Abstract
The article provides an overview of current views on the role of biomechanical forces in the pathogenesis of atherosclerosis. The importance of biomechanical forces in maintaining vascular homeostasis is considered. We provide descriptions of mechanosensing and mechanotransduction. The roles of wall shear stress and circumferential wall stress in the initiation, progression and destabilization of atherosclerotic plaque are described. The data on the possibilities of assessing biomechanical factors in clinical practice and the clinical significance of this approach are presented. The article concludes with a discussion on current therapeutic approaches based on the modulation of biomechanical forces.
Keywords: Atherosclerosis, wall shear stress, peripheral artery disease, circumferential wall stress, mechanosensing, plaque progression
1. INTRODUCTION
Atherosclerosis is a systemic chronic inflammatory disease predominantly affecting muscular and elastic arteries, characterized by autoimmune response to arterial wall injury with development of subintimal accumulation of lipids, immune cells, and Smooth Muscle Cells (SMCs) [1].
Despite the systemic nature of the process, the onset of atherosclerosis in the form of atherosclerotic plaque formation is focal in nature and is determined by hemodynamic factors acting locally [2]. The most important among them are circumferential wall tension and stress, acting perpendicular to the vessel wall, initiated by blood pressure, as well as wall shear stress, directed tangentially and arising under the action of viscous forces on the Endothelial Cells (ECs) generated by the moving stream of blood inside the vessel [3].
2. Definitions of circumferential wall stress and wall shear stress
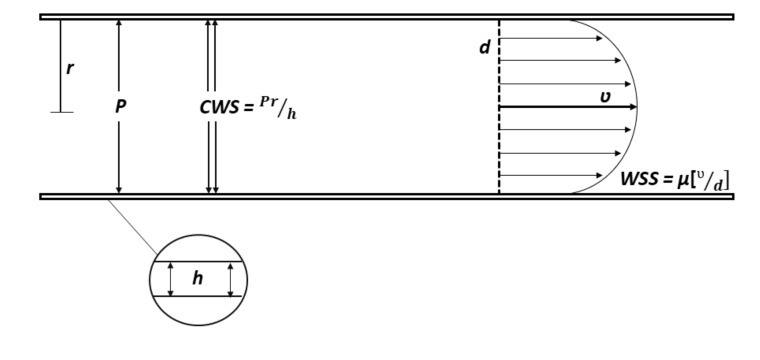
Circumferential Wall Stress (CWS), arising from the effect of blood pressure on the vascular wall, has as its point of application at all the layers of the artery wall (intima, media and adventitia). According to the Laplace law (T = Pr), the tension of the vascular wall (T) is proportional to the pressure (P) and the radius of the vessel (r). CWS (CWS = Pr/h), in turn, is directly proportional to pressure (P) and vessel radius (r), and inversely proportional to vascular wall thickness (h). Currently, synonymous terms are often used to refer to CWS: “circumferential wall stress”, “tensile stress”, “structural stress” or “plaque structural stress” [2]. Under physiological conditions, CWS values are 1-2 × 106 dyne/cm2 or 100-200 kPa [3].
Wall Shear Stress (WSS), generated by a moving blood stream, acts primarily on the endothelial lining of the artery. Before proceeding to the description of the WSS, it is necessary to take into account such a concept as the Wall Shear Rate (WSR). WSR can be defined as the speed at which nearby fluid layers move relative to each other (measured in inverse seconds, с-1) [4]. In other words, WSR is a gradient of blood flow velocity along the radial direction, increasing in the direction from the vessel wall to its center [5]. Knowing WSR (WSR = V / d), we can get WSS according to the Hagen-Poiseuille law: WSS is directly proportional to WSR and blood viscosity (WSS = µ × WSR) (Fig. 1).
Fig. (1).
Circumferential wall stress and wall shear stress.
The literature describes various options for calculating the WSS in accordance with the Hagen-Poiseuille law [6]. When using the Hagen-Poiseuille formula for assessing WSS in vivo, a number of assumptions of this model should be kept in mind: blood is treated as Newtonian fluid; blood flow is considered as laminar and constant; the vessel is considered as a cylindrical tube with rigid walls.
Under physiological conditions, WSR and WSS vary widely depending on the localization of the vessel in the circulation system. For example, WSS is 0.3-0.5 Pa in the femoral arteries, 0.4-0.5 Pa in the brachial arteries and 1.1-1.3 in the carotid arteries [7]. WSR (max) in these vessels is on average 735 с-1, 914 с-1 and 947 с-1 respectively [8].
3. Mechanosensing and mechanotransduction
Despite the systemic nature of the process, the onset of atherosclerosis in the form of atherosclerotic plaque formation is focal in nature and is determined by hemodynamic factors acting locally [2]. The most important among them are circumferential wall tension and stress, acting perpendicular to the vessel wall, initiated by blood pressure, as well as wall shear stress, directed tangentially and arising under the action of viscous forces on the Endothelial Cells (ECs) generated by the moving stream of blood inside the vessel [3].
Biomechanical forces are involved in maintaining vascular homeostasis and are the most important participants in the processes of vascular remodeling under various pathological conditions: hypertension, atherosclerosis, etc. This is made possible by two phenomena - mechanosensing and mechanotransduction.
Mechanosensing is the ability of biological cells, including ECs, to detect and respond to the effects of external mechanical forces that make up their microenvironment [8]. Mechanotransduction is a set of molecular and cellular mechanisms for converting biomechanical forces into biochemical stimuli [9]. Mechanotransduction is carried out by means of mechanosensors - cellular structures, often of a protein nature, which convert the mechanical “input” into the biochemical “output” [10].
Thus, CWS and WSS are the most important regulators of vascular homeostasis through their mechanosensing and mechanotransinduction. ECs, being the interface of interaction between the blood flow and the vascular wall, play a key role in it.
In recent years, the сonducted research aimed at describing the mechanisms by which ECs convert mechanical effects into biological reactions. According to a review by Y. Fang et al. today, we can distinguish the following types of mechanosensors [11]:
Mechanically activated potassium and calcium ion channels;
Integrins;
Cell junction molecules;
Elements of the cytoskeleton and its associated molecules;
Glycocalyx and cilia; etc.
Intracellular signaling pathways involved in mechanotransduction are also described in detail in the literature [12, 13].
4. Biomechanical forces in the regulation of vascular homeostasis
The participation of biomechanical forces in the regulation of normal physiology of ECs and vascular homeostasis is called mechanoregulation [14].
One of the key signaling pathways that implement mechano-regulatory mechanisms is the eNOS (endothelial NO Synthase)/ NO Pathway. ENOS is an enzyme that generates NO from L-arginine.
Exposure of ECs with a laminar blood flow with high WSR and WSS values, activates eNOS and leads to an increase in NO synthesis in several ways [15]:
Akt activation with subsequent Ser1177 phosphorylation;
Stimulation of AMP-activated Protein Kinase (AMPK) followed by Ser633 phosphorylation;
KLF2 transcription factor stimulation;
PI3K/Akt and Protein Kinase C (PKC) stimulation with subsequent nuclear factor (erythroid-derived 2)-like 2 (NRF2) activation.
In turn, NO performs a large number of functions that ensure the survival of ECs, vascular tone maintenance, anti-inflammatory and antithrombotic properties of the endothelial monolayer, and also takes part in angiogenesis [15].
WSS is an extremely important stimulus for angiogenesis. So, it is established that high WSS values at the microcirculatory level (more than 10 dynes/cm2 or more than 1 Pa) are a sprouting trigger for ECs [16]. At the same time, angiogenesis stimulated by the WSS depends on the NO bioavailability and is provided by mechanisms associated with VEGF [17].
One of the key mechanisms by which WSS affects the physiology of ECs is the regulation of gene expression. Thus, more than 350 genes regulated by the WSS have been identified [18]. At the same time, about 190 genes are upregulated under the conditions of laminar flow with high WSS (atheroprotective blood flow profile), and about 166 genes - under the conditions of the proatherogenic blood flow profile. Accordingly, WSS directly determines the transcriptome and the proteome of ECs [19]. This in turn determines the phenotype of ECs and their role in a number of pathological processes (inflammation, thrombosis, oxidative stress, etc.) [11, 20].
The influence of biomechanical factors on the immune system, both systemic and local, also plays a crucial role in the maintenance of vascular homeostasis. Above the examples the influence of WSS on ECs have been described, however, at present, the mechanisms by which WSS affects various immunocompetent cells are also established. In turn, the interaction of ECs and immune cells determines the vessel physiology.
The paper of A.A. Kadam et al., has been demonstrated that the supra-physiological increase in WSS, observed, for example, at the sites of vessel stenosis, leads to increase in expression of TNFα, ICAM-1, MCP-1 and VCAM-1 genes monocytes [21]. Thus, there is the increase in adhesion of monocytes and their pro-inflammatory transformation. Earlier in the study of M.J. Mitchell et al., the ability of WSS to activate circulating neutrophils via the PAF-dependent pathway was established [22]. Later studies have investigated and described the mechanisms of WSS-induced transendothelial migration of neutrophils [23, 24]. W.D. Qin et al., described the enhancement of translocation and release into the extracellular compartment by the endotheliocytes of the High Mobility Group Box 1 (HMGB1), which is the archetypical Damage-Associated Molecular Pattern (DAMP), under the influence of low WSS [25]. HMGB1, which is a ligand for TLR2, TLR4 and RAGE, in turn, activates the cells of innate immunity and triggers a cascade of inflammatory reactions in the vascular wall. The influence of biomechanical forces on the circulating lymphocytes is currently being studied and the available data are limited [26]. It is known that the nature and size of WSS and WSR can modulate the migration of T-lymphocytes and the direction of their movement [27].
While the physiological effects of the WSS are being studied quite actively, the role of CWS in maintaining vascular homeostasis is not well described [3]. However, it is known that CWS has a significant impact not only on the functioning of ECs, but also on SMCs and the extracellular matrix. The current state of the problem is described in sufficient detail in the reviews of Y. Fang et al. and N.F. Jufri et al. [11, 28].
5. Biomechanical forces in atherosclerosis
5.1. Plaque Initiation
Atherosclerosis is a systemic disease characterized by focal manifestation. In this case, biomechanical factors acting locally determine the localization of atheroma. For a long time, it is known that atheromas are localized mainly in places of vascular bifurcations, lateral branch discharge or in the bends of vessels [29]. These areas of the arterial tree are characterized by the following features of the distribution of biomechanical forces:
Low WSS (< 5 dyne/cm2);
Oscillatory WSS with significant variations in the direction and amplitudes of WSS.
In this case, the least favorable profile of the blood flow (proatherogenic) is observed along the inner curvature of the vessel bend and along the outer wall of the Y-shaped bifurcations [30, 31]. In addition, the increase in the angle of separation of vessels in the bifurcation is also associated with the increase in the area subject to blood flow with low oscillatory WSS [32, 33].
Cause-effect relationship of the pro-atherogenic blood flow pattern with characteristic changes in the WSS has been proven in a number of experimental studies. Thus, it was shown that in regions with low oscillatory WSS, atheromas develop significantly more often [2, 34-36]. Moreover, atheromas in these regions are characterized by their large size, large volume of the lipid-necrotic core and a lower content of collagen and SMCs. In prospective human clinical studies, similar results were obtained. So, according to a 12-year prospective study by C. Carallo et al. decrease in carotid WSS during the observation period was an independent predictor of atherosclerotic plaque formation in the carotid arteries [37].
The pro-atherogenic role of low WSS is usually considered from several different perspectives [3]. First, the decrease in WSS and WSR means, among other things, that time during which atherogenic blood components are retained over area of the vascular wall increases. The increase in stagnation time in turn may indicate the increase in near-wall transport, for example, of apo-B-containing lipoproteins in the subintimal space [38]. Second, the reduction of WSS per se through mechanosensing and mechanotransduction initiates a complex of reactions, the result of which is the phenotypic transformation of ECs and the development of atherosclerosis. Among these reactions the following can be noted:
Up-regulation of gene networks determining the phenotypic transformation of ECs into an “inflammatory” phenotype [39];
Change of the phenotype of ECs and endothelial-to-mesenchymal transition [40];
Up-regulation of NF-kB and an increase in the expression of adhesion molecules by endotheliocytes (MCP-1, VCAM-1, ICAM-1, E-selectin) [41];
Decreased synthesis of Nicotinamide Adenine Dinucleotide (NADH) in ECs, increased production of free oxygen forms and activation of oxidative stress [41];
Reduction of NO synthesis [42];
Up-regulation of SREBP2 with subsequent activation of NRLP3-inflamasome and increased synthesis of pro-inflammatory cytokines [43].
5.2. Plaque Progression
Currently, there is convincing evidence of the important role of WSS in the growth of atheroma and its transformation into an “vulnerable” plaque. In a number of clinical studies, it has been established that a decrease in WSS is a necessary condition for the growth of atherosclerotic plaque [44]. In a study of P.H. Stone et al. using intravascular ultrasound, it has been demonstrated that in segments of the coronary arteries subject to low WSS (less than 12 dynes/cm2), the increase in the volume of atheroma over 8 ± 2 months of observation was significantly more frequent [45]. In their later study, which included 506 patients with acute coronary syndrome, decrease in WSS was an independent predictor of both the increase in atheroma volume and the degree of coronary artery stenosis [46]. Similar results were obtained in the works of X. Liu et al. and O. Parodi et al. [47, 48]. Moreover, in addition to the increase in the volume of atherosclerotic lesions, decrease in WSS was also associated with the increase in the volume of the lipid-necrotic plaque core and decrease in the volume of fibrous tissue, which indicates its transformation into a phenotypically unstable atheroma [49].
5.3. Plaque Destabilization and Rupture
Data on which hemodynamic profile is associated with destabilization of atheroma are contradictory. It was found in the study by D. Shishikura et al., that in the segments of the coronary arteries with high WSS, atherosclerotic plaques more often transformed into phenotypically vulnerable [50]. Similar results were obtained in studies of M.T. Corban et al. and N. Murata et al. [51, 52]. It is assumed that in atheromas exposed to high WSS, up-regulation of apoptosis of SMCs, up-regulation of macrophages and the increase in the activity of matrix metalloproteinases occurs [53]. This leads to thinning of plaque cap, its erosion or rupture.
On the other hand, in the study of A.M. Kok et al. predictors of atheroma growth and adverse changes in its composition were low and multidirectional WSS [54]. In atheroma areas prone to low WSS, activation of the inflammatory cascade, increase in lipid accumulation and expansion of the lipid-necrotic core, predominance of the processes of biodegradation of the extracellular matrix can be observed [55]. The result of these processes is the formation of thin-cap fibroatheroma that is at high risk of atherothrombosis.
It was essential to recall that the effects of WSS can be modified by other factors and the role of various WSS patterns in destabilizing atheroma can depend both on the local microenvironment and on system factors. For example, it was demonstrated in the work of C. Costopoulos et al., that the combination of low WSS and high CWS (or plaque structural stress) was the most unfavorable in terms of destabilization of the plaque [56]. In addition, the interpretation of heterogeneous research results makes it difficult for WSS to evolve over time, as atheroma grows and develops, as well as WSS heterogeneity in space - low WSS values proximal to atheroma, with an increase in the proximal shoulder area, reaching supraphysiological values at the site of maximum narrowing of the vessel lumen a subsequent decrease in the region of the distal shoulder [55]. In conclusion, it should be noted that with large assumptions it can be assumed that WSS low values initiate the development and further growth of atheroma, which leads to stenosis of the vessel lumen and, respectively, growth of WSS and CWS, which trigger destabilization of atheroma with its rupture or erosion [56].
6. DIAGNOSTIC METHODS FOR ASSESSING BIO-MECHANICAL FORCES IN VIVO AND CLINICAL IMPLICATIONS
6.1. Diagnostic Tools for Biomechanical Forces Assessment
Currently, the clinical studies use the following methods for assessing WSS and other biomechanical factors involved in the pathogenesis of atherosclerosis:
Methods based on the use of duplex ultrasound scanning of blood vessels, allowing to determine the blood flow velocity, an diameter of the vessel, and, consequently, WSR [57-59]. According to Hagen-Poiseuille law, WSS can be calculated after measuring blood viscosity;
Ultrasound Doppler velocimetry and particle image velocimetry [60];
Phase-Contrast Magnetic Resonance Imaging (PC-MRI) and 4D PC-MRI [61-63]. These techniques are among the most widely used WSS measurement methods in the recent years. They combine optimal spatial and temporal resolution, high measurement accuracy, low operator dependence and the absence of ionizing radiation;
Intravascular ultrasound, including in combination with other imaging techniques, such as computed tomography or invasive angiography [64];
Computational Fluid Dynamics (CFD) methods based on the Navier-Stokes equations system [65, 66].
To date, the assessment of biomechanical forces and factors involved in atherogenesis is used mainly in the clinical studies. Nevertheless, various practical implications can be distinguished, the introduction of which in clinical practice seems to be justified and highly probable.
6.2. Carotid WSR and WSS as Markers of Vascular Remodeling and Atherosclerotic Cardiovascular Diseases
Progressive reduction in carotid WSS and WSR was observed in the experimental work of R. Xing et al., after carotid artery stenosis in vivo was established. Interestingly that WSS and WSR were decreased during the follow-up period of 9 weeks, despite the fact that the degree of stenosis remained unchanged [67]. The decrease in WSS and the increase in Carotid Intimal Medial Thickness (CIMT) of the Common Carotid Artery (CCA) was demonstrated in a study by B. Zhang et al., as atherosclerosis progressed against a fat-rich diet. At the same time, decrease in WSS was observed already from the first week of the experiment, while the increase in the CIMT was recorded only at week 5 of observation. It is evident that the diagnostic value of carotid WSS and WSR is quite high, and may exceed the traditional indicators, such as CIMT of the CCA [68].
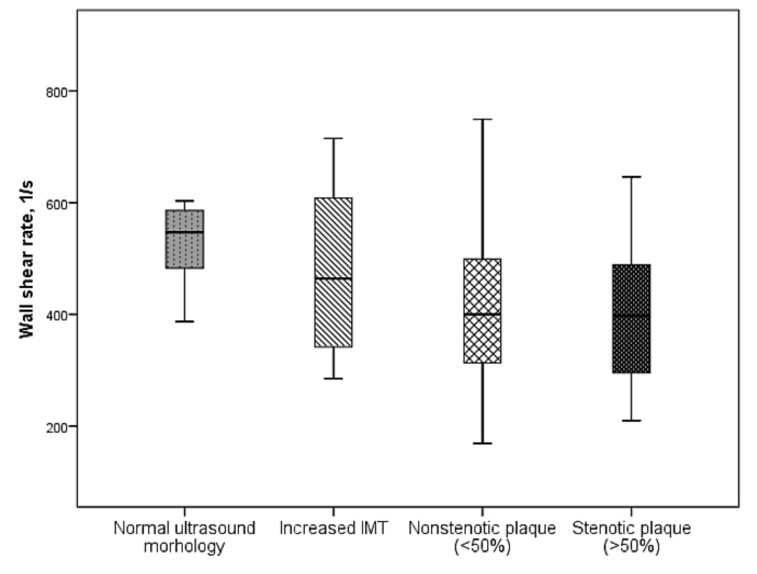
It has been found in our study that as the severity of atherosclerotic lesions of peripheral arteries increases, a significant decrease in carotid WSR was observed (Fig. 2) [69].
Fig. (2).
Changes in carotid WSR, depending on the severity of atherosclerotic lesions of peripheral arteries.
This may indicate that the carotid WSR is capable of representing systemic vascular remodeling associated with atherosclerosis and hypertension.
In the study by KI Cho et al., carotid WSS was evaluated in patients with suspected Coronary Artery Disease (CAD). It was found that the patients with verified diagnosis of CAD differed significantly as they have lower WSS values compared with patients without CAD. Also, the decrease in carotid WSS was associated with the severity of atherosclerotic lesions of the carotid arteries and was an independent predictor of the patient’s angiographically confirmed CAD. It is important that the predictive value of WSS was higher in comparison with CIMT and the area of carotid plaque [70].
6.3. Diagnostic Tools for Biomechanical Forces Assessment
Data reflecting the ability of the WSS to serve as a predictor of the development and progression of atherosclerosis in the corresponding vascular pool - from carotid arteries to the coronary arteries as presented in sections 5.1., 5.2. and 5.3.
A number of clinical studies have demonstrated an inverse correlation relationship between the WSS value after stenting of the arteries and neointima thickness during the observation period [71]. These connections are more characteristic of the implantation of bare-metal stents [72]. A decrease in WSS in the stent area was also associated with the increase in the volume of neoatherosclerotic lesions [73]. In the vascular wall areas exposed to WSS less than 1 Pa, neointimal hyperplasia and thrombosis are developed significantly more often. Further study of changes associated with stent implantation may contribute to the development of new stents and grafts, as well as prognostic models that minimize the risks associated with stent restenosis [74].
6.4. WSS as a Predictor of Adverse Events Associated with Cardiovascular Risk Factors and Atherosclerosis
Carotid WSS can serve as a marker not only of atherosclerosis and atherosclerotic CVD, but also of other pathological conditions associated with cardiovascular risk factors and taking their place in the cardiovascular continuum. So, according to Y. Guo et al., carotid WSS is directly correlated with glomerular filtration rate in the elderly patients [75].
Z. Liu et al. found that the decrease in carotid WSS is associated with the presence and extent of white-matter hyperintensities [76]. In addition, the carotid WSS directly correlated with the MMSE score. At the same time, a decrease in carotid WSS was an independent predictor of the decrease in the MMSE score for 5.4 years of observation [77].
6.5. Other
Outside the scope of the work, other clinical areas were left, in which the study of biomechanical factors is progressing very actively and contributes to the translation of the results of these studies into clinical practice. So, it should be noted that there is great importance of research on biomechanical factors in the development and progression of vascular aneurysms and aortopathies [78, 79].
7. Current therapeutic approaches based on the modulation of biomechanical forces
7.1. Flow Modification Stents
The stent implantation leads to a number of adverse changes in the hemodynamic profile of the blood flow, among which are the slowing of blood flow, the appearance of recirculation sites, areas with reduced, elevated or oscillatory WSS. On the one hand, these factors may contribute to stent restenosis, on the other, to the progression of atherosclerosis distal to the location of the stent [80, 81].
The question of the effect of WSS changes after the stent installation on the risk of its thrombosis is extremely important. It is known that reduction in WSS observed at the site of stent implantation, contributes to the reduction in the expression of nitrogen oxide, prostacyclin and tissue-type plasminogen activator [82]. In addition, low WSS inhibits reendothelialization within the stent, which may also increase the risk of stent thrombosis. On the other hand, the heterogeneity of WSS arising over and between the struts, causes platelet activation and also plays a role in thrombus formation [82, 83].
A number of stents' manufacturers are actively pursuing the development and clinical studies of stents that modify the flow and change the local hemodynamic profile in a favorable direction [84].
Among these stents should be noted Spiral FlowTM (VFT, Dundee, United Kingdom) and BioMimics 3DTM (Veryan Medical Ltd, West Sussex, United Kingdom). Spiral FlowTM has an original design, according to which there is a ridge at its distal end, which forms a spiral flow distal to the stent. Satisfactory results were shown in the small clinical study regarding the patency in the stent implanted for peripheral artery disease in the proximal or distal arteries of the lower limbs. Obviously, further research is needed in this direction [85]. A similar graft concept - SwirlGraftTM - was developed by C.G. Caro et al. [86]. However, clinical trial data is also limited [87].
BioMimics 3DTM was created for endovascular interventions for stenosis of the popliteal and superficial femoral arteries (Fig. 3).
Fig. (3).
BioMimics 3DTM (source: http://www.medicalexpo.com). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The stent has a spiral shape. According to a two-year prospective study where 50 patients were implanted with this stent, it was shown that the primary patency of BioMimics 3DTM was higher compared to the control group - 72% against 55% (p=0.05) [88].
The concept of developing stents and grafts forming a spiral flow is that the spiral flow of blood is physiological and is characteristic of arteries under physiological conditions, which is associated with the twisting and unwinding of the left ventricle during the cardiac cycle. The beneficial effects of spiral blood flow include reducing flow turbulence, reducing stagnation and normalizing WSS - increasing its absolute values and reducing oscillations of WSS [89]. In CFD studies, it was found that stents generating a spiral flow of blood lead to the increase in WSS, which depends directly on the size and position of the ridge [89]. For example, it was shown according to BioMimics 3DTM CFD-studies of the stent, that WSS was significantly higher inside a spiral stent compared to the control group - 1.13 ± 0.13 Pa vs. 1.06 ± 0.12 Pa (p = 0.05) [90]. In this case, according to the study of F. Kabinejadian et al., the helical stents allowed for optimal modification of the flow compared with spiral stents [91]. Thus, there were significantly fewer portions of the vascular wall exposed to WSS with high oscillatory index, and average WSS values were significantly higher in BioMimics 3DTM-type stents.
The development and introduction into clinical practice of stents and flow-modifying grafts is a perspective and promising direction, however, requiring further clinical studies.
7.2. Individual Shear Rate Therapy
Given the above data on the role of WSS in angiogenesis, the idea of stimulating angiogenesis seems logical and promising by artificial increase of WSS [92]. In addition, physical training-induced angiogenesis, mediated predominantly by WSS, is a natural model demonstrating the effectiveness of this approach [93].
The pioneers in this field are the team of authors who developed the Individual Shear Rate Therapy (ISRT) technique, adapting an external counterpulsation system [94].
Previously, the study results obtained indicate that the use of Enhanced External Counterpulsation (EECP) may contribute to the development of new collateral blood flow networks [95, 96]. It was found in the study on the dogs that the use of EECP is associated with the increase in the density of microcirculation vessels per square millimeter of myocardial tissue in the infarction areas [97, 98]. Clinical prospective studies have also demonstrated a reduction in myocardial ischemia according to perfusion scan after 1 month of EECP [99].
Initially, ISRT was conceived as a therapeutic angiogenesis system for the patients with Peripheral Arterial Disease (PAD) and intermittent claudication. The efferent part of the ISRT-system consists of two pairs of cuffs located on the hip and thighs. Compression of the cuffs is coordinated by synchronous ECG recording - it starts in the late systole phase and ends in the early diastole phase. Selection of the required therapy regimen occurs individually by measuring and analyzing the parameters of the Doppler study.
According to the studies conducted both on healthy volunteers and on the patients with intermittent claudication, it was found that long-term ISRT led to a significant increase in blood nitrites in the patients with PAD, indicating an increase in NO synthesis and improvement in endothelial function [100]. In the ISRT study, which included 14 patients with PAD IIb stage according to Fontein, the effect of ISRT on endothelial function and walking distance was studied. It was found that long-term ISRT led to a significant increase in the flow-mediated dilation of brachial artery - from 0.13 ± 0.09 mm to 0.38±0.05 mm (p<0.05) [101].
An increase in painless walking distance (from 92.6 ± 8.2 meters to 280 ± 101.3 meters, p <0.05) and absolute walking distance (from 167.8 ± 18.1 meters to 446.7 ± 133.3 meters; p < 0.05) was also recorded. In addition, the authors found that long-term ISRT led to a significant increase in the activity of telomerases of peripheral blood mononuclear cells in the patients with PAD, which, according to the authors, could indicate improved monocyte and macrophage regeneration, which is a condition for successful angiogenesis [102].
The study of F. Picard et al. studied the effect of ISRT on the course of stable CAD. It has been shown that ISRT for 6 weeks increased the walking distance in the 6-minute walking test for 78 meters (p = 0.007). In addition, decrease in pulse wave velocity by 1.2 m/s (p = 0.004) and the central arterial pressure by 12 mmHg (p = 0.008) was noted. Decrease in systolic blood pressure by 15 mm Hg (p < 0.001) and diastolic blood pressure by 8 mm Hg (p = 0.033) was also observed according to daily blood pressure monitoring [103].
Thus, it can be assumed that ISRT, due to a variety of systemic effects, may in the future be the effective method for treating various atherosclerotic CVD, and not just PAD.
7.3. Therapeutic Ability to Influence on WSS
In accordance with Hagen-Poiseuille law, WSS is directly proportional to blood viscosity and WSR. Accordingly, the effect on WSS is possible both by changing the WSR (for example, during the physical exercise or ISRT), and by influencing the blood viscosity.
At the same time, a decrease in blood viscosity should lead to a decrease in WSS, and increase - to increase in WSS. Thus, the increase in WSS, which is hypothetically an atheroprotective factor, is possible with an increase in blood viscosity. On the other hand, according to large epidemiological studies, an increase in the viscosity of whole blood and plasma in the general population is associated with increase of relative risk of adverse cardiovascular events [104]. At the same time, according to B.Y. Salazar Vázquez et al., moderate increase in blood viscosity may be associated with improved endothelial function and may have positive effects on cardiovascular health. It is especially noted that currently there is not enough data to determine the optimal level of hematocrit and blood viscosity to maintain vascular and metabolic homeostasis [105].
In this context, the results of the study of SGLT2-inhibitor empagliflozin, which demonstrated a decrease in the relative risk of cardiovascular complications in the patients with type 2 diabetes, are extremely interesting. According to the study of C. Irace et al., administration of empagliflozin for 3 months led to significant changes in hemorheology and carotid artery structure [106]. Thus, there was a statistically significant increase in blood viscosity, and a decrease in the diameter of the CCA, which led to a significant increase in carotid WSS. These changes were not observed in the liraglutide treatment group. Thus, according to the outcome of a short-term study, empagliflozin increased blood viscosity and carotid WSS, and according to the outcome of a long-term study (EMPA-REG Outcome) significantly reduced the risk of adverse cardiovascular events and the risk of all-cause death. How these phenomena are related to each other and what the nature of these relationships should answer future research.
However, problematisation of this issue is extremely important and interesting from the point of view of possible ways of pharmacological influence on WSS and the associated risks of benefits.
7.4. Therapeutic Targeting of Mechanosensitive Transcription Factors
The mechanisms by which the biomechanical forces realize their pro-atherogenic and atheroprotective effects were described in Section 3. The development and study of the agents that affect the mechanosensing and subsequent expression of genes that regulate vascular homeostasis are very promising.
One of the most promising targets is KLF2 and KLF4, whose activation has a number of anti-atherogenic effects [107]. The ability to regulate these factors has been identified in several pharmacological agents: statins, resveratrol, vorinostat (suberanilohydroxamic acid), tannic acid, etc. Thus, for example, it was found that statins up-regulate KLF2 via the mevalonate pathway and MEF2 (myocyte enhancer factor 2)-dependent pathway [108]. For the other agents listed above, the mechanisms of KLF2 up-regulation are also described [107].
Another possible direction is the up-regulation of nuclear factor (erythroid-derived 2)-like 2 NRF2. One of the activators of NRF2 is sulforaphane substance isolated from broccoli [109]. The up-regulation of NRF2 by sulforaphane leads to the inhibition of the activation of ECs in places of the vessel that are subject to disorganized blood flow through the suppression of p38/VCAM1 signaling pathway [109]. Other promising areas are inhibition of NF-kB (for example, Vinpocetine), HIF-1a (evodiamine, digoxin) and YAP/TAZ/ TEAD-signaling pathway.
Conclusion
Biomechanical forces are the most important factors in maintaining vascular homeostasis and are directly involved in its violation within the framework of pathological vascular remodeling, for example, in atherosclerosis. Studying the role of these factors in the pathogenesis of atherosclerosis can enhance the understanding of the mechanisms of atherosclerosis, as well as contribute to the discovery of fundamentally new methods of prevention and treatment of atherosclerotic CVD. Currently, the translation of the results of fundamental and clinical researches in this field into clinical practice has led to the development of new diagnostic and therapeutic approaches.
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- CAD
Coronary Artery Disease
- CCA
Common Carotid Artery
- CIMT
Carotid Intimal Medial Thickness
- CWS
Circumferential Wall Stress
- ECs
Endothelial Cells
- ISRT
Individual Shear Rate Therapy
- PAD
Peripheral Arterial Disease
- SMCs
Smooth Muscle Cells
- WSR
Wall Shear Rate
- WSS
Wall Shear Stress
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Bäck M., Hansson G.K. Anti-inflammatory therapies for atherosclerosis. Nat. Rev. Cardiol. 2015;12(4):199–211. doi: 10.1038/nrcardio.2015.5. [DOI] [PubMed] [Google Scholar]
- 2.Brown A.J., Teng Z., Evans P.C., Gillard J.H., Samady H., Bennett M.R. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat. Rev. Cardiol. 2016;13(4):210–220. doi: 10.1038/nrcardio.2015.203. [DOI] [PubMed] [Google Scholar]
- 3.Kwak BR, Bäck M, Bochaton-Piallat ML, et al. 2014.
- 4.Papaioannou T.G., Stefanadis C. Vascular wall shear stress: Basic principles and methods. Hellenic J. Cardiol. 2005;46(1):9–15. [PubMed] [Google Scholar]
- 5.Ricci S., Swillens A., Ramalli A., Segers P., Tortoli P. Wall shear rate measurement: Validation of a new method through multiphysics simulations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2017;64(1):66–77. doi: 10.1109/TUFFC.2016.2608442. [DOI] [PubMed] [Google Scholar]
- 6.Parker B.A., Trehearn T.L., Meendering J.R. Pick your poiseuille: Normalizing the shear stimulus in studies of flow-mediated dilation. J. Appl. Physiol. 2009;107(4):1357–1359. doi: 10.1152/japplphysiol.91302.2009. [DOI] [PubMed] [Google Scholar]
- 7.Reneman R.S., Hoeks A.P. Wall shear stress as measured in vivo: Consequences for the design of the arterial system. Med. Biol. Eng. Comput. 2008;46(5):499–507. doi: 10.1007/s11517-008-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martino F., Perestrelo A.R., Vinarský V., Pagliari S., Forte G. Cellular mechanotransduction: From tension to function. Front. Physiol. 2018;9:824. doi: 10.3389/fphys.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holle A.W., Engler A.J. More than a feeling: Discovering, understanding, and influencing mechanosensing pathways. Curr. Opin. Biotechnol. 2011;22(5):648–654. doi: 10.1016/j.copbio.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedorchak G.R., Kaminski A., Lammerding J. Cellular mechanosensing: Getting to the nucleus of it all. Prog. Biophys. Mol. Biol. 2014;115(2-3):76–92. doi: 10.1016/j.pbiomolbio.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y., Wu D., Birukov K.G. Mechanosensing and mechanoregulation of endothelial cell functions. Compr. Physiol. 2019;9(2):873–904. doi: 10.1002/cphy.c180020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y.S., Lee O.K. In search of the pivot point of mechanotransduction: Mechanosensing of stem cells. Cell Transplant. 2014;23(1):1–11. doi: 10.3727/096368912X659925. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee S. Endothelial mechanotransduction, redox signaling and the regulation of vascular inflammatory pathways. Front. Physiol. 2018;9:524. doi: 10.3389/fphys.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe J., Berk B.C. Novel mechanisms of endothelial mechanotransduction. Arterioscler. Thromb. Vasc. Biol. 2014;34(11):2378–2386. doi: 10.1161/ATVBAHA.114.303428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Effects of shear stress on endothelial cells: Go with the flow. Acta Physiol. (Oxf.) 2017;219(2):382–408. doi: 10.1111/apha.12725. [DOI] [PubMed] [Google Scholar]
- 16.Galie P.A., Nguyen D.H., Choi C.K., Cohen D.M., Janmey P.A., Chen C.S. Fluid shear stress threshold regulates angiogenic sprouting. Proc. Natl. Acad. Sci. USA. 2014;111(22):7968–7973. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez I., González M. Physiological mechanisms of vascular response induced by shear stress and effect of exercise in systemic and placental circulation. Front. Pharmacol. 2014;5:209. doi: 10.3389/fphar.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wragg J.W., Durant S., McGettrick H.M., Sample K.M., Egginton S., Bicknell R. Shear stress regulated gene expression and angiogenesis in vascular endothelium. Microcirculation. 2014;21(4):290–300. doi: 10.1111/micc.12119. [DOI] [PubMed] [Google Scholar]
- 19.Ajami N.E., Gupta S., Maurya M.R., et al. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proc. Natl. Acad. Sci. USA. 2017;114(41):10990–10995. doi: 10.1073/pnas.1707517114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moskovtsev AA, Kolesov DV, Mylnikova AN, et al. 2017.
- 21.Kadam A.A., Gersch R.P., Rosengart T.K., Frame M.D. Inflammatory monocyte response due to altered wall shear stress in an isolated femoral artery model. J. Biol. Methods. 2019;6(1):e109. doi: 10.14440/jbm.2019.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell M.J., Lin K.S., King M.R. Fluid shear stress increases neutrophil activation via platelet-activating factor. Biophys. J. 2014;106(10):2243–2253. doi: 10.1016/j.bpj.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niethammer P. Neutrophil mechanotransduction: A GEF to sense fluid shear stress. J. Cell Biol. 2016;215(1):13–14. doi: 10.1083/jcb.201609101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine N., Dimitriou I.D., Rullo J., et al. GEF-H1 is necessary for neutrophil shear stress-induced migration during inflammation. J. Cell Biol. 2016;215(1):107–119. doi: 10.1083/jcb.201603109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin W.D., Mi S.H., Li C., et al. Low shear stress induced HMGB1 translocation and release via PECAM-1/PARP-1 pathway to induce inflammation response. PLoS One. 2015;10(3):e0120586. doi: 10.1371/journal.pone.0120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison D.L., Fang Y., Huang J. T-Cell mechanobiology: Force sensation, potentiation, and translation. Front. Phys. 2019;7:45. doi: 10.3389/fphy.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dominguez G.A., Anderson N.R., Hammer D.A. The direction of migration of T-lymphocytes under flow depends upon which adhesion receptors are engaged. Integr. Biol. 2015;7(3):345–355. doi: 10.1039/C4IB00201F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jufri N.F., Mohamedali A., Avolio A., Baker M.S. Mechanical stretch: physiological and pathological implications for human vascular endothelial cells. Vasc. Cell. 2015;7:8. doi: 10.1186/s13221-015-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caro C.G., Fitz-Gerald J.M., Schroter R.C. Arterial wall shear and distribution of early atheroma in man. Nature. 1969;223(5211):1159–1160. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- 30.Li X., Yang Q., Wang Z., Wei D. Shear stress in atherosclerotic plaque determination. DNA Cell Biol. 2014;33(12):830–838. doi: 10.1089/dna.2014.2480. [DOI] [PubMed] [Google Scholar]
- 31.Harloff A, Markl M. 2012.
- 32.Otero-Cacho A., Aymerich M., Flores-Arias M.T., et al. Determination of hemodynamic risk for vascular disease in planar artery bifurcations. Sci. Rep. 2018;8(1):2795. doi: 10.1038/s41598-018-21126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markl M., Wegent F., Zech T., et al. In vivo wall shear stress distribution in the carotid artery: Effect of bifurcation geometry, internal carotid artery stenosis, and recanalization therapy. Circ Cardiovasc Imaging. 2010;3(6):647–655. doi: 10.1161/CIRCIMAGING.110.958504. [DOI] [PubMed] [Google Scholar]
- 34.Dhawan S.S., Avati Nanjundappa R.P., Branch J.R., et al. Shear stress and plaque development. Expert Rev. Cardiovasc. Ther. 2010;8(4):545–556. doi: 10.1586/erc.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Giessen A.G., Wentzel J.J., Meijboom W.B., et al. Plaque and shear stress distribution in human coronary bifurcations: A multislice computed tomography study. EuroIntervention. 2009;4(5):654–661. doi: 10.4244/EIJV4I5A109. [DOI] [PubMed] [Google Scholar]
- 36.Kuhlmann M.T., Cuhlmann S., Hoppe I., et al. Implantation of a carotid cuff for triggering shear-stress induced atherosclerosis in mice. J. Vis. Exp. 2012;13(59):3308. doi: 10.3791/3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carallo C., Tripolino C., De Franceschi M.S., Irace C., Xu X.Y., Gnasso A. Carotid endothelial shear stress reduction with aging is associated with plaque development in twelve years. Atherosclerosis. 2016;251:63–69. doi: 10.1016/j.atherosclerosis.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 38.Arzani A., Gambaruto A.M., Chen G., Shadden S.C. Wall shear stress exposure time: A Lagrangian measure of near-wall stagnation and concentration in cardiovascular flows. Biomech. Model. Mechanobiol. 2017;16(3):787–803. doi: 10.1007/s10237-016-0853-7. [DOI] [PubMed] [Google Scholar]
- 39.Vozzi F., Campolo J., Cozzi L., et al. Computing of low shear stress-driven endothelial gene network involved in early stages of atherosclerotic process. BioMed Res. Int. 2018;2018:5359830. doi: 10.1155/2018/5359830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahmoud M.M., Serbanovic-Canic J., Feng S., et al. Shear stress induces endothelial-to-mesenchymal transition via the transcription factor Snail. Sci. Rep. 2017;7(1):3375. doi: 10.1038/s41598-017-03532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu J.J., Usami S., Chien S. Vascular endothelial responses to altered shear stress: Pathologic implications for atherosclerosis. Ann. Med. 2009;41(1):19–28. doi: 10.1080/07853890802186921. [DOI] [PubMed] [Google Scholar]
- 42.Simmons R.D., Kumar S., Thabet S.R., Sur S., Jo H. Omics-based approaches to understand mechanosensitive endothelial biology and atherosclerosis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016;8(5):378–401. doi: 10.1002/wsbm.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baeyens N., Bandyopadhyay C., Coon B.G., Yun S., Schwartz M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Invest. 2016;126(3):821–828. doi: 10.1172/JCI83083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hung O.Y., Brown A.J., Ahn S.G., Veneziani A., Giddens D.P., Samady H. Association of wall shear stress with coronary plaque progression and transformation. Interv. Cardiol. Clin. 2015;4(4):491–502. doi: 10.1016/j.iccl.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Stone P.H., Coskun A.U., Kinlay S., et al. Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: An in vivo serial study. Eur. Heart J. 2007;28(6):705–710. doi: 10.1093/eurheartj/ehl575. [DOI] [PubMed] [Google Scholar]
- 46.Stone P.H., Saito S., Takahashi S., et al. PREDICTION Investigators Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: The prediction study. Circulation. 2012;126(2):172–181. doi: 10.1161/CIRCULATIONAHA.112.096438. [DOI] [PubMed] [Google Scholar]
- 47.Liu X., Wu G., Xu C., et al. Prediction of coronary plaque progression using biomechanical factors and vascular characteristics based on computed tomography angiography. Comput. Assist. Surg. (Abingdon) 2017;22(1):286–294. doi: 10.1080/24699322.2017.1389407. [DOI] [PubMed] [Google Scholar]
- 48.Parodi O., Exarchos T.P., Marraccini P., et al. Patient-specific prediction of coronary plaque growth from CTA angiography: A multiscale model for plaque formation and progression. IEEE Trans. Inf. Technol. Biomed. 2012;16(5):952–965. doi: 10.1109/TITB.2012.2201732. [DOI] [PubMed] [Google Scholar]
- 49.Samady H., Eshtehardi P., McDaniel M.C., et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124(7):779–788. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
- 50.Shishikura D., Sidharta S.L., Honda S., et al. The relationship between segmental wall shear stress and lipid core plaque derived from near-infrared spectroscopy. Atherosclerosis. 2018;275:68–73. doi: 10.1016/j.atherosclerosis.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 51.Corban M.T., Eshtehardi P., Suo J., et al. Combination of plaque burden, wall shear stress, and plaque phenotype has incremental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis. 2014;232(2):271–276. doi: 10.1016/j.atherosclerosis.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 52.Murata N., Hiro T., Takayama T., et al. High shear stress on the coronary arterial wall is related to computed tomography-derived high-risk plaque: A three-dimensional computed tomography and color-coded tissue-characterizing intravascular ultrasonography study. Heart Vessels. 2019;34(9):1429–1439. doi: 10.1007/s00380-019-01389-y. [DOI] [PubMed] [Google Scholar]
- 53.Boyle J.J., Weissberg P.L., Bennett M.R. Human macrophage-induced vascular smooth muscle cell apoptosis requires NO enhancement of Fas/Fas-L interactions. Arterioscler. Thromb. Vasc. Biol. 2002;22(10):1624–1630. doi: 10.1161/01.ATV.0000033517.48444.1A. [DOI] [PubMed] [Google Scholar]
- 54.Kok A.M., Molony D.S., Timmins L.H., et al. The influence of multidirectional shear stress on plaque progression and composition changes in human coronary arteries. EuroIntervention. 2019;15(8):692–699. doi: 10.4244/EIJ-D-18-00529. [DOI] [PubMed] [Google Scholar]
- 55.Koskinas K.C., Chatzizisis Y.S., Baker A.B., Edelman E.R., Stone P.H., Feldman C.L. The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Curr. Opin. Cardiol. 2009;24(6):580–590. doi: 10.1097/HCO.0b013e328331630b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Costopoulos C., Timmins L.H., Huang Y., et al. Impact of combined plaque structural stress and wall shear stress on coronary plaque progression, regression, and changes in composition. Eur. Heart J. 2019;40(18):1411–1422. doi: 10.1093/eurheartj/ehz132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widlansky M.E. Shear stress and flow-mediated dilation: All shear responses are not created equally. Am. J. Physiol. Heart Circ. Physiol. 2009;296(1):H31–H32. doi: 10.1152/ajpheart.01187.2008. [DOI] [PubMed] [Google Scholar]
- 58.Nishiyama S.K., Walter Wray D., Berkstresser K., Ramaswamy M., Richardson R.S. Limb-specific differences in flow-mediated dilation: The role of shear rate. J. Appl. Physiol. 2007;103(3):843–851. doi: 10.1152/japplphysiol.00273.2007. [DOI] [PubMed] [Google Scholar]
- 59.Zhang B., Ma Y., Ding F. Evaluation of spatial distribution and characterization of wall shear stress in carotid sinus based on two-dimensional color Doppler imaging. Biomed. Eng. Online. 2018;17(1):141. doi: 10.1186/s12938-018-0589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gates P.E., Gurung A., Mazzaro L., et al. Measurement of wall shear stress exerted by flowing blood in the human carotid artery: Ultrasound doppler velocimetry and echo particle image velocimetry. Ultrasound Med. Biol. 2018;44(7):1392–1401. doi: 10.1016/j.ultrasmedbio.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng S.L., Shih C.T., Huang C.W., Chiu S.C., Shen W.C. Optimized analysis of blood flow and wall shear stress in the common carotid artery of rat model by phase-contrast MRI. Sci. Rep. 2017;7(1):5253. doi: 10.1038/s41598-017-05606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pantos I., Patatoukas G., Efstathopoulos E.P., Katritsis D. In vivo wall shear stress measurements using phase-contrast MRI. Expert Rev. Cardiovasc. Ther. 2007;5(5):927–938. doi: 10.1586/14779072.5.5.927. [DOI] [PubMed] [Google Scholar]
- 63.Ha H., Kim G.B., Kweon J., et al. Hemodynamic measurement using four-dimensional phase-contrast MRI: Quantification of hemodynamic parameters and clinical applications. Korean J. Radiol. 2016;17(4):445–462. doi: 10.3348/kjr.2016.17.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Giessen A.G., Schaap M., Gijsen F.J., et al. 3D fusion of intravascular ultrasound and coronary computed tomography for in-vivo wall shear stress analysis: A feasibility study. Int. J. Cardiovasc. Imaging. 2010;26(7):781–796. doi: 10.1007/s10554-009-9546-y. [DOI] [PubMed] [Google Scholar]
- 65.Itatani K., Miyazaki S., Furusawa T., et al. New imaging tools in cardiovascular medicine: Computational fluid dynamics and 4D flow MRI. Gen. Thorac. Cardiovasc. Surg. 2017;65(11):611–621. doi: 10.1007/s11748-017-0834-5. [DOI] [PubMed] [Google Scholar]
- 66.Steinman D.A. Image-based computational fluid dynamics: A new paradigm for monitoring hemodynamics and atherosclerosis. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004;4(2):183–197. doi: 10.2174/1568006043336302. [DOI] [PubMed] [Google Scholar]
- 67.Xing R., Moerman A.M., Ridwan Y., et al. Temporal and spatial changes in wall shear stress during atherosclerotic plaque progression in mice. R. Soc. Open Sci. 2018;5(3):171447. doi: 10.1098/rsos.171447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang B., Gu J., Qian M., Niu L., Zhou H., Ghista D. Correlation between quantitative analysis of wall shear stress and intima-media thickness in atherosclerosis development in carotid arteries. Biomed. Eng. Online. 2017;16(1):137. doi: 10.1186/s12938-017-0425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Genkel V.V., Salashenko A.O., Shamaeva T.N., Sumerkina V.A., Shaposhnik I.I. Association between carotid wall shear rate and arterial stiffness in patients with hypertension and atherosclerosis of peripheral arteries. Int. J. Vasc. Med. 2018;2018:6486234. doi: 10.1155/2018/6486234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cho K.I., Kim B.H., Kim H.S., Heo J.H. Low carotid artery wall shear stress is associated with significant coronary artery disease in patients with chest pain. J. Atheroscler. Thromb. 2016;23(3):297–308. doi: 10.5551/jat.31377. [DOI] [PubMed] [Google Scholar]
- 71.Ng J., Bourantas C.V., Torii R., et al. Local hemodynamic forces after stenting: Implications on restenosis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017;37(12):2231–2242. doi: 10.1161/ATVBAHA.117.309728. [DOI] [PubMed] [Google Scholar]
- 72.Papafaklis M.I., Bourantas C.V., Theodorakis P.E., et al. The effect of shear stress on neointimal response following sirolimus- and paclitaxel-eluting stent implantation compared with bare-metal stents in humans. JACC Cardiovasc. Interv. 2010;3(11):1181–1189. doi: 10.1016/j.jcin.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 73.Torii R., Stettler R., Räber L., et al. Implications of the local hemodynamic forces on the formation and destabilization of neoatherosclerotic lesions. Int. J. Cardiol. 2018;272:7–12. doi: 10.1016/j.ijcard.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 74.Tenekecioglu E., Torii R., Katagiri Y., et al. Post-implantation shear stress assessment: An emerging tool for differentiation of bioresorbable scaffolds. Int. J. Cardiovasc. Imaging. 2019;35(3):409–418. doi: 10.1007/s10554-018-1481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Y., Wei F., Wang J., et al. Carotid artery wall shear stress is independently correlated with renal function in the elderly. Oncotarget. 2018;9(4):5251–5262. doi: 10.18632/oncotarget.23825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Z., Zhao Y., Wang X., et al. Low carotid artery wall shear stress is independently associated with brain white-matter hyperintensities and cognitive impairment in older patients. Atherosclerosis. 2016;247:78–86. doi: 10.1016/j.atherosclerosis.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H., Liu H., Dong Y., et al. Low carotid wall shear stress independently accelerates the progression of cognitive impairment and white matter lesions in the elderly. Oncotarget. 2017;9(13):11402–11413. doi: 10.18632/oncotarget.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valen-Sendstad K., Bergersen A.W., Shimogonya Y., et al. Real-world variability in the prediction of intracranial aneurysm wall shear stress: The 2015 international aneurysm CFD Challenge. Cardiovasc. Eng. Technol. 2018;9(4):544–564. doi: 10.1007/s13239-018-00374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grewal N., Gittenberger-de Groot A.C. Wall shear stress directional abnormalities in BAV aortas: Toward a new hemodynamic predictor of aortopathy? Front. Physiol. 2019;10(10):225. doi: 10.3389/fphys.2019.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen H.Y., Hermiller J., Sinha A.K., Sturek M., Zhu L., Kassab G.S. Effects of stent sizing on endothelial and vessel wall stress: Potential mechanisms for in-stent restenosis. J. Appl. Physiol. 2009;106(5):1686–1691. doi: 10.1152/japplphysiol.91519.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wentzel J.J., Gijsen F.J.H., Schuurbiers J.C.H., van der Steen A.F.W., Serruys P.W. The influence of shear stress on in-stent restenosis and thrombosis. EuroIntervention. 2008;4(Suppl. C):C27–C32. [PubMed] [Google Scholar]
- 82.Koskinas K.C., Chatzizisis Y.S., Antoniadis A.P., Giannoglou G.D. Role of endothelial shear stress in stent restenosis and thrombosis: Pathophysiologic mechanisms and implications for clinical translation. J. Am. Coll. Cardiol. 2012;59(15):1337–1349. doi: 10.1016/j.jacc.2011.10.903. [DOI] [PubMed] [Google Scholar]
- 83.Chesnutt J.K., Han H.C. Computational simulation of platelet interactions in the initiation of stent thrombosis due to stent malapposition. Phys. Biol. 2016;13(1):016001. doi: 10.1088/1478-3975/13/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kokkalis E., Aristokleous N., Houston J.G. Haemodynamics and flow modification stents for peripheral arterial disease: A review. Ann. Biomed. Eng. 2016;44(2):466–476. doi: 10.1007/s10439-015-1483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stonebridge P.A., Vermassen F., Dick J., Belch J.J.F., Houston G. Spiral laminar flow prosthetic bypass graft: Medium-term results from a first-in-man structured registry study. Ann. Vasc. Surg. 2012;26(8):1093–1099. doi: 10.1016/j.avsg.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Caro C.G., Cheshire N.J., Watkins N. Preliminary comparative study of small amplitude helical and conventional ePTFE arteriovenous shunts in pigs. J. R. Soc. Interface. 2005;2(3):261–266. doi: 10.1098/rsif.2005.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huijbregts H.J., Blankestijn P.J., Caro C.G., et al. A helical PTFE arteriovenous access graft to swirl flow across the distal anastomosis: Results of a preliminary clinical study. Eur. J. Vasc. Endovasc. Surg. 2007;33(4):472–475. doi: 10.1016/j.ejvs.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 88.Zeller T., Gaines P.A., Ansel G.M., Caro C.G. Helical centerline stent improves patency: Two-year results from the randomized mimics trial. Circ. Cardiovasc. Interv. 2016;9(6):002930. doi: 10.1161/CIRCINTERVENTIONS.115.002930. [DOI] [PubMed] [Google Scholar]
- 89.Ruiz-Soler A., Kabinejadian F., Slevin M.A., Bartolo P.J., Keshmiri A. Optimisation of a novel spiral-inducing bypass graft using computational fluid dynamics. Sci. Rep. 2017;7(1):1865. doi: 10.1038/s41598-017-01930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sullivan T.M., Zeller T., Nakamura M., Caro C.G., Lichtenberg M. Swirling flow and wall shear: Evaluating the biomimics 3d helical centerline stent for the femoropopliteal segment. Int. J. Vasc. Med. 2018;2018:9795174. doi: 10.1155/2018/9795174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kabinejadian F., McElroy M., Ruiz-Soler A., et al. Numerical assessment of novel helical/spiral grafts with improved hemodynamics for distal graft anastomoses. PLoS One. 2016;11(11):e0165892. doi: 10.1371/journal.pone.0165892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bondke Persson A., Buschmann E.E., Lindhorst R., et al. Therapeutic arteriogenesis in peripheral arterial disease: Combining intervention and passive training. Vasa. 2011;40(3):177–187. doi: 10.1024/0301-1526/a000092. [DOI] [PubMed] [Google Scholar]
- 93.Padilla J., Simmons G.H., Bender S.B., Arce-Esquivel A.A., Whyte J.J., Laughlin M.H. Vascular effects of exercise: Endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 2011;26(3):132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Picard F., Panagiotidou P., Wolf-Pütz A., et al. Individual shear rate therapy (ISRT)-further development of external counterpulsation for decreasing blood pressure in patients with symptomatic coronary artery disease (CAD). Hypertens. Res. 2020;43(3):186–196. doi: 10.1038/s41440-019-0380-x. [DOI] [PubMed] [Google Scholar]
- 95.Qin X., Deng Y., Wu D., Yu L., Huang R. Does Enhanced External Counterpulsation (EECP) Significantly affect myocardial perfusion?: A systematic review meta-analysis. PLoS One. 2016;11(4):e0151822. doi: 10.1371/journal.pone.0151822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bonetti P.O., Holmes D.R., Jr, Lerman A., Barsness G.W. Enhanced external counterpulsation for ischemic heart disease: What’s behind the curtain? J. Am. Coll. Cardiol. 2003;41(11):1918–1925. doi: 10.1016/s0735-1097(03)00428-5. [DOI] [PubMed] [Google Scholar]
- 97.Wu G., Du Z., Hu C., et al. Angiogenic effects of long-term enhanced external counterpulsation in a dog model of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2006;290(1):H248–H254. doi: 10.1152/ajpheart.01225.2004. [DOI] [PubMed] [Google Scholar]
- 98.Wu G.F., Du Z.M., Hu C.H., et al. Microvessel angiogenesis: A possible cardioprotective mechanism of external counterpulsation for canine myocardial infarction. Chin. Med. J. (Engl.) 2005;118(14):1182–1189. [PubMed] [Google Scholar]
- 99.Eslamian F., Aslanabadi N., Mahmoudian B., Shakouri S.K. Therapeutic effects of enhanced external counterpulsation on clinical sumptoms, echocardiographic measurements, perfusion scan parameters and exercise tolerance test in coronary artery disease patients with refractory angina. Int. J. Med. Sci. Public Health. 2013;2:187–195. doi: 10.5455/ijmsph.2013.2.179-187. [DOI] [Google Scholar]
- 100.Brix M., Buschmann E.E., Zietzer A., et al. Long-term individual shear rate therapy counterpulsation enhances plasma nitrite release in patients with PAD. Vasa. 2017;46(1):37–45. doi: 10.1024/0301-1526/a000600. [DOI] [PubMed] [Google Scholar]
- 101.Buschmann E.E., Brix M., Li L., et al. Adaptation of external counterpulsation based on individual shear rate therapy improves endothelial function and claudication distance in peripheral artery disease. Vasa. 2016;45(4):317–324. doi: 10.1024/0301-1526/a000544. [DOI] [PubMed] [Google Scholar]
- 102.Zietzer A., Buschmann E.E., Janke D., et al. Acute physical exercise and long-term individual shear rate therapy increase telomerase activity in human peripheral blood mononuclear cells. Acta Physiol. (Oxf.) 2017;220(2):251–262. doi: 10.1111/apha.12820. [DOI] [PubMed] [Google Scholar]
- 103.Picard F., Panagiotidou P., Wolf-Pütz A., et al. Usefulness of individual shear rate therapy, new treatment option for patients with symptomatic coronary artery disease. Am. J. Cardiol. 2018;121(4):416–422. doi: 10.1016/j.amjcard.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 104.Peters S.A., Woodward M., Rumley A., Tunstall-Pedoe H.D., Lowe G.D. Plasma and blood viscosity in the prediction of cardiovascular disease and mortality in the Scottish Heart Health Extended Cohort Study. Eur. J. Prev. Cardiol. 2017;24(2):161–167. doi: 10.1177/2047487316672004. [DOI] [PubMed] [Google Scholar]
- 105.Salazar Vázquez B.Y., Martini J., Chávez Negrete A., et al. Cardiovascular benefits in moderate increases of blood and plasma viscosity surpass those associated with lowering viscosity: Experimental and clinical evidence. Clin. Hemorheol. Microcirc. 2010;44(2):75–85. doi: 10.3233/CH-2010-1261. [DOI] [PubMed] [Google Scholar]
- 106.Irace C., Casciaro F., Scavelli F.B., et al. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc. Diabetol. 2018;17(1):52. doi: 10.1186/s12933-018-0695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Niu N., Xu S., Xu Y., Little P.J., Jin Z.G. Targeting mechanosensitive transcription factors in atherosclerosis. Trends Pharmacol. Sci. 2019;40(4):253–266. doi: 10.1016/j.tips.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parmar K.M., Nambudiri V., Dai G., Larman H.B., Gimbrone M.A., Jr, García-Cardeña G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 2005;280(29):26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 109.Zakkar M., Van der Heiden K., Luong A., et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler. Thromb. Vasc. Biol. 2009;29(11):1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]