Abstract
Background
There is a growing interest in the observed significant incidence of transthyretin cardiac amyloidosis in elderly patients with aortic stenosis. Approximately, 16% of patients with severe aortic stenosis undergoing aortic valve replacement have transthyretin cardiac amyloidosis. Outcomes after aortic valve replacement appear to be worst in patients with concomitant transthyretin cardiac amyloidosis.
Methods
Publications in PubMed, Cochrane Library, and Embase databases were systematically searched from January 2012 to September 2018 using the keywords transthyretin, amyloidosis, and aortic stenosis. All studies published in English that reported the prevalence, association and outcomes of transthyretin cardiac amyloidosis in patients with aortic stenosis undergoing were included.
Results/Conclusion
The relationship between aortic stenosis and transthyretin cardiac amyloidosis is not well understood. A few studies have proven successful surgical management when both conditions coexist. This systematic review suggests that transthyretin cardiac amyloidosis is common in elderly patients with aortic stenosis and tend to have high mortality rates after AVR. The significant incidence of the two diseases occurring simultaneously warrants further investigation to improve management strategies in the future.
Keywords: Aortic stenosis, transthyretin, cardiac amyloidosis, aortic valve replacement, left ventricular myocardium, hereditary amyloidosis
1. INTRODUCTION
Aortic Stenosis (AS) is characterized by failure of the aortic valve to open completely, impeding forward flow which subjects the left ventricular myocardium to increased pressures [1, 2]. The current theory of the most common etiology is calcification and sclerosis of anatomically normal or bicuspid aortic valves, although this process is not well understood [1, 2]. There are other known inflammatory and deposition disorders known to cause AS, although they too are not well understood [2-5].
Cardiac Amyloidosis is the deposition of misfolded proteins in the myocardium which can lead to a variety of functional disorders of the heart. Transthyretin (TTR) is a serum protein that normally binds and transports thyroxine and retinol [3]. TTR is the most common cause of Cardiac Amyloidosis. Both TTR Cardiac Amyloidosis and AS are diseases that are positively correlated with age, and fairly common in the elderly [5-8].
There is a growing interest in the observed significant incidence of Transthyretin Cardiac Amyloidosis (TTR-CA) in patients with aortic stenosis. Aortic stenosis is the most common valvular heart disease in the developed world and it is expected to further impact healthcare due to the aging Western population [5]. According to the American Heart Association (AHA) guidelines for the management of patients with valvular heart disease, the standard management of severe symptomatic AS is to consider Aortic Valve Replacement (AVR) [9]. It is estimated that 16% of patients with severe aortic stenosis receiving AVR have TTR-CA, which is associated with a severe phenotype of low-flow low-gradient resulting in a poor prognosis [6]. In a recent study, patients with TTR-CA with concurrent aortic stenosis had a 56% 1-year all-cause mortality compared with 20% 1-year all-cause mortality in patients with isolated aortic stenosis [10]. It is now recognized that patients with coexistent AS and TTR-CA have worse prognosis when compared patients with isolated AS [10]. This associated worsened prognosis has increased the demand for research regarding TTR-CA and AS. The purpose of this article is to review the available research on TTR-CA and AS.
2. Epidemiology and relevance
Aortic stenosis is the most common valvular heart disease in the developed world. There is a positive correlation between age and AS prevalence [7]. Aortic stenosis most commonly affects the elderly population predominantly elderly men. The prevalence of AS in the elderly greater than 75 years of age is estimated to be 12.4%, and the estimated prevalence of severe AS is 3.4% [5]. By the year 2050, it is estimated that the United States elderly population (>65 years-old) will reach 84 million which is nearly double the estimated elderly population in 2012 which was 43 million [11]. This increase in the elderly population is expected to correlate with an increased total prevalence of AS [5]. Elderly patients with symptomatic AS have average survival rates of 1.8 years [7].
Cardiac Amyloidosis is a heterogenous group of conditions involving proteinaceous infiltration of the myocardium [1]. It is difficult to estimate the prevalence of the condition due to unestablished standards for screening and detection. This stems from the invasive nature of confirming TTR-CA diagnosis on biopsy [12]. However, there is promising data suggesting non-invasive alternatives to detect TTR-CA. A 2015 nuclear medicine study used myocardial radiotracer uptake on bone scintigraphy to estimate the prevalence of TTR-CA in the elderly population, one of the first studies of its kind; they found 13.9% prevalence in elderly males and 2.7% in elderly females, and an estimated 2.59% prevalence in the general population [13]. Additionally, this study found myocardial radiotracer uptake on bone scintigraphy was 99% sensitive and 86% specific for TTR-CA screening and diagnosis [13]. A more recent 2018 meta-analysis found that bone scintigraphy in the assessment of TTR-CA has a sensitivity of 92.2% and specificity of 95.4% [14]. Although this recent data demonstrates the potential for non-invasive detection of TTR-CA, it is worth noting this non-invasive technique is relatively new and has not formally been accepted as the gold standard of TTR-CA screening. Our current estimates of cardiac amyloidosis incidence come from pre-operative referrals for individuals who have severe AS and are being considered for AVR [15]. The study by Scully et al. found a TTR-CA prevalence of 16% in men and 12% in women via bone scintigraphy which was confirmed via biopsy, in patients referred for AVR [15]. However, this makes the estimation of cardiac amyloidosis biased toward patients with severe AS, while the incidence of isolated cardiac amyloidosis may be different.
Overall, both AS and TTR-CA have an age-correlated prevalence. A significant portion of the elderly population is affected by these diseases, and AS concurrent with TTR-CA seems to occur in a significant portion of patients referred for AVR.
3. Type of amyloidosis affecting the heart
Any type of amyloid protein can deposit and affect the heart (Fig. 1); this includes primary light chain (AL) amyloid, secondary amyloid A (AA) amyloid, age-related or hereditary type transthyretin amyloid, etc. [3]. The most common amyloid types affecting the heart are described below.
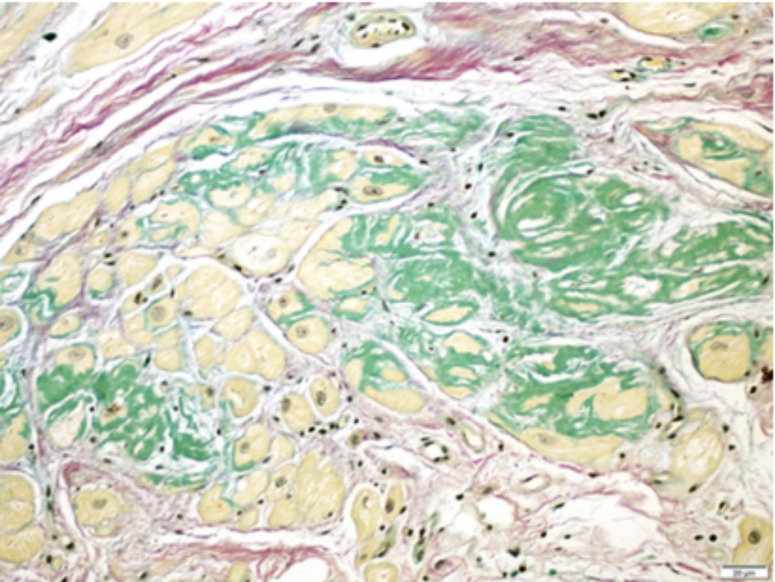
Fig. (1).
Example of TTR-CA. This photo-micrograph of myocardial biopsy shows discrete pericellular deposits of amyloid protein (green) in the interstitium between the atrial myocytes (yellow). Java et al. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.1. Senile
Wild-type transthyretin (w-TTR) is normally a transport protein synthesized in the liver and choroid plexus. When misfolded it can form amyloid deposits. TTR-CA is typically seen in men >70 years old and is prevalent in about 25% of people older than 80 years. Extensive amyloid deposition leads to clinically significant heart failure and median survival is 75 months [12, 15].
3.2. Hereditary Amyloidosis (Familial)
An autosomal dominant disease with mutated proteins that form amyloid fibrils. More than 120 mutations have been described for familial TTR amyloidosis [4]. It causes a myriad of clinical manifestations including polyneuropathy, cardiac involvement, and mixed patterns. An isoleucine 122 gene mutation of TTR DNA causes familial amyloidosis primarily affecting the heart and it is unique to elderly black patients [12, 15]. Overall this type of TTR is less common.
If tissue sampling (cardiac or non-cardiac tissue) confirms the presence of TTR in the amyloid deposits, then Isoelectric focusing (IEF) electrophoresis of serum distinguishes the hereditary TTR variant from the wild-type TTR [16]. Genetic testing can then identify the polymorphism and the type of mutation. In a series of pathologic studies of 33 patients, mutant TTR was found in 36% and w-TTR in 64%, noting that w-TTR is more common [17].
Java et al. reported that in patients that underwent AVR, the type of amyloidosis found on cardiac tissue specimen examination was AL amyloid in 6 patients (38%), w-TTR in 6 patients (38%), and localized in 4 patients (25%) [18]. Patients with AL amyloid versus w-TTR amyloid had a median survival of 6 months versus 3.5 years after the onset of heart failure in untreated patients [18]. No hereditary type was seen in this series of cases [18]. Java et al. results suggest an association between the specific type of amyloid deposition and varying prognosis. Similarly, Longhi et al. studied 43 patients with severe aortic stenosis with possible amyloidosis features and confirmed that 5 (11.6%) of them had w-TTR amyloidosis [19]. Scully et al. found w-TTR amyloidosis in his patient population that underwent TAVR, and no cases of hereditary type [20].
4. Clinical Features and Exam
The clinical characteristics of patients with aortic stenosis and coexisting amyloidosis include a diverse range of symptoms that can be difficult to distinguish from cases of isolated AS or cardiac amyloidosis. Java et al. utilized the New York Heart Association (NYHA) functional class of heart failure to classify the patients by symptom severity; about 50% of the patients had NYHA class II, 44% had NYHA class III/IV, and one patient presented with cardiogenic shock requiring inotropes [18]. In this report, two patients (13%) had atrial arrhythmias. Longhi et al. also utilized the NYHA classification system, and all patients with w-TTR and severe AS had advanced heart failure (NYHA III/IV). When compared with patients with isolated AS, Castano et al. reported that coexistence of amyloidosis correlates to more severe NYHA class (III/IV in 78% vs. 59% p=0.1), more atrial arrhythmias (67% vs. 20% p=0.006), and more Society of Thoracic Surgery (STS) risk score (6.9% vs. 3.8% p=0.024) [10].
5. Investigations
The gold standard for investigating TTR-CA is an endo-myocardial biopsy followed by Isoelectric Focusing (IEF) electrophoresis [12]. However, performing a biopsy on the typical elderly patient population that is affected by TTR-CA and AS comes at a cost due to the invasive nature of the procedure. Non-invasive methods including 2D-echocardiogram with speckle tracking, Cardiac Magnetic Resonance (CMR) and bone scintigraphy are increasingly being used to screen and diagnose patients with TTR-CA.
Multiple Electrocardiographic (EKG) patterns have been associated with cardiac amyloidosis, such as low voltage criteria, Left Ventricular Hypertrophy (LVH) and a pseudo-infarct pattern. However, these EKG findings are not sensitive nor specific for amyloidosis. In a retrospective study by Murtagh et al., these EKG findings were only seen in approximately 45% of patients with biopsy-proven cardiac amyloidosis [21]. In patients with cardiac amyloidosis and concomitant AS, these EKG findings have even less association. In a retrospective study by Cavalcante, none of the 9 patients with both diseases met the criteria for low voltage [7]. Normal EKG in the setting of severe LVH on echocardiogram is another prevalent finding in this population [19]. This discrepancy is likely secondary to the interaction between the myocardial hypertrophy from increased afterload and the voltage-attenuating amyloid tissue infiltration.
Echocardiographic characteristics associated with cardiac amyloidosis include thickened Atrio-Ventricular (AV) valves, interatrial septum or ventricular wall, as well as pericardial effusion and myocardial granular sparkling (Fig. 2) [22]. These findings remain prevalent in patients with TTR-CA with concomitant AS. Longhi et al. prospectively performed bone scintigraphy (Technetium-DPD scans) in patients with these “red flag”-echocardiographic findings in which 5 out of 5 such patients were confirmed to have TTR-CA on tissue biopsy [19]. In contrast, compared to isolated AS, patients with both diseases tend to have higher Left Ventricular (LV) mass index, thicker interventricular septal wall, and lower stroke volume [10, 19]. Other unique hemodynamic features in this subgroup include higher E/A ratio, lower deceleration time and lower average mitral annular systolic velocity (mitral annular S’), which are likely secondary to more severe diastolic and systolic dysfunction [4, 10].
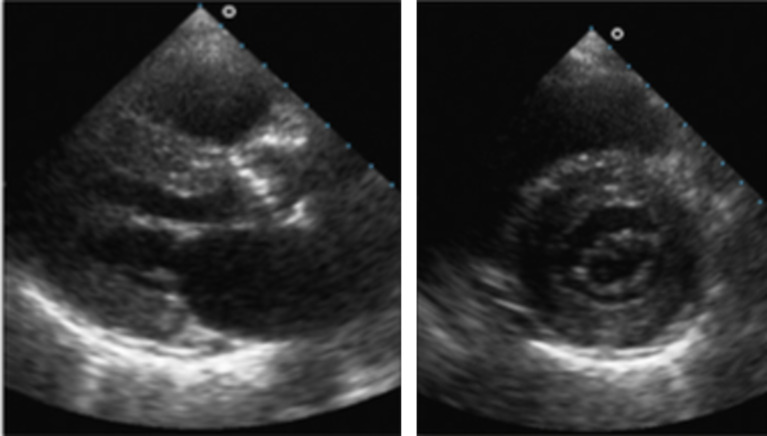
Fig. (2).
This is an example of an echocardiogram showing features of cardiac amyloidosis including ventricular myocardium granular sparkling, atrioventricular valve thickening, and mild pericardial effusion. Longhi et al.
Aortic stenosis is graded A to D, A being at risk for AS, and D being symptomatic severe AS [9]. Stage D is further divided into D1 to D3. D1 includes normal left ventricular Ejection Fraction (EF) and normal flow with a high-gradient; D2 includes low-flow low-gradient with low EF; and D3 includes low-flow low-gradient with normal EF. Low-flow, low-gradient AS (stage D2 and D3) is remarkably more prevalent in patients with concomitant TTR-CA compared to isolated AS cases [10]. Castano et al. found that 30% of the patients with both diseases had the low-flow low-gradient subtype compared to 10% in isolated AS cases [10]. Cavalante et al. also reported that 7 out of 9 patients with TTR-CA and AS had D2 or D3 subtypes. It is a clinically important finding as D2 and D3 AS have a significantly worse outcome both in the natural course of the disease and in the outcomes with treatments [4, 10, 19]. The higher prevalence of low-flow low-gradient AS in TTR-CA patients may partially be explained by the observation that TTR-CA induced similar pathological changes to that of low-flow low-gradient AS, including severe concentric remodeling, diastolic dysfunction and reduced longitudinal shortening (Fig. 3).
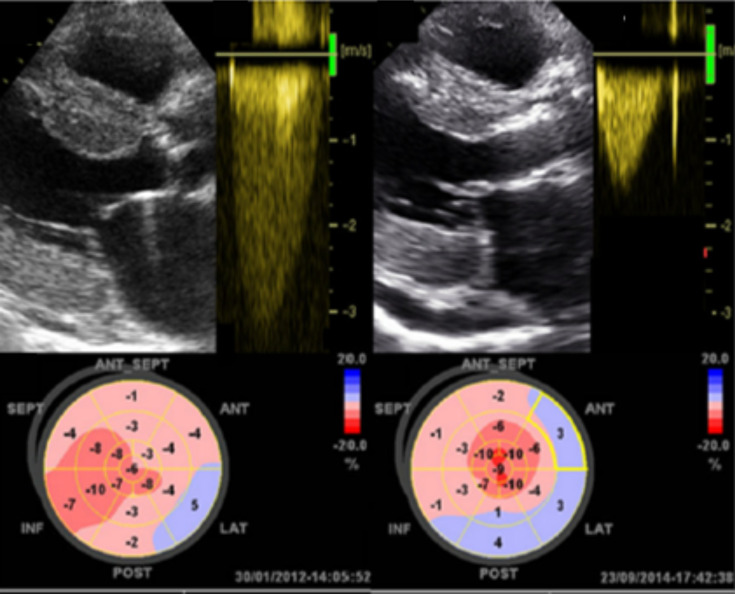
Fig. (3).
This is an example of the low flow low gradient pathophysiology seen in TTR-CA. On the left, echocardiogram shows aortic stenosis, transaortic Doppler shows a low flow low gradient curve, and a global LV longitudinal strain shows severe decrease in LV contractibility. On the right, the same patient 33 months post-TAVR showing improved aortic gradient and slight decrease in LV contractility. Castano et al. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Speckle Tracking Echocardiogram (STE) (Fig. 4) with strain measurement is commonly used along with conventional 2D-echocardiogram to aid the diagnosis of cardiac amyloid. Classical findings include decreased longitudinal strain pattern with apical sparing giving rise to the hallmark appearance on a “bull’s eye” plot [21]. “Bull’s eye” plots are circular maps of cardiac function superimposed on an AHA standard 17-segment model of LV-anatomy. They are commonly used to display pathology through characteristic patterns observed on the plots. Interestingly, patients with ATTR and severe symptomatic AS do not demonstrate typical apical sparing pattern on the STE [10]. This may be secondary to extensive diffuse remodeling in the ventricle due to the increased afterload secondary to AS.
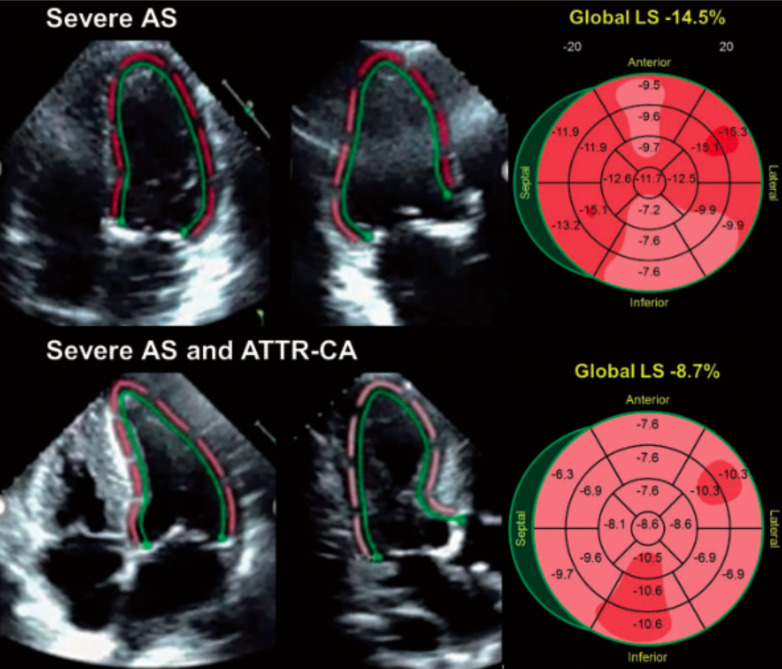
Fig. (4).
This is an example of speckle-strain imaging in elderly patients with severe symptomatic aortic stenosis with and without TTR-CA. The bullseye plots (right) demonstrate relative apical sparing independent of the presence of amyloid. Castano et al. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
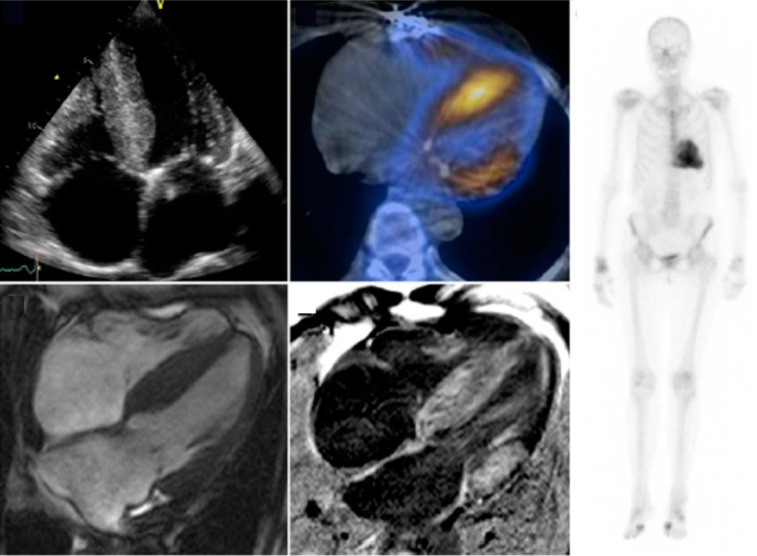
Cardiac Magnetic Resonance imaging (CMR) is another non-invasive imaging method that is widely used to diagnose cardiac amyloidosis. Diffuse delayed gadolinium enhancement of sub-endocardial tissue with abnormal myocardial and blood-pool gadolinium kinetics is the key characteristic pattern of cardiac amyloidosis [23]. A Meta-analysis evaluating the diagnostic value of CMR in detecting cardiac amyloidosis revealed a sensitivity of 85% and specificity of 92% [22]. However, in patients with both TTR-CA and AS, CMR seems to be a poor diagnostic tool for detecting amyloidosis. Treibel et al. pre-operatively performed CMR in patients undergoing Surgical Aortic Valve Replacement (SAVR) for severe AS, and of the 6 patients that had TTR-CA on the intra-op biopsy, only 2 patients showed features suggestive of cardiac amyloid on CMR [24]. Fig. (5) for an example or multimodality imaging inf a patient with TTR-CA.
Fig. (5).
This is an example of multimodality imaging of a patient with TTR-CA and aortic stenosis. The echocardiogram (top left) demonstrates LVH. Cardiac scintigraphy (right) demonstrates cardiac amyloidosis which is more obvious via single-photon emission on CT (upper middle). Cardiovascular MRI with and without gadolinium enhancement on the bottom. Treibel et al. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Bone scintigraphy using radio-active tracer is an increasingly utilized imaging tool to diagnose TTR amyloidosis. The nuclear tracers (99mTc-DPD, 99mTc-PYP or pyrophosphate, among others) are taken up by TTR and the extent of uptake is visually graded on the Perugini scale [25]. The Perugini scale was devised to grade the intensity of the uptake of tracer by the myocardium, relative to that in the bones. The scale ranges from zero to three. Briefly, grade 0 is no cardiac uptake and normal bone uptake, grade 1 is cardiac uptake which is less intense than bone signal, grade 2 is cardiac uptake with intensity similar to bone, and grade 3 is cardiac uptake with increased cardiac intensity compared to bone [25]. Grade 2 or higher myocardial radiotracer uptake on bone scintigraphy has been demonstrated to have >99% sensitivity and 86% specificity for diagnosing TTR-CA. Almost all false positives are exclusive due to AL amyloidosis with cardiac involvement. A combined finding of positive scintigraphy and absence of monoclonal protein in serum or urine, had 100% specificity and 100% positive predictive value for TTR-CA [12].
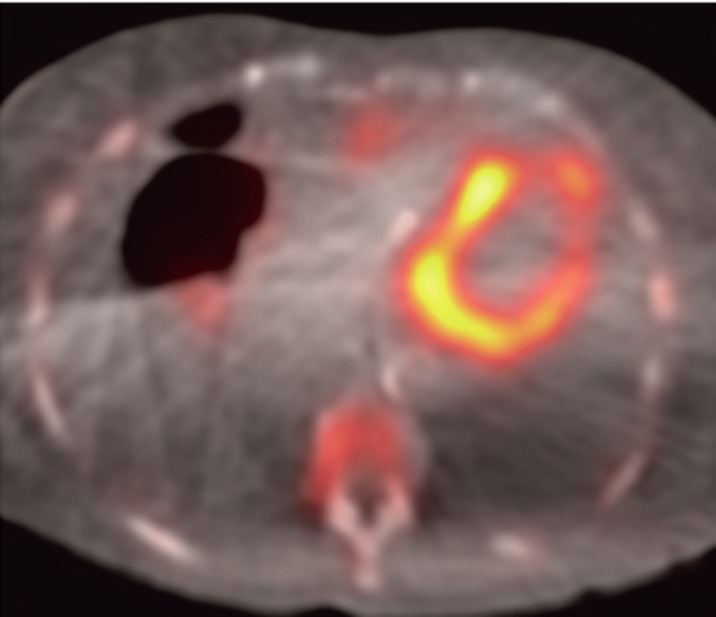
Bone scintigraphy is equally effective in diagnosing TTR-CA patients with concomitant AS. Treibel et al. demonstrated that 99mTc-DPD bone scintigraphy showed positive uptake in all cases in this subgroup [8]. Scully et al. also found that, in contrast to typical pyramidal distribution of Perugini grade 1>2>3, DPD Grade 2 was predominant in patients with concomitant AS [20]. This may suggest an association between higher amyloid burden with severe, symptomatic AS. See Fig. (6) for an example of a positive DPD scan.
Fig. (6).
This is an example of DPD scintigraphy illustrating cardiac tracer retention suggestive of cardiac amyloid. Scully et al. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
6. Treatment/Management
Prior studies have shown significantly poor outcome in patients with both AS and cardiac amyloidosis. Please see Table 1 for a summary of the current evidence. Currently, there is limited evidence on the safety and outcomes of AVR in patients with severe AS with concomitant cardiac amyloidosis.
Table 1.
Summary of the available studies addressing cardiac amyloidosis and aortic stenosis coexistence and outcomes.
| Study/Authors | Year | Study Design | Patients (n) | Age (years) | Intervention | Prevalence | Follow-up | Results | Complications | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| Castaño et al. | 2017 | Prospective study of patients receiving TAVR screened for ATTR-CA with 99mTc-PYP cardiac scintigraphy. | 151 | 84±6 | TAVR with 99mTc-PYP cardiac scintigraphy screening for ATTR-CA. | ATTR in 16% of post-TAVR patients | n/a | Patients with ATTR-CA had a thicker interventricular septum, higher LV mass index, lower SV index, advanced diastolic dysfunction with a high E/A ratio, lower EF, and more impaired global longitudinal strain. | Post-TAVR 17.4% of patients with ATTR-CA needed a permanent pacemaker, versus 14.4% without ATTR-CA. | ATTR-CA is prevalent in 16% of patients with severe AS undergoing TAVR and is associated with severe AS phenotype of low-flow low-gradient with a reduced EF. |
| Cavalcante J et al. | 2017 | Retrospective study of patients with AS referred for CMR, and assessment of the associations between CA & AS and all-cause mortality. | 113 | 74±14 | Transthoracic echocardiogram, CMR, LGE and identification of CA. 59 patients received AVR. | 9/96 (9.4%) patients with severe AS had CA. | Median follow-up was 18 months. | 8% patients had combined CA-AS, 16% patients >74 years-old had combined CA-AS, excluding women 32% of men >74 years-old had CA-AS, combined CA-AS had low flow low gradient physiology, 1 year all-cause mortality in AS-CA patients was 56% versus 20% in isolated AS. | 40 patients died within the median 18-month follow-up, | Significant CA prevalence in patients with moderate-severe AS, particularly among older males. Combined CA-AS often presents with A-fib and a low flow low gradient physiology. Combined CA-AS has an increased 1-year all cause mortality. |
| Java AP et al. | 2018 | Retrospective study of patients with diagnosed ATTR-CA who underwent AVR. | 16 | 76±6 | 11/16 patients had surgical AVR, 5/16 patients had TAVR. | n/a | Median follow-up was 1.9 year. | No 30-day mortality post-op. First post-op mortality occurred 1.5 years after surgery. 4 total deaths during the follow-up period all occurring >1 year post-op ranging from 1.5 to 7.4 years. | 3/16 patients had procedure related complications including femoral artery pseudoaneurysm, post-op tamponade, and low-output syndrome. | AVR has low risk of operative morbidity and mortality in patients with combined CA-AS, TAVR has a reduced hospital stay, 1 year survival rate is excellent. |
| Longhi S et al. | 2016 | Prospective study of patients referred for treatment of AS. Screening for ATTR-CA with echocardiogram and confirmed with DPD scintigraphy. | 43 | 84±6 | DPD scintigraphy in patients with red flags for ATTR-CA on echocardiogram, all patients underwent balloon aortic valvuloplasty and currently undergoing clinical follow-up. | 11.6% patients had combined ATTR-CA and AS. | Currently under-going clinical follow-up | 5 patients had combined ATTR-CA and AS, 4 were men, all had advanced HF, 3 had carpal tunnel syndrome, 4 patients had low flow low gradient physiology. | None reported, pending clinical follow-up. | Combined ATTR-CA & AS can be suspected on the basis of clinical and echocardiographic features, and effectively diagnosed with DPD scintigraphy. |
| Study/Authors | Year | Study Design | Patients (n) | Age (years) | Intervention | Prevalence | Follow-up | Results | Complications | Conclusions |
| Scully PR et al. | 2018 | Prospective study of DPD screening pre-TAVR for patients with severe AS. Patients will be followed post-op through April 2019. | 125 | 86±5 | DPD bone scintigraphy, TAVR, patients positive for ATTR-CA referred to National Amyloidosis Center. | ATTR in 12.8% of patients pre-TAVR. | Patients will under-go follow-up through April 2019. Most recent update was May 2018 [34]. | ATTR in 12.8% of patients pre-TAVR. These patients had lower mean AV gradient and stroke volume index. | 1 periprocedural permanent pacemaker placed, 1 implantable cardiac defibrillator placed, 1 spinal cord infarction. 2 deaths pre-TAVR. | ATTR-CA is prevalent in 12.8% of patients undergoing TAVR and is likely to alter clinical presentation and mortality. |
| Seki T | 2017 | Case report of a 77 year-old patient with 19 year history of hemodialysis, mixed systemic amyloidosis, and carpal tunnel syndrome who underwent AVR. | 1 | 77 | Surgical AVR. | n/a | n/a | After significant hemodynamic instability, metabolic acidosis, and cardiac arrest the patient expired. Autopsy revealed a mixed systemic amyloidosis in virtually all major organs and extensive whole body edema. | Hemodynamic deterioration unresponsive to aggressive therapy, metabolic acidosis, cardiac arrest, and eventual death. | Systemic amyloidosis may be a risk factor for hemodynamic deterioration due to increased vascular permeability after AVR. |
| Treibel TA et al. | 2016 | Prospective study of patients receiving AVR surgery who underwent magnetic resonance and intraoperative biopsies. ATTR was confirmed histologically and typed with immune-histochemistry. | 146 | 75±6 | AVR with intra-operative biopsy. | ATTR in 4.1% of all patients pre-AVR and 5.6% patients >65 years-old. | Median follow-up was 2.3 years. | 6 patients undergoing AVR surgery tested positive for ATTR. These patients had severe hypertrophy and left ventricular impairment. | 3 of the ATTR positive patients died at follow-up (50%), versus 8 ATTR-negative who died at follow-up (7.5%). | ATTR is an important prognostic indicator for elderly patients with AS receiving AVR. |
Note: Transcatheter Aortic Valve Replacement (TAVR), Transthyretin Cardiac Amyloidosis (ATTR-CA), Technetium-99m Pyrophosphate (99mTc-PYP) cardiac scintigraphy, Monoclonal gammopathy of undetermined significance (MGUS), Cardiovascular Magnetic Resonance (CMR), Late Gadolinium Enhancement (LGE), Dihosphono-1,2-Propanodicarboxylic acid (DPD) scintigraphy.
Management of cardiac amyloidosis consists primarily of heart failure symptom alleviation and arrhythmia management; specifically, careful use of diuretics and beta-blockers for optimization of fluid status and liberal placement of pacemakers for arrhythmia prevention [22, 26-29]. Meanwhile, ACE-Inhibitors, Angiotensin-Receptor blockers, Calcium antagonists and Digoxin have no proven mortality benefit [26-29]. Additionally, other management options that need more investigation include amyloid production prevention via liver transplantation and amyloid stabilization (with medications such as tafamidis or diflunisal) [12, 26-30].
Generally, valve replacement for AS is recommended for symptomatic patients with severe high-gradient AS, asymptomatic patients with severe AS and LVEF <50%, and patients with severe or moderate AS undergoing other cardiac surgery [9]. The prognosis of patients with TTR-CA is worse compared with other cardiomyopathy etiologies. The survival is usually 4 to 5 years after symptom onset [3, 16, 28]. However, the outcomes of aortic valve replacement when there is TTR-CA and AS coexistence are recently being reported.
Cavalcante J et al. studied 113 patients with AS and he found a prevalence of TTR-CA of 9% (9 patients). Four of these patients underwent TAVR and 2 of them (50%) died within 3 months. Patients with TTR-CA had significantly higher 1-year all-cause mortality than patients with isolated AS (56% vs. 20%, P <0.001) regardless of valve replacement or conservative management [7].
Another prospective study including 146 patients undergoing surgical AVR, showed a prevalence of TTR-CA on cardiac biopsy of 6%. In this study, TTR-CA was associated with death with a hazard ratio of 9.5 (95% CI 2.5-35.8, P=0.001) [24].
In the prospective study by Castaño et al., 16% of 151 patients (over 65 years of age and 68% were males) receiving TAVR for AS had a diagnosis of TTR-CA on nuclear imaging. All the patients had a successful procedure at 30 days follow-up. The rate of pacemaker implantation after TAVR, which is a known complication of the procedure, was not higher in the TTR-CA group [10]. Additionally, Java et al. published an excellent 1-year survival rate on his 16 patients with TTR-CA and AS treated with either TAVR or surgical AVR (all patients alive at 1 year) [18]. Another ongoing study by Scully et al. will follow up on 101 patients that underwent TAVR and 14% of them had TTR-CA [20, 31].
In the study by Longhi et al, 5 (12%) of the 43 patients with severe AS had TTR-CA and conservative management with balloon valvuloplasty was reported pending therapeutic decision [19].
7. Future directions
Managing patients with concurrent AS and CA poses a challenge for providers. Currently, there is no literature addressing the direct pathophysiology of CA and AS, and how these two processes may interact with each other. An understanding of the underlying pathophysiology seems vital to the future understanding of how to address patients with concurrent disease. The theory of isolated AS pathophysiology is well documented in the literature. Isolated AS is thought to be the end result of an inflammatory process over time involving endothelial damage due to mechanical stress, lipid penetration, fibrosis, leaflet thickening, and calcification [5]. Regarding the CA pathophysiology, the literature is less abundant. There is sufficient published work showing that amyloid, specifically TTR and AL amyloid, can deposit in the aortic valve [32]. Additionally, there is literature that addresses the pathophysiology of amyloid deposition and an inflammatory response that ensues. Multiple studies have demonstrated beta-amyloid deposition in the brain results in an inflammatory process that is present in even early-stage Alzheimer’s disease, and progressive inflammation parallels progressive disease [33-35]. This could be relevant when considering TTR deposition in aortic valves. We propose that AS and CA are both chronic inflammatory diseases in nature, and when present concurrently they augment each other resulting in a more progressive inflammatory change in the aortic valve. This has not been documented in the literature, but we believe it is a reasonable theory to why concurrent AS and CA results in progressive disease. Future studies aimed at the pathophysiology of concurrent CA and AS will help illuminate processes to target in disease management and treatment.
There has already been promising research exemplifying how targeting a component of the process of CA can have beneficial effects on patient outcomes. A 2018 study in the New England Journal of Medicine looked at Tafamidis use in CA; Maurer et al. concluded that patients with TTR-CA treated with Tafamidis was associated with a reduced all-cause mortality, reduced cardiovascular-related hospitalizations, and reduced the decline of functional capacity [36]. This sounds promising, but future larger studies are needed to assess the efficacy and safety in this patient population in order to confidently incorporate it into AS-CA patient management. And again, future studies aimed at the inflammatory pathophysiology of concurrent CA and AS may reveal other various targets for treatment and management.
Another challenge with concurrent AS and CA is the lack consensus regarding patient management. As presented in this article, there are a few case studies that exist that give insight into case-by-case management. However, there are no set guidelines for AS-CA patients. Our review of AS and CA literature has given insight into future research to influence the management of patients. Concurrent AS and CA have been observed to manifest as a more severe progressive clinical and pathologic disease. Recent literature demonstrates sensitive and specific non-invasive techniques to diagnose CA, such as bone scintigraphy. And although research has demonstrated the relative safety of TAVR in these patients with concurrent AS and CA [18], case studies reveal many providers are hesitant to perform TAVR on these patients. In summary, current literature reveals AS and CA results in severe progressive disease, non-invasive methods for CA diagnosis exist, and TAVR is relatively safe in these patients. Future studies that look at patients with CA could identify specific risk factors that put AS patients at risk for CA. Such studies could set the stage for the development of a guideline that would help providers decide when to utilize non-invasive diagnostics and screen for CA in patients with AS. For example, early screening and identification of concurrent AS and CA could affect management, such as performing TAVR or SAVR earlier in the disease course to avoid progression. Currently AS stage is what dictates the decision for TAVR. A future guideline could result in a more dynamic approach to AS-CA patients, including screening for CA, timing of valve replacement (or valve replacement at all), and potential medical management (ex. Tafamidis).
Conclusion
The relationship between aortic stenosis and TTR-CA is not well understood. This makes the management of patients with coexistent aortic stenosis and TTR-CA very difficult. A few studies have proven success in the surgical management of elderly patients with AS and TTR-CA. However, coexistence of both conditions seems to worsen prognosis of these patients whether they undergo valve intervention or not. The significant incidence of the two diseases occurring concurrently warrants further research to better the understanding and potential for improved management in the future.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
STANDARD OF REPORTING
PRISMA guidelines and methodologies were followed.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Kumar V., Abbas A.K., Fausto N., Robbins S.L., Cotran R.S. Robbins and cotran pathologic basis of disease. Philadelphia. Elsevier Saunders. 2005;258-259:554–556. [Google Scholar]
- 2.Lindman B.R., Clavel M.A., Mathieu P., et al. Calcific aortic stenosis. Nat. Rev. Dis. Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wechalekar A.D., Gillmore J.D., Hawkins P.N. Systemic amyloidosis. Lancet. 2016;387(10038):2641–2654. doi: 10.1016/S0140-6736(15)01274-X. [DOI] [PubMed] [Google Scholar]
- 4.Galat A., Guellich A., Bodez D., et al. Aortic stenosis and transthyretin cardiac amyloidosis: the chicken or the egg? Eur. Heart J. 2016;37(47):3525–3531. doi: 10.1093/eurheartj/ehw033. [DOI] [PubMed] [Google Scholar]
- 5.2013.
- 6.Thaden J.J., Nkomo V.T., Enriquez-Sarano M. The global burden of aortic stenosis. Prog. Cardiovasc. Dis. 2014;56(6):565–571. doi: 10.1016/j.pcad.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Cavalcante João L., et al. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J. Cardiovasc. Magn. Reson. 2017;191:98. doi: 10.1186/s12968-017-0415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed-Salem L., Santos-Mateo J.J., Sanchez-Serna J., et al. Prevalence of wild type ATTR assessed as myocardial uptake in bone scan in the elderly population. Int. J. Cardiol. 2018;270:192–196. doi: 10.1016/j.ijcard.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura R.A., Otto C.M., Bonow R.O., et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63(22):e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 10.Castaño A., Narotsky D.L., Hamid N., et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. 2017;38(38):2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonow Robert O. Greenland Philip. Population-wide trends in aortic stenosis incidence and outcomes. Circulation. 2015;••• doi: 10.1161/CIRCULATIONAHA.115.014846. [DOI] [PubMed] [Google Scholar]
- 12.Shah K.B., Inoue Y., Mehra M.R. Amyloidosis and the heart: A comprehensive review. Arch. Intern. Med. 2006;166(17):1805–1813. doi: 10.1001/archinte.166.17.1805. [DOI] [PubMed] [Google Scholar]
- 13.Maurer M.S. Noninvasive identification of ATTRwt cardiac amyloid: the re-emergence of nuclear cardiology. Am. J. Med. 2015;128(12):1275–1280. doi: 10.1016/j.amjmed.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treglia G., Glaudemans A.W.J.M., Bertagna F., et al. Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: A bivariate meta-analysis. Eur. J. Nucl. Med. Mol. Imaging. 2018;45(11):1945–1955. doi: 10.1007/s00259-018-4013-4. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson D.R., Pastore R., Pool S., et al. Revised transthyretin Ile122allele frequency in African-Americans. Hum. Genet. 1996;98:236–238. doi: 10.1007/s004390050199. [DOI] [PubMed] [Google Scholar]
- 16.Pinney Jennifer H., et al. Senile systemic amyloidosis: Clinical features at presentation and outcome. J. Am. Heart Assoc. 2013;22:e000098. doi: 10.1161/JAHA.113.000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson M., Büttner J., Todorov T., et al. Prevalence of germline mutations in the TTR gene in a consecutive series of surgical pathology specimens with ATTR amyloid. Am. J. Surg. Pathol. 2009;33(1):58–65. doi: 10.1097/PAS.0b013e3181788566. [DOI] [PubMed] [Google Scholar]
- 18.Java A.P., Greason K.L., Dispenzieri A., et al. Aortic valve replacement in patients with amyloidosis. J. Thorac. Cardiovasc. Surg. 2018;156(1):98–103. doi: 10.1016/j.jtcvs.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 19.Longhi S., Lorenzini M., Gagliardi C., et al. Coexistence of degenerative aortic stenosis and wild-type transthyretin-related cardiac amyloidosis. JACC Cardiovasc. Imaging. 2016;9(3):325–327. doi: 10.1016/j.jcmg.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Scully P.R., Treibel T.A., Fontana M., et al. Prevalence of cardiac amyloidosis in patients referred for transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2018;71(4):463–464. doi: 10.1016/j.jacc.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murtagh B., Hammill S.C., Gertz M.A., Kyle R.A., Tajik A.J., Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am. J. Cardiol. 2005;95(4):535–537. doi: 10.1016/j.amjcard.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Gertz M.A., Benson M.D., Dyck P.J., et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J. Am. Coll. Cardiol. 2015;66(21):2451–2466. doi: 10.1016/j.jacc.2015.09.075. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L., Tian Z., Fang Q. Diagnostic accuracy of cardiovascular magnetic resonance for patients with suspected cardiac amyloidosis: a systematic review and meta-analysis. BMC Cardiovasc. Disord. 2016;16(1):129. doi: 10.1186/s12872-016-0311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treibel T.A., Fontana M., Gilbertson J.A., et al. Occult transthyretin cardiacamyloid in severe calcific aortic stenosis: Prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging. 2016;9(8):e005066. doi: 10.1161/CIRCIMAGING.116.005066. [DOI] [PubMed] [Google Scholar]
- 25.Gillmore J.D., Maurer M.S., Falk R.H., et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins P.N., Ando Y., Dispenzeri A., Gonzalez-Duarte A., Adams D., Suhr O.B. Evolving landscape in the management of transthyretin amyloidosis. Ann. Med. 2015;47(8):625–638. doi: 10.3109/07853890.2015.1068949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruberg F.L., Berk J.L. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286–1300. doi: 10.1161/CIRCULATIONAHA.111.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quarta C.C., Kruger J.L., Falk R.H. Cardiac amyloidosis. Circulation. 2012;126(12):e178–e182. doi: 10.1161/CIRCULATIONAHA.111.069195. [DOI] [PubMed] [Google Scholar]
- 29.González-López E., López-Sainz Á., Garcia-Pavia P. Diagnosis and treatment of transthyretin cardiac amyloidosis. Progress and hope. Rev. Esp. Cardiol. (Engl. Ed.) 2017;70(11):991–1004. doi: 10.1016/j.rec.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 30.Dahm Cherie N., Cornell R. Frank, Lenihan Daniel J. Advances in treatment of cardiac amyloid. Curr. Treat. Options Cardiovasc. Med. 2018;20(5):37. doi: 10.1007/s11936-018-0631-1. [DOI] [PubMed] [Google Scholar]
- 31.Scully P.R., Treibel T.A., Fontana M., et al. 1 A multi-centre study of cardiac amyloidosis in tavi patients. Heart. 2018;104:A15. [Google Scholar]
- 32.Nemshah Yaser Amyloid heart disease. US Cardiol Rev. 2018;12(2) doi: 10.15420/usc.2018.5.1. [DOI] [Google Scholar]
- 33.Hommet C., Mondon K., Camus V., et al. Neuroinflammation and β amyloid deposition in Alzheimer’s disease: In vivo quantification with molecular imaging. Dement. Geriatr. Cogn. Disord. 2014;37(1-2):1–18. doi: 10.1159/000354363. [DOI] [PubMed] [Google Scholar]
- 34.Gorevic P.D. Amyloid and Inflammation. Proc. Natl. Acad. Sci. USA. 2013;110(41):16291–16292. doi: 10.1073/pnas.1315112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGeer P.L., McGeer E.G. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: Implications for therapy. Acta Neuropathol. 2013;126(4):479–497. doi: 10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- 36.Maurer M.S., Schwartz J.H., Gundapaneni B., et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N. Engl. J. Med. 2018;379(11):1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]