Abstract
Coronary collateral vessels supply blood to areas of myocardium at risk after arterial occlusion. Flow through these channels is driven by a pressure gradient between the donor and the occluded artery. Concomitant with increased collateral flow is an increase in shear force, a potent stimulus for collateral development (arteriogenesis). Arteriogenesis is self-limiting, often ceasing prematurely when the pressure gradient is reduced by the expanding lumen of the collateral vessel. After the collateral has reached its self-limited maximal conductance, the only way to drive further increases is to re-establish the pressure gradient.
During exercise, the myocardial oxygen demand is increased, subsequently increasing coronary flow. Therefore, exercise may represent a means of driving augmented arteriogenesis in patients with stable coronary artery disease. Studies investigating the ability of exercise to drive collateral development in humans are inconsistent. However, these inconsistencies may be due to the heterogeneity of assessment methods used to quantify change. This article summarises current evidence pertaining to the role of exercise in the development of coronary collaterals, highlighting areas of future research.
Keywords: Chronic total occlusion, angiogenesis, arteriogenesis, shear force, collateral, coronary, artery
1. INTRODUCTION
Coronary collateral vessels are pre-existing anastomotic pathways interconnecting epicardial coronary arteries [1]. The ability of these vessels to connect proximal and distal portions of the same artery (antegrade) or divergent adjacent vessels (retrograde) can provide an alternative blood supply to areas of the myocardium inadequately perfused by their native artery (Fig. 1). Whilst these collaterals reduce the ischaemic burden, they are seldom sufficient to completely overcome ischaemia in patients with stable Coronary Artery Disease (CAD), particularly during exercise [2]. Furthermore, in the acute setting, the presence of collaterals is associated with an improved rate of survival following myocardial infarction [3]. The development of collaterals is, therefore, an important protective process in patients with cardiovascular disease. However, the processes underpinning the development of coronary collaterals are complex and the extent of development varies widely amongst individuals in clinical practice. In this review, we evaluate the current evidence regarding the development of coronary collaterals with a particular focus on the potential role of exercise.
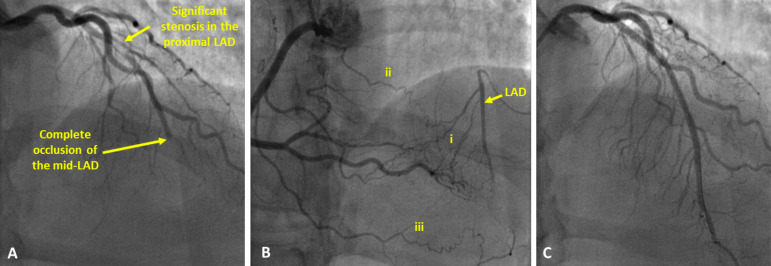
Fig. (1).
Angiography images of a patient with a Chronic Total Occlusion (CTO) of the Left Anterior Descending artery (LAD). (A): contrast injected into the left coronary artery demonstrates severe stenosis in the proximal part of the LAD, contrast flow stops abruptly at the point of complete occlusion in the mid-LAD. (B): contrast injected into the right coronary demonstrating retrograde filling of the LAD via multiple collateral channels, both septal (i) as well as epicardial (ii, iii) channels can be identified. (C): contrast injected into the left coronary artery after successful revascularisation of the LAD. Two stents have been implanted (one in the proximal LAD and a second at the site of the occlusion). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2. METHODS
We performed a literature search of online databases (PubMed & Science Direct) using the Boolean search terms, “Collateral”, “Coronary collateral”, “Collateral artery” And “Exercise”. The selected articles were reviewed first by title, then abstract and finally by full text, before ‘snowballing’ was used to find further relevant articles from reference lists.
3. REVIEW OF THE LITERATURE
Post mortem studies have been used to demonstrate the presence of coronary collaterals even in individuals with healthy hearts [4], most likely related to remnants of the embryonic vascular network [1]. In the presence of obstructive CAD, the extent of collateral development is related to both the severity and duration of obstruction. A more extensive network and larger calibre collateral vessels are evident in patients who have a long history of angina. Interestingly, coronary collateral blood vessels are evident in 63% of hearts with significant CAD as opposed to 95% of hearts with a completely occluded coronary artery [5, 6].
Human coronary arteries are not end arteries, they are instead interconnected by a network of pre-existing arteriolar anastomosis [7]. Blood within this system flows from an area of high to an area of low pressure, traveling across the path of least resistance. If the main coronary arteries are patent, then they indeed represent the most efficient path for coronary flow and little flow will likely pass through the anastomosis. However, if the coronary arteries become stenotic and ultimately occluded, the pressure in the vessel distal to the occlusion will become significantly lower than that in the proximal portion. This pressure gradient will drive flow via the native collateral circuit (Ohm’s Law).
Increasing flow through these arterioles augments the physical forces acting upon the vessel wall (Fluid Shear Stress (FSS)) [8, 9]. This enhanced physical stimulus causes the intimal endothelial layer of the collateral arteriole to become activated, initiating a process of gene expression that results in cellular proliferation and structural remodelling of the vessel [10, 11]. Once the endothelium is activated, the vessel passes through four stages of vascular remodelling, including an increase in vascular permeability, digestion of extracellular scaffolding, reconstruction of the Smooth Muscle Cell (SMC) coat along with the production of a larger vascular scaffolding and pruning of additional collateral vessels [12]. Over the course of one week, arterioles with a diameter of 30-50 µm [13], ensheathed in one to two layers of SMC’s [14], develop into arterial structures up to 20-fold larger and more robust than their previous forms [15]. These vessels become capable of supporting demonstrably greater levels of blood flow, with some researchers reporting a 25-fold increase above baseline levels [13]. This mechanism by which pre-existing collateral vessels mature into more substantial connections has been termed as arteriogenesis [16].
Under pathologically occlusive conditions, coronary collateral vessels typically demonstrate compensatory maturation sufficient enough to provide only 30-40% of the flow supplied by the artery they bypass [12]. This is due to the impact that arteriogenesis has on the collateral pressure gradient. As the collateral vessel matures, its luminal diameter increases, providing more flow to the distal portion of the occluded vessel. This reduces the pressure gradient, eventually reaching a nadir at which FSS no longer surpasses the threshold stimulation for collateral growth [17]. At this point, the collateral vessel does not continue to mature without increased provocation [12].
To test this assumption, Pipp and co-workers undertook a study using the swine hind limb model of occlusion in which the distal section of the ligated femoral artery was surgically grafted to the venous system [9]. This graft acted as a pressure release valve, shunting blood from the distal portion of the occluded vessel. Consequently, the pressure in the vessel distal to the occlusion remained reduced, thereby maintaining the pressure gradient across the collateral. The arteriovenous shunt model produced a 2.3-fold increase in maximal collateral flow compared to the standard ligature model. A similar study by Eitenmüller and colleagues demonstrated consistent results in a rabbit model. After 4 weeks, the collateral vessels were sufficiently developed to provide 199±19% recovery of natural flow in animals with an arteriovenous shunt, in contrast to the non-shunted arteries which demonstrated no increase in flow beyond the initial 7 days [17].
3.1. Clinical Studies
The aforementioned animal models are not suitable for human studies on coronary collateral development. However, an alternative mechanism could be established to drive collateral development by increasing the arterial pressure proximal to the site of an occlusion. One method that has been evaluated is Enhanced External Counterpulsation (EECP). During EECP, lower limb pressure cuffs are timed to synchronously inflate to an external hydraulic pressure of 300 mmHg during the diastolic phase of the cardiac cycle. This cuff inflation augments diastolic blood pressure and cardiac output, increasing pressure across all the branches of the coronary tree [16]. In principle, this transiently replicates the changes in physical forces (FSS) that are usually localised to collaterals terminating distal to arterial stenosis. Clinical studies have demonstrated that EECP does improve myocardial perfusion in CAD patients [18]. Furthermore, results of a randomised controlled trial demonstrate that EECP increases the Collateral Flow Index (CFI), indicating that clinical improvements are related to the development of coronary collateral vessels [19]. Whilst it remains a promising avenue for clinical arteriogenesis, EECP procedures require expensive specialised equipment, trained staff and numerous visits to medical facilities.
3.2. Exercise Training
An alternative means of transiently increasing a collateral vessel’s FSS without specialist equipment is physical exercise (Fig. 2). During physical exercise training, heart rate and blood pressure are augmented in order to manage increased cellular respiration [20]. The peripheral increase in oxygen demand during exercise is mirrored centrally by an increase in myocardial oxygen demand, subsequently driving an approximate 5-fold increase in coronary blood flow [20]. This increase results in an amplification of the FSS disseminated across the endothelium of the coronary vasculature and thus is hypothesised to augment arteriogenesis.
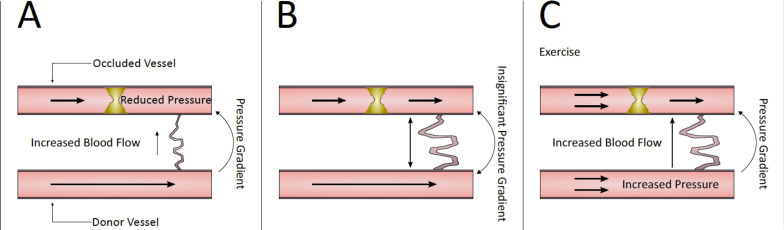
Fig. (2).
Pressure gradient of a developing collateral. Early after the development of arterial stenosis, low pressure distal to the stenosis draws flow through the collateral and increases shear stress on the endothelium (A). Collateral development reaches a plateau; collateral diameter increases, reducing collateral shear stress (B). An increase in donor artery pressure during exercise training reintroduces a pressure gradient across the collateral; fluid shear stress again increases across the collateral endothelium (C). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Coronary perfusion pressure is the outcome of aortic diastolic pressure minus left ventricular end-diastolic pressure [21]. The majority of coronary flow occurs during diastole, and an increase in heart rate negatively impacts diastole to a greater extent than systole [21]. Current research suggests that the diastolic function of some CAD patients may be impaired [22], however exactly how this influences collateral development is yet to be fully understood. Regular exercise can reduce the resting heart rate, leading to an increase in diastolic time and, therefore, an increase in shear stress. This hypothesis was evaluated in relation to arteriogenesis by using ivabradine medication which lowers the heart rate by blocking the if-channel of the sino-atrial node. Following positive data from animal studies [23], a randomised controlled trial of 46 patients with stable CAD was undertaken [24]. At 6 months, patients who received ivabradine had a significant increase in CFI (p= 0.05) while those who received a placebo showed a decrease in the CFI.
In 1957, Eckstein [25] reported that an exercise intervention successfully augmented collateral development in dogs with surgically occluded left circumflex coronary arteries. The researchers determined which animals developed ECG changes when the vessel was ligated, signifying a lack of collateral development at baseline. These animals had the ligation relaxed and, after recovering for one week, were randomised into exercising versus non-exercising groups. Exercise training was undertaken for 15-20 minutes, 4 times per day, 5 days per week for 6 to 8 weeks. A subsequent assessment demonstrated that exercise-trained animals had higher retrograde coronary flow, indicative of collateral development. In 1981, Scheel and colleagues [26] undertook a similar study in dogs with ligated circumflex arteries. They too found that 8 weeks of exercise training augmented collateral growth compared to a group of sedentary animals. Although intriguing, these results must be interpreted with caution before being extrapolated to human coronary pathophysiology, as there are substantial differences between species [14].
We have identified nine studies relating to exercise-induced augmentation of the coronary collateral circulation that are included in this narrative review. Whilst our search strategy was not systematic, the apparent dearth of studies is indicative of the sparseness of evidence in this field. The nine studies we identified can be divided into two groups (Tables 1 and 2) depending on their use of CFI. The Collateral Flow Index is now considered the “gold standard” measurement of collateral flow and is much more sensitive than other modalities at detecting changes in collateral flow [27]. Each of the five studies using CFI reported a relationship between CFI and exercise. In contrast, only two of the four studies not using CFI reported significant changes. Whist the heterogeneous methodology of these studies precludes meta-analysis, it is evident that introducing CFI into this field will result in a more robust and accurate assessment of collateral channel conductance, compared to qualitative assessment alone.
Table 1.
Summary of human studies examining exercise-induced changes in collateral flow, prior to the introduction of the coronary flow index measurement.
| Study | Population | Design | Duration | Intervention | Control | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| Nolewajka et al., 1979 [27] | n = 20 men Mean age 48 years MI previous 3-6 months |
RCT | 7 months | Exercise = 60-70% max HR, 2x per week for 1 hour (Group session), supervised exercise session x2 per week, unsupervised home exercise x1 per week. | x2 supervised sessions per week (Group) = Light calisthenics & volleyball. | Angiography, Scintigraphy, CPET with stress ECG | The exercise group significantly increased angina threshold and decreased HR at a set workload (p < 0.01). No improvement in collateralization, perfusion or ventricular function. |
| Fujita et al., 1988 [29] | n =16 (4 men/2 women) Mean age 58 years ≥1 major coronary artery obstruction (16 CTO’s) |
RCT | 22 days | Exercise (treadmill) 2x daily until angina pain was 60-80% max previously felt. Patients were pre-treated with 5000 IU intravenous heparin. | Exercise (treadmill) 2x daily until angina pain was 60-80% max previously felt. | ECG treadmill test (total exercise time, Rate pressure product (RPP) to time of angina), Radionuclide ventriculography, Angiography | The pre-exercise heparin group demonstrated a significant increase (p < 0.001) in exercise duration and RPP, as well as an increase in RPP at angina onset & ST depression (p < 0.01). None of the above were changed in the exercise-only group. Angiography demonstrated improved collateralization (however this was only evaluated in the exercise & heparin group). No significant increase in ventricular function. |
| Niebauer et al., 1995 [28] | n = 113 men Mean age 53.5 years Documented coronary stenosis Left ventricular ejection fraction > 35% |
RCT | 1 year | Initial 3 weeks on a metabolic ward learning to reduce the fat content of their diet (<20% total energy). 30 min exercise daily on a cycle ergometer at a target heart rate close to 75% max (achieved). ≥2 group training sessions per week (60min). |
Initial week on metabolic ward receiving identical instructions on diet and the importance of regular physical exercise. Adherence to these instructions was left to their initiative (usual care given by physician). | Angiography, ventriculography, symptom limited exercise test with thallium-201 scintigraphy | Reduction in stenosis severity in the intervention group (p<0.05 versus control). No significant change in collateral formation for either group at 1 year. When both the groups of patients were combined, there was a correlation between stenosis progression and an increase in collaterals (p<0.00001). No significant correlation between collateral formation and exercise performance. No significant difference between collateral formation and stress-induced myocardial ischaemia on thallium-201 scintigraphy. |
| Belardinelli et al., 1998 [30] | n = 46 (42 men / 4 women) Mean age 57 years Chronic coronary artery disease and impaired left ventricular function (ejection fraction < 40%) |
RCT | 8 weeks | Supervised exercise (cycle ergometer) at 60% of peak oxygen uptake for 60 min 3x per week for 8 weeks. | Avoid regular exercise and activity with caloric expenditure over 80% peak oxygen consumption. Given a list of acceptable and unacceptable activities. | CPET, Stress Echocardiography, Scintigraphy, Angiography | VO2 peak increased in exercise group versus control (p < 0.001 versus control). Oxygen pulse for a set relative intensity was improved in the exercise group (p < 0.01). Collateral score significantly increased in the exercise group. Ejection fraction significantly improved in the exercise group (p< 0.001 versus control). Thallium activity (scintigraphy) significantly improved in the exercise group (p < 0.001 versus control). The mean collateral score significantly increased only in the exercise group (p < 0.001 versus control). |
Abbreviations: RCT: Randomised Control Trial; HR: Heart Rate.
Table 2.
Summary of human studies examining exercise-induced changes in collateral flow through the measurement of the collateral flow index.
| Study | Population | Design | Duration | Intervention | Control | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| Zbinden et al., 2004 [32] | n =1 male Age 46 years Healthy amateur long-distance runner |
Case study | 3 years | Angiography performed during 3 phases of endurance training: 1 = Baseline 2 = Intermediate 3 = High |
N/A | Echocardiogram, angiogra-phy, CPET, CFR, CFI |
Left ventricular ejection fraction increased from phase 1 to phase 2 but dropped below baseline levels during phase 3. Peak exercise capacity (W) increased during every subsequent phase. VO2 (ml/min/kg) max increased during every subsequent phase. CFR increased during every subsequent phase. CFI increased from phase 2 to phase 3. |
| Zbinden et al., 2007 [33] | n = 40 (35 male / 5 female) Mean age 61 years Referred for coronary angiography |
Retrospective cohort study | 3 months | Cardiac Rehabilitation (jogging / cycling) 3x per week for >60 minutes at a target heart rate of 80% heart rate at VO2 max. | A retrospective sedentary group that did not adhere to prescribed exercise programme. | Angio-graphy, CFI, CPET | CFI in the occluded vessel significantly increased in the exercise group (p < 0.03). CFI in the normal vessel significantly increased in the exercise group (p < 0.0002). Significantly correlation between change in CFI and VO2 max (p < 0.007) in the exercise group. |
| Togni et al., 2010 [36] | n = 30 (28 men / 2 women) Mean age 59 years Chronic stable, non-occlusive CAD |
Intra-individual comparison randomised crossover study | N/A | CFI comparatively measured during exercise and rest for each patient. | N/A | CFI, angiogram |
CFI increased significantly from rest to peak exercise (p < 0.0002). |
| Lin et al., 2012 [37] | n = 65 Mean age 60.2 years Single vessel CAD undergoing PCI |
RCT | N/A | CFI during 1 minute of isometric handgrip exercise (50% maximal voluntary contraction). | CFI at rest. | CFI, angiogram |
∆CFI (CFI post occlusion – CFI pre occlusion) significantly increased in the exercising group (p < 0.01). |
| Möbius-Winkler et al., 2016 [34] | n = 60 (45 men / 15 women) Median age 64 years Significant CAD (FFR ≤ 0.75) |
Open-label RCT | 4 weeks | High intensity versus moderate-intensity training versus usual care . | Usual care | CPET, CFI, angiogram |
CFI increased significantly for both exercising groups when compared with usual care (high-intensity p= 0.005, moderate-intensity p= 0.004) VO2 peak increased significantly for both exercising groups (high-intensity p= 0.036, moderate-intensity p= 0.008). |
Abbreviations: CAD: Coronary Artery Disease; CFR: Coronary Flow Reserve; CFI: Collateral Flow Index; CPET: Cardiopulmonary Exercise Test; FFR: Fractional Flow Reserve; HIT: High Intensity Interval Training; PCI: Percutaneous Coronary Intervention; RCT: Randomised Control Trial.
In 1979, Nolewajka and colleagues recruited 20 men with a mean age of 48 years, who had recently suffered from Myocardial Infarction (MI) (3-6 months) and randomly allocated them to an exercise training intervention (n=10) or control group (n=10) [28]. Participants underwent a baseline angiogram before those in the exercise group undertook an exercise training regimen. Exercise training consisting of one-hour, group-based exercise was performed at a 60-70% maximum heart rate twice per week. Participants conducted further two individual supervised sessions per week and one unsupervised training at home. After 7 months, no difference was observed between the groups for collateral development (based on angiographic visualisation), myocardial perfusion or left ventricular function. However, the exercise-trained group experienced an increase in their angina threshold, indicating that some physiological adaptation had occurred. However, it is plausible that these adaptations took place in the peripheral musculoskeletal system and not in the coronary vasculature. One limitation of the research was that controls also undertook group exercise sessions that included calisthenics and volleyball.
A lack of collateral development was also reported in a study of 133 patients with CAD, randomised to a low-fat diet and exercise training, or control [29]. Patients in the intervention group undertook ≥ 2 group training sessions (one hour each) per week, as well as 30 minutes of home-based exercise per day (target heart rate ‘close’ to 75% of max achieved). Unfortunately, this study was also confounded by the fact that the control group received the same educational sessions, focused on the benefits of healthy eating and exercise training, as the intervention group. Follow-up angiography was performed at 1 year in 92 patients (81%) that demonstrated no difference in collateralisation between the groups. Notably, adherence to the exercise training programme was only 68% (range 39-92%) for group sessions and 60% self-reported participating in home-based training. It was also not possible to confirm whether the prescribed exercise intensity of group- or home-based exercise was maintained.
In contrast, a small study suggested that collaterals did increase if patients underwent treadmill exercise [30]. However, the authors suggested that this effect was related to the use of IV heparin administered prior to each exercise session (based on the results of animal experiments). A larger randomised study was subsequently undertaken by Belardinelli and colleagues [31] who demonstrated augmented collateral development in patients with CAD (n=50) who underwent an eight-week exercise training programme. Following baseline assessments, including maximal Cardiopulmonary Exercise Testing (CPET), and angiography, participants were randomised to either exercise training (n=26) or control (n=24). Contrary to the previous studies, control subjects were advised to avoid regular physical exercise, whilst those allocated to the intervention group performed approximately one hour of exercise training 3 times per week for 8 weeks using a cycle ergometer. The intensity of exercise was personalised to 60% of peak oxygen uptake (VO2peak) achieved during the CPET. After 8 weeks, 12 (46%) of the exercise-trained patients, and 11 (46%) controls underwent follow-up angiography. Collateral development significantly augmented in the exercise-trained group, with no change in controls. Notably, the cohorts recruited in these studies were comparatively diverse. All the patients recruited by Nolewajka et al. [28] and Belardinelli et al. [31] had a history of MI, compared to only 66% of patients recruited by Niebauer and colleagues [29]. These differences could be indicative of lower lesion severity in patients participating in the study by Niebauer et al.
The aforementioned studies relied on visual assessment of coronary angiograms to measure collateral development. Unfortunately, the limited spatial resolution of angiography means that small diameter changes in collateral networks may have been overlooked [32]. CFI is a more accurate direct measurement of collateral flow. Zbinden and colleagues [33] used CFI to evaluate collateral conductance in a healthy 46-year-old male cardiologist with angiographically normal coronary arteries and no cardiovascular risk factors. The objective of the study was to invasively assess the non-pathological collateral flow response during three phases of exercise training. The participant had a 25-year history of amateur distance running and was performing 2 hours of endurance exercise training per week at the time of enrolment. He underwent coronary angiography during both intermediate (4 hours training per week for 4 months) and high phases of training (8-9 hours training per week for four months). Hyperaemic coronary flow reserve (CFR) was measured in the left anterior descending artery as the maximum flow velocity after 18mg intra-coronary adenosine, divided by the baseline flow velocity. The CFI was determined by simultaneously measuring mean aortic pressure and mean distal coronary pressure at the end of a one-minute balloon occlusion.
The CFI increased by more than 60% during the high training versus the intermediate training phase and coincided with the eradication of symptoms felt during balloon occlusion. Given that the participant had normal coronary arteries, these changes were not driven by prolonged myocardial ischaemia.
Recently Zbinden and colleagues [34] recruited 40 patients undergoing percutaneous coronary intervention, measuring CFI in both the diseased and angiographically normal coronary artery at baseline. One day later, participants completed a CPET to establish their power to weight ratio (W·kg-1) and VO2max (ml·kg-1·min-1). For three months, the cohort exercised for at least 60 minutes 3 times per week by jogging or cycling at an intensity individualised to 80% of their heart rate at VO2max. After three months, patients were allocated to an exercise training group (n=24) or sedentary group (n=16), according to whether or not they adhered to the exercise program. Repeated angiography and CFI were performed in 12 exercising (50%), and 10 sedentary patients (62%). Those in the exercise group were more resilient to ischaemia caused by balloon occlusion in the normal and previously stenotic vessels, whereas there was no significant improvement in either vessel in the sedentary group. CFI significantly increased in the exercise-trained group for both the previously stenosed (0.155 ± 0.081 to 0.204 ± 0.056 (p= 0.03)), and normal vessel (0.176 ± 0.075 to 0.227 ± 0.070 (p= 0.0002)). By contrast, CFI did not change significantly in either vessel in the sedentary group. As expected, the exercise-trained group showed significant improvements in VO2max (p < 0.0001) and power to weight ratio (p= 0.001). Although this was not a randomised controlled trial, it does suggest that exercise training can augment collateral development, as evidenced by a “gold standard” method of quantification.
The largest and perhaps most notable human study on exercise induced collateral development is the EXCITE Trial [35]. The trial recruited 60 stable angina patients with physiologically significant coronary stenosis (defined as a Fractional Flow Reserve (FFR) of ≤ 0.75 [36]). After baseline testing (spiroergometry, CFI, FFR), patients were randomly allocated to receive four weeks of high-intensity training (group A), multimodal lifestyle intervention (group B), or usual care (group C) in a 1:1:1 ratio. High-intensity training consisted of 4 bouts of 30 minutes supervised exercise training at 70% angina free capacity, interspersed with 1 hour of recovery, 5 times per week for one month. In contrast, group B performed 6-8, 20-minute sessions at 60% of angina threshold per day, alongside lifestyle modification training. Usual care participants were advised to perform physical activity 2-3 times per week, for 20-30 minutes, as per current recommendations. After 1 month, FFR did not significantly change in any group (p= 0.148). However, CFI values did significantly increase post-training in both groups A and B (p= 0.001), and this coincided with significant improvements in VO2peak and an improved ischaemic threshold. The EXCITE Trial provided further evidence of improved collateral flow in response to exercise training and the absence of a change in FFR indicated that this was not a result of lesion regression.
The aforementioned studies investigated medium to long term exercise training by measuring CFI at rest. Whilst CFI is considered the gold standard measure of collateral flow, it is methodologically limited as it does not account for the potential effects of exercise on collateral vasomotion. This limitation was evaluated by two novel studies published in 2010 and 2012 that investigated the acute effects of exercise on collateral flow in patients with non-occlusive CAD. Togni and colleagues recruited 30 patients with stable, non-occlusive CAD undergoing coronary angiography. Previous research demonstrated that the coronary vasculature may be subject to ischaemic preconditioning via repeated balloon occlusion [37], or through physical exercise [38]. Therefore, the cohorts were randomly allocated to either rest/exercise or exercise/rest groups [39]. The rest/exercise group had baseline CFI measured during 1-minute balloon occlusion. Then, following 10 minutes of rest, they were asked to pedal a supine bike for 6 minutes, the intensity of which increased every 2 minutes (low/medium/high). Prior to the 6th minute, the balloon catheter was again inflated and CFI was measured. In contrast the exercise/rest group underwent the process in reverse. CFI increased significantly from 0.168 ± 0.118 during resting conditions to 0.262 ± 0.166 during exercise (p= 0.0002). There was no statistical difference in this increase irrespective of group allocation.
Lin et al. [40] recruited 65 single vessel CAD patients, randomly allocating them to either an isometric exercise training (n=33) or sedentary group (n=32). CFI was measured pre and post 1-minute balloon occlusion at the site of stenosis. During occlusion, the exercise group performed isometric handgrip exercise at 50% maximal voluntary contraction, whilst the sedentary group remained still. CFI post occlusion was significantly greater than pre-occlusion in the isometric exercise group, whilst there was no difference in the sedentary group (p < 0.002).
4. DISCUSSION
Arteriogenesis is an important process to protect against the effects of ischaemia in both stable and acute clinical settings. Current evidence indicate that collateral development through arteriogenesis is shear force-mediated. Shear stress is implicated in atherosclerotic development with areas of high shear stress being relatively protected from build-up of plaque. It also transiently increased during physical activity, and regular exercise is associated with a reduction in cardiovascular events and is thus recommended inprimary and secondary cardiovascular disease prevention.
The primary driver of shear stress in patients with a coronary lesion is the pressure gradient. Therefore, even if study participants exercise at the same intensity, the change in their individual pressure gradient will be diverse owing to heterogeneous lesion location and severity. In order to reduce these disparities, one strategy could be to evaluate a group of patients with a single vessel chronic total occlusion. By definition, the level of stenosis would be standardised and each patient would potentially have a more comparable level of collateral development. Therefore, patients exercising at or just below their individual ischemic threshold would ensure they were exercising at the same physiological limit. Additionally, research has linked transient and systemic increases in diastolic blood pressure to greater collateral development [41]. A method for increasing diastolic pressure that has yet to be investigated in this regard is resistance training. Resistance training may be a potent arteriogenic trigger, as it has been shown to generate significantly larger increases in diastolic blood pressure compared to standard ergometer based training, which has little or very little impact on diastolic blood pressure [42]. Furthermore, resistance exercise has been shown to result in a lower heart rate during training sessions; this may favourably increase coronary perfusion pressure and filling time [43]. Therefore, future research should also seek to individualise exercise intensity, frequency and modality in order to elicit enhanced collateral development.
4.1. Application to Clinical Practice
The development of coronary collaterals is a crucial mechanism to preserve the function of myocardium and protect against ischaemia. This is particularly important in patients with severe occlusive coronary disease as well as those with a diffuse disease that is not treatable by either PCI or Coronary Artery Bypass Graft surgery (CABG). Indeed, the current treatment options for patients with unrevascularizable coronary lesions are limited. Exercise may represent a cost-effective and viable pro-arteriogenic treatment option in this patient group.
CONCLUSION
Coronary collateral vessels supply blood to ischaemic myocardium, therefore, protecting against damage. Exercise increases coronary flow and, since the introduction of CFI measurements, has consistently been shown to stimulate collateral development. However, fundamental questions relating to the aetiology of collateral development, such as identifying the inter-relationship between ischaemia, shear force and the collateral network, remain unresolved. Exercise training interventions represent a valid and viable means of increasing our current understanding of collateral development.
Acknowledgements
Mr. Thomas Nickolay was awarded a Ph.D. scholarship from the University of Hull during the writing of this manuscript.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Zimarino M., D’Andreamatteo M., Waksman R., Epstein S.E., De Caterina R. The dynamics of the coronary collateral circulation. Nat. Rev. Cardiol. 2014;11(4):191–197. doi: 10.1038/nrcardio.2013.207. [DOI] [PubMed] [Google Scholar]
- 2.Werner G.S., Surber R., Ferrari M., Fritzenwanger M., Figulla H.R. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur. Heart J. 2006;27(20):2406–2412. doi: 10.1093/eurheartj/ehl270. [DOI] [PubMed] [Google Scholar]
- 3.Cui K., Lyu S., Song X., et al. Effect of coronary collaterals on prognosis in patients undergoing primary percutaneous coronary intervention for acute st-segment elevation myocardial infarction: A Meta-analysis. Angiology. 2018;69(9):803–811. doi: 10.1177/0003319718768399. [DOI] [PubMed] [Google Scholar]
- 4.Baroldi G., Mantero O., Scomazzoni G. The collaterals of the coronary arteries in normal and pathologic hearts. Circ. Res. 1956;4(2):223–229. doi: 10.1161/01.RES.4.2.223. [DOI] [PubMed] [Google Scholar]
- 5.Fulton W.F.M. The time factor in the enlargement of anastomoses in coronary artery disease. Scott. Med. J. 1964;9(1):18–23. doi: 10.1177/003693306400900104. [DOI] [PubMed] [Google Scholar]
- 6.Zoll P.M., Wessler S., Schlesinger M.J. Interarterial coronary anastomoses in the human heart, with particular reference to anemia and relative cardiac anoxia. Circulation. 1951;4(6):797–815. doi: 10.1161/01.CIR.4.6.797. [DOI] [PubMed] [Google Scholar]
- 7.Meier P., Hemingway H., Lansky A.J., Knapp G., Pitt B., Seiler C. The impact of the coronary collateral circulation on mortality: A meta-analysis. Eur. Heart J. 2012;33(5):614–621. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- 8.Koerselman, van der Graaf, de Jaegere, Grobbee. Coronary collaterals. Circulation. 2003;107(19):2507–2511. doi: 10.1161/01.CIR.0000065118.99409.5F. [DOI] [PubMed] [Google Scholar]
- 9.Pipp F., Boehm S., Cai W.J., et al. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler. Thromb. Vasc. Biol. 2004;24(9):1664–1668. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- 10.Van Royen N., Piek J.J., Schaper W., Bode C., Buschmann I. Arteriogenesis: Mechanisms and modulation of collateral artery development. J. Nucl. Cardiol. 2001;8(6):687–693. doi: 10.1067/mnc.2001.118924. [DOI] [PubMed] [Google Scholar]
- 11.Baeyens N., Bandyopadhyay C., Coon B.G., Yun S., Schwartz M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Invest. 2016;126(3):821–828. doi: 10.1172/JCI83083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaper. Collateral circulation. Basic Res. Cardiol. 2009;104(1):5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholz D., Cai W.J., Schaper W. Arteriogenesis, a new concept of vascular adaptation in occlusive disease. Angiogenesis. 2001;4(4):247–257. doi: 10.1023/A:1016094004084. [DOI] [PubMed] [Google Scholar]
- 14.Seiler C., Stoller M., Pitt B., Meier P. The human coronary collateral circulation: Development and clinical importance. Eur. Heart J. 2013;34(34):2674–2682. doi: 10.1093/eurheartj/eht195. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 16.Degen A., Millenaar D., Schirmer S.H. Therapeutic approaches in the stimulation of the coronary collateral circulation. Curr. Cardiol. Rev. 2014;10(1):65–72. doi: 10.2174/1573403X113099990027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eitenmüller I., Volger O., Kluge A., et al. The range of adaptation by collateral vessels after femoral artery occlusion. Circ. Res. 2006;99(6):656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- 18.Qin X., Deng Y., Wu D., Yu L., Huang R. Does Enhanced External Counterpulsation (EECP) significantly affect myocardial perfusion?: A systematic review & meta-analysis. PLoS One. 2016;11(4):e0151822. doi: 10.1371/journal.pone.0151822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gloekler S., Meier P., de Marchi S.F., et al. Coronary collateral growth by external counterpulsation: A randomised controlled trial. Heart. 2010;96(3):202–207. doi: 10.1136/hrt.2009.184507. [DOI] [PubMed] [Google Scholar]
- 20.Duncker D.J., Bache R.J. Regulation of coronary blood flow during exercise. Physiol. Rev. 2008;88(3):1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 21.Telkova I.L., Tepliakov A.T. Relationships between changes of coronary blood flow, energy metabolism of the myocardium, and hyperinsulinemia in patients with ischemic heart disease. Kardiologiia. 2005;45(8):61–68. [PubMed] [Google Scholar]
- 22.Magnani S., Roberto S., Sainas G., et al. Metaboreflex-mediated hemodynamic abnormalities in individuals with coronary artery disease without overt signs or symptoms of heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018;314(3):H452–H463. doi: 10.1152/ajpheart.00436.2017. [DOI] [PubMed] [Google Scholar]
- 23.Schirmer S.H., Degen A., Baumhäkel M., et al. Heart-rate reduction by If-channel inhibition with ivabradine restores collateral artery growth in hypercholesterolemic atherosclerosis. Eur. Heart J. 2012;33(10):1223–1231. doi: 10.1093/eurheartj/ehr255. [DOI] [PubMed] [Google Scholar]
- 24.Gloekler S., Traupe T., Stoller M., et al. The effect of heart rate reduction by ivabradine on collateral function in patients with chronic stable coronary artery disease. Heart. 2014;100(2):160–166. doi: 10.1136/heartjnl-2013-304880. [DOI] [PubMed] [Google Scholar]
- 25.Eckstein R.W. Effect of exercise and coronary artery narrowing on coronary collateral circulation. Circ. Res. 1957;5(3):230–235. doi: 10.1161/01.RES.5.3.230. [DOI] [PubMed] [Google Scholar]
- 26.Scheel K.W., Ingram L.A., Wilson J.L. Effects of exercise on the coronary and collateral vasculature of beagles with and without coronary occlusion. Circ. Res. 1981;48(4):523–530. doi: 10.1161/01.RES.48.4.523. [DOI] [PubMed] [Google Scholar]
- 27.Heaps C.L., Parker J.L. Effects of exercise training on coronary collateralization and control of collateral resistance. J. Appl. Physiol. 2011;111(2):587–598. doi: 10.1152/japplphysiol.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolewajka A.J., Kostuk W.J., Rechnitzer P.A., Cunningham D.A. Exercise and human collateralization: An angiographic and scintigraphic assessment. Circulation. 1979;60(1):114–121. doi: 10.1161/01.CIR.60.1.114. [DOI] [PubMed] [Google Scholar]
- 29.Niebauer J., Hambrecht R., Marburger C., et al. Impact of intensive physical exercise and low-fat diet on collateral vessel formation in stable angina pectoris and angiographically confirmed coronary artery disease. Am. J. Cardiol. 1995;76(11):771–775. doi: 10.1016/S0002-9149(99)80224-0. [DOI] [PubMed] [Google Scholar]
- 30.Fujita M., Sasayama S., Asanoi H., Nakajima H., Sakai O., Ohno A. Improvement of treadmill capacity and collateral circulation as a result of exercise with heparin pretreatment in patients with effort angina. Circulation. 1988;77(5):1022–1029. doi: 10.1161/01.CIR.77.5.1022. [DOI] [PubMed] [Google Scholar]
- 31.Belardinelli R., Georgiou D., Ginzton L., Cianci G., Purcaro A. Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation. 1998;97(6):553–561. doi: 10.1161/01.CIR.97.6.553. [DOI] [PubMed] [Google Scholar]
- 32.Werner G.S. The role of coronary collaterals in chronic total occlusions. Curr. Cardiol. Rev. 2014;10(1):57–64. doi: 10.2174/1573403X10666140311123814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zbinden R., Zbinden S., Windecker S., Meier B., Seiler C. Direct demonstration of coronary collateral growth by physical endurance exercise in a healthy marathon runner. Heart. 2004;90(11):1350–1351. doi: 10.1136/hrt.2003.023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zbinden R., Zbinden S., Meier P., et al. Coronary collateral flow in response to endurance exercise training. Eur. J. Cardiovasc. Prev. Rehabil. 2007;14(2):250–257. doi: 10.1097/HJR.0b013e3280565dee. [DOI] [PubMed] [Google Scholar]
- 35.Möbius-Winkler S., Uhlemann M., Adams V., et al. Coronary collateral growth induced by physical exercise: Results of the impact of intensive exercise training on coronary collateral circulation in patients with stable coronary artery disease (EXCITE) trial. Circulation. 2016;133(15):1438–1448. doi: 10.1161/CIRCULATIONAHA.115.016442. [DOI] [PubMed] [Google Scholar]
- 36.Pijls N.H.J., De Bruyne B., Peels K., et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N. Engl. J. Med. 1996;334(26):1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 37.Laskey W.K. Brief repetitive balloon occlusions enhance reperfusion during percutaneous coronary intervention for acute myocardial infarction: A pilot study. Catheter. Cardiovasc. Interv. 2005;65(3):361–367. doi: 10.1002/ccd.20397. [DOI] [PubMed] [Google Scholar]
- 38.Crisafulli A., Melis F., Tocco F., et al. Exercise-induced and nitroglycerin-induced myocardial preconditioning improves hemodynamics in patients with angina. Am. J. Physiol. Heart Circ. Physiol. 2004;287(1):H235–H242. doi: 10.1152/ajpheart.00989.2003. [DOI] [PubMed] [Google Scholar]
- 39.Togni M., Gloekler S., Meier P., et al. Instantaneous coronary collateral function during supine bicycle exercise. Eur. Heart J. 2010;31(17):2148–2155. doi: 10.1093/eurheartj/ehq202. [DOI] [PubMed] [Google Scholar]
- 40.Lin S., Lu X., Chen S., et al. Human coronary collateral recruitment is facilitated by isometric exercise during acute coronary occlusion. J. Rehabil. Med. 2012;44(8):691–695. doi: 10.2340/16501977-0989. [DOI] [PubMed] [Google Scholar]
- 41.Shu W. The relationship between diastolic pressure and coronary collateral circulation in patients with stable angina pectoris and chronic total occlusion. Am. J. Hypertens. 2013;26(5):630–635. doi: 10.1093/ajh/hps096. [DOI] [PubMed] [Google Scholar]
- 42.MacDougall J.D., Tuxen D., Sale D.G., Moroz J.R., Sutton J.R. Arterial blood pressure response to heavy resistance exercise. J. Appl. Physiol. 1985;58(3):785–790. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- 43.McCartney N. Acute responses to resistance training and safety. Med. Sci. Sports Exerc. 1999;31(1):31–37. doi: 10.1097/00005768-199901000-00007. [DOI] [PubMed] [Google Scholar]