Abstract
The cannabinoids, Δ9 tetrahydrocannabinol and its analogue, nabilone, have been found to reliably attenuate the intensity and frequency of post-traumatic nightmares. This essay examines how a traumatic event is captured in the mind, after just a single exposure, and repeatedly replicated during the nights that follow. The adaptive neurophysiological, endocrine and inflammatory changes that are triggered by the trauma and that alter personality and behavior are surveyed. These adaptive changes, once established, can be difficult to reverse. But cannabinoids, uniquely, have been shown to interfere with all of these post-traumatic somatic adaptations. While cannabinoids can suppress nightmares and other symptoms of post-traumatic stress disorder, they are not a cure. There may be no cure. The cannabinoids may best be employed, alone, but more likely in conjunction with other agents, in the immediate aftermath of a trauma to mitigate or even abort the metabolic changes which are set in motion by the trauma and which may permanently alter the reactivity of the nervous system. Steps in this direction have already been taken.
Keywords: Nightmares, sleep, episodic memory, sharp wave ripple complex, cannabinoids, post-traumatic stress disorder
1. Introduction
The large number of drugs and psychological interventions recommended for the treatment of nightmares attest to the relative ineffectiveness of these therapies as well as to the resistance of nightmares to their elimination [1] Three recent studies have found that nabilone, the synthetic dimethylheptyl analogue of Δ9-tetrahydrocannabinol (THC) and an agonist at both the CB1 and CB2 cannabinoid receptors, can be used with a high degree of success for long periods of time to effectively suppress nightmares in patients with the post-traumatic stress disorder and to not otherwise interfere with dreaming [2-5]. THC, the only other clinically available therapeutic cannabinoid and the major psychoactive ingredient of marijuana, has also been shown to effectively suppress nightmares [6]. How do these cannabinoids do this? In a recent review, Hindocha et al., [7] have written that given the widespread use of marijuana products to treat the sleep disturbances in post-traumatic stress disorder, understanding their mechanism of action has become imperative.
2. Replicative Nightmares
According to the International Classification of Sleep Disorders, nightmares refer to recurrent episodes of awakening from sleep with a recall of intensely disturbing dreams, usually involving fear or anxiety but also anger, sadness, disgust and other dysphoric emotions. There is full alertness on awakening with little mental confusion or disorientation. Recall of the distressing dreams is immediate and clear, but the continuity of sleep is disrupted and the return to sleep is delayed after each episode [8]. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [9], adds that nightmares are typically elaborate story like sequences of dream imagery that focus on attempts to avoid or cope with imminent danger. Trauma can lead to unique nightmares that vividly and recurrently replicate the what, where and when of the traumatic event and its associated mood, usually fear. Remarkably, the entire traumatic event can be registered in the mind after even a single exposure. The re-experiencing of this event in the form of repetitive dreams, memories or flashbacks is recognized as a cardinal feature of post-traumatic stress disorder [9].
Memories of autobiographical events that have a specific trajectory in time and space and that describe the event and its context, i.e., what happened, where it happened and when, are referred to as episodic memories [10]. An episodic memory is defined as a memory of an actual waking event (or episode) that is recalled as an integrated whole [11]. Episodic memories are examples of autonoetic consciousness in which one feels that one is reliving a past event [12]. Post-traumatic replicative nightmares come under this definition [13]. Post-traumatic nightmares are reported by up to 70% of individuals suffering from post-traumatic stress disorders and about 50% of these post-traumatic dreams replicate the traumatic event [13]. Recurrent replicative nightmares occur frequently in civilian life following exposure to life-threatening events, assaults, workplace injuries and car accidents. Replicative nightmares bring back all the imagery of the event, i.e., the headlights of the oncoming car and the intense fear about the impending collision. Replicative nightmares can be difficult to treat although they fortunately most often resolve in time on their own [13]. On the other hand, they can become a persistent and a lifetime source of distress. Many war veterans and Holocaust survivors can attest to this [14, 15]. In this essay, we focus on the nature of the replicative nightmares which emerge against a background of fear and pain in the aftermath of physical injury. The brain mechanisms which mediate their nightly replay have become a subject of great interest.
3. The hippocampal-neocortical dialogue
According to the dual-stage theory of memory formation, events or episodes experienced during wakefulness are thought to be initially encoded in parallel neocortical and hippocampal networks [16-19]. In subsequent periods of NREM sleep, the newly acquired memory traces are repeatedly reactivated and gradually redistributed over days and weeks to strengthen the synaptic connections within the neocortex where the memory of the episode is consolidated and rendered less vulnerable to amnestic treatments. According to some investigators, the hippocampus has a time-limited role and becomes disengaged after the memory trace is transferred to the neocortex. Others, however, believe that the hippocampus never becomes entirely disengaged and that the retrieval of memories continues to require the activation of both their hippocampal and cortical representations [20-22]. In either case, the reactivation and redistribution of memories during sleep may be viewed as a dialogue between the hippocampus and the neocortex which takes place off-line, during NREM sleep, when the brain is not occupied with processing current sensory information and is essentially disconnected from the environment. New information is more easily assimilated if it is compatible with an existing set of ideas or ‘schema’ and is more rapidly transferred from hippocampus to neocortex in the presence of such a schema [23]. It appears that memories which are relevant to the future behavior of the individual are preferentially consolidated. How this is done is not known, but it has been proposed that processing through hippocampal circuits determines the importance or relevance of incoming information and subsequently influences the nature of the synaptic modifications made within the neocortex. As we shall see, salient events often elicit neuroendocrine and inflammatory responses which promote their long-term registration. The dual stage model is consistent with clinical observations that damage to the hippocampus does not affect long term memories but severely impairs the acquisition of new memories and the susceptibility of memories to post-acquisition manipulation [24].
4. REM vs. NREM
Thus, this dual-stage theory of memory formation would propose that post-traumatic replicative nightmares are NREM phenomena and the role, if any, of REM sleep in the encoding or consolidation of episodic memories is not clear [16, 25-27]. In clinical studies in normal subjects, residues from waking experience are incorporated into about 50% of dreams, but do so in new and unrelated contexts and verified memories for episodes of recent life are only found in about 1.5% of dreams [11, 28, 29]. Dream content elicited during REM sleep is largely unrelated to experiences from the preceding day and does not accurately represent episodic memories available during wakefulness. Awakenings from NREM sleep are more likely to be concerned with current issues. The lack of episodic memories in REM dreams goes along with experimental findings in the rat to be described shortly, which demonstrate that information does not flow from the hippocampus to the neocortex during REM sleep as it does during NREM sleep [24, 28, 30, 31]. Suppression of REM sleep with antidepressant drugs and even its complete elimination with monoamine oxidase inhibitors does not reliably alleviate post-traumatic nightmares [1]. REM sleep has been proposed to have a role in the consolidation of nondeclarative (nonepisodic)/procedural memory and skill learning but convincing evidence for this has not been forthcoming [16, 32-34]. However, there is evidence that NREM sleep and its spindles are critically engaged in the consolidation of both nondeclarative and procedural memory [35, 36]. Nevertheless, many still believe that REM sleep plays a role in memory formation and specifically in the consolidation of emotional memories and possibly also non-emotional memory as optogenetic suppression of hippocampal theta activity during REM sleep interferes with the consolidation of fear-conditioned contextual memory and object place memory [37-40]. A ‘sequential hypothesis’ has been proposed which suggests that all memory is optimally consolidated when NREM and REM stages take place in succession [26, 41].
In practice, however, it has been difficult to capture post-traumatic nightmares in the sleep laboratory and assign them to either REM or NREM sleep [13, 42]. The alterations in sleep architecture in post-traumatic stress disorder have recently been reviewed [43]. Replicative and non-replicative nightmares are found to arise from both REM and NREM sleep in patients with this disorder. The matter is further complicated because a single nightmare in a patient with post-traumatic stress disorder may have both replicative and non-replicative features [42]. The findings in this study suggest that nightmares may be triggered by awakenings during sleep caused by breathing irregularities, aberrant movements or hyperarousal and that the more frequent the arousals, the more likely a dream recall. However, Schredl et al., [44] found no significant correlation between the respiratory disturbance index or the periodic limb movements index and nightmare frequency. Phelps [42] and Schredl [44] agree that arousal alone is insufficient and that pre-existing stress is required for nightmares but their work suggests that terrifying dreams at night and flashbacks during the day may be related waking phenomena. Both are susceptible to suppression by cannabinoids [2, 45].
There have only been 6 studies on the effects of cannabinoids on the polysomnographically recorded stages of sleep and no consistent effects on the stages of sleep have been documented [46]. None of these studies were conducted on subjects suffering from post-traumatic sleep disorder. In the most recent of these studies, a double-blind, placebo-controlled cross over trial, a 15 mg dose of THC administered as an oromucosal spray at bedtime had no effect on the recorded stages of sleep [47]. Some morning drowsiness was observed. When studies on the effects of cannabinoids on sleep are considered, no definite consensus has emerged on their effects on sleep architecture and their beneficial effects on sleep appear limited to patients with conditions like pain and spasticity that disrupt sleep [46, 48]. The effects of nabilone on the stages of sleep have not been examined.
5. Memory Processing
In contrast to these uncertainties, a body of sophisticated experimental work reveals a central role for NREM sleep in the formation of episodic memories and in the transfer of information between the hippocampus and other brain regions [49-53]. Episodic memory formation in man is proposed to have evolved from the hippocampal mechanisms mediating spatial navigation [54]. Spatial navigation and episodic memory, both require the integration of spatial and temporal data. Early studies on rodents demonstrated that spatial information acquired during active waking behavior is replayed in hippocampal circuits during the NREM sleep periods which follow. In these studies, simultaneous recordings were made from large ensembles of hippocampal place cells during waking spatial behavioral tasks and in NREM sleep preceding and following these tasks. Cells that fired together when the animal occupied a particular location in the environment showed an increased tendency to fire together subsequently during NREM sleep in comparison to sleep preceding the behavioral tasks [55]. Moreover, cell pairs active together in the neocortex and in the hippocampus during waking behaviors continued to fire together subsequently during NREM sleep. Thus, reactivation occurred within each of these regions and between the two regions suggesting that reactivation was a global and coordinated phenomenon [21, 56, 57]. Blocking the replay during sleep prevents subsequent memory retrieval [58, 59]. Using cerebral blood flow measurements, this phenomenon has been demonstrated in humans as well where it has been shown that hippocampal areas that are activated during route learning in a virtual town are reactivated during subsequent slow-wave sleep [60].
6. Sharp Waves and Ripples
The reactivation of these waking patterns in NREM sleep is most common during the population bursts known as sharp wave-ripples (SPW-R). Sharp waves emerge from the recurrent excitatory system of the hippocampal CA3 region and are transmitted along CA3 axonal (Schaffer) collaterals to the hippocampal CA1 region where they induce sharp waves (0.01 Hz to 3 Hz) in the CA1 apical dendritic layer. The synchronous discharging CA3 pyramidal cells not only activate the CA1 pyramidal cells but also perisomatic basket cell interneurons and the interaction between the pyramidal cells and these inhibitory interneurons gives rise to short-lived fast oscillations (140 Hz to 200 Hz) confined to the CA1 pyramidal cell layer known as ‘ripples’ [19, 54, 61]. Despite their super-synchronous appearance, neurons participating in this SPW-R activity are sequentially organized and this orderly structure reflects a temporally compressed version of the sequential neuronal firing patterns in the waking animal. These remarkable features of SPW-R activity have led to the hypothesis that these organized neuronal assemblies serve as a mechanism that transfers compressed spike sequences from the hippocampal CA1 region back to the neocortical regions from which they originated for long term storage when the brain is disengaged from environmental stimuli.
In its simplest version, this ‘two-stage’ memory consolidation model posits that during learning the neocortex provides the hippocampus with novel information leading to the transient synaptic reorganization of its circuits (waking theta phase in rodents) followed by the transfer of the modified hippocampal content back to the neocortex during NREM sleep where the memories may be consolidated. The formation of a permanent memory trace is not immediate with the transfer of information and repeated reactivation of hippocampal CA3-CA1 networks over time is usually required before it can take place [20, 62].
The hippocampal SPW-R complex has unique features that facilitate the transfer of neuronal patterns and the consolidation of synaptic plasticity. One of its major features is its widespread effect. In the approximately 100 msec. time window of a hippocampal sharp wave, 50,000-100,000 neurons discharge together in the CA3-CA1-suborbicular complex-entorhinal axis of the rat qualifying it as the most synchronous network pattern in the brain. This massive synchronous discharge would almost certainly alter synaptic connectivity within the neocortex [19, 54].
Additional evidence for the critical role of the SPW-R complex in the transfer of information from the hippocampus to the neocortex and its function in learning and memory consolidation comes from the impaired performance of rats trained on hippocampal-dependent spatial memory tasks following the selective elimination of SPW-R activity during sleep or even during quiet wakefulness with electrical stimulation during post-training consolidation periods [58, 59, 63].
Studies on rodents, in vivo and in vitro, have demonstrated that spatial memory can also be impaired by suppressing SPW-R activity with cannabinoid receptor agonists [64]. Activation of the cannabinoid receptor CB1 using THC, as well as synthetic agonists and even the endogenous endocannabinoid (eCB), anandamide (AEA), all disrupt the tightly organized spiking of SPW-R discharges and reduce their power and incidence [64-66]. THC appears to impair memory encoding by functionally isolating CA1 from CA3 [67]. Experimental work suggests that reduced transmitter release from glutamatergic terminals may be the primary cause of this effect. Decreased excitation of principal cells reduces their excitability and consequently the excitatory drive of interneurons. Under the influence of cannabinoids, neurons fail to organize into temporally coordinated assemblies. The upshot of this decreased synchrony is reduced effectiveness in the acquisition, consolidation and, as we shall see, even the retrieval of information. The cannabinoids impair neuronal synchronization within the hippocampus but also impair the tight neuronal integration between the hippocampus and other brain regions such as the prefrontal cortex and amygdala.
SPW-R activity has been found during sleep in all mammals investigated, including humans, as well as at a reduced rate during quiet wakefulness, i.e., during periods of immobility or consummatory behaviors such as eating and drinking which follow exploratory learning [19, 54, 68]. In humans, in a study that used macro electrodes to record the intracranial electroencephalogram from the hippocampus and rhinal cortex contralateral to the seizure onset zone, ripples occurred at the highest incidence during periods when the subject lay awake during a scheduled nap time [69]. The frequency of rhinal ripples was highly correlated with memory consolidation, measured as the number of correctly recollected items (post-nap) learned prior to sleep. More recent work in patients with epilepsy using subdural electrodes found that awake memory retrieval in humans involves a significant increase in ripple oscillations in the 80 Hz to 120 Hz frequency range in the medial temporal lobe and that this increased ripple activity is coupled with ripples in the temporal association cortex. Moreover, coupled ripples appear to reinstate cortical neural activity that was present during encoding. Coupled ripples, therefore, appear to constitute a neural mechanism for actively retrieving memory representations in the human brain [70]. These observations replicate corresponding studies in rodents and establish a role for both sleeping and waking SPW-R in memory formation and retrieval [27, 63, 71].
SPW-R discharges are the ultimate self-organized and endogenous hippocampal events because they most often appear when the animal has minimal or no contact with the environment. Sharp waves and associated neuronal burst discharges persist when the hippocampus is completely isolated from the environment, for example, after being transplanted into the brain cavity or the anterior chamber of the eye [19, 54]. SPW-R activity is an emergent event. It is not triggered but rather is “released” when the balance between the aminergic and cholinergic subcortical neuromodulators is altered and the suppressive effects on the excitability of the hippocampus are decreased.
7. Acetylcholine: Encoding and Retrieval
The initial encoding of new memories during the waking exploratory state and their subsequent consolidation during sleep are modulated by acetylcholine [30, 72]. A primary function of acetylcholine in the hippocampus is to reduce interference in the learning process by adaptively timing and separating memory encoding and retrieval. Cholinergic innervation of the hippocampus is achieved through the release of acetylcholine from septohippocampal nerve terminals and the vertical limb of the diagonal band. The amount released reflects the novelty detected in the environment. During active wakefulness, high levels of acetylcholine in the hippocampus favor sensory processing and the predominant flow of information from the entorhinal cortex to the hippocampal CA3 region where the episodic representations of events are registered [30]. During active wakefulness, neurons in layers II and III of the entorhinal cortex convey multimodal information into the hippocampus but neuronal activity in the output layers V and VI of the entorhinal cortex is reduced [24]. Feedback connections from CA3 are still functional and can mediate retrieval but do not dominate over the flow of information from the entorhinal cortex to the hippocampal CA3 region. Acetylcholine suppresses glutamatergic synaptic transmission at the recurrent excitatory collaterals in the CA3 region as well as at the Schaffer collaterals connecting the CA3 and CA1 regions and thus inhibits output from the CA1 region by inhibiting the connections between CA1 and the subiculum which constitute the first step to the entorhinal cortex and neocortex. Muscarinic receptor agonists have similarly been shown to suppress SPW-R complexes in vivo and in-vitro [73]. In essence, acetylcholine protects the encoding of new information from proactive interference arising from the activation of information stored in the CA3.
On the other hand, during quiet wakefulness or NREM sleep, the loss of cholinergic tone evident from the striking decrease in the concentration of acetylcholine found in the hippocampus with micro-dialysis releases the hippocampal SPW-R circuits from inhibition and allows the synchronous depolarization of the pyramidal cell population in the CA3 region and the accurate transmission of episodic memories to the entorhinal cortex and on to the neocortex. CA3, CA1, subicular and deep layer (V-VI) neurons have been shown to participate in a synchronized population burst at this time [24].
Micro-dialysis measurements during REM sleep demonstrate that acetylcholine levels in the hippocampus rise to levels above those seen during active wakefulness [30]. These high levels are consistent with the observed decrease in transmission through the hippocampus during REM sleep compared with the high levels of transmission from the hippocampus to the neocortex during NREM sleep. Thus, the transmission of episodic memories such as contextual fears and replicative nightmares from the hippocampus to the neocortex would not be expected during REM sleep.
While the reduction in cholinergic tone during quiet wakefulness and the even greater decline during NREM sleep releases SPW-R activity and facilitates the transfer of information and memory to the neocortex, acetylcholine also mediates the generation of the gamma (30-100 HZ) and theta (4-12 HZ) hippocampal oscillations present during active wakefulness and REM sleep that encodes sensory information, the first step in the acquisition of memory [61, 74-76]. Cholinergically induced gamma oscillations in the hippocampus are generated by a recurrent feedback loop composed of CA3 pyramidal cells and fast-spiking GABAergic parvalbumin containing basket cells while theta rhythms, in part, are generated by pacemaking GABAergic parvalbumin containing interneurons in the medial septum where cholinergic inputs from this region contribute to their generation [61, 74, 75, 77]. Cannabinoids may owe their capacity to impair working or short-term memory, in part, to the inhibition of acetylcholine release [78]. Cannabinoids have been shown to decrease acetylcholine release in the hippocampus through a CB1 receptor-mediated mechanism [79]. Activation of the CB1 receptor by cannabinoids appears to have the more generalized effect of interfering with the temporal coordination of cell assemblies in the hippocampus and this is the likely immediate cause for the impairment of all hippocampus-dependent memory be it memory encoding or the transfer of information from the hippocampus to the neocortex. This is mirrored by the reduction in power of all hippocampal oscillations by cannabinoids, gamma, theta and sharp wave associated ripples [64, 80]. Cannabinoids reduce the power of gamma, theta and ripple oscillations and reduce their spike timing coordination. These properties are held responsible for the memory impairments they induce.
8. An Attractor Network
The hippocampal CA3 region operates as a single attractor or auto-association network. In an auto-association network, a pattern is associated with itself by using recurrent collaterals [81]. In the rat, for example, there are approximately 12,000 recurrent collateral synapses on each of the 300,000 CA3 neurons. The CA3 network became known as an attractor network when it was recognized that partial patterns could be attracted to a basin of attraction. A cue would then be sufficient to elicit the entire memory. The hippocampal CA3 network receives information flowing to it from visual, auditory, parietal and prefrontal cortical association areas as well as from olfactory, taste and somatosensory areas. Information flowing to the hippocampal CA3 neurons from the amygdala and orbitofrontal cortex provides an emotional tone to the incoming information. SPW-R carries the information about the setting, temporal course and emotional response to this event. All high order cortical regions converge into a single network in the hippocampal CA3 region and make it possible for the hippocampus to accurately construct the panorama of sensory experience and emotion that constitute an episodic memory. Auto-association makes it possible to integrate this diverse information and makes one trial or ‘one shot’ learning possible. Auto-association also makes it possible to recall the entire memory from any of its parts. A traumatic event can be recorded in the mind after a single exposure and any subtle reminder, even the mood at the time, can bring it back to consciousness.
9. The Pain/Fear Circuit
The reciprocal connections between the hippocampus, the medial prefrontal cortex (mPFC) and the amygdala constitute a ‘fear/pain circuit’ that encodes and consolidates traumatic memories but that also engages in the extinction of these memories (Woodhams et al, 2017). Failure to extinguish the memory of a traumatic event all too often leaves chronic fear and its close companion, chronic pain, in its wake. The amygdala is hyperactive in fearful and painful states and mediates the expression of the emotional, affective aspects of these states. This hyperactivity may interfere with the functions of the mPFC and with this comes the loss of fear and pain extinction and the emergence of the cognitive impairment that accompanies states of chronic fear and pain [82, 83].
The hippocampus and mPFC have complementary roles in episodic memory processing and interact bidirectionally through oscillatory synchrony [84]. The hippocampus is crucial for organizing memories within the context in which they are experienced whereas the mPFC has an essential role in the retrieval of context-appropriate memories. There is also strong evidence in humans and rodents that the mPFC and the hippocampus are jointly involved during the integration of new information into new schemas. The mPFC and other cortical areas become more active during the retrieval of remotely acquired memories while the hippocampus becomes less activated at this time. Damage to the mPFC, in contrast to damage to the hippocampus, selectively blocks remotely acquired memories.
The mPFC receives monosynaptic input from the hippocampus as well as other bidirectional indirect input through thalamic and cortical regions and the hippocampal replay of the spatiotemporal sequences of an event has been shown to be strongly synchronized with the neural activity of the mPFC [84]. During waking states, phase locking of theta rhythms between the hippocampus and PFC mediates working memory and the acquisition of learned behaviors [85-87]. Cannabinoids disrupt these integrated rhythms and induce deficits in both the hippocampal theta rhythms and prefrontal gamma oscillations during working memory tasks [80]. Neurons that fire together in the mPFC during waking are also reactivated during subsequent NREM sleep in synchrony with hippocampal SPW-R activity, which then mediates the transfer of this cortical activity back to the mPFC during NREM sleep [61, 88]. Neural networks in the hippocampus and mPFC operate in tandem during NREM sleep as the SPW-R discharges during NREM are closely coupled to the cerebral slow oscillations and spindles and serve to transfer reactivated hippocampal memory information to respective neocortical and striatal sites [38]. This coupling requires very fine temporal precision as introducing a random delay as brief as 200 msec between hippocampal and cortical events is sufficient to prevent memory consolidation [27, 89, 90]. SPW-R activity occurs during both quiet wakefulness and sleep and experimental evidence suggests that hippocampal-PFC synchronization is even stronger during awake SPW-R activity than during sleep despite the absence of coupling to slow oscillations and spindles. Awake reactivation may support initial memory formation, retrieval and planning while sleep reactivation may have a broader role in memory consolidation [63, 91].
It remains to be determined how the contextual and emotional aspects of episodic memory, the site, the fear and the pain, for example, are combined to fully represent the event [92]. The amygdala and hippocampal circuits are believed to process the emotional and spatial components of an event. In rats, in the course of learning a contextual threat or spatial memory task, a small set of neurons become active in both the basolateral nucleus of the amygdala (BLA) and the hippocampus during post-training NREM sleep. These unique BLA neurons are recognized because they increase their firing rate during hippocampal SPW-R. Thus, SPW-R appears to be instrumental in establishing functional connections between the hippocampus and BLA to consolidate place threat associations. An earlier study demonstrated that suppression of sleep hippocampal SPW-R, known to consolidate spatial memories, also impairs contextual fear conditioning [93]. The hippocampus records the context of the event and the amygdala is remarkably able to identify the emotions associated with an event, be they rewarding or punitive [94, 95].
The importance of the amygdala in the control of social and emotional behaviour has been known for many years. Bilateral amygdalectomy in rhesus monkeys has long been known to impair the acquisition of avoidance behaviour, reduce fear responses and tame the animal [96]. In humans as well, damage to the amygdala appears to reduce fear sensitivity. A 14-year-old girl with selective bilateral amygdala damage due to Urbach-Wiethe disease had significantly lower fear sensitivity and impaired fear recognition, i.e., impaired recognition of fearful facial expression but the exact nature of the damage to the amygdala that leads to the loss of fear sensitivity remains to be determined [96, 97].
10. Fear and Pain: Acquisition and Extinction
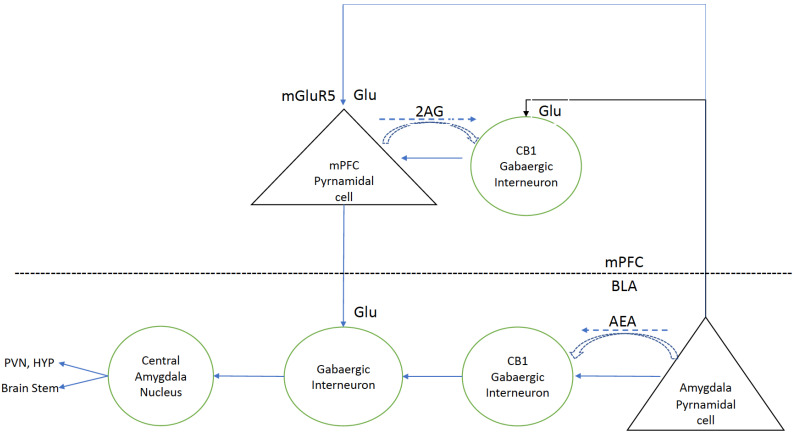
Fear learning and its extinction are controlled by the activity of three interconnected regions, the amygdala, prefrontal cortex and hippocampus [98]. Sensory inputs such as fear and pain are integrated in the BLA. The BLA, in turn, projects to the central amygdala (CeA) which is thought to be the main output structure of the amygdala. Downstream projections from the CeA trigger the peripheral manifestations of fear, freezing or fleeing, increased heart rate and adrenaline release (Fig. 1). Following the acquisition of a fear response, the memory of this response is transferred to the prelimbic (PL) region of the mPFC where further consolidation of the memory takes place. The PL has a central role in the expression and recall of learned fear. Stimuli which trigger a fear response can gradually lose their power if not reinforced. Memory for the fear triggering event can gradually be lost through a learning process known as extinction. The infralimbic (IL) region of the mPFC, ventral to the PL region, harbours these extinguishing fear memories. IL is engaged in both the consolidation and retrieval of these extinction memories [99]. Thus, it is proposed that fearful stimuli enter the BLA and are also transmitted to the PL. Signals from both the BLA and PL activate the CeA to trigger the fear response. Following extinction, the fearful stimulus again enters the BLA but also go on to excite neurons in the IL which project to intercalated cells (ITC) in the amygdala which, in turn, send inhibitory projections to the CeA to dampen its activity and the fear response and to extinguish aversive behavior [100].
Fig. (1).
Modified and published with permission from Woodhams et al. Neuropharmacology 124: 105-120, 2017. Medial pre-frontal Cortex: BLA pyramidal neurons, carrying signals encoding aversive stimuli or nociceptive signals, synapse at mGluR5 receptors on mPFC pyramidal cells as well as on inhibitory CB1 gabaergic interneurons. Normally, medial mPFC pyramidal neurons project back to ITC in the amygdala to inhibit the CeA central nucleus of the amygdala to inhibit the stress response. But, excessive concomitant activation of mPFC CB1 gabaergic interneurons prevents this. Release of 2AG or cannabinoids inhibits the actions of CB1 gabaergic interneurons. Basolateral Amygdala: Inhibition of CB1 gabaergic interneurons in the BLA reduces the activation of the CEA and this, in turn, reduces the activation of the HPA axis. Stress induced reduction in AEA levels in BLA neurons releases CB1 gabaergic interneurons from inhibition and this, in turn, activates the CEA and the HPA axis. See text for further discussion. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Cannabinoids are known to play an important role in the extinction of aversive/traumatic memories by activating CB1 receptors in the IL region of the mPFC [101-104]. Fig. 1 provides a simple model that explains how this comes about [105]. BLA pyramidal neurons carrying fear or pain stimuli synapse at mGluR5 receptors on mPFC pyramidal neurons and on gabaergic interneurons in the infralimbic mPFC and these interneurons, in turn, inhibit the prefrontal pyramidal neurons that normally project back to the ITCs in the CeA to inhibit the output of these cells. The CeA, in turn, projects to the hypothalamus and brain stem and, in the absence of infralimbic inhibition, elicits a stress response and systemic manifestations of fear such as trembling, sweating, tachycardia and increased blood pressure. Endogenous cannabinoids, released from pyramidal cells expressing mGluR5 receptors, or synthetic cannabinoids acting on CB1 receptors on the GABAergic interneurons, inhibit these interneurons and release the inhibition of the infralimbic pyramidal cells projecting to the ITC. Releasing this inhibition blocks the expression of fear and pain [102, 103].
The modulation of these neural circuits by cannabinoids in humans during fear extinction learning in humans has been demonstrated by fMRI studies which reveal that THC attenuated amygdala reactivity to a conditioned stimulus that had previously been paired to an unconditioned stimulus and that participants who received THC during extinction learning showed significantly more activation in the mPFC and hippocampus during extinction recall than subjects that had received a placebo. The effects of THC on extinction memory retention are evident even a week after learning and are associated with increased functional coupling between the mPFC and the hippocampus [106-108].
11. Stress and Memory: Consolidation, Retrieval and Extinction
Under stressful conditions, these closely integrated neural circuits may fail. In rodents, chronic stress alters the dendritic structure of PFC neurons and reduces their length, branching and spine density [109]. Neuroimaging studies in humans with post-traumatic stress also show signs of dendritic loss and impaired synaptogenesis. These studies, although not definitive, reveal increased amygdala activation in response to aversive emotional stimuli but smaller amygdala volumes, inconsistent changes in hippocampal activation but smaller volumes and both reduced activation and volume of the frontal cortex [110]. In chronic pain conditions, brain imaging has consistently demonstrated decreased grey matter density in the mPFC and, again, this decrease has been related to a decrease in dendritic length, volume and branching. Stress in the forms of high cortisol levels, inflammation and the release of immune mediators or alterations in neurotransmitter release are thought to account for these changes in dendritic morphology [111]. Failure of the integrated activities of the amygdala, hippocampus and mPFC under conditions of chronic stress may account for the persistence of fear and pain in these states [105].
Initially, under normal conditions, the synergistic actions of noradrenaline (NA) and cortisol (corticosterone in rats) made possible by the rapid rise in brain levels of these agents in response to stressful and traumatic events increase the probability of permanently registering the memory of these events in the brain’s fear and pain circuits [112]. Emotionally significant experiences, good and bad, tend to be well remembered. Under experimental conditions, these agents are most effective when given just before or after the event to be remembered. However, their effects on memory consolidation follow an inverse U-shaped dose-response curve. Moderate doses enhance memory consolidation but lower or higher doses may be less effective and may even impair consolidation. Memory consolidation is initiated by the actions of norepinephrine and glucocorticoids at the BLA and while inactivating the amygdala prevents memory consolidation, the BLA is not the permanent site of the consolidated memory. Extensive study has demonstrated that the release of NE into the BLA specifically influences the process by which a fragile short-term memory trace is transferred and consolidated into a stable long-term memory in many target areas such as the hippocampus, caudate and cortical regions [113, 114]. The rise in NE levels in the BLA in response to emotionally arousing stimuli selectively enhances the memory of these stimuli compared with neutral stimuli [115]. Patients with Urbach-Wiethe disease who have bilateral damage to the amygdala demonstrate selective memory impairment for emotional material in the presence of otherwise normal memory [116].
The enhancing effect of adrenal stress hormones on memory consolidation depends on the integrity of the amygdala noradrenergic system. Adrenaline, released from the adrenal gland together with cortisol in response to stress, activates β-adrenoreceptor (β-AR) on vagal afferents that project to the nucleus tractus solitarius (NTS) and on to locus coeruleus in the hindbrain to release noradrenaline (NA) into the amygdala. Infusions of β-AR antagonists into the amygdala block the memory-enhancing effects of peripherally released adrenaline or the effects of NA introduced directly into the amygdala. Glucocorticoids may act on the NTS to increase NA release but also require noradrenergic activation within the amygdala to promote the consolidation of emotionally arousing memories. Thus, glucocorticoid actions can also be blocked by β-AR antagonists.
β-AR agonists improve memory consolidation by stimulating the cAMP/PKA pathway and glucocorticoids appear to act by potentiating this pathway. Experimental work has demonstrated that NA acting on β-AR in the amygdala regulates both the acquisition and consolidation phases of aversive memory formation through distinct, temporally regulated signaling pathways [117]. NA binds to G-protein coupled β-ARs to produce cyclic AMP (cAMP) which, in turn, activates a variety of signaling pathways such as cAMP-dependent protein kinase and extracellular regulated kinase (ERK). During aversive memory acquisition, these kinases rapidly phosphorylate GluA1 subunits of AMPA receptors to enhance their function and recruit their insertion into postsynaptic sites. ERK, which induces gene transcription and protein synthesis is subsequently required for memory consolidation. Weak memory formation does not require AMPA receptors or ERK activation, unlike the formation of salient memories in response to a strong shock. GC acts at intranuclear receptors to affect gene transcription but also has more immediate non-genomic actions at membrane-associated variant(s) of the steroid receptor which result in a rapid increase in the probability of glutamate release from presynaptic sites and rapid insertion of AMPA-receptor subunits into postsynaptic terminals. GC and NE thus appear to act synergistically to rapidly enhance AMPA receptor function. The combined action of these agents has been shown to increase glutaminergic transmission within the BLA. GC and NE acting together may also affect cellular epigenetics by altering histone acetylation and, in this way, influence memory storage.
While exposure to emotionally arousing events and the combined actions of noradrenaline and GC may facilitate the consolidation and storage of new information, high circulating levels of glucocorticoids have also been shown to impair the retrieval of memory and particularly hippocampal-dependent memories, i.e., contextual, spatial and declarative memory [118]. This effect has been demonstrated both in man and rats and, again, requires the integrity of the BLA and the contribution of NA [119-122]. In man, GC administration to stress levels shortly before retention testing impairs hippocampal-dependent recall of previously learned verbal material. This effect can be blocked by the concurrent use of β-AR blockers [120, 123]. Similarly, in rats, the systemic injection of corticosteroids has been shown to impair tasks that rely on spatial or contextual information as well as other learned behaviors. The infusion of glucocorticoid receptor agonists directly into the hippocampus has the same effect but, again, can be blocked by β-AR antagonists administered either systemically or directly into the hippocampus or BLA shortly before testing [121]. These effects of GC on memory retrieval are temporary and dissipate when GC levels return to baseline. The release of endocannabinoids in response to stress and the ensuing increase in noradrenaline levels can be shown to work in tandem with GC to impair memory retrieval by GC. CB1 agonists can duplicate this effect on memory retrieval and local infusions of β-AR antagonists can again be shown to prevent this effect [118].
The endocannabinoid system appears to play an essential role in buffering the interactions between GC and the noradrenergic system in memory consolidation and retrieval and can induce distinct and even opposite effects on anxiety and cognition depending on the stress level and aversiveness of the context [124, 125]. Endocannabinoids bind to CB1 receptors on gabaergic interneurons and suppresses the release of GABA and this, in turn, leads to an increase in NE and promotes the consolidation of the memory of aversive events [125, 126]. This consolidating effect can be duplicated by the administration of synthetic CB1 agonists directly into the amygdala immediately after inhibitory avoidance training. However, much like the effects of GC on memory consolidation, cannabinoids appear to have an inverted U-shaped dose-response relationship on memory consolidation as higher doses, actions at different sites and different experimental paradigms reveal that cannabinoids can interfere with memory consolidation [127-129].
Cannabinoids, as discussed earlier, also promote the extinction of aversive memories. GC share this capacity. GC promotes the consolidation of memory and, thus, the consolidation of extinction memory [114]. In man, a recent fMRI study demonstrated that cortisol administration before extinction training diminished conditioned fear responses during extinction learning. It reduced neural activation in the amygdala-hippocampal complex and increased functional connectivity of the parahippocampal gyrus to the IL during early extinction learning. These changes were associated with the inhibited fear recall. Thus, the concerted action of the hippocampus and IL during extinction recall was associated with activation of the extinction memory trace and the suppression of the original fear memory trace. This is in line with the proposed role of the hippocampus in conveying contextual information and that of the IL in inhibiting conditioned fear responses by projections to inhibitory intercalated cells in the amygdala [130].
12. The Endocannabinoid System
The eCB are a group of naturally occurring members of the eicasanoid superfamily that can activate cannabinoid receptors. As such, eCB can act like exogenously delivered cannabinoids to impact memory functions and the brain’s response to stress. eCB are derivatives of long-chain fatty acids, primarily arachidonic acid. They are released from neurons in response to neurotransmitters and steroids and from immune cells in response to inflammatory agents [5]. There are two major lipid signaling agents in the eCB system, anandamide (AEA) and 2-arachidonyl glycerol (2-AG) together with the enzymes engaged in their synthesis and metabolism, two cannabinoid receptors, CB1 and CB2, reuptake mechanisms and the ionotropic transient receptor potential vanilloid receptor type 1 [131]. The eCB, AEA and 2-AG, are short-lived messengers [132]. They are synthesized in postsynaptic neurons in response to specific signals but each one responds differently to these signals in different species and at different sites in the brain [133]. They act retrogradely on presynaptic cannabinoid receptors to affect both the short and long-term suppression of neurotransmitter release and to counteract the release of inflammatory mediators. AEA is a partial agonist at these receptors, while 2-AG is a full agonist [131]. Most research suggests that acute stress produces a mobilization of 2-AG while concurrently reducing the tissue content of AEA [133]. Nevertheless, they appear to act together to control the activity of the hypothalamic-pituitary axis (HPA). For example, the release of eCB within the paraventricular nucleus (PVN) of the hypothalamus in response to rising levels of GC suppresses incoming excitatory neurotransmission to corticotropin releasing hormone neurosecretory cells and serves as a means by which GC can rapidly feedback to shut down the HPA axis and the release of GC [134]. This effect can be duplicated by CB1 agonists acting at the PVN. The inhibition of the HPA axis is supported by eCB acting on CB1 receptors on gabaergic interneurons in the BLA to reduce the release of GABA which, in turn, reduces the inhibition gabaergic interneurons in the intercalated nuclei and this in turn increases the inhibition the CeA normally exerts on the excitatory pyramidal neurons projecting to the HPA (Fig. 1). CB1 agonists have also been shown to decrease the excitability of projection neurons in the BLA. The end result again is the reduced activation of the HPA axis, a reduction in the stress induced increase in GC levels and, thus, the prevention of the enhancing effect of stress on emotional memory [129]. On the other hand, while 2-AG acts directly on CB1 receptors in the PVN to turn off the HPA axis, a decrease in AEA levels in the amygdala in response to stress can release projection neurons to the HPA from inhibition and ultimately increases neural activity within the PVN and HPA axis. High basal levels of AEA normally maintain inhibitory control over these projection neurons. Thus, eCB signaling is involved in the homeostatic control, both activation and inhibition, of the HPA axis [133]. In humans, imaging studies reveal that cannabinoid agonists can significantly reduce amygdala reactivity to signals of social threat [135]. The diverse effects of eCB and the cannabinoids at different sites helps explain their apparent capacity to either enhance or interfere with memory.
Cannabinoid receptors have been identified in both the central nervous system and peripheral organs. CB1 receptors are principally expressed in the brain in multiple regions including the thalamus, basal ganglia, prefrontal cortex, amygdala and hippocampus and generally to a lesser degree in many peripheral organs. CB1 receptors are expressed at an especially high density in the CA1 and CA3 regions of the hippocampus [124, 136]. They can be found at presynaptic sites predominantly on inhibitory gabaergic interneurons expressing the anxiety-inducing peptide, cholecystokinin, but not on parvalbumin-positive fast-spiking basket cells. They are also present on the terminals of glutamatergic neurons but at a much lower density than on inhibitory gabaergic interneurons. Activation of CB1 receptors leads to the suppression of GABA and glutamate release and as well as to the inhibition of acetylcholine, noradrenaline, dopamine and serotonin release [137]. Thus, while altered neurotransmitter release may impair learning and memory by disturbing the coordination of the hippocampus within its own circuits and with its projections to other vital brain centres, gathering evidence indicates that CB1 agonists may also regulate memory formation by engaging signal transduction pathways [137-140]. The activation of the mTOR pathway by CB-1 receptor agonists is one example. Activation of this pathway impairs object recognition and context recognition-two cognitive functions associated with the hippocampus. Inhibiting this pathway with rapamycin prevents these memory deficits.
CB2 receptors are mostly located peripherally on immunological tissue. Activation of CB2 receptors leads to the increased production and release of pro-resolving eicosanoids and the eventual resolution of inflammation [5]. In the central nervous system, CB2 receptors under physiological conditions have been identified on neurons and glia in many regions of the brain, such as the cerebellum and hippocampus [124]. However, CB2 expression markedly increases on reactive microglia in the central nervous system in response to inflammation or injury [141]. Reactive microglia release several inflammatory cytokines and chemokines that can be damaging to adjacent neurons and that can generate pain. To this end, new synthetic cannabinoids are being developed targeting the CB2 receptor. Ajulemic acid, a synthetic cannabinoid with 12 times greater affinity for CB2 than CB1, targets a diverse array of inflammatory processes with potent associated analgesic effects in both animal models and human subjects and without the psychotropic effects found with THC [5].
13. Stress, Memory and Inflammation
Even in the absence of physical injury, stress can elicit an inflammatory response in the brain in critical regions such as the hippocampus, amygdala and prefrontal cortex which independently of other factors can interfere with learning and memory. In mice, stress induced by subchronic immobilization and by acoustic stress results in the massive release of the excitatory amino acid glutamate together with pro-inflammatory cytokines such as TNF-α as well as the release of NF-κB and pro-inflammatory enzymes like NOS-2 and COX-2 and consequent cellular oxidative and nitrosative damage (lipid peroxidation). CB2 agonists prevent this stress-induced excitotoxic and neuroinflammatory response without alteration in plasma corticosterone levels. Activation of CB1 receptors also elicited a neuroprotective response in the same experimental paradigm [142]. In rats, single prolonged stress, a forced 20-minute prolonged swim, induced mechanical allodynia and anxiety-like behavior accompanied by increased the expression of microglia in the hippocampal CA1 and CA3 regions and significantly increased levels of the proinflammatory cytokines TNF-α and IL-1β. The results were thought to model the common co-development of PTSD and chronic pain [143]. Muhie et al. [144] used a social stress mouse model based on the resident intruder paradigm to simulate features of post-traumatic stress disorder in man. However, evidence suggests that IL-1β may be required for the normal physiological regulation of hippocampal-dependent memory [145, 146]. IL-1β gene expression is induced in the hippocampus 24 hours following fear conditioning and blocking its actions impairs stress enhanced fear learning [147]. IL-1β is upregulated by context fear conditioning and small increases (1 ng) of IL-1β injected centrally enhance context fear conditioning while IL-1 receptor antagonists block context fear conditioning and spatial memory. However, acute intrahippocampal injection of IL-1β impairs context fear conditioning and its chronic overexpression in the hippocampus impairs spatial memory. Thus, the exact function of IL-1β remains to be determined and its actions appear to depend on timing, site of injection and dose [145]. The evidence suggests that although a basal level of IL-1β is required for memory formation, both excessive and insufficient amounts of IL-1β impair memory formation. The involvement of IL-1β in hippocampal dependent memory appears to follow an inverted U-shaped dose-response curve in which a slight increase in brain IL-1β levels can improve memory while any deviation from the physiological range, either high or low, impairs memory [146]. Targeted deletion of IL-1β in animals leads to severely impaired hippocampal memory [146]. However, manipulating a single cytokine alters inflammatory signaling by regulating the levels of other cytokines. Furthermore, relatively short-acting cytokine activity cannot easily explain the long-lasting memory and cognitive changes which develop after a traumatic event. Memory and inflammatory signaling cascades must intersect to mediate the long-lasting effects of inflammation on memory and cognitive functions. Memory and inflammatory signaling could intersect to produce epigenetic modifications that mediate the long-lasting effects of inflammation on memory and cognitive function [145]. Thus, cytokines share with cannabinoids and steroids an inverted U-shaped dose-response homeostatic system which interacts with neurotransmitters, glucocorticoids and inflammatory mediators to control the flow of information into the brain and its response to physical and psychological stress [146]. This complex interaction makes it difficult to define an exact dose-response relationship to any of its elements.
14. Nightmares, Cannabinoids and the Post-traumatic Stress Disorder
The ability of cannabinoids to interfere with the retrieval of episodic memories and to promote the extinction of memories should make them ideal drugs for treating replicative post-traumatic nightmares as these nightmares reflect the nightly cycle of episodic memory retrieval and reconsolidation of the fearful event as well as the failure to extinguish this fearful memory. Thus, it is not surprising that nabilone and THC have proven to be remarkably effective in eliminating or mitigating post-traumatic nightmares. In 2009, Fraser treated 47 patients with post-traumatic stress disorder (PTSD) and treatment-resistant nightmares with nabilone in doses ranging from 0.2 mg to 4.0 mg nightly. A majority of the patients, (72%), experienced either a total cessation of nightmares or a reduction in their intensity. Some patients noted improved sleep and fewer daytime flashbacks. These findings were corroborated in a larger retrospective study in a correctional institution [3]. Treatment with nabilone led to a significant improvement in PTSD symptoms and the associated insomnia and nightmares. These improvements were maintained over a mean period of 11 weeks. There was also a subjective improvement in chronic pain whether musculoskeletal or other. Subsequently, a randomized, double-blind, placebo-controlled cross-over designed study was conducted in military personnel with PTSD [4]. Nabilone produced a significant reduction in the Clinician-Administered PTSD Scale (CAPS) Recurring and Distressing Dream Score and a significant improvement in the Clinical Global Impression of Change. Subjects in this study did not report the suppression of normal dreams. In a preliminary open-label study, 5 mg of oral THC given as an add-on treatment to subjects with PTSD also significantly reduced nightmares and hyperarousal and improved the quality of sleep [6].
Conclusion
With the widespread use of many different types of cannabinoid formulations, a growing consensus has emerged that while cannabinoids can palliate many of the symptoms of post-traumatic stress disorder to varying degrees, they cannot cure the disorder [7, 148-150]. In the case of nabilone, for example, nightmares may return when the drug is discontinued even after a year [2]. Since cannabinoids appear to be able to eliminate nightmares and other post-traumatic symptoms in patients in whom these symptoms have existed for long periods of time, how soon after a traumatic event would they become effective? The data reviewed earlier indicate that cannabinoids can interfere with the initial acquisition and registration of a traumatic event and can also suppress the stress, the pain and the inflammatory response, which enhance the salience of these events [151, 152]. Could cannabinoids be used to interfere with the development of post-traumatic-stress disorders by treating patients, where possible, with these agents as soon as possible after a traumatic event? In the particular case of motor vehicle accidents and whiplash injuries where pain and fear are often mild or even absent in the immediate aftermath of the collision but grow in severity in the hours and days that follow, could the immediate post-accident application of a cannabinoid abort or mitigate the development of post-traumatic symptomatology? This is a critical matter as once PTSD takes hold, it can be very difficult to effectively treat or even palliate the chronic pain, fear, nightmares and memory problems that may persist for years [153]. Pain critically influences the severity of post-traumatic symptomatology that follows an injury. In whiplash injuries, the initial severity of the pain and the associated anxiety have been identified as the major factors determining long term outcome [154]. In Iraq, soldiers treated with morphine within 48 hours of combat injuries were found less likely to develop PTSD [155]. Morphine has been shown to prevent stress enhanced fear learning. It also reduces the expression of IL-1β levels in the hippocampus [147]. Steroids can also inhibit the production and secretion of Il-1β by microglia and have also been used to prevent the onset of PTSD after trauma and mitigate its severity [156, 157]. Although there are no reported studies on the effects of steroids on brain cytokine levels in stressed animals, in man, steroid doses that can interfere with the retrieval of emotional memories, reduce plasma cytokine levels [158]. Should THC or nabilone, agonists at both CB1 and CB2 receptors with known anti-stress, anti-nociceptive and anti-inflammatory properties be started prophylactically immediately after an accident injury to prevent or mitigate the development of post-traumatic stress disorders? Might the judicious use of steroids or opiates be combined with cannabinoids to prevent or mitigate this disorder?
Acknowledgements
Declared none.
list of Abbreviations
- 2-AG
2-arachidonyl glycerol
- AEA
Anandamide
- AMPA
Alpha-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid
- BLA
Basolateral nucleus of the amygdala
- CA1
Cornu ammonis 1
- CA3
Cornu ammonis 3
- CB1
Cannabinoid 1 receptor
- CB2
Cannabinoid 2 receptor
- CeA
Central nucleus of the amygdala
- COX-2
Cyclo oxygenase-2
- eCB
Endocannabinoids
- ERK
Extracellular regulated kinase
- fMRI
Functional magnetic resonance imaging
- GC
Glucocorticoid
- GluA
Glutamate ionotropic receptor AMPR type
- HYP
Hypothalamus
- IL
Infralimbic
- IL-β1
Interleukin beta 1
- ITC
Intercalated cells
- mPFC
Media prefrontal cortex
- NA
Noradrenaline
- NF-kB
Nuclear factor kappa-light-chain enhancer of activated B cells
- NOS-2
Nicric oxide synthase-2
- NREM
Non-rapid eye movement sleep
- NTS
Nucleus tractus solitarius
- PL
Prelimbic
- PVN
Paraventricular nucleus
- REM
Rapid eye movement sleep
- SPW-R
Sharp wave - ripple
- THC
Δ9 tetrahydrocannabinol
- TNF-α
Tumor necrosis factor-alpha
- vmPFC
Ventro medial (infralimbic) prefrontal cortex
- β-AR
Beta adrenergic receptor
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Morgenthaler T.I., Auerbach S., Casey K.R., Kristo D., Maganti R., Ramar K., Zak R., Kartje R. Position paper for the treatment of nightmare disorder in adults: an american academy of sleep medicine position paper. J. Clin. Sleep Med. 2018;14(6):1041–1055. doi: 10.5664/jcsm.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser G.A. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci. Ther. 2009;15(1):84–88. doi: 10.1111/j.1755-5949.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron C., Watson D., Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J. Clin. Psychopharmacol. 2014;34(5):559–564. doi: 10.1097/JCP.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jetly R., Heber A., Fraser G., Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–588. doi: 10.1016/j.psyneuen.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Zurier R.B., Burstein S.H. Cannabinoids, inflammation, and fibrosis. FASEB J. 2016;30(11):3682–3689. doi: 10.1096/fj.201600646R. [DOI] [PubMed] [Google Scholar]
- 6.Roitman P., Mechoulam R., Cooper-Kazaz R., Shalev A. Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin. Drug Investig. 2014;34(8):587–591. doi: 10.1007/s40261-014-0212-3. [DOI] [PubMed] [Google Scholar]
- 7.Hindocha C., Cousijn J., Rall M., Bloomfield M.A.P. the effectiveness of cannabinoids in the treatment of posttraumatic stress disorder (PTSD): A systematic review. J. Dual Diagn. 2019;•••:1–20. doi: 10.1080/15504263.2019.1652380. [DOI] [PubMed] [Google Scholar]
- 8.The International Classification of Sleep Disorders . Diagnostic and Coding Manual. Illinois: Westchester; 2005. [Google Scholar]
- 9.Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA: American Psychiatric Publishing; 2013. [DOI] [PubMed] [Google Scholar]
- 10.Corballis M.C. In: Cambridge Handb. Conscious; Zelazo, P.D.; Moscovitch, M. Thompson E., editor. pp. 571–596. [Google Scholar]
- 11.Stickgold R. Learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- 12.Tulving E. Memory and consciousness. Can. Psychol. 1985;26:1–12. doi: 10.1037/h0080017. [DOI] [Google Scholar]
- 13.Wittmann L., Schredl M., Kramer M. Dreaming in posttraumatic stress disorder: A critical review of phenomenology, psychophysiology and treatment. Psychother. Psychosom. 2007;76(1):25–39. doi: 10.1159/000096362. [DOI] [PubMed] [Google Scholar]
- 14.Freyberger H.J., Freyberger H. Sechzig Jahre danach: Posttraumatische Belastungsstörungen, salutogene Faktoren und gutachterliche Einschätzungen bei Holocaust-Uberlebenden im Langzeitverlauf. Z. Psychosom. Med. Psychother. 2007;53(4):380–392. doi: 10.13109/zptm.2007.53.4.380. [DOI] [PubMed] [Google Scholar]
- 15.Schreuder B.J.N., Kleijn W.C., Rooijmans H.G.M. Nocturnal re-experiencing more than forty years after war trauma. J. Trauma. Stress. 2000;13(3):453–463. doi: 10.1023/A:1007733324351. [DOI] [PubMed] [Google Scholar]
- 16.Born J., Wilhelm I. System consolidation of memory during sleep. Psychol. Res. 2012;76(2):192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31(3):551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 18.Buzsáki G. The hippocampo-neocortical dialogue. Cereb. Cortex. 1996;6(2):81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 19.Buzsáki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25(10):1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura T., Ogawa S.K., Roy D.S., Okuyama T., Morrissey M.D., Smith L.M., Redondo R.L., Tonegawa S. 2017. [DOI] [PMC free article] [PubMed]
- 21.O’Neill J., Pleydell-Bouverie B., Dupret D., Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33(5):220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Sekeres M.J., Winocur G., Moscovitch M. The hippocampus and related neocortical structures in memory transformation. Neurosci. Lett. 2018;680:39–53. doi: 10.1016/j.neulet.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Lewis P.A., Durrant S.J. 2011. [Google Scholar]
- 24.Chrobak J.J., Buzsáki G. Selective activation of deep layer (V-VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J. Neurosci. 1994;14(10):6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyce R., Williams S., Adamantidis A. REM sleep and memory. Curr. Opin. Neurobiol. 2017;44:167–177. doi: 10.1016/j.conb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Giuditta A. Sleep memory processing: the sequential hypothesis. Front. Syst. Neurosci. 2014;8:219. doi: 10.3389/fnsys.2014.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang W., Jadhav S.P. Sharp-wave ripples as a signature of hippocampal-prefrontal reactivation for memory during sleep and waking states. Neurobiol. Learn. Mem. 2019;160:11–20. doi: 10.1016/j.nlm.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fosse M.J., Fosse R., Hobson J.A., Stickgold R.J. Dreaming and episodic memory: a functional dissociation? J. Cogn. Neurosci. 2003;15(1):1–9. doi: 10.1162/089892903321107774. [DOI] [PubMed] [Google Scholar]
- 29.Nir Y., Tononi G. 2010. [Google Scholar]
- 30.Hasselmo M.E. 1999. [Google Scholar]
- 31.Mizuseki K., Miyawaki H. Hippocampal information processing across sleep/wake cycles. Neurosci. Res. 2017;118:30–47. doi: 10.1016/j.neures.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Rasch B., Pommer J., Diekelmann S., Born J. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat. Neurosci. 2009;12(4):396–397. doi: 10.1038/nn.2206. [DOI] [PubMed] [Google Scholar]
- 33.Siegel J.M. REM sleep: a biological and psychological paradox. Sleep Med. Rev. 2011;15(3):139–142. doi: 10.1016/j.smrv.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vertes R.P., Eastman K.E. The case against memory consolidation in REM sleep. Behav. Brain Sci. 2000;23(6):867–876. doi: 10.1017/S0140525X00004003. [DOI] [PubMed] [Google Scholar]
- 35.Fogel S.M., Smith C.T. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci. Biobehav. Rev. 2011;35(5):1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Gulati T., Ramanathan D.S., Wong C.C., Ganguly K. Reactivation of emergent task-related ensembles during slow-wave sleep after neuroprosthetic learning. Nat. Neurosci. 2014;17(8):1107–1113. doi: 10.1038/nn.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyce R., Glasgow S.D., Williams S., Adamantidis A. 2016.
- 38.Klinzing J.G., Niethard N., Born J. Publisher Correction: Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 2019;22(10):1743–1744. doi: 10.1038/s41593-019-0507-z. [DOI] [PubMed] [Google Scholar]
- 39.Nishida M., Pearsall J., Buckner R.L., Walker M.P. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb. Cortex. 2009;19(5):1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sopp M.R., Michael T., Weeß H.G., Mecklinger A. Remembering specific features of emotional events across time: The role of REM sleep and prefrontal theta oscillations. Cogn. Affect. Behav. Neurosci. 2017;17(6):1186–1209. doi: 10.3758/s13415-017-0542-8. [DOI] [PubMed] [Google Scholar]
- 41.Xia Z., Storm D. Role of circadian rhythm and REM sleep for memory consolidation. Neurosci. Res. 2017;118:13–20. doi: 10.1016/j.neures.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phelps A.J., Kanaan R.A.A., Worsnop C., Redston S., Ralph N., Forbes D. An ambulatory polysomnography study of the post-traumatic nightmares of post-traumatic stress disorder. Sleep (Basel) 2018;41(1):zsx188. doi: 10.1093/sleep/zsx188. [DOI] [PubMed] [Google Scholar]
- 43.Richards A., Kanady J.C., Neylan T.C. Sleep disturbance in PTSD and other anxiety-related disorders: an updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms. Neuropsychopharmacology. 2020;45(1):55–73. doi: 10.1038/s41386-019-0486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schredl M., Schmitt J., Hein G., Schmoll T., Eller S., Haaf J. Nightmares and oxygen desaturations: is sleep apnea related to heightened nightmare frequency? Sleep Breath. 2006;10(4):203–209. doi: 10.1007/s11325-006-0076-8. [DOI] [PubMed] [Google Scholar]
- 45.Passie T., Emrich H.M., Karst M., Brandt S.D., Halpern J.H. Mitigation of post-traumatic stress symptoms by Cannabis resin: a review of the clinical and neurobiological evidence. Drug Test. Anal. 2012;4(7-8):649–659. doi: 10.1002/dta.1377. [DOI] [PubMed] [Google Scholar]
- 46.Gates P.J., Albertella L., Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep Med. Rev. 2014;18(6):477–487. doi: 10.1016/j.smrv.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Nicholson A.N., Turner C., Stone B.M., Robson P.J. Effect of Δ-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J. Clin. Psychopharmacol. 2004;24(3):305–313. doi: 10.1097/01.jcp.0000125688.05091.8f. [DOI] [PubMed] [Google Scholar]
- 48.Babson K.A., Sottile J., Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr. Psychiatry Rep. 2017;19(4):23. doi: 10.1007/s11920-017-0775-9. [DOI] [PubMed] [Google Scholar]
- 49.Destexhe A., Hughes S.W., Rudolph M., Crunelli V. Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 2007;30(7):334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girardeau G., Zugaro M. Hippocampal ripples and memory consolidation. Curr. Opin. Neurobiol. 2011;21(3):452–459. doi: 10.1016/j.conb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Payne J.D. Sleep on it!: stabilizing and transforming memories during sleep. Nat. Neurosci. 2011;14(3):272–274. doi: 10.1038/nn0311-272. [DOI] [PubMed] [Google Scholar]
- 52.Schwindel C.D., McNaughton B.L. Hippocampal-cortical interactions and the dynamics of memory trace reactivation. Prog. Brain Res. 2011;193:163–177. doi: 10.1016/B978-0-444-53839-0.00011-9. [DOI] [PubMed] [Google Scholar]
- 53.Sirota A., Csicsvari J., Buhl D., Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl. Acad. Sci. USA. 2003;100(4):2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buzsáki G. Rhythm. Brain. Oxford University Press; 2006. pp. 334–356. [Google Scholar]
- 55.Wilson M., McNaughton B. 1994.
- 56.Ji D., Wilson M.A. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 2007;10(1):100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 57.Qin Y-L., McNaughton B.L., Skaggs W.E., Barnes C.A. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997;352(1360):1525–1533. doi: 10.1098/rstb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ego-Stengel V., Wilson M.A. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20(1):1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Girardeau G., Benchenane K., Wiener S.I., Buzsáki G., Zugaro M.B. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 2009;12(10):1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 60.Peigneux P., Laureys S., Fuchs S., Collette F., Perrin F., Reggers J., Phillips C., Degueldre C., Del Fiore G., Aerts J., Luxen A., Maquet P. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44(3):535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Colgin L.L. Rhythms of the hippocampal network. Nat. Rev. Neurosci. 2016;17(4):239–249. doi: 10.1038/nrn.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakashiba T., Buhl D.L., McHugh T.J., Tonegawa S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron. 2009;62(6):781–787. doi: 10.1016/j.neuron.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jadhav S.P., Kemere C., German P.W., Frank L.M. 2012. [DOI] [PMC free article] [PubMed]
- 64.Robbe D., Montgomery S.M., Thome A., Rueda-Orozco P.E., McNaughton B.L., Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat. Neurosci. 2006;9(12):1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- 65.Maier N., Morris G., Schuchmann S., Korotkova T., Ponomarenko A., Böhm C., Wozny C., Schmitz D. Cannabinoids disrupt hippocampal sharp wave-ripples via inhibition of glutamate release. Hippocampus. 2012;22(6):1350–1362. doi: 10.1002/hipo.20971. [DOI] [PubMed] [Google Scholar]
- 66.Sun Y., Norimoto H., Pu X-P., Matsuki N., Ikegaya Y. Cannabinoid receptor activation disrupts the internal structure of hippocampal sharp wave-ripple complexes. J. Pharmacol. Sci. 2012;118(2):288–294. doi: 10.1254/jphs.11199FP. [DOI] [PubMed] [Google Scholar]
- 67.Sandler R.A., Fetterhoff D., Hampson R.E., Deadwyler S.A., Marmarelis V.Z. Cannabinoids disrupt memory encoding by functionally isolating hippocampal CA1 from CA3. PLOS Comput. Biol. 2017;13(7):e1005624. doi: 10.1371/journal.pcbi.1005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bragin A., Engel J., Jr, Wilson C.L., Fried I., Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999;9(2):137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137:AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 69.Axmacher N., Elger C.E., Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131(Pt 7):1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 70.Vaz A.P., Inati S.K., Brunel N., Zaghloul K.A. 2019.
- 71.Wu C-T., Haggerty D., Kemere C., Ji D. Hippocampal awake replay in fear memory retrieval. Nat. Neurosci. 2017;20(4):571–580. doi: 10.1038/nn.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Easton A., Douchamps V., Eacott M., Lever C. A specific role for septohippocampal acetylcholine in memory? Neuropsychologia. 2012;50(13):3156–3168. doi: 10.1016/j.neuropsychologia.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norimoto H., Mizunuma M., Ishikawa D., Matsuki N., Ikegaya Y. Muscarinic receptor activation disrupts hippocampal sharp wave-ripples. Brain Res. 2012;1461:1–9. doi: 10.1016/j.brainres.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 74.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- 75.Colgin L.L. Mechanisms and functions of theta rhythms. Annu. Rev. Neurosci. 2013;36:295–312. doi: 10.1146/annurev-neuro-062012-170330. [DOI] [PubMed] [Google Scholar]
- 76.Holderith N., Németh B., Papp O.I., Veres J.M., Nagy G.A., Hájos N. Cannabinoids attenuate hippocampal γ oscillations by suppressing excitatory synaptic input onto CA3 pyramidal neurons and fast spiking basket cells. J. Physiol. 2011;589(Pt 20):4921–4934. doi: 10.1113/jphysiol.2011.216259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gulyás A.I., Szabó G.G., Ulbert I., Holderith N., Monyer H., Erdélyi F., Szabó G., Freund T.F., Hájos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J. Neurosci. 2010;30(45):15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goonawardena A.V., Robinson L., Hampson R.E., Riedel G. Cannabinoid and cholinergic systems interact during performance of a short-term memory task in the rat. Learn. Mem. 2010;17(10):502–511. doi: 10.1101/lm.1893710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carta G., Nava F., Gessa G.L. Inhibition of hippocampal acetylcholine release after acute and repeated Δ9-tetrahydrocannabinol in rats. Brain Res. 1998;809(1):1–4. doi: 10.1016/S0006-8993(98)00738-0. [DOI] [PubMed] [Google Scholar]
- 80.Kucewicz M.T., Tricklebank M.D., Bogacz R., Jones M.W. Dysfunctional prefrontal cortical network activity and interactions following cannabinoid receptor activation. J. Neurosci. 2011;31(43):15560–15568. doi: 10.1523/JNEUROSCI.2970-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rolls E.T. The storage and recall of memories in the hippocampo-cortical system. Cell Tissue Res. 2018;373(3):577–604. doi: 10.1007/s00441-017-2744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thompson J.M., Neugebauer V. Amygdala plasticity and pain. Pain Res. Manag. 2017;2017:8296501. doi: 10.1155/2017/8296501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thompson J.M., Neugebauer V. Cortico-limbic pain mechanisms. Neurosci. Lett. 2019;702:15–23. doi: 10.1016/j.neulet.2018.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eichenbaum H. Prefrontal-hippocampal interactions in episodic memory. Nat. Rev. Neurosci. 2017;18(9):547–558. doi: 10.1038/nrn.2017.74. [DOI] [PubMed] [Google Scholar]
- 85.Benchenane K., Peyrache A., Khamassi M., Tierney P.L., Gioanni Y., Battaglia F.P., Wiener S.I. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66(6):921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 86.Jones M.W., Wilson M.A. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siapas A.G., Lubenov E.V., Wilson M.A. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46(1):141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 88.Colgin L.L. Oscillations and hippocampal-prefrontal synchrony. Curr. Opin. Neurobiol. 2011;21(3):467–474. doi: 10.1016/j.conb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inostroza M., Born J. Sleep for preserving and transforming episodic memory. Annu. Rev. Neurosci. 2013;36:79–102. doi: 10.1146/annurev-neuro-062012-170429. [DOI] [PubMed] [Google Scholar]
- 90.Maingret N., Girardeau G., Todorova R., Goutierre M., Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 2016;19(7):959–964. doi: 10.1038/nn.4304. [DOI] [PubMed] [Google Scholar]
- 91.Tang W., Shin J.D., Frank L.M., Jadhav S.P. Hippocampal-prefrontal reactivation during learning is stronger in awake compared with sleep states. J. Neurosci. 2017;37(49):11789–11805. doi: 10.1523/JNEUROSCI.2291-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Girardeau G., Inema I., Buzsáki G. Reactivations of emotional memory in the hippocampus-amygdala system during sleep. Nat. Neurosci. 2017;20(11):1634–1642. doi: 10.1038/nn.4637. [DOI] [PubMed] [Google Scholar]
- 93.Wang D.V., Yau H-J., Broker C.J., Tsou J-H., Bonci A., Ikemoto S. Mesopontine median raphe regulates hippocampal ripple oscillation and memory consolidation. Nat. Neurosci. 2015;18(5):728–735. doi: 10.1038/nn.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burgos-Robles A., Kimchi E.Y., Izadmehr E.M., Porzenheim M.J., Ramos-Guasp W.A., Nieh E.H., Felix-Ortiz A.C., Namburi P., Leppla C.A., Presbrey K.N., Anandalingam K.K., Pagan-Rivera P.A., Anahtar M., Beyeler A., Tye K.M. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 2017;20(6):824–835. doi: 10.1038/nn.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Namburi P., Beyeler A., Yorozu S., Calhoon G.G., Halbert S.A., Wichmann R., Holden S.S., Mertens K.L., Anahtar M., Felix-Ortiz A.C., Wickersham I.R., Gray J.M., Tye K.M. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520(7549):675–678. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hortensius R., Terburg D., Morgan B., Stein D.J., van Honk J., de Gelder B. The dynamic consequences of amygdala damage on threat processing in Urbach-Wiethe Disease. A commentary on Pishnamazi et al. (2016). Cortex. 2017;88:192–197. doi: 10.1016/j.cortex.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 97.Pishnamazi M., Tafakhori A., Loloee S., Modabbernia A., Aghamollaii V., Bahrami B., Winston J.S. Attentional bias towards and away from fearful faces is modulated by developmental amygdala damage. Cortex. 2016;81:24–34. doi: 10.1016/j.cortex.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marek R., Sun Y., Sah P. Neural circuits for a top-down control of fear and extinction. Psychopharmacology (Berl.) 2019;236(1):313–320. doi: 10.1007/s00213-018-5033-2. [DOI] [PubMed] [Google Scholar]
- 99.Milad M.R., Quirk G.J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 100.Senn V., Wolff S.B., Herry C., Grenier F., Ehrlich I., Gründemann J., Fadok J.P., Müller C., Letzkus J.J., Lüthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81(2):428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 101.Lisboa S.F., Vila-Verde C., Rosa J., Uliana D.L., Stern C.A.J., Bertoglio L.J., Resstel L.B., Guimaraes F.S. Tempering aversive/traumatic memories with cannabinoids: a review of evidence from animal and human studies. Psychopharmacology (Berl.) 2019;236(1):201–226. doi: 10.1007/s00213-018-5127-x. [DOI] [PubMed] [Google Scholar]
- 102.Kiritoshi T., Sun H., Ren W., Stauffer S.R., Lindsley C.W., Conn P.J., Neugebauer V. Modulation of pyramidal cell output in the medial prefrontal cortex by mGluR5 interacting with CB1. Neuropharmacology. 2013;66:170–178. doi: 10.1016/j.neuropharm.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kiritoshi T., Ji G., Neugebauer V. Rescue of impaired mGluR5-driven endocannabinoid signaling restores prefrontal cortical output to inhibit pain in arthritic rats. J. Neurosci. 2016;36(3):837–850. doi: 10.1523/JNEUROSCI.4047-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marsicano G., Wotjak C.T., Azad S.C., Bisogno T., Rammes G., Cascio M.G., Hermann H., Tang J., Hofmann C., Zieglgänsberger W., Di Marzo V., Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 105.Woodhams S.G., Chapman V., Finn D.P., Hohmann A.G., Neugebauer V. The cannabinoid system and pain. Neuropharmacology. 2017;124:105–120. doi: 10.1016/j.neuropharm.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hammoud M.Z., Peters C., Hatfield J.R.B., Gorka S.M., Phan K.L., Milad M.R., Rabinak C.A. Influence of Δ9-tetrahydrocannabinol on long-term neural correlates of threat extinction memory retention in humans. Neuropsychopharmacology. 2019;44(10):1769–1777. doi: 10.1038/s41386-019-0416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rabinak C.A., Angstadt M., Lyons M., Mori S., Milad M.R., Liberzon I., Phan K.L. Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiol. Learn. Mem. 2014;113:125–134. doi: 10.1016/j.nlm.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rabinak C.A., Peters C., Marusak H.A., Ghosh S., Phan K.L. Effects of acute Δ9-tetrahydrocannabinol on next-day extinction recall is mediated by post-extinction resting-state brain dynamics. Neuropharmacology. 2018;143:289–298. doi: 10.1016/j.neuropharm.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arnsten A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henigsberg N., Kalember P., Petrović Z.K., Šečić A. Neuroimaging research in posttraumatic stress disorder - Focus on amygdala, hippocampus and prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;90:37–42. doi: 10.1016/j.pnpbp.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 111.Kang D., McAuley J.H., Kassem M.S., Gatt J.M., Gustin S.M. What does the grey matter decrease in the medial prefrontal cortex reflect in people with chronic pain? Eur. J. Pain. 2019;23(2):203–219. doi: 10.1002/ejp.1304. [DOI] [PubMed] [Google Scholar]
- 112.McIntyre C., Roozendaal B. Neural Plast. Mem. CRC Press; 2007. pp. 265–283. [Google Scholar]
- 113.Beldjoud H., Barsegyan A., Roozendaal B. Noradrenergic activation of the basolateral amygdala enhances object recognition memory and induces chromatin remodeling in the insular cortex. Front. Behav. Neurosci. 2015;9:108. doi: 10.3389/fnbeh.2015.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de Quervain D., Wolf O.T., Roozendaal B. Glucocorticoid-induced enhancement of extinction-from animal models to clinical trials. Psychopharmacology (Berl.) 2019;236(1):183–199. doi: 10.1007/s00213-018-5116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roozendaal B., Luyten L., de Voogd L.D., Hermans E.J. Importance of amygdala noradrenergic activity and large-scale neural networks in regulating emotional arousal effects on perception and memory. Behav. Brain Sci. 2016;39:e222. doi: 10.1017/S0140525X15001934. [DOI] [PubMed] [Google Scholar]
- 116.Cahill L., Babinsky R., Markowitsch H.J., McGaugh J.L. The amygdala and emotional memory. Nature. 1995;377(6547):295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 117.Schiff H.C., Johansen J.P., Hou M., Bush D.E.A., Smith E.K., Klein J.E., LeDoux J.E., Sears R.M. β-Adrenergic receptors regulate the acquisition and consolidation phases of aversive memory formation through distinct, temporally regulated signaling pathways. Neuropsychopharmacology. 2017;42(4):895–903. doi: 10.1038/npp.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Atsak P., Hauer D., Campolongo P., Schelling G., McGaugh J.L., Roozendaal B. Glucocorticoids interact with the hippocampal endocannabinoid system in impairing retrieval of contextual fear memory. Proc. Natl. Acad. Sci. USA. 2012;109(9):3504–3509. doi: 10.1073/pnas.1200742109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Quervain D.J-F., Roozendaal B., Nitsch R.M., McGaugh J.L., Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat. Neurosci. 2000;3(4):313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 120.de Quervain D.J-F., Aerni A., Roozendaal B. Preventive effect of β-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am. J. Psychiatry. 2007;164(6):967–969. doi: 10.1176/ajp.2007.164.6.967. [DOI] [PubMed] [Google Scholar]
- 121.Roozendaal B., Hahn E.L., Nathan S.V., de Quervain D.J., McGaugh J.L. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J. Neurosci. 2004;24(37):8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roozendaal B., Okuda S., de Quervain D.J-F., McGaugh J.L. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138(3):901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 123.Schwabe L., Römer S., Richter S., Dockendorf S., Bilak B., Schächinger H. Stress effects on declarative memory retrieval are blocked by a β-adrenoceptor antagonist in humans. Psychoneuroendocrinology. 2009;34(3):446–454. doi: 10.1016/j.psyneuen.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 124.Akirav I. The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus. Front. Behav. Neurosci. 2011;5:34. doi: 10.3389/fnbeh.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morena M., Roozendaal B., Trezza V., Ratano P., Peloso A., Hauer D., Atsak P., Trabace L., Cuomo V., McGaugh J.L., Schelling G., Campolongo P. Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proc. Natl. Acad. Sci. USA. 2014;111(51):18333–18338. doi: 10.1073/pnas.1420285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Campolongo P., Roozendaal B., Trezza V., Hauer D., Schelling G., McGaugh J.L., Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc. Natl. Acad. Sci. USA. 2009;106(12):4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Atsak P., Roozendaal B., Campolongo P. Role of the endocannabinoid system in regulating glucocorticoid effects on memory for emotional experiences. Neuroscience. 2012;204:104–116. doi: 10.1016/j.neuroscience.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 128.Pamplona F.A., Takahashi R.N. WIN 55212-2 impairs contextual fear conditioning through the activation of CB1 cannabinoid receptors. Neurosci. Lett. 2006;397(1-2):88–92. doi: 10.1016/j.neulet.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 129.Ramot A., Akirav I. Cannabinoid receptors activation and glucocorticoid receptors deactivation in the amygdala prevent the stress-induced enhancement of a negative learning experience. Neurobiol. Learn. Mem. 2012;97(4):393–401. doi: 10.1016/j.nlm.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 130.Merz C.J., Hamacher-Dang T.C., Stark R., Wolf O.T., Hermann A. Neural underpinnings of cortisol effects on fear extinction. Neuropsychopharmacology. 2018;43(2):384–392. doi: 10.1038/npp.2017.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morena M., Campolongo P. The endocannabinoid system: an emotional buffer in the modulation of memory function. Neurobiol. Learn. Mem. 2014;112:30–43. doi: 10.1016/j.nlm.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 132.Atsak P., Morena M., Schoenmaker C., Tabak E., Oomen C.A., Jamil S., Hill M.N., Roozendaal B. Glucocorticoid-endocannabinoid uncoupling mediates fear suppression deficits after early - Life stress. Psychoneuroendocrinology. 2018;91:41–49. doi: 10.1016/j.psyneuen.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 133.Hill M.N., McEwen B.S. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(5):791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Di S., Malcher-Lopes R., Halmos K.C., Tasker J.G. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 2003;23(12):4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Phan K.L., Angstadt M., Golden J., Onyewuenyi I., Popovska A., de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J. Neurosci. 2008;28(10):2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Puighermanal E., Busquets-Garcia A., Maldonado R., Ozaita A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1607):3254–3263. doi: 10.1098/rstb.2011.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Al-Zoubi; Morales; Reggio Structural insights into CB1 receptor biased signaling. Int. J. Mol. Sci. 2019;20:1837. doi: 10.3390/ijms20081837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoeffer C.A., Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33(2):67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nogueras-Ortiz C., Yudowski G.A. The Multiple Waves of Cannabinoid 1 Receptor Signaling. Mol. Pharmacol. 2016;90(5):620–626. doi: 10.1124/mol.116.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bie B., Wu J., Foss J.F., Naguib M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr. Opin. Anaesthesiol. 2018;31(4):407–414. doi: 10.1097/ACO.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zoppi S., Madrigal J.L., Caso J.R., García-Gutiérrez M.S., Manzanares J., Leza J.C., García-Bueno B. Regulatory role of the cannabinoid CB2 receptor in stress-induced neuroinflammation in mice. Br. J. Pharmacol. 2014;171(11):2814–2826. doi: 10.1111/bph.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun R., Zhang Z., Lei Y., Liu Y., Lu C., Rong H., Sun Y., Zhang W., Ma Z., Gu X. Hippocampal activation of microglia may underlie the shared neurobiology of comorbid posttraumatic stress disorder and chronic pain. Mol. Pain. 2016;12:1–13. doi: 10.1177/1744806916679166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Muhie S., Gautam A., Chakraborty N., Hoke A., Meyerhoff J., Hammamieh R., Jett M. Molecular indicators of stress-induced neuroinflammation in a mouse model simulating features of post-traumatic stress disorder. Transl. Psychiatry. 2017;7(5):e1135–e1135. doi: 10.1038/tp.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Donzis E.J., Tronson N.C. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiol. Learn. Mem. 2014;115:68–77. doi: 10.1016/j.nlm.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goshen I., Kreisel T., Ounallah-Saad H., Renbaum P., Zalzstein Y., Ben-Hur T., Levy-Lahad E., Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32(8-10):1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 147.Jones M.E., Lebonville C.L., Barrus D., Lysle D.T. The role of brain interleukin-1 in stress-enhanced fear learning. Neuropsychopharmacology. 2015;40(5):1289–1296. doi: 10.1038/npp.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Loflin M.J.E., Babson K.A., Bonn-Miller M.O. Cannabinoids as therapeutic for PTSD. Curr. Opin. Psychol. 2017;14:78–83. doi: 10.1016/j.copsyc.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 149.Orsolini; Chiappini; Volpe; Berardis; Latini; Papanti; Corkery Use of Medicinal Cannabis and Synthetic Cannabinoids in Post-Traumatic Stress Disorder (PTSD): A Systematic Review. Medicina (B. Aires) 2019;55:525. doi: 10.3390/medicina55090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ney L.J., Matthews A., Bruno R., Felmingham K.L. Cannabinoid interventions for PTSD: Where to next? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;93:124–140. doi: 10.1016/j.pnpbp.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 151.Conti S., Costa B., Colleoni M., Parolaro D., Giagnoni G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br. J. Pharmacol. 2002;135(1):181–187. doi: 10.1038/sj.bjp.0704466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsang C.C., Giudice M.G. Nabilone for the Management of Pain. Pharmacotherapy. 2016;36(3):273–286. doi: 10.1002/phar.1709. [DOI] [PubMed] [Google Scholar]
- 153.Tournier C., Hours M., Charnay P., Chossegros L., Tardy H. 2016. [DOI] [PMC free article] [PubMed]
- 154.Sarrami P., Armstrong E., Naylor J.M., Harris I.A. Factors predicting outcome in whiplash injury: a systematic meta-review of prognostic factors. J. Orthop. Traumatol. 2017;18(1):9–16. doi: 10.1007/s10195-016-0431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Holbrook T.L., Galarneau M.R., Dye J.L., Quinn K., Dougherty A.L. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N. Engl. J. Med. 2010;362(2):110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 156.Amos T., Stein D.J., Ipser J.C. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD). Cochrane Database Syst. Rev. 2014;(7):CD006239. doi: 10.1002/14651858.CD006239.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yao J., Johnson R.W. Induction of interleukin-1 β-converting enzyme (ICE) in murine microglia by lipopolysaccharide. Brain Res. Mol. Brain Res. 1997;51(1-2):170–178. doi: 10.1016/S0169-328X(97)00235-0. [DOI] [PubMed] [Google Scholar]
- 158.Rohleder N., Wolf J.M., Kirschbaum C., Wolf O.T. Effects of cortisol on emotional but not on neutral memory are correlated with peripheral glucocorticoid sensitivity of inflammatory cytokine production. Int. J. Psychophysiol. 2009;72(1):74–80. doi: 10.1016/j.ijpsycho.2008.03.010. [DOI] [PubMed] [Google Scholar]