of insomnia: difficulty initiating sleep, difficulty maintaining sleep, and having non-restorative sleep (HR 2.38; 95% CI 1.11-5.09) [82]. A retrospective study encompassing 11021 patients with insomnia detected an increased risk of breast cancer as compared with the population of age-matched women who did not complain of insomnia, adjusted HR 1.43; 95% CI 1.10-1.84. This study also found no relationship to hypnotic use [83].
On the contrary, the association of insomnia and increased risk of cancer was found in patients using sleeping pills in Korea. A study showed significantly increased risk for thyroid cancer, breast cancer, ovarian cancer, and lung cancer in women using any sedative-hypnotic drug as compared to non-users. There also was a significantly increased risk for prostate cancer, brain cancer, and lung cancer in men using any sedative-hypnotic drug as compared with non-users [84].
Finally, in a recent retrospective case-control study encompassing 7355 patients, the authors revealed that insomnia diagnosed prior to the diagnosis of oncologic disease was associated significantly with colorectal cancer (adjusted OR 1.54; 95% CI 1.35-1.75) [85].
6. Sleep Breathing Disorders, Nocturnal Hypoxia, and Cancer Risk
Typical features of OSA include repetitive episodes of complete (apnea) or partial (hypopnea) collapse of the upper airway. The apnea-hypopnea index (AHI) is used to gauge severity based on the mean number of events per hour of sleep: AHI >30/h indicates severe, >15/h indicates moderate, and AHI>5/h indicates mild form of OSA [86]. Although the main symptom of OSA is excessive daytime somnolence, about one-third of patients complain of disturbed sleep and about one-fourth of patients neglect any important symptoms despite the presence of OSA. Most sufferers of OSA remain undiagnosed and untreated [87].
Increasing attention has been paid to the possible link between OSA and cancer risk [88-92], although the first information linking intermittent hypoxia with cancer onset was reported earlier [93].
Experimental studies with mice showed that intermittent hypoxia (mimicking OSA) led to increased tumor growth compared with mice breathing room air. This effect was mediated by vascular endothelial growth factor [94, 95]. The study revealed that OSA with concomitant intermittent hypoxia and sleep fragmentation promoted tumor growth [96]. In mice bearing human subcutaneous melanoma xenografts, intermittent hypoxia exposure accelerated tumor progression and was associated positively with both metastases formation and resistance to treatment [97]. This effect is considered to be through the activation of the hypoxia-inducible factor (HIF) 1-alpha pathway [98]. In a group of 443 patients with melanoma who subsequently underwent polysomnography, the more invasive form of melanoma was associated with higher AHI and oxygen desaturation index, especially in patients younger than 56 years [99].
Diminished immune function and increased sympathetic tone may be responsible for increased oncogenesis in subjects submitted to intermittent hypoxia [89]. The injection of lung cancer cells in mice exposed to intermittent hypoxia led to the increased expression of programmed death-ligand 1 (PD-L1) as compared with the lung cancer cells injected in mice living in normal conditions [100]. Intermittent hypoxia mimicking OSA resulted in increased PD-L1 expression on splenocytes in young mice, but not in older mice [101]. In OSA patients, programmed cell death-1 (PD-1) receptor and its ligand (PD-L1) are overexpressed on the cells taking part in immune reactions, leading to a reduction in CD8+ T cells and thereby facilitating cancer growth [102]. Intermittent hypoxia in patients with OSA is shown by an increase in PD-L1 expression on monocytes and an increase in PD-L1 concentration in plasma. Again an age-related factor was revealed only in relatively young subjects (i.e. <55 years of age) [101].
Intermittent hypoxia also reduces serum concentrations of adiponectin as shown in experimental animal studies [103, 104]. Adiponectin is considered an anti-proliferative adipokine, preventing cancer growth [105, 106]. Thus, lowered concentrations of adiponectin in the serum of OSA patients partially may explain the increased susceptibility to cancer development. Concentrations of adiponectin are lower in OSA patients than in the controls [107]. These are associated with the severity of OSA [108] and independent from obesity presence [109].
The proteome of circulating extracellular vesicles (EV) was detected on sleep apnea models [110, 111] and in patients with sleep-disordered breathing [112, 113]. The biological function of EV may be associated with altered immune response in the course of cancer development and progression [114]. Heterogeneous EV composition encompasses proteins, RNA and DNA material that can be transported to remote body cells [115]. EV may be submitted to the changes in their proteome compositions in response to multiple stimuli, including oxidative stress and hypoxic stress, as well as heat shock and nutrient stress [116]. Chronic intermittent hypoxia, as seen in OSA patients, mainly contributes to the increase of inflammatory proteins in circulating microvesicles [117]. Experimental data from studies using human adenocarcinoma cells as well as exosomes from mice submitted to intermittent hypoxia and from OSA patients revealed that intermittent hypoxia led to the release of exosomes into the bloodstream thus enhancing carcinogenic potential through cell proliferation and migration. These results strongly suggest that circulating exosomes in OSA patients may influence the progression of cancer [110].
The association of OSA and cancer has been explored in epidemiological and clinical studies over the past few years. The results are equivocal. In a pioneering study, the observation of 1522 patients from the Wisconsin Sleep Cohort observed for 22 years revealed a significantly increased risk of cancer mortality associated with OSA, especially in patients with severe OSA and high ODI (HR 4.8; 95% CI 1.7-13.2) [118]. In a 20-years retrospective study of 386 persons without a history of cancer or stroke, cancer mortality was significantly increased in moderate to severe OSA (HR 3.4; 95% CI 1.1, 10.2) [119]. Another study encompassing 5427 patients diagnosed with suspected OSA at the time of observation (median of 4.5 years), showed that cancer mortality was associated with OSA severity (both with AHI and hypoxemia), especially in patients younger than 65 years of age. For the logarithmically transformed percent of the time during sleep spent with SaO2 below 90% (TSat90) HR was 1.73 (95% CI 1.23-2.4) and for upper versus lower TSat90 tertile HR was as high as 14.4 (95% CI 1.85-116.6) [120]. Some studies, however, did not show increased mortality associated with the occurrence of OSA in cancer patients [121]. Most of the studies focused on an increased incidence of cancer in OSA patients than controls [122-124]. The main symptom of OSA (i.e. daytime sleepiness) was associated with an increased risk of cancer study in the persons <50 years of age (HR 4.09; 95% CI 1.58-10.55) [125]. Moreover, there is a high incidence of OSA among newly diagnosed lung cancer patients [126] and positive results of lung cancer screening are significantly associated with nocturnal hypoxemia resulting from OSA [127].
Recently, a group of 66 patients with lung cancer with sleep apnea revealed moderate-to-severe OSA (AHI>15/h) in 50 percent of patients [128]. In a case-control study based on the results of colonoscopy, the risk of colorectal cancer was significantly increased in OSA patients (OR 3.03; 95% CI 1.44-6.340 [129]. Furthermore, a study encompassing 7355 patients revealed that sleep apnea present before the diagnosis of oncologic disease was significantly associated with colorectal cancer (adjusted OR 1.76; 95% CI 1.54-2.00). In addition, any sleep disorder with concomitant depression was the strongest factor associated with colorectal cancer (adjusted OR 5.69; 95% CI 4.01-6.98) [85].
Intermittent hypoxia in OSA may increase cancer risk, as shown by meta-analysis [130]. A study of around 5.6 million persons revealed an increased risk of selected neoplasms (e.g., pancreatic, kidney, and melanoma), but a decreased risk of other cancers (e.g., colorectal, breast, and prostate cancers) in OSA patients [121]. A Canadian study with 9629 patients, followed-up for a median of 7.8 years, showed that there was no increase in the risk of cancer in association with OSA; neither with AHI or with desaturation [131]. Finally, a recent case-cohort study in a group of 1162 patients did not find an increased risk of cancer in moderate to severe OSA patients [132]. These combined results strongly suggest a relationship between severe OSA and sleep apnea with the incidence of several types of cancer.
Hypotheses, Future Directions, and Conclusion
Research studies and meta-analyses reveal a possible link between obesity, sleep disorders, and increased risk of cancer. These factors, along with other pathologies, warrant additional studies, especially in high-risk groups. The aim is to formulate diagnostic and therapeutic procedures that are informed by these correlated factors.
In addition, some recent studies reported an inverse association between neuro-degenerative disorders like Alzheimer’s disease with cancer incidence or mortality [133, 134]. Thus, additional studies regarding possible markers of early stages of neurodegenerative or proliferative disorders in persons with chronic sleep disturbances or obesity are needed in order to establish a clear correlation between neuro-degeneration and cancer development.
Another question is related to the possible accumulation of co-occurrence risk factors of cancer with other diseases. For example, patients with chronic obstructive pulmonary diseases (COPD), often smokers or ex-smokers, are at risk of developing lung cancer independently from the degree of bronchial obstruction [135-137]. COPD patients are especially at risk of developing lung cancer if they are obese, as recently observed in a study with a group of 433 patients followed up for 9 years (HR 3.3; 95% CI 1.3-8.5) [138]. Obesity in COPD patients, in turn, may be associated with non-diagnosed sleep disorders [139, 140]. The potential additive risk factors such as a combination of smoking, COPD, and obesity remain unaddressed in current literature.
Acknowledgements
Declared none.
AUTHORS' CONTRIBUTIONS
Anna Brzecka (AB), Karolina Sarul (KS), Tomasz Dyła (TD), Marco Avila-Rodriguez (MAR), Ricardo Cabezas-Perez (RCP), Vladimir N. Chubarev (VNC), Nina N. Minyaeva (NNM), Sergey G. Klochkov (SGK), Margarita E.Neganova (MEN), Liudmila M. Mikhaleva (LMM), Siva G. Somasundaram (SGS), Cecil E. Kirkland (CEK), Vadim V. Tarasov (VVT), Gjumrakch Aliev (GA) conceptualized and designed the study. AB, KS, TD, MAR, RCP, and GA collected and analyzed the data. AB, KS, TD, MAR, RCP, VNC, SGK, MEN, LMM, SGS, CEK, VVT, and GA discussed the analyses, the results, and their interpretation, and wrote the original manuscript draft. All authors reviewed and approved the manuscript before submission.
Consent for Publication
Not applicable.
Funding
This research was financially supported by the Ministry of Science and High Education of the Russian Federation; State task 0090_2019_0006; unique identifier of the project: RFMEFI61318X0086, and RFBR under scientific project No. 18-33-20209”. This work also supported by the Russian Academic Excellence Project “5-100” for Sechenov University, Moscow, Russia.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Danaei G., Vander Hoorn S., Lopez A.D., Murray C.J., Ezzati M., Comparative Risk Assessment collaborating group (Cancers). Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 2.Lewandowska A.M., Rudzki M., Rudzki S., Lewandowski T., Laskowska B. Environmental risk factors for cancer - review paper. Ann. Agric. Environ. Med. 2019;26(1):1–7. doi: 10.26444/aaem/94299. [DOI] [PubMed] [Google Scholar]
- 3.Murphy N., Moreno V., Hughes D.J., Vodicka L., Vodicka P., Aglago E.K., Gunter M.J., Jenab M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Aspects Med. 2019;69:2–9. doi: 10.1016/j.mam.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Kyrgiou M., Kalliala I., Markozannes G., Gunter M.J., Paraskevaidis E., Gabra H., Martin-Hirsch P., Tsilidis K.K. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele C.B., Thomas C.C., Henley S.J., Massetti G.M., Galuska D.A., Agurs-Collins T., Puckett M., Richardson L.C. Vital signs: Trends in incidence of cancers associated with overweight and obesity- United States, 2005-2014. MMWR Morb. Mortal. Wkly. Rep. 2017;66(39):1052–1058. doi: 10.15585/mmwr.mm6639e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold M., Pandeya N., Byrnes G., Renehan P.A.G., Stevens G.A., Ezzati P.M., Ferlay J., Miranda J.J., Romieu I., Dikshit R., Forman D., Soerjomataram I. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manna P., Jain S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015;13(10):423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Włodarczyk M., Nowicka G. Obesity, DNA damage, and development of obesity-related diseases. Int. J. Mol. Sci. 2019;20(5):E1146. doi: 10.3390/ijms20051146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Zazzo E., Polito R., Bartollino S., Nigro E., Porcile C., Bianco A., Daniele A., Moncharmont B. Adiponectin as link factor between adipose tissue and cancer. Int. J. Mol. Sci. 2019;20(4):839. doi: 10.3390/ijms20040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J., Hotta K., Shimomura I., Nakamura T., Miyaoka K., Kuriyama H., Nishida M., Yamashita S., Okubo K., Matsubara K., Muraguchi M., Ohmoto Y., Funahashi T., Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 11.Riondino S., Roselli M., Palmirotta R., Della-Morte D., Ferroni P., Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014;20(18):5177–5190. doi: 10.3748/wjg.v20.i18.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon Y.S., Kwon A.R., Lee Y.K., Oh S.W. Circulating adipokines and risk of obesity related cancers: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2019;13(4):329–339. doi: 10.1016/j.orcp.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Considine R.V., Sinha M.K., Heiman M.L., Kriauciunas A., Stephens T.W., Nyce M.R., Ohannesian J.P., Marco C.C., McKee L.J., Bauer T.L., Caro J.F. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q., Sun Y., Fei Z., Yang Z., Duan K., Zi J., Cui Q., Yu M., Xiong W. Leptin promotes fatty acid oxidation and OXPHOS via the c-Myc/PGC-1 pathway in cancer cells. Acta Biochim. Biophys. Sin. (Shanghai) 2019;51(7):707–714. doi: 10.1093/abbs/gmz058. [DOI] [PubMed] [Google Scholar]
- 15.Modzelewska P., Chludzińska S., Lewko J., Reszeć J. The influence of leptin on the process of carcinogenesis. Contemp. Oncol. (Pozn.) 2019;23(2):63–68. doi: 10.5114/wo.2019.85877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray A., Cleary M.P. The potential role of leptin in tumor invasion and metastasis. Cytokine Growth Factor Rev. 2017;38:80–97. doi: 10.1016/j.cytogfr.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bifulco M., Ciaglia E. Updates on “adiponcosis”: More new incoming evidence strengthening the obesity-cancer link. Eur. J. Intern. Med. 2017;41:e19–e20. doi: 10.1016/j.ejim.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Ogilvie R.P., Patel S.R. The epidemiology of sleep and obesity. Sleep Health. 2017;3(5):383–388. doi: 10.1016/j.sleh.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatima Y., Doi S.A.R., Al Mamun A. Sleep problems in adolescence and overweight/obesity in young adults: is there a causal link? Sleep Health. 2018;4(2):154–159. doi: 10.1016/j.sleh.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto N., Gozal D., Smith D.L., Yang L., Morimoto N., Wada H., Maruyama K., Ikeda A., Suzuki Y., Nakayama M., Horiguchi I., Tanigawa T. Sleep duration, snoring prevalence, obesity, and behavioral problems in a large cohort of primary school students in Japan. Sleep (Basel) 2017;40(3):zsw082. doi: 10.1093/sleep/zsw082. [DOI] [PubMed] [Google Scholar]
- 21.Ryan S., Arnaud C., Fitzpatrick S.F., Gaucher J., Tamisier R., Pépin J-L. Adipose tissue as a key player in obstructive sleep apnoea. Eur. Respir. Rev. 2019;28(152):190006. doi: 10.1183/16000617.0006-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinzer R., Vat S., Marques-Vidal P., Marti-Soler H., Andries D., Tobback N., Mooser V., Preisig M., Malhotra A., Waeber G., Vollenweider P., Tafti M., Haba-Rubio J. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir. Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep Med. Rev. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Javaheri S., Barbe F., Campos-Rodriguez F., Dempsey J.A., Khayat R., Javaheri S., Malhotra A., Martinez-Garcia M.A., Mehra R., Pack A.I., Polotsky V.Y., Redline S., Somers V.K. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J. Am. Coll. Cardiol. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cowie M.R. Sleep apnea: state of the art. Trends Cardiovasc. Med. 2017;27(4):280–289. doi: 10.1016/j.tcm.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Ryan S. Mechanisms of cardiovascular disease in obstructive sleep apnoea. J. Thorac. Dis. 2018;10(Suppl. 34):S4201–S4211. doi: 10.21037/jtd.2018.08.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding C., Lim L.L., Xu L., Kong A.P.S. Sleep and obesity. J. Obes. Metab. Syndr. 2018;27(1):4–24. doi: 10.7570/jomes.2018.27.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuelsson L.B., Bovbjerg D.H., Roecklein K.A., Hall M.H. Sleep and circadian disruption and incident breast cancer risk: An evidence-based and theoretical review. Neurosci. Biobehav. Rev. 2018;84:35–48. doi: 10.1016/j.neubiorev.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang W., Shi Y., Ke X., Sun H., Guo J., Wang X. Long-term sleep habits and the risk of breast cancer among Chinese women: a case-control study. Eur. J. Cancer Prev. 2019;28(4):323–329. doi: 10.1097/CEJ.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 30.Cao J., Eshak E.S., Liu K., Muraki I., Cui R., Iso H., Tamakoshi A., JACC Study Group Sleep duration and risk of breast cancer: The JACC Study. Breast Cancer Res. Treat. 2019;174(1):219–225. doi: 10.1007/s10549-018-4995-4. [DOI] [PubMed] [Google Scholar]
- 31.Nelson B. Dying for a good night of sleep?: Researchers are investigating how poor sleep can increase the risk of cancer or worsen the course of the disease. Cancer Cytopathol. 2019;127(5):273–274. doi: 10.1002/cncy.22142. [DOI] [PubMed] [Google Scholar]
- 32.Shen J., Chrisman M., Wu X., Chow W.H., Zhao H. Sleep duration and risk of cancer in the Mexican American Mano-a-Mano Cohort. Sleep Health. 2019;5(1):78–83. doi: 10.1016/j.sleh.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Nayak M.G., George A., Vidyasagar M.S., Mathew S., Nayak S., Nayak B.S., Shashidhara Y.N., Kamath A. Symptoms experienced by cancer patients and barriers to symptom management. Indian J. Palliat. Care. 2015;21(3):349–354. doi: 10.4103/0973-1075.164893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akman T., Yavuzsen T., Sevgen Z., Ellidokuz H., Yilmaz A.U. Evaluation of sleep disorders in cancer patients based on Pittsburgh Sleep Quality Index. Eur. J. Cancer Care (Engl.) 2015;24(4):553–559. doi: 10.1111/ecc.12296. [DOI] [PubMed] [Google Scholar]
- 35.Daroszewski C., Stasiewicz M., Jaźwińska-Tarnawska E., Rachwalik A., Mura E., Luboch-Kowal J., Dryś A., Bogucki Z.A., Brzecka A. Quality of life in patients with advanced non-small-cell lung cancer receiving palliative chemotherapy. Adv. Exp. Med. Biol. 2019;1160:11–18. doi: 10.1007/5584_2019_346. [DOI] [PubMed] [Google Scholar]
- 36.Reinsel R.A., Starr T.D., O’Sullivan B., Passik S.D., Kavey N.B. Polysomnographic study of sleep in survivors of breast cancer. J. Clin. Sleep Med. 2015;11(12):1361–1370. doi: 10.5664/jcsm.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palesh O., Aldridge-Gerry A., Zeitzer J.M., Koopman C., Neri E., Giese-Davis J., Jo B., Kraemer H., Nouriani B., Spiegel D. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep (Basel) 2014;37(5):837–842. doi: 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garfield V., Joshi R., Garcia-Hernandez J., Tillin T., Chaturvedi N. The relationship between sleep quality and all-cause, CVD and cancer mortality: the Southall and Brent REvisited study (SABRE). Sleep Med. 2019;60:230–235. doi: 10.1016/j.sleep.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker W.H., II, Borniger J.C. Molecular mechanisms of cancer-induced sleep disruption. Int. J. Mol. Sci. 2019;20(11):E2780. doi: 10.3390/ijms20112780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besedovsky L., Lange T., Haack M. The sleep-immune crosstalk in health and disease. Physiol. Rev. 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shearer W.T., Reuben J.M., Mullington J.M., Price N.J., Lee B.N., Smith E.O., Szuba M.P., Van Dongen H.P., Dinges D.F. Soluble TNF-α receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J. Allergy Clin. Immunol. 2001;107(1):165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 42.Irwin M.R., Wang M., Campomayor C.O., Collado-Hidalgo A., Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med. 2006;166(16):1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 43.Irwin M.R., Wang M., Ribeiro D., Cho H.J., Olmstead R., Breen E.C., Martinez-Maza O., Cole S. Sleep loss activates cellular inflammatory signaling. Biol. Psychiatry. 2008;64(6):538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Born J., Lange T., Hansen K., Mölle M., Fehm H.L. Effects of sleep and circadian rhythm on human circulating immune cells. J. Immunol. 1997;158(9):4454–4464. [PubMed] [Google Scholar]
- 45.De Lorenzo B.H.P., Novaes E. Brito, R.R.; Paslar Leal, T.; Piqueira Garcia, N.; Martins Dos Santos, R.M.; Alvares-Saraiva, A.M.; Perez Hurtado, E.C.; Braga Dos Reis, T.C.; Duarte Palma, B. Chronic sleep restriction impairs the antitumor immune response in mice. Neuroimmunomodulation. 2018;25(2):59–67. doi: 10.1159/000490352. [DOI] [PubMed] [Google Scholar]
- 46.Gu F., Xiao Q., Chu L.W., Yu K., Matthews C.E., Hsing A.W., Caporaso N.E. Sleep duration and cancer in the NIH-AARP diet and health study cohort. PLoS One. 2016;11(9):e0161561. doi: 10.1371/journal.pone.0161561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen P., Wang C., Song Q., Chen T., Jiang J., Zhang X., Xu J., Cui J., Cheng Y. Impacts of sleep duration and snoring on the risk of esophageal squamous cell carcinoma. J. Cancer. 2019;10(9):1968–1974. doi: 10.7150/jca.30172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kakizaki M., Kuriyama S., Sone T., Ohmori-Matsuda K., Hozawa A., Nakaya N., Fukudo S., Tsuji I. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br. J. Cancer. 2008;99(9):1502–1505. doi: 10.1038/sj.bjc.6604684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao Q., Signorello L.B., Brinton L.A., Cohen S.S., Blot W.J., Matthews C.E. Sleep duration and breast cancer risk among black and white women. Sleep Med. 2016;20:25–29. doi: 10.1016/j.sleep.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang P., Ren F.M., Lin Y., Su F.X., Jia W-H., Su X.F., Tang L.Y., Ren Z.F. Night-shift work, sleep duration, daytime napping, and breast cancer risk. Sleep Med. 2015;16(4):462–468. doi: 10.1016/j.sleep.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 51.Zhao H., Yin J.Y., Yang W.S., Qin Q., Li T.T., Shi Y., Deng Q., Wei S., Liu L., Wang X., Nie S.F. Sleep duration and cancer risk: a systematic review and meta-analysis of prospective studies. Asian Pac. J. Cancer Prev. 2013;14(12):7509–7515. doi: 10.7314/APJCP.2013.14.12.7509. [DOI] [PubMed] [Google Scholar]
- 52.Shigesato M., Kawai Y., Guillermo C., Youkhana F., Shvetsov Y.B., Setiawan V.W., Haiman C.A., Marchand L.L., Maskarinec G. Association between sleep duration and breast cancer incidence: the multiethnic cohort. Int. J. Cancer. 2019;146(3):664–670. doi: 10.1002/ijc.32292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao Q., Arem H., Pfeiffer R., Matthews C. Prediagnosis sleep duration, napping, and mortality among colorectal cancer survivors in a large US cohort. Sleep (Basel) 2017;40(4):zsx010. doi: 10.1093/sleep/zsx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNeil J., Barberio A.M., Friedenreich C.M., Brenner D.R. Sleep and cancer incidence in Alberta’s Tomorrow Project cohort. Sleep (Basel) 2019;42(3):zsx010. doi: 10.1093/sleep/zsy252. [DOI] [PubMed] [Google Scholar]
- 55.Li Y., Cai S., Ling Y., Mi S., Fan C., Zhong Y., Shen Q. Association between total sleep time and all cancer mortality: non-linear dose-response meta-analysis of cohort studies. Sleep Med. 2019;60:211–218. doi: 10.1016/j.sleep.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Zhang S., Xie L., Yu H., Zhang W., Qian B. Association between nighttime-daytime sleep patterns and chronic diseases in Chinese elderly population: a community-based cross-sectional study. BMC Geriatr. 2019;19(1):124. doi: 10.1186/s12877-019-1136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gery S., Komatsu N., Baldjyan L., Yu A., Koo D., Koeffler H.P. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell. 2006;22(3):375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 58.Li H.X. The role of circadian clock genes in tumors. OncoTargets Ther. 2019;12:3645–3660. doi: 10.2147/OTT.S203144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu C.C., Chen L.C., Chiou C.Y., Chang Y.J., Lin V.C., Huang C.Y., Lin I.L., Chang T.Y., Lu T.L., Lee C.H., Huang S.P., Bao B.Y. Genetic variants in the circadian rhythm pathway as indicators of prostate cancer progression. Cancer Cell Int. 2019;19:87. doi: 10.1186/s12935-019-0811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu M., Chen Y.B., Jin S., Fang X.F., He X.X., Xiong Z.F., Yang S.L. Research on circadian clock genes in non-small-cell lung carcinoma. Chronobiol. Int. 2019;36(6):739–750. doi: 10.1080/07420528.2018.1509080. [DOI] [PubMed] [Google Scholar]
- 61.Qiu M.J., Liu L.P., Jin S., Fang X.F., He X.X., Xiong Z-F., Yang S.L. Research on circadian clock genes in common abdominal malignant tumors. Chronobiol. Int. 2019;36(7):906–918. doi: 10.1080/07420528.2018.1477792. [DOI] [PubMed] [Google Scholar]
- 62.Amaral F.G.D., Cipolla-Neto J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018;62(4):472–479. doi: 10.20945/2359-3997000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardeland R. Aging, melatonin, and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 2019;20(5):1223–E1223. doi: 10.3390/ijms20051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giudice A., Crispo A., Grimaldi M., Polo A., Bimonte S., Capunzo M., Amore A., D’Arena G., Cerino P., Budillon A., Botti G., Costantini S., Montella M. The effect of light exposure at night (LAN) on carcinogenesis via decreased nocturnal melatonin synthesis. Molecules. 2018;23(6):E1308. doi: 10.3390/molecules23061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pourhanifeh M.H., Sharifi M., Reiter R.J., Davoodabadi A., Asemi Z. Melatonin and non-small cell lung cancer: new insights into signaling pathways. Cancer Cell Int. 2019;19:131. doi: 10.1186/s12935-019-0853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan D.X., Hardeland R., Back K., Manchester L.C., Alatorre-Jimenez M.A., Reiter R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. J. Pineal Res. 2016;61(1):27–40. doi: 10.1111/jpi.12336. [DOI] [PubMed] [Google Scholar]
- 67.Park S.Y., Jang W.J., Yi E.Y., Jang J.Y., Jung Y., Jeong J.W., Kim Y.J. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1α stabilization under hypoxia. J. Pineal Res. 2010;48(2):178–184. doi: 10.1111/j.1600-079X.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- 68.Tamtaji O.R., Mirhosseini N., Reiter R.J., Behnamfar M., Asemi Z. Melatonin and pancreatic cancer: Current knowledge and future perspectives. J. Cell. Physiol. 2019;234(5):5372–5378. doi: 10.1002/jcp.27372. [DOI] [PubMed] [Google Scholar]
- 69.Veiga E.C.D.A., Simões R., Valenti V.E., Cipolla-Neto J., Abreu L.C., Barros E.P.M., Sorpreso I.C.E., Baracat M.C.P., Baracat E.C., Junior J. M.S. 2019.
- 70.Pourhanifeh M.H., Mahdavinia M., Reiter R.J., Asemi Z. Potential use of melatonin in skin cancer treatment: A review of current biological evidence. J. Cell. Physiol. 2019;234(8):12142–12148. doi: 10.1002/jcp.28129. [DOI] [PubMed] [Google Scholar]
- 71.Zare H., Shafabakhsh R., Reiter R.J., Asemi Z. Melatonin is a potential inhibitor of ovarian cancer: molecular aspects. J. Ovarian Res. 2019;12(1):26. doi: 10.1186/s13048-019-0502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chok K.C., Ng C.H., Koh R.Y., Ng K.Y., Chye S.M. The potential therapeutic actions of melatonin in colorectal cancer. Horm. Mol. Biol. Clin. Investig. 2019;••• doi: 10.1515/hmbci-2019-0001. [DOI] [PubMed] [Google Scholar]
- 73.Reiter R.J., Rosales-Corral S.A., Tan D.X., Acuna-Castroviejo D., Qin L., Yang S.F., Xu K. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017;18(4):E843. doi: 10.3390/ijms18040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Straif K., Baan R., Grosse Y., Secretan B., El Ghissassi F., Bouvard V., Altieri A., Benbrahim-Tallaa L., Cogliano V., WHO International Agency For Research on Cancer Monograph Working Group Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 75.Stevens R.G., Hansen J., Costa G., Haus E., Kauppinen T., Aronson K.J., Castaño-Vinyals G., Davis S., Frings-Dresen M.H.W., Fritschi L., Kogevinas M., Kogi K., Lie J.A., Lowden A., Peplonska B., Pesch B., Pukkala E., Schernhammer E., Travis R.C., Vermeulen R., Zheng T., Cogliano V., Straif K. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup. Environ. Med. 2011;68(2):154–162. doi: 10.1136/oem.2009.053512. [DOI] [PubMed] [Google Scholar]
- 76.Erren T.C., Lewis P. Hypothesis: ubiquitous circadian disruption can cause cancer. Eur. J. Epidemiol. 2019;34(1):1–4. doi: 10.1007/s10654-018-0469-6. [DOI] [PubMed] [Google Scholar]
- 77.Rosa D., Terzoni S., Dellafiore F., Destrebecq A. Systematic review of shift work and nurses’ health. Occup. Med. (Lond.) 2019;69(4):237–243. doi: 10.1093/occmed/kqz063. [DOI] [PubMed] [Google Scholar]
- 78.Jones M.E., Schoemaker M.J., McFadden E.C., Wright L.B., Johns L.E., Swerdlow A.J. Night shift work and risk of breast cancer in women: the Generations Study cohort. Br. J. Cancer. 2019;121(2):172–179. doi: 10.1038/s41416-019-0485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leung L., Grundy A., Siemiatycki J., Arseneau J., Gilbert L., Gotlieb W.H., Provencher D.M., Aronson K.J., Koushik A. Shift work patterns, chronotype, and epithelial ovarian cancer risk. Cancer Epidemiol. Biomarkers Prev. 2019;28(5):987–995. doi: 10.1158/1055-9965.EPI-18-1112. [DOI] [PubMed] [Google Scholar]
- 80.Fang H.F., Miao N.F., Chen C.D., Sithole T., Chung M.H. Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: a nationwide nested case-control study. J. Cancer. 2015;6(11):1140–1147. doi: 10.7150/jca.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fleming L., Randell K., Stewart E., Espie C.A., Morrison D.S., Lawless C., Paul J. Insomnia in breast cancer: a prospective observational study. Sleep (Basel) 2019;42(3):zsy245. doi: 10.1093/sleep/zsy245. [DOI] [PubMed] [Google Scholar]
- 82.Sen A., Opdahl S., Strand L.B., Vatten L.J., Laugsand L.E., Janszky I. Insomnia and the risk of breast cancer. Psychosom. Med. 2017;79(4):461–468. doi: 10.1097/PSY.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 83.Chiu H.Y., Huang C.J., Fan Y.C., Tsai P.S. Insomnia but not hypnotics use associates with the risk of breast cancer: a population-based matched cohort study. J. Womens Health (Larchmt.) 2018;27(10):1250–1256. doi: 10.1089/jwh.2017.6626. [DOI] [PubMed] [Google Scholar]
- 84.Jung S.J., Lee J., Choi J.W., Kim S., Shin A., Lee Y.J. Association between sedative-hypnotic medication use and incidence of cancer in Korean Nation Health Insurance Service data. Sleep Med. 2019;60:159–164. doi: 10.1016/j.sleep.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 85.Lin C.L., Liu T.C., Wang Y.N., Chung C.H., Chien W.C. The association between sleep disorders and the risk of colorectal cancer in patients: a population-based nested case-control study. In Vivo. 2019;33(2):573–579. doi: 10.21873/invivo.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berry R.B., Budhiraja R., Gottlieb D.J., Gozal D., Iber C., Kapur V.K., Marcus C.L., Mehra R., Parthasarathy S., Quan S.F., Redline S., Strohl K.P., Davidson Ward S.L., Tangredi M.M. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ye L., Pien G.W., Ratcliffe S.J., Björnsdottir E., Arnardottir E.S., Pack A.I., Benediktsdottir B., Gislason T. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur. Respir. J. 2014;44(6):1600–1607. doi: 10.1183/09031936.00032314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lévy P., Godin-Ribuot D., Pèpin J-L. Sleep apnoea and cancer: the new challenge. Eur. Respir. J. 2014;43(6):1567–1570. doi: 10.1183/09031936.00065714. [DOI] [PubMed] [Google Scholar]
- 89.Gozal D., Farré R., Nieto F.J. Putative links between sleep apnea and cancer. Chest. 2015;148(5):1140–1147. doi: 10.1378/chest.15-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gozal D., Farré R., Nieto F.J. Obstructive sleep apnea and cancer: Epidemiologic links and theoretical biological constructs. Sleep Med. Rev. 2016;27:43–55. doi: 10.1016/j.smrv.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Almendros I., Gozal D. Intermittent hypoxia and cancer: Undesirable bed partners? Respir. Physiol. Neurobiol. 2018;256:79–86. doi: 10.1016/j.resp.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 92.Martinez-Garcia M.A., Campos-Rodriguez F., Almendros I., Garcia-Rio F., Sanchez-De-La-Torre M., Farre R., Gozal D. Cancer and sleep apnea: Cutaneous melanoma as a case study. Am. J. Respir. Crit. Care Med. 2019;200(11):1345–1353. doi: 10.1164/rccm.201903-0577PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Toffoli S., Michiels C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J. 2008;275(12):2991–3002. doi: 10.1111/j.1742-4658.2008.06454.x. [DOI] [PubMed] [Google Scholar]
- 94.Almendros I., Montserrat J.M., Torres M., Bonsignore M.R., Chimenti L., Navajas D., Farré R. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med. 2012;13(10):1254–1260. doi: 10.1016/j.sleep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 95.Almendros I., Wang Y., Becker L., Lennon F.E., Zheng J., Coats B.R., Schoenfelt K.S., Carreras A., Hakim F., Zhang S.X., Farré R., Gozal D. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am. J. Respir. Crit. Care Med. 2014;189(5):593–601. doi: 10.1164/rccm.201310-1830OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akbarpour M., Khalyfa A., Qiao Z., Gileles-Hillel A., Almendros I., Farré R., Gozal D. Altered CD8 T-cell lymphocyte function and TC1 cell stemness contribute to enhanced malignant tumor properties in murine models of sleep apnea. Sleep (Basel) 2017;40(2):zsw040. doi: 10.1093/sleep/zsw040. [DOI] [PubMed] [Google Scholar]
- 97.Almendros I., Montserrat J.M., Torres M., Dalmases M., Cabañas M.L., Campos-Rodríguez F., Navajas D., Farré R. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir. Physiol. Neurobiol. 2013;186(3):303–307. doi: 10.1016/j.resp.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 98.Yoon D.W., So D., Min S., Kim J., Lee M., Khalmuratova R., Cho C.H., Park J.W., Shin H.W. Accelerated tumor growth under intermittent hypoxia is associated with hypoxia-inducible factor-1-dependent adaptive responses to hypoxia. Oncotarget. 2017;8(37):61592–61603. doi: 10.18632/oncotarget.18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez-Garcia M.A., Campos-Rodriguez F., Nagore E., Martorell A., Rodriguez-Peralto J.L., Riveiro-Falkenbach E., Hernandez L., Bañuls J., Arias E., Ortiz P., Cabriada V., Gardeazabal J., Montserrat J.M., Carrera C., Corral J., Masa J.F., de Terreros J.G., Abad J., Boada A., Mediano O., de Eusebio E., Chiner E., Landete P., Mayos M., Fortuño A., Barbé F., Sánchez de la Torre M., Sanchez de la Torre A., Cano I., Gonzalez C., Pérez-Gil A., Gómez-García T., Cullen D., Somoza M., Formigón M., Aizpuru F., Navarro C., Selma-Ferrer M.J., Garcia-Ortega A., de Unamuno B., Almendros I., Farré R., Gozal D. Spanish Sleep Network. Sleep-disordered breathing is independently associated with increased aggressiveness of cutaneous melanoma: a multicenter observational study in 443 patients. Chest. 2018;154(6):1348–1358. doi: 10.1016/j.chest.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 100.Huang M.H., Zhang X.B., Wang H.L., Li L.X., Zeng Y.M., Wang M., Zeng H.Q. Intermittent hypoxia enhances the tumor programmed death ligand 1 expression in a mouse model of sleep apnea. Ann. Transl. Med. 2019;7(5):97. doi: 10.21037/atm.2019.01.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cubillos-Zapata C., Balbás-García C., Avendaño-Ortiz J., Toledano V., Torres M., Almendros I., Casitas R., Zamarrón E., García-Sánchez A., Feliu J., Aguirre L.A., Farre R., López-Collazo E., García-Rio F. Age-dependent hypoxia-induced PD-L1 upregulation in patients with obstructive sleep apnoea. Respirology. 2019;24(7):684–692. doi: 10.1111/resp.13470. [DOI] [PubMed] [Google Scholar]
- 102.Cubillos-Zapata C., Avendaño-Ortiz J., Hernandez-Jimenez E., Toledano V., Casas-Martin J., Varela-Serrano A., Torres M., Almendros I., Casitas R., Fernández-Navarro I., Garcia-Sanchez A., Aguirre L.A., Farre R., López-Collazo E., García-Rio F. Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in sleep apnoea. Eur. Respir. J. 2017;50(4):1700833. doi: 10.1183/13993003.00833-2017. [DOI] [PubMed] [Google Scholar]
- 103.Fu C., Jiang L., Zhu F., Liu Z., Li W., Jiang H., Ye H., Kushida C.A., Li S. Chronic intermittent hypoxia leads to insulin resistance and impaired glucose tolerance through dysregulation of adipokines in non-obese rats. Sleep Breath. 2015;19(4):1467–1473. doi: 10.1007/s11325-015-1144-8. [DOI] [PubMed] [Google Scholar]
- 104.He Q., Yang Q.C., Zhou Q., Zhu H., Niu W.Y., Feng J., Wang Y., Cao J., Chen B.Y. Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes. PLoS One. 2014;9(1):e86326. doi: 10.1371/journal.pone.0086326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Amin M.N., Hussain M.S., Sarwar M.S., Rahman Moghal M.M., Das A., Hossain M.Z., Chowdhury J.A., Millat M.S., Islam M.S. How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab. Syndr. 2019;13(2):1213–1224. doi: 10.1016/j.dsx.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 106.Tumminia A., Vinciguerra F., Parisi M., Graziano M., Sciacca L., Baratta R., Frittitta L. Adipose tissue, obesity and adiponectin: role in endocrine cancer risk. Int. J. Mol. Sci. 2019;20(12):E2863. doi: 10.3390/ijms20122863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hargens T.A., Guill S.G., Kaleth A.S., Nickols-Richardson S.M., Miller L.E., Zedalis D., Gregg J.M., Gwazdauskas F., Herbert W.G. Insulin resistance and adipose-derived hormones in young men with untreated obstructive sleep apnea. Sleep Breath. 2013;17(1):403–409. doi: 10.1007/s11325-012-0708-0. [DOI] [PubMed] [Google Scholar]
- 108.Al Mutairi S., Mojiminiyi O.A., Al Alawi A., Al Rammah T., Abdella N. Study of leptin and adiponectin as disease markers in subjects with obstructive sleep apnea. Dis. Markers. 2014;2014:706314. doi: 10.1155/2014/706314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolk R., Svatikova A., Nelson C.A., Gami A.S., Govender K., Winnicki M., Somers V.K. Plasma levels of adiponectin, a novel adipocyte-derived hormone, in sleep apnea. Obes. Res. 2005;13(1):186–190. doi: 10.1038/oby.2005.24. [DOI] [PubMed] [Google Scholar]
- 110.Almendros I., Khalyfa A., Trzepizur W., Gileles-Hillel A., Huang L., Akbarpour M., Andrade J., Farré R., Gozal D. Tumor cell malignant properties are enhanced by circulating exosomes in sleep apnea. Chest. 2016;150(5):1030–1041. doi: 10.1016/j.chest.2016.08.1438. [DOI] [PubMed] [Google Scholar]
- 111.Khalyfa A., Almendros I., Gileles-Hillel A., Akbarpour M., Trzepizur W., Mokhlesi B., Huang L., Andrade J., Farré R., Gozal D. Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget. 2016;7(34):54676–54690. doi: 10.18632/oncotarget.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bikov A., Kunos L., Pállinger É., Lázár Z., Kis A., Horváth G., Losonczy G., Komlósi Z.I. Diurnal variation of circulating microvesicles is associated with the severity of obstructive sleep apnoea. Sleep Breath. 2017;21(3):595–600. doi: 10.1007/s11325-017-1464-y. [DOI] [PubMed] [Google Scholar]
- 113.Khalyfa A., Gozal D., Masa J.F., Marin J.M., Qiao Z., Corral J., González M., Marti S., Kheirandish-Gozal L., Egea C., Sánchez-Quiroga M.Á., de Terreros F.J.G., Barca F.J. Sleep-disordered breathing, circulating exosomes, and insulin sensitivity in adipocytes. Int. J. Obes. 2018;42(6):1127–1139. doi: 10.1038/s41366-018-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tarasov V.V., Svistunov A.A., Chubarev V.N., Dostdar S.A., Sokolov A.V., Brzecka A., Sukocheva O., Neganova M.E., Klochkov S.G., Somasundaram S.G., Kirkland C.E., Aliev G. Extracellular vesicles in cancer nanomedicine. 2019. [DOI] [PubMed]
- 115.Vagner T., Chin A., Mariscal J., Bannykh S., Engman D.M., Di Vizio D. Protein composition reflects extracellular vesicle heterogeneity. Proteomics. 2019;19(8):e1800167. doi: 10.1002/pmic.201800167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abramowicz A., Widłak P., Pietrowska M. Different types of cellular stress affect the proteome composition of small extracellular vesicles: a mini review. Proteomes. 2019;7(2):E23. doi: 10.3390/proteomes7020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang H., Yang F., Guo Y., Wang L., Fang F., Wu H., Nie S., Wang Y., Fung M.L., Huang Y., Deng H., Qin Y., Ma X., Wei Y. The contribution of chronic intermittent hypoxia to OSAHS: From the perspective of serum extracellular microvesicle proteins. Metabolism. 2018;85:97–108. doi: 10.1016/j.metabol.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 118.Nieto F.J., Peppard P.E., Young T., Finn L., Hla K.M., Farré R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2012;186(2):190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marshall N.S., Wong K.K., Cullen S.R., Knuiman M.W., Grunstein R.R. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J. Clin. Sleep Med. 2014;10(4):355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martínez-García M.A., Campos-Rodriguez F., Durán-Cantolla J., de la Peña M., Masdeu M.J., González M., Del Campo F., Serra P.C., Valero-Sánchez I., Ferrer M.J., Marín J.M., Barbé F., Martínez M., Farré R., Montserrat J.M. Spanish Sleep Network. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15(7):742–748. doi: 10.1016/j.sleep.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 121.Gozal D., Ham S.A., Mokhlesi B. Sleep apnea and cancer: Analysis of a nationwide population sample. Sleep (Basel) 2016;39(8):1493–1500. doi: 10.5665/sleep.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Campos-Rodriguez F., Martinez-Garcia M.A., Martinez M., Duran-Cantolla J. Peña, Mde.L.; Masdeu, M.J.; Gonzalez, M.; Campo, Fd.; Gallego, I.; Marin, J.M.; Barbe, F.; Montserrat, J.M.; Farre, R. Spanish Sleep Network. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am. J. Respir. Crit. Care Med. 2013;187(1):99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 123.Chen J.C., Hwang J.H. Sleep apnea increased incidence of primary central nervous system cancers: a nationwide cohort study. Sleep Med. 2014;15(7):749–754. doi: 10.1016/j.sleep.2013.11.782. [DOI] [PubMed] [Google Scholar]
- 124.Chang W.P., Liu M.E., Chang W.C., Yang A.C., Ku Y.C., Pai J.T., Lin Y.W., Tsai S.J. Sleep apnea and the subsequent risk of breast cancer in women: a nationwide population-based cohort study. Sleep Med. 2014;15(9):1016–1020. doi: 10.1016/j.sleep.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 125.Christensen A.S., Clark A., Salo P., Nymann P., Lange P., Prescott E., Rod N.H. Symptoms of sleep disordered breathing and risk of cancer: a prospective cohort study. Sleep (Basel) 2013;36(10):1429–1435. doi: 10.5665/sleep.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dreher M., Krüger S., Schulze-Olden S., Keszei A., Storre J.H., Woehrle H., Arzt M., Müller T. Sleep-disordered breathing in patients with newly diagnosed lung cancer. BMC Pulm. Med. 2018;18(1):72. doi: 10.1186/s12890-018-0645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pérez-Warnisher M.T., Cabezas E., Troncoso M.F., Gómez T., Melchor R., Pinillos E.J., El Hachem A., Gotera C., Rodriguez P., Mahíllo I., González-Mangado N., Peces-Barba G., Seijo L.M. Sleep disordered breathing and nocturnal hypoxemia are very prevalent in a lung cancer screening population and may condition lung cancer screening findings: results of the prospective Sleep Apnea In Lung Cancer Screening (SAILS) study. Sleep Med. 2019;54:181–186. doi: 10.1016/j.sleep.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 128.Cabezas E., Pérez-Warnisher M.T., Troncoso M.F., Gómez T., Melchor R., Pinillos E.J., El Hachem A., Gotera C., Rodríguez P., Mahillo Fernández I., Martínez-García M.Á., Peces-Barba G., Seijo L.M., Seijo L.M. González-Mangado. Sleep disordered breathing is highly prevalent in patients with lung cancer: results of the sleep apnea in lung cancer study. Respiration. 2019;97(2):119–124. doi: 10.1159/000492273. [DOI] [PubMed] [Google Scholar]
- 129.Lee S., Kim B.G., Kim J.W., Lee K.L., Koo D.L., Nam H., Im J.P., Kim J.S., Koh S.J. Obstructive sleep apnea is associated with an increased risk of colorectal neoplasia. Gastrointest. Endosc. 2017;85(3):568–573.e1. doi: 10.1016/j.gie.2016.07.061. [DOI] [PubMed] [Google Scholar]
- 130.Palamaner Subash Shantha G., Kumar A.A., Cheskin L.J., Pancholy S.B. Association between sleep-disordered breathing, obstructive sleep apnea, and cancer incidence: a systematic review and meta-analysis. Sleep Med. 2015;16(10):1289–1294. doi: 10.1016/j.sleep.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 131.Kendzerska T., Leung R.S., Hawker G., Tomlinson G., Gershon A.S. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ. 2014;186(13):985–992. doi: 10.1503/cmaj.140238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sillah A., Watson N.F., Gozal D., Phipps A.I. Obstructive sleep apnea severity and subsequent risk for cancer incidence. Prev. Med. Rep. 2019;15:100886. doi: 10.1016/j.pmedr.2019.100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brzecka A., Leszek J., Ashraf G.M., Ejma M., Ávila-Rodriguez M.F., Yarla N.S., Tarasov V.V., Chubarev V.N., Samsonova A.N., Barreto G.E., Aliev G. Sleep disorders associated with alzheimer’s disease: a perspective. Front. Neurosci. 2018;12:330. doi: 10.3389/fnins.2018.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Okereke O.I., Meadows M.E. More evidence of an inverse association between cancer and alzheimer disease. JAMA Netw. Open. 2019;2(6):e196167. doi: 10.1001/jamanetworkopen.2019.6167. [DOI] [PubMed] [Google Scholar]
- 135.Ställberg B., Janson C., Johansson G., Larsson K., Stratelis G., Telg G., Lisspers K.H. Management, morbidity and mortality of COPD during an 11-year period: an observational retrospective epidemiological register study in Sweden (PATHOS). Prim. Care Respir. J. 2014;23(1):38–45. doi: 10.4104/pcrj.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eapen M.S., Hansbro P.M., Larsson-Callerfelt A.K., Jolly M.K., Myers S., Sharma P., Jones B., Rahman M.A., Markos J., Chia C., Larby J., Haug G., Hardikar A., Weber H.C., Mabeza G., Cavalheri V., Khor Y.H., McDonald C.F., Sohal S.S. Chronic obstructive pulmonary disease and lung cancer: underlying pathophysiology and new therapeutic modalities. Drugs. 2018;78(16):1717–1740. doi: 10.1007/s40265-018-1001-8. [DOI] [PubMed] [Google Scholar]
- 137.Greulich T., Weist B.J.D., Koczulla A.R., Janciauskiene S., Klemmer A., Lux W., Alter P., Vogelmeier C.F. Prevalence of comorbidities in COPD patients by disease severity in a German population. Respir. Med. 2017;132:132–138. doi: 10.1016/j.rmed.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 138.Husebø G.R., Nielsen R., Hardie J., Bakke P.S., Lerner L., D’Alessandro-Gabazza C., Gyuris J., Gabazza E., Aukrust P., Eagan T. Risk factors for lung cancer in COPD - results from the Bergen COPD cohort study. Respir. Med. 2019;152:81–88. doi: 10.1016/j.rmed.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 139.Raveendran R., Wong J., Singh M., Wong D.T., Chung F. Obesity hypoventilation syndrome, sleep apnea, overlap syndrome: perioperative management to prevent complications. Curr. Opin. Anaesthesiol. 2017;30(1):146–155. doi: 10.1097/ACO.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 140.Pissulin F.D.M., Pacagnelli F.L., Aldá M.A., Beneti R., Barros J.L., Minamoto S.T., Weber S.A.T. The triad of obstructive sleep apnea syndrome, COPD, and obesity: sensitivity of sleep scales and respiratory questionnaires. J. Bras. Pneumol. 2018;44(3):202–206. doi: 10.1590/s1806-37562016000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]