Abstract
The endocannabinoid system participates in the regulation of CNS homeostasis and functions, including neurotransmission, cell signaling, inflammation and oxidative stress, as well as neuronal and glial cell proliferation, differentiation, migration and survival. Endocannabinoids are produced by multiple cell types within the CNS and their main receptors, CB1 and CB2, are expressed in both neurons and glia. Signaling through these receptors is implicated in the modulation of neuronal and glial alterations in neuroinflammatory, neurodegenerative and psychiatric conditions, including Alzheimer’s, Parkinson’s and Huntington’s disease, multiple sclerosis, amyotrophic lateral sclerosis, stroke, epilepsy, anxiety and depression. The therapeutic potential of endocannabinoid receptors in neurological disease has been hindered by unwelcome side effects of current drugs used to target them; however, due to their extensive expression within the CNS and their involvement in physiological and pathological process in nervous tissue, they are attractive targets for drug development. The present review highlights the potential applications of the endocannabinoid system for the prevention and treatment of neurologic and psychiatric disorders.
Keywords: Endocannabinoids, endocannabinoid receptors, neuroinflammation, neurodegeneration, psychiatric disease, drug targets
1. INTRODUCTION
The term “endocannabinoid” (eCB) derives from the similarities between the main active compound found in the plant Cannabis sativa, Δ9-tetrahydrocannabinol (Δ9-THC), and endogenous substances produced within the organism. The eCB system was first detected in the 1980’s, with the identification of anandamide (from the Sanskrit ananda, bliss) in the brain of pigs, followed by the identification of its main receptor in the 1990’s. Since its discovery and characterization, eCBs have been a subject of constant study due to their relevance for the regulation of systemic and tissue-specific homeostasis. In the nervous system, eCBs participate in a wide range of biological activities, particularly in the modulation of neurotransmission, neuronal survival, synaptic plasticity and inflammatory responses. Therefore, alterations in eCB functions have been related to the development of neuropathology ranging from Parkinson’s disease to stroke and depression and may thus be relevant targets for therapeutic intervention in these pathologies.
2. THE ENDOCANNABINOID SYSTEM
The eCB system consists of two main ligands, anadamide (N-arachidonoylethanolamine, AEA) and 2-arachidonoylglycerol (2-AG). AEA is derived from the cell membrane phospholipid arachidonic acid by the action of the enzymes N-acylphosphatidylethanolamine-phospholipase D (NAPE-PLD), which produces AEA and phosphatidic acid. 2-AG is produced by the action of phospholipase C (PLC) and diacylglycerol lipase (DAGL), generating also diacylglycerol (DAG) [1, 2]. eCBs are produced as required by cells in response to changes in intracellular calcium concentrations and are subsequently re-uptaked and inactivated principally by the enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), which degrade AEA and 2-AG, respectively [3, 4]. 2-AG is considered the main ligand for cannabinoid receptors and appears to be present at higher concentrations than AEA in nervous tissue [5].
Cannonical eCB receptors belong to the G protein-coupled receptor (GPCR) family. The main receptor, CB1, has been detected in multiple tissues, including skeletal muscle, vascular endothelium, gastrointestinal system, liver, pancreas, adipose tissue, spleen, heart and immune cells, and it is particularly abundant at neuronal synapses in the central nervous system (CNS). CB1 is considered the most abundant GPCR and can be found in multiple areas, including the cortex, hippocampus, hypothalamus, basal ganglia, cerebellum and brainstem [6-9]. CB1 expression can also be found in the peripheral nervous system, mainly at sensory neuron synaptic terminals [10-12]. CB1 is found in both glutamatergic and GABAergic circuits, although it modulates release of other neurotransmitters, including dopamine, serotonin, acetylcholine and norepinephrine, through regulation of glutamate or GABA release from pre- or postsynaptic neurons. This receptor acts predominantly through retrograde modulation of neurotransmitter release in the presynaptic neuron [11, 13-17]. In contrast, CB2 is expressed at higher densities in leukocytes and lymphoid tissues, including the spleen, thymus and lymph nodes, although it is also expressed in the gastrointestinal and cardiovascular systems, liver and adipose tissue. This receptor is also expressed in low levels in the brain, both in neurons and glial cells. Its expression increases in glial cells upon activation and recent studies are beginning to elucidate its neuromodulatory and homeostatic functions [10-12, 17]. Stimulation of both CB1 and CB2 receptors usually promotes activation of Gi/Go subunits, inhibiting adenylate cyclase activity and decreasing intracellular cyclic AMP concentrations. This causes inhibition of protein kinase A (PKA) and mitogen-activated protein kinases (MAPKs) activity, as well as modulation of voltage-gated Ca2+ channels and inwardly rectifying K+ channels [11, 12, 17].
3. ENDOCANNABINOID-DEPENDENT REGULATION OF SYNAPTIC PLASTICITY
As CB1 receptors are the most abundant type of GPCRs in the brain, their effects on the activity of Ca2+ and K+ channels promote significant alterations in neuronal functions. eCBs are known to participate in both short- and long-term changes in synaptic plasticity. Fast-acting (in the order of seconds) CB1-dependent inhibition of voltage-gated Ca2+ channels in presynaptic neurons affects short-term synaptic plasticity in GABAergic and glutamatergic circuits by modifying the activity of depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE), respectively [16, 18]. DSI and DSE are based on transient and reversible inhibition of afferent synaptic signals caused by depolarization of postsynaptic neurons, which inhibits neurotransmitter release by the presynaptic neuron. In contrast, long-term modulation of synaptic plasticity is achieved by sustained (in the order of minutes) or repetitive CB1-dependent stimulation of neuronal circuits. This leads to the inhibition of adenylate cyclase activity, reduction in cAMP concentrations and PKA activity, as well as induction of long-term depression (LTD) in GABAergic and glutamatergic circuits [16, 18, 19]. In addition, there is accumulating evidence demonstrating CB1 expression at the mitochondrial membrane in hippocampal and hypothalamic neurons and its relationship to regulation of mitochondrial respiration and ATP production, which may have significant effects on neuronal functions [12, 19, 20].
Integration of synaptic activity and eCB signaling is achieved by the activation of eCB synthesis in the postsynaptic neuron in response to neurotransmitter release by the presynaptic neuron. Group I metabotropic glutamate receptors (mGluRs 1/5), along with m1/m3 muscarinic receptors, are known to promote activation of phospholipase C (PLC), increasing intracellular concentrations of DAG and synthesis of 2-AG, the main ligand for CB1 receptors [17]. Importantly, 2-AG appears to be produced constitutively by post-synaptic neurons, with its effects being regulated by expression of MAGL in presynaptic neurons [20]. Anadamide, on the other hand, is synthesized in response to the activation of neurotransmitter receptors, such as the dopamine D2 receptors, which are also necessary for induction of changes in long-term synaptic plasticity [17]. Anandamide also modulates constitutive 2-AG signaling by activation of transient receptor potential vanilloid-1 (TRPV1) receptors in GABAergic neurons [21].
Control of the duration of eCB stimulation is controlled by regulated expression of their degradative enzymes. MAGL, the main enzyme for degradation of 2-AG, is expressed predominantly in the presynaptic neuron, whereas the presence of the fatty acid carrier proteins FABP5 and FABP7 allows transport of AEA into intracellular compartments containing FAAH, for its degradation [22]. Continuous activation of CB1 receptors decreases its signaling efficacy by inducing tolerance to eCBs, as well as activating β–arrestin-dependent internalization of membrane-bound CB1 receptors, reducing the overall density of CB1 receptors at the cell surface [10, 19]. In contrast, repetitive activation of neuronal synapses enhances the expression of CB1 receptors in the presynaptic neuron, thus regulating neurotransmission and preventing overexcitation of the neuronal circuit [19]. Furthermore, neural stem cells responsible for the maintenance of neurogenesis at specific areas of the nervous system express both CB1 and CB2 receptors and treatment with AEA or CB1-specific agonists, but not by CB2 agonists, promotes their differentiation to mature neurons by inhibiting ERK 1/2 signaling, demonstrating the importance of CB1-dependent signals for neurogenesis [23].
In contrast to CB1, CB2-dependent modulation of synaptic activity has not been as extensively studied, due to the limited expression of CB2 receptors in neurons. However, previous studies have demonstrated that CB2 receptors are expressed at low levels in hippocampal and cortical neurons, where they regulate synaptic plasticity by inducing 2-AG-dependent hyperpolarization of pyramidal neurons, altering the activity of the sodium-bicarbonate co-transporter and the intracellular concentrations of Na+ and Cl-, promoting long-lasting neuronal hyperpolarization at the CA2 and CA3 areas in vivo [24, 25]. In vitro studies in transfected cell cultures have demonstrated that 2-AG activation of presynaptic CB2 receptors can induce DSE in CB1-knockout hippocampal neurons, maintaining tonic inhibition of neurotransmission that could be reversed by specific CB2 antagonists or DAGL inhibitors [26]. There is also evidence for pre- and postsynaptic neuronal expression of CB2 at the prefrontal cortex, basal ganglia, thalamus, brain stem and cerebellum, as well as in oligodendrocytes, astrocytes, microglia and endothelial cells of the blood-brain barrier (BBB) and its expression is upregulated under inflammatory conditions [27, 28].
Although most of the functions of 2-AG appear to depend on the activation of CB receptors in the CNS, AEA also plays a very important role in the modulation of synaptic activity, particularly through activation of other receptors, such as TRPV1. TRPV1 receptors are part of the transient receptor potential channel family and are found particularly in the peripheral nervous system, where they are related to transmission of painful stimuli [29]. They are involved in the induction of AEA-dependent postsynaptic LTD in neurons expressing D2 dopamine receptors, after activation of mGluR5, thus being related to the regulation of synaptic plasticity in glutamatergic circuits [30].
eCBs have multiple effects in different areas of the brain. In the hippocampus, for example, CB1 receptors appear to be preferentially expressed in GABAergic neurons and their activity has been related to enhancement of LTP and contributing to learning and memory processes [31]. The cortex presents abundant CB1 expression in GABAergic interneurons and is also found in glutamatergic fibers and modulates both excitatory and inhibitory neurotransmission at specific cortical layers [19, 24]. In the somatosensory cortex in rodents, LTP stimulation by eCBs is related to activation of NMDA receptors and increased secretion of brain-derived neurotrophic factor (BDNF), which may in part be dependent of eCB activation of astrocytes and release of glutamate [24]. At the basal ganglia, activation of NMDARs, mGluRs and dopamine D2 receptors promote the release of eCBs that modulate both LTP and depotentiation in the striatum and have significant effects on behavior and dopamine-dependent motor control and there is evidence of potential interaction between serotonin 5-HT2, µ-opiod and CB1 receptors in the nucleus accumbens [32].
eCB-dependent modulation of neuronal functions does not rely only on eCB synthesis and signaling on neurons. Both 2-AG and AEA can be produced by astrocytes and microglia and these cells, along with oligodendrocytes, express proteins involved in eCB synthesis [33-37]. Astrocytes, in particular, respond to eCBs via CB1-dependent activation of PLC, releasing glutamate at neuronal synapses and participating in the modulation of neurotransmission [38]. Astrocyte-dependent glutamate release in response to eCB signaling stimulates NMDARs on hippocampal neurons and promotes neuronal excitability and neurotransmitter release, leading to modulation of synaptic plasticity, including induction of LTD in cortical neurons and inhibition of neurotransmission in the hippocampus [39], although further studies also report that glutamate and D-serine release by astrocytes in response to eCB signaling are also involved in the induction of LTP in glutamatergic circuits in the hippocampus, striatum and cortex, possibly by enhancing expression or activation of NMDARs and mGluRs, as well as release of BDNF and nitric oxide in response to astrocyte activity at the synapse [40]. The relevance of eCB-dependent astrocyte activity for neuronal functions has been demonstrated in animal models of selective CB1 deficiency in astrocytes, which suggest a possible role for astrocytes in the regulation of learning and memory processes after Cannabis use [41]. As evidenced here, the ample distribution of the eCB system within the CNS allows it to modulate a variety of neuronal functions, therefore making it relevant for the study of neuronal homeostasis and dysfunction.
4. ENDOCANNABINOIDS AND NEUROLOGICAL DYSFUNCTION
The eCB system participates in the regulation of multiple physiologic processes in the nervous system, including the regulation of neurotransmission, neurogenesis and synaptic plasticity, thus affecting multiple cognitive processes, particularly learning and memory, as well as the control of behavior and emotions, sleep, appetite, nociception, inflammation and oxidative stress [42]. As its main mechanism of action on neurons is thought to depend on CB1-dependent modulation of neurotransmitter release via retrograde signaling at neuronal synapses, the system is a key component in the maintenance of nervous system homeostasis. Following this paradigm, it has been demonstrated that eCB receptor expression is altered during neuroinflammatory conditions and the expression patterns may vary in different conditions and during disease evolution. In general, alterations in CB1 and CB2 expression in nervous and immune system cells and tissues, as well as plasma concentrations of eCBs, are observed in neuroinflammatory and neurodegenerative conditions, including multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, stroke, traumatic brain injury and epilepsy [42-48]. Furthermore, altered eCB signaling is involved in the pathological characteristics of patients with psychiatric disorders such as schizophrenia, anxiety, depression and chronic stress [49-52].
In addition, eCBs may also affect neuronal functions through interaction with glial cells. In particular, astrocytes are known to express functional CB1 receptors and respond to neuronal eCB production by releasing neurotransmitters, in turn modulating neurotransmission and neuronal functions even beyond the originating synapse [53]. Although the contribution of glial-derived eCB-dependent signals to development of neurological dysfunction has not been properly determined, there is evidence for their involvement in cognitive dysfunction, including learning and memory deficits and in the pathogenesis of epilepsy [53].
4.1. eCB-dependent Modulation of the HPA Axis
eCBs have been implicated in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis, participating in the modulation of the stress response by altering production of relevant systemic signaling molecules, such as glucocorticoids, which usually promote anti-inflammatory effects. It has been demonstrated that repeated stressful stimuli decrease AEA concentrations in the limbic system while increasing 2-AG production in the amygdala, leading to adaptive stress responses in vivo [54]. Release of corticosterone decreases hypothalamic 2-AG concentrations in vivo, in models of acute stress, which is in turn negatively regulated under chronic stress by increased 2-AG production in the CNS, suggesting a model in which eCBs modulate the production of stress hormones differentially under acute and chronic stress conditions, hence maintaining CNS homeostasis [55].
eCB-dependent regulation of glucocorticoid production is a relevant aspect in CNS homeostasis, as both types of compounds have shown neuroprotective effects in various experimental models of neurodegeneration, promoting anti-inflammatory and anti-oxidative functions individually; however, there is also evidence for negative effects when both types of compounds act together on the same cell populations [54]. Of note, alterations in HPA axis functions have been related to development of neurological alterations observed in patients with depression and inhibition of CB1-mediated signaling is related to increased concentrations of ACTH and development of depressive-like behavior in animals [56].
4.2. Regulation of BBB Integrity by eCB Receptors
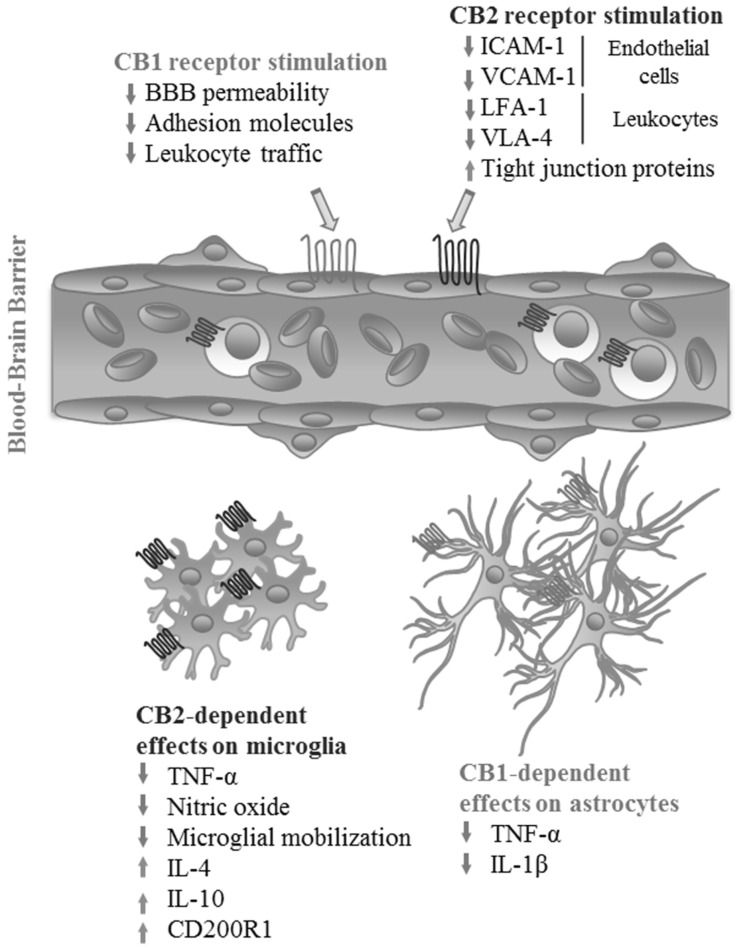
Maintenance of the structural integrity of the BBB is critical for CNS homeostasis, as it prevents the free flow of potentially detrimental substances, inflammatory mediators and activated immune cells into the CNS. However, under inflammatory conditions, the endothelial cells lining the BBB become activated, decreasing their expression of tight junction proteins while increasing their expression of chemokines and adhesion molecules, thus enhancing the diffusion of soluble mediators across the endothelial barrier, as well as leukocyte adhesion and diapedesis [57-60]. eCBs contribute to the maintenance of BBB functions, particularly during neuroinflammatory conditions. AEA-dependent activation of CB1 receptors downregulates the expression of the endothelial adhesion molecule VCAM-1, also decreasing leukocyte traffic and microglial activation in experimental models of viral encephalitis, an effect that could be blocked by administration of a specific CB1 receptor antagonist, therefore demonstrating their dependence on CB1 activation [61]. In addition, CB1-deficient animals showed enhanced microgliosis and BBB expression of VCAM-1 after viral infection compared to wild-type animals, exposing the role of CB1 in the regulation of neuroinflammation [61]. Increased expression of VCAM-1 has also been observed in cerebrospinal fluid and brain tissue from patients with multiple sclerosis and other neuroinflammatory conditions [62-64]. In addition, eCBs inhibit the adhesion of encephalitogenic T cells to brain venules in an in vivo model of neuroinflammatory pathology [65]. This effect is achieved in an adhesion molecule-independent manner, by CB1-dependent increase in cAMP and PKA activation that can be reversed by inhibition of CB1 signaling, thus demonstrating the relevance of eCBs for the regulation of leukocyte adhesion to the BBB under inflammatory conditions [65].
Supporting the anti-inflammatory effects of CB2-dependent signaling in other cell types, administration of selective CB2 agonists JWH133 or O-1966 reduces BBB permeability and expression of ICAM-1 and VCAM-1 in vivo in an animal model after administration of lipopolysaccharide (LPS), as well as increasing the expression of tight junction proteins in an in vitro endothelial barrier model, suggesting that CB2 activation inhibits leukocyte migration across the BBB [47]. Additionally, activation of CB2 receptors in leukocytes also decreases their adhesion to LPS-activated BBB endothelial cells in vivo and in vitro by down-regulating leukocyte expression of the adhesion molecules LFA-1 and VLA-4 [66] (Fig. 1).
Fig. (1).
Effects of eCB receptor activation on the blood-brain barrier and glial inflammatory response. Activation of CB1 and CB2 receptors by their endogenous ligands or exogenous agonists at the BBB decreases endothelial permeability and leukocyte infiltration into the CNS by reducing the expression of intercellular adhesion molecules both at the endothelial layer and on leukocytes, while also increasing the expression of tight junction proteins. On glial cells, signaling through CB2 decreases production of pro-inflammatory cytokines and nitric oxide by microglia, while increasing the production of inhibitory molecules. In astrocytes, a similar effect is achieved by activation of CB1. (Figure created by the authors). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
These data show that specific activation of CB2 at endothelial cells is useful for the maintenance of BBB integrity under neuroinflammatory conditions. Interestingly, CB1 and CB2 may present opposing functions at the BBB under certain conditions. For example, production of nitric oxide and expression of proteins used for transport of AEA at the BBB are induced by activation of CB1 receptors and inhibited by activation of CB2 [67]. Furthermore, eCBs affect BBB permeability not only through CB receptor-dependent signaling, but also via TRPV1, calcitonin gene-related peptide (CGRP) receptor and peroxisome proliferator-activated receptors (PPARs), although the latter two appear to react specifically to AEA and the endocannabinoid-like N-oeoylethanolamide (OEA), respectively, decreasing BBB permeability under ischemia-reperfusion conditions in vitro [68].
4.3. Modulation of Glial Inflammatory Responses by eCB Receptors
As mentioned previously, eCB receptors have been detected in all glial cell types in the CNS and astrocytes and microglia are known to produce both AEA and 2-AG [12, 69]. Microglial activation and acquisition of a M1-like (pro-inflammatory) phenotype is a common feature of neuroinflammatory conditions and pathologies like Alzheimer’s disease, Parkinson’s disease and multiple sclerosis [70]. Microglia are considered an important source of AEA in the CNS and express both CB1 and CB2 receptors, with CB2 expression in particular increasing after microglial activation in animal models [71]. Recent studies demonstrate that CB1-dependent signaling is involved in the maintenance of a resting phenotype in microglia in the hippocampus through an indirect mechanism involving eCB control of GABAergic neurons, as microglia from mice with a targeted deletion of CB1 on this neuronal population presented a pre-activated phenotype resembling LPS-stimulated microglia, with increased production of TNF-α and decreased expression of CX3CL1 by neurons [72].
Likewise, administration of AEA or 2-AG to cultured microglia before antigenic stimulation with LPS decreases microglial activation and production of TNF-α and nitric oxide and use of a CB1 antagonist did not change the observed effect, indicating a CB1-independent mechanism, [71]. Nonetheless, microglial response to AEA was blocked by the use of a CB2 antagonist, supporting the CB2-dependent anti-inflammatory effects of eCBs on leukocytes in the CNS [71]. Additional studies have shown that microglial responses to CB2 include the mitogen-activated protein kinase-phosphatase (MKP) 1 and 3-dependent inhibition of extracellular signal-regulated kinase (ERK) activity after in vitro LPS stimulation [73]. Use of a CB2 agonist increases microglial MKP expression and causes a reduction in the production of pro-inflammatory cytokines and microglial mobilization after LPS stimulation [73]. Activation of CB2 enhances production of both IL-4 and IL-10, while decreasing production of nitric oxide and response to chemokines like CCL2, suggesting that activation of this receptor in microglia promotes phenotype switching to alternative M2-like cells, reducing classical pro-inflammatory responses [74]. An additional mechanism that has been proposed to explain the anti-inflammatory properties of eCBs in neuroinflammation is an AEA and CB2-dependent enhancement of the expression of the immune suppressor protein CD200R1 in antigen-stimulated microglia, leading to decreased production of pro-inflammatory cytokines, increased production of IL-10 and increased neuronal survival in an animal model of virus-induced demyelination [75]. AEA supplementation increases the expression of both CD200 and CD200R1 in the brain, decreasing the severity of disease in this model [75].
Similarly, astrocyte activation is an essential component of the neuroinflammatory response observed in multiple pathologic conditions and there is solid evidence demonstrating that eCBs also inhibit astrocyte activation and production of pro-inflammatory mediators. In vitro studies have shown that stimulation with AEA decreases production of TNF-α and IL-1β by astrocytes after infection with Theiler’s virus [73]. Deletion of MAGL, the enzyme responsible for degradation of 2-AG, in astrocytes, causes accumulation of this molecule in the CNS in vivo, leading to reduced production of prostaglandins and pro-inflammatory cytokines after antigenic challenge [76]. Importantly, these effects are only partially reversed by administration of CB1 antagonists, demonstrating not only the relevance of eCB signaling in astrocytes as mediator of the inflammatory response within the CNS, but the existence of CB1-independent pathways for eCB regulation of astrocyte functions [76]. eCB-dependent neuroprotective effects appear to be variable and rely on their interaction with other signaling molecules. For example, eCBs have shown significant capacity to decrease production of pro-inflammatory cytokines by activated leukocytes by signaling through CB2, but this capacity is lost when combined with glucocorticoids, an effect that can be reversed by blocking CB2-dependent signals [77] (Fig. 1).
4.4. eCB Receptors in the Ageing Brain
Neurons and glial cells undergo physiological changes during ageing that modify their responses to various stimuli. eCB signaling in the CNS changes throughout ageing, with studies in rodents and humans demonstrating that CB1 expression reaches its highest level in puberty and adulthood, declining as individuals grow older. Comparisons between young (3 months) and old (more than 2 years) rats found alterations in CB1 mRNA and protein levels in the cortex, hippocampus, cerebellum and hypothalamus, with the most significant being decreased receptor-agonist binding in the cerebellum and a very significant increase (over 211%) in CB1 mRNA in the brainstem of aged rats [78]. In humans, decreased CB1 expression was evident in adult brains, compared to fetal and neonatal tissue, particularly in the basal ganglia, substantia nigra and cerebellar cortex [8]. CB1 mRNA decreases by as much as 50% in the prefrontal cortex of adults [79] CNS AEA concentrations increase with age into adulthood, when they reach their peak in the hypothalamus [80]. Changes in eCB signaling related to age have been shown to have significant effects on cognition, affecting learning and memory performance in various experimental settings, whereas use of THC is known to promote cognitive impairment in humans and has been suggested to promote neurological alterations that may promote development of psychiatric pathologies [81].
Absence of CB1 receptors in GABAergic neurons promotes development of inflammation at the hippocampus, dependent on activation of astrocytes and microglia, with increased neuronal apoptosis [82]. In contrast, lack of CB2 receptors appears to enhance the deleterious effects of ageing in other tissues, such as bone, although these receptors may also be important in the regulation of neuroinflammatory conditions that arise as a consequence of ageing [81]. Importantly, enhancing AEA-dependent functions by use of FAAH inhibitors or in FAAH-knockout mice increases hippocampal neurogenesis in adult animals, which may be reduced by as much as 50% in old individuals and may also be reduced by knocking out CB1 receptors or decreasing 2-AG production in the CNS, supporting the relevance for eCB-dependent signaling in maintenance of CNS homeostasis and cognitive functions in aged organisms [81].
Glial reactivity is a prominent characteristic among the cellular changes that appear in aged nervous tissue, an effect that is enhanced by absence of CB1 receptors. Aged astrocytes and microgllia from CB1-knockout mice become more susceptible to activation and production of pro-inflammatory cytokines, even in the absence of antigenic stimulation [82, 83]. Although astrocytes do not express eCB receptors at high levels under normal conditions, they are known to respond to eCBs via activation of CB1 receptors, as well as producing both AEA and 2-AG [83]. Interestingly, studies in mice with specific deletion of the CB1 receptor on GABAergic neurons demonstrate that absence of CB1 expression on these cells promotes an activated phenotype in astrocytes, with characteristic morphological changes and increased expression of GFAP and TNF-α by these cells in the hippocampus of aged animals [82, 83].
Aged microglia undergo similar changes to those observed in aged astrocytes. They become more susceptible to activation and acquisition of a pro-inflammatory phenotype, and while they also express low levels of CB1 receptors in normal conditions, they express high amounts of CB2, which increases after activation [84]. Microglia are the main producers of 2-AG within the brain, therefore being a relevant part in eCB-dependent modulation of neuronal and glial activity [36]. CB1/CB2-dependent eCB signaling on microglia promotes anti-inflammatory effects in multiple models of neuroinflammation [85, 86] and is also susceptible to CB1-activated functions of neurons, as studies in the GABAergic CB1-knockout model demonstrate that absence of CB1 signaling in these cells enhances the activated pro-inflammatory phenotype of microglia in the hippocampus in vivo [72]. Neuron-dependent alterations in glial phenotypes have been attributed to a decreased production of eCBs, particularly 2-AG, in the ageing brain, which may in turn decrease its efficacy as an anti-inflammatory mediator and promote microglial and astrocyte activation [72, 83], Increased activation of these cells promotes pro-inflammatory responses, thus contributing to neuronal dysfunction and possibly to the development of neurological pathologies, although there may be additional as yet unknown factors contributing to the alterations described here.
4.5. eCB Receptors in Alzheimer’s Disease
Pathological features of Alzheimer disease include the accumulation of amyloid beta (Aβ) and hyper-phosphorylation of Tau proteins, leading to the formation of amyloid plaques and neurofibrillary tangles that promote CNS inflammation and neurotoxicity [87]. The inflammatory response in this pathology depends mostly on activation of microglia and astrocytes, promoting production of pro-inflammatory cytokines and nitric oxide, affecting neuronal functions [88, 89]. Under pathologic conditions, damage to neurons usually increases eCBs production [90]. Due to their role in the regulation of neurotransmission and neuronal homeostasis, eCBs may be relevant in the development and progression of Alzheimer’s disease. This has been demonstrated in studies reporting decreased 2-AG degradation in areas of Aβ accumulation in late-stage Alzheimer’s patients [91]. In contrast, there is evidence for decreased production of AEA in areas of Aβ accumulation in the cerebral cortex of patients [92]. Furthermore, decreased AEA levels may be associated with enhanced microglial expression of FAAH, which has been detected in neuritic plaques [93].
Despite well-documented alterations in eCB production in Alzheimer’s disease patients, there is no clear evidence regarding changes to CB1 expression in the brain in this pathology. Some studies have reported decreased expression in specific brain areas of patients, whereas others have not found significant changes related to the pathology [94, 95]. Nevertheless, enhanced CB2 expression corresponding with Aβ plaque formation and microglial activation, has been well established in animal models and humans [91, 96]. Supporting the relevance for CB2 in Alzheimer’s pathology, in vitro studies have shown that administration of AEA, AEA analogs, FAAH inhibitors (which maintain AEA concentrations in the culture medium) and CB2 agonists, prevents Aβ accumulation, production of inflammatory lipid mediators and cell death in human neuronal cell lines, while these effects can be reversed using CB1 and CB2 receptor antagonists [97]. Similarly, CB2 agonists enhance phagocytosis of amyloid peptides which is compromised by a CD40-dependent mechanism in animal models and they also decrease amyloid peptide-dependent microglial activation and release of pro-inflammatory cytokines [98].
Direct administration of eCBs, including AEA, 2-AG and noladin ether, has demonstrated significant protective effects in in vitro studies and animal models of Alzheimer’s disease. These agonists are thought to inhibit Aβ–induced neuroinflammation by reducing the production of pro-inflammatory cytokines, chemokines and nitric oxide, as well as decreasing neuronal apoptosis and improving cognitive performance by stimulating receptors on neurons and glial cells [48, 99-101]. Similar effects have been reported using exogenous cannabinoids or synthetic CB receptor agonists [99]. Supplementation of neuronal cell lines engineered to accumulate Aβ with eCBs or eCB analogs has demonstrated beneficial effects, preventing intracellular accumulation of toxic protein and inhibiting eicosanoid production, promoting cell survival [97]. Additionally, AEA has been proposed as an enhancer of neurogenesis in conditions of Aβ toxicity in vitro [102], and hippocampal neurogenesis can be improved by PPARγ activation by cannabidiol in an animal model of Alzheimer’s disease [103].
CB2-specific agonists have demonstrated beneficial effects in animal models of this pathology, not only by decreasing microglial activation, but also by ameliorating Aβ deposition in the cerebral cortex and decreasing cognitive dysfunction [104]. CB2 activation has also shown positive effects in enhancing eCB-dependent Aβ clearance across the blood-brain barrier both in vitro and in vivo [105], as well as by decreasing Tau hyper-phorphorylation and cognitive deficits in animal models of Alzhemier’s disease [106]. Furthermore, stimulation of CB1 receptors with selective agonists decreases nitric oxide-dependent oxidative stress and also reduces neuronal Tau hyper-phosphorylation and toxicity both in vitro and in vivo [107, 108]. In addition, there is evidence for decreased astrocyte activation and production of interferon-gamma after CB1 stimulation with selective agonists in animal models [108].
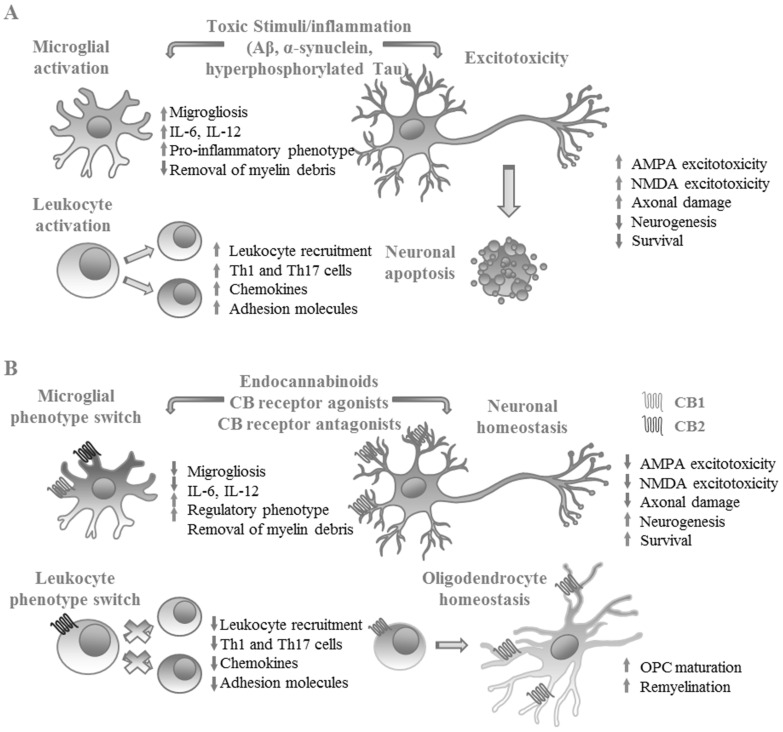
Studies in humans have focused mostly on the use of THC analogs for amelioration of the clinical characteristics of Alzheimer’s patients. These studies have been limited by low sample sizes and the most significant findings reported are related to improvement in behavioral abnormalities in patients, without significant effects on cognitive decline parameters [109, 110]. Nevertheless, the accumulated data demonstrates that eCBs are relevant for protection from amyloid neurotoxicity and may be useful therapeutic alternatives for the prevention of Alzheimer’s disease (Fig. 2).
Fig. (2).
Beneficial effects of eCB receptor activation in experimental models of neurological disease. A. In neurological pathologies such as Alzheimer’s and Parkinson’s disease, pathologic accumulation of misfolded or aggregated proteins (Amyloid β, α-synuclein, hyperphosphorylated Tau) promotes neuronal damage and development of inflammatory responses. In multiple sclerosis, aberrant recognition of self-antigens promotes demyelination and neuronal damage, causing CNS inflammation. Inflammatory responses in the CNS lead to activation of glial cells that produce proinflammatory mediators, promoting leukocyte recruitment and in some cases inducing differentiation of Th1 and Th17 cells that cause neuronal cell death. B. Activation of CB1 and CB2 receptors in leukocytes, glia and neurons prevent neuronal damage by decreasing classical microglial activation, improving the removal of myelin, decreasing the differentiation of Th1 and Th17 cells and promoting the differentiation of oligodendrocyte precursor cells (OPCs) to mature oligodendrocytes, improving remyelination and decreasing excitotoxic neuronal death. (Figure created by the authors). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.6. eCB Receptors in Parkinson’s Disease
Parkinson’s disease is characterized by the loss of dopaminergic neurons in neuronal circuits responsible for the regulation of motor functions, leading to movement dysfunction like dyskinesia, bradykinesia, rigidity and constant muscle tremors. Although previous studies have not been able to show clear beneficial effects of cannabinoids in the treatment of Parkinson’s disease [111], there is evidence that their therapeutic targeting may be worthwhile for the treatment of this disease, since data gathered from animal models and human patients demonstrate altered CB1 expression and eCB concentrations in the CSF and specific areas of the brain in this pathology, although their involvement has been suggested to be mostly in the regulation of the neuroinflammatory response [112].
Regarding eCBs, AEA production is elevated more than two-fold in untreated Parkinson’s disease patients compared to healthy controls, and its levels respond to dopamine replace therapy [113]. AEA is known as an endogenous regulator of motor activity in vivo and thus, seems to have an essential role in the pathogenesis of Parkinson’s disease [114, 115]. eCBs regulate motor circuits in part through indirect control of neuronal dopamine release via regulation of glutamate- and GABA-dependent neurotransmission. GABAergic neurons in basal ganglia circuits co-express CB1 eCB receptors and D1 and D2 dopaminergic receptors, thus being able to respond to both types of stimuli [116, 117]. Increased CB1 expression and signaling has been detected in the basal ganglia of Parkinson’s disease patients, as well as in animal models [118-120]. In contrast, there are reports on decreased CB1 expression in the substantia nigra in patients with Parkinson’s [121]. The relevance of CB1 in the regulation of motor circuits in the brain is also exemplified in animal models of CB1 deficiency, which display reductions in dopaminergic neuronal synapses in the nucleus accumbens [121]. In addition, CB1-deficient animals show decreased motor signs in experimental models of Parkinson’s disease [122].
Similar to other neurodegenerative conditions, CNS inflammation is a characteristic of this disease, with activated microglia and enhanced production of multiple pro-inflammatory cytokines [48]. In accordance with this, it has been demonstrated that CB2 expression is increased in the brain of Parkinson’s disease patients, particularly in activated microglia that produce pro-inflammatory cytokines in the substantia nigra, the area where dopaminergic neuron loss is more evident in this pathology [123]. Studies in animal models of Parkinson’s-like disease have shown that use of CB2 agonists reduces microglial activation and production of pro-inflammatory cytokines, contributing to the survival of tyrosine hydroxylase positive neurons in the striatum and the maintenance of neurological functions, while lack of CB2 receptors exacerbates neuroinflammation and neuronal dysfunction [124, 125]. Notably, neuroprotection in these models appears to be independent of CB1 signaling, as use of selective CB2 agonists protects dopaminergic neurons in the substantia nigra even in CB1-deficient mice and the effect is blocked by CB2 antagonists [124] (Fig. 2). In addition, transgenic overexpression of CB2 receptors promotes survival or dopaminergic neurons and ameliorates motor and behavioral alterations in animal models of Parkinson’s disease [126].
Studies in animal models have shown that administration of exogenous cannabinoids prevents dopaminergic neuron loss in vitro and in vivo [127]. However, studies in humans have reported contradictory findings, with significant amelioration of motor deficits reported in Parkinson’s disease patients using exogenous cannabinoids, improving bradykinesia, muscle rigidity and tremors [128, 129], and no significant effects able to be replicated in studies with stricter controls [111]. Use of the CB1/CB2 receptor agonist nabilone has demonstrated potential benefits reducing the severity of levodopa-induced dyskinesia in Parkinson’s patients [130], while use of the CB1 antagonist rimonabant did not show significant effects [131]. The contrast between the results obtained for the effects of cannabinoids in uncontrolled and controlled studies is striking. These contradictory results highlight the need for further research to determine the specific contributions of eCB receptors in this pathology.
4.7. eCB Receptors in Multiple Sclerosis
Multiple sclerosis is considered a primordially autoimmune neuroinflammatory pathology characterized by demyelination and neuronal death. Production of pro-inflammatory cytokines by glial cells is a major factor promoting BBB disruption, CNS leukocyte infiltration and activation of adaptive responses against oligodendrocytes and neurons [57]. Due to their anti-inflammatory properties, eCB receptors have been explored as regulators of the immune response in multiple sclerosis patients. There is evidence showing that cannabis and other CB receptor agonists improve the clinical signs and symptoms of these patients, although the precise mechanisms of action are not fully understood [73, 132]; however, previous studies have shown that both THC and cannabidiol are able to inhibit production of IL-6 and IL-17 by antigen-stimulated encephalitogenic T cells in a dose-dependent manner in animal models [133]. Enhanced eCB production in this pathology is thought to act as a modulatory process to limit neuroinflammation and support neuronal function and survival through activation of both CB1 and CB2 receptors, although their specific functions may vary, with CB1 being more related to neuronal homeostasis and regulation of neurotransmission, whereas CB2 would be involved in regulation of microglial and astrocyte activation and decreased inflammatory responses [132].
Clinical improvement in experimental models and human patients has been attributed mainly to activation of CB1 receptors [132], as well as alterations in both AEA and 2-AG production in different stages of disease that may be mediated by production of pro-inflammatory cytokines in the CNS [134]. In support for the role of CB1 in the regulation of pathology, studies in CB1 knockout models have demonstrated that neurological pathology is exacerbated in the absence of CB1, corresponding with increased excitotoxic neuronal damage [132]. Production of AEA has been related to neuronal protection from excitotoxic damage by modulation of neuronal calcium influx via signaling through CB1 receptors in vivo [134] and to prevention of AMPA and NMDA-dependent neuronal excitotoxicity in vitro [135]. Similarly, 2-AG has been shown to have neuroprotective effects through enhanced expression of CB1 receptors in neurons and glial cells, reducing the severity of disease in the animal model experimental autoimmune encephalomyelitis [136]. In addition, there is evidence for direct CB1-dependent anti-inflammatory effects in the CNS, decreasing microglial activation and production of pro-inflammatory cytokines in animal models [132].
The immune response in this pathology is considered to be dependent on the activation and maintenance of an autoimmune Th1 and Th17 responses, and these responses are often attained by microglial- and astrocyte-dependent production of pro-inflammatory cytokines like IL-12 and IFN-γ [137]. Enhanced CB2 expression has been detected in microglia at lesion sites in multiple sclerosis patients [73]. Studies in animal models of virally-induced MS-like disease have shown significant reductions in disease severity and neuroinflammation, along with enhanced remyelination, after activation of CB2 receptors using selective agonists, correlated to decreased IL-12 production by microglia, macrophages and T cells after antigenic stimulation, therefore decreasing Th1 polarization [73]. Similarly, cannabidiol supplementation decreases disease severity by preventing axonal damage, leukocyte recruitment and inflammation within the CNS in the same model, although these effects appear to be independent of the classical CB receptors [138]. A recent study has demonstrated that supplementation with 2-AG in this models promotes significant beneficial effects on remyelination and functional recovery by enhancing microglia-dependent removal of myelin debris and improving differentiation of oligodendrocyte precursor cells, demonstrating the importance of eCB signaling for recovery of CNS homeostasis after virus-induced demyelination [139] (Fig. 2).
Furthermore, CB2-deficient animals are more susceptible to induction of experimental autoimmune encephalomyelitis than wild-type animals and the disease shows increased severity and mortality [140]. In this model, disease severity correlates specifically with CB2 expression on encephalitogenic T cells, rather than microglia or CNS cells, although CB1 deficiency on neurons promotes a similar effect to that of CB2 deficiency on T cells, hence demonstrating the relevance of concerted CB1 and CB2 signaling in the regulation of neuroinflammation [140]. Furthermore, use of highly selective CB2 agonists in this model has shown that CB2-dependent leukocyte activation is useful in decreasing disease severity, reducing neuronal damage and demyelination by preventing Th1 and Th17 cell differentiation in secondary lymphoid organs [141]. CB2 agonists also decrease CNS accumulation of encephalitogenic T cells by inhibiting expression of chemokine receptors and adhesion molecules by leukocytes, thus demonstrating the relevance of CB2 signaling for disease pathogenesis in this model [141] (Fig. 2).
In spite of the extensive evidence for potential therapeutic benefits of CB receptor stimulation in experimental models of multiple sclerosis, studies regarding the therapeutic potential of the eCB system in multiple sclerosis have focused on the use of exogenous cannabinoids. Significant amelioration of spasticity and pain has been reported in patients after a 15-week regime of THC or Cannabis extracts administration, although no effects were observed in the levels of inflammatory cytokines or T cell activity or in the modulation of disease progression [142].
4.8. eCB Receptors in Psychiatric Disorders
Although the precise mechanisms involved in development and evolution of psychiatric disorders are still a matter of controversy, there is extensive evidence linking eCB system dysfunction with the development and clinical characteristics of psychiatric disease. eCB receptors can be found in multiple brain areas related to the control of emotion and behavior, including the prefrontal cortex, hippocampus and amygdala, particularly on GABAergic CCK-positive neurons, although they are also present in glutamate-, dopamine-, serotonine- and acetylcholine-producing neurons and are thus essential for the global regulation of neurotransmission [6-8]. Regarding behavioral and emotional responses, the eCB system has been well established as an important regulator of these processes. CB1-deficient mice show heightened anxiety responses compared to wild-type animals, although this effect may be reversed by specific CB1 deletion in GABAergic neurons in the forebrain, demonstrating opposing roles for CB1-dependent signaling at specific brain areas for the control of anxiety-like behavior [143]. This is also relevant in the context of dose-dependent effects for exogenous cannabinoids in the control of anxiety, since research in animal models and humans has shown that low doses of cannabinoids promote anxiolytic effects by activating CB1 receptors on cortical glutamatergic cells, whereas high doses activate CB1 receptors on GABAergic neurons in the forebrain and are anxiogenic [144]. Decreased production of 2-AG has also been involved in development of anxiety-like behavior that can be prevented by inhibition of MAGL [145].
eCB signaling is also important in the pathogenesis of depression. Decreased production of AEA and 2-AG have been detected in the circulation and cerebrospinal fluid of patients with depression [146]. Similarly, absence of CB1 expression in knockout models or pharmacological inhibition of CB1-dependent signaling increase the incidence of depressive-like behavior, whereas inhibition of eCB degradation leading to increased AEA levels in the CNS has shown anti-depressant effects [74]. eCB-dependent anti-depressant effects are thought to rely at least in part in modulation of serotonin production and signaling and may be affected in turn by serotonin reuptake inhibitors [147]. Similarly, although there is no solid evidence for alterations in eCB receptor expression in schizophrenic patients, there are reports relating increased eCB concentrations in the CSF and clinical symptoms of disease [49]. Data is contradictory on the effects of THC and cannabidiol in schizophrenic patients, with the former being related to worse symptomatology and the latter to amelioration of symptoms [148, 149].
Concerning stress responses, it has been demonstrated that acute stress inhibits CNS production of both AEA and 2-AG, particularly in the prefrontal cortex and amygdala, which in turn promote activation of the HPA axis and production of glucocorticoids [150]. This response, in turn, enhances eCB production within the CNS, achieving mutual regulation of the stress response and maintenance of CNS homeostasis [143, 151]. In a similar way, chronic stress alters hippocampal CB1 expression in animal models, decreasing AEA production and CB1-dependent signaling in the prefrontal cortex, amygdala, hippocampus and hypothalamus [152]. In general, stressful conditions are thought to promote eCB synthesis, which in turn decreases the negative effects of adrenocortical hormones on the CNS, ameliorating the cognitive and emotional effects of stress on the organism [143]. Importantly, it has been determined that stress-induced cognitive impairments are significantly related to CB1 expression on β-hydroxylase-expressing adrenergic neurons and that inhibition of CB1-dependent signals either in the peripheral or central nervous systems prevents stress-induced cognitive dysfunction in vivo [153].
Regarding CB2, there is evidence that CB2 gene polymorphisms and decreased expression are related to psychiatric disorders like schizophrenia, depression, anxiety, autism spectrum disorders, chronic stress and alcohol dependence [154]. Use of CB2 agonists decreases anxiety-like behavior in animal models of chronic stress, whereas CB2 antagonists exacerbate the symptomatology [154]. CB2-dependent effects on stress-related behavior may be due to its previously mentioned properties for the regulation of the HPA axis and the production of glucocorticoids [54]. Similarly, enhanced CB2-dependent signaling induced by transgenic overexpression of this receptor or by the use of CB2 agonists reduces anxiety and depressive-like behavior in animal models, demonstrating that this receptor participates in the regulation of behavior and emotional responses in vivo [74]. In addition, there are multiple studies showing a strong relationship between chronic, low-grade inflammation and the neurological alterations observed in psychiatric pathologies. Patients with major depression show increased production of IL-1β, IL-6, TNF-α and C-reactive protein in circulation [155, 156]. It has also been demonstrated that exogenous administration of pro-inflammatory cytokines like IFN-α or -γ promotes the appearance of depressive-like behavior in humans [157]. Similarly, chronic inflammatory conditions like autoimmune disease positively correlate to development of depression that can be ameliorated by use of anti-inflammatory therapies [158]. In animal models, depression also correlates with increased microglial activation [159]. Although the extent of their involvement in the pathogenesis of this disease has not been properly determined, it is possible that CB2 receptors are involved in the control of microgliosis in neuropsychiatric conditions, as CB2 expression is upregulated by microglia under neuroinflammatory conditions [71]. In support of this, recent studies have demonstrated that antidepressants promote microglial phenotype switching to M2-like cells, which are able to secrete anti-inflammatory cytokines, an effect that is also observed by activation of CB2 receptors on these cells [74, 160].
Clinical use of CB receptor agonists (THC, nabilone) and antagonists (rimonabant, taranabant) for therapeutic intervention in psychiatric disorders has been severy hampered by their extensive distribution within the CNS. Although significant beneficial effects have been observed with the use of these compounds for treatment of anxiety and depression in experimental models, their use in humans has presented unwanted side effects, inducing psychosis, anxiety attacks, severe depression and development of suicidal thoughts [161]. Therefore, their therapeutic application requires caution.
4.9. eCB Receptors in Pain Perception
Cannabinoids have long been related to control of pain perception in experimental models and clinical settings. The potential for the use of cannabinoids as analgesics is exemplified by recent evidence showing that genetic alterations promoting decreased expression of FAAH increase AEA concentrations in circulation and inhibit pain sensitivity in humans [162] and animal models [163, 164], demonstrating that eCBs are important regulators of pain perception. Nonetheless, clinical use of FAAH inhibitors has failed to produce similar effects, showing no significant effects in reducing pain in arthritic patients and causing severe and permanent neurological damage in some individuals [165, 166]. In animal models, administration of cannabinoids and endocannabinoids can have anti-nociceptive effects [167, 168]. Similarly, a synthetic agonist that activates both CB1 and CB2 receptors in peripheral neurons is able to decrease the hyperalgesic response in humans with neuropathic pain [170].
The expression and functions of eCB receptors in neuropathic pain has been studied in various experimental models. Expression of CB1 receptors in the peripheral nervous system, in nociceptive neurons in particular, has been related to analgesic effects after administration of painful stimuli [170]. Use of eCB agonists inhibits the activation of nociceptive neurons in the dorsal horn of the spinal cord and also in the thalamus, in a CB1-dependent manner. Furthermore, intradermal administration of AEA prevents the release of the pro-inflammatory factor CGRP from nerve terminals, decreasing the nociceptive response elicited by injection of painful stimuli [171]. In contrast, use of CB1-specific antagonists abrogates the anti-nociceptive responses [171]. In addition to the effects of CB1 on the induction of analgesia, enhanced expression of CB2 receptors in the CNS has also been described in animal models of neuropathic pain, where its presence has been involved in the regulation of allodynia and neuroinflammation [172].
Development of hyperalgesia and allodynia in experimental models of neuropathic pain is related to microglial activation and production of pro-inflammatory cytokines [173]. As mentioned previously, CB2 activation reduces microglial activation and release of pro-inflammatory mediators [71], which makes these receptors an intriguing therapeutic target for the treatment of neuropathic pain. Studies of selective CB2 deficiency in myeloid cells (microglia and macrophages) have shown that CB2 deletion on these cells promotes development of mirror-image pain and enhanced contralateral inflammatory responses, similar to those observed in constitutive CB2 knockout animals, demonstrating the role for CB2 receptors in the regulation of microglial activation and neuroinflammation after sciatic nerve injury in vivo [172, 174]. In contrast, transgenic overexpression of CB2 receptors in neurons and glial cells decreases the severity of neuropathic pain, as well as microglia and astrocyte activation, after sciatic nerve injury [172], supporting the role of CB2-dependent signaling in the control of the severity of the inflammatory response and clinical signs of disease in this model.
Regarding the potential for CB2 receptors in the control of neuropathic pain, research in animal models has demonstrated that AEA inhibits the activity of neurons in the spinal cord via CB2 receptor activation [175] Furthermore, AEA administration on carrageenan hypersensitivity can be blocked by CB2 selective antagonists [176]. Administration of CB2 selective agonists is useful in reducing nociceptive responses in several animal models of inflammatory pain [177-179], supporting the significant role of CB2 receptors in the regulation of pain sensitivity. The possibility of using CB2 receptor agonists for treatment of human pain are still under study.
4.10. Non-classical eCB Receptors
Besides CB receptors, eCBs are capable of binding additional proteins at the cell surface. Among them, different G protein-coupled receptors, such as GPR55, have been proposed as additional targets for eCB functions. In a similar way to CB1, GPR55 is expressed in multiple areas within the CNS, including the frontal cortex, hippocampus, cerebellum and hypothalamus and has thus been involved in the regulation of multiple neuronal and cognitive functions. Although data on this issue is still controversial, various studies have demonstrated that GPR55 binds both AEA and 2-AG, as well as THC, at a similar rate than CB1 or CB2, promoting activation of RhoA, PLC and NF-AT in various models in vitro [180-182]. However, other studies have failed to replicate these results and have instead suggested other endogenous ligands for this receptor [183, 184]. Nonetheless, decreased CNS expression of GPR55 has been linked to development of anxiety-like behavior that can be alleviated by use of the GPR55 agonist O-1602, a cannabidiol analog, in animal models, which may be dependent on inhibition of glutamate signaling at specific areas of the brain [185].
Additional evidence shows that eCBs and cannabinoids can modulate ligand binding in a variety of ionotropic and metabotropic neurotransmitter receptors, including nicotinic and muscarinic acetylcholine receptors, NMDA receptors, glycine receptors, µ- and δ–opiod receptors, adenosine A1 and A3 receptors, β-adrenergic receptors and 5-HT receptors, although their binding to these receptors usually has low affinity and their effects probably depend more on allosteric changes than on direct activation/inactivation of the corresponding receptors [186-189]. Nonetheless, these interactions should be taken into account when inducing alterations in eCB-dependent functions in vivo. Indirect regulation of neurotransmitter release by modulation of neurotransmitter receptor functions via eCBs has great potential for therapeutic intervention, since preventing the release of specific neurotransmitters might be useful for the reduction of excitotoxic and oxidative neuronal damage in pathologies like Alzheimer’s and Parkinson’s diseases, traumatic brain injury and stroke, as well as potentially ameliorating clinical symptoms of disease in psychiatric conditions like depression and anxiety [190].
In addition, ionotropic TRPV proteins, particularly TRPV1, also act as functional eCB receptors. These molecules are highly expressed in sensory neurons involved in nociception and are activated by various noxious stimuli, including temperature and pH changes [191]. They also show a similar expression pattern to CB1 receptors in the brain and are found in the cerebral cortex, hippocampus, cerebellum and hypothalamus [192]. These receptors are often co-localized with CB1 receptors in the neurons both in the peripheral and central nervous systems and can bind selectively to AEA, but not 2-AG, as well as binding to exogenous cannabinoids like cannabidiol [193, 194]. These receptors have been implicated in the regulation of behavioral and emotional responses in models of depression, anxiety and stress, as well as in the regulation of cognitive processes for learning and memory, and may also be involved in the regulation of glutamate-induced excitotoxic damage to neurons and glial cells, although their dependence on eCB activation has not been firmly established [195]. eCB-induced reduction of pain sensitivity is thought to depend at least in part on activation and subsequent desensitization of TRPV1 receptors at nociceptive neurons [196]. Moreover, there is evidence for eCB-mediated regulation of other relevant ionic channels in neurons and other cell types, including calcium, potassium and sodium channels, further demonstrating the relevance of eCB functions in the regulation of neuronal depolarization and neurotransmission [197-199].
Finally, previous studies have demonstrated that AEA and 2-AG can bind to and activate PPARs, including PPARα, PPARβ/δ and PPARγ, which are considered metabolic sensors involved in the regulation of systemic homeostasis [200]. These receptors can be activated by eCBs and byproducts of eCB metabolism and are suggested to promote neuroprotective effects against exitotoxicity in vitro [201, 202], and may have relevance in the inhibition of inflammatory pain in vivo [203, 204]. Activation of PPARs appears to regulate eCB function by altering the expression of relevant enzymes, like FAAH, or CB receptors in different cell types [77]. Supporting this notion, previous studies have shown that activation of PPARγ has a beneficial effect in experimental models of Alzheimer’s disease and decreases expression of genes involved in the inflammatory response [205]. Suppression of the neuroinflammatory response has been linked to 2-AG signaling via CB1- and CB2-independent mechanisms, raising the possibility that PPARs may be involved in neuroprotection under these circumstances [206].
CONCLUDING REMARKS
As evidenced in this review, eCBs are highly relevant potential targets for therapeutic intervention in a multitude of neurological conditions due to their role in the modulation of excitotoxicity, neuroinflammation and neuronal and glial homeostasis, either by using natural compounds or synthetic agonists/antagonists for eCB receptors, which have been assayed for the treatment of neuroinflammatory and neurodegenerative conditions, along with psychiatric conditions [207]. Nevertheless, as the eCB system participates in the modulation of a great variety of physiological functions, either directly through CB receptors or indirectly through a multitude of additional mechanisms, its manipulation must be performed with caution to avoid unwanted effects. Case in point, use of CB1 receptor antagonists for the treatment of obesity and cardiovascular disease, on the basis of the effects of eCBs on the regulation of energy metabolism and inflammation, showed promising results, until they had to be withdrawn from use due to unexpected side effects including development of mood alterations, depression and suicidal behavior in patients [35]; therefore, therapeutic manipulation of the eCB system must be carried out with caution, to minimize risks and maximize its potential benefits for human health.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Di Marzo V., Fontana A., Cadas H., Schinelli S., Cimino G., Schwartz J.C., Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372(6507):686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 2.Stella N., Schweitzer P., Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388(6644):773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 3.Deutsch D.G., Chin S.A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem. Pharmacol. 1993;46(5):791–796. doi: 10.1016/0006-2952(93)90486-G. [DOI] [PubMed] [Google Scholar]
- 4.Blankman J.L., Simon G.M., Cravatt B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007;14(12):1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn K., McKinney M.K., Cravatt B.F. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem. Rev. 2008;108(5):1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen E.M., Haycock D.A., Ward S.J., Seybold V.S. Distribution of cannabinoid receptors in rat brain determined with aminoalkylindoles. Brain Res. 1992;575(1):93–102. doi: 10.1016/0006-8993(92)90428-C. [DOI] [PubMed] [Google Scholar]
- 8.Glass M., Dragunow M., Faull R.L. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77(2):299–318. doi: 10.1016/S0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 9.Mato S., Del Olmo E., Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci. 2003;17(9):1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- 10.Kendall D.A., Yudowski G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017;10:294. doi: 10.3389/fncel.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chanda D., Neumann D., Glatz J.F.C. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target. Prostaglandins Leukot. Essent. Fatty Acids. 2019;140:51–56. doi: 10.1016/j.plefa.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Zou S., Kumar U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018;19(3):e833. doi: 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreitzer A.C., Regehr W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29(3):717–727. doi: 10.1016/S0896-6273(01)00246-X. [DOI] [PubMed] [Google Scholar]
- 14.Ohno-Shosaku T., Maejima T., Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29(3):729–738. doi: 10.1016/S0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 15.Wilson R.I., Nicoll R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410(6828):588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 16.Castillo P.E., Younts T.J., Chávez A.E., Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76(1):70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuboi K., Uyama T., Okamoto Y., Ueda N. Endocannabinoids and related N-acylethanolamines: biological activities and metabolism. Inflamm. Regen. 2018;38:28. doi: 10.1186/s41232-018-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diana M.A., Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br. J. Pharmacol. 2004;142(1):9–19. doi: 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busquets-Garcia A., Bains J., Marsicano G. CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology. 2018;43(1):4–20. doi: 10.1038/npp.2017.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fišar Z., Singh N., Hroudová J. Cannabinoid-induced changes in respiration of brain mitochondria. Toxicol. Lett. 2014;231(1):62–71. doi: 10.1016/j.toxlet.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.H., Ledri M., Tóth B., Marchionni I., Henstridge C.M., Dudok B., Kenesei K., Barna L., Szabó S.I., Renkecz T., Oberoi M., Watanabe M., Limoli C.L., Horvai G., Soltesz I., Katona I. Multiple forms of endocannabinoid and endovanilloid signaling regulate the tonic control of GABA release. J. Neurosci. 2015;35(27):10039–10057. doi: 10.1523/JNEUROSCI.4112-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaczocha M., Glaser S.T., Deutsch D.G., Lennarz W.J. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. USA. 2009;106(15):6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Compagnucci C., Di Siena S., Bustamante M.B., Di Giacomo D., Di Tommaso M., Maccarrone M., Grimaldi P., Sette C. Type-1 (CB1) cannabinoid receptor promotes neuronal differentiation and maturation of neural stem cells. PLoS One. 2013;8(1):e54271. doi: 10.1371/journal.pone.0054271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araque A., Castillo P.E., Manzoni O.J., Tonini R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology. 2017;124:13–24. doi: 10.1016/j.neuropharm.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stempel A.V., Stumpf A., Zhang H.Y., Özdoğan T., Pannasch U., Theis A.K., Otte D.M., Wojtalla A., Rácz I., Ponomarenko A., Xi Z.X., Zimmer A., Schmitz D. Cannabinoid Type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron. 2016;90(4):795–809. doi: 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atwood B.K., Straiker A., Mackie K. CB2 cannabinoid receptors inhibit synaptic transmission when expressed in cultured autaptic neurons. Neuropharmacology. 2012;63(4):514–523. doi: 10.1016/j.neuropharm.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro G., Morales P., Rodríguez-Cueto C., Fernández-Ruiz J., Jagerovic N., Franco R. Targeting cannabinoid CB2 receptors in the central nervous system. medicinal chemistry approaches with focus on neurodegenerative disorders. Front. Neurosci. 2016;10:406. doi: 10.3389/fnins.2016.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong J.P., Onaivi E.S., Ishiguro H., Liu Q.R., Tagliaferro P.A., Brusco A., Uhl G.R. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071(1):10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 30.Chávez A.E., Chiu C.Q., Castillo P.E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 2010;13(12):1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Younts T.J., Castillo P.E. Endogenous cannabinoid signaling at inhibitory interneurons. Curr. Opin. Neurobiol. 2014;26:42–50. doi: 10.1016/j.conb.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manduca A., Lassalle O., Sepers M., Campolongo P., Cuomo V., Marsicano G., Kieffer B., Vanderschuren L.J., Trezza V., Manzoni O.J. interacting cannabinoid and opioid receptors in the nucleus accumbens core control adolescent social play. Front. Behav. Neurosci. 2016;10:211. doi: 10.3389/fnbeh.2016.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter L., Franklin A., Witting A., Moller T., Stella N. Astrocytes in culture produce anandamide and other acylethanolamides. J. Biol. Chem. 2002;277(23):20869–20876. doi: 10.1074/jbc.M110813200. [DOI] [PubMed] [Google Scholar]
- 34.Walter L., Stella N. Endothelin-1 increases 2-arachidonoyl glycerol (2-AG) production in astrocytes. Glia. 2003;44(1):85–90. doi: 10.1002/glia.10270. [DOI] [PubMed] [Google Scholar]
- 35.Gabrielli M., Battista N., Riganti L., Prada I., Antonucci F., Cantone L., Matteoli M., Maccarrone M., Verderio C. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 2015;16(2):213–220. doi: 10.15252/embr.201439668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witting A., Walter L., Wacker J., Möller T., Stella N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc. Natl. Acad. Sci. USA. 2004;101(9):3214–3219. doi: 10.1073/pnas.0306707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernal-Chico A., Canedo M., Manterola A., Victoria Sánchez-Gómez M., Pérez-Samartín A., Rodríguez-Puertas R., Matute C., Mato S. Blockade of monoacylglycerol lipase inhibits oligodendrocyte excitotoxicity and prevents demyelination in vivo. Glia. 2015;63(1):163–176. doi: 10.1002/glia.22742. [DOI] [PubMed] [Google Scholar]
- 38.Navarrete M., Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57(6):883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Navarrete M., Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68(1):113–126. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 40.Andrade-Talavera Y., Duque-Feria P., Paulsen O., Rodríguez-Moreno A. Presynaptic spike timing-dependent long-term depression in the mouse hippocampus. Cereb. Cortex. 2016;26(8):3637–3654. doi: 10.1093/cercor/bhw172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han J., Kesner P., Metna-Laurent M., Duan T., Xu L., Georges F., Koehl M., Abrous D.N., Mendizabal-Zubiaga J., Grandes P., Liu Q., Bai G., Wang W., Xiong L., Ren W., Marsicano G., Zhang X. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148(5):1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 42.Gorzkiewicz A., Szemraj J. Brain endocannabinoid signaling exhibits remarkable complexity. Brain Res. Bull. 2018;142:33–46. doi: 10.1016/j.brainresbull.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Panikashvili D., Shein N.A., Mechoulam R., Trembovler V., Kohen R., Alexandrovich A., Shohami E. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol. Dis. 2006;22(2):257–264. doi: 10.1016/j.nbd.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Wettschureck N., van der Stelt M., Tsubokawa H., Krestel H., Moers A., Petrosino S., Schütz G., Di Marzo V., Offermanns S. Forebrain-specific inactivation of Gq/G11 family G proteins results in age-dependent epilepsy and impaired endocannabinoid formation. Mol. Cell. Biol. 2006;26(15):5888–5894. doi: 10.1128/MCB.00397-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Laere K., Casteels C., Dhollander I., Goffin K., Grachev I., Bormans G., Vandenberghe W. Widespread decrease of type 1 cannabinoid receptor availability in Huntington disease in vivo. J. Nucl. Med. 2010;51(9):1413–1417. doi: 10.2967/jnumed.110.077156. [DOI] [PubMed] [Google Scholar]
- 46.Westlake T.M., Howlett A.C., Bonner T.I., Matsuda L.A., Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience. 1994;63(3):637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- 47.Ramirez S.H., Haskó J., Skuba A., Fan S., Dykstra H., McCormick R., Reichenbach N., Krizbai I., Mahadevan A., Zhang M., Tuma R., Son Y.J., Persidsky Y. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J. Neurosci. 2012;32(12):4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassano T., Calcagnini S., Pace L., De Marco F., Romano A., Gaetani S. Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front. Neurosci. 2017;11:30. doi: 10.3389/fnins.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giuffrida A., Leweke F.M., Gerth C.W., Schreiber D., Koethe D., Faulhaber J., Klosterkötter J., Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29(11):2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- 50.Haller J., Bakos N., Szirmay M., Ledent C., Freund T.F. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur. J. Neurosci. 2002;16(7):1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- 51.Beyer C.E., Dwyer J.M., Piesla M.J., Platt B.J., Shen R., Rahman Z., Chan K., Manners M.T., Samad T.A., Kennedy J.D., Bingham B., Whiteside G.T. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol. Dis. 2010;39(2):148–155. doi: 10.1016/j.nbd.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Cota D., Steiner M.A., Marsicano G., Cervino C., Herman J.P., Grübler Y., Stalla J., Pasquali R., Lutz B., Stalla G.K., Pagotto U. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology. 2007;148(4):1574–1581. doi: 10.1210/en.2005-1649. [DOI] [PubMed] [Google Scholar]
- 53.Navarrete M., Díez A., Araque A. Astrocytes in endocannabinoid signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369(1654):20130599. doi: 10.1098/rstb.2013.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill M.N., McLaughlin R.J., Bingham B., Shrestha L., Lee T.T., Gray J.M., Hillard C.J., Gorzalka B.B., Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Natl. Acad. Sci. USA. 2010;107(20):9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viveros M.P., Marco E.M., Llorente R., López-Gallardo M. Endocannabinoid system and synaptic plasticity: implications for emotional responses. Neural Plast. 2007;2007:52908. doi: 10.1155/2007/52908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barna I., Zelena D., Arszovszki A.C., Ledent C. The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in vivo and in vitro studies in CB1 receptor knockout mice. Life Sci. 2004;75(24):2959–2970. doi: 10.1016/j.lfs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Abbott N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 2000;20(2):131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huber J.D., Witt K.A., Hom S., Egleton R.D., Mark K.S., Davis T.P. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am. J. Physiol. Heart Circ. Physiol. 2001;280(3):H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- 59.Hickey W.F. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991;1(2):97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 60.Ek M., Engblom D., Saha S., Blomqvist A., Jakobsson P.J., Ericsson-Dahlstrand A. Inflammatory response: pathway across the blood-brain barrier. Nature. 2001;410(6827):430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- 61.Mestre L., Iñigo P.M., Mecha M., Correa F.G., Hernangómez-Herrero M., Loría F., Docagne F., Borrell J., Guaza C. Anandamide inhibits Theiler’s virus induced VCAM-1 in brain endothelial cells and reduces leukocyte transmigration in a model of blood brain barrier by activation of CB(1) receptors. J. Neuroinflammation. 2011;8:102. doi: 10.1186/1742-2094-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Droogan A.G., McMillan S.A., Douglas J.P., Hawkins S.A. Serum and cerebrospinal fluid levels of soluble adhesion molecules in multiple sclerosis: predominant intrathecal release of vascular cell adhesion molecule-1. J. Neuroimmunol. 1996;64(2):185–191. doi: 10.1016/0165-5728(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 63.Matsuda M., Tsukada N., Miyagi K., Yanagisawa N. Increased levels of soluble vascular cell adhesion molecule-1 (VCAM-1) in the cerebrospinal fluid and sera of patients with multiple sclerosis and human T lymphotropic virus type-1-associated myelopathy. J. Neuroimmunol. 1995;59(1-2):35–40. doi: 10.1016/0165-5728(95)00023-U. [DOI] [PubMed] [Google Scholar]
- 64.Rothoerl R.D., Schebesch K.M., Kubitza M., Woertgen C., Brawanski A., Pina A.L. ICAM-1 and VCAM-1 expression following aneurysmal subarachnoid hemorrhage and their possible role in the pathophysiology of subsequent ischemic deficits. Cerebrovasc. Dis. 2006;22(2-3):143–149. doi: 10.1159/000093243. [DOI] [PubMed] [Google Scholar]
- 65.Rossi B., Zenaro E., Angiari S., Ottoboni L., Bach S., Piccio L., Pietronigro E.C., Scarpini E., Fusco M., Leon A., Constantin G. Inverse agonism of cannabinoid CB1 receptor blocks the adhesion of encephalitogenic T cells in inflamed brain venules by a protein kinase A-dependent mechanism. J. Neuroimmunol. 2011;233(1-2):97–105. doi: 10.1016/j.jneuroim.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Rom S., Zuluaga-Ramirez V., Dykstra H., Reichenbach N.L., Pacher P., Persidsky Y. Selective activation of cannabinoid receptor 2 in leukocytes suppresses their engagement of the brain endothelium and protects the blood-brain barrier. Am. J. Pathol. 2013;183(5):1548–1558. doi: 10.1016/j.ajpath.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maccarrone M., Fiori A., Bari M., Granata F., Gasperi V., De Stefano M.E., Finazzi-Agrò A., Strom R. Regulation by cannabinoid receptors of anandamide transport across the blood-brain barrier and through other endothelial cells. Thromb. Haemost. 2006;95(1):117–127. doi: 10.1160/TH05-06-0413. [DOI] [PubMed] [Google Scholar]
- 68.Hind W.H., Tufarelli C., Neophytou M., Anderson S.I., England T.J., O’Sullivan S.E. Endocannabinoids modulate human blood-brain barrier permeability in vitro. Br. J. Pharmacol. 2015;172(12):3015–3027. doi: 10.1111/bph.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Correa F., Docagne F., Mestre L., Loría F., Hernangómez M., Borrell J., Guaza C. Cannabinoid system and neuroinflammation: implications for multiple sclerosis. Neuroimmunomodulation. 2007;14(3-4):182–187. doi: 10.1159/000110644. [DOI] [PubMed] [Google Scholar]
- 70.Tang Y., Le W. Differential roles of m1 and m2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016;53(2):1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 71.Malek N., Popiolek-Barczyk K., Mika J., Przewlocka B., Starowicz K. Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast. 2015;2015:130639. doi: 10.1155/2015/130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ativie F., Komorowska J.A., Beins E., Albayram Ö., Zimmer T., Zimmer A., Tejera D., Heneka M., Bilkei-Gorzo A. Cannabinoid 1 receptor signaling on hippocampal gabaergic neurons influences microglial activity. Front. Mol. Neurosci. 2018;11:295. doi: 10.3389/fnmol.2018.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romero-Sandoval E.A., Horvath R., Landry R.P., DeLeo J.A. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol. Pain. 2009;5:25. doi: 10.1186/1744-8069-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boorman E., Zajkowska Z., Ahmed R., Pariante C.M., Zunszain P.A. Crosstalk between endocannabinoid and immune systems: a potential dysregulation in depression? Psychopharmacology (Berl.) 2016;233(9):1591–1604. doi: 10.1007/s00213-015-4105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hernangómez M., Mestre L., Correa F.G., Loría F., Mecha M., Iñigo P.M., Docagne F., Williams R.O., Borrell J., Guaza C. CD200-CD200R1 interaction contributes to neuroprotective effects of anandamide on experimentally induced inflammation. Glia. 2012;60(9):1437–1450. doi: 10.1002/glia.22366. [DOI] [PubMed] [Google Scholar]
- 76.Grabner G.F., Eichmann T.O., Wagner B., Gao Y., Farzi A., Taschler U., Radner F.P., Schweiger M., Lass A., Holzer P., Zinser E., Tschöp M.H., Yi C.X., Zimmermann R. Deletion of monoglyceride lipase in astrocytes attenuates lipopolysaccharide-induced neuroinflammation. J. Biol. Chem. 2016;291(2):913–923. doi: 10.1074/jbc.M115.683615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bisicchia E., Chiurchiù V., Viscomi M.T., Latini L., Fezza F., Battistini L., Maccarrone M., Molinari M. Activation of type-2 cannabinoid receptor inhibits neuroprotective and antiinflammatory actions of glucocorticoid receptor α: when one is better than two. Cell. Mol. Life Sci. 2013;70(12):2191–2204. doi: 10.1007/s00018-012-1253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berrendero F., Romero J., García-Gil L., Suarez I., De la Cruz P., Ramos J.A., Fernández-Ruiz J.J. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim. Biophys. Acta. 1998;1407(3):205–214. doi: 10.1016/S0925-4439(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 79.Long L.E., Lind J., Webster M., Weickert C.S. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci. 2012;13:87. doi: 10.1186/1471-2202-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wenger T., Gerendai I., Fezza F., González S., Bisogno T., Fernandez-Ruiz J., Di Marzo V. The hypothalamic levels of the endocannabinoid, anandamide, peak immediately before the onset of puberty in female rats. Life Sci. 2002;70(12):1407–1414. doi: 10.1016/S0024-3205(01)01516-8. [DOI] [PubMed] [Google Scholar]
- 81.Di Marzo V., Stella N., Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015;16(1):30–42. doi: 10.1038/nrn3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Albayram O., Alferink J., Pitsch J., Piyanova A., Neitzert K., Poppensieker K., Mauer D., Michel K., Legler A., Becker A., Monory K., Lutz B., Zimmer A., Bilkei-Gorzo A. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc. Natl. Acad. Sci. USA. 2011;108(27):11256–11261. doi: 10.1073/pnas.1016442108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bilkei-Gorzo A., Albayram O., Ativie F., Chasan S., Zimmer T., Bach K., Zimmer A. Cannabinoid 1 receptor signaling on GABAergic neurons influences astrocytes in the ageing brain. PLoS One. 2018;13(8):e0202566. doi: 10.1371/journal.pone.0202566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58(9):1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ribeiro R., Yu F., Wen J., Vana A., Zhang Y. Therapeutic potential of a novel cannabinoid agent CB52 in the mouse model of experimental autoimmune encephalomyelitis. Neuroscience. 2013;254:427–442. doi: 10.1016/j.neuroscience.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Palazuelos J., Aguado T., Pazos M.R., Julien B., Carrasco C., Resel E., Sagredo O., Benito C., Romero J., Azcoitia I., Fernández-Ruiz J., Guzmán M., Galve-Roperh I. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain. 2009;132(Pt 11):3152–3164. doi: 10.1093/brain/awp239. [DOI] [PubMed] [Google Scholar]
- 87.Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J. Neurochem. 2009;110(4):1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 88.Sastre M., Klockgether T., Heneka M.T. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int. J. Dev. Neurosci. 2006;24(2-3):167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 89.White J.A., Manelli A.M., Holmberg K.H., Van Eldik L.J., Ladu M.J. Differential effects of oligomeric and fibrillar amyloid-beta 1-42 on astrocyte-mediated inflammation. Neurobiol. Dis. 2005;18(3):459–465. doi: 10.1016/j.nbd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 90.Marsicano G., Goodenough S., Monory K., Hermann H., Eder M., Cannich A., Azad S.C., Cascio M.G., Gutiérrez S.O., van der Stelt M., López-Rodriguez M.L., Casanova E., Schütz G., Zieglgänsberger W., Di Marzo V., Behl C., Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302(5642):84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 91.Mulder J., Zilberter M., Pasquaré S.J., Alpár A., Schulte G., Ferreira S.G., Köfalvi A., Martín-Moreno A.M., Keimpema E., Tanila H., Watanabe M., Mackie K., Hortobágyi T., de Ceballos M.L., Harkany T. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain. 2011;134(Pt 4):1041–1060. doi: 10.1093/brain/awr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jung K.M., Astarita G., Yasar S., Vasilevko V., Cribbs D.H., Head E., Cotman C.W., Piomelli D. An amyloid β42-dependent deficit in anandamide mobilization is associated with cognitive dysfunction in Alzheimer’s disease. Neurobiol. Aging. 2012;33(8):1522–1532. doi: 10.1016/j.neurobiolaging.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benito C., Núñez E., Tolón R.M., Carrier E.J., Rábano A., Hillard C.J., Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J. Neurosci. 2003;23(35):11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramírez B.G., Blázquez C., Gómez del Pulgar T., Guzmán M., de Ceballos M.L. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005;25(8):1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahmad R., Goffin K., Van den Stock J., De Winter F.L., Cleeren E., Bormans G., Tournoy J., Persoons P., Van Laere K., Vandenbulcke M. In vivo type 1 cannabinoid receptor availability in Alzheimer’s disease. Eur. Neuropsychopharmacol. 2014;24(2):242–250. doi: 10.1016/j.euroneuro.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Solas M., Francis P.T., Franco R., Ramírez M.J. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol. Aging. 2013;34(3):805–808. doi: 10.1016/j.neurobiolaging.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 97.Currais A., Quehenberger O.M., Armando A., Daugherty D., Maher P., Schubert D. Amyloid proteotoxicity initiates an inflammatory response blocked by cannabinoids. NPJ Aging Mech. Dis. 2016;2:16012. doi: 10.1038/npjamd.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ehrhart J., Obregon D., Mori T., Hou H., Sun N., Bai Y., Klein T., Fernandez F., Tan J., Shytle R.D. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J. Neuroinflammation. 2005;2:29. doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janefjord E., Mååg J.L., Harvey B.S., Smid S.D. Cannabinoid effects on β amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell. Mol. Neurobiol. 2014;34(1):31–42. doi: 10.1007/s10571-013-9984-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harvey B.S., Ohlsson K.S., Mååg J.L., Musgrave I.F., Smid S.D. Contrasting protective effects of cannabinoids against oxidative stress and amyloid-β evoked neurotoxicity in vitro. Neurotoxicology. 2012;33(1):138–146. doi: 10.1016/j.neuro.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 101.van der Stelt M., Mazzola C., Esposito G., Matias I., Petrosino S., De Filippis D., Micale V., Steardo L., Drago F., Iuvone T., Di Marzo V. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell. Mol. Life Sci. 2006;63(12):1410–1424. doi: 10.1007/s00018-006-6037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tanveer R., Gowran A., Noonan J., Keating S.E., Bowie A.G., Campbell V.A. The endocannabinoid, anandamide, augments Notch-1 signaling in cultured cortical neurons exposed to amyloid-β and in the cortex of aged rats. J. Biol. Chem. 2012;287(41):34709–34721. doi: 10.1074/jbc.M112.350678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Esposito G., Scuderi C., Valenza M., Togna G.I., Latina V., De Filippis D., Cipriano M., Carratù M.R., Iuvone T., Steardo L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011;6(12):e28668. doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martín-Moreno A.M., Brera B., Spuch C., Carro E., García-García L., Delgado M., Pozo M.A., Innamorato N.G., Cuadrado A., de Ceballos M.L. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers β-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J. Neuroinflammation. 2012;9:8. doi: 10.1186/1742-2094-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bachmeier C., Beaulieu-Abdelahad D., Mullan M., Paris D. Role of the cannabinoid system in the transit of beta-amyloid across the blood-brain barrier. Mol. Cell. Neurosci. 2013;56:255–262. doi: 10.1016/j.mcn.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 106.Aso E., Juvés S., Maldonado R., Ferrer I. CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AβPP/PS1 mice. J. Alzheimers Dis. 2013;35(4):847–858. doi: 10.3233/JAD-130137. [DOI] [PubMed] [Google Scholar]
- 107.Esposito G., De Filippis D., Steardo L., Scuderi C., Savani C., Cuomo V., Iuvone T. CB1 receptor selective activation inhibits beta-amyloid-induced iNOS protein expression in C6 cells and subsequently blunts tau protein hyperphosphorylation in co-cultured neurons. Neurosci. Lett. 2006;404(3):342–346. doi: 10.1016/j.neulet.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 108.Aso E., Palomer E., Juvés S., Maldonado R., Muñoz F.J., Ferrer I. CB1 agonist ACEA protects neurons and reduces the cognitive impairment of AβPP/PS1 mice. J. Alzheimers Dis. 2012;30(2):439–459. doi: 10.3233/JAD-2012-111862. [DOI] [PubMed] [Google Scholar]
- 109.Walther S., Schüpbach B., Seifritz E., Homan P., Strik W. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia. J. Clin. Psychopharmacol. 2011;31(2):256–258. doi: 10.1097/JCP.0b013e31820e861c. [DOI] [PubMed] [Google Scholar]
- 110.Krishnan S., Cairns R., Howard R. Cannabinoids for the treatment of dementia. Cochrane Database Syst. Rev. 2009;2(2):CD007204. doi: 10.1002/14651858.CD007204.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carroll C.B., Bain P.G., Teare L., Liu X., Joint C., Wroath C., Parkin S.G., Fox P., Wright D., Hobart J., Zajicek J.P. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004;63(7):1245–1250. doi: 10.1212/01.WNL.0000140288.48796.8E. [DOI] [PubMed] [Google Scholar]
- 112.Xu J.Y., Chen C. Endocannabinoids in synaptic plasticity and neuroprotection. Neuroscientist. 2015;21(2):152–168. doi: 10.1177/1073858414524632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pisani V., Moschella V., Bari M., Fezza F., Galati S., Bernardi G., Stanzione P., Pisani A., Maccarrone M. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Mov. Disord. 2010;25(7):920–924. doi: 10.1002/mds.23014. [DOI] [PubMed] [Google Scholar]
- 114.de Lago E., de Miguel R., Lastres-Becker I., Ramos J.A., Fernández-Ruiz J. Involvement of vanilloid-like receptors in the effects of anandamide on motor behavior and nigrostriatal dopaminergic activity: in vivo and in vitro evidence. Brain Res. 2004;1007(1-2):152–159. doi: 10.1016/j.brainres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 115.Crawley J.N., Corwin R.L., Robinson J.K., Felder C.C., Devane W.A., Axelrod J. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol. Biochem. Behav. 1993;46(4):967–972. doi: 10.1016/0091-3057(93)90230-Q. [DOI] [PubMed] [Google Scholar]
- 116.Benarroch E. Endocannabinoids in basal ganglia circuits: implications for Parkinson disease. Neurology. 2007;69(3):306–309. doi: 10.1212/01.wnl.0000267407.79757.75. [DOI] [PubMed] [Google Scholar]
- 117.Adermark L., Talani G., Lovinger D.M. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur. J. Neurosci. 2009;29(1):32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lastres-Becker I., Cebeira M., de Ceballos M.L., Zeng B.Y., Jenner P., Ramos J.A., Fernández-Ruiz J.J. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur. J. Neurosci. 2001;14(11):1827–1832. doi: 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed] [Google Scholar]
- 119.van der Stelt M., Fox S.H., Hill M., Crossman A.R., Petrosino S., Di Marzo V., Brotchie J.M. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson’s disease. FASEB J. 2005;19(9):1140–1142. doi: 10.1096/fj.04-3010fje. [DOI] [PubMed] [Google Scholar]
- 120.Van Laere K., Casteels C., Lunskens S., Goffin K., Grachev I.D., Bormans G., Vandenberghe W. Regional changes in type 1 cannabinoid receptor availability in Parkinson’s disease in vivo. Neurobiol. Aging. 2012;33(3):620.e1–620.e8. doi: 10.1016/j.neurobiolaging.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 121.Lane D.A., Chan J., Lupica C.R., Pickel V.M. Cannabinoid-1 receptor gene deletion has a compartment-specific affect on the dendritic and axonal availability of μ-opioid receptors and on dopamine axons in the mouse nucleus accumbens. Synapse. 2010;64(12):886–897. doi: 10.1002/syn.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pérez-Rial S., García-Gutiérrez M.S., Molina J.A., Pérez-Nievas B.G., Ledent C., Leiva C., Leza J.C., Manzanares J. Increased vulnerability to 6-hydroxydopamine lesion and reduced development of dyskinesias in mice lacking CB1 cannabinoid receptors. Neurobiol. Aging. 2011;32(4):631–645. doi: 10.1016/j.neurobiolaging.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 123.Gómez-Gálvez Y., Palomo-Garo C., Fernández-Ruiz J., García C. Potential of the cannabinoid CB(2) receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:200–208. doi: 10.1016/j.pnpbp.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 124.Price D.A., Martinez A.A., Seillier A., Koek W., Acosta Y., Fernandez E., Strong R., Lutz B., Marsicano G., Roberts J.L., Giuffrida A. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur. J. Neurosci. 2009;29(11):2177–2186. doi: 10.1111/j.1460-9568.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.García C., Palomo-Garo C., García-Arencibia M., Ramos J., Pertwee R., Fernández-Ruiz J. Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson’s disease. Br. J. Pharmacol. 2011;163(7):1495–1506. doi: 10.1111/j.1476-5381.2011.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ternianov A., Pérez-Ortiz J.M., Solesio M.E., García-Gutiérrez M.S., Ortega-Álvaro A., Navarrete F., Leiva C., Galindo M.F., Manzanares J. Overexpression of CB2 cannabinoid receptors results in neuroprotection against behavioral and neurochemical alterations induced by intracaudate administration of 6-hydroxydopamine. Neurobiol. Aging. 2012;33(2):421.e1–421.e16. doi: 10.1016/j.neurobiolaging.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 127.Lastres-Becker I., Molina-Holgado F., Ramos J.A., Mechoulam R., Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiol. Dis. 2005;19(1-2):96–107. doi: 10.1016/j.nbd.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 128.Venderová K., Růzicka E., Vorísek V., Visnovský P. Survey on cannabis use in Parkinson’s disease: subjective improvement of motor symptoms. Mov. Disord. 2004;19(9):1102–1106. doi: 10.1002/mds.20111. [DOI] [PubMed] [Google Scholar]
- 129.Kindred J.H., Li K., Ketelhut N.B., Proessl F., Fling B.W., Honce J.M., Shaffer W.R., Rudroff T. Cannabis use in people with Parkinson’s disease and Multiple Sclerosis: A web-based investigation. Complement. Ther. Med. 2017;33:99–104. doi: 10.1016/j.ctim.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 130.Sieradzan K.A., Fox S.H., Hill M., Dick J.P., Crossman A.R., Brotchie J.M. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology. 2001;57(11):2108–2111. doi: 10.1212/WNL.57.11.2108. [DOI] [PubMed] [Google Scholar]
- 131.Mesnage V., Houeto J.L., Bonnet A.M., Clavier I., Arnulf I., Cattelin F., Le Fur G., Damier P., Welter M.L., Agid Y. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin. Neuropharmacol. 2004;27(3):108–110. doi: 10.1097/00002826-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 132.de Lago E., Moreno-Martet M., Cabranes A., Ramos J.A., Fernández-Ruiz J. Cannabinoids ameliorate disease progression in a model of multiple sclerosis in mice, acting preferentially through CB1 receptor-mediated anti-inflammatory effects. Neuropharmacology. 2012;62(7):2299–2308. doi: 10.1016/j.neuropharm.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 133.Kozela E., Juknat A., Kaushansky N., Rimmerman N., Ben-Nun A., Vogel Z. Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J. Neuroimmune Pharmacol. 2013;8(5):1265–1276. doi: 10.1007/s11481-013-9493-1. [DOI] [PubMed] [Google Scholar]
- 134.Sido J.M., Nagarkatti P.S., Nagarkatti M. Role of Endocannabinoid Activation of Peripheral CB1 Receptors in the Regulation of Autoimmune Disease. Int. Rev. Immunol. 2015;34(5):403–414. doi: 10.3109/08830185.2014.921165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Loría F., Petrosino S., Hernangómez M., Mestre L., Spagnolo A., Correa F., Di Marzo V., Docagne F., Guaza C. An endocannabinoid tone limits excitotoxicity in vitro and in a model of multiple sclerosis. Neurobiol. Dis. 2010;37(1):166–176. doi: 10.1016/j.nbd.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 136.Lourbopoulos A., Grigoriadis N., Lagoudaki R., Touloumi O., Polyzoidou E., Mavromatis I., Tascos N., Breuer A., Ovadia H., Karussis D., Shohami E., Mechoulam R., Simeonidou C. Administration of 2-arachidonoylglycerol ameliorates both acute and chronic experimental autoimmune encephalomyelitis. Brain Res. 2011;1390:126–141. doi: 10.1016/j.brainres.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 137.Arellano G., Acuña E., Reyes L.I., Ottum P.A., De Sarno P., Villarroel L., Ciampi E., Uribe-San Martín R., Cárcamo C., Naves R. Th1 and Th17 Cells and Associated Cytokines Discriminate among Clinically Isolated Syndrome and Multiple Sclerosis Phenotypes. Front. Immunol. 2017;8:753. doi: 10.3389/fimmu.2017.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kozela E., Lev N., Kaushansky N., Eilam R., Rimmerman N., Levy R., Ben-Nun A., Juknat A., Vogel Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmacol. 2011;163(7):1507–1519. doi: 10.1111/j.1476-5381.2011.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mecha M., Yanguas-Casás N., Feliú A., Mestre L., Carrillo-Salinas F., Azcoitia I., Yong V.W., Guaza C. The endocannabinoid 2-AG enhances spontaneous remyelination by targeting microglia. Brain Behav. Immun. 2019;77:110–126. doi: 10.1016/j.bbi.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 140.Maresz K., Pryce G., Ponomarev E.D., Marsicano G., Croxford J.L., Shriver L.P., Ledent C., Cheng X., Carrier E.J., Mann M.K., Giovannoni G., Pertwee R.G., Yamamura T., Buckley N.E., Hillard C.J., Lutz B., Baker D., Dittel B.N. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 2007;13(4):492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- 141.Kong W., Li H., Tuma R.F., Ganea D. Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell. Immunol. 2014;287(1):1–17. doi: 10.1016/j.cellimm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zajicek J., Fox P., Sanders H., Wright D., Vickery J., Nunn A., Thompson A. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet. 2003;362(9395):1517–1526. doi: 10.1016/S0140-6736(03)14738-1. [DOI] [PubMed] [Google Scholar]
- 143.Lutz B., Marsicano G., Maldonado R., Hillard C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015;16(12):705–718. doi: 10.1038/nrn4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rubino T., Guidali C., Vigano D., Realini N., Valenti M., Massi P., Parolaro D. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology. 2008;54(1):151–160. doi: 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 145.Shonesy B.C., Bluett R.J., Ramikie T.S., Báldi R., Hermanson D.J., Kingsley P.J., Marnett L.J., Winder D.G., Colbran R.J., Patel S. Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep. 2014;9(5):1644–1653. doi: 10.1016/j.celrep.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wyrofsky R., McGonigle P., Van Bockstaele E.J. Drug discovery strategies that focus on the endocannabinoid signaling system in psychiatric disease. Expert Opin. Drug Discov. 2015;10(1):17–36. doi: 10.1517/17460441.2014.966680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tao R., Ma Z. Neural circuit in the dorsal raphe nucleus responsible for cannabinoid-mediated increases in 5-HT efflux in the nucleus accumbens of the rat brain. ISRN Pharmacol. 2012;2012:276902. doi: 10.5402/2012/276902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Large M., Sharma S., Compton M.T., Slade T., Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch. Gen. Psychiatry. 2011;68(6):555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- 149.Fusar-Poli P., Crippa J.A., Bhattacharyya S., Borgwardt S.J., Allen P., Martin-Santos R., Seal M., Surguladze S.A., O’Carrol C., Atakan Z., Zuardi A.W., McGuire P.K. Distinct effects of delta9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch. Gen. Psychiatry. 2009;66(1):95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- 150.Hill M.N., McLaughlin R.J., Morrish A.C., Viau V., Floresco S.B., Hillard C.J., Gorzalka B.B. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34(13):2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Di S., Malcher-Lopes R., Marcheselli V.L., Bazan N.G., Tasker J.G. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and γ-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146(10):4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- 152.Hill M.N., Kumar S.A., Filipski S.B., Iverson M., Stuhr K.L., Keith J.M., Cravatt B.F., Hillard C.J., Chattarji S., McEwen B.S. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol. Psychiatry. 2013;18(10):1125–1135. doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Busquets-Garcia A., Gomis-González M., Srivastava R.K., Cutando L., Ortega-Alvaro A., Ruehle S., Remmers F., Bindila L., Bellocchio L., Marsicano G., Lutz B., Maldonado R., Ozaita A. Peripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidation. Proc. Natl. Acad. Sci. USA. 2016;113(35):9904–9909. doi: 10.1073/pnas.1525066113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ishiguro H., Horiuchi Y., Tabata K., Liu Q.R., Arinami T., Onaivi E.S. Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders. Molecules. 2018;23(8):e1836. doi: 10.3390/molecules23081836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 156.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 157.Asnis G.M., De La Garza R.I.I. Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches. J. Clin. Gastroenterol. 2006;40(4):322–335. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- 158.Tyring S., Gottlieb A., Papp K., Gordon K., Leonardi C., Wang A., Lalla D., Woolley M., Jahreis A., Zitnik R., Cella D., Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 159.Hinwood M., Morandini J., Day T.A., Walker F.R. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb. Cortex. 2012;22(6):1442–1454. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- 160.Su F., Yi H., Xu L., Zhang Z. Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia in vitro. Neuroscience. 2015;294:60–68. doi: 10.1016/j.neuroscience.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 161.Moreira F.A., Grieb M., Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23(1):133–144. doi: 10.1016/j.beem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 162.Habib A.M., Okorokov A.L., Hill M.N., Bras J.T., Lee M.C., Li S., Gossage S.J., van Drimmelen M., Morena M., Houlden H., Ramirez J.D., Bennett D.L.H., Srivastava D., Cox J.J. Microdeletion in a FAAH pseudogene identified in a patient with high anandamide concentrations and pain insensitivity. Br. J. Anaesth. 2019;123(2):e249–e253. doi: 10.1016/j.bja.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cravatt B.F., Demarest K., Patricelli M.P., Bracey M.H., Giang D.K., Martin B.R., Lichtman A.H. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA. 2001;98(16):9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lichtman A.H., Shelton C.C., Advani T., Cravatt B.F. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109(3):319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 165.Huggins J.P., Smart T.S., Langman S., Taylor L., Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153(9):1837–1846. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 166.Kerbrat A., Ferré J.C., Fillatre P., Ronzière T., Vannier S., Carsin-Nicol B., Lavoué S., Vérin M., Gauvrit J.Y., Le Tulzo Y., Edan G. Acute neurologic disorder from an inhibitor of fatty acid amide hydrolase. N. Engl. J. Med. 2016;375(18):1717–1725. doi: 10.1056/NEJMoa1604221. [DOI] [PubMed] [Google Scholar]
- 167.Calignano A., La Rana G., Giuffrida A., Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394(6690):277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 168.Guindon J., Desroches J., Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br. J. Pharmacol. 2007;150(6):693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dziadulewicz E.K., Bevan S.J., Brain C.T., Coote P.R., Culshaw A.J., Davis A.J., Edwards L.J., Fisher A.J., Fox A.J., Gentry C., Groarke A., Hart T.W., Huber W., James I.F., Kesingland A., La Vecchia L., Loong Y., Lyothier I., McNair K., O’Farrell C., Peacock M., Portmann R., Schopfer U., Yaqoob M., Zadrobilek J. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: a potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration. J. Med. Chem. 2007;50(16):3851–3856. doi: 10.1021/jm070317a. [DOI] [PubMed] [Google Scholar]
- 170.Agarwal N., Pacher P., Tegeder I., Amaya F., Constantin C.E., Brenner G.J., Rubino T., Michalski C.W., Marsicano G., Monory K., Mackie K., Marian C., Batkai S., Parolaro D., Fischer M.J., Reeh P., Kunos G., Kress M., Lutz B., Woolf C.J., Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007;10(7):870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Walker J.M., Hohmann A.G., Martin W.J., Strangman N.M., Huang S.M., Tsou K. The neurobiology of cannabinoid analgesia. Life Sci. 1999;65(6-7):665–673. doi: 10.1016/S0024-3205(99)00289-1. [DOI] [PubMed] [Google Scholar]
- 172.Racz I., Nadal X., Alferink J., Baños J.E., Rehnelt J., Martín M., Pintado B., Gutierrez-Adan A., Sanguino E., Manzanares J., Zimmer A., Maldonado R. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J. Neurosci. 2008;28(46):12125–12135. doi: 10.1523/JNEUROSCI.3400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Clark A.K., Yip P.K., Grist J., Gentry C., Staniland A.A., Marchand F., Dehvari M., Wotherspoon G., Winter J., Ullah J., Bevan S., Malcangio M. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc. Natl. Acad. Sci. USA. 2007;104(25):10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nent E., Nozaki C., Schmöle A.C., Otte D., Zimmer A. CB2 receptor deletion on myeloid cells enhanced mechanical allodynia in a mouse model of neuropathic pain. Sci. Rep. 2019;9(1):7468. doi: 10.1038/s41598-019-43858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sokal D.M., Elmes S.J.R., Kendall D.A., Chapman V. Intraplantar injection of anandamide inhibits mechanically-evoked responses of spinal neurones via activation of CB2 receptors in anaesthetised rats. Neuropharmacology. 2003;45(3):404–411. doi: 10.1016/S0028-3908(03)00195-3. [DOI] [PubMed] [Google Scholar]
- 176.Nackley A.G., Zvonok A.M., Makriyannis A., Hohmann A.G. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J. Neurophysiol. 2004;92(6):3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- 177.LaBuda C.J., Koblish M., Little P.J. Cannabinoid CB2 receptor agonist activity in the hindpaw incision model of postoperative pain. Eur. J. Pharmacol. 2005;527(1-3):172–174. doi: 10.1016/j.ejphar.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 178.Hohmann A.G., Farthing J.N., Zvonok A.M., Makriyannis A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J. Pharmacol. Exp. Ther. 2004;308(2):446–453. doi: 10.1124/jpet.103.060079. [DOI] [PubMed] [Google Scholar]
- 179.Beltramo M., Bernardini N., Bertorelli R., Campanella M., Nicolussi E., Fredduzzi S., Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur. J. Neurosci. 2006;23(6):1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 180.Pertwee R.G., Howlett A.C., Abood M.E., Alexander S.P., Di Marzo V., Elphick M.R., Greasley P.J., Hansen H.S., Kunos G., Mackie K., Mechoulam R., Ross R.A. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 2010;62(4):588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N.O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007;152(7):1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Waldeck-Weiermair M., Zoratti C., Osibow K., Balenga N., Goessnitzer E., Waldhoer M., Malli R., Graier W.F. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J. Cell Sci. 2008;121(Pt 10):1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Oka S., Nakajima K., Yamashita A., Kishimoto S., Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007;362(4):928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 184.Kapur A., Zhao P., Sharir H., Bai Y., Caron M.G., Barak L.S., Abood M.E. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J. Biol. Chem. 2009;284(43):29817–29827. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shi Q.X., Yang L.K., Shi W.L., Wang L., Zhou S.M., Guan S.Y., Zhao M.G., Yang Q. The novel cannabinoid receptor GPR55 mediates anxiolytic-like effects in the medial orbital cortex of mice with acute stress. Mol. Brain. 2017;10(1):38. doi: 10.1186/s13041-017-0318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lagalwar S., Bordayo E.Z., Hoffmann K.L., Fawcett J.R., Frey W.H., II Anandamides inhibit binding to the muscarinic acetylcholine receptor. J. Mol. Neurosci. 1999;13(1-2):55–61. doi: 10.1385/JMN:13:1-2:55. [DOI] [PubMed] [Google Scholar]
- 187.Savinainen J.R., Saario S.M., Niemi R., Järvinen T., Laitinen J.T. An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors. Br. J. Pharmacol. 2003;140(8):1451–1459. doi: 10.1038/sj.bjp.0705577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kimura T., Ohta T., Watanabe K., Yoshimura H., Yamamoto I. Anandamide, an endogenous cannabinoid receptor ligand, also interacts with 5-hydroxytryptamine (5-HT) receptor. Biol. Pharm. Bull. 1998;21(3):224–226. doi: 10.1248/bpb.21.224. [DOI] [PubMed] [Google Scholar]
- 189.Hillard C.J., Bloom A.S. delta 9-Tetrahydrocannabinol-induced changes in beta-adrenergic receptor binding in mouse cerebral cortex. Brain Res. 1982;235(2):370–377. doi: 10.1016/0006-8993(82)91016-2. [DOI] [PubMed] [Google Scholar]
- 190.Croxford J.L. Therapeutic potential of cannabinoids in CNS disease. CNS Drugs. 2003;17(3):179–202. doi: 10.2165/00023210-200317030-00004. [DOI] [PubMed] [Google Scholar]
- 191.Venkatachalam K., Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Starowicz K., Nigam S., Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007;114(1):13–33. doi: 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 193.Di Marzo V., De Petrocellis L., Fezza F., Ligresti A., Bisogno T. Anandamide receptors. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66(2-3):377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- 194.Akopian A.N., Ruparel N.B., Jeske N.A., Patwardhan A., Hargreaves K.M. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol. Sci. 2009;30(2):79–84. doi: 10.1016/j.tips.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ryskamp D.A., Redmon S., Jo A.O., Križaj D. TRPV1 and endocannabinoids: emerging molecular signals that modulate mammalian vision. Cells. 2014;3(3):914–938. doi: 10.3390/cells3030914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lowin T., Straub R.H. Cannabinoid-based drugs targeting CB1 and TRPV1, the sympathetic nervous system, and arthritis. Arthritis Res. Ther. 2015;17:226. doi: 10.1186/s13075-015-0743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Oz M., Tchugunova Y.B., Dunn S.M. Endogenous cannabinoid anandamide directly inhibits voltage-dependent Ca(2+) fluxes in rabbit T-tubule membranes. Eur. J. Pharmacol. 2000;404(1-2):13–20. doi: 10.1016/S0014-2999(00)00396-4. [DOI] [PubMed] [Google Scholar]
- 198.Oz M., Yang K.H., Dinc M., Shippenberg T.S. The endogenous cannabinoid anandamide inhibits cromakalim-activated K+ currents in follicle-enclosed Xenopus oocytes. J. Pharmacol. Exp. Ther. 2007;323(2):547–554. doi: 10.1124/jpet.107.125336. [DOI] [PubMed] [Google Scholar]
- 199.Nicholson R.A., Liao C., Zheng J., David L.S., Coyne L., Errington A.C., Singh G., Lees G. Sodium channel inhibition by anandamide and synthetic cannabimimetics in brain. Brain Res. 2003;978(1-2):194–204. doi: 10.1016/S0006-8993(03)02808-7. [DOI] [PubMed] [Google Scholar]
- 200.Michalik L., Auwerx J., Berger J.P., Chatterjee V.K., Glass C.K., Gonzalez F.J., Grimaldi P.A., Kadowaki T., Lazar M.A., O’Rahilly S., Palmer C.N., Plutzky J., Reddy J.K., Spiegelman B.M., Staels B., Wahli W. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006;58(4):726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 201.Bouaboula M., Hilairet S., Marchand J., Fajas L., Le Fur G., Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur. J. Pharmacol. 2005;517(3):174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 202.Ghosh M., Wang H., Ai Y., Romeo E., Luyendyk J.P., Peters J.M., Mackman N., Dey S.K., Hla T. COX-2 suppresses tissue factor expression via endocannabinoid-directed PPARdelta activation. J. Exp. Med. 2007;204(9):2053–2061. doi: 10.1084/jem.20070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.D’Agostino G., La Rana G., Russo R., Sasso O., Iacono A., Esposito E. Acute intracerebroventricular administration of palmitoylethanolamide, an endogenous PPAR-agonist, modulates carrageenan-Induced paw edema in mice. J. Pharmacol. Exp. Ther. 2007;322:1137–1143. doi: 10.1124/jpet.107.123265. [DOI] [PubMed] [Google Scholar]
- 204.LoVerme J., Russo R., La Rana G., Fu J., Farthing J., Mattace-Raso G., Meli R., Hohmann A., Calignano A., Piomelli D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J. Pharmacol. Exp. Ther. 2006;319(3):1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- 205.Landreth G., Jiang Q., Mandrekar S., Heneka M. PPARgamma agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics. 2008;5(3):481–489. doi: 10.1016/j.nurt.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Altamura C., Ventriglia M., Martini M.G., Montesano D., Errante Y., Piscitelli F., Scrascia F., Quattrocchi C., Palazzo P., Seccia S., Vernieri F., Di Marzo V. Elevation of Plasma 2-Arachidonoylglycerol Levels in Alzheimer’s Disease patients as a potential protective mechanism against neurodegenerative decline. J. Alzheimers Dis. 2015;46(2):497–506. doi: 10.3233/JAD-142349. [DOI] [PubMed] [Google Scholar]
- 207.Pertwee R.G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009;156(3):397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]