Abstract
In the scenario of global warming and climate change, an outbreak of new pests and pathogens has become a serious concern owing to the rapid emergence of arms races, their epidemic infection, and the ability to break down host resistance, etc. Fusarium head blight (FHB) is one such evidence that depredates major cereals throughout the world. The symptomatological perplexity and aetiological complexity make this disease very severe, engendering significant losses in the yield. Apart from qualitative and quantitative losses, mycotoxin production solemnly deteriorates the grain quality in addition to life endangerment of humans and animals after consumption of toxified grains above the permissible limit. To minimize this risk, we must be very strategic in designing sustainable management practices constituting cultural, biological, chemical, and host resistance approaches. Even though genetic resistance is the most effective and environmentally safe strategy, a huge genetic variation and unstable resistance response limit the holistic deployment of resistance genes in FHB management. Thus, the focus must shift towards the editing of susceptible (S) host proteins that are soft targets of newly evolving effector molecules, which ultimately could be exploited to repress the disease development process. Hence, we must understand the pathological, biochemical, and molecular insight of disease development in a nutshell. In the present time, the availability of functional genomics, proteomics, and metabolomics information on host-pathogen interaction in FHB have constructed various networks which helped in understanding the pathogenesis and coherent host response(s). So now translation of this information for designing of host defense in the form of desirable resistant variety/genotype is the next step. The insights collected and presented in this review will be aiding in the understanding of the disease and apprise a solution to the multi-faceted problems which are related to FHB resistance in wheat and other cereals to ensure global food safety and food security.
Keywords: FHB, FGSC, mycotoxin, host defense, wheat, RNA interference, genome editing
1. Introduction
Fusarium head blight (FHB) or scab is one of the destructive cereal diseases that have emerged as a severe threat in most of the wheat and barley-based cereal growing regions of the world. Apart from infecting the plant parts, the mycotoxin produced from the pathogen causes a detrimental effect on humans and animals [1, 2]. Since the 1880s, the disease has appeared in a sporadic form. After that, several significant epidemics have been documented in various parts of the world, especially in the United States, during the year 1917, 1919, 1928, 1932, and 1935 [3]. After a long time, this disease again re-emerged in the early 1990s [4, 5]. Since then, a chronic outbreak of this disease is reported in various parts of the world, especially in North-Central USA, Eastern Canada, Eastern, and Western Europe, former USSR, China, Brazil, Romania, etc. [6-8]. At present, this disease affects many small portions of cereal viz., maize, millet, sorghum, oats, triticale, rye, etc. It is prevalent in humid to the semi-humid condition [9, 10], particularly in regions where natural precipitation occurs frequently and high atmospheric moisture prevails throughout the crop growing season, specifically during and after anthesis thus directly affecting the crop yield.
The disease has received much attention during the past decades owing to the unavoidable potential risk to food production [11]. It is accountable for both qualitative as well as quantitative loss in the infected cereal crops. FHB has brought about a 35-61% reduction in wheat yield in the Pacific Northwest of the United States, with an estimated loss of $2.7 billion during 1998-2000 [12, 13]. Similarly, the yield losses in wheat growing regions viz. North Dakota, South Dakota, and Minnesota of USA, have exceeded 13 billion kg since the 1990s with an estimated economic loss of about $2.5 billion [14]. The head blight of wheat and barley has now become an emerging concern to Indian agriculture as well as many other agriculturally dominated economies bearing countries. In India, it was a disease of minor importance. Still, changes in the global climate have led to capricious rainfall and frequent precipitation during anthesis that makes wheat vulnerable to head blight [15]. Therefore, this disease becomes a vital constraint in wheat production in Punjab with its frequent epidemic outbreak on bread wheat cultivar PBW 343 at 10-50% incidence and durum wheat cultivar PDW 274 at > 90% incidence [16, 17]. The aetiological complexity and more extensive adaptability of pathogens in a diverse climatic situation and newer ecological niche help the disease to spread on a larger scale and make it very difficult to manage.
A possible FHB epidemic may cause extensive damage and substantial loss to wheat and barley production, which ultimately will be a threat to national food and nutritional security. On the other side, it deteriorates grain quality by contaminating the cereal grain with mycotoxins produced by several species of the fungal pathogen. Nowadays, FHB becomes a significant concern for grain quality due to the production and accumulation of deoxynivalenol (DON) mycotoxin produced by Fusarium spp. Most notably, the toxins contaminate valuable grain reserve materials leading to consumer’s health-associated problems [8, 18]. DON contaminated cereal crop results into both rejections of sale or price deduction by grain buyers in domestic as well as export markets [19]. Thus, this disease has got great research importance globally to focus on its impacts on economic concern as well as food safety and associated human and animal health risk issues [20]. To decipher the pathological, biochemical, and molecular aspects of the host-pathogen interaction in FHB disease, various omics approaches were adopted in different temporal scales to enlighten us about the overall pathogenomics, including the biochemical alterations starting from pathogenesis to host immunity. Now, it is time to translate this necessary information into functional shape, so that host defense can desirably be structured to obtain durable, resistant genotypes. Moreover, in addition to the development of resistant cultivars from the desired donor sources, an integrative approach for the management of disease outbreak is the need of the hour owing to the complex nature of resistance to semi-biotrophic pathogens [21]. In this review, we try to delineate all the omics information available from the previous studies to formulate the disease management strategies sustainably.
2. Symptomatological perplexity
This disease is diagnosed primarily by pre-mature blighting of head of wheat and barley that can easily be confused with other floral infections like a wheat blast, glume blotch, black chaff, etc. [22-24]. In all these cases, partial or complete bleaching of spikelet accompanied by glume discoloration (Fig. 1A) and shriveling of grains is most common, creating uncertainty in accurate disease diagnostic. Thus, a proper symptomatological characterization is necessary to avoid perplexity.
Fig. (1A).
Partial bleaching of spikelet accompanied with glume discoloration. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The symptoms of FHB first appear around the middle of a spike where the flowering begins [25]. In this region, the spikelets have higher moisture content than basipetal or antipetal spikelets [26]. Initially, it commonly appears as small, water-soaked spots in the middle of glumes, rachis, or on the first floret and gradually spread within the head wherever the pathogen grows from the point of infection, resulting in drying up of the spike that is visualized as premature whitened or bleached ear head (Fig. 1B). As stated, before these symptoms of FHB are often confused with the wheat blast and glume blotch but can be differentiated upon close examination of infected heads. In moist condition, pathogen grows profusely as a white mass of mycelia with pink or salmon-colored conidia on edges of infected glumes that can be distinguished from the grey to dark brown growth of blast, glume blotch and spot pathogens [27-30]. Furthermore, the pathogen gradually colonizes on the developing grain, resulting in the formation of typically shrunken, shriveled, discolored, rough-surfaced and lightweight kernels with pinkish chalky white appearance, commonly called as Fusarium-damaged kernels (FDK) or ‘tombstones’ because they resembled light-colored stone pieces [31]. In the late season, bluish-black spherical sexual bodies known as perithecia may also get produced on the surface of the affected spikelet. Usually, infection remains confined to the head and/or grain, but sometimes it reaches the neck or peduncle region, also causing a brown or purple discoloration of peduncle bearing sterile ear-head [7]. The tombstone grain formation and peduncle discoloration are also absent in black chaff and glume blotch. In case of severe infections, premature blighting or bleaching of some spikelets or the entire ear head after emergence is also commonly evident. Finally, the infected grains become shriveled with a floury discolored interior content.
Fig. (1B).
Symptomatological development of fusarium head blight in wheat. It commonly starts in the middle of glumes, rachis, or on the first floret and gradually spread within the head in upward and downward direction resulting in drying up of the spike with whitened or bleached ear head. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. Aetiological complexity
Head blight of wheat and barley is a complex disease. Several phytopathogenic fungal species are associated with this disease. The disease complex is associated with at least seventeen Fusarium species, and two Microdochium causal agents; F. graminearum, F. culmorum, and F. avenaceum, are the most pathogenic and widely distributed species in most infected areas worldwide. Among the less frequently encountered species are several others, which are less pathogenic or opportunistic. These include F. poae, F. cereal, F. equiseti, F. sporotrichioides, F. tricinctum, and, to a lesser extent, F. acuminatu, F. subglutinans, F. solani, F. oxysporum, F. verticillioides, F. semitectum, and F. proliferatum [32-34]. Of all FHB causing pathogens, total 16 species formally classified under Fusarium graminearum species complex (FGSC) based on the geographical distribution, production of trichothecene (type B) with slight changes in the degree of acetylation, hydroxylation and their biological activity in causing the disease. Multi-locus sequence typing approach is used for their identification. Among FGSC, F. graminearum sensu stricto is the most prevalent and has the cosmopolitan distribution along with other Fusarium sp. [35], whereas, the snow mould pathogen Microdochium nivale is more serious in various wheat-growing low temperature regime, viz., USA, Canada, and UK. In India, various Fusarium spp. like F. graminearum, F. avenaceum, F. compactum, F. equiseti, F. moniliforme, F. oxysporum, F. pallidoroseum, F. semitectum, F. solani, and F. subglutinans are found to be associated with this disease in different situations and in different locations [36-40]. But the most prevalent species are F. graminearum, F. oxysporum, F. pallidoroseum, and F. solani, but M. nivale (syn. Fusarium nivale) is not reported yet in India [2, 41, 42]. Compared to other Fusarium species, F. graminearum is highly prominent and pathogenic and is found to be associated with this disease in most of the occasions and spread all over the world [43]. Canadian wheat, including Alberta and Saskatchewan, almost infected by F. graminearum as the most abundant species than F. poae where Nerbaska is prominent with F. boothi [2]. Further, the FHB associated pathogen diversity from all over the world states that F. graminearum is more abundant, and infecting all most all cereals like wheat, maize, and barley (Fig. 2). Earlier, F. graminearum used to be minor species infecting the wheat but changing climate and adoption of new susceptible cultivars leading the minor Fusarium species to become major and shifting too many host plants [2]. The introduction of new species to the FGSC from the transportation of food grains become a serious impact as the F. asiaticum was earlier infecting to rice fields but introduced to wheat crop in North America [44]. There is a need to concern about the dynamics of mycotoxin produced from these pathogens, which can create a hunger strike for the human population under a climate change scenario [45]. In this filamentous fungus, physiologically and genetically distinct individuals of Fusarium spp. can fuse asexually by the process of the fungal anastomosis to form a stable heterokaryon. These heterokaryons produce genetically new haploid individuals through genetic recombination during the parasexual cycle. Thus, creating a huge genetic diversity within the population. This huge pathogenic diversity and aetiological complexity make the disease even more critical. Although the interaction between Fusarium species is mostly competitive [46]. Some positive interactions among FHB associated species have also been recorded during population surveys [47-49]. These synergistic interactions among Fusarium species enrich the aetiological complexity of the disease. The positive association among Fusarium species might be due to their adaptability in similar climatic conditions. In the present climate change scenario, the shift in Fusarium species’ composition on cereal grain is the main liming factor. Researchers predicted the change in the population dynamics of mycotoxins producing Fusarium sp. in Northern Europe by 2050 [50]. The wider adaptability, huge genetic variability, and more pathogenic complexity help FHB disease to establish easily in new climatic conditions and appear in epidemic form in the future. Therefore, global food security, as well as nutritional security, will be at higher risk.
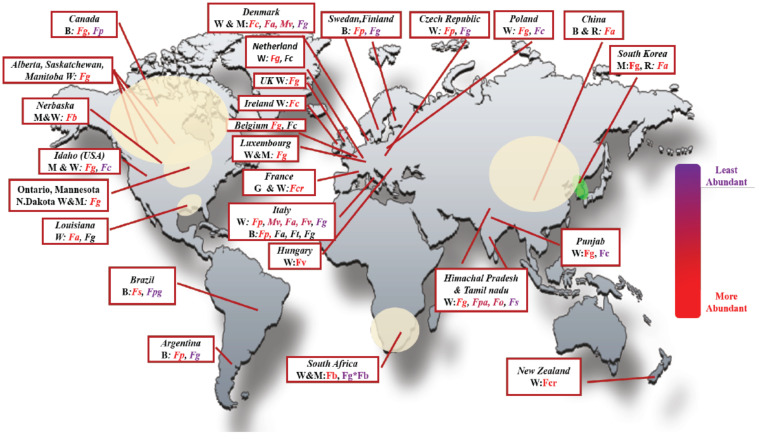
Fig. (2).
Worldwide distribution of Fusarium head blight associated pathogens. The predominance of F. graminearum from tropical to temperate climate is indicated. Fpa: F. pallidoroseum, Fg: F. graminearum, Fc: F. culmorum, Fo: F. oxysporum, Fe: F. equiseti, Fa: F. asiaticum, Fsa: Fusarium semitectum, Fcr: F. cortaderiae, Fb: F. boothii, Fv: F. vorosii, Fp: F. poae, Mv: M. nivale; W:Wheat, B: Barley, M: Maize, R: Rice. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. Pathogens’ biology and its survivability
Fusarium graminearum (Gibberella zeae) can occur both in a sexual and asexual forming the infected field. The disease cycle of FHB is initiated by the landing of airborne spores (ascospores and macroconidia) on flowering spikelets. Basically, the pathogen survives in soil or crop residues [51] as dormant mycelium, macroconidia, chlamydospores, and ascospores, which serve as the primary source of inoculum [52]. In the winter/spring season, when frequent precipitation occurs during the flowering stage, the ascospores or conidia are released from crop residues and get spread by wind and splashing water, which finally landed on the ear-head where they germinate and start infecting the floral parts [7]. In the initial phase, the fungus produces asexual spores (macroconidia) on sporodochia (asexual fruiting body) developed in infected floral parts. It bears slender, thick-walled, curved to straight, end tapered macroconidia (41-60 x 4.5-5.0 μm) with five to seven septa. The macroconidia are dispersed to the floral parts of healthy plants by rain-splash or wind where they germinate and start the disease by entering inside the plant tissues through degenerating anther or natural opening such as palea and lemma base. Later, pathogen starts developing globose, thick-walled asexual resting spores called chlamydospore. Chlamydospores are produced mainly from macroconidia but may also form from mycelia in soil and crop debris [51], which act as the survival structure for pathogen under unfavorable conditions. The pathogen also produces dark purple or black sexual fruiting body known as perithecia in its teleomorphic phase (G. zeae). It can survive for more than 16 months on corn and wheat residue under field conditions [14, 52].
The sexual stage of the fungus develops bluish-black perithecia on the infested plant debris when conditions are warm, humid, and wet. The sexual spores (ascospores) are forcibly discharged into the air from the surface of these residues. F. graminearum is a haploid organism belonging to phylum Ascomycota. In this phylum, sexual development begins with hyphal formation (with binucleate cells), which is a characteristic of Ascomycota. F. graminearum is homothallic (does not require a sexual partner to produce ascospores). This is due to the subsistence of genes in the haploid genome associated with Mat1-1 and Mat1-2 mating types in fungus. The binucleate cells of the fungus develop small-coiled cells, which are the fruiting body. The life cycle of F. graminearum takes two weeks in the laboratory. In the FHB disease epidemiology, the contribution of conidia against ascospores still remains unresolved [53, 54].
5. Events of pathogenesis
The FHB pathogen generally has a wide host range and infects small grain cereals like wheat, barley, oat, triticale, rye, corn, canary seed, and forage grasses [55]. Infection occurs primarily during anthesis, which is the most vulnerable phase [56, 57] and may continue until the soft dough stage [52]. Although open florets provide an opportunity for the pathogen entry and to come in contact at primary penetration sites [58], F. culmorum and F. graminearum produce surface-active molecules or hydrophobins [59]. The location of hydrophobins in F. graminearum spore surfaces and hyphal cell walls propose that they might support spore attachment, hence facilitating in hyphal invasion [26].
During pathogenesis events and whole infection process, FHB pathogens stay in the interplay between biotrophic and necrotrophic phase. During the initial stages of infection, the fungus grows intracellularly at the infection site and proliferates through the pith and xylem. This indicates the biotrophic lifestyle of F. graminearum [60]. Underneath the infection site, the F. graminearum spreads gradually and grows intracellularly, resulting in cell death (Fig. 3). As the necrosis of the floral tissue begins, the pathogen subsequently colonizes as necrotroph or hemibiotroph. But the exact route of infection is not clearly understood yet. However, several studies revealed that after deposition, spores germinate on the abaxial surface of the glumes or in the floral cavity the pathogen produces unbranched hyphae and penetrate either through natural openings such as stomata of the floral brackets like the glume, lemma, and palea [25, 53, 61-63] or penetrate directly by rupturing epidermal cell walls with short infection hyphae. Initially, it was thought that FHB pathogens do not form any kind of specialized infection structures like appressorium [61, 62, 64-69]. However, some recent evidence of lobed, highly septate, coralloid hyphal structures, compound, and lobate appressoria and infection cushions involved in the penetration process indicate towards complex penetration strategy of these fungi [58, 62, 63, 70]. Following the entry, they invade into the head by degrading the cells around their path [56, 61, 65] and finally reach to the ovary, where the hypha forms a dense mycelial network. After a short intercellular growth, they start developing both inter and intracellular hyphae to colonize throughout the ovary and floral brackets [62, 65]. Ultimately they contaminate the developing young grains by invading the parenchyma of the pericarp close to the embryo [71], disintegrating nuclei, the disappearance of cytoplasm and breaking of the cell wall [56]. In cases of severe infections, they also spread to the rachis and cortex [65, 72, 73], invade the phloem, chlorenchyma tissue of ear head, eventually leading to the death of parenchymal cells [74, 75]. Overall, tissue bleaching of infected heads is a characteristic symptom, and the blighting of the head is the ultimate outcome.
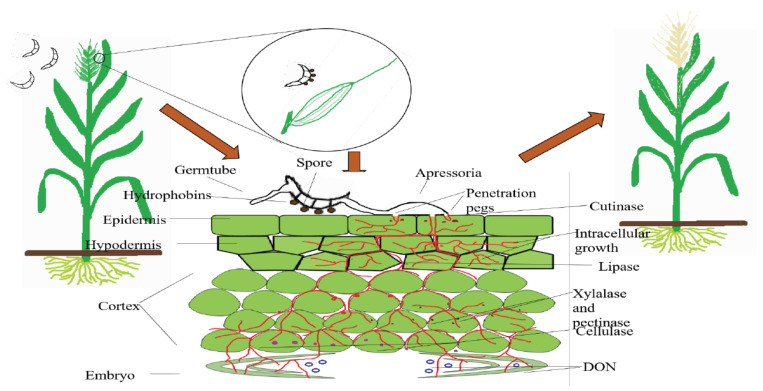
Fig. (3).
Biochemical basis of Fusarium-Wheat interaction in FHB. After landing of spores, the fungal hydrophobins support their attachment on the host surface and facilitate subsequent germination and hyphal invasion, followed by release of various extracellular enzymes, and toxins involved in the pathogenesis event. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
6. Biochemical consequences of disease development
Biochemical investigations of FHB disease are crucially important to understand pathogenesis events. Many studies have been carried out in this direction with more prevalent disease-carrying species F. graminearum and F. culmorum along with disease susceptible and resistant plant varieties. Biochemically, FHB pathogenesis can be divided for an investigation into two parts, i.e., first to understand the biomolecules involved in pathogenesis and infection of floret tissue and second to decipher the process of colonization and further spread of fungal infection with the aid of trichothecene production. The infection by Fusarium fungi on plants occurs through floret either passively through natural openings or actively by hypha penetration activity through the production of hydrolytic enzymes. So far, 24 different kinds of cell wall degrading enzymes have been identified which are involved in the complete digestion of plant cell wall during the pathogenesis events of F. graminearum which acts as an essential virulence factor whereas, mycotoxins produced during the circumstances are aggressiveness factor [65, 76]. During host penetration, the role of cutinases secreted by F. graminearum and F. culmorum is well accounted. After host penetration, lipases may have significant action for the sub-cuticular growth of F. graminearum [62]. Therefore, altogether these enzymes are an important pathogenicity factor. Other cell wall-degrading enzymes like cellulases, lipase, xylanases, and pectinases are also the important virulence determinants of F. avenaceum, F. culmorum, and F. graminearum for rapid colonization in wheat spikes at early stages of infection [61, 66, 77].
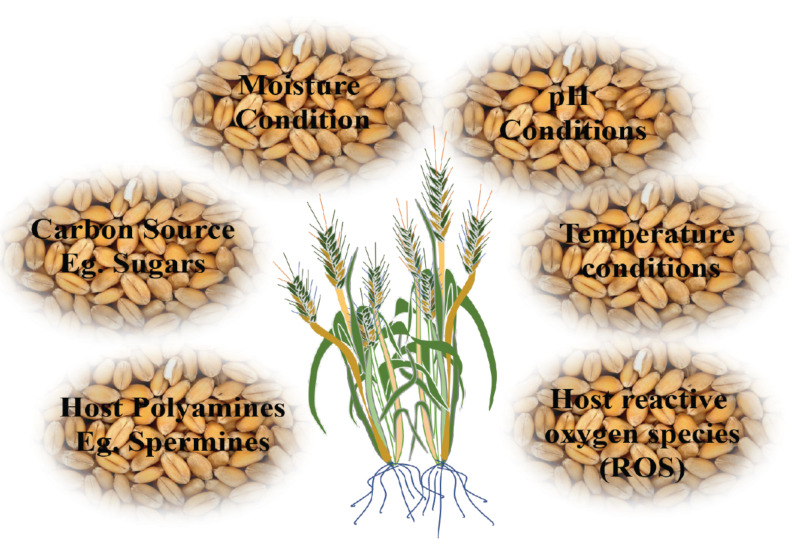
As per the different previous studies, the main feature of FHB disease is the production of trichothecene mycotoxins in developing floral parts of cereals. These mycotoxins are phytotoxic and necessarily essential for the infection, spread, and full aggressiveness of the pathogen [78]. One of the most important trichothecenes having toxicity for the animal system, including humans, is DON whose presence in grains is highly investigated in feed and food products before marketing and consumption. There are several factors reported to influence DON production viz., H2O2, sugars, pH conditions, host polyamines, and host reactive oxygen species (ROS) [79-81]. Several studies related to trichothecene production, especially DON, indicate the role of these mycotoxins in fungal colonization on rachis and progression of disease within and between plants [82]. After floral infection, the fungus F. graminearum expresses genes for DON biosynthesis. DON induces tissue necrosis and allows the F. graminearum to spread from florets into the rachis. This trichothecene is not an essential pathogenicity factor for the initial infection but acts as the essential virulence factor for the post-infection spread and symptom development. Due to DON accumulation and colonization, the developing seeds become wrinkled, shriveled, and small-sized hence referred to as ‘tombstones’ [54, 83]. The toxin production is also influenced by several host factors (Fig. 4), thus aggravating the disease development and pathogenicity.
Fig. (4).
Factors affecting the mycotoxin production by Fusarium sp. The mycotoxin (DON) acts as the aggressiveness factor that is essential for the post-infection spread and symptom development. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
7. Signalling pathways of host-pathogen interaction
The infection starts from the physical binding of fungal spore on floral tissue and subsequent recognition of signaling molecules by both counterparts, i.e., host and pathogen. It triggers complex biochemical and metabolic pathways both in plants and fungi. It is the basis of host-pathogen interaction resulting in resistance, semi-resistance, and susceptible outcome of plants disease expression. During host-pathogen interactions, the host plant tries to recognize the fungal intruder by employing a large number of pattern recognition receptors (PRRs). In the Fusarium-wheat pathosystem, plant chitin-binding proteins act as PRRs, which either directly or indirectly bind to the pathogen-associated molecular patterns (PAMPs) like chitin, chitosan, glucan, etc. and induce basal defense system by activating the MAPK pathways. In the early stage of infection, F. graminearum infected wheat spike express elevated levels of pathogenesis-related (PR) genes like chitinases, glucanases, thaumatin-like proteins, etc. [62, 84, 85]. The expression of PR proteins is always associated with the induction of salicylic acid (SA) signaling pathways [86, 87]. Salicylic acid is one of the key signaling molecules involved in the expression induction of PR1, NPR1, Pdf1.2, and PR4 genes that are involved in the regulation of plant resistance to diverse pathogens. This clearly indicates the role of SA in basal resistance to F. graminearum during the early stages of infection. There is also the evidence of activation of Jasmonic acid (JA) mediated defense pathways following F. graminearum infection in Arabidopsis thaliana and wheat, which runs parallel to SA signaling [84, 88]. Interestingly, a complex signal transduction pathway does not follow the conversational rule, and crosstalk between SA and JA mediated defense signaling pathways keeps fine-tuning itself in this pathosystem. This is evident from the overlapping expression of SA and JA pathways in maize against F. graminearum [89] and different phases of SA and JA pathways expression, i.e., induction of SA in very early stage followed by induction of JA in the later stage of F. graminearum infection in wheat [84]. The synergistic interaction between SA and JA signally pathways is a major determinant for basal resistance against head blight pathogen.
8. Phytoimmmunological system of host plant
After sensing the presence of Fusarium infection, the host plant starts remodeling its own defense system. Basically, cereal hosts express its resistance mechanism at different stages of infection, i.e. resistance to the initial infection (type I), resistance to spread and colonization of fungal hyphae within the host following infection (type II), resistance to kernel infection, (type III), host tolerance to infection (type IV), and resistance to toxin accumulation (type V) [90]. These different types of resistance to fungus development and trichothecenes accumulation are controlled by different genes [91]. The gene expression in Wangshuibai and its FHB susceptible mutant NAUH117 were analyzed at different infection stages of F. graminearum. Pathogen-related proteins like PR5, PR14, and ABC transporter and JA signaling pathways were important for FHB resistance, which is mediated by Fhb1. Ethylene pathway, calcium signaling pathway, reactive oxygen species, and nitric oxide pathway were found not to be activated in Wangshuibai and may not be crucial in defense to FHB. However, in Wangshuibai, twenty out of eighty-nine genes showed changed expression patterns upon the infection of F. graminearum [92]. Three types of heritable genetic resistance have been discussed viz., initial infection resistance, defense to pathogen spread, defense based on mycotoxin degradation ability [93].
The minimum specificity of Microdochium and Fusarium is due to polygenic resistance from genes with several degrees of dominance in a plant. Combinations of these genes, coupled with the environmental effects on these genes confer genetic resistance in the host plant [94]. The inheritance of resistance to FHB can be explained by the model called the dominance additive effect in which an essential factor is an additive effect. Resistance to wheat head blight is non-specific (horizontal) that provides resistance against all species of Microdochium and Fusarium. Thus, a given cultivar might be susceptible to FHB at the seedling stage but resistant at the adult stage or vice-versa. Following infection with FHB pathogens, the expression of host plant resistance genes varies with the wheat developmental stages. The peak level of toxin accumulation also depends on the cultivar grown [95]. It is worth mentioning that the induction period and response intensity to induced protective mechanism also determines the plant defense. Furthermore, tetraploid wheat species are found to be more resistant to FHB than diploid wheat species. The plant defense mechanism depends on the growth stage, air temperature, humidity, and genetic capability of the wheat cultivar [96]. However, no histological or macroscopical defense reaction of the host plant has ever been found on the outer side of glumes. Moreover, no anatomical, cellular, or histological characteristics have been found associated with susceptibility or resistance. This implies the existence of various mechanisms of resistance or defense in plants, both including active (physiological processes) and passive (morphological features) tolerance [91]. Finally, FHB resistance is designed to avoid fungal infection to floral parts and decrease mycotoxin contamination in the kernel, thereby ensuring food safety.
Re-enforcement of the host defense system is mediated by various structural and biochemical changes in the plant. Among structural changes, thickening of the cell wall, formation of cell wall appositions, papillae formation, and lignin deposition might be associated with a restricted influx of Fusarium toxins and efflux of host nutrients [60, 61]. The plant also possesses numerous biochemical compounds which can act as an active defense mechanism during initial infections and prevent pathogen entry. In cereals, the primary cell wall contains phenolic compounds like ferulic acid and p-coumaric acid [97], whereas the secondary cell wall is enriched with lignin layers, which are very recalcitrant plant compound and precludes pathogen invasion [98]. The phenolics act as cell wall reinforcers and antifungal molecules. During Fusarium infection, antifungal compounds and volatile organic chemicals are synthesized in plants by polyphenol oxidase and peroxidase enzymes. The peroxidases promote oxidation of phenols to polyphenol whereas polyphenol oxidases catalyse the oxidation of polyphenols to quinones and lignin’s. Therefore, enhanced activity of peroxidase and polyphenol oxidase was recorded in the F. graminearum infected wheat heads of resistance cultivars compared to susceptible ones [99]. In a study to find the effect of sea-weed extract on this disease, it was observed that the peroxidase and polyphenol oxidase activity was enhanced in wheat plant showing reduced severity of the phytopathogen [100]. Deposition of lignin and phenolics forms a mechanical barrier by modifying the primary and secondary cell walls that prevent activities of cell wall degrading enzymes. Siranidou et al. [101] observed higher lignification in the cell walls of F. graminearum infected resistant cultivar ‘Frontana’ compared to susceptible cultivar ‘Agent’ and concluded that phenolics were involved in resistance mechanism to FHB disease in wheat. Similarly, the role of antifungal proteins in cell wall re-enforcement of monocot plant is well established. Kang et al. [102] reported an enhanced accumulation of some antifungal proteins like thionins and hydroxyproline glycoproteins in FHB resistant cultivar ‘Arina’. In addition to that, some volatile compounds may also be directly or indirectly involved in the resistance mechanism against FHB in wheat. This can be evident from the production of 16 volatile organic compounds in greenhouse-grown wheat leaves namely: dimethylamine, 2-methyl-1-propanol, octanoic acid-ethyl ester, acetic acid, 2-ethyl-1-hexanol, nonanoic acid-ethyl ester, nonanol, N-ethyl-benzenamine, naphthalene, butylated hydroxytoluene, dimethoxy methane, phenol, 3-methyl-phenol, 3,4-dimethoxy-phenol, 2,4-bis (1,1-dimethyl ethyl)-phenol, and 1,4,7,10,13,16-hexaoxacyclooc- ta-decane [103]. Similarly, phytoanticipins also play an important role in the inhibition of growth of Fusarium pathogens [104]. Therefore, a strong correlation between accumulation of cyclic hydroxamic acids in wheat head and resistance response to FHB is proved by [105]. In this way, cereal hosts re-module their host defense system by certain structural and biochemical changes.
Plant defense mechanism also counteracts pathogen invasion through detoxification of toxic compounds produced by pathogens during host-pathogen interactions. Chemical modification and sequestering of toxins are key steps of the detoxification system [106]. Similarly, the conjugation process of chemical transformation of toxins to polar compounds like sulphates, amino acids, or sugars is indirectly involved in the resistance mechanism of plants [107]. Distinct metabolic transformation rates of 14C DON in the FHB resistant wheat cultivar ‘Frontana’ (18%) compared to susceptible cultivar ‘Casavant’ (5%) were observed [108].
Metabolic profiling of resistant and susceptible genotypes was performed by several research groups. [Bollina et al. [109] and Kumaraswamy et al. [110] found a high level of resistance-related metabolites in four resistant barley genotypes and suggested their association with antimicrobial, signaling and cell wall re-enforcement properties. Association of resistance-related metabolites with a resistance mechanism of FHB can serve as potential biomarkers. Similarly, Kumaraswamy et al. [110] identified a significantly higher level of constitutive and induced resistance-related metabolites in resistant barley genotypes compared to susceptible ones and suggested that they belonged to different chemical groups such as linolenic acid, p-cumaric, sinapic acid, naringenin, kaempferol glucoside, and catechol glucoside. A proteomics approach to find out the protein molecules that are involved in the pathogenicity and resistance of host-plants would also be very useful for a better understanding of processes underlying during interaction of FHB pathogen and host plant [111]. This information will help us to understand and re-program highly complex structural, biochemical, and molecular aspects of the defense network system of cereals against Fusarium pathogen.
9. Remodeling Host Defense
9.1. Conventional and Molecular Breeding Strategies
Disease management through host resistance is economically the cheapest and environmentally safe approach. Fungicides may not be the best option to reduce the threat of mycotoxin contamination in cereal food and feed. Therefore, growing resistant cultivars is always the best option to manage FHB disease. Resistance to FHB in wheat and barley is quantitative, and its expression is dependent on the genetic background of germplasm [112], which becomes the main constrains for the breeding programs. Due to poor genetic background [113] and less variance in breeding materials [114, 115], getting success in resistance breeding to FHB is a herculean task. In order to achieve that collection and characterization of several germplasms for the development of mini-core against biotic stress would be the best way to enrich the source of resistance for combating FHB. According to the Food and Agriculture Organization (FAO), more than 70 lakh plant accessions are held worldwide in national and international gene banks. Wheat stands first [61, 68, 85], and barley [40, 49, 77] stand third in term of the number of accessions stored in gene banks [116]. This large collection of wheat and barley possesses so much diversity with many of them pertaining to biotic stress, and the collection continues to increase in size. Such a huge collection hinders their proper characterization and subsequent utilization; hence using a core and mini-core size concept, we can reduce the collection size upto 5-10% without affecting the gene diversity [117]. Recently, core of wheat and barley has been developed by different national and international collections. Further, this core can be utilized for screening of germplasm related to biotic chiefly for FHB disease. Identification of the novel QTL, QFhb-5A distributed within a widespread wheat core collection containing 229 accessions (including germplasm, cultivars, landraces) from different countries signify the importance of core and minicore, and facilitates easy selection of the germplasm containing both QTLs, Fhb1 and QFhb -5A for better FHB resistance [118].
The major goal of the breeding program is to develop genotypes through the introgression of FHB resistance genes from different wild sources, followed by selection for desirable agronomic traits. The availability of molecular markers for FHB resistance speeds up the selection process more rapidly with a few generations earlier and on a relatively large scale. This minimizes the chance of leaving out an elite line with improved resistance traits to FHB disease. Molecular markers allow fast screening of FHB resistance before testing them for all agronomic characters in the field. Marker-assisted selection of the germplasms with FHB resistance can be employed for the successful breeding program. Recently, it has been reported that wheat cultivars bred in west Japan displayed high resistance to FHB worldwide. From these germplasms, elite genotypes can be produced, and introgression efficiency of favorable genes would increase with the help of molecular markers [119]. Therefore, our main aim should be screening and evaluating the FHB resistance in an existing germplasm collection of wheat, barley, and other small grain cereals [10]. All the intensive pursuits to find completely resistant germplasm against FHB are still unsuccessful, as only few resistance sources identified from China (Sumai 3, Wangshuibai, Hongheshang, Yangmai158, and Baishanyuehuang, Yangangfangzhu), Italy (Funo), Japan (Nobeokabozu, and Nyu Bai), Brazil (Frontana) carrying the resistance gene, and recognized to be the desirable resource for resistant breeding programs [120, 121]. Earlier, Sumai 3 carrying the resistant gene Fhb1 was only the available donor used all over the world in breeding program due to its highest level of resistance against FHB disease [120]. Later, few more released cultivars, viz., Yangmai 30, Ningmai 26 or Shengxuan 6, Ning 8331, Pioneer 25R18, Pioneer 25R42 and Pioneer 25R51containing the Fhb1 resistance gene were developed as the resistant donor apart from Sumai 3 [122]. Even, Ningmai 9 containing the Fhb1 gene shown better results in breeding program and further utilized as the potential donar which resulted in the development of resistant cultivars i.e. Ningmai 13, Ningmai 1, Yangmai 21, and Zhenmai 5 [122].
More recently, marker-assisted selection has also been implemented due to the availability of reliable, co-dominant, closely linked, and cost-effective molecular markers that enhances the efficacy and performance of FHB breeding programs. Genotyping of mapping populations using several DNA markers facilitates in locating the position of FHB resistance genes/Quantitative Trait Loci (QTLs) in the wheat germplasm across the globe. Fine mapping leads the identification of more than 100 QTLs associated with head blight resistance from various resistant donar, viz., Sumai 3 (Fhb1, Fhb2, Rht1, Qfha.ifa-5Ac, Qfha.ifa.5AS, Qfhs.ndsu-3BS), Wangshuibai (Fhb1, Fhb2, Fhb4 Fhb5, Qfhi.nau-4B), Canas (Qfhs.lfl-1BL), Ning 8026 (Fhb2), W14 (Fhb1, Qfhs.nau-2DL), Triticum dicoccoides (Qfhs.ndsu-3A), Nanda 2419 (Qfh.nau-2B), Shanghai-3/Catbird (Rht-B1b), Alsen (Fhb1, Fhb2), Baishanyuehuang (Qfhb.hwwg-3BSc, Qfhb.hwwg-5A), Ningmai 9 (Fhb1, QFhb -3B.1), Yangmai 158 (QFhb -5A), Frontana (QTL on chromosome 3A, 5A, 7A), Freedom (on 2A chromosome), Ernie (on 3B, 4B, and 5A), Dream (Chromosome 2BL, 6AL, 7BS), as well as other wild wheat relatives to enrich our existing germplasm collection and their utilization [8, 114, 117, 121-124]. The map based positional cloning of few QTLs, viz., Fhb1, QFhb.mgb-2A helps to decipher their sequence and function. The QTL Fhb1 of Sumai 3 contains a pore-forming toxin-like (PFT) gene which encodes a chimeric lectin with two agglutinin domains and a pore-forming toxin-like domain to confer resistance against FHB [125]. Silimarly, QFhb.mgb-2A locus contains a wall-associated receptor-like kinase (WAK2) gene that acts as a cell wall receptors. In the presence of the fungal pathogens, it activates the intracellular signaling to trigger the negative regulation of the pectin methylesterase (PME) genes, subsequent methylation of cell wall pectin that makes the wall more rigid to fungal penetration [126].
Most of QTLs in wheat confers type II resistance, i.e., limiting fungal spread from the site of infection [112]. The best FHB resistance QTL, Fhb1 can show disease reduction upto 50%, but on average, about 20-25% disease reduction occurs depending upon the genetic background [127]. Novel resistant germplasm evaluation and QTL analysis have been a priority in resistance breeding to minimize yield and quality losses due to FHB disease and delay the possible epidemic. Therefore, combining major QTLs with other traits would be the best approach for enhancing host resistance against this disease.
Mapping of the chromosomal region involved in resistance to FHB has also been a high priority in barley growing areas of the world to minimize disease severity. Several studies with QTL mapping using different resistance or susceptible sources have identified a few genomic stretches that can be useful for barley resistance breeding programs. Numerous studies have also reported for QTL mapping of FHB severity, DON accumulation, and morphological traits in barley. However, very few studies have been carried out to validate QTL identified for FHB resistance. The linkage of morphological traits with FHB resistance loci provides the advantage of morphological marker-assisted selection for resistance breeding in barely. Additionally, molecular markers linked to major QTL in different genetic backgrounds resistance to FHB can be useful in marker-assisted breeding in barley. A combination of molecular and morphological markers can also help in the selection of desirable progenies in barley FHB resistance breeding programs.
9.2. Transgenic Strategies
In addition to classical and molecular breeding methods, transgenic approaches have also been used by various researchers around the world to hasten FHB resistance breeding programs in cereals. It might be due to missing good resistance source is missing or breeding for a polygenic trait is very challenging. In such cases, the insertion of antifungal and antitoxin genes through the process of genetic transformation has the potential to develop resistant wheat and barley [128]. For the development of transgenic wheat and barley, selection and transformation of transgenes derived from either host-plant or non-host plant origin are of paramount significance. Although the genetic transformation of wheat and barley is quite difficult, there are various reports suggesting overexpression of defense response genes like chitinases, β-1,3-glucanase, α-1-purothionin, thaumatin-like protein 1, RIPs (Ribosome-Inactivating Proteins), lipid transfer protein gene(TaLTP5/PR14), permatin (oat), hordothionin (barley), mammalian lactoferrin gene. The transgenics exhibit a high level of resistance to Fusarium infection and toxin accumulation in wheat and barley under the greenhouse condition [129-132], but most are unsuccessful in the field [130]. Thus, these FHB transgenics are yet to be released on a commercial scale.
Targeting regulatory genes that control the expression of multiple defense genes involved in signaling pathways offers an alternative strategy for controlling plant diseases. It can provide a broad-spectrum and durable resistance against pathogens [133], which can be achieved either by the application of synthetic salicylic acid or salicylic acid analogs (benzothiadiazole), overexpression of At-NPR1 in transgenic wheat which would embellish the basal defense [134, 135]. Expression of Fusarium-specific antibodies in wheat plants is also another mechanism that can be utilized to restrict the effect of FHB on wheat as the pathogen-specific antibodies showed to significantly enhance the resistance in T2 and T3 generation of wheat plants against F. asiaticum. In addition to this (type II resistance), type I resistance in the transgenic wheat also remarkably increased when the inoculants were further sprayed on the plants [136].
Apart from inserting foreign genes, the interference of pathogens biology via RNAi also holds a great promise for managing the disease. RNAi is highly useful for the regulation of protein-coding genes at the transcriptional and post-transcriptional levels [137]. This technology is used to modify the host defense via Host Induced Gene Silencing (HIGS). HIGS is carried out through the integration of fungal transgene in the antisense or hairpin orientation and their expression in planta for silencing the fungal genes during the infection process (Table 1). The efficiency of the HIGS strategy depends on the production of small interfering RNA molecules that are complementary to targeted fungal genes (Fig. 5). Being a transgenic strategy, it is a very cumbersome, costly, and time-consuming process. Further, biosafety and other regulatory issues restrict its widescale field applicability [138]. Thus, various non-transgenic approaches are prioritized for their future perspective.
Table 1.
RNA interference mediated defense against fusarium head bight.
| Strategy | Pathogen | Targeted Gene | Gene Function | Impairment of Function | Host Response | References |
| RNAi via HIGS (transgenic) |
F. graminearum | Protein kinase genes (Fg00677, Fg08731) |
Encode alpha catalytic subunit of casein kinase 2 (CK2) and casein kinase 1 (CK1), which are necessary for infection in wheat spikes | Signal pathway during plant-pathogen interaction | Enhanced resistance in Brachypodium | [137] |
| F. graminearum | Cytochrome P450 lano-sterol C14-α-demethylase encoding genes (FgCYP51A, FgCYP 51B, FgCYP 51C) | Encode 14-α demethylase enzyme for the biosynthesis of ergosterol | Affect the fungal cell membrane integrity | Enhanced resistance in Arabidopsis, barley, Brachypodium | [137, 140] | |
| F. graminearum | FgPKS12 | Encode polyketide synthatase | Hamper the pigment biosynthesis via polyketide pathway | In-vitro | [141] | |
| F. graminearum | FgTRI6 transcription factor | Regulates DON biosynthesis | Reduces the toxin production and pathogenesis | Reduced spike infection in wheat and barley | [142] | |
| F. graminearum |
Chs3b (Chitin synthase 3b) |
Chitin biosynthesis and cell wall formation | Affect disease development and virulence | Reduced virulence of FHB in wheat | [143] | |
| F. culmorum | Fgl1(Secreted lipase) | Plant cell wall degradation | Affect infection process | Moderated symptom reduction in wheat | [144] | |
| F. culmorum |
Fmk1 (MAP kinase) |
Signal transduction in pathogen | Affect pathogenicity event | Moderated symptom reduction in wheat | [144] | |
| F. culmorum |
FcGls1 (glucan synthase 1) |
Role in 1,3- Beta glucan synthesis | hyphal cell wall defects | Stronger symptom reduction in wheat | [144] | |
| F. culmorum | TaArf2 (auxin response factor 2-like protein) | Auxin biosynthesis | Impair the host defense | Significant reduction in disease symptoms | [144] | |
| RNAi via SIGS (Exogenous application) |
F. graminearum |
DICER genes (FgDCL1 and FgDCL2) |
Contribute to vegetative growth and disease progress | - | Barley disease resistance | [145] |
| F. graminearum | ARGONAUTE genes (FgAGO1, FgAGO2) |
Contribute to vegetative growth and disease progress | - | Barley disease resistance | [145] | |
| F. graminearum | Cytochrome P450 lanosterol C-14𝛼-demethylase (FgCYP51) |
Biosynthesis of fungal ergosterol | Inhibits fungal growth in the infected tissue | Resistance in Arabidopsis, Barley, Wheat | [146, 147] |
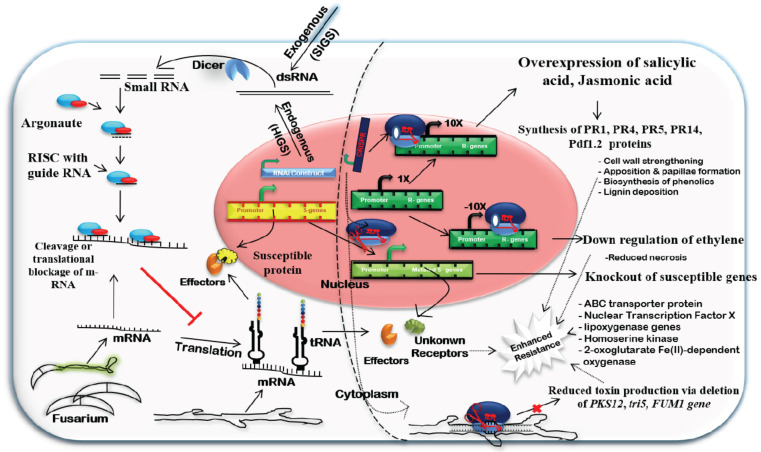
Fig. (5).
Remodelling host defence through RNA interference and CRISPR interference. Application of RNAi applied through HIGS or SIGS leads to the knocking down of the expression of pathogenicity genes in Fusarium. Whereas the CRISPR tool can be used either to knock down the host genes as well as pathogen genes or to regulate the transcriptional expression of various host defense genes to promote the durable host defense. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
9.3. Non-transgenic Genome Engineering Strategies
The recent technological advancement in the area of gene silencing introduces the products based on spraying techniques, which will regulate the silencing mechanism, which ultimately ousting the pesticide industries. The use of double-stranded RNA molecules, i.e., hairpin RNA (hpRNA), synthetic double-stranded RNA (dsRNA), and short RNA (sRNA), can be commercially produced and applied exogenously through spraying to induced gene silencing (SIGS) (Fig. 5) [139]. The advantage of SIGS based RNAi over other technology is to develop resistance source without converting the plant material to transgenic [139]. The SIGS approach is well suited for managing the FHB disease and reducing the mycotoxin contamination (Table 1).
Nowadays, the focus is shifted from gene knockdown to knockout strategy to design host defense desirably. With the rapid evolution of genome editing technology such as CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)/ CAS9, TALEN, it becomes plausible to edit mono or multi-host genes simultaneously leading to knockout of trait [148, 149]. Because of easy operation, handling, and efficient results, they are popularised in various research activities to make plant resistance from harmful pathogens and their arms races. Gene knockout will be done at the specific site of double-strand breaks (DSB) due to insertion or deletion (InDels) derived by the CAS9 endonuclease activity [150]. Utilization of genome editing/ CRISPR in the field of plant disease management evolved at a much faster rate due to the occurrence of minor diseases becoming major due to the impact of climate change, breakdown of resistance, and with the identification of susceptible genes in the host plants through omics approach. Genome editing can be targeted in the host as well as a pathogen to regulate the pest/pathogen population. Validated pieces of evidence are available for CRISPR/CAS9 genome editing for plant disease resistance against fungi, bacteria, and viruses in the targeted host such as rice (Oryza sativa) editing the specific genes leads to enhancement of defense/resistance response, OsMPK5, OsERF922 and OsSEC3A-Magnaporthe oryzae [150-152]. Similar works reported in tomato (Solanum lycopersicum), targeted deletion of SIDMR6-1/2, increased the levels of salicylic acids, Citrus sinensis – CsWRKY22 targeted deletion triggered immunity against canker [153]. Further targeted deletion of susceptible genes in rice OsSWEET11,13,14 against Xanthomonas oryzae pv oryzae and M. oryzae [154] and TaMlo-A1 and TaEDR1 against powdery mildew in
wheat improved the resistance response in wheat [155]. A similar kind of genome editing can be done in phytopathogens, where it leads to being less efficient strain compared to wild types. Several studies had shown the comparative results of CRISPR/CAS9 editing in fungal pathogens, i.e., Alternaria alternata deletion of pyrG and URA5 in F. oxysporum restrict their ability to grow in the medium [156]. The fungal pathogens produce pigmentation linked to virulence property. But, targeted editing the polyketide synthase gene Sspks12 (Sclerotinia sclerotiorum) [157] BIK1 (F. oxysporum) [158] and SCD1 (Colletotrichum sansevieriae) [159] resulting the lack of melanin biosynthesis in the fungal mycelium affected the pathogenesis nature of fungal pathogens. Establishment of such successful technology was also made in FHB disease infecting the host plant where genome editing with the help of polycistronic tRNA (PTG) or CAS9 protein of lipoxygenase (TaLpx-1) developed resistance response against FHB disease mediated the production of JA synthesis leading the suppression of salicylic acid defense response in wheat [160]. Genome editing the toxin-producing gene also harbor the ability of a pathogen to infect the host plant where deletion of Lox3 in FHB affected the conidia production and toxin synthesis. However, the deletion of tri5 and tri6 in F. graminearum reduced the disease incidence in wheat and maize [161] whereas, the deletion of map1leads to reduced perithecia formation and mycotoxin production [162].
The resistance gene FHB1 governs the highest level of resistance in wheat against FHB were, TaHRC-encoding the histidine calcium-rich protein candidate for FHB1, but the TaHRC also present in susceptible cultivar (TaHRC-S) it might likely to be active as the susceptible gene for FHB [163]. The knockdown of TaHRC-S improved the resistance response against FHb under the climate change scenario [163]. The protein BAX-inhibitor (BI-1) known for cell death suppression can also act as a susceptible gene against FHB and stripe rust also to various necrotrophs (Botrytis cinerea) [164]. In addition, mutation of WheatPME-1 (pectin methylesterase) gene leads the production of highly methylated cell wall pectin, and prevents the fungal penetration [126]. Thus, would be a promising target. A recent study on enhancement of resistance response against FHB in barley identified two important susceptible genes ethylene insensitive 2 (EIN2) and 2-oxoglutarate Fe(II)-dependent oxygenase (2OGO) is a plant immunity suppressor [164]. The targeted deletion of EIN2 hijacks the ethylene synthesis pathway, and the plant becomes susceptible [164]. Similarly, targeted deletion of TansLTP9.4, TaABCC6, and TaNFXL1enhanced the defense response against FHB in wheat [165]. AlsoOs1 (Histidine Kinase1) deletion shows a reduction in disease [166].
Similarly, genome editing in virulent strain ITEM124 of F. graminearum containing the polyketide synthase gene (PKS12) reduced the growth and sporulation rate in medium containing hygromycin [148] which state the ability of technology to edit/manipulate at the genome level benefits the host plants to withstand the pathogen stress at the minimal levels instead of applying the chemicals for disease management. FUM1 is polyketide synthase gene necessary for fumonisin synthesis that was knockout through CRISPR-CAS9 affected the toxin synthesis in pathogen [167]. Thus, CRISPR interference of both susceptible genes of the host as well as pathogenicity genes of pathogens makes it feasible to remodel host defense via non-transgenic strategy (Fig. 5) and exemplified in many circumstances (Table 2).
Table 2.
CRISPR interference mediated defense against fusarium head bight.
| Strategy | Organism | Targeted Genes | Gene Function | Phenotypic Changes | References |
| CRISPRi of host gene (Gene knockout) | Wheat | ABC transporter gene (TaABCC6), | Encode DON-induced ABC transporter protein associated with FHB susceptibility in wheat | Expected higher resistance response against FHB | [168] |
| Wheat | Nuclear Transcription Factor X box-binding-Like 1 (TaNFXL1) | Susceptible gene for FHB disease induced by DON | Expected higher resistance response against FHB | [168] | |
| Wheat | TansLTP9.4, a type 1 Non-specific Lipid Transfer Protein (nsLTP) | Contribute to resistance to FHB disease | Expected increase in disease susceptible | [168] | |
| Wheat | Lipoxygenase genes (TaLpx1, TaLox2) | Induces the jasmonic acid-mediated defence signaling pathways | Expected higher resistance response against FHB | [158, 169, 170] | |
| Arabidopsis, Barley | Homoserine kinase (HSK) | Key enzyme for the biosynthesis of Threonine, Methionine | Enhanced disease resistance against FHB via accumulation of homoserine | [171] | |
| Arabidopsis, Barley | 2-oxoglutarate Fe(II)-dependent oxygenase (2OGO) | Host metabolism | Slow FHB disease development | [171] | |
| Arabidopsis, Barley | Ethylene insensitive 2 (EIN2) | Ethylene signaling | Slow FHB disease development | [171] | |
| CRISPRi of pathogen gene (Gene knockout) | F. graminearum | Histidine kinase 1 (Fgos1) | Encode histidine kinase for amino acid metabolism | Minimal impact on fungal growth, but leads to the fungicide tolerance | [166] |
| F. graminearum | Trichodiene synthase (tri5) | Involve in trichothecene biosynthesis | - | [166] | |
| F. graminearum | Polyketide-synthase (PKS12) | Toxin production and sporulation via Polyketide synthesis pathway | Slow growth and reduced sporulation | [148] | |
| F. proliferatum | Polyketide synthase gene (FUM1) | Fumonisin biosynthesis | - | [167] |
10. Challenges and Future Prospects
Although a lot of research is already carried out in different aspects of FHB, still the disease is very difficult to manage in the field. To tackle this challenge, we must focus on disease prediction and its management aspects mainly. Several prediction-based models are available linking various
climatic factors to disease development. There is a need to develop models predicting climate-mycotoxins relationship, which would enable farmers and buyers to anticipate future mycotoxin contamination incidents and fungal disease occurrences. This would allow for both long and short-term planning of mycotoxin control ensuring economic stability as well as safe food and feed supply. The use of simulation models for predicting future climate scenarios would provide a strategic option about climatic conditions that can be tested in a laboratory. Use of existing data and performing research to provide new data on how climatic variations in the future will influence the associated insect herbivory, fungal population, and ultimately mycotoxin concentration is very difficult owing to the regular changes. Development and validation of models that can predict DON, aflatoxin, and fumonisin levels under future climatic scenarios would be of practical importance. Additionally, they can help to determine the impacts of climate change on future food production and security in terms of mycotoxin related health and economic risks. Furthermore, it is important to study the life cycle, mycotoxin production, genetic structure, and population dynamics of FHB pathogen. In this climate change scenario, our research program should also be directed to predict the changes in aetiological complexity, genomic structure, and. population dynamics of the pathogen. There is an urgent need to keep continuous vigilance on the occurrence, distribution. and the spread of this disease by surveying the vulnerable areas. A strict regulatory approach should be enforced to prevent the entry and establishment of a new variant of Fusarium spp. in a new climatic zone. Moreover, the lack of suitable source against FHB is one of the main limiting factors. For that, we also must be focused on the enhancement of host resistance. The transgenic approaches viz., RNAi via HIGS are very popular in this regard, but due to some biosafety and ethical issues, the technologies are lagging. To solve this issue, RNAi mediated through SIGS is very popularized, but the durability and large-scale applicability in the field is debatable. Thus, a paradigm shift from transgenic to non-transgenic is focused. Newly evolved genome editing tools like TALEN, CAS-9 are employed to eliminate the host susceptible genes and enrich the resistance for achieving strong host defense. Further, they can also be exploited to regulate the host defense against multiple pathogens. Along with these, the synthetic gene circuit can also be introduced as part of a system biology approach to synthesize host defense mechanisms. These noble technological advancements will strengthen the host system to combat this disease.
Conclusion
FHB is one of the most threatening diseases of wheat, barley, and other cereals globally. The major factors contributing to its epidemics are cultivation practices, extensive cultivation of susceptible cultivars, and favorable environment during flowering and grain filling period. The occasional occurrence of FHB has led to another issue for the breeders to implement the breeding program based on natural infection, which is not always possible. There is a need for immediate attention for wheat disease resistance, whether it is accomplished by a transgenic approach, conventional breeding, molecular marker, or a combination of these approaches. Disease forecasting models can also help the grain producers to evaluate the risk of disease outbreak and spread. Integrated management of Fusarium head blight could be an important part of disease management, which incorporates various approaches together synergistically. Higher genetic disease resistance levels will pledge lower risks of crop loss to the farmers and might be helpful to solve crop production associated problems such as reduced grain size, mycotoxin contamination, and yield reduction. Therefore, there is a need to be prepared against this potential threat that may destroy our global food security system.
Acknowledgements
The authors acknowledge Dr. Kapil K Tiwari, Dr. Amit Verma, Dr. N.K. Singh, Mr. L. Maharaja for helpful discussions, guidance and providing symptoms pictures during the preparation of this manuscript.
Consent for Publication
Not applicable.
Funding
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Dean R., Van Kan J.A., Pretorius Z.A., Hammond-Kosack K.E., Di Pietro A., Spanu P.D., Rudd J.J., Dickman M., Kahmann R., Ellis J., Foster G.D. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012;13(4):414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valverde-Bogantes E., Bianchini A., Herr J.R., Rose D.J., Wegulo S.N., Hallen-Adams H.E. Recent population changes of Fusarium head blight pathogens: drivers and implications. Can. J. Plant Pathol. 2019;1-5 doi: 10.1080/07060661.2019.1680442. [DOI] [Google Scholar]
- 3.Stack R.W., Leonard K., Bushnell W. History of Fusarium head blight with emphasis on North America. In: Leonard K.J., Bushnell W.R., editors. Fusarium head blight of wheat and barley. The American Phytopathological Society; 2003. pp. 1–34. [Google Scholar]
- 4.McMullen M., Jones R., Gallenberg D. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis. 1997;81(12):1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- 5.Wegulo S.N., Bockus W.W., Nopsa J.F., Peiris K.H., Dowell F.E. Integration of fungicide application and cultivar resistance to manage Fusarium head blight in wheat. In: Nita M., editor. Fungicides- Showcases of Integrated Plant Disease Management from Around the World. Rijeka, Croatia: Intech; 2013. pp. 35–54. [Google Scholar]
- 6.Saulescu N.N. An evaluation of present wheat breeding priorities for Romania. Lucrãri Stiintifice USAB. 1993;24(1):39–47. [Google Scholar]
- 7.Parry D.W., Jenkinson P. McLeod. Fusarium ear blight (scab) in small grain cereals-a review. Plant Pathol. 1995;44:207–238. doi: 10.1111/j.1365-3059.1995.tb02773.x. [DOI] [Google Scholar]
- 8.Gilbert J., Tekauz A. Recent developments in research on Fusarium head blight of wheat in Canada. Can. J. Plant Pathol. 2000;22(1):1–8. doi: 10.1080/07060660009501155. [DOI] [Google Scholar]
- 9.Russell C.T., Luhmann J.G., Jian L.K. How unprecedented a solar minimum? Rev. Geophys. 2010;48(2):1–16. doi: 10.1029/2009RG000316. [DOI] [Google Scholar]
- 10.Kubo K., Kawada N., Fujita M. Evaluation of Fusarium head blight resistance in wheat and the development of a new variety by integrating type I and II resistance. Japan Agri. Res. Quarterly: JARQ. 2013;47(1):9–19. doi: 10.6090/jarq.47.9. [DOI] [Google Scholar]
- 11.Magan N., Medina A., Aldred D. Possible climate‐change effects on mycotoxin contamination of food crops pre‐and postharvest. Plant Pathol. 2011;60(1):150–163. doi: 10.1111/j.1365-3059.2010.02412.x. [DOI] [Google Scholar]
- 12.Goswami R.S., Kistler H.C. Heading for disaster: Fusarium graminearum on cereal crops. . Mol. Plant Pathol. 2004;5(6):515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 13.Smiley R.W., Gourlie J.A., Easley S.A., Patterson L.M., Whittaker R.G. Crop damage estimates for crown rot of wheat and barley in the Pacific Northwest. Plant Dis. 2005;89(6):595–604. doi: 10.1094/PD-89-0595. [DOI] [PubMed] [Google Scholar]
- 14.Windels C.E. Economic and social impacts of fusarium head blight: changing farms and rural communities in the northern great plains. Phytopathology. 2000;90(1):17–21. doi: 10.1094/PHYTO.2000.90.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Saharan M.S., Kumar J., Sharma A.K., Nagarajan S. Fusarium head blight (FHB) or head scab of wheat- a review. Proc. Nat. Acad. Sci. India. 2004;3:255–268. [Google Scholar]
- 16.Bagga P.S., Saharan M.S. 2005. [Google Scholar]
- 17.Bagga P. Fusarium head blight (FHB) of wheat: role of host resistance, wheat aphids, insecticide and strobilurin fungicide in disease control in Punjab, India. Cereal Res. Commun. 2008;36(Suppl. 6):667–670. doi: 10.1556/CRC.36.2008.Suppl.B.57. [DOI] [Google Scholar]
- 18.Hornick S.B. Factors affecting the nutritional quality of crops. Am. J. Altern. Agric. 1992;7(1-2):63–68. doi: 10.1017/S0889189300004471. [DOI] [Google Scholar]
- 19.Schaafsma A.W., Ilinic L.T., Miller J.D., Hooker D.C. Agronomic considerations for reducing deoxynivalenol in wheat grain. Can. J. Plant Pathol. 2001;23(3):279–285. doi: 10.1080/07060660109506941. [DOI] [Google Scholar]
- 20.Teli B., Chattopadhyay A., Meena S.C., Gangwar G.P., Pandey S.K. Present status of Fusarium head blight of wheat and barley in India. In: Vaish S.S., editor. Diseases of wheat and their management. New Delhi: Astral International; 2016. pp. 79–92. [Google Scholar]
- 21.Ma Z., Xie Q., Li G., Jia H., Zhou J., Kong Z., Li N., Yuan Y. Germplasms, genetics and genomics for better control of disastrous wheat Fusarium head blight. Theor. Appl. Genet. 2020;133(5):1541–1568. doi: 10.1007/s00122-019-03525-8. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Espinoza A.D., Youmans J., Vermeer C.B., Buck J.W. Evaluation of fungicides for control of stripe rust and Fusarium head blight of winter wheat in southwest Georgia, 2015. Plant Disease Management Reports PDMR. 2016;10:CF034. [Google Scholar]
- 23.Singh D.P. Wheat blast—a new challenge to wheat production in South Asia. Indian Phytopathol. 2017;70(2):169–177. doi: 10.24838/ip.2017.v70.i2.70609. [DOI] [Google Scholar]
- 24.Del Ponte E.M., Valent B., Bergstrom G.C. A special issue on Fusarium head blight and wheat blast. Trop. Plant Pathol. 2017;42(3):143–145. doi: 10.1007/s40858-017-0166-0. [DOI] [Google Scholar]
- 25.Bushnell W.R., Hazen B.E., Pritsch C., Leonard K. 2003. [Google Scholar]
- 26.Walter S., Nicholson P., Doohan F.M. Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 2010;185(1):54–66. doi: 10.1111/j.1469-8137.2009.03041.x. [DOI] [PubMed] [Google Scholar]
- 27.Wise K., Woloshuk C., Freije A. Fusarium Head Blight (Head Scab). Diseases of Wheat. C. Woloshunk. West Layfayette, Indiana: Purdue Extension; 2015. [Google Scholar]
- 28.Freije A.N., Wise K.A. Impact of Fusarium graminearum inoculum availability and fungicide application timing on Fusarium head blight in wheat. . Crop Prot. 2015;77:139–147. doi: 10.1016/j.cropro.2015.07.016. [DOI] [Google Scholar]
- 29.Islam M.T., Croll D., Gladieux P., Soanes D.M., Persoons A., Bhattacharjee P., Hossain M.S., Gupta D.R., Rahman M.M., Mahboob M.G., Cook N., Salam M.U., Surovy M.Z., Sancho V.B., Maciel J.L. NhaniJúnior, A.; Castroagudín, V.L.; Reges, J.T.; Ceresini, P.C.; Ravel, S.; Kellner, R.; Fournier, E.; Tharreau, D.; Lebrun, M.H.; McDonald, B.A.; Stitt, T.; Swan, D.; Talbot, N.J.; Saunders, D.G.; Win, J.; Kamoun, S. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol. 2016;14(1):84. doi: 10.1186/s12915-016-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta D.R., Avila C.S.R., Win J., Soanes D.M., Ryder L.S., Croll D., Bhattacharjee P., Hossain M.S., Mahmud N.U., Mehebub M.S., Surovy M.Z., Rahman M.M., Talbot N.J., Kamoun S., Islam M.T. Cautionary notes on use of the MoT3 diagnostic assay for Magnaporthe oryzae wheat and rice blast isolates. . Phytopathology. 2019;109(4):504–508. doi: 10.1094/PHYTO-06-18-0199-LE. [DOI] [PubMed] [Google Scholar]
- 31.Loughman R., Thomas G., Wright D. Fusarium head blight of cereals and stalk rot of maize, millet and sorghum and their identification. 2004.
- 32.Bottalico A., Perrone G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Mycotoxins in plant disease. Dordrecht: Springer; 2002. pp. 611–624. [Google Scholar]
- 33.Boutigny A.L., Ward T.J., Van Coller G.J., Flett B., Lamprecht S.C., O’Donnell K., Viljoen A. Analysis of the Fusarium graminearum species complex from wheat, barley and maize in South Africa provides evidence of species-specific differences in host preference. Fungal Genet. Biol. 2011;48(9):914–920. doi: 10.1016/j.fgb.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Xu X.M., Parry D.W., Nicholson P., Thomsett M.A., Simpson D., Edwards S.G., Cooke B.M., Doohan F.M., Brennan J.M., Moretti A., Tocco G. Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. Eur. J. Plant Pathol. 2005;112(2):143–154. doi: 10.1007/s10658-005-2446-7. [DOI] [Google Scholar]
- 35.Wiese M.V. Compendium of wheat diseases. American Phytopathological Society; 1987. [Google Scholar]
- 36.Roy A.K. Ear blight and scab of wheat in Arunachal Pradesh. Curr. Sci. 1973 [Google Scholar]
- 37.Brahma R.N., Singh S.D. Occurrence of scab of wheat in the Nilgiri hills. Curr. Sci. 1985;54(22):1184–1185. [Google Scholar]
- 38.Singh P.J., Aujla S.S. Effect of lodging on the development of head scab of wheat. Indian Phytopathol. 1994;47(3):256–257. [Google Scholar]
- 39.Mann S.K., Nanda G.S. A new leaf spot disease of wheat from India. Indian Phytopathol. 1999;52(4):425–426. [Google Scholar]
- 40.Kaur S., Chahal S.S., Singh N. Effect of temperature on the development of the pathogen and head blight disease in wheat. Plant Dis. Res. 1999;14(2):191–194. [Google Scholar]
- 41.Dev U., Agarwal P.C., Baleshwar S. 2005. [Google Scholar]
- 42.Khetarpal R.K., Gupta K. Plant biosecurity in India-Preparedness. Indian J. Plant Prot. 2007;35(2):168–178. [Google Scholar]
- 43.Saharan M.S., Saharan M.S. Current status of resistant source to Fusarium head blight disease of wheat: a review. Indian Phytopathol. 2019;73:3–9. [Google Scholar]
- 44.Gale L.R., Harrison S.A., Ward T.J., O’Donnell K., Milus E.A., Gale S.W., Kistler H.C. Nivalenol-type populations of Fusarium graminearum and F. asiaticum are prevalent on wheat in southern Louisiana. . Phytopathology. 2011;101(1):124–134. doi: 10.1094/PHYTO-03-10-0067. [DOI] [PubMed] [Google Scholar]
- 45.Starkey D.E., Ward T.J., Aoki T., Gale L.R., Kistler H.C., Geiser D.M., Suga H., Tóth B., Varga J., O’Donnell K. Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genet. Biol. 2007;44(11):1191–1204. doi: 10.1016/j.fgb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Siou D., Gélisse S., Laval V., Suffert F., Lannou C. Mutual exclusion between fungal species of the Fusarium head blight complex in a wheat spike. Appl. Environ. Microbiol. 2015;81(14):4682–4689. doi: 10.1128/AEM.00525-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X.M., Nicholson P., Thomsett M.A., Simpson D., Cooke B.M., Doohan F.M., Brennan J., Monaghan S., Moretti A., Mule G., Hornok L., Beki E., Tatnell J., Ritieni A., Edwards S.G. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology. 2008;98(1):69–78. doi: 10.1094/PHYTO-98-1-0069. [DOI] [PubMed] [Google Scholar]
- 48.Reid L.M., Nicol R.W., Ouellet T., Savard M., Miller J.D., Young J.C., Stewart D.W., Schaafsma A.W. Interaction of Fusarium graminearum and F. moniliforme in maize ears: disease progress, fungal biomass, and mycotoxin accumulation. . Phytopathology. 1999;89(11):1028–1037. doi: 10.1094/PHYTO.1999.89.11.1028. [DOI] [PubMed] [Google Scholar]
- 49.Picot A., Hourcade‐Marcolla D., Barreau C., Pinson‐Gadais L., Caron D., Richard‐Forget F., Lannou C. Interactions between Fusarium verticillioides and Fusarium graminearum in maize ears and consequences for fungal development and mycotoxin accumulation. . Plant Pathol. 2012;61(1):140–151. doi: 10.1111/j.1365-3059.2011.02503.x. [DOI] [Google Scholar]
- 50.Parikka P., Hakala K., Tiilikkala K. Expected shifts in Fusarium species’ composition on cereal grain in Northern Europe due to climatic change. . Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012;29(10):1543–1555. doi: 10.1080/19440049.2012.680613. [DOI] [PubMed] [Google Scholar]
- 51.Sutton J.C. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. . Can. J. Plant Pathol. 1982;4(2):195–209. doi: 10.1080/07060668209501326. [DOI] [Google Scholar]
- 52.Wegulo S., Jackson T.A., Baenziger P.S., Carlson M.P., Nopsa J.H. Fusarium head blight of wheat. 2008.
- 53.Trail F. For blighted waves of grain : Fusarium graminearum in the postgenomics era. . Plant Physiol. 2009;149(1):103–110. doi: 10.1104/pp.108.129684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert J., Haber S. Overview of some recent research developments in Fusarium head blight of wheat. Can. J. Plant Pathol. 2013;35(2):149–174. doi: 10.1080/07060661.2013.772921. [DOI] [Google Scholar]
- 55.Booth C. The genus Fusarium. 1971.
- 56.Pugh G.W., Johann H., Dickson J.G. Factors affecting infection of Wheat heads by Gibberella saubiuetii. . J. Agric. Res. 1933;46(9):771–797. [Google Scholar]
- 57.Strange R.N., Smith H. A fungal growth stimulant in anthers which predisposes wheat to attack by Fusarium graminearum. . Physiol. Plant Pathol. 1971;1(2):141–150. doi: 10.1016/0048-4059(71)90023-3. [DOI] [Google Scholar]
- 58.Boenisch M.J., Schäfer W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. . BMC Plant Biol. 2011;11(1):110. doi: 10.1186/1471-2229-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarlin T., Vilpola A., Kotaviita E., Olkku J., Haikara A. Fungal hydrophobins in the barley‐to‐beer chain. J. Inst. Brew. 2007;113(2):147–153. doi: 10.1002/j.2050-0416.2007.tb00271.x. [DOI] [Google Scholar]
- 60.Brown N.A., Urban M., van de Meene A.M., Hammond-Kosack K.E. The infection biology of Fusarium graminearum : defining the pathways of spikelet to spikelet colonisation in wheat ears. . Fungal Biol. 2010;114(7):555–571. doi: 10.1016/j.funbio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Kang Z., Buchenauer H. Ultrastructural and immunocytochemical investigation of pathogen development and host responses in resistant and susceptible wheat spikes infected by Fusarium culmorum. . Physiol. Mol. Plant Pathol. 2000;57(6):255–268. doi: 10.1006/pmpp.2000.0305. [DOI] [Google Scholar]
- 62.Pritsch C., Muehlbauer G.J., Bushnell W.R., Somers D.A., Vance C.P. Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. . Mol. Plant Microbe Interact. 2000;13(2):159–169. doi: 10.1094/MPMI.2000.13.2.159. [DOI] [PubMed] [Google Scholar]
- 63.Boddu J., Cho S., Kruger W.M., Muehlbauer G.J. Transcriptome analysis of the barley- Fusarium graminearum interaction. . Mol. Plant Microbe Interact. 2006;19(4):407–417. doi: 10.1094/MPMI-19-0407. [DOI] [PubMed] [Google Scholar]
- 64.Kikot G.E., Hours R.A., Alconada T.M. Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum : a review. . J. Basic Microbiol. 2009;49(3):231–241. doi: 10.1002/jobm.200800231. [DOI] [PubMed] [Google Scholar]
- 65.Wanjiru W.M., Zhensheng K., Buchenauer H. Importance of cell wall degrading enzymes produced by Fusarium graminearum during infection of wheat heads. . Eur. J. Plant Pathol. 2002;108(8):803–810. doi: 10.1023/A:1020847216155. [DOI] [Google Scholar]
- 66.Cuomo C.A., Güldener U., Xu J.R., Trail F., Turgeon B.G., Di Pietro A., Walton J.D., Ma L.J., Baker S.E., Rep M., Adam G., Antoniw J., Baldwin T., Calvo S., Chang Y.L., Decaprio D., Gale L.R., Gnerre S., Goswami R.S., Hammond-Kosack K., Harris L.J., Hilburn K., Kennell J.C., Kroken S., Magnuson J.K., Mannhaupt G., Mauceli E., Mewes H.W., Mitterbauer R., Muehlbauer G., Münsterkötter M., Nelson D., O’donnell K., Ouellet T., Qi W., Quesneville H., Roncero M.I., Seong K.Y., Tetko I.V., Urban M., Waalwijk C., Ward T.J., Yao J., Birren B.W., Kistler H.C. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. . Science. 2007;317(5843):1400–1402. doi: 10.1126/science.1143708. [DOI] [PubMed] [Google Scholar]
- 67.Mendgen K., Hahn M., Deising H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 1996;34(1):367–386. doi: 10.1146/annurev.phyto.34.1.367. [DOI] [PubMed] [Google Scholar]
- 68.Bluhm B.H., Zhao X., Flaherty J.E., Xu J.R., Dunkle L.D. RAS2 regulates growth and pathogenesis in Fusarium graminearum. . Mol. Plant Microbe Interact. 2007;20(6):627–636. doi: 10.1094/MPMI-20-6-0627. [DOI] [PubMed] [Google Scholar]
- 69.Rittenour W.R., Harris S.D. An in vitro method for the analysis of infection-related morphogenesis in Fusarium graminearum. . Mol. Plant Pathol. 2010;11(3):361–369. doi: 10.1111/j.1364-3703.2010.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang Z., Buchenauer H. Studies on the infection process of Fusarium culmorum in wheat spikes: degradation of host cell wall components and localization of trichothecene toxins in infected tissue. . Eur. J. Plant Pathol. 2002;108(7):653–660. doi: 10.1023/A:1020627013154. [DOI] [Google Scholar]
- 71.Schroeder H.W., Christensen J.J. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology. 1963;53(71):831–838. [Google Scholar]
- 72.Miller S.S., Chabot D.M., Ouellet T., Harris L.J., Fedak G. Use of a Fusarium graminearum strain transformed with green fluorescent protein to study infection in wheat ( Triticum aestivum ). . Can. J. Plant Pathol. 2004;26(4):453–463. doi: 10.1080/07060660409507165. [DOI] [Google Scholar]
- 73.Jansen C., von Wettstein D., Schäfer W., Kogel K.H., Felk A., Maier F.J. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. . Proc. Natl. Acad. Sci. USA. 2005;102(46):16892–16897. doi: 10.1073/pnas.0508467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ribichich K.F., Lopez S.E., Vegetti A.C. Histopathological spikelet changes produced by Fusarium graminearum in susceptible and resistant wheat cultivars. . Plant Dis. 2000;84(7):794–802. doi: 10.1094/PDIS.2000.84.7.794. [DOI] [PubMed] [Google Scholar]
- 75.Guenther J.C., Trail F. The development and differentiation of Gibberella zeae (anamorph: Fusarium graminearum ) during colonization of wheat. . Mycologia. 2005;97(1):229–237. doi: 10.1080/15572536.2006.11832856. [DOI] [PubMed] [Google Scholar]
- 76.Phalip V., Delalande F., Carapito C., Goubet F., Hatsch D., Leize-Wagner E., Dupree P., Dorsselaer A.V., Jeltsch J.M. Diversity of the exoproteome of Fusarium graminearum grown on plant cell wall. . Curr. Genet. 2005;48(6):366–379. doi: 10.1007/s00294-005-0040-3. [DOI] [PubMed] [Google Scholar]
- 77.Wanyoike M.W., Walker F., Buchenauer H. Relationship between virulence, fungal biomass and mycotoxin production by Fusarium graminearum in winter wheat head blight. Z. Pflanzenkr. Pflanzenschutz. 2002;109(6):589–600. [Google Scholar]
- 78.Proctor R.H., Hohn T.M., McCormick S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant Microbe Interact. 1995;8(4):593–601. doi: 10.1094/mpmi-8-0593. [DOI] [PubMed] [Google Scholar]
- 79.Ochiai N., Tokai T., Nishiuchi T., Takahashi-Ando N., Fujimura M., Kimura M. Involvement of the osmosensor histidine kinase and osmotic stress-activated protein kinases in the regulation of secondary metabolism in Fusarium graminearum. . Biochem. Biophys. Res. Commun. 2007;363(3):639–644. doi: 10.1016/j.bbrc.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 80.Ponts N., Couedelo L., Pinson-Gadais L., Verdal-Bonnin M.N., Barreau C., Richard-Forget F. Fusarium response to oxidative stress by H2O2 is trichothecene chemotype-dependent. . FEMS Microbiol. Lett. 2009;293(2):255–262. doi: 10.1111/j.1574-6968.2009.01521.x. [DOI] [PubMed] [Google Scholar]
- 81.Jiao F., Kawakami A., Nakajima T. Effects of different carbon sources on trichothecene production and Tri gene expression by Fusarium graminearum in liquid culture. . FEMS Microbiol. Lett. 2008;285(2):212–219. doi: 10.1111/j.1574-6968.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- 82.Gardiner D.M., Kazan K., Praud S., Torney F.J., Rusu A., Manners J.M. Early activation of wheat polyamine biosynthesis during Fusarium head blight implicates putrescine as an inducer of trichothecene mycotoxin production. BMC Plant Biol. 2010;10(1):289. doi: 10.1186/1471-2229-10-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wegulo S.N. Factors influencing deoxynivalenol accumulation in small grain cereals. Toxins (Basel) 2012;4(11):1157–1180. doi: 10.3390/toxins4111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ding L., Xu H., Yi H., Yang L., Kong Z., Zhang L., Xue S., Jia H., Ma Z. Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. . PLoS One. 2011;6(4):e19008. doi: 10.1371/journal.pone.0019008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Theis T., Stahl U. Antifungal proteins: targets, mechanisms and prospective applications. Cell. Mol. Life Sci. 2004;61(4):437–455. doi: 10.1007/s00018-003-3231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaturvedi R., Shah J. Salicylic acid in plant disease resistance. Salicylic acid: A Plant Hormone. Dordrecht: Springer; 2007. pp. 335–370. [Google Scholar]
- 87.Durrant W.E., Dong X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 88.Makandar R., Nalam V., Chaturvedi R., Jeannotte R., Sparks A.A., Shah J. Involvement of salicylate and jasmonate signaling pathways in Arabidopsis interaction with Fusarium graminearum. . Mol. Plant Microbe Interact. 2010;23(7):861–870. doi: 10.1094/MPMI-23-7-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mika A., Boenisch M.J., Hopff D., Lüthje S. Membrane-bound guaiacol peroxidases from maize ( Zea mays L.) roots are regulated by methyl jasmonate, salicylic acid, and pathogen elicitors. . J. Exp. Bot. 2010;61(3):831–841. doi: 10.1093/jxb/erp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mesterhazy A. Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 1995;114(5):377–386. doi: 10.1111/j.1439-0523.1995.tb00816.x. [DOI] [Google Scholar]
- 91.Champeil A., Doré T., Fourbet J.F. Fusarium head blight: epidemiological origin of the effects of cultural practices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains. . Plant Sci. 2004;166(6):1389–1415. doi: 10.1016/j.plantsci.2004.02.004. [DOI] [Google Scholar]
- 92.Xiao J., Jin X., Jia X., Wang H., Cao A., Zhao W., Pei H., Xue Z., He L., Chen Q., Wang X. Transcriptome-based discovery of pathways and genes related to resistance against Fusarium head blight in wheat landrace Wangshuibai. BMC Genomics. 2013;14(1):197. doi: 10.1186/1471-2164-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hollins T.W., Ruckenbauer P., De Jong H. Progress towards wheat varieties with resistance to Fusarium head blight. Food Control. 2003;14(4):239–244. doi: 10.1016/S0956-7135(03)00013-6. [DOI] [Google Scholar]
- 94.Ruckenbauer P., Buerstmayr H., Lemmens M. Present strategies in resistance breeding against scab ( Fusarium spp.). . Euphytica. 2001;119(1-2):123–129. doi: 10.1023/A:1017598523085. [DOI] [Google Scholar]
- 95.Hart L.P., Pestka J.J., Liu M.T. Effect of kernel development and wet periods on production of deoxynivalenol in wheat infected with Gibberella zeae. . Phytopathology. 1984;74(12):1415–1418. doi: 10.1094/Phyto-74-1415. [DOI] [Google Scholar]
- 96.Andersen A.L. The development of Gibberella zeae head blight of wheat. . Phytopathology. 1948;38:595–611. [Google Scholar]
- 97.Klepacka J., Fornal Ł. Ferulic acid and its position among the phenolic compounds of wheat. Crit. Rev. Food Sci. Nutr. 2006;46(8):639–647. doi: 10.1080/10408390500511821. [DOI] [PubMed] [Google Scholar]
- 98.Blanchette R.A. Delignification by wood-decay fungi. Annu. Rev. Phytopathol. 1991;29(1):381–403. doi: 10.1146/annurev.py.29.090191.002121. [DOI] [Google Scholar]
- 99.Mohammadi M., Kazemi H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. . Plant Sci. 2002;162(4):491–498. doi: 10.1016/S0168-9452(01)00538-6. [DOI] [Google Scholar]
- 100.Gunupuru L.R., Patel J.S., Sumarah M.W., Renaud J.B., Mantin E.G., Prithiviraj B. A plant biostimulant made from the marine brown algae Ascophyllum nodosum and chitosan reduce Fusarium head blight and mycotoxin contamination in wheat. . PLoS One. 2019;14(9):e0220562. doi: 10.1371/journal.pone.0220562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siranidou E., Kang Z., Buchenauer H. Studies on symptom development, phenolic compounds and morphological defense responses in wheat cultivars differing in resistance to Fusarium head blight. J. Phytopathol. 2002;150(4‐5):200–208. doi: 10.1046/j.1439-0434.2002.00738.x. [DOI] [Google Scholar]
- 102.Kang Z., Buchenauer H. Immunocytochemical localization of cell wall‐bound thionins and hydroxyproline‐rich glycoproteins in Fusarium culmorum ‐infected wheat spikes. . J. Phytopathol. 2003;151(3):120–129. doi: 10.1046/j.1439-0434.2003.00693.x. [DOI] [Google Scholar]
- 103.Cruz A.F., Hamel C., Yang C., Matsubara T., Gan Y., Singh A.K., Kuwada K., Ishii T. Phytochemicals to suppress Fusarium head blight in wheat-chickpea rotation. Phytochemistry. 2012;78:72–80. doi: 10.1016/j.phytochem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 104.McKeehen J.D., Busch R.H., Fulcher R.G. Evaluation of wheat ( Triticum aestivum L.) phenolic acids during grain development and their contribution to Fusarium resistance. . J. Agric. Food Chem. 1999;47(4):1476–1482. doi: 10.1021/jf980896f. [DOI] [PubMed] [Google Scholar]
- 105.Soltoft M., Jørgensen L.N., Svensmark B., Fomsgaard I.S. Benzoxazinoid concentrations show correlation with Fusarium head blight resistance in Danish wheat varieties. Biochem. Syst. Ecol. 2008;36:245–259. doi: 10.1016/j.bse.2007.10.008. [DOI] [Google Scholar]
- 106.Coleman J., Blake-Kalff M., Davies E. Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997;2(4):144–151. doi: 10.1016/S1360-1385(97)01019-4. [DOI] [Google Scholar]
- 107.Berthiller F., Dall’Asta C., Schuhmacher R., Lemmens M., Adam G., Krska R. Masked mycotoxins: determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2005;53(9):3421–3425. doi: 10.1021/jf047798g. [DOI] [PubMed] [Google Scholar]
- 108.Miller D.J., Arnison P.G. Degradation of deoxynivalenol by suspension cultures of the Fusarium head blight resistant wheat cultivar Frontana. Can. J. Plant Pathol. 1986;8(2):147–150. doi: 10.1080/07060668609501818. [DOI] [Google Scholar]
- 109.Bollina V., Kumaraswamy G.K., Kushalappa A.C., Choo T.M., Dion Y., Rioux S., Faubert D., Hamzehzarghani H. Mass spectrometry-based metabolomics application to identify quantitative resistance-related metabolites in barley against Fusarium head blight. Mol. Plant Pathol. 2010;11(6):769–782. doi: 10.1111/j.1364-3703.2010.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kumaraswamy G.K., Kushalappa A.C., Choo T.M., Dion Y., Rioux S. Differential metabolic response of barley genotypes, varying in resistance, to trichothecene‐producing and‐nonproducing (tri5-) isolates of Fusarium graminearum. . Plant Pathol. 2012;61(3):509–521. doi: 10.1111/j.1365-3059.2011.02528.x. [DOI] [Google Scholar]
- 111.Yang F., Jacobsen S., Jørgensen H.J., Collinge D.B., Svensson B., Finnie C. Fusarium graminearum and its interactions with cereal heads: studies in the proteomics era. . Front. Plant Sci. 2013;4:37. doi: 10.3389/fpls.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bai G., Shaner G. Management and resistance in wheat and barley to fusarium head blight. Annu. Rev. Phytopathol. 2004;42:135–161. doi: 10.1146/annurev.phyto.42.040803.140340. [DOI] [PubMed] [Google Scholar]
- 113.Chen P., Liu D., Sun W. New countermeasures of breeding wheat for scab resistance. 1997.
- 114.Buerstmayr H., Ban T., Anderson J.A. QTL mapping and marker‐assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breed. 2009;128(1):1–26. doi: 10.1111/j.1439-0523.2008.01550.x. [DOI] [Google Scholar]
- 115.Miedaner T., Wilde F., Korzun V., Ebmeyer E., Schmolke M., Hartl L., Schön C.C. Marker selection for Fusarium head blight resistance based on quantitative trait loci (QTL) from two European sources compared to phenotypic selection in winter wheat. Euphytica. 2009;166(2):219–227. doi: 10.1007/s10681-008-9832-0. [DOI] [Google Scholar]
- 116.Allender C. The second report on the state of the world’s plant genetic resources for food and agriculture. Rome: Food and Agriculture Organization of the United Nations; 2010. p. 370. [Google Scholar]
- 117.Tiwari K.K., Singh A., Pattnaik S., Sandhu M., Kaur S., Jain S., Tiwari S., Mehrotra S., Anumalla M., Samal R., Bhardwaj J. Identification of a diverse mini‐core panel of Indian rice germplasm based on genotyping using microsatellite markers. Plant Breed. 2015;134(2):164–171. doi: 10.1111/pbr.12252. [DOI] [Google Scholar]
- 118.Jiang P., Zhang X., Wu L., He Y., Zhuang W., Cheng X., Ge W., Ma H., Kong L. A novel QTL on chromosome 5AL of Yangmai 158 increases resistance to Fusarium head blight in wheat. Plant Pathol. 2020;69:249–258. doi: 10.1111/ppa.13130. [DOI] [Google Scholar]
- 119.Snijders C.H. Resistance in wheat to Fusarium infection and trichothecene formation. . Toxicol. Lett. 2004;153(1):37–46. doi: 10.1016/j.toxlet.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 120.Bai G., Su Z., Cai J. Wheat resistance to Fusarium head blight. Can. J. Plant Pathol. 2018;40(3):336–346. doi: 10.1080/07060661.2018.1476411. [DOI] [Google Scholar]
- 121.Hao Y., Rasheed A., Zhu Z., Wulff B.B.H., He Z. Harnessing wheat Fhb1 for Fusarium resistance. Trends Plant Sci. 2020;25(1):1–3. doi: 10.1016/j.tplants.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 122.Zhang X., Pan H., Bai G. Quantitative trait loci responsible for Fusarium head blight resistance in Chinese landrace Baishanyuehuang. Theor. Appl. Genet. 2012;125(3):495–502. doi: 10.1007/s00122-012-1848-0. [DOI] [PubMed] [Google Scholar]
- 123.Steiner B., Buerstmayr M., Michel S., Schweiger W., Lemmens M., Buerstmayr H. Breeding strategies and advances in line selection for Fusarium head blight resistance in wheat. Trop. Plant Pathol. 2017;42(3):165–174. doi: 10.1007/s40858-017-0127-7. [DOI] [Google Scholar]
- 124.Eckard J.T., Gonzalez-Hernandez J.L., Caffe M., Berzonsky W., Bockus W.W., Marais G.F., Baenziger P.S. Native Fusarium head blight resistance from winter wheat cultivars ‘Lyman,’ ‘Overland,’ ‘Ernie,’ and ‘Freedom’ mapped and pyramided onto ‘Wesley’-Fhb1 backgrounds. Mol. Breed. 2015;35(1):6. doi: 10.1007/s11032-015-0200-1. [DOI] [Google Scholar]
- 125.Rawat N., Pumphrey M.O., Liu S., Zhang X., Tiwari V.K., Ando K., Trick H.N., Bockus W.W., Akhunov E., Anderson J.A., Gill B.S. Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight. Nat. Genet. 2016;48(12):1576–1580. doi: 10.1038/ng.3706. [DOI] [PubMed] [Google Scholar]
- 126.Gadaleta A., Colasuonno P., Giove S.L., Blanco A., Giancaspro A. Map-based cloning of QFhb.mgb-2A identifies a WAK2 gene responsible for Fusarium Head Blight resistance in wheat. Sci. Rep. 2019;9(1):6929. doi: 10.1038/s41598-019-43334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pumphrey M.O., Bernardo R., Anderson J.A. Validating the Fhb1 QTL for Fusarium head blight resistance in near-isogenic wheat lines developed from breeding populations. Crop Sci. 2007;47(1):200–206. doi: 10.2135/cropsci2006.03.0206. [DOI] [Google Scholar]
- 128.Dahleen L.S., Okubara P.A., Blechl A.E. Transgenic approaches to combat Fusarium head blight in wheat and barley. Crop Sci. 2001;41(3):628–637. doi: 10.2135/cropsci2001.413628x. [DOI] [Google Scholar]
- 129.Chen D., Ma H., Hong H., Koh S.S., Huang S.M., Schurter B.T., Aswad D.W., Stallcup M.R. Regulation of transcription by a protein methyltransferase. Science. 1999;284(5423):2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 130.Anand A., Zhou T., Trick H.N., Gill B.S., Bockus W.W., Muthukrishnan S. Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J. Exp. Bot. 2003;54(384):1101–1111. doi: 10.1093/jxb/erg110. [DOI] [PubMed] [Google Scholar]
- 131.Mackintosh C.A., Lewis J., Radmer L.E., Shin S., Heinen S.J., Smith L.A., Wyckoff M.N., Dill-Macky R., Evans C.K., Kravchenko S., Baldridge G.D., Zeyen R.J., Muehlbauer G.J. Overexpression of defense response genes in transgenic wheat enhances resistance to Fusarium head blight. Plant Cell Rep. 2007;26(4):479–488. doi: 10.1007/s00299-006-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Han J., Lakshman D.K., Galvez L.C., Mitra S., Baenziger P.S., Mitra A. Transgenic expression of lactoferrin imparts enhanced resistance to head blight of wheat caused by Fusarium graminearum. BMC Plant Biol. 2012;12(1):33. doi: 10.1186/1471-2229-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stuiver M.H., Custers J.H. Engineering disease resistance in plants. Nature. 2001;411(6839):865–868. doi: 10.1038/35081200. [DOI] [PubMed] [Google Scholar]
- 134.Makandar R., Essig J.S., Schapaugh M.A., Trick H.N., Shah J. Genetically engineered resistance to Fusarium head blight in wheat by expression of Arabidopsis NPR1. Mol. Plant Microbe Interact. 2006;19(2):123–129. doi: 10.1094/MPMI-19-0123. [DOI] [PubMed] [Google Scholar]
- 135.Makandar R., Nalam V.J., Lee H., Trick H.N., Dong Y., Shah J. Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol. Plant Microbe Interact. 2012;25(3):431–439. doi: 10.1094/MPMI-09-11-0232. [DOI] [PubMed] [Google Scholar]
- 136.Li H.P., Zhang J.B., Shi R.P., Huang T., Fischer R., Liao Y.C. Engineering Fusarium head blight resistance in wheat by expression of a fusion protein containing a Fusarium-specific antibody and an antifungal peptide. Mol. Plant Microbe Interact. 2008;21(9):1242–1248. doi: 10.1094/MPMI-21-9-1242. [DOI] [PubMed] [Google Scholar]
- 137.He F., Zhang R., Zhao J., Qi T., Kang Z., Guo J. Host-induced silencing of Fusarium graminearum genes enhances the resistance of Brachypodium distachyon to Fusarium head blight. . Front. Plant Sci. 2019;10:1362. doi: 10.3389/fpls.2019.01362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chattopadhyay A., Purohit J., Tiwari K.K., Deshmukh R. Targeting transcription factors for plant disease resistance: shifting paradigm. Curr. Sci. 2019;117(10):1598. doi: 10.18520/cs/v117/i10/1598-1607. [DOI] [Google Scholar]
- 139.Cagliari D., Dias N.P., Galdeano D.M., Dos Santos E.Á., Smagghe G., Zotti M.J. Management of pest insects and plant diseases by non-transformative RNAi. Front. Plant Sci. 2019;10:1319. doi: 10.3389/fpls.2019.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Koch A., Kumar N., Weber L., Keller H., Imani J., Kogel K.H. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase-encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA. 2013;110(48):19324–19329. doi: 10.1073/pnas.1306373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Y., Gao Q., Huang M., Liu Y., Liu Z., Liu X., Ma Z. Characterization of RNA silencing components in the plant pathogenic fungus Fusarium graminearum. . Sci. Rep. 2015;5:12500. doi: 10.1038/srep12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baldwin T., Islamovic E., Klos K., Schwartz P., Gillespie J., Hunter S., Bregitzer P. Silencing efficiency of dsRNA fragments targeting Fusarium graminearum TRI6 and patterns of small interfering RNA associated with reduced virulence and mycotoxin production. . PLoS One. 2018;13(8):e0202798. doi: 10.1371/journal.pone.0202798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheng W., Song X.S., Li H.P., Cao L.H., Sun K., Qiu X.L., Xu Y.B., Yang P., Huang T., Zhang J.B., Qu B., Liao Y.C. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 2015;13(9):1335–1345. doi: 10.1111/pbi.12352. [DOI] [PubMed] [Google Scholar]
- 144.Chen W., Kastner C., Nowara D., Oliveira-Garcia E., Rutten T., Zhao Y., Deising H.B., Kumlehn J., Schweizer P. Host-induced silencing of Fusarium culmorum genes protects wheat from infection. . J. Exp. Bot. 2016;67(17):4979–4991. doi: 10.1093/jxb/erw263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Werner B., Gaffar F.Y., Biedenkopf D., Koch A. RNA-spray-mediated silencing of Fusarium graminearum AGO and DCL genes improve barley disease resistance. . Front. Plant Sci. 2020;11:476. doi: 10.3389/fpls.2020.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koch A., Biedenkopf D., Furch A., Weber L., Rossbach O., Abdellatef E., Linicus L., Johannsmeier J., Jelonek L., Goesmann A., Cardoza V., McMillan J., Mentzel T., Kogel K.H. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016;12(10):e1005901. doi: 10.1371/journal.ppat.1005901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Höfle L., Biedenkopf D., Werner B.T., Shrestha A., Jelonek L., Koch A. Study on the efficiency of dsRNAs with increasing length in RNA-based silencing of the Fusarium CYP51 genes. RNA Biol. 2020;17(4):463–473. doi: 10.1080/15476286.2019.1700033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Malfatti L. Use of CRISPR cas9 for the genome editing of Fusarium graminearum, one of the main causal agents of Fusarium head blight. ETD System: Electronic Theses and Dissertations. 2018.
- 149.Chaudhary K., Chattopadhyay A., Pratap D. The evolution of CRISPR/Cas9 and their cousins: hope or hype? Biotechnol. Lett. 2018;40(3):465–477. doi: 10.1007/s10529-018-2506-7. [DOI] [PubMed] [Google Scholar]
- 150.Wang X., Pan C., Gong J., Liu X., Li H. Enhancing the enrichment of pharmacophore-based target prediction for the polypharmacological profiles of drugs. J. Chem. Inf. Model. 2016;56(6):1175–1183. doi: 10.1021/acs.jcim.5b00690. [DOI] [PubMed] [Google Scholar]
- 151.Xie K., Yang Y. RNA-guided genome editing in plants using a CRISPR-Cas system. Mol. Plant. 2013;6(6):1975–1983. doi: 10.1093/mp/sst119. [DOI] [PubMed] [Google Scholar]
- 152.Ma H.X., Bai G.H., Zhang X., Lu W.Z. Main effects, epistasis, and environmental interactions of quantitative trait Loci for fusarium head blight resistance in a recombinant inbred population. Phytopathology. 2006;96(5):534–541. doi: 10.1094/PHYTO-96-0534. [DOI] [PubMed] [Google Scholar]
- 153.Wang N. The Citrus Huanglongbing Crisis and Potential Solutions. . Mol. Plant. 2019;12(5):607–609. doi: 10.1016/j.molp.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 154.Oliva R., Ji C., Atienza-Grande G., Huguet-Tapia J.C., Perez-Quintero A., Li T., Eom J.S., Li C., Nguyen H., Liu B., Auguy F., Sciallano C., Luu V.T., Dossa G.S., Cunnac S., Schmidt S.M., Slamet-Loedin I.H., Vera Cruz C., Szurek B., Frommer W.B., White F.F., Yang B. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019;37(11):1344–1350. doi: 10.1038/s41587-019-0267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Y., Cheng X., Shan Q., Zhang Y., Liu J., Gao C., Qiu J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014;32(9):947–951. doi: 10.1038/nbt.2969. [DOI] [PubMed] [Google Scholar]
- 156.Wenderoth M., Pinecker C., Voß B., Fischer R. Establishment of CRISPR/Cas9 in Alternaria alternata. . Fungal Genet. Biol. 2017;101:55–60. doi: 10.1016/j.fgb.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 157.Li J., Zhang Y., Zhang Y., Yu P.L., Pan H., Rollins J.A. Introduction of large sequence inserts by CRISPR-Cas9 to create pathogenicity mutants in the multinucleate filamentous pathogen Sclerotinia sclerotiorum. . MBio. 2018;9(3):e00567–e18. doi: 10.1128/mBio.00567-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang W., Pan Q., He F., Akhunova A., Chao S., Trick H., Akhunov E. Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. CRISPR J. 2018;1(1):65–74. doi: 10.1089/crispr.2017.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nakamura M., Okamura Y., Iwai H. Plasmid-based and-free methods using CRISPR/Cas9 system for replacement of targeted genes in Colletotrichum sansevieriae. . Sci. Rep. 2019;9(1):1. doi: 10.1038/s41598-019-55302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nalam V.J., Alam S., Keereetaweep J., Venables B., Burdan D., Lee H., Trick H.N., Sarowar S., Makandar R., Shah J. Facilitation of Fusarium graminearum infection by 9-lipoxygenases in Arabidopsis and wheat. . Mol. Plant Microbe Interact. 2015;28(10):1142–1152. doi: 10.1094/MPMI-04-15-0096-R. [DOI] [PubMed] [Google Scholar]
- 161.Ravensdale M., Rocheleau H., Wang L., Nasmith C., Ouellet T., Subramaniam R. Components of priming-induced resistance to Fusarium head blight in wheat revealed by two distinct mutants of Fusarium graminearum. . Mol. Plant Pathol. 2014;15(9):948–956. doi: 10.1111/mpp.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Urban M., Mott E., Farley T., Hammond-Kosack K. The Fusarium graminearum MAP1 gene is essential for pathogenicity and development of perithecia. . Mol. Plant Pathol. 2003;4(5):347–359. doi: 10.1046/j.1364-3703.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 163.Su Z., Bernardo A., Tian B., Chen H., Wang S., Ma H., Cai S., Liu D., Zhang D., Li T., Trick H., St Amand P., Yu J., Zhang Z., Bai G. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019;51(7):1099–1105. doi: 10.1038/s41588-019-0425-8. [DOI] [PubMed] [Google Scholar]
- 164.van Schie C.C., Takken F.L. Susceptibility genes 101: how to be a good host. Annu. Rev. Phytopathol. 2014;52:551–581. doi: 10.1146/annurev-phyto-102313-045854. [DOI] [PubMed] [Google Scholar]
- 165.Low Y.C. Applications of biotechnology for crop enhancement in disease resistance and nutrition.
- 166.Gardiner D.M., Kazan K. Selection is required for efficient Cas9-mediated genome editing in Fusarium graminearum. . Fungal Biol. 2018;122(2-3):131–137. doi: 10.1016/j.funbio.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 167.Ferrara M., Haidukowski M., Logrieco A.F., Leslie J.F., Mulè G. CRISPR-Cas9 system for genome editing of Fusarium proliferatum. . Sci. Rep. 2019;9(1):19836. doi: 10.1038/s41598-019-56270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cui X., Balcerzak M., Schernthaner J., Babic V., Datla R., Brauer E.K., Labbé N., Subramaniam R., Ouellet T. An optimised CRISPR/Cas9 protocol to create targeted mutations in homoeologous genes and an efficient genotyping protocol to identify edited events in wheat. Plant Methods. 2019;15(1):1–2. doi: 10.1186/s13007-019-0500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shan Q., Wang Y., Li J., Gao C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 2014;9(10):2395–2410. doi: 10.1038/nprot.2014.157. [DOI] [PubMed] [Google Scholar]
- 170.Zhang Y., Liang Z., Zong Y., Wang Y., Liu J., Chen K., Qiu J.L., Gao C. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 2016;7(1):12617. doi: 10.1038/ncomms12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Di R. https://portal.nifa.usda.gov/web/crisproject pages/1007598-mycotoxins-biosecurity-food-safety-and-biofuels-byproducts-nc129F-nc1025.html