Abstract
Alzheimer’s is an insidious, progressive, chronic neurodegenerative disease which causes the devastation of neurons. Alzheimer's possesses complex pathologies of heterogeneous nature counting proteins as one major factor along with enzymes and mutated genes. Proteins such as amyloid precursor protein (APP), apolipoprotein E (ApoE), presenilin, mortalin, calbindin-D28K, c-reactive protein, heat shock proteins (HSPs), and prion protein are some of the chief elements in the foremost hypotheses of AD like amyloid-beta (Aβ) cascade hypothesis, tau hypothesis, cholinergic neuron damage, etc. Disturbed expression of these proteins results in synaptic dysfunction, cognitive impairment, memory loss, and neuronal degradation. On the therapeutic ground, attempts of developing anti-amyloid, anti-inflammatory, anti-tau therapies are on peak, having APP and tau as putative targets. Some proteins, e.g., HSPs, which ameliorate oxidative stress, calpains, which help in regulating synaptic plasticity, and calmodulin-like skin protein (CLSP) with its neuroprotective role are few promising future targets for developing anti-AD therapies. On diagnostic grounds of AD C-reactive protein, pentraxins, collapsin response mediator protein-2, and growth-associated protein-43 represent the future of new possible biomarkers for diagnosing AD. The last few decades were concentrated over identifying and studying protein targets of AD. Here, we reviewed the physiological/pathological roles and therapeutic significance of nearly all the proteins associated with AD that addresses putative as well as probable targets for developing effective anti-AD therapies.
Keywords: Alzheimer's, neurodegeneration, proteins, pathological role, therapeutics
1. Introduction
Alzheimer’s disease (AD) with a long preclinical period of ~20 years is a chronic, insidious, and progressive neurodegenerative disease of old age. AD mostly affects people over the age of 65 years categorized as “late-onset AD”, however, AD before 65 refers to “early-onset AD”. Neuropathologically, AD is characterized by the presence of accumulated amyloid-beta (Aβ) in plaques (in extracellular spaces, and walls of blood vessels), as well as aggregated tau protein in neurofibrillary tangles (NFTs). AD is an irreversible disorder which sources abnormalities in the brain tissues and triggers a decline in cognition, thinking and thus affects daily routine. AD is rapidly growing, with a rate of 10 million people per year globally. According to the WHO Dementia Fact sheet, 2017, an estimated population of demented people will reach 82 million by 2030 and 152 million by 2050 [1-5]. AD turned out to be an expensive disease in 2015 with a total estimated worldwide cost of US$ 818 billion and currently, it is a trillion dollar disease [6, 7]. The exact cause of AD is not known yet, however, it is believed to be a complex multifactorial disease resulting from complex crosstalk between different pathophysiological processes. The foremost hypotheses behind the pathology of AD are the Aβ cascade hypothesis, tau hypothesis, inflammation hypothesis, synapse-centered hypothesis, cholinergic neuron damage, oxidative stress hypothesis etc. AD begins with a long and complex phase that consists of feedback and feedforward responses of astrocytes, microglia, and vasculature. Aβ has long been studied as a potential target for AD because of its accumulation in the brain (senile plaques) that causes cognitive deficits. GWAS and sequencing approaches revolutionized the genetics of AD and these data support the amyloid cascade hypothesis with the additional role of microglia in Aβ peptide clearance and neuroinflammation. From CNS, Aβ is cleared out via perivascular circulation and glymphatic pathway, where P-glycoprotein and LRP-1, along with SORLA, facilitate its transportation. However, inflammation and synaptic failure, along with their associated proteins (e.g., Interleukins, MCP-1, ApoE, etc.), lead to vascular problems such as cerebral amyloid angiopathy [8-11]. Furthermore, the hyperphosphorylation of tau proteins gives rise to NFTs, which plays a pivotal role in the commencement of early symptoms of AD. Altogether, these hypotheses complement each other through protein talk, and this complex network of protein interactions constantly plays a significant role in the progression of AD pathogenesis [12, 13].
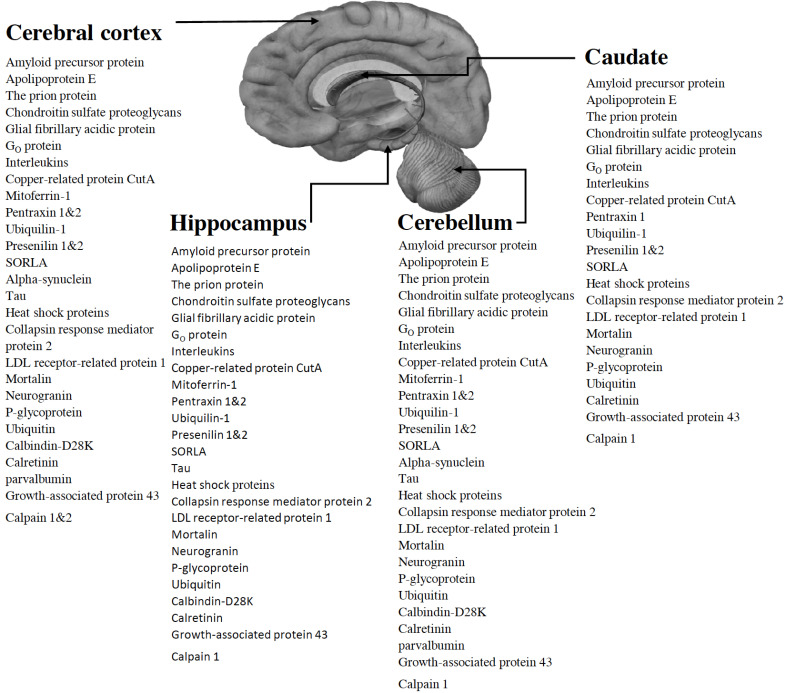
Besides the foremost contributing factors in the progression of AD, such as amyloid precursor protein (APP), tau, and presenilins, numerous additional proteins either potentiate or ameliorate the pathology of AD. For example, proteins such as mitoferrin-1, Interleukins, GO protein, C- reactive protein, prion protein, etc. play their role in the progression of AD either in a dependent or independent manner with admiration to Aβ and tau hypothesis. Whereas, proteins like neurogranin, P-glycoprotein, ubiquitin, insulin, calbindin-D28K, calretinin, parvalbumin, and many other aid neuroprotection. In addition, proteins such as calmodulin-like skin protein interact with Heterotrimeric Humanin receptors (htHNR) and help to abolish neurodegeneration, though some other proteins can initiate it, e.g., mutated presenilin-1 can potentiate Aβ production through presenilin-dependent γ-secretase degradation of APP [14, 15]. Furthermore, proteins like ubiquilin-1 interact only with APP and potentiate the production of insoluble Aβ peptides, whereas interactions of pentraxins are found with both NFTs and senile plaques [16, 17]. For the treatment of AD, U.S. Food and Drug Administration (FDA) has approved two types of medications; cholinesterase inhibitors (Donepezil, Galantamine, and Rivastigmine) and N-methyl-D-aspartate (NMDA) receptor antagonist (Memantine). These drugs show beneficial effects on cognitive, functional, and behavioral symptoms though none of them are capable of inhibiting the advancement of neurodegeneration, and hence providing the immense need as well as an opportunity to identify new targets in AD pathology [18, 19]. In recent AD treatment pipeline 2019, a total 132 of agents are there, out of which 28 agents are getting assessed in 42 Phase III clinical trials, 74 agents managed to reach Phase II clinical trials (in 83 different trials), and 30 agents in 31 Phase I trials. 73% of these agents are having a mechanism of action as disease-modifying agents, 14% are symptomatic cognitive enhancers, and 11% are symptomatic agents addressing neuropsychiatric and behavioral changes. Out of all these agents, 55 agents are targeting Aβ (38 agents) and tau (17 agents) proteins. This data shows a narrow approach for treating AD by means of targeting AD-associated proteins and it is also limited to Aβ and tau proteins. Moreover, agents targeting these two proteins also failed to show significant results in their clinical trials, e.g., agents targeting amyloid protein such as Aducanumab and crenezumab (development of both the drugs has been halted recently). Furthermore, a clinical trial for an anti-tau agent TRx0237 revealed negative results, although its new Phase 2/3 trial started in 2018 [20]. Continuous failure of these clinical trials from the last couple of years somehow indicates an essential diversion towards the assessment of new targets associated with AD. From the literature, we found that proteins like C-reactive protein, GFAP, and RANTES shall further be explored to establish them as promising early diagnostic markers of AD so that chronic degradation of the nervous system can be prevented or at least slowed down. Furthermore, proteins such as calmodulin-like skin protein may act as a new target for the development of new preventive/curative therapies to decelerate the neurodegeneration. These proteins are found to be present in major brain areas like Cerebral cortex, Hippocampus, Cerebellum and Caudate (Fig. 1). The present review highlights various unfamiliar or less familiar proteins associated with AD that have a significant role in either progression or amelioration of pathological processes responsible for the clinical manifestation of AD.
Fig. (1).
Locations of the proteins in major brain areas (Cerebral cortex, Hippocampus, Cerebellum, Caudate). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
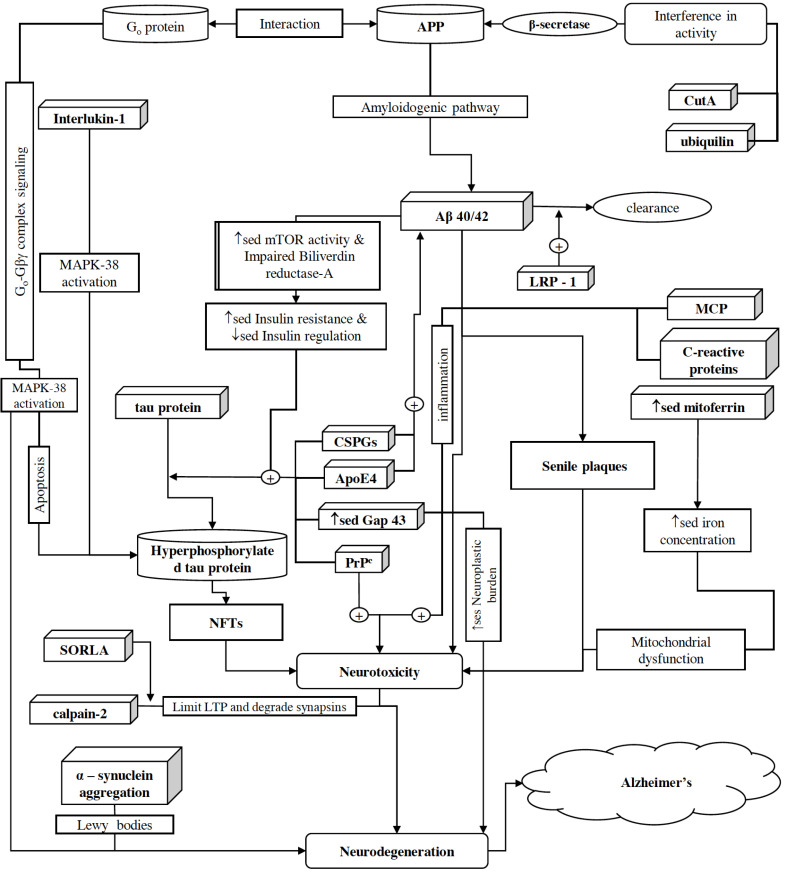
2. Proteins associated with neuro- toxicity and neurodegeneration [Fig. 2]
Fig. (2).
Schematic representation of proteins associated with neurotoxicity and neurodegeneration. AD is a disease caused by a complex talk between various pathologies and proteins associated with them. For example, amyloidogenic processing of APP results in the secretion of one major form of Aβ, i.e., Aβ-40, along with few minor species containing 42 or 43 amino acid residues, which later on gets aggregated in the brain . Increased Aβ accumulation in the brain enhances APP/Go protein complex formation, which then initiates Go-GβΥ complex signaling and as a result, irregular activation of the MPAK-p38 pathway causes neurotoxicity. MCP-1 is primarily expressed by glial cells, i.e., astrocytes and microglia. Astrocytes rapidly express MCP-1 in ipsilateral thalamus as a primary response to brain injury. Later on, MCP-1 acts as a potent chemoattractant for monocytes, memory T cells, and dendritic cells, as a result, it initiates a cascade of secondary responses in inflammation and causes neurodegeneration through direct apoptosis. ILs are also secreted during various pathological processes and further enhance neuroinflammation, e.g., IL-1 activates T cells and other inflammatory mediators, which initiate an inflammatory response and activate p38-MAPK. With the initiation of inflammation, neutrophils and macrophages secrete IL-6, which in turn, facilitates the production of C-reactive protein. C-reactive protein further promotes inflammation and increases the production of Aβ-42. Despite this, ubiquilin-1 facilitates the accumulation of insoluble Aβ peptides by affecting BACE1 activity and via assisting presenilin 1 accumulation, which afterward, results in presenilin-dependent γ-secretase degradation of APP. Copper-related protein CutA also interferes with BACE1 activity and indirectly affects APP processing. In AD, iron levels have been found to be elevated and facilitating cognitive decline. Mitoferrin-1 is associated with iron homeostasis and its up-regulation contributes to the excessive accumulation of iron in mitochondria, resulting in increased mitochondria induced oxidative stress. Moreover, aggregation of alpha-synuclein as Lewy bodies is potentiated by ganglioside lipids, which results in increased alpha-synuclein-mediated oxidative stress, neurotoxicity, and ultimately neurodegeneration. From CNS, Aβ is cleared out via perivascular circulation and glymphatic pathway where P-glycoprotein and LRP-1 along with SORLA facilitate its transportation. Disrupted functioning of SORLA prevents Aβ clearance from the brain. Moreover, SORLA facilitates calpain mediated proteolytic degradation of synapsins and thus, potentiating impaired regulation of neurotransmitters release at synapsis. In addition, overactivation of calpain-2 puts restrictions to the extent of LTP and aids Aβ accumulation via supplementing the expression level of β-secretase. On the other hand, CIP2A and CDK5RAP2 facilitate hyperphosphorylation of tau protein; as a result, disruption of nucleocytoplasmic transport causes neuronal death and NFTs. In addition, ApoE4’s isoform-specific interactions with tau, PrPC-induced neurotoxicity, and disrupted Gap43 activities (such as synaptic transmission, membrane permeability, neuronal growth, etc.) also cause an upsurge in NFTs level in the brain. However, the role of CSPGs is remained unexplored yet; they are also found abundantly in Aβ-plaques and NFTs. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.1. Amyloid Precursor Protein
APP is a highly conserved ancient protein which belongs to the family of type 1 transmembrane glycoprotein, mainly positioned at synapses with a large extracellular domain and a short cytoplasmic domain. The functions of APP are still not clearly acknowledged. The entire APP complex (accompanied with its fragments) is derived from the non-amyloidogenic pathway for various neural functions such as neuroprotection, neurotrophic action, regulation of cell excitability, synaptic plasticity, etc., whereby amyloidogenic pathway results in the production of Aβ which exerts opposite effects as compared to APP. Although, lack of APP in the younger brain is beneficial as it upsurges total synapses, it plays a crucial role in the commencement and increment of tau pathology and pathogenesis of AD. Membrane-bound APP is processed by α-, β-, and γ-secretases, which release soluble APP fragments as well as the non-soluble sticky fragment of Aβ peptides [21-24]. APP also interacts with various membrane proteins, including G protein-coupled receptors (GPCRs) such as type-1 cannabinoid receptor (CB1 is the most abundant GPCR in the brain) and interferes with its membrane localization and inhibitory signaling activity [11]. Amyloidogenic processing of APP results in the secretion of one major form of Aβ, i.e., Aβ-40, along with few minor species containing 42 or 43 amino acid residues, which, in turn, plays a crucial role in colossal tau deposition in distant cortical regions [25]. Accumulation of Aβ is directly associated with the progression of AD and it has an upstream accumulation in the brain prior to processing of tau proteins. Moreover, the accumulation of Aβ-42 is much greater than Aβ-40 along with its toxicity which includes pore formation and ion leakage, the disintegration of cellular calcium balance, disturbed membrane potential, apoptosis, synaptic loss, and, ultimately disruption of the cytoskeleton [26]. Thus, APP has an association with AD through the production of numerous sticky Aβ peptides that ultimately generate senile plaques (i.e., a classical hallmark of AD). Various new therapeutic agents, e.g., CNP520, CAD106 & CNP520, and Gantenerumab (ClinicalTrials.gov ID; NCT03444870, NCT03443973) are getting assessed in different clinical trials for their anti-amyloidal activity. The majority of these agents are having an approach for immunotherapy whereas, others are targeting beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibition and Aβ-aggregation. In total, 10 anti-amyloidal agents are there in phase III clinical trials of AD drug development pipeline 2019 [20]. APP has significant possibilities for further exploration to prevent abnormal Aβ accumulation in the brain, which can help in ameliorating dementia-like symptoms in AD. In addition, genetic and biochemical exploration of APP processing could be beneficial for the development of therapeutic targets for treating AD in the coming years.
2.2. Apolipoprotein E
Apolipoprotein E (ApoE), a major apoprotein of chylomicron, is a major cholesterol carrier, encoded by the APOE gene [27]. ApoE has 299 amino acids with a molecular weight of 34,200. It is synthesized and secreted by astrocytes. ApoE transports lipids and repairs injuries in the brain. Three common isoforms of ApoE, i.e., apoE2, apoE3, and apoE4 are produced by three different alleles of the APOE gene. The human APOE gene exists as three polymorphic alleles, i.e., ε2, ε3, and ε4. People carrying ε4 allele possess the enhanced risk of developing AD as compared to those carrying the more common ε3 allele. Despite this, the ε2 allele declines the risk of AD [28, 29]. ApoE presents abundantly in the liver and one-third of its levels present in the brain as compared to the liver [30]. Patients with AD were found to have an association between Aβ deposition and ApoE4. ApoE4 increases the formation of senile plaques and NFTs. Secondarily, it is also associated with the reduction of choline acetyltransferase activity in the temporal cortex of AD individuals [31]. The ApoE in some neurons may influence their metabolic activities, and isoform-specific interactions with tau and microtubule-associated protein 2 (MAP2C) can influence the rate of AD pathogenesis [32]. Moreover, allele ε4 also affects SNAP levels in patients with mild cognitive impairment causing synaptic dysfunction [33]. In addition, ApoE ε4 has a greater impact on females as compared to male individuals. This information can help in interpreting the failure or deviates conclusions of the clinical interventions targeting Aβ peptides [34]. In conclusion, there is a need to
further explore the role of ApoE to AD pathogenesis as ApoE protein and APOE gene; both have remarkable contributions in the development of AD. Both of these directly influence AD and help worsening its symptoms, which suggest a tremendous scope for research in the respective field.
2.3. The Prion Protein
The prion protein (PrPC) is an extracellular glycoprotein present on the cell membrane surface with the help of glycophosphatidylinositol and abundantly expressed in neurons. Human PrPC is a 208 residue protein. PrPC is responsible for causing various neurodegenerative diseases like prion disease and AD. Recent studies indicate its role as a mediator of Aβ neurotoxicity by acting as an Aβ oligomer receptor [35]. PrPC plays a crucial role in memory processing through the activation of PKA and ERK1⁄2 signaling pathways [36]. Misfolded PrPC is not responsible for the formation of hyperphosphorylated tau though PrPC-induced neurotoxicity is responsible for the abnormal processing of tau [37]. Even though not having a direct association with the pathogenesis of AD, PrPC plays a crucial role in memory impairment and shows an impetus for future research. Association between PrPC and Aβ can be utilized for further clinical relevance and therapeutic potential in AD.
2.4. C-reactive Protein
C- reactive protein is an immune response protein that belongs to the pentraxin family. It is predominantly synthesized in the liver as a response to Interleukin-6 (IL-6) secretion, and this synthesis is favored synergistically by IL-1β, as IL-1β mediate IL-6 induction [38, 39]. With the initiation of inflammation, neutrophils and macrophages secrete IL-6, which, in turn, facilitates the production of C- reactive protein, serum amyloid P and fibrinogen. IL-6 also aids the transformation of neutrophil-mediated immune response to macrophage-mediated immune response along with the differentiation of T-cells and B-cells, which ultimately results in concentrating the immune response towards infected cells. On the other hand, secreted C- reactive protein follows a positive feedback loop and enhances the secretion of IL-6, IL-1β, and TNF-α. Even though, the interaction of IL-6 and C- reactive protein promotes inflammation, they also participate in anti-inflammatory and repair-oriented processes [40]. Numerous studies suggest that increased levels of C- reactive protein can nonspecifically serve as blood biomarkers of AD, especially in patients over 90 years old age [41]. During inflammatory reactions, increased C- reactive protein’s levels were found in serum, CSF, and brain tissue of AD patients. Moreover, mRNA expressions of APP, BACE-1, and presenilin 1&2 were found to be significantly increased after incubation with C- reactive protein. In addition, Aβ-42 production also found to be enhanced in the presence of C- reactive protein. Although these findings were supported by several population-based epidemiological studies, few contradict them [42]. Despite wanting literature, various researches suggest dual possibilities of C- reactive protein as a biomarker as well as a participant in AD pathogenesis and, therefore, C- reactive protein can be a field of interest for further studies in the coming years. We assume that further research on C- reactive protein may be translated into promising measures for the diagnosis and treatment of AD in the future.
2.5. Chondroitin Sulfate Proteoglycans
Chondroitin sulfate proteoglycans (CSPGs) consist of two structural parts, i.e., a protein core and a chondroitin sulfate side chain. CSPGs compose highly organized structures of perineuronal nets (PNNs) along with hyaluronan, link proteins, and tenascin-R. CSPGs help in memory and neuroplasticity after getting digested with Chondroitin sulfate-digesting enzyme. CSPGs are present abundantly in Aβ-plaques and NFTs in AD [43]. Proteoglycans affect Aβ aggregation and neurotoxicity, but their pathophysiological role is still not well known. CSPGs are present at many stages of Aβ pathology though their role, as well as the effect of Aβ-Proteoglycans interactions, is not clear. Proteoglycans mediated accumulation of Aβ results in the generation of endolysosomal vesicles, which ultimately can lead to great neural toxicity [44]. The reduction of CSPGs can significantly help in improving memory and neural plasticity [45]. Moreover, Neurocan (a brain CSPG) expression is also increased by Aβ with the help of transcription factor Sox9, which contributes to AD pathology [46, 47]. As it is clear that CSPGs surely participate in the AD pathogenesis, their role has yet to be discovered, which potentiates dedicated research in this field. CSPGs also provide an idea for new strategies to treat AD.
2.6. Glial Fibrillary Acidic Protein
Glial fibrillary acidic protein (GFAP) is an intermediate filament protein in astrocytes (a type of glial cells in the central nervous system (CNS)). Astrocytes help in supervising various functions of CNS as well as maintaining the homeostasis and help in acute CNS trauma, ischemia, and in neurodegenerative diseases. For the purpose, astrocytes increase expression and rearrangement of the intermediate filaments to form a highly complex system consisting of GFAP, vimentin, synemin, and nestin [48]. After degeneration, GFAP and its byproducts in the cerebrospinal fluid (CSF), serve as biomarkers for diagnosing various neurologic disorders like stroke and spinal cord injuries [49]. Expressions of different isoforms of GFAP, i.e., GFAPδ, GFAP+1 occur differently in distant astrocytes during AD progression and are highly associated with Aβ plaques as compared to NFTs [50]. In AD, more acidic isoforms (phosphorylated and N-glycosylated) of the GFAP show significant quantitative amplification compared to more basic (O-glycosylated) isoforms which did not show any rise in their concentration [51]. The possible role of GFAP in AD pathology and underlying mechanisms concerning the functions of GFAP in astrocytes needs to be explored in the future. GFAP could be a promising diagnostic marker in AD and can be further examined and investigated for developing an effective diagnostic tool.
2.7. GO Protein
GPCRs are the prime family of cell surface molecules that help in signal transmission in various physiologies and their failure can lead to severe pathologies in a body, including the brain. GPCRs activate the Heterotrimeric guanine nucleotide-binding proteins, i.e., G protein [52]. G proteins are ancient proteins and were conserved throughout the evolution. G proteins are composed of an alpha subunit (Gα) and a beta-gamma Complex (Gβγ) subunit. Go is a key GTP binding protein in the brain. Nishimoto et al., 1993, firstly reported the complex formation between APP and Go. The cytoplasmic APP sequence His657-Lys 676 specifically activates Go protein and thus assists APP in complex formation with Go. This study concludes that APP is a receptor coupled with Go protein whose abnormal signaling takes part in the pathogenesis of AD. Moreover, increased accumulation of Aβ in brain favors APP/Go protein complex formation, which, in turn, induces Aβ assisted degeneration by Go-Gβγ complex signaling and p38-MAPK activation [53, 54]. Literature suggests that this Go-Gβγ complex formation is a signaling target which shall further be explored as it may serve as a potential target for developing possible anti-AD therapy in the near future.
2.8. Interleukins
Interleukins (ILs) are proteins produced by white blood cells, helper CD4+ T lymphocytes, as well as through monocytes, macrophages, and endothelial cells. Over 50 interleukins can be encoded by the human genome [55]. IL-1 is a pluripotent, proinflammatory cytokine that helps in inflammation and forms a defense system in the body. IL-1 activates T cells and other inflammatory mediators that ultimately initiate an inflammatory response. IL-1 activates p38-MAPK and thus leads to tau hyperphosphorylation and formation of NFTs [56]. Polymorphism in the IL-1A gene results in increasing AD risk by three folds, especially for onset of AD in early age [57]. Moreover, IL- lβ and IL-6 are also found to be increased in CSF in the brains of AD patients [58, 59]. Along with it, IL-18 and IL-7 are also involved in the pathology of AD [60, 61]. Interleukins are inflammatory mediators and can cause damage to the brain by inflammatory cascades. Thus, inflammatory pathways involving interleukins signaling could be potential targets for future research so that new therapies can be evolved for the amelioration of their effects, which can be helpful in the symptomatic cure of AD.
2.9. Copper-related Protein CutA
CutA is a protein found in bacteria, plants, and animals encoded by the CUTA1 gene. Human CutA has different variants and can be expressed in different isoforms [62, 63]. β-secretase is an enzyme responsible for the abnormal cleavage of APP, resulting in pathogenic insoluble Aβ peptides, CutA interacts and interferes with intracellular levels of the BACE1 (i.e., β-secretase 1), and indirectly plays an important role in the mechanism of APP processing [64]. Increased expression of CutA facilitates the intracellular accumulation of copper as well as, elevated intracellular copper promotes CutA expression. Although overexpressed CutA diminishes copper-induced Aβ secretion, it does not affect APP expression. Moreover, copper also contributes to both amyloid and tau pathology and its excessive levels can affect CNS by an unknown mechanism and acts as a neurotoxic agent to initiate neurodegeneration [65, 66]. As the role of CutA is still not thoroughly studied in AD, it provides an opportunity to further investigate its role in the pathology of AD.
2.10. Mitoferrin-1
Mitoferrin-1 and mitoferrin-2 are two homologous proteins responsible for iron homeostasis by transporting iron to the mitochondria in humans. Mitoferrin-1 is encoded by the SLC25A37 gene, having a molecular weight of 38kDa [67, 68]. Overaccumulation of iron in the mitochondria, oxidative stress, and mitochondrial dysfunction are some of the cofactors that play a role in AD pathology. Moreover, in dementia, iron overload contributes to an accelerated decline in cognition [69]. Mitoferrin-1 is associated with iron homeostasis and its up-regulation contributes to the overaccumulation of iron in mitochondria. Although, its down-regulation diminishes mitochondrial iron levels, alters mitochondrial morphology, and reduces mitochondria induced oxidative stress [70]. The role of iron in AD is still understudied and needs further exploration. As iron levels in AD have been found to be elevated and facilitating cognitive decline (in some cases), there must be a possibility of mitoferrin-1 contribution in it. Therefore, further study is needed on mitoferrin-1 to further identify its role in AD pathology.
2.11. Monocyte Chemoattractant Protein 1
Monocyte chemoattractant protein 1 (MCP-1), also known as chemokine (C-C motif) ligand 2 (CCL2), is positioned on chromosome 17 with a molecular weight of approximately 13 kDa. Composed of 76 amino acids, MCP-1 is a monomeric polypeptide and a member of the C-C chemokine family [71, 72]. MCP-1 binds to C-C chemokine receptor type 2 (CCR2), and acts as a potent chemoattractant for monocytes, memory T cells, and dendritic cells [73]. MCP-1 and its respective receptor CCR2 are primarily expressed by glial cells, i.e., astrocytes and microglia. Astrocytes rapidly express MCP-1 mRNA and protein in ipsilateral thalamus as a primary response to an injury in the brain. Later on, MCP-1 initiates a cascade of secondary responses in inflammation and causes neurodegeneration through direct apoptosis [74, 75]. Astrocytes and microglia are responsible for remodeling and repairing of CNS, and cytokines act as communication messengers in between. Moreover, the role of MCP-1 is also reported in HIV dementia, multiple sclerosis, and AD, suggesting its potential role as a molecular mediator of response towards an injury to CNS [76, 77]. Eradication of MCP-1 can suspend neuronal degeneration, decreases microglial activation, and alters cell death through apoptotic factors [78]. This suggests that developing a functional therapy for the MCP-1 can decelerate the impact of inflammation-mediated neurodegeneration in AD.
2.12. Pentraxin
Pentraxins or pentaxins are a family of acute phase proteins composed of five or ten identical subunits organized in pentameric radial symmetry. Pentraxins protein family is an anciently conserved family of proteins, where pentraxin I and II are primarily expressed in the human nervous system. Pentraxin I is encoded by the NPTX1 gene located over chromosome 17 whereas, pentraxin II is encoded by the NPTX2 gene located on chromosome 7 [79, 80]. Proteins of the pentraxin family are involved in acute immunological responses and show association with NFTs and Aβ plaques [81]. Expression of neuronal pentraxin 1 (NP1) and its receptor neuronal pentraxin receptor (NPR) occurs primarily in excitatory neurons, and its axonal secretion is mediated by astrocyte-secreted glypican 4 for the formation of the synapse. Normal expression of neuronal pentraxin I is disrupted in AD; moreover, it is also modulated by the Aβ expression representing its potential role in synapse-derived plasma biomarker in early-stage AD detection [82, 83]. Neuronal pentraxin II is a pro-inflammatory CSF biomarker whose quantification can help in the detection of chronic neuroinflammation in the brain, which is responsible for the medial temporal lobe atrophy and memory decline [84]. Neuronal pentraxin receptor quantification from plasma also represents a potential biomarker for synaptic dysfunction as its accumulation in cerebral along with Aβ occurs with similar fashion in temporal and regional patterns [85]. Some other members of the pentraxins family, like serum amyloid P component and C-reactive protein, are also associated with the pathology of AD and can serve as potential biomarkers for the detection of AD. Aβ deposits constantly contain serum amyloid P component whereas, both serum amyloid P component and D protein are associated with NFTs [86]. Although the role of these pentraxin proteins in the pathogenesis of AD is not completely understood, they still possess the potential to serve as biomarkers for mild cognitive impairment and early-stage AD detection.
2.13. Ubiquilin-1
In humans, ubiquilin-1 is encoded by the UBQLN1 gene over chromosome 9 [87]. Although the relationship of ubiquilin-1 has already been reported in AD, its mechanism is still mysterious. Polymorphisms in the ubiquilin-1 gene (i.e., UBQLN1) may increase the risk of late-onset AD as it is associated with nearly all the major pathological hallmarks of AD, i.e., APP, presenilin, and Aβ accumulation. Moreover, the mutated UBQLN2 gene is reported to supplement amyotrophic lateral sclerosis (ALS) and ALS with frontotemporal lobar dementia. Ubiquilin-1 plays a central role in maintaining neuronal proteostasis as well as mediates the regulation of APP biosynthesis, its trafficking, degradation, and neural toxicity [88, 89]. Ubiquilin-1 also affects BACE1 (β–secretase) activity, thus promotes abnormal processing of APP via the amyloidogenic pathway and accumulation of insoluble Aβ peptides. Overexpression of ubiquilin-1 correlates with the endogenous expression of BACE1, suggesting there mutual sharing of a dependent type of relationship [90]. Presenilin 1 accumulation and aggresome formation are also aided by ubiquilin-1 transcript variant 1 and ubiquilin-1 transcript variant 2. In addition, presenilin potentiates Aβ peptides generation through presenilin-dependent γ-secretase degradation of APP [91]. Ubiquilin-1 is already reported as an associated protein in many AD-related hallmarks, which has a great importance of its further investigation to know about the mechanistic pathways by which ubiquilin-1 affects the pathogenesis and contributes to AD.
2.14. Presenilin
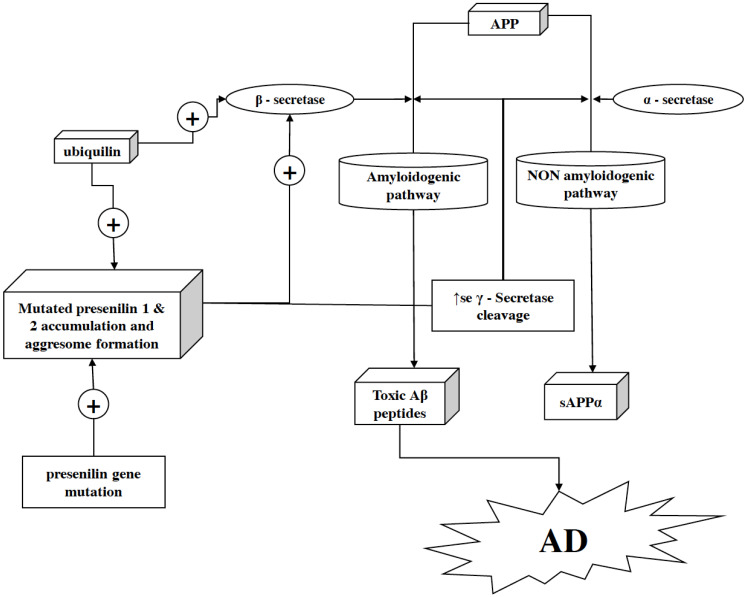
Presenilins belong to the family of transmembrane proteins, which play a crucial role in the pathogenesis of AD [92]. A human possesses two presenilin proteins, i.e., presenilin 1 and presenilin 2 encoded by PSEN1 located on chromosome 14 and PSEN2 positioned over chromosome 1 [93]. Presenilin is associated with the γ-secretase complex activity, Ca2+ signaling, and cellular differentiation/development. Presenilin proteins (i.e., presenilin 1 and presenilin 2) constitute a γ-secretase complex, which aids APP proteolytic cleavage. This also suggests that γ-secretase is essential for Aβ-42 production [94, 95]. In the pathogenesis of familial AD, mutated presenilin 1 and 2 potentiate γ-secretase cleavage of APP, which results in the increased burden of Aβ-42 specifically (Fig. 3). Aβ-42 is more amyloidogenic and toxic than Aβ-40, resulting in its accumulation as senile plaques. Mutation in the PSEN gene upsurges the risk of mutated presenilin protein production and thus increases Aβ-42 inside the brain. Whereby depletion of presenilin 1 and presenilin 2 mediated γ-secretase cleavage of APP aids reduction in the production of Aβ endogenously [96]. Conclusively, discovering new anti-AD therapies targeting presenilins or therapies promoting the inhibition of γ-secretase activity can be an effective approach for treating AD. However in clinical trials, γ-secretase inhibitors such as avagacestat and semagacestat showed dose dependent alterations in Aβ isoforms and CSF biomarkers of AD but, because of their narrow therapeutic window, larger doses cause an upsurge in the rate of skin cancers, diarrhoea, nausea, rashes etc., which at last lead to termination of their respective clinical trials [211].
Fig. (3).
Role of presenilin in neurodegeneration. Presenilin proteins, i.e., presenilin 1 and presenilin 2 (along with other proteins), are structural units of γ-secretase complex. In the γ-secretase protein complex, presenilin acts as an active site after being cleaved into an N- and C-terminal fragment. Moreover, it helps the γ-secretase complex to exert its activity by letting the substrate pass through the hydrophilic pocket in the membrane formed by two presenilin fragments. Within the hydrophobic environment of the plasma membrane, γ-secretase cleaves the C-terminal fragment of APP after it gets cleaved by α- or β-secretase (in non-amyloidogenic and amyloidogenic pathways, respectively) to release the APP’s cytoplasmic domain. As a key factor in γ-secretase activity, the mutation in presenilin can facilitate overactivation of the amyloidogenic pathway and as a result, the production of Aβ–42 gets enhanced in the brain. In addition, ubiquilin-1 fuels this pathology more aggressively as it facilitates the presenilin 1 accumulation and aggresome formation by ubiquilin-1 transcript variant 1 and ubiquilin-1 transcript variant 2. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.15. SORLA
SORLA or sortilin-related receptor is a neuronal receptor primarily expressed in human CNS and encodes by gene SORLA1. SORLA is a type 1 membrane protein [97]. SORLA persists an interaction with low-density lipoprotein receptor-related protein (LRP) within the perinuclear compartments of neurons. Both SORLA and LRP potentiate Aβ generation as well as affect APP trafficking [98, 99]. SORLA plays a crucial role in calpain mediated proteolytic degradation of synapsins and, thus, potentiating impaired regulation of neurotransmitters release at synapsis, resulting in various CNS problems, including neurodegeneration [100]. Furthermore, the impaired function of SORLA prevents its binding with APP and thus its intracellular trafficking, which averts its deviation from amyloidogenic processing. This dysregulation of APP intracellular trafficking then fuels amyloidogenic processing and generation of Aβ [101]. Although SORLA is found to be abundant in neurons of the hippocampus, brain stem, and Purkinje fibers, its amount is relatively low in neurons of thalamus and hypothalamus [102]. As SORLA is present in few most crucial sites inside the brain, its dysregulation and impaired functioning can cause immense amelioration in the regulation of APP. This then can increase the amyloidogenic cleavage of APP and generate neurotoxic Aβ peptides. Its direct interaction with the key factor of AD makes it an important protein to investigate further and find out its possibility of becoming a target for an anti-AD therapy.
2.16. Alpha-synuclein
Alpha-synuclein is a protein encoded by the SNCA gene in humans located over chromosome 4 [103]. Alpha-synuclein facilitates the release of neurotransmitters from presynaptic terminals by binding to some specific presynaptic proteins and thus, helping in neurotransmission in the CNS [104, 105]. Aggregation of hyperphosphorylated alpha-synuclein in neuronal cytoplasm in the form of insoluble fibrils, i.e., Lewy bodies, potentiates neurodegeneration. Parkinson’s disease, dementia with Lewy bodies, and familial AD are some of the neurodegenerative diseases, which possess the existence of Lewy bodies [106]. CSF alpha-synuclein is found to exhibit a direct association with the AD pathology and potentiate the fibrilization of tau protein and Aβ [107]. Conclusively, this suggests that the presence of alpha-synuclein in CSF represents as an AD biomarker and could be a reliable diagnostic strategy [108, 109]. Aggregation of alpha-synuclein is potentiated by ganglioside lipids, however, extracellular chaperones reduce the membrane permeability of the alpha-synuclein oligomers and thus, reducing alpha-synuclein-mediated oxidative stress and neurotoxicity [110, 111]. In addition, alpha-synuclein influences the ApoE4 and tau-mediated pathogenesis of AD and acts as an enhancer [112, 113]. Having a direct influence on various neurodegenerative diseases and participation in the pathogenesis of AD, alpha-synuclein could be a potential target for developing new strategies against AD as well as for its diagnosis.
2.17. Tau
Hyperphosphorylation of tau via various factors results in the disruption of a neuronal skeleton, as it helps in stabilizing and binding microtubules in the axon. Tau presents abundantly in neurons and is encoded by the MAPT gene located over chromosome 17 [114, 115]. Recent studies suggest that reduced aerobic glycolysis with aging impairs neuroplasticity and neuroprotection, causing potentiation in tau pathology [116]. Cancerous Inhibitor of Protein phosphatase 2A (CIP2A) plays a crucial role in the hyperphosphorylation of the tau protein via inhibiting the expression of Protein phosphatase 2A and represents a possible target for halting tauopathy [117]. Moreover, the CDK5 regulatory subunit associated protein 2 gene (CDK5RAP2) also correlates with tauopathy as it helps in the phosphorylation of the tau protein. Amplified expression of the CDK5RAP2 gene potentiates the hyperphosphorylation of tau [118]. In addition, the presence of Aβ peptides, microglial activation, and thereafter inflammation correlate with each other, whereby this correlation does not coexist with tauopathy, suggesting the independent nature of tauopathy from inflammation [119]. Moreover, tau gathering and distribution in the entorhinal cortex of AD-affected brain precede neocortical deposition of Aβ peptides but, the presence of Aβ is essential for potentiation of substantial tau accumulation [120]. A study revealing the disruption of the nuclear pore complex via direct interaction of tau and nucleoporins advocates malfunctioning of nucleocytoplasmic transport by tau protein, which, in turn, leads into neuronal death and NFTs in AD [121]. Although tau serves as a factor of AD progression, it could also be used in diagnostic purposes by utilizing the ability of plasma pTau181 as an effective predictor towards Aβ presence in the brain [122]. Tau proteins also exist in saliva and salivary glands, but they cannot be utilized as an AD marker because of their lack of correlation with CSF tau levels [123]. Tau is one of the major hallmarks as well as an inducing factor of AD pathology in the human brain. Unfortunately, just a single anti-tau agent, i.e., TRx0237 (LMTX) managed to reach phase III clinical trial, however, the primary examination for this study also exhibits negative results. Besides this, one more agent ANAVEX2-73 with multiple mechanisms of actions, i.e., anti-tau, anti-amyloid, and anti-inflammatory is also getting assessed in phase III clinical trial. Moreover, a total of 10 agents are getting assessed in phase II clinical trial where 2 agents are targeting both tau and amyloid protein. These agents possess a mechanism of action as anti-tau aggregation, immunotherapeutic action, and MAPT RNA inhibition [20]. Tauopathy has a major pathological role in the progression of AD, although various agents are getting assessed in the clinical trials, none of them have been approved as an effective therapy. This suggests an immense need for investigation in the progression of AD via tauopathy.
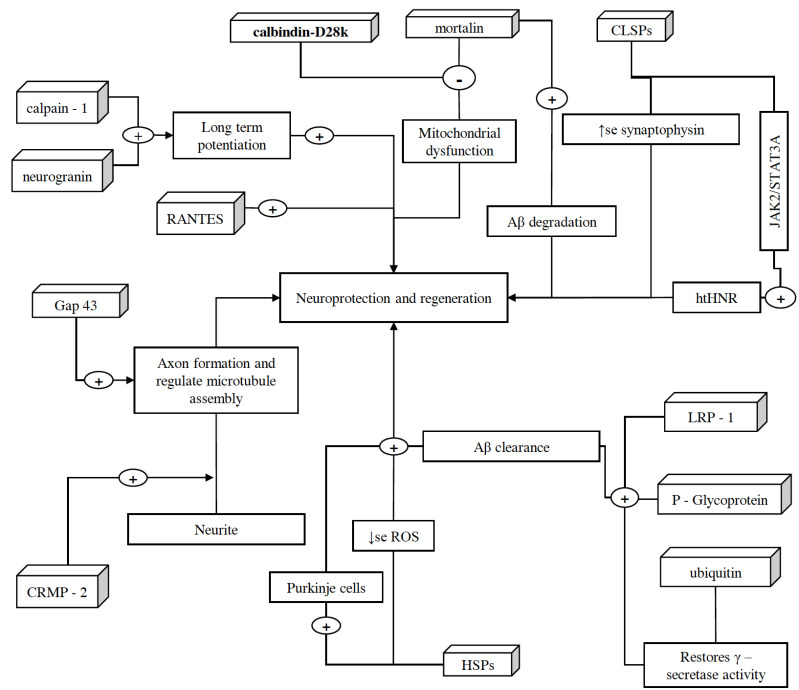
3. Proteins associated with neuro- protection and regeneration [Fig. 4]
Fig. (4).
Schematic representation of proteins associated with neuroprotection and regeneration. These proteins help in the growth, regulation, and protection of neurons. CRMP-2, after getting phosphorylated by Rho-kinase and glycogen synthase, assists axon development, and binds/regulate microtubules assembly, respectively. Along with CRMP-2, GAP-43 also contributes to the growth of axons and modulates the formation of new connections. However, because of high lipid content and oxygen consumption in the brain, there is an increased amount of oxidative stress. In order to counteract this situation, brain expresses HSPs in different regions of CNS such as cortical region (especially in astrocytes). These HSPs also ameliorate the decline in Purkinje cells of the brain and halt neurodegeneration. CLSP exhibits neuroprotection by interacting with htHNR and inhibiting the loss of synaptophysin, which indeed facilitate detoxification and synaptic transmission via playing a crucial role in the biogenesis of secretory vesicles and prompting the targeting of VMATs towards these vesicles. In addition, calpain-1 also plays an important role in the initiation of LTP. On the other hand, ubiquitin helps in restoring the γ-secretase activity, clearing denatured protein fragments and insoluble aggregates such as Aβ plaques and NFTs via ubiquitin-dependent protein degradation system. P-glycoprotein and LRP-1, along with SORLA, also assist Aβ clearance and facilitate its transportation across the BBB. However, Aβ mediated microglia activation and therefore the expression of TNF-α, IL-1β, and NF-κB cause reduction in the expression of P-glycoprotein, which later is halted by Mortalin overexpression, as it extenuates Aβ mediated cell damage and apoptosis via inhibiting the mPTP, caspase-3 activation, and release of pro-apoptotic factor cytochrome C. Moreover, overexpressed mortalin, along with calbindin-D28k, regulates intracellular calcium concentrations, which help in reducing the oxidative stress and glutamate neurotoxicity. Moreover, increased RANTES expression is associated with the activation of PI-3K and MAPK signaling pathways and this phenomenon exhibits neuroprotection. However, the role of neurogranin in neuroprotection is yet to be discovered, it is believed to be facilitating the hippocampal synaptic plasticity and spatial learning. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.1. Calmodulin-like Skin Protein
Calmodulin-like skin protein (CLSP) is a 146 residue (15.92 kDa) protein that shares 49% of identity with Calmodulin. CLSP is secreted only in the epithelial cells of the thyroid, mammary gland, prostate, kidney, skin and barely in the brain [124, 125]. Heterotrimeric Humanin receptor (htHNR), comprised of ciliary neurotrophic factor receptor α, cytokine receptor WSX-1, and Glycoprotein 130, shows a neuroprotective effect after interacting with humanin [126, 127]. Humanin itself is not a potent agonist of htHNR and has a maximal effective concentration (EC50) of 1–10 µM. Humanin is also expressed poorly in CNS. In comparison to humanin, CLSP secreted by skin keratinocytes shows as a potent htHNR agonist with an EC50 of 10–100 µM against AD-related neuronal death, which suggests that CLSP is 104–105 times more effective than humanin [128]. CLSP-mediated neuroprotection and amelioration of memory impairment are independent of Aβ. CLSP neither affects levels of Aβ, soluble Aβ oligomers, or gliosis. Moreover, CLSP inhibits the loss of synaptic marker synaptophysin and inactivation of STAT3 in the hippocampus. Moreover, a gene expressing CLSP, i.e., CLSP-1, also helps in reducing spatial learning impairment, loss of presynaptic marker synaptophysin, and activates the JAK2/STAT3-mediated pro-survival signaling pathway in neurons via htHNR [128]. CLSP transports across the CNS by crossing the blood-brain barrier and blood-CSF barrier efficiently [129]. Thus having an indirect association with the AD pathogenesis, CLSP shows a promising future of alternative therapy to reduce the pace of neuronal degeneration in the early stages of AD.
3.2. Heat Shock Proteins
Heat shock proteins (HSPs) are produced by the cells to counter stressful conditions like exposure to heat shock, cold, UV light, and during wound healing or tissue remodeling and serve as healers. HSPs are termed according to their molecular weight, e.g., Hsp60, Hsp70 and Hsp90, are most studied HSPs [130-133]. The brain consists of high lipid content and consumes a high amount of oxygen, which make it susceptible to oxidative stress. Moreover, increased protein oxidation levels are observed in the senile plaque-dense regions of the AD brain [134, 135]. One of the various mechanisms of the brain to counteract oxidative stress is to express the HSPs. Patients carrying one or two copies of the HSP70-2 A2 allele, which is responsible for the expression of HSP70-2 protein, show noncognitive alterations in AD [136]. The expression of HSP27 is also found to be significantly increased in the cortex, especially in astrocytes of the AD brain [137, 138]. In AD, expression levels of HSP60, HSP70, and HSP90 used to get downregulated in the cerebellum of the brain. Furthermore, heat shock factor 1 (HSF1), a key transcription factor responsible for the expression of HSPs genes, also gets significantly reduced in the cerebellum. Although, increased expression of HSF1 has a contribution in ameliorating the decline in Purkinje cells of the brain, which got significantly reduced in AD [139]. Thus having an association of these HSPs in amelioration of oxidative stress, which indeed is one of the key factors involved in AD pathogenesis, they may act as new targets to prevent early symptoms as well as AD pathogenesis. Moreover, amplification in the expression of HSF1 can serve as a new therapy.
3.3. Collapsin Response Mediator Protein 2
Collapsin response mediator protein 2 (CRMP-2) is a phosphoprotein which belongs to the collapsin response mediator family. CRMP-2 possesses almost similar size (60–66 kDa) and identical amino acid sequences (50–70%) with CRMP-1, CRMP-3, CRMP-4, and CRMP-5. CRMP-2 assists the development of axon form neurites and is present abundantly in the early stages. CRMP-2 induces growth cone collapse after being phosphorylated by Rho-kinase. CRMP-2 also binds and regulates the microtubules assembly, which is regulated by its phosphorylation via glycogen synthase [140]. Although CRMP-2 plays a crucial role in axonal growth, its hyperphosphorylation at Ser522 (Cdk5-mediated) exists in AD. In addition, hyperphosphorylation of CRMP-2 represents a very early event in the AD and can be served as a valuable marker for diagnostic purposes as well as the
generation of new anti-AD therapy, which could possibly inhibit the pathology of AD in its early stages [141].
3.4. LDL Receptor-related Protein 1
Low-density lipoprotein receptor-related protein 1 (LRP1) (also termed as alpha-2-macroglobulin receptor (A2MR) and apolipoprotein E receptor (APOER)) mediated transport helps the Blood-Brain Barrier (BBB) in removing the Aβ from the brain [142, 143]. LRP1 also helps in regulating the ApoE and Cholesterol Metabolism inside the brain via APP. As the metabolism of both ApoE and cholesterol is linked with the AD, LRP1 helps in their physiologic processing [144]. Association of LRP1 has already been reported with ApoE, alpha-2-macroglobulin, APP, and presenilins, which are some of the major influencing factors in AD. Mutated presenilin-1 expression affects the expression of LRP1 [145]. Although LRP1 helps in transporting Aβ outside the brain, Aβ oxidizes the LRP1, alters this pathway, and initiates further deposition of its own in the brain [146]. As LRP1 interactions are already known with various AD-related factors, their further investigation is strongly needed, so this may also serve as a possible future target for developing anti-AD therapies.
3.5. Mortalin
Mortalin is an anciently conserved mitochondrial heat shock chaperone protein having a molecular weight of 74 kDa and in humans, it is encoded by the HSPA9 gene. Mortalin is also known as mtHsp70, PBP74, and GRP75 [147-149]. Mortalin interacts with different proteins, e.g., APP, ApoE, Fibroblast growth factor 1, HSP60, p53, 94 kDa glucose-regulated protein, and Protein Dj-1, which in turn, influences its different activities, i.e., cell survival, control of cell proliferation, mitochondrial biogenesis, intracellular protein trafficking, and stress response [150]. Mortalin isoforms are regulated by the APOE genotype and get differentially expressed, and phosphorylate in the hippocampus of patients with AD as a cellular defense mechanism to oxidative stress [151]. Mortalin overexpression reduces Aβ induced cell damage and apoptosis as well as protects the cell from Aβ induced neurotoxicity. Furthermore, mortalin overexpression helps in reducing Aβ induced mitochondrial dysfunction [152]. This reduction of Aβ induced neurotoxicity after mortalin overexpression occurs because of the inhibition of mitochondrial permeability transition pore opening (mPTP). Mortalin induced mPTP inhibition results in attenuation of Aβ induced caspase-3 activation and release of pro-apoptotic factor Cytochrome C. Moreover, overexpressed mortalin also attenuates intracellular calcium concentration and oxidative stress, which, in turn, prevents the cell from toxicity and degeneration [153]. Although mortalin overexpression attenuates Aβ induced neurotoxicity, its suppressed expression results in Aβ induced oxidative stress and apoptosis, which ultimately lead to neurodegeneration. Mortalin and its activities are directly associated with AD pathology and further studies can possibly yield a potential anti AD therapy.
3.6. Neurogranin
Neurogranin is an important protein expressed primarily in dendritic spines of the brain. It is a calmodulin-binding protein and helps in long-term potentiation (LTP) and learning abilities through Ca2+/calmodulin dependent signal transduction in neurons [154, 155]. Neurogranin is a postsynaptic protein and involved in neuroplasticity. The presence of 15 neurogranin peptides has already been reported in CSF as short C-terminal peptide species. All these neurogranin peptides can be quantified and used as synaptic biomarkers in AD [156-158]. Although neurogranin is reported as an important biomarker of injuries, it also indicates the repair and regeneration of neurons and dendrites [159]. Moreover, Huang et al., 2004, reported the deficits in hippocampal synaptic plasticity and spatial learning impairments in mice lacking the neurogranin gene [160]. This suggests that neurogranin plays a major role in memory through neuroplasticity and, detection of its increased CSF concentration can help in the early detection of AD as there are several studies supporting it as a potential biomarker for AD.
3.7. P-glycoprotein
P-glycoprotein (permeability glycoprotein) belongs to the ATP-binding cassette (ABC) super-families and encoded by gene ABCB1 situated at chromosome 7q36. P-glycoprotein is widely distributed in the intestine, liver, kidney, and brain. In the brain, it is present in capillary endothelial cells of the Blood-Brain Barrier [161-163]. P-glycoprotein helps in clearing Aβ-peptides from the brain through the BBB by acting as an efflux pump. Initially, Danielle M et al., 2011, reported diminished P-glycoprotein activity because of faulty BBB activity in sporadic AD [163, 164]. Aβ mediated microglia activation results in amplified expression of inflammatory mediators like TNF-α, IL-1β, and NF-κB, which facilitate the reduction in expression and functioning of P-glycoprotein, though its mechanism is still mysterious [165]. Upregulation in the P-glycoprotein expression can possibly accelerate the Aβ clearance and thus, reducing the pathogenic levels of the Aβ peptides [166]. Whereas, its downregulation can potentiate Aβ mediated neurodegeneration in AD [167]. P-glycoprotein plays a crucial role in preventing Aβ generated neurotoxicity and therefore, therapy targeting either its reduced expression or factors liable for the reduction in its expression, which could be considered for treating AD.
3.8. Ubiquitin
Ubiquitin is a small and highly conserved protein (8.6 kDa) encoded by UBB, UBC, UBA52, and RPS27A genes in humans. This protein is found in most of the body tissues [168]. Wang et al., 1991, firstly reported a significant upsurge in the levels of ubiquitin along with the increased hyperphosphorylated tau in the cerebral cortex of the brain having AD, suggesting a correlation between ubiquitin and NFTs [169]. Recent studies also report its crucial role in partially restoring the γ-secretase activity and clearing the Aβ burden in the brain [170]. Denatured protein fragments and insoluble aggregates such as Aβ plaques and NFTs are cleared by the help of the ubiquitin-dependent protein degradation system. A disturbed ubiquitin system may cause failure in reducing insoluble pathogenic fragments burden in the brain, which ultimately leads to neurotoxicity and pathogenesis of AD [171]. Having a role in clearance and prevention of neurodegeneration via pathogenic factors, ubiquitin may prove as a target for developing anti-AD treatments.
3.9. Insulin and Insulin-like Growth Factor-I
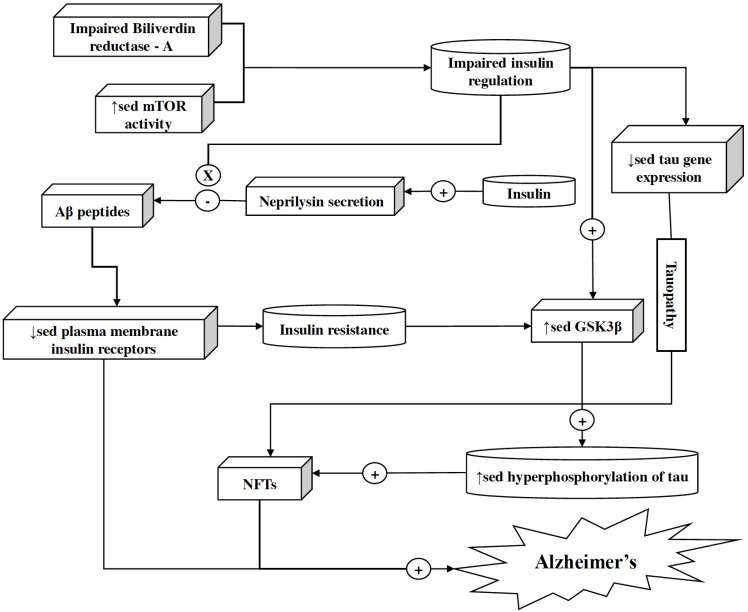
Reduced consumption of glucose and reduced energy metabolism appear early in AD. These mechanisms are mediated by insulin and insulin-like growth factor-I & II (IGF-I & IGF-II). Impairment in the signaling mediated by insulin and IGFs accompanied by reduced energy metabolism cause serious abnormalities [172-174]. Accumulation of Aβ oligomers is related to insulin resistance, although molecular mechanisms underlying this association are still partially known. However, impairment of Biliverdin reductase-A (which is a unique Ser/Thr/Tyr kinase responsible for the regulation of insulin signaling) and increased activity of mammalian target of rapamycin (mTOR) may serve as a link for an association between brain insulin resistance and Aβ accumulation (Fig. 5). Aβ oligomers mediate the downregulation of plasma membrane insulin receptors (IRs) via mechanism sensitive to calcium Calmodulin-dependent kinase II and casein kinase II inhibition. Moreover, this downregulation of surface IRs can be prevented by insulin [175-177]. Insulin helps in Aβ degradation by increasing neprilysin secretion and insulin-degrading enzyme expression in astrocytes, via activating the ERK-mediated pathway [178]. Insulin and IGF stimulation regulate tau gene expression and phosphorylation. Thus, insulin resistance results in reduced tau gene expression causing tauopathy. Insulin resistance also results in the overactivation of glycogen synthase kinase-3β, i.e., GSK-3β, which is partially responsible for the hyperphosphorylation of Tau, resulting in neurofibrillary tangles [179-182]. Reduction in the IGF-I level relates to mild cognitive impairment [183, 184]. IGF-I signaling is essential to regulate astrocytic mitochondrial functioning as well as coordinate hippocampal-dependent spatial learning. Age-related astrocytic dysfunction by reduced IGF-I signaling may lead to AD [185]. Currently, various agents such as Insulin glulisine, Dapagliflozin, and Insulin aspart are getting assessed in different phases of clinical trials. These agents are based on the mechanism of action, i.e., increasing insulin signaling in the brain to ameliorate cognitive deficits [20]. Thus elevating insulin and IGF levels inside the brain can be beneficial in attenuating the risk of AD and may provide promising therapy.
Fig. (5).
Role of insulin in neuroprotection. Insulin helps in halting Aβ degradation by increasing neprilysin secretion and expression of insulin-degrading enzyme in astrocytes via the activation of the ERK-mediated pathway. However, impaired Biliverdin reductase-A, increased mTOR activity, and Aβ accumulation in the brain result in impaired insulin regulation and downregulation of plasma membrane insulin receptors, respectively. Insulin resistance and impaired insulin regulation also result in overactivation of GSK-3β, which ultimately takes part in the hyperphosphorylation of tau protein and generation of NFTs. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.10. Calbindin-D28K, Calretinin, and Parvalbumin
Calbindin-D28K, calretinin, and parvalbumin are three calcium-binding proteins (CBPs). Though calcium is vital for brain growth and its normal functioning, its sustained high concentration in the brain can cause neuronal toxicity and cell apoptosis [186]. These CBPs play a role in calcium buffer and their failure in calcium regulation can promote various neurodegenerative disorders [187]. Calbindin-D28K and calretinin possess 58% similarity in their homology, RNAs of both of these proteins, i.e., calbindin-D28K and calretinin, are present abundantly in the retina and in many areas of the brain, whereas calretinin RNA is present mostly in neural tissues and particularly present in auditory neurons. Calbindin-D28K interacts with a number of proteins, including IMPase, RanBPM, caspase-3, 3’: 5’-cyclic nucleotide phosphodiesterase and the plasma membrane ATPase and affects their activity [188-190]. Calbindin-D28k protects neurons from glutamate neurotoxicity by reducing the intracellular calcium levels [191]. Specific and significant reduction in gene expression of the calbindin-D28K in aging and neurodegenerative diseases results in the failure of calcium buffering or intraneuronal calcium homeostasis, which causes calcium-mediated cytotoxic events [192]. Neurons with different calcium-binding proteins are subjected to differential susceptibility in AD. In mild cases of AD, parvalbumin and calbidin-D28K containing neurons undergo degeneration in layer II of the lateral, intermediate and caudal subfields, and layer III in the most severe cases of AD. Calretinin neurons of the entorhinal cortex are not that prone to degeneration in AD as compared to parvalbumin-containing neurons [193]. These calcium binding proteins partially have an indirect association with AD pathology and by regulating the intracellular calcium, a certain degree of the halt in disease progression can be achieved [194]. Loss of calretinin and parvalbumin-positive interneurons might be related to the age-dependent progression of AD [195]. Thus a new therapy can be established for restoring the functionality of these CBPs to slow down the pathology of the AD in earlier days.
4. Other proteins associated with Alzheimer’s disease
4.1. RANTES
RANTES (regulated on activation, normal T cell expressed and secreted) or Chemokine (C-C motif) ligand 5 (CCL5) is a chemokine protein encoded by the CCL5 gene located over chromosome 17qll-q12 and 5q31-q34 [196]. Although RANTES, with its respective G protein-coupled receptors, resides and facilitates inflammation in endothelial cells, glia, and neurons in nearly the entire brain; it also plays a crucial role in the survival and differentiation of the neurons [197, 198]. RANTES is a neuroprotective peptide that helps in the development and regeneration of neurons. Expression of RANTES is arbitrated by Pituitary adenylate cyclase activating polypeptide (PACAP) and thus, this intracellular signaling pathway displays neuroprotective effect [199]. Increased RANTES expression is associated with the activation of PI-3K and MAPK signaling pathways and this phenomenon exhibits neuroprotection [200]. By presenting double functions, i.e., supplementing neuroprotection and lessening inflammatory initiation, RANTES works as a chemokine and thus, alterations in its brain concentration levels can be examined for identifying early signs of AD pathology [201, 202].
4.2. Growth-associated Protein 43
Growth-associated protein 43 (Gap43) is an intracellular membrane-bound presynaptic protein encoded by the GAP43 gene positioned in the nervous tissue. Gap43 shows its role in the synaptic transmission, membrane permeability, neuronal growth, and sprouting by stabilizing actin filaments. GAP-43 contributes to the growth of axons and modulates the formation of new connections [203, 204]. Although moderate overexpression of Gap43 helps in memory enhancement, its excessive overexpression can cause a neuroplasticity burden and can cause neurodegeneration [205]. A rise in the CSF levels of Gap43 occurs with an upsurge in NFTs levels. Therefore, pathogenesis, responsible for the formation of NFTs, has a great impact on the Gap43 expression in the hippocampus, amygdala, and cortex. This upsurge in the levels of Gap43 can help in the early diagnosis of AD and, thus can aid delay in the commencement of its symptoms [206].
4.3. Calpain
Calpain is an intracellular Ca2+-dependent protein present in the cytosol as the inactive proenzyme. The recent extension of sequence data across the species shows that calpain has been present throughout evolution; calpains are found in almost all eukaryotes and some bacteria, but not in archaebacteria. Calpain like protease is encoded by more than one gene (specifically fifteen genes) in humans. A small number of human calpains, predominantly those having non-classical domain structures, are very much identical to calpain homologs acknowledged in evolutionarily distant organisms [207]. Calpains are found to exhibit opposite functions in both synaptic plasticity and neuronal degeneration. Activation of calpain-1 is essential for the initiation of LTP and in general, it is neuroprotective, while calpain-2 activation puts restrictions to the extent of potentiation and takes part in neurodegeneration. This difference in functionality is related to their interaction with different PDZ-binding proteins, resulting in differential subcellular localization, and offers new therapeutic opportunities [208]. Calpain-induced protein degradation is supplemented by calpastatin in the brain, which suggests a role of calpastatin in reducing the AD pathology. Promoting calpastatin expression may be used to ameliorate some manifestations of AD [209]. Sustained activation of protein kinase C is required to underlie persistent changes associated with memory formation. Reduced levels of calpains influence PKCs activity, which may affect cellular mechanisms during memory processes [210]. Accumulation of Aβ peptide is facilitated by overactivation of μ-calpain via supplementing the expression level of β-secretases. Moreover, it also induces hyperphosphorylation of tau and establishment of NFTs by mediating p35 cleavage into p25, both of which are the key mechanisms of neurodegeneration AD.
Conclusion
In this review, we have discussed and summarized our findings about the physiological/pathological roles and therapeutic significance of nearly all the proteins associated with AD, which address putative as well as probable targets for developing effective anti-AD therapies. AD consists of a complex pathology which includes disturbed functioning of proteins that are associated with oxidative stress, immune response, and neurotransmission. Insufficient knowledge about AD is allocating hindrance in the development of its effective therapies. Despite the failure of existing therapies, numerous noble compounds are going through distinct phases of clinical trials targeting Aβ removal, Aβ degradation, preventing Aβ toxicity, and tau hyperphosphorylation, aggregation, etc. Literature suggests that there is an urgent need to explore proteins like HSPs, calpains and CLSP in AD as these could be promising future targets for developing anti-AD therapies while C-reactive protein, pentraxins, collapsin response mediator protein-2, and growth-associated protein-43 represent the future of new possible biomarkers for diagnosing AD. The present comprehensive review of AD-associated proteins highlights nearly all the proteins that could serve as targets for developing new anti-AD therapies as well as biomarkers in diagnosing early symptoms of AD to deaccelerate its growth. Approach to target these proteins for the diagnosis and treatment of AD could be a novel and viable therapeutic or disease modifying strategy in the future.
Acknowledgements
Declared none.
list of Abbreviations
- ABC
ATP-binding cassette
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- ApoE
Apolipoprotein E
- APP
Amyloid precursor protein
- Aβ
Amyloid-beta
- BACE1
Beta-site amyloid precursor protein cleaving enzyme 1
- BBB
Blood Brain Barrier
- CBPs
Calcium-binding proteins
- CCR2
C-C chemokine receptor type 2
- CDK5RAP2
CDK5 regulatory subunit associated protein 2
- CIP2A
Cancerous Inhibitor of Protein phosphatase 2A
- CLSP
Calmodulin-like skin protein
- CNS
Central nervous system
- CRMP-2
Collapsin response mediator protein 2
- CSPGs
Chondroitin sulfate proteoglycans
- ERK1 ⁄ 2
Extracellular signal-regulated kinase1/2
- Gap43
Growth-associated protein 43
- GFAP
Glial fibrillary acidic protein
- GPCRs
G protein-coupled receptors
- HSPs
Heat shock proteins
- htHNR
Heterotrimeric Humanin receptors
- IGF-I & IGF-II
Insulin-like growth factor-I & II
- ILs
Interleukin
- IRs
Insulin receptors
- JAK2
Janus kinase 2
- LRP
Low-density lipoprotein receptor-related protein
- LRP1
Low-density lipoprotein receptor-related protein 1
- LTP
Long-term potentiation
- MAP2C
Microtubule-associated protein 2
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemoattractant protein 1
- mPTP
Permeability transition pore opening
- NFTs
Neurofibrillary tangles
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NP1
Neuronal pentraxin
- NPR
Neuronal pentraxin receptor
- PI-3K
Phosphoinositide 3-kinase
- PKA
Protein kinase A
- PNNs
Perineuronal nets
- PrPC
The prion protein
- RANTES
Regulated on activation normal T cell expressed and secreted
- SNAP
Synaptosomal nerve-associated protein
- STAT3
Signal transducer and activator of transcription 3
- TNF-α
Tumor necrosis factor alpha
- VMATs
Vesicular monoamine transporters
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S., Van der Flier W.M. Alzheimer’s disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization and Alzheimer’s Disease International Dementia: a public health priority. 2012.
- 3.WHO Dementia Fact sheet. 2017 https://www.who.int/en/news-room/fact-sheets/detail/dementia
- 4.Alzheimer’s & Related Disorders Society of India . The Dementia India Report: prevalence, impact, costs and services for Dementia: Executive Summary. New Delhi: ARDSI; 2010. [Google Scholar]
- 5.Alzheimer’s Association 2018 Alzheimer’s disease Facts and Figures. Alzheimers Dement. 2018;14:367–429. doi: 10.1016/j.jalz.2018.02.001. [DOI] [Google Scholar]
- 6.Prince M., Wimo A., Guerchet M., Ali G.C., Wu Y.T., Prina M. 2015. [Google Scholar]
- 7.Patterson C. The State of the Art of Dementia Research: new frontiers. 2018. [Google Scholar]
- 8.Schultz S.A., Gordon B.A., Mishra S., Su Y., Perrin R.J., Cairns N.J., Morris J.C., Ances B.M., Benzinger T.L.S. Widespread distribution of tauopathy in preclinical Alzheimer’s disease. Neurobiol. Aging. 2018;72:177–185. doi: 10.1016/j.neurobiolaging.2018.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du X., Wang X., Geng M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018;7:2. doi: 10.1186/s40035-018-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings J., Aisen P.S., DuBois B., Frölich L., Jack C.R., Jr, Jones R.W., Morris J.C., Raskin J., Dowsett S.A., Scheltens P. Drug development in Alzheimer’s disease: the path to 2025. Alzheimers Res. Ther. 2016;8:39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maccarrone M., Totaro A., Leuti A., Giacovazzo G., Scipioni L., Mango D., Coccurello R., Nisticò R., Oddi S. Early alteration of distribution and activity of hippocampal type-1 cannabinoid receptor in Alzheimer’s disease-like mice overexpressing the human mutant amyloid precursor protein. Pharmacol. Res. 2018;130:366–373. doi: 10.1016/j.phrs.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Brier M.R., Gordon B., Friedrichsen K., McCarthy J., Stern A., Christensen J., Owen C., Aldea P., Su Y., Hassenstab J., Cairns N.J., Holtzman D.M., Fagan A.M., Morris J.C., Benzinger T.L., Ances B.M. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci. Transl. Med. 2016;8(338):338ra66. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmadi S., Ebralidze I., She Z., Kraatz H.B. Electrochemical studies of tau protein-iron interactions − potential implications for Alzheimer’s disease. Electrochim. Acta. 2017;236:384–393. doi: 10.1016/j.electacta.2017.03.175. [DOI] [Google Scholar]
- 14.Hashimoto Y., Nawa M., Kurita M., Tokizawa M., Iwamatsu A., Matsuoka M. Secreted calmodulin-like skin protein inhibits neuronal death in cell-based Alzheimer’s disease models via the heterotrimeric Humanin receptor. Cell Death Dis. 2013;4:e555. doi: 10.1038/cddis.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toda T., Noda Y., Ito G., Maeda M., Shimizu T. Presenilin-2 mutation causes early amyloid accumulation and memory impairment in a transgenic mouse model of Alzheimer’s disease. J. Biomed. Biotechnol. 2011;2011:617974. doi: 10.1155/2011/617974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abad M.A., Enguita M., DeGregorio-Rocasolano N., Ferrer I., Trullas R. Neuronal pentraxin 1 contributes to the neuronal damage evoked by amyloid-beta and is overexpressed in dystrophic neurites in Alzheimer’s brain. J. Neurosci. 2006;26(49):12735–12747. doi: 10.1523/JNEUROSCI.0575-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiltunen M., Lu A., Thomas A.V., Romano D.M., Kim M., Jones P.B., Xie Z., Kounnas M.Z., Wagner S.L., Berezovska O., Hyman B.T., Tesco G., Bertram L., Tanzi R.E. Ubiquilin 1 modulates amyloid precursor protein trafficking and Abeta secretion. J. Biol. Chem. 2006;281(43):32240–32253. doi: 10.1074/jbc.M603106200. [DOI] [PubMed] [Google Scholar]
- 18.Scarpini E., Scheltens P., Feldman H. Treatment of Alzheimer’s disease: current status and new perspectives. Lancet Neurol. 2003;2(9):539–547. doi: 10.1016/S1474-4422(03)00502-7. [DOI] [PubMed] [Google Scholar]
- 19.Alzheimer’s Association Medications for Memory. 2018 https://alz.org/alzheimers-dementia/treatments/medications-for-memory
- 20.Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. (N. Y.) 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priller C., Bauer T., Mitteregger G., Krebs B., Kretzschmar H.A., Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J. Neurosci. 2006;26(27):7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tharp W.G., Sarkar I.N. Origins of amyloid-β. BMC Genomics. 2013;14:290. doi: 10.1186/1471-2164-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanden Dries V., Stygelbout V., Pierrot N., Yilmaz Z., Suain V., De Decker R., Buée L., Octave J.N., Brion J.P., Leroy K. Amyloid precursor protein reduction enhances the formation of neurofibrillary tangles in a mutant tau transgenic mouse model. Neurobiol. Aging. 2017;55:202–212. doi: 10.1016/j.neurobiolaging.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Zheng H., Koo E.H. Biology and pathophysiology of the amyloid precursor protein. Mol. Neurodegener. 2011;6(1):27. doi: 10.1186/1750-1326-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho H., Lee H.S., Choi J.Y., Lee J.H., Ryu Y.H., Lee M.S., Lyoo C.H. Predicted sequence of cortical tau and amyloid-β deposition in Alzheimer disease spectrum. Neurobiol. Aging. 2018;68:76–84. doi: 10.1016/j.neurobiolaging.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Hamley I.W. The amyloid beta peptide: a chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 2012;112(10):5147–5192. doi: 10.1021/cr3000994. [DOI] [PubMed] [Google Scholar]
- 27.Entrez Gene https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=348
- 28.Liu C.C., Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y. Apolipoprotein E and Alzheimer disease. Neurology. 2006;66(2) Suppl. 1:S79–S85. doi: 10.1212/01.wnl.0000192102.41141.9e. [DOI] [PubMed] [Google Scholar]
- 30.Elshourbagy N.A., Liao W.S., Mahley R.W., Taylor J.M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc. Natl. Acad. Sci. USA. 1985;82(1):203–207. doi: 10.1073/pnas.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beffert U., Poirier J. Apolipoprotein E, plaques, tangles and cholinergic dysfunction in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1996;777:166–174. doi: 10.1111/j.1749-6632.1996.tb34415.x. [DOI] [PubMed] [Google Scholar]
- 32.Han S.H., Einstein G., Weisgraber K.H., Strittmatter W.J., Saunders A.M., Pericak-Vance M., Roses A.D., Schmechel D.E. Apolipoprotein E is localized to the cytoplasm of human cortical neurons: a light and electron microscopic study. J. Neuropathol. Exp. Neurol. 1994;53(5):535–544. doi: 10.1097/00005072-199409000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Wang S., Zhang J., Pan T. for Alzheimer’s Disease Neuroimaging Initiative. APOE ε4 is associated with higher levels of CSF SNAP-25 in prodromal Alzheimer’s disease. Neurosci. Lett. 2018;685:109–113. doi: 10.1016/j.neulet.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Nyarko J.N.K., Quartey M.O., Pennington P.R., Heistad R.M., Dea D., Poirier J., Baker G.B., Mousseau D.D. Profiles of β-amyloid peptides and key secretases in brain autopsy samples differ with sex and apoe ε4 status: impact for risk and progression of alzheimer disease. Neuroscience. 2018;373:20–36. doi: 10.1016/j.neuroscience.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Nieznanska H., Bandyszewska M., Surewicz K., Zajkowski T., Surewicz W.K., Nieznanski K. Identification of prion protein-derived peptides of potential use in Alzheimer’s disease therapy. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864(6 Pt A):2143–2153. doi: 10.1016/j.bbadis.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Coitinho A.S., Freitas A.R., Lopes M.H., Hajj G.N., Roesler R., Walz R., Rossato J.I., Cammarota M., Izquierdo I., Martins V.R., Brentani R.R. The interaction between prion protein and laminin modulates memory consolidation. Eur. J. Neurosci. 2006;24(11):3255–3264. doi: 10.1111/j.1460-9568.2006.05156.x. [DOI] [PubMed] [Google Scholar]
- 37.Piccardo P., King D., Brown D., Barron R.M. Variable tau accumulation in murine models with abnormal prion protein deposits. J. Neurol. Sci. 2017;383:142–150. doi: 10.1016/j.jns.2017.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurlimann J., Thorbecke G.J., Hochwald G.M. The liver as the site of C-reactive protein formation. J. Exp. Med. 1966;123(2):365–378. doi: 10.1084/jem.123.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marnell L., Mold C., Du Clos T.W. C-reactive protein: ligands, receptors and role in inflammation. Clin. Immunol. 2005;117(2):104–111. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Del Giudice M., Gangestad S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Kravitz B.A., Corrada M.M., Kawas C.H. Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old. Alzheimers Dement. 2009;5(4):318–323. doi: 10.1016/j.jalz.2009.04.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bi B.T., Lin H.B., Cheng Y.F., Zhou H., Lin T., Zhang M.Z., Li T.J., Xu J.P. Promotion of β-amyloid production by C-reactive protein and its implications in the early pathogenesis of Alzheimer’s disease. Neurochem. Int. 2012;60(3):257–266. doi: 10.1016/j.neuint.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Yang S., Hilton S., Alves J.N., Saksida L.M., Bussey T., Matthews R.T., Kitagawa H., Spillantini M.G., Kwok J.C.F., Fawcett J.W. Antibody recognizing 4-sulfated chondroitin sulfate proteoglycans restores memory in tauopathy-induced neurodegeneration. Neurobiol. Aging. 2017;59:197–209. doi: 10.1016/j.neurobiolaging.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Wesén E., Gallud A., Paul A., Lindberg D.J., Malmberg P., Esbjörner E.K. Cell surface proteoglycan-mediated uptake and accumulation of the Alzheimer’s disease peptide Aβ(1-42). Biochim. Biophys. Acta Biomembr. 2018;1860(11):2204–2214. doi: 10.1016/j.bbamem.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Richard A.D., Tian X.L., El-Saadi M.W., Lu X.H. Erasure of striatal chondroitin sulfate proteoglycan-associated extracellular matrix rescues aging-dependent decline of motor learning. Neurobiol. Aging. 2018;71:61–71. doi: 10.1016/j.neurobiolaging.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Yan H., Zhu X., Xie J., Zhao Y., Liu X. β-amyloid increases neurocan expression through regulating Sox9 in astrocytes: A potential relationship between Sox9 and chondroitin sulfate proteoglycans in Alzheimer’s disease. Brain Res. 2016;1646:377–383. doi: 10.1016/j.brainres.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Rauch U., Feng K., Zhou X.H. Neurocan: a brain chondroitin sulfate proteoglycan. Cell. Mol. Life Sci. 2001;58(12-13):1842–1856. doi: 10.1007/PL00000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hol E.M., Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015;32:121–130. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z., Wang K.K. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38(6):364–374. doi: 10.1016/j.tins.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamphuis W., Middeldorp J., Kooijman L., Sluijs J.A., Kooi E.J., Moeton M., Freriks M., Mizee M.R., Hol E.M. Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiol. Aging. 2014;35(3):492–510. doi: 10.1016/j.neurobiolaging.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 51.Korolainen M.A., Auriola S., Nyman T.A., Alafuzoff I., Pirttilä T. Proteomic analysis of glial fibrillary acidic protein in Alzheimer’s disease and aging brain. Neurobiol. Dis. 2005;20(3):858–870. doi: 10.1016/j.nbd.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Marinissen M.J., Gutkind J.S. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci. 2001;22(7):368–376. doi: 10.1016/S0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 53.Nishimoto I., Okamoto T., Matsuura Y., Takahashi S., Okamoto T., Murayama Y., Ogata E. Alzheimer amyloid protein precursor complexes with brain GTP-binding protein G(o). Nature. 1993;362(6415):75–79. doi: 10.1038/362075a0. [DOI] [PubMed] [Google Scholar]
- 54.Bignante E.A., Ponce N.E., Heredia F., Musso J., Krawczyk M.C., Millán J., Pigino G.F., Inestrosa N.C., Boccia M.M., Lorenzo A. APP/Go protein Gβγ-complex signaling mediates Aβ degeneration and cognitive impairment in Alzheimer’s disease models. Neurobiol. Aging. 2018;64:44–57. doi: 10.1016/j.neurobiolaging.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Brocker C., Thompson D., Matsumoto A., Nebert D.W., Vasiliou V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum. Genomics. 2010;5(1):30–55. doi: 10.1186/1479-7364-5-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheng J.G., Jones R.A., Zhou X.Q., McGinness J.M., Van Eldik L.J., Mrak R.E., Griffin W.S. Interleukin-1 promotion of MAPK-p38 overexpression in experimental animals and in Alzheimer’s disease: potential significance for tau protein phosphorylation. Neurochem. Int. 2001;39(5-6):341–348. doi: 10.1016/S0197-0186(01)00041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mrak R.E., Griffin W.S. Interleukin-1, neuroinflammation, and Alzheimer’s disease. Neurobiol. Aging. 2001;22(6):903–908. doi: 10.1016/S0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 58.Blum-Degen D., Müller T., Kuhn W., Gerlach M., Przuntek H., Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett. 1995;202(1-2):17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 59.Bauer J., Strauss S., Schreiter-Gasser U., Ganter U., Schlegel P., Witt I., Yolk B., Berger M. Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 1991;285(1):111–114. doi: 10.1016/0014-5793(91)80737-N. [DOI] [PubMed] [Google Scholar]
- 60.Bossù P., Ciaramella A., Salani F., Vanni D., Palladino I., Caltagirone C., Scapigliati G. Interleukin-18, from neuroinflammation to Alzheimer’s disease. Curr. Pharm. Des. 2010;16(38):4213–4224. doi: 10.2174/138161210794519147. [DOI] [PubMed] [Google Scholar]
- 61.Kilic U., Elibol B., Uysal O., Kilic E., Yulug B., Sayin Sakul A., Babacan Yildiz G. Specific alterations in the circulating levels of the SIRT1, TLR4, and IL7 proteins in patients with dementia. Exp. Gerontol. 2018;111:203–209. doi: 10.1016/j.exger.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 62.Entrez Gene https://www.ncbi.nlm.nih.gov/gene/?term=A8I832
- 63.Arnesano F., Banci L., Benvenuti M., Bertini I., Calderone V., Mangani S., Viezzoli M.S. The evolutionarily conserved trimeric structure of CutA1 proteins suggests a role in signal transduction. J. Biol. Chem. 2003;278(46):45999–46006. doi: 10.1074/jbc.M304398200. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Y., Wang Y., Hu J., Zhang X., Zhang Y.W. CutA divalent cation tolerance homolog (Escherichia coli) (CUTA) regulates β-cleavage of β-amyloid precursor protein (APP) through interacting with β-site APP cleaving protein 1 (BACE1). J. Biol. Chem. 2012;287(14):11141–11150. doi: 10.1074/jbc.M111.330209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hou P., Liu G., Zhao Y., Shi Z., Zheng Q., Bu G., Xu H., Zhang Y.W. Role of copper and the copper-related protein CUTA in mediating APP processing and Aβ generation. Neurobiol. Aging. 2015;36(3):1310–1315. doi: 10.1016/j.neurobiolaging.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Kitazawa M., Cheng D., Laferla F.M. Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J. Neurochem. 2009;108(6):1550–1560. doi: 10.1111/j.1471-4159.2009.05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Entrez Gene https://www.ncbi.nlm.nih.gov/gene/51312
- 68.Hentze M.W., Muckenthaler M.U., Galy B., Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 69.Hanmanthraya B., Byrne A. Haemochromatosis and dementia: cause or contributor. Prog. Neurol. Psychiatry. 2015;19:5–8. doi: 10.1002/pnp.378. [DOI] [Google Scholar]
- 70.Huang J., Chen S., Hu L., Niu H., Sun Q., Li W., Tan G., Li J., Jin L., Lyu J., Zhou H. Mitoferrin-1 is involved in the progression of Alzheimer’s Disease through targeting mitochondrial iron metabolism in a Caenorhabditis elegans Model of Alzheimer’s Disease. Neuroscience. 2018;385:90–101. doi: 10.1016/j.neuroscience.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 71.Carr M.W., Roth S.J., Luther E., Rose S.S., Springer T.A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA. 1994;91(9):3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu L.L., Warren M.K., Rose W.L., Gong W., Wang J.M. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J. Leukoc. Biol. 1996;60(3):365–371. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- 73.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muessel M.J., Berman N.E., Klein R.M. Early and specific expression of monocyte chemoattractant protein-1 in the thalamus induced by cortical injury. Brain Res. 2000;870(1-2):211–221. doi: 10.1016/S0006-8993(00)02450-1. [DOI] [PubMed] [Google Scholar]
- 75.Kalehua A.N., Nagel J.E., Whelchel L.M., Gides J.J., Pyle R.S., Smith R.J., Kusiak J.W., Taub D.D. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-2 are involved in both excitotoxin-induced neurodegeneration and regeneration. Exp. Cell Res. 2004;297(1):197–211. doi: 10.1016/j.yexcr.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 76.Persidsky Y., Ghorpade A., Rasmussen J., Limoges J., Liu X.J., Stins M., Fiala M., Way D., Kim K.S., Witte M.H., Weinand M., Carhart L., Gendelman H.E. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am. J. Pathol. 1999;155(5):1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coughlan C.M., McManus C.M., Sharron M., Gao Z., Murphy D., Jaffer S., Choe W., Chen W., Hesselgesser J., Gaylord H., Kalyuzhny A., Lee V.M., Wolf B., Doms R.W., Kolson D.L. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97(3):591–600. doi: 10.1016/S0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 78.Muessel M.J., Klein R.M., Wilson A.M., Berman N.E. Ablation of the chemokine monocyte chemoattractant protein-1 delays retrograde neuronal degeneration, attenuates microglial activation, and alters expression of cell death molecules. Brain Res. Mol. Brain Res. 2002;103(1-2):12–27. doi: 10.1016/S0169-328X(02)00158-4. [DOI] [PubMed] [Google Scholar]
- 79.Gewurz H., Zhang X.H., Lint T.F. Structure and function of the pentraxins. Curr. Opin. Immunol. 1995;7(1):54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 80.Omeis I.A., Hsu Y.C., Perin M.S. Mouse and human neuronal pentraxin 1 (NPTX1): conservation, genomic structure, and chromosomal localization. Genomics. 1996;36(3):543–545. doi: 10.1006/geno.1996.0503. [DOI] [PubMed] [Google Scholar]
- 81.McGeer E.G., Yasojima K., Schwab C., McGeer P.L. The pentraxins: possible role in Alzheimer’s disease and other innate inflammatory diseases. Neurobiol. Aging. 2001;22(6):843–848. doi: 10.1016/S0197-4580(01)00288-3. [DOI] [PubMed] [Google Scholar]
- 82.Farhy-Tselnicker I., van Casteren A.C.M., Lee A., Chang V.T., Aricescu A.R., Allen N.J. Astrocyte-secreted glypican 4 regulates release of neuronal pentraxin 1 from axons to induce functional synapse formation. Neuron. 2017;96(2):428–445.e13. doi: 10.1016/j.neuron.2017.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma Q.L., Teng E., Zuo X., Jones M., Teter B., Zhao E.Y., Zhu C., Bilousova T., Gylys K.H., Apostolova L.G., LaDu M.J., Hossain M.A., Frautschy S.A., Cole G.M. Neuronal pentraxin 1: A synaptic-derived plasma biomarker in Alzheimer’s disease. Neurobiol. Dis. 2018;114:120–128. doi: 10.1016/j.nbd.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swanson A., Willette A.A. Alzheimer’s Disease Neuroimaging Initiative. Neuronal Pentraxin 2 predicts medial temporal atrophy and memory decline across the Alzheimer’s disease spectrum. Brain Behav. Immun. 2016;58:201–208. doi: 10.1016/j.bbi.2016.07.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bilousova T., Taylor K., Emirzian A., Gylys R., Frautschy S.A., Cole G.M., Teng E. Parallel age-associated changes in brain and plasma neuronal pentraxin receptor levels in a transgenic APP/PS1 rat model of Alzheimer’s disease. Neurobiol. Dis. 2015;74:32–40. doi: 10.1016/j.nbd.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duong T., Acton P.J., Johnson R.A. The in vitro neuronal toxicity of pentraxins associated with Alzheimer’s disease brain lesions. Brain Res. 1998;813(2):303–312. doi: 10.1016/S0006-8993(98)00966-4. [DOI] [PubMed] [Google Scholar]
- 87.Entrez Gene https://www.ncbi.nlm.nih.gov/gene/?term=29979
- 88.El Ayadi A., Stieren E.S., Barral J.M., Boehning D. Ubiquilin-1 and protein quality control in Alzheimer disease. Prion. 2013;7(2):164–169. doi: 10.4161/pri.23711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jantrapirom S., Lo Piccolo L., Yoshida H., Yamaguchi M. A new Drosophila model of Ubiquilin knockdown shows the effect of impaired proteostasis on locomotive and learning abilities. Exp. Cell Res. 2018;362(2):461–471. doi: 10.1016/j.yexcr.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 90.Natunen T., Takalo M., Kemppainen S., Leskelä S., Marttinen M., Kurkinen K.M.A., Pursiheimo J.P., Sarajärvi T., Viswanathan J., Gabbouj S., Solje E., Tahvanainen E., Pirttimäki T., Kurki M., Paananen J., Rauramaa T., Miettinen P., Mäkinen P., Leinonen V., Soininen H., Airenne K., Tanzi R.E., Tanila H., Haapasalo A., Hiltunen M. Relationship between ubiquilin-1 and BACE1 in human Alzheimer’s disease and APdE9 transgenic mouse brain and cell-based models. Neurobiol. Dis. 2016;85:187–205. doi: 10.1016/j.nbd.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 91.Viswanathan J., Haapasalo A., Böttcher C., Miettinen R., Kurkinen K.M., Lu A., Thomas A., Maynard C.J., Romano D., Hyman B.T., Berezovska O., Bertram L., Soininen H., Dantuma N.P., Tanzi R.E., Hiltunen M. Alzheimer’s disease-associated ubiquilin-1 regulates presenilin-1 accumulation and aggresome formation. Traffic. 2011;12(3):330–348. doi: 10.1111/j.1600-0854.2010.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sherrington R., Rogaev E.I., Liang Y., Rogaeva E.A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K., Tsuda T., Mar L., Foncin J.F., Bruni A.C., Montesi M.P., Sorbi S., Rainero I., Pinessi L., Nee L., Chumakov I., Pollen D., Brookes A., Sanseau P., Polinsky R.J., Wasco W., Da Silva H.A., Haines J.L., Perkicak-Vance M.A., Tanzi R.E., Roses A.D., Fraser P.E., Rommens J.M., St George-Hyslop P.H. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 93.Levy-Lahad E., Wasco W., Poorkaj P., Romano D.M., Oshima J., Pettingell W.H., Yu C.E., Jondro P.D., Schmidt S.D., Wang K. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 94.Duncan R.S., Song B., Koulen P. Presenilins as Drug Targets for Alzheimer’s Disease-Recent Insights from Cell Biology and Electrophysiology as Novel Opportunities in Drug Development. Int. J. Mol. Sci. 2018;19(6):E1621. doi: 10.3390/ijms19061621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ebke A., Luebbers T., Fukumori A., Shirotani K., Haass C., Baumann K., Steiner H. Novel γ-secretase enzyme modulators directly target presenilin protein. J. Biol. Chem. 2011;286(43):37181–37186. doi: 10.1074/jbc.C111.276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iwatsubo T. Aβ42, Presenilins, and Alzheimer’s Disease. Neurobiol. Aging. 1998;19:11–13. doi: 10.1016/S0197-4580(98)00027-X. [DOI] [PubMed] [Google Scholar]
- 97.Entrez Gene https://www.ncbi.nlm.nih.gov/gene/6653
- 98.Spoelgen R., Adams K.W., Koker M., Thomas A.V., Andersen O.M., Hallett P.J., Bercury K.K., Joyner D.F., Deng M., Stoothoff W.H., Strickland D.K., Willnow T.E., Hyman B.T. Interaction of the apolipoprotein E receptors low density lipoprotein receptor-related protein and sorLA/LR11. Neuroscience. 2009;158(4):1460–1468. doi: 10.1016/j.neuroscience.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andersen O.M., Schmidt V., Spoelgen R., Gliemann J., Behlke J., Galatis D., McKinstry W.J., Parker M.W., Masters C.L., Hyman B.T., Cappai R., Willnow T.E. Molecular dissection of the interaction between amyloid precursor protein and its neuronal trafficking receptor SorLA/LR11. Biochemistry. 2006;45(8):2618–2628. doi: 10.1021/bi052120v. [DOI] [PubMed] [Google Scholar]
- 100.Hartl D., Nebrich G., Klein O., Stephanowitz H., Krause E., Rohe M. SORLA regulates calpain-dependent degradation of synapsin. Alzheimers Dement. 2016;12(9):952–963. doi: 10.1016/j.jalz.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 101.Gill R.L., Jr, Wang X., Tian F. A membrane proximal helix in the cytosolic domain of the human APP interacting protein LR11/SorLA deforms liposomes. Biochim. Biophys. Acta. 2015;1848(1 Pt B):323–328. doi: 10.1016/j.bbamem.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Motoi Y., Aizawa T., Haga S., Nakamura S., Namba Y., Ikeda K. Neuronal localization of a novel mosaic apolipoprotein E receptor, LR11, in rat and human brain. Brain Res. 1999;833(2):209–215. doi: 10.1016/S0006-8993(99)01542-5. [DOI] [PubMed] [Google Scholar]
- 103.Entrez Gene https://www.ncbi.nlm.nih.gov/gene/?term=6622
- 104.Wong Y.C., Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat. Med. 2017;23(2):1–13. doi: 10.1038/nm.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fang F., Yang W., Florio J.B., Rockenstein E., Spencer B., Orain X.M., Dong S.X., Li H., Chen X., Sung K., Rissman R.A., Masliah E., Ding J., Wu C. Synuclein impairs trafficking and signaling of BDNF in a mouse model of Parkinson’s disease. Sci. Rep. 2017;7(1):3868. doi: 10.1038/s41598-017-04232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Colom-Cadena M., Pegueroles J., Herrmann A.G., Henstridge C.M., Muñoz L., Querol-Vilaseca M., Martín-Paniello C.S., Luque-Cabecerans J., Clarimon J., Belbin O., Núñez-Llaves R., Blesa R., Smith C., McKenzie C.A., Frosch M.P., Roe A., Fortea J., Andilla J., Loza-Alvarez P., Gelpi E., Hyman B.T., Spires-Jones T.L., Lleó A. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain. 2017;140(12):3204–3214. doi: 10.1093/brain/awx275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vergallo A., Bun R.S., Toschi N., Baldacci F., Zetterberg H., Blennow K., Cavedo E., Lamari F., Habert M.O., Dubois B., Floris R., Garaci F., Lista S., Hampel H., INSIGHT-preAD study group Alzheimer Precision Medicine Initiative (APMI). Association of cerebrospinal fluid α-synuclein with total and phospho-tau181 protein concentrations and brain amyloid load in cognitively normal subjective memory complainers stratified by Alzheimer’s disease biomarkers. Alzheimers Dement. 2018;14(12):1623–1631. doi: 10.1016/j.jalz.2018.06.3053. [DOI] [PubMed] [Google Scholar]
- 108.Shi M., Tang L., Toledo J.B., Ginghina C., Wang H., Aro P., Jensen P.H., Weintraub D., Chen-Plotkin A.S., Irwin D.J., Grossman M., McCluskey L., Elman L.B., Wolk D.A., Lee E.B., Shaw L.M., Trojanowski J.Q., Zhang J. Cerebrospinal fluid α-synuclein contributes to the differential diagnosis of Alzheimer’s disease. Alzheimers Dement. 2018;14(8):1052–1062. doi: 10.1016/j.jalz.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Slaets S., Vanmechelen E., Le Bastard N., Decraemer H., Vandijck M., Martin J.J., De Deyn P.P., Engelborghs S. Increased CSF α-synuclein levels in Alzheimer’s disease: correlation with tau levels. Alzheimers Dement. 2014;10(5) Suppl.:S290–S298. doi: 10.1016/j.jalz.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 110.Gaspar R., Pallbo J., Weininger U., Linse S., Sparr E. Ganglioside lipids accelerate α-synuclein amyloid formation. Biochim. Biophys. Acta. Proteins Proteomics. 2018;1866:1062–1072. doi: 10.1016/j.bbapap.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Whiten D.R., Cox D., Horrocks M.H., Taylor C.G., De S., Flagmeier P., Tosatto L., Kumita J.R., Ecroyd H., Dobson C.M., Klenerman D., Wilson M.R. Single-molecule characterization of the interactions between extracellular chaperones and toxic α-synuclein oligomers. Cell Rep. 2018;23(12):3492–3500. doi: 10.1016/j.celrep.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bar R., Boehm-Cagan A., Luz I., Kleper-Wall Y., Michaelson D.M. The effects of apolipoprotein E genotype, α-synuclein deficiency, and sex on brain synaptic and Alzheimer’s disease-related pathology. Alzheimers Dement. (Amst.) 2017;10:1–11. doi: 10.1016/j.dadm.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Castillo-Carranza D.L., Guerrero-Muñoz M.J., Sengupta U., Gerson J.E., Kayed R. α-Synuclein oligomers induce a unique toxic tau strain. Biol. Psychiatry. 2018;84(7):499–508. doi: 10.1016/j.biopsych.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goedert M., Spillantini M.G., Jakes R., Rutherford D., Crowther R.A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 115.Goedert M., Wischik C.M., Crowther R.A., Walker J.E., Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc. Natl. Acad. Sci. USA. 1988;85(11):4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vlassenko A.G., Gordon B.A., Goyal M.S., Su Y., Blazey T.M., Durbin T.J., Couture L.E., Christensen J.J., Jafri H., Morris J.C., Raichle M.E., Benzinger T.L. Aerobic glycolysis and tau deposition in preclinical Alzheimer’s disease. Neurobiol. Aging. 2018;67:95–98. doi: 10.1016/j.neurobiolaging.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shentu Y.P., Huo Y., Feng X.L., Gilbert J., Zhang Q., Liuyang Z.Y., Wang X.L., Wang G., Zhou H., Wang X.C., Wang J.Z., Lu Y.M., Westermarck J., Man H.Y., Liu R. CIP2A causes tau/app phosphorylation, synaptopathy, and memory deficits in alzheimer’s disease. Cell Rep. 2018;24(3):713–723. doi: 10.1016/j.celrep.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miron J., Picard C., Nilsson N., Frappier J., Dea D., Théroux L., Poirier J. alzheimer’s disease neuroimaging initiative; united kingdom brain expression consortium. cdk5rap2 gene and tau pathophysiology in late-onset sporadic Alzheimer’s disease. Alzheimers Dement. 2018;14(6):787–796. doi: 10.1016/j.jalz.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 119.Parbo P., Ismail R., Sommerauer M., Stokholm M.G., Hansen A.K., Hansen K.V., Amidi A., Schaldemose J.L., Gottrup H., Brændgaard H., Eskildsen S.F., Borghammer P., Hinz R., Aanerud J., Brooks D.J. Does inflammation precede tau aggregation in early Alzheimer’s disease? A PET study. Neurobiol. Dis. 2018;117:211–216. doi: 10.1016/j.nbd.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 120.Alam J., Sharma L. Potential enzymatic targets in Alzheimer’s: A comprehensive review. Curr. Drug Targets. 2019;20(3):316–339. doi: 10.2174/1389450119666180820104723. [DOI] [PubMed] [Google Scholar]
- 121.Eftekharzadeh B., Daigle J.G., Kapinos L.E., Coyne A., Schiantarelli J., Carlomagno Y., Cook C., Miller S.J., Dujardin S., Amaral A.S., Grima J.C., Bennett R.E., Tepper K., DeTure M., Vanderburg C.R., Corjuc B.T., DeVos S.L., Gonzalez J.A., Chew J., Vidensky S., Gage F.H., Mertens J., Troncoso J., Mandelkow E., Salvatella X., Lim R.Y.H., Petrucelli L., Wegmann S., Rothstein J.D., Hyman B.T. Tau protein disrupts nucleocytoplasmic transport in Alzheimer’s Disease. Neuron. 2019;101(2):349. doi: 10.1016/j.neuron.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mielke M.M., Hagen C.E., Xu J., Chai X., Vemuri P., Lowe V.J., Airey D.C., Knopman D.S., Roberts R.O., Machulda M.M., Jack C.R., Jr, Petersen R.C., Dage J.L. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14(8):989–997. doi: 10.1016/j.jalz.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pekeles H., Qureshi H.Y., Paudel H.K., Schipper H.M., Gornistky M., Chertkow H. Development and validation of a salivary tau biomarker in Alzheimer’s disease. Alzheimers Dement. (Amst.) 2018;11:53–60. doi: 10.1016/j.dadm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Babini E., Bertini I., Capozzi F., Chirivino E., Luchinat C. A structural and dynamic characterization of the EF-hand protein CLSP. Structure. 2006;14(6):1029–1038. doi: 10.1016/j.str.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 125.Matsuoka M. Protective effects of Humanin and calmodulin-like skin protein in Alzheimer’s disease and broad range of abnormalities. Mol. Neurobiol. 2015;51(3):1232–1239. doi: 10.1007/s12035-014-8799-1. [DOI] [PubMed] [Google Scholar]
- 126.Hashimoto Y., Kurita M., Aiso S., Nishimoto I., Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol. Biol. Cell. 2009;20(12):2864–2873. doi: 10.1091/mbc.e09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hashimoto Y., Kurita M., Matsuoka M. Identification of soluble WSX-1 not as a dominant-negative but as an alternative functional subunit of a receptor for an anti-Alzheimer’s disease rescue factor Humanin. Biochem. Biophys. Res. Commun. 2009;389(1):95–99. doi: 10.1016/j.bbrc.2009.08.095. [DOI] [PubMed] [Google Scholar]
- 128.Kusakari S., Nawa M., Sudo K., Matsuoka M. Calmodulin-like skin protein protects against spatial learning impairment in a mouse model of Alzheimer disease. J. Neurochem. 2018;144(2):218–233. doi: 10.1111/jnc.14258. [DOI] [PubMed] [Google Scholar]
- 129.Hashimoto Y., Umahara T., Hanyu H., Iwamoto T., Matsuoka M. Calmodulin-like skin protein is downregulated in human cerebrospinal fluids of Alzheimer’s disease patients with apolipoprotein E4; a pilot study using postmortem samples. Neurol. Res. 2017;39(9):767–772. doi: 10.1080/01616412.2017.1335458. [DOI] [PubMed] [Google Scholar]
- 130.Ritossa F.M. A New puffing pattern induced by temperature shock and dnp in Drosophila. Cell. Mol. Life Sci. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- 131.Matz J.M., Blake M.J., Tatelman H.M., Lavoi K.P., Holbrook N.J. Characterization and regulation of cold-induced heat shock protein expression in mouse brown adipose tissue. Am. J. Physiol. 1995;269(1 Pt 2):R38–R47. doi: 10.1152/ajpregu.1995.269.1.R38. [DOI] [PubMed] [Google Scholar]
- 132.Cao Y., Ohwatari N., Matsumoto T., Kosaka M., Ohtsuru A., Yamashita S. TGF-beta1 mediates 70-kDa heat shock protein induction due to ultraviolet irradiation in human skin fibroblasts. Pflugers Arch. 1999;438(3):239–244. doi: 10.1007/s004240050905. [DOI] [PubMed] [Google Scholar]
- 133.Laplante A.F., Moulin V., Auger F.A., Landry J., Li H., Morrow G., Tanguay R.M., Germain L. Expression of heat shock proteins in mouse skin during wound healing. J. Histochem. Cytochem. 1998;46(11):1291–1301. doi: 10.1177/002215549804601109. [DOI] [PubMed] [Google Scholar]
- 134.Abdul H.M., Calabrese V., Calvani M., Butterfield D.A. Acetyl-L-carnitine-induced up-regulation of heat shock proteins protects cortical neurons against amyloid-beta peptide 1-42-mediated oxidative stress and neurotoxicity: implications for Alzheimer’s disease. J. Neurosci. Res. 2006;84(2):398–408. doi: 10.1002/jnr.20877. [DOI] [PubMed] [Google Scholar]
- 135.Hensley K., Hall N., Subramaniam R., Cole P., Harris M., Aksenov M., Aksenova M., Gabbita S.P., Wu J.F., Carney J.M. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J. Neurochem. 1995;65(5):2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 136.Clarimón J., Bertranpetit J., Boada M., Tàrraga L., Comas D. HSP70-2 (HSPA1B) is associated with noncognitive symptoms in late-onset Alzheimer’s disease. J. Geriatr. Psychiatry Neurol. 2003;16(3):146–150. doi: 10.1177/0891988703256051. [DOI] [PubMed] [Google Scholar]
- 137.Renkawek K., Bosman G.J., de Jong W.W. Expression of small heat-shock protein hsp 27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol. 1994;87(5):511–519. doi: 10.1007/BF00294178. [DOI] [PubMed] [Google Scholar]
- 138.Renkawek K., Bosman G.J., Gaestel M. Increased expression of heat-shock protein 27 kDa in Alzheimer disease: a preliminary study. Neuroreport. 1993;5(1):14–16. doi: 10.1097/00001756-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 139.Jiang Y.Q., Wang X.L., Cao X.H., Ye Z.Y., Li L., Cai W.Q. Increased heat shock transcription factor 1 in the cerebellum reverses the deficiency of Purkinje cells in Alzheimer’s disease. Brain Res. 2013;1519:105–111. doi: 10.1016/j.brainres.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 140.Arimura N., Menager C., Fukata Y., Kaibuchi K. Role of CRMP-2 in neuronal polarity. J. Neurobiol. 2004;58(1):34–47. doi: 10.1002/neu.10269. [DOI] [PubMed] [Google Scholar]
- 141.Cole A.R., Noble W., van Aalten L., Plattner F., Meimaridou R., Hogan D., Taylor M., LaFrancois J., Gunn-Moore F., Verkhratsky A., Oddo S., LaFerla F., Giese K.P., Dineley K.T., Duff K., Richardson J.C., Yan S.D., Hanger D.P., Allan S.M., Sutherland C. Collapsin response mediator protein-2 hyperphosphorylation is an early event in Alzheimer’s disease progression. J. Neurochem. 2007;103(3):1132–1144. doi: 10.1111/j.1471-4159.2007.04829.x. [DOI] [PubMed] [Google Scholar]
- 142.Entrez Gene https://www.ncbi.nlm.nih.gov/gene/4035
- 143.Shibata M., Yamada S., Kumar S.R., Calero M., Bading J., Frangione B., Holtzman D.M., Miller C.A., Strickland D.K., Ghiso J., Zlokovic B.V. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 2000;106(12):1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu Q., Zerbinatti C.V., Zhang J., Hoe H.S., Wang B., Cole S.L., Herz J., Muglia L., Bu G. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56(1):66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Van Uden E., Carlson G., St George-Hyslop P., Westaway D., Orlando R., Mallory M., Rockenstein E., Masliah E. Aberrant presenilin-1 expression downregulates LDL receptor-related protein (LRP): is LRP central to Alzheimer’s disease pathogenesis? Mol. Cell. Neurosci. 1999;14(2):129–140. doi: 10.1006/mcne.1999.0772. [DOI] [PubMed] [Google Scholar]
- 146.Owen J.B., Sultana R., Aluise C.D., Erickson M.A., Price T.O., Bu G., Banks W.A., Butterfield D.A. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Aβ accumulation in AD brain. Free Radic. Biol. Med. 2010;49(11):1798–1803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Entrez Gene https://www.ncbi.nlm.nih.gov/gene/3313
- 148.Domanico S.Z., DeNagel D.C., Dahlseid J.N., Green J.M., Pierce S.K. Cloning of the gene encoding peptide-binding protein 74 shows that it is a new member of the heat shock protein 70 family. Mol. Cell. Biol. 1993;13(6):3598–3610. doi: 10.1128/MCB.13.6.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Deocaris C.C., Kaul S.C., Wadhwa R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones. 2006;11(2):116–128. doi: 10.1379/CSC-144R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Londono C., Osorio C., Gama V., Alzate O. Mortalin, apoptosis, and neurodegeneration. Biomolecules. 2012;2(1):143–164. doi: 10.3390/biom2010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Osorio C., Sullivan P.M., He D.N., Mace B.E., Ervin J.F., Strittmatter W.J., Alzate O. Mortalin is regulated by APOE in hippocampus of AD patients and by human APOE in TR mice. Neurobiol. Aging. 2007;28(12):1853–1862. doi: 10.1016/j.neurobiolaging.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 152.Qu M., Zhou Z., Xu S., Chen C., Yu Z., Wang D. Mortalin overexpression attenuates beta-amyloid-induced neurotoxicity in SH-SY5Y cells. Brain Res. 2011;1368:336–345. doi: 10.1016/j.brainres.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 153.Qu M., Zhou Z., Chen C., Li M., Pei L., Yang J., Wang Y., Li L., Liu C., Zhang G., Yu Z., Wang D. Inhibition of mitochondrial permeability transition pore opening is involved in the protective effects of mortalin overexpression against beta-amyloid-induced apoptosis in SH-SY5Y cells. Neurosci. Res. 2012;72(1):94–102. doi: 10.1016/j.neures.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 154.Martínez de Arrieta C., Morte B., Coloma A., Bernal J. The human RC3 gene homolog, NRGN contains a thyroid hormone-responsive element located in the first intron. Endocrinology. 1999;140(1):335–343. doi: 10.1210/endo.140.1.6461. [DOI] [PubMed] [Google Scholar]
- 155.Díez-Guerra F.J. Neurogranin, a link between calcium/calmodulin and protein kinase C signaling in synaptic plasticity. IUBMB Life. 2010;62(8):597–606. doi: 10.1002/iub.357. [DOI] [PubMed] [Google Scholar]
- 156.Kvartsberg H., Duits F.H., Ingelsson M., Andreasen N., Öhrfelt A., Andersson K., Brinkmalm G., Lannfelt L., Minthon L., Hansson O., Andreasson U., Teunissen C.E., Scheltens P., Van der Flier W.M., Zetterberg H., Portelius E., Blennow K. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimers Dement. 2015;11(10):1180–1190. doi: 10.1016/j.jalz.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 157.De Vos A., Jacobs D., Struyfs H., Fransen E., Andersson K., Portelius E., Andreasson U., De Surgeloose D., Hernalsteen D., Sleegers K., Robberecht C., Van Broeckhoven C., Zetterberg H., Blennow K., Engelborghs S., Vanmechelen E. C-terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer’s disease. Alzheimers Dement. 2015;11(12):1461–1469. doi: 10.1016/j.jalz.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 158.Thorsell A., Bjerke M., Gobom J., Brunhage E., Vanmechelen E., Andreasen N., Hansson O., Minthon L., Zetterberg H., Blennow K. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer’s disease. Brain Res. 2010;1362:13–22. doi: 10.1016/j.brainres.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 159.Li L., Li Y., Ji X., Zhang B., Wei H., Luo Y. The effects of retinoic acid on the expression of neurogranin after experimental cerebral ischemia. Brain Res. 2008;1226:234–240. doi: 10.1016/j.brainres.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 160.Huang K.P., Huang F.L., Jäger T., Li J., Reymann K.G., Balschun D. Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J. Neurosci. 2004;24(47):10660–10669. doi: 10.1523/JNEUROSCI.2213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ueda K., Clark D.P., Chen C.J., Roninson I.B., Gottesman M.M., Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J. Biol. Chem. 1987;262(2):505–508. [PubMed] [Google Scholar]
- 162.Bell D.R., Trent J.M., Willard H.F., Riordan J.R., Ling V. Chromosomal location of human P-glycoprotein gene sequences. Cancer Genet. Cytogenet. 1987;25(1):141–148. doi: 10.1016/0165-4608(87)90169-5. [DOI] [PubMed] [Google Scholar]
- 163.van Assema D.M., Lubberink M., Bauer M., van der Flier W.M., Schuit R.C., Windhorst A.D., Comans E.F., Hoetjes N.J., Tolboom N., Langer O., Müller M., Scheltens P., Lammertsma A.A., van Berckel B.N. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain. 2012;135(Pt 1):181–189. doi: 10.1093/brain/awr298. [DOI] [PubMed] [Google Scholar]
- 164.Jeynes B., Provias J. P-Glycoprotein altered expression in alzheimer’s disease: regional anatomic variability. J. Neurodegener. Dis. 2013;2013257953 doi: 10.1155/2013/257953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang C., Qin H., Zheng R., Wang Y., Yan T., Huan F., Han Y., Zhu W., Zhang L. A new approach for Alzheimer’s disease treatment through P-gp regulation via ibuprofen. Pathol. Res. Pract. 2018;214(11):1765–1771. doi: 10.1016/j.prp.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 166.Mohamed L.A., Keller J.N., Kaddoumi A. Role of P-glycoprotein in mediating rivastigmine effect on amyloid-β brain load and related pathology in Alzheimer’s disease mouse model. Biochim. Biophys. Acta. 2016;1862(4):778–787. doi: 10.1016/j.bbadis.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chiu C., Miller M.C., Monahan R., Osgood D.P., Stopa E.G., Silverberg G.D. P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: preliminary observations. Neurobiol. Aging. 2015;36(9):2475–2482. doi: 10.1016/j.neurobiolaging.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 168.Kimura Y., Tanaka K. Regulatory mechanisms involved in the control of ubiquitin homeostasis. J. Biochem. 2010;147(6):793–798. doi: 10.1093/jb/mvq044. [DOI] [PubMed] [Google Scholar]
- 169.Wang G.P., Khatoon S., Iqbal K., Grundke-Iqbal I. Brain ubiquitin is markedly elevated in Alzheimer disease. Brain Res. 1991;566(1-2):146–151. doi: 10.1016/0006-8993(91)91692-T. [DOI] [PubMed] [Google Scholar]
- 170.Verheijen B.M., Stevens J.A.A., Gentier R.J.G., van ’t Hekke C.D., van den Hove D.L.A., Hermes D.J.H.P., Steinbusch H.W.M., Ruijter J.M., Grimm M.O.W., Haupenthal V.J., Annaert W., Hartmann T., van Leeuwen F.W. Paradoxical effects of mutant ubiquitin on Aβ plaque formation in an Alzheimer mouse model. Neurobiol. Aging. 2018;72:62–71. doi: 10.1016/j.neurobiolaging.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 171.Cole G.M., Timiras P.S. Ubiquitin-protein conjugates in Alzheimer’s lesions. Neurosci. Lett. 1987;79(1-2):207–212. doi: 10.1016/0304-3940(87)90698-7. [DOI] [PubMed] [Google Scholar]
- 172.Rivera E.J., Goldin A., Fulmer N., Tavares R., Wands J.R., de la Monte S.M. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J. Alzheimers Dis. 2005;8(3):247–268. doi: 10.3233/JAD-2005-8304. [DOI] [PubMed] [Google Scholar]
- 173.Steen E., Terry B.M., Rivera E.J., Cannon J.L., Neely T.R., Tavares R., Xu X.J., Wands J.R., de la Monte S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J. Alzheimers Dis. 2005;7(1):63–80. doi: 10.3233/JAD-2005-7107. [DOI] [PubMed] [Google Scholar]
- 174.Talbot K., Wang H.Y., Kazi H., Han L.Y., Bakshi K.P., Stucky A., Fuino R.L., Kawaguchi K.R., Samoyedny A.J., Wilson R.S., Arvanitakis Z., Schneider J.A., Wolf B.A., Bennett D.A., Trojanowski J.Q., Arnold S.E. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.De Felice F.G., Vieira M.N., Bomfim T.R., Decker H., Velasco P.T., Lambert M.P., Viola K.L., Zhao W.Q., Ferreira S.T., Klein W.L. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc. Natl. Acad. Sci. USA. 2009;106(6):1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Triani F., Tramutola A., Di Domenico F., Sharma N., Butterfield D.A., Head E., Perluigi M., Barone E. Biliverdin reductase-A impairment links brain insulin resistance with increased Aβ production in an animal model of aging: Implications for Alzheimer disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864(10):3181–3194. doi: 10.1016/j.bbadis.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 177.Caccamo A., Belfiore R., Oddo S. Genetically reducing mTOR signaling rescues central insulin dysregulation in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2018;68:59–67. doi: 10.1016/j.neurobiolaging.2018.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 178.Yamamoto N., Ishikuro R., Tanida M., Suzuki K., Ikeda-Matsuo Y., Sobue K. Insulin-signaling Pathway Regulates the Degradation of Amyloid β-protein via Astrocytes. Neuroscience. 2018;385:227–236. doi: 10.1016/j.neuroscience.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 179.de la Monte S.M. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr. Alzheimer Res. 2012;9(1):35–66. doi: 10.2174/156720512799015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schubert M., Brazil D.P., Burks D.J., Kushner J.A., Ye J., Flint C.L., Farhang-Fallah J., Dikkes P., Warot X.M., Rio C., Corfas G., White M.F. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J. Neurosci. 2003;23(18):7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schubert M., Gautam D., Surjo D., Ueki K., Baudler S., Schubert D., Kondo T., Alber J., Galldiks N., Küstermann E., Arndt S., Jacobs A.H., Krone W., Kahn C.R., Brüning J.C. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. USA. 2004;101(9):3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xu H., Chen X., Wang J., Yang T., Liu N., Cheng J., Gao R., Liu J., Xiao H. Involvement of insulin signalling pathway in methamphetamine-induced hyperphosphorylation of Tau. Toxicology. 2018;408:88–94. doi: 10.1016/j.tox.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 183.Doi T., Shimada H., Makizako H., Tsutsumimoto K., Hotta R., Nakakubo S., Suzuki T. Association of insulin-like growth factor-1 with mild cognitive impairment and slow gait speed. Neurobiol. Aging. 2015;36(2):942–947. doi: 10.1016/j.neurobiolaging.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 184.Gasperi M., Castellano A.E. Growth hormone/insulin-like growth factor I axis in neurodegenerative diseases. J. Endocrinol. Invest. 2010;33(8):587–591. doi: 10.1007/BF03346653. [DOI] [PubMed] [Google Scholar]
- 185.Logan S., Pharaoh G.A., Marlin M.C., Masser D.R., Matsuzaki S., Wronowski B., Yeganeh A., Parks E.E., Premkumar P., Farley J.A., Owen D.B., Humphries K.M., Kinter M., Freeman W.M., Szweda L.I., Van Remmen H., Sonntag W.E. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-β uptake in astrocytes. Mol. Metab. 2018;9:141–155. doi: 10.1016/j.molmet.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Blaustein M.P. Calcium transport and buffering in neurons. Trends Neurosci. 1988;11(10):438–443. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- 187.Heizmann C.W., Braun K. Changes in Ca(2+)-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992;15(7):259–264. doi: 10.1016/0166-2236(92)90067-I. [DOI] [PubMed] [Google Scholar]
- 188.Kojetin D.J., Venters R.A., Kordys D.R., Thompson R.J., Kumar R., Cavanagh J. Structure, binding interface and hydrophobic transitions of Ca2+-loaded calbindin-D(28K). Nat. Struct. Mol. Biol. 2006;13(7):641–647. doi: 10.1038/nsmb1112. [DOI] [PubMed] [Google Scholar]
- 189.Rogers J.H. Calretinin: a gene for a novel calcium-binding protein expressed principally in neurons. J. Cell Biol. 1987;105(3):1343–1353. doi: 10.1083/jcb.105.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Altobelli G.G., Cimini D., Esposito G., Iuvone T., Cimini V. Analysis of calretinin early expression in the rat hippocampus after beta amyloid (1-42) peptide injection. Brain Res. 2015;1610:89–97. doi: 10.1016/j.brainres.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 191.Mattson M.P., Rychlik B., Chu C., Christakos S. Evidence for calcium-reducing and excito-protective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron. 1991;6(1):41–51. doi: 10.1016/0896-6273(91)90120-O. [DOI] [PubMed] [Google Scholar]
- 192.Iacopino A.M., Christakos S. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proc. Natl. Acad. Sci. USA. 1990;87(11):4078–4082. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mikkonen M., Alafuzoff I., Tapiola T., Soininen H., Miettinen R. Subfield- and layer-specific changes in parvalbumin, calretinin and calbindin-D28K immunoreactivity in the entorhinal cortex in Alzheimer’s disease. Neuroscience. 1999;92(2):515–532. doi: 10.1016/S0306-4522(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 194.Hof P.R., Nimchinsky E.A., Celio M.R., Bouras C., Morrison J.H. Calretinin-immunoreactive neocortical interneurons are unaffected in Alzheimer’s disease. Neurosci. Lett. 1993;152(1-2):145–148. doi: 10.1016/0304-3940(93)90504-E. [DOI] [PubMed] [Google Scholar]
- 195.Zallo F., Gardenal E., Verkhratsky A., Rodríguez J.J. Loss of calretinin and parvalbumin positive interneurones in the hippocampal CA1 of aged Alzheimer’s disease mice. Neurosci. Lett. 2018;681:19–25. doi: 10.1016/j.neulet.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 196.Donlon T.A., Krensky A.M., Wallace M.R., Collins F.S., Lovett M., Clayberger C. Localization of a human T-cell-specific gene, RANTES (D17S136E), to chromosome 17q11.2-q12. Genomics. 1990;6(3):548–553. doi: 10.1016/0888-7543(90)90485-D. [DOI] [PubMed] [Google Scholar]
- 197.Rostene W., Buckingham J.C. Chemokines as modulators of neuroendocrine functions. J. Mol. Endocrinol. 2007;38(3):351–353. doi: 10.1677/JME-07-0006. [DOI] [PubMed] [Google Scholar]
- 198.Valerio A., Ferrario M., Martinez F.O., Locati M., Ghisi V., Bresciani L.G., Mantovani A., Spano P. Gene expression profile activated by the chemokine CCL5/RANTES in human neuronal cells. J. Neurosci. Res. 2004;78(3):371–382. doi: 10.1002/jnr.20250. [DOI] [PubMed] [Google Scholar]
- 199.Sanchez A., Tripathy D., Grammas P. RANTES release contributes to the protective action of PACAP38 against sodium nitroprusside in cortical neurons. Neuropeptides. 2009;43(4):315–320. doi: 10.1016/j.npep.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lin M.S., Hung K.S., Chiu W.T., Sun Y.Y., Tsai S.H., Lin J.W., Lee Y.H. Curcumin enhances neuronal survival in N-methyl-d-aspartic acid toxicity by inducing RANTES expression in astrocytes via PI-3K and MAPK signaling pathways. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(4):931–938. doi: 10.1016/j.pnpbp.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 201.Haskins M., Jones T.E., Lu Q., Bareiss S.K. Early alterations in blood and brain RANTES and MCP-1 expression and the effect of exercise frequency in the 3xTg-AD mouse model of Alzheimer’s disease. Neurosci. Lett. 2016;610:165–170. doi: 10.1016/j.neulet.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 202.Tripathy D., Thirumangalakudi L., Grammas P. RANTES upregulation in the Alzheimer’s disease brain: a possible neuroprotective role. Neurobiol. Aging. 2010;31(1):8–16. doi: 10.1016/j.neurobiolaging.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kosik K.S., Orecchio L.D., Bruns G.A., Benowitz L.I., MacDonald G.P., Cox D.R., Neve R.L. Human GAP-43: its deduced amino acid sequence and chromosomal localization in mouse and human. Neuron. 1988;1(2):127–132. doi: 10.1016/0896-6273(88)90196-1. [DOI] [PubMed] [Google Scholar]
- 204.Benowitz L.I., Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20(2):84–91. doi: 10.1016/S0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 205.Holahan M.R., Honegger K.S., Tabatadze N., Routtenberg A. GAP-43 gene expression regulates information storage. Learn. Mem. 2007;14(6):407–415. doi: 10.1101/lm.581907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sandelius Å., Portelius E., Källén Å., Zetterberg H., Rot U., Olsson B., Toledo J.B., Shaw L.M., Lee V.M.Y., Irwin D.J., Grossman M., Weintraub D., Chen-Plotkin A., Wolk D.A., McCluskey L., Elman L., Kostanjevecki V., Vandijck M., McBride J., Trojanowski J.Q., Blennow K. Elevated CSF GAP-43 is Alzheimer’s disease specific and associated with tau and amyloid pathology. Alzheimers Dement. 2019;15(1):55–64. doi: 10.1016/j.jalz.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ono Y., Sorimachi H. Calpains: an elaborate proteolytic system. Biochim. Biophys. Acta. 2012;1824(1):224–236. doi: 10.1016/j.bbapap.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 208.Baudry M., Bi X. Calpain-1 and Calpain-2: The Yin and Yang of Synaptic Plasticity and Neurodegeneration. Trends Neurosci. 2016;39(4):235–245. doi: 10.1016/j.tins.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vaisid T., Kosower N.S., Katzav A., Chapman J., Barnoy S. Calpastatin levels affect calpain activation and calpain proteolytic activity in APP transgenic mouse model of Alzheimer’s disease. Neurochem. Int. 2007;51(6-7):391–397. doi: 10.1016/j.neuint.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 210.Touyarot K., Poussard S., Cortes-Torrea C., Cottin P., Micheau J. Effect of chronic inhibition of calpains in the hippocampus on spatial discrimination learning and protein kinase C. Behav. Brain Res. 2002;136(2):439–448. doi: 10.1016/S0166-4328(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 211.Mehta D., Jackson R., Paul G., Shi J., Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin. Investig. Drugs. 2017;26(6):735–739. doi: 10.1080/13543784.2017.1323868. [DOI] [PMC free article] [PubMed] [Google Scholar]