Abstract
Objectives
To assess the relationship between surgeon and hospital volume on mortality following radical cystectomy (RC).
Patients and Methods
We queried the National Cancer Database (NCDB) for adult patients undergoing RC from 2010–2013. We calculated average volume for each surgeon (SV) and hospital (HV). Using propensity-scored weights for combined volume groups with a proportional hazards regression model, we compared association between HV and SV on 90-day survival following RC.
Results
19,346 RC were performed at 927 hospitals by 2,927 surgeons from 2010 – 2013. Median HV and SV were 12.3 (IQR 5.0–35.5) and 4.3 (IQR 1.3–12.3) cases, respectively. For HV, 90 day unadjusted mortality was 8.5% in centers with <5 cases/year (95% CI 7.7–9.3) and 5.6% in those with >30 cases/year (95% CI 5.0–6.2). For SV, 90 day mortality was 8.1% for surgeons with <5 cases/year (95% CI 7.6–8.6) and 4.0% for those with >30 cases/year (95% CI 2.8–5.2; all p<0.05). 30-day mortality was lowest for the combined HV-SV groups with HV>30, ranging from 1.6% to 2.1%.
Conclusions
In hospitals reporting to the NCDB, volume is associated with improved mortality after RC. These associations appear to be driven by hospital- rather than surgeon-level effects. Increased SV provides a beneficial effect on mortality at the highest volume hospitals. These findings inform efforts to regionalize complex surgical care and improve quality at community and safety net hospitals.
Keywords: Volume, Outcomes, Cystectomy, Regionalization, Bladder
Introduction
The volume-outcome relationship has been well described for a number of surgical procedures, both at the hospital-(1) and surgeon- level.(2) This relationship has been repeatedly demonstrated for radical cystectomy (RC), where having surgery performed at a higher volume hospital results in a small but demonstrable reduction in short term mortality.(1–17) However, the relative impact of SV and HV on short term mortality outcomes remains unclear.
The differential impact of individual surgeon versus hospital influence on outcomes becomes more relevant with national attention being directed towards publicly reported outcomes information.(18) Selection of a high volume surgeon appears to be important, as data suggest that higher SV is associated with improved odds of survival, decreased rates of reintervention,(12) complications,(13) cost,(13) and increased patient satisfaction.(19) Nevertheless, the perioperative care of bladder cancer patients extends beyond the direct control of the individual surgeon, and includes perioperative medical assessment and optimization; post-operative nursing, education, continued post-discharge rehabilitation, and acute care in the setting of readmission. Each of these features is a surrogate for both structural and process metrics, which impact the overall quality of care being delivered.(20) High volume centers, which are associated with lower failure to rescue rates, tend to excel at providing the aforementioned spectrum of perioperative care.(15, 21–23) Indeed, a better understanding of the relative importance and interplay between SV and HV, as they relate to cystectomy outcomes, could have important policy implications. For instance, if SV is proven to be the driver of outcome, minimum volume standards or provider-based pooled referrals may be advisable. On the other hand, if high HV is necessary for outcome optimization, large-scale attempts for regionalization of care could be justified.(17)
As such, we set out to further characterize the volume-outcomes relationship in patients undergoing cystectomy for muscle-invasive bladder cancer. Specifically, we utilized a national tumor registry to assess the relative impact of SV and HV on cystectomy outcomes by using a nested volume modeling approach in order to identify the impact of volume on short-term mortality following RC.
Patients and Methods
Analytic Cohort
The NCDB is a collaborative project of the American College of Surgeons and the American Cancer Society. The NCDB prospectively collects cancer data, including patient and hospital characteristics, from more than 1,500 Commission on Cancer facilities representing approximately 70% of all cancer cases in the United States.(24) As Physician National Provider Identification (NPI) reporting became mandatory in 2009, we included the diagnosis years 2010–2013 in our analysis of all patients older than 18 years of age who underwent radical cystectomy, resulting in 20,713 patients in 1,003 facilities.
After excluding 6 facilities where surgeon NPIs were attributed to the facility, there were 20,569 patients in 998 facilities. An additional 1,223 patients were excluded from 71 hospitals where no NPIs were reported, resulting in 19,346 patients in 927 facilities, with 2,927 distinct surgeon NPI numbers. Among these patients, 1,210 resections (6.3%) were missing Surgeon NPIs. Average annual surgical and hospital volume was calculated for all patients, and the methods of Raghunathan and colleagues (25) were used to impute average annual surgical volume for the 1,210 physicians with missing NPIs.
Table 1 displays surgical volume by hospital volume, where it can be seen that very few high volume surgeons practice in low volume hospitals, and vice-versa. For this reason, it was not possible to use similar surgeon groupings within each hospital, since the number of patients in some groups would be quite small and the statistical models would not be identifiable. Combinations of surgeon and hospital volume groups were based on having a sufficient number of cases in each group that would allow for reliable estimation. Hospitals performing fewer than 10 cases per year were defined as low volume, those performing 10–29 were moderate volume, and 30+ were considered high volume. Within low volume hospitals, SV were defined as <2 (low), 2–4 (moderate), and >=5 (high); within moderate volume hospitals, low, moderate, and high SV were defined as <5, 5–9, >=10, respectively; and within high volume hospitals, SV were defined as <10 (low), 10–19(moderate), and 20–29 (high).
Table 1.
Average Annual Hospital Volume by Average Annual Surgeon Volume 2010–2013 (N=18,136)1
| Average Annual Hospital Volume |
Average Annual Surgeon Volume | |||||
|---|---|---|---|---|---|---|
| <2 | 2–4 | 5–9 | 10–19 | >=20 | Total | |
| < 2 | 1,306 | 44 | 10 | 2 | 1 | 1,363 |
| 2–4 | 2,047 | 840 | 47 | 20 | 0 | 2,954 |
| 5–9 | 1,398 | 1,442 | 602 | 109 | 0 | 3,551 |
| 10–19 | 683 | 727 | 1,071 | 320 | 0 | 2,801 |
| 20–29 | 248 | 333 | 341 | 683 | 175 | 1,780 |
| >=30 | 235 | 393 | 691 | 2,046 | 2,322 | 5,687 |
| Total | 5,917 | 3,779 | 2,762 | 3,180 | 2,498 | 18,136 |
Excludes 1,210 patients with missing surgical volume.
A non-imputed file, including hospitals where at least 70% of Surgeon NPIs were reported, was used to compare hospital and surgeon volume results with the imputed volume results. This file included 18,176 patients in 864 hospitals, with 548 patients missing surgeon NPIs.
Statistical Analyses
Propensity scores for SV and HV combined groups were generated in a logistic model with the following variables included: age, sex, race, stage, insurance type, median income of the patient zip code in the year 2012, surgical approach, WHO grade, lymphovascular invasion, neoadjuvant chemotherapy utilization, Elixhauser comorbid condition groups (excluding cancer), and census region of the facility where the procedure was performed. Supplemental table 1 demonstrates balance among covariates after weighting by propensity-scores. Cox proportional hazards regression—weighted by the inverse probability of treatment generated in the logistic model(26)—was used to generate weighted survival probabilities for mortality between 0 and 90 days, and survival curves were created from these generated probabilities(27). Cumulative unadjusted and adjusted 30-, 60- and 90-day mortality rates and 95% confidence intervals were generated by the HV and SV groups. The adjusted rates were obtained from the propensity score-adjusted survival probabilities. Interaction between SV and HV weighted by the propensity scores was assessed for multiple ordinal groupings, as well as for continuous volume.
Results
Among the 19,346 RC patients in the final dataset, the median age was 69 (IQR 61–75). 90% of patients were Caucasian, 75% were male, and 59% had Medicare insurance. Fifty one percent had pathologic stage III or IV cancer and 87% had high-grade disease. Ninety-three percent of patients had a Charlson score of 0 or 1. An open approach was utilized in 72% of cases, and 28% of cases were performed after receipt of neoadjuvant chemotherapy. Median HV was 12.3 cases per year (IQR 5.0–35.5; range <1 – 122) (Table 2). Description of case frequency by hospital type is detailed in supplemental table 2.
Table 2.
Descriptive Patient, Tumor and Hospital Characteristics
| All Hospitals (927 hospitals, 19,346 patients) |
|
|---|---|
| Mean Age 95% CI | 67.8 (67.6–67.9) |
| Percent Distribution | |
| Age | |
| 18–45 | 0.7 (142) |
| 45–54 | 4.1 (788) |
| 55–64 | 16.6 (3,216) |
| 65–74 | 32.0 (6,184) |
| 75–84 | 33.8 (6,533) |
| >=85 | 12.8 (2,483) |
| Race | |
| White | 90.4 (17,492) |
| Black | 6.7 (1,292) |
| Other | 2.9 (562) |
| Sex | |
| Male | 74.6 (14,426) |
| Female | 25.4 (4,920) |
| Insurance | |
| Private | 25.9 (5,017) |
| Not Insured | 3.1 (604) |
| Insurance NOS | 4.6 (888) |
| Medicaid | 5.0 (965) |
| Medicare | 58.8 (11,385) |
| Other government | 1.1 (223) |
| Unknown | 1.4 (264) |
| Median Income Quintiles2 | |
| Lowest Quintile | 7.3 (1,410) |
| 21–40%ile | 13.7 (2,648) |
| 41–60%ile | 18.5 (3,589) |
| 61–80%ile | 23.8 (4,613) |
| > 80%ile | 33.1 (6,401) |
| Unknown | 3.5 (685) |
| Stage1 | |
| 0A,0IS, I | 17.8 (3,449) |
| II | 28.6 (5,527) |
| III | 26.0 (5,039) |
| IV | 25.1 (4,853) |
| Missing/Unknown | 2.5 (478) |
| WHO Grade | |
| Low Grade | 2.3 (443) |
| High Grade | 87.4 (16,910) |
| Unknown | 10.3 (1,993) |
| Cancer Sequence | |
| Only primary | 55.2 (10,671) |
| 1st of multiple primaries | 21.7 (4,202) |
| 2nd Primary | 18.6 (3,596) |
| 3rd or higher Primary | 4.5 (877) |
| Diagnosis Year | |
| 2010 | 25.5 (4,926) |
| 2011 | 25.3 (4,890) |
| 2012 | 24.6 (4,766) |
| 2013 | 24.6 (4,764) |
| Surgical Approach | |
| Robotic Assisted | 19.1 (3,695) |
| Robotic converted to open | 0.8 (161) |
| Endoscopic or laparoscopic | 7.3 (1,422) |
| Endoscopic or laparoscopic converted to open | 0.6 (126) |
| Open or approach unspecified | 72.1 (13,942) |
| Neo Adjuvant chemotherapy | |
| No | 71.4 (13,818) |
| Yes | 27.6 (5,332) |
| Unknown if chemotherapy administered | 1.0 (196) |
| Lymph Vascular Invasion | |
| No | 51.1 (9,881) |
| Yes | 34.4 (6,650) |
| Unknown | 14.5 ((2,815) |
| Hospital Type | |
| Academic | 60.4 (11,685) |
| Comprehensive Community | 30.9 (5,979) |
| Community, Other | 8.7 (1,682) |
| Hospital Average Annual Volume | |
| < 2 | 7.4 (1,439) |
| 2–4 | 16.6 (3,220) |
| 5–9 | 19.6 (3,796) |
| 10–19 | 16.0 (3,090) |
| 20–29 | 9.7 (1,874) |
| >=30 | 30.6 (5,927) |
| Surgeon Average Annual Volume3 | |
| < 2 | 32.6 (5,917) |
| 2–4 | 20.8 (3,779) |
| 5–9 | 15.2 (2,762) |
| 10–19 | 17.5 (3,180) |
| 20–29 | 7.8 (1,407) |
| >=30 | 6.0 (1,091) |
| Hospital Census Region | |
| Northeast | 23.8 (4,613) |
| South | 36.1 (6,992) |
| Midwest | 27.8 (5,383) |
| West | 4.8 (934) |
| Pacific | 7.4 (1,424) |
| Mortalities | |
| 30 day | 475 (2.5%) |
| 90 day | 1316 (6.8%) |
Pathologic Stage; if missing then clinical stage assigned.
Median household income from patient zip code area of residence from 2012 American Community Survey data.
Excludes 1,210 missing Surgeon NPIs, for all hospitals, and 548 missing Surgeon NPIs for hospitals with 70% of NPIs reported.
Based on SV without imputation (6% missing NPIs), 33% of RC were performed by surgeons with average annual SV of less than two cases, and 53% of cystectomies were performed by surgeons with SV less than 5 cases per year. These distributions were similar before and after imputation (both median = SV 4.3) (range <1 – 63) (Table 3). In addition, propensity score weighted and unadjusted Hazard Ratios by Surgeon and Hospital volume were similar based on the imputed and non- imputed data (data not shown). As such, the remaining analysis is described using imputation.
Table 3.
Percent Distribution of Imputed Surgical Volume, Surgical volume with missing NPIs excluded
| Imputed (N=19,346) |
Non Imputed (N=18,136) |
|
|---|---|---|
| < 2 | 32.7 | 32.6 |
| 2–4 | 20.3 | 20.8 |
| 5–9 | 15.6 | 15.2 |
| 10–19 | 18.1 | 17.5 |
| 20–29 | 7.6 | 7.8 |
| >=30 | 5.7 | 6.0 |
(Median Average Annual Surgeon Volume Non Imputed=4.3, imputed=4.3)
Unadjusted and adjusted mortality rates are shown in Table 4. Unadjusted comparison of the highest and lowest volume groups for both hospitals and surgeons demonstrate a significant inverse relationship between volume and mortality at 30-, 60-, and 90-days. For example, centers with HV<5 had a 90-day mortality rate of 8.5% (95% CI 7.7–9.3), compared to HV>30 which had a mortality rate of 5.6% (95% CI 5.0–6.2). Similarly, SV<5 demonstrated a 90-day mortality rate of 8.1% (95% CI 7.6–8.6), compared to SV>30 which had a mortality rate of 4.0% (95% CI 2.8–5.2). After adjustment, HV remained significant at 60 and 90 days, suggesting that the hospital effect is more salient than the surgeon effect on short-term mortality. Further evidence of this is seen in Figures 1–3, which demonstrates propensity score weighted mortality rates for each combined volume group; with crossing SV confidence intervals and a clear HV-outcome relationship, it appears that HV accounts for most of the differences in 30-, 60-, and 90-day mortality. However in high volume hospitals, relative to surgeons in the highest volume group (HV>30 SV>30), surgeons with 10–19 cases (HV>30 SV10–19) demonstrated a higher 90-day mortality (HR 1.21, 95% CI 1.07–1.37, p=.0032). This effect becomes evident after postoperative day 50. Similarly, surgeons with fewer than 10 cases in high HV centers (HV>30 SV<10) were associated with higher risk of mortality, however this difference was not significant (HR=1.13, 95% CI 1.00–1.29, p=.055; Figure 4, Table 5).
Table 4.
Unadjusted and Propensity Score Weighted 30, 60, 90 day cumulative mortality Percent, 95% Confidence Intervals, by Hospital and Surgeon Average Annual Volume, 2010–2013 Diagnosis Years
| 30 Day Mortality, 95% CI | 60 Day Mortality 95% CI | 90 Day Mortality 95% CI | |
|---|---|---|---|
| Hospital Volume | Unadjusted | Unadjusted | Unadjusted |
| < 5 (n=4,630) | 3.3 (2.7–3.8) | 5.7 (5.1–6.4) | 8.5 (7.7–9.3) |
| 5–9 (n=3,762) | 2.9 (2.4–3.4) | 5.7 (4.9–6.4) | 8.4 (7.4–9.3) |
| 10–19 (n=3,064) | 2.4 (1.8–2.9) | 5.0 (4.2–5.8) | 7.2 (6.3–8.1) |
| 20–29 (n=1,859) | 2.2 (1.5–2.9) | 4.1 (3.2–5.0) | 6.4 (5.2–7.5) |
| >=30 (n=5,887) | 1.9 (1.5–2.2) | 3.8 (3.3–4.3) | 5.6 (5.0–6.2) |
| Hospital Volume | Weighted | Weighted | Weighted |
| < 5 (n=4,630) | 2.8 (2.2–3.5) | 5.1 (4.3–5.9) | 7.8 (6.7–8.9) |
| 5–9 (n=3,762) | 2.8 (2.1–3.6) | 5.5 (4.4–6.6) | 8.0 (6.7–9.2) |
| 10–19 (n=3,064) | 2.5 (1.8–3.3) | 5.3 (4.2–6.4) | 7.6 (6.2–8.9) |
| 20–29 (n=1,859) | 2.4 (1.5–3.2) | 4.1 (3.0–5.2) | 7.0 (5.5–8.5) |
| >=30 (n=5,887) | 1.9 (1.4–2.4) | 3.6 (2.9–4.3) | 5.5 (4.6–6.3) |
| Surgeon Volume | Unadjusted | Unadjusted | Unadjusted |
| <5 (n=10,191) | 2.9 (2.6–3.2) | 5.5 (5.1–6.0) | 8.1 (7.6–8.6) |
| 5–9 (n=2,992) | 2.2 (1.6–2.8) | 4.6 (3.8–5.4) | 6.9 (5.9–7.8) |
| 10–19 (n=3,456) | 2.1 (1.6–2.6) | 4.1 (3.3 −4.8) | 6.3 (5.4–7.2) |
| 20–29 (n=1,463) | 2.2 (1.4–2.9) | 4.0 (2.9–5.1) | 5.7 (4.4–7.0) |
| >=30 (n=1,100) | 1.8 (1.0–2.6) | 2.7 (1.7–3.7) | 4.0 (2.8–5.2) |
| Surgeon Volume | Weighted | Weighted | Weighted |
| <5 (n=10,191) | 2.5 (2.2–2.9) | 5.0 (4.5–5.5) | 7.3 (6.7–8.0) |
| 5–9 (n=3,762) | 2.4 (1.5–3.3) | 4.9 (3.8–6.0) | 7.0 (5.7–8.3) |
| 10–19 (n=3,064) | 2.1 (1.5–2.8) | 4.1 (3.2–5.1) | 6.8 (5.6–8.1) |
| 20–29 (n=1,859) | 2.3 (1.1–3.6) | 4.2 (2.5–5.8) | 5.9 (4.1–7.8) |
| >=30 (n=5,887) | 2.1 (0.6–3.6) | 2.9 (1.2–4.5) | 4.9 (2.6–7.3) |
| Total | |||
| All Patients | 2.5 (2.3–2.7) | 4.8 (4.5–5.1) | 7.2 (6.8–7.6) |
Figure 1:
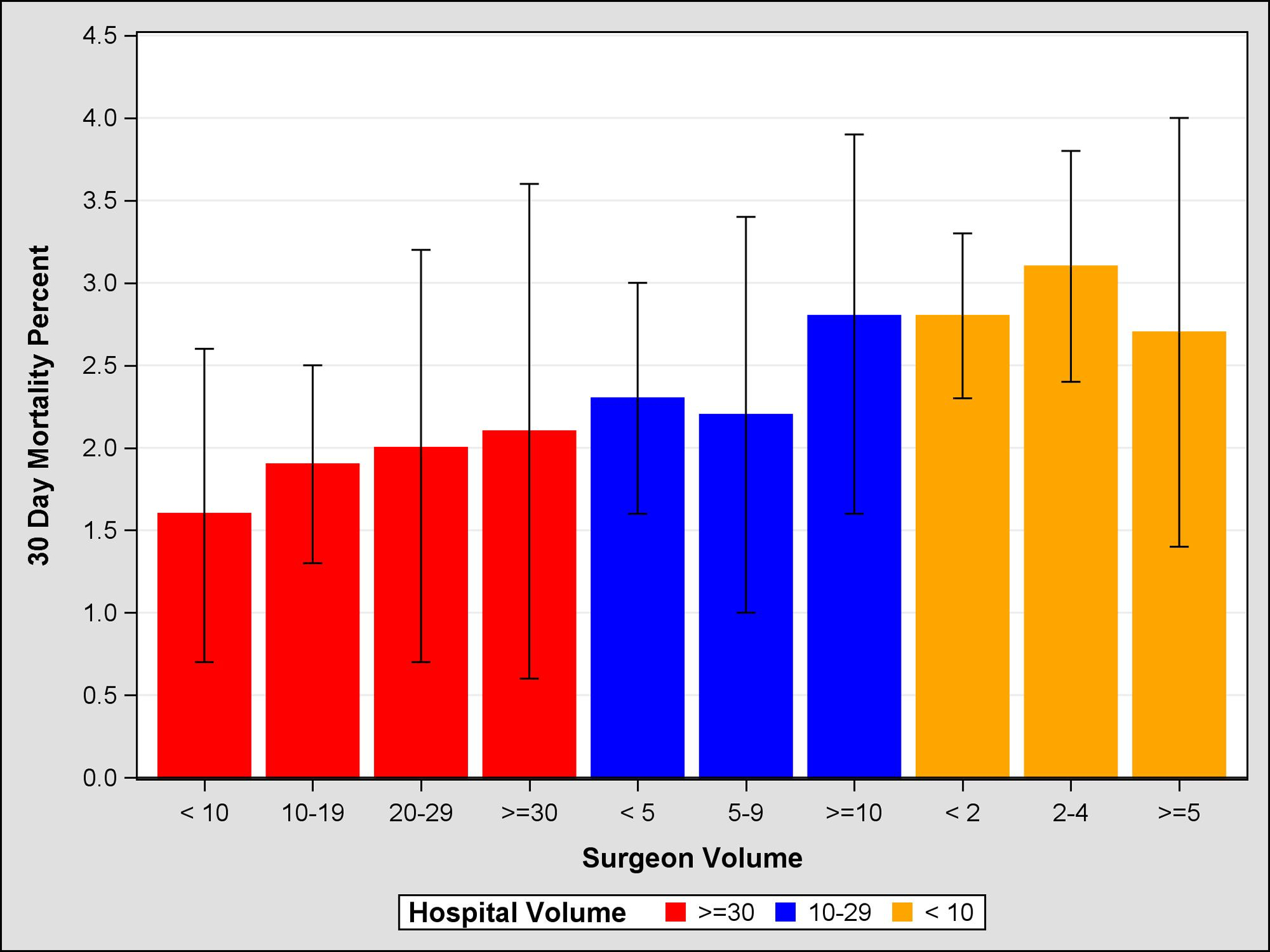
Propensity Score Weighted 30 Day Mortality, 95% Confidence Intervals, by Surgeon and Hospital Volume
Figure 3:
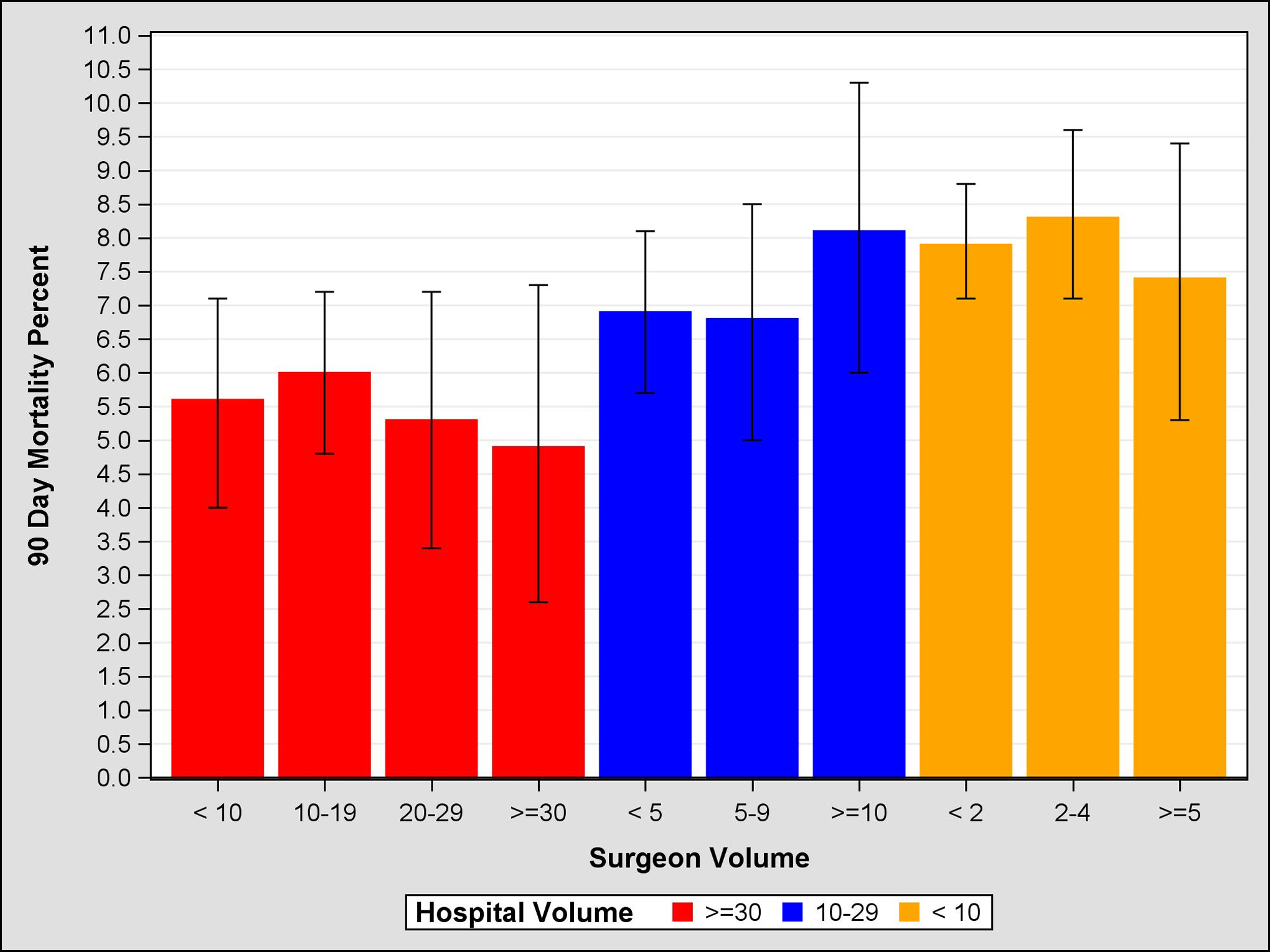
Propensity Score Weighted 90 Day Mortality, 95% Confidence Intervals, by Surgeon and Hospital Volume
Figure 4:
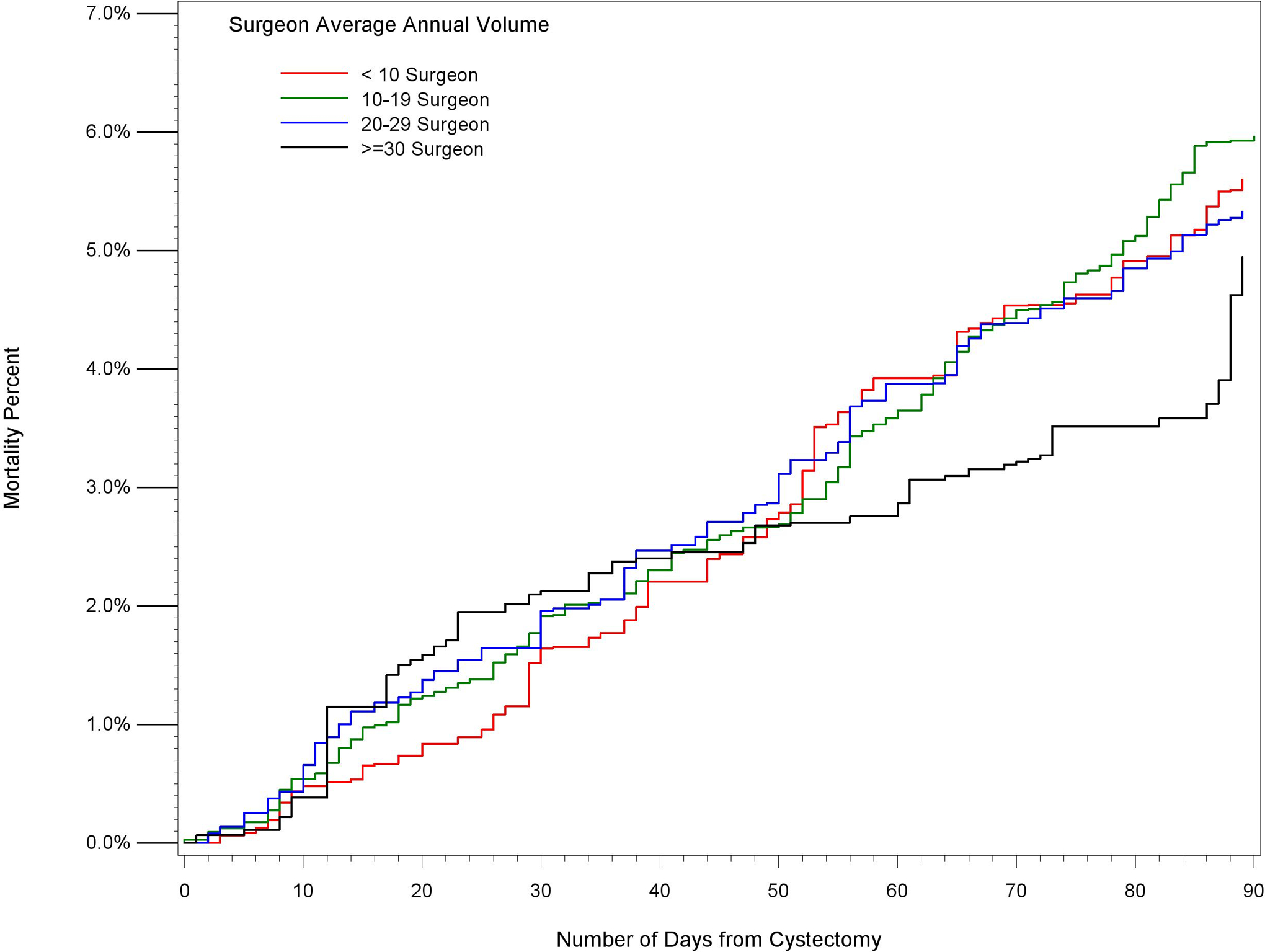
Propensity Score Weighted Cumulative Mortality Estimates 2010–2013, by Surgeon Volume in High Volume Hospitals (Average Annual Volume >=30)
Table 5:
Propensity score-weighted, Imputed Hazard Ratios for 90 day Mortality
| Hospital Surgeon Average Annual Volume Groups | Propensity Score Weighted, Imputed Hazard Ratios, 95% Confidence Intervals |
|---|---|
| Reference Group Hospital Volume >=30, Surgeon volume >=30 | |
| Hospital volume >=30 | |
| Surgeon Volume < 10 | 1.13 (1.00–1.29) |
| Surgeon Volume 10–19 | 1.21 (1.07–1.37) |
| Surgeon Volume 20–29 | 1.08( 0.85–1.37) |
| Hospital Volume 10–29 | |
| Surgeon Volume < 5 | 1.42 (1.28–1.58) |
| Surgeon Volume 5–9 | 1.39 (1.23–1.57) |
| Surgeon Volume >=10 | 1.69 (1.46–1.95) |
| Hospital Volume < 10 | |
| Surgeon Volume < 2 | 1.64 (1.48–1.81) |
| Surgeon Volume 2–4 | 1.73 (1.55–1.94) |
| Surgeon Volume >=5 | 1.51 (1.27–1.79) |
Discussion
Two constructs have been proposed that attempt to explain the volume-outcome relationship in medicine: the “practice makes perfect” hypothesis, and the “selective referral” theory. The “practice makes perfect” hypothesis suggests that hospitals and physicians with more exposure to a specific clinical scenario develop skill sets that enable them to treat patients more efficiently and effectively. (28) In urologic surgery this concept is supported by the abundance of data documenting the existence of operative learning curves.(29) In contrast, the “selective referral” theory suggests that the volume-outcome relationship may be driven by reputation, general community knowledge of acceptable outcomes, or by status as a provider or center for specialized care. In this model, physicians are preferentially channeling patients to select hospitals or specialists without explicit knowledge of objective outcomes.(30) Both theories are independent, but not mutually exclusive, and drive regionalization through different mechanisms. Parsing of the volume-outcome relationship by volume source can crystallize the importance of each theoretical concept and, in turn, its policy implications.
While a number of investigators have described a volume-outcome relationship for radical cystectomy, only a few have simultaneously examined both HV and SV. Siemens and colleagues used a population-based registry of 2800 patients who underwent cystectomy and found that SV and HV were both individually associated with improved CSS. When incorporated into the same model, the effect of each was attenuated. Moreover, there was no significant interaction effect between each volume type for OS or CSS.(32)
In their investigation of 14,000 patients in the Nationwide Inpatient Sample, Konety and associates similarly found that despite increased odds of in-hospital mortality of 96% and 41% in low and moderate volume hospitals relative to high volume centers, the impact was mitigated with the addition of SV to the model. The authors hypothesized that this may be due to collinearity between volume subtypes, citing Birkmeyer and colleagues’ finding that ~40% of the SV effect was due to HV.(2) Alternatively, a lack of complete data (including surgeon identifiers) may have impacted the combined model.(33)
Morgan and associates found in a SEER-Medicare analysis of 7,100 patients that increased SV and HV were associated with decreased mortality, and that when both covariates were included in the model, only the HV relationship persisted. Moreover, while survival was associated with HV in each SV stratum, there was no association between survival and SV within each HV stratum. They concluded that the relationship between SV and survival after radical cystectomy is accounted for by HV, and that structure and process characteristics of high volume hospitals drive long-term outcomes after radical cystectomy.(34)
In our study utilizing a large national tumor registry, we confirmed a volume-outcomes relationship for both HV and SV groups at 30-, 60-, and 90-days. Moreover, we demonstrated that HV, and not SV, appears to be the critical component in this relationship. As seen in figures 1–3, HV exhibits a “dose-dependent” effect, while SV appears to have a variable effect within each HV group. Kaplan Meier survival curves for each combined stratum (not shown) similarly demonstrate clear organization by HV. The exception to this pattern occurs between 50 and 90 days, where even within high volume hospitals, high volume surgeons have significantly lower mortality than low volume surgeons (Figure 4). The significance of this finding is unclear, but at more than 2 months post-operatively may be related more to disease-associated case mix than surgical quality. Some have suggested that volume is associated with performance of a more oncologically sound operation,(6) however, this is unlikely to substantially influence short term mortality outcomes.
In terms of policy implications, our data suggest that incentives to regionalize cystectomy patients to high volume hospitals may produce improvements in short term mortality; pooling of cases to high volume surgeons within this centralized framework may yield further gains. Yet, survival benefits are only one criterion on which health policy decisions must pivot. Indeed, access to care remains a critical consideration. While centralization of cystectomy has been demonstrated at the regional level,(35) it risks exacerbation of disparity in access to care at high-volume centers that already exists for minority, elderly, and Medicaid populations.(35, 36) Structural and process improvements of low HV centers and safety net hospitals—while easier said than done—is necessary to level the playing field and increase access of high value care. Initiatives such as the National Surgical Quality Improvement Program have a proven track record for improving care in other disease sites and may help to bridge the gap in quality between low and high HV hospitals.(14, 37) In the meantime, improved networking –including utilization of telemedicine, nurse navigators, and collaborative care access programs –between low HV centers or safety net hospitals and centers of excellence may provide expedient access for those in need.
As is the case with other analyses of large data registries, our study is limited by the potential for missing data. Within our dataset, surgeon NPI was missing in 6% of cases, and this was addressed using imputation. Our imputed and non-imputed analyses had similar distributions and results. Second, our findings can only be generalized to hospitals reporting to the NCDB; while our dataset did not include all cystectomies performed in the United States, our conclusions -- drawn from a sample of approximately 70% of all cases in the US -- can be considered externally valid. Third, while our dataset includes surgeons who operate at multiple hospitals, it is possible to have SV > HV in some centers. Similarly, it is possible that surgeons may have a “true” SV that is greater than what is represented in our data if they also operated at non-NCDB hospitals. Fourth, we selected short-term mortality as an outcome metric for perioperative quality, but did not include other quality metrics such as complications, readmissions, and failure to rescue. Inclusion of such variables could provide a more complete assessment of quality, which could further inform the debate on hospital-based regionalization vs. provider-based pooling of cases. Finally, there exists a degree of collinearity between surgeon and hospital volume that, regardless of the statistical methodology employed, is likely not possible to untangle. In our study, for example, low volume surgeons in a high HV center had equivalent volumes as high volume surgeons in low HV and moderate volume surgeons in moderate HV centers. Strengths of our study include the robust sample size of over 19,000 patients of all ages. This large number of patients, combined with the low rate of missing data, provides the ability to compare large hospital and surgeon volume subgroups. Methodologically, our assessment of main effects and interaction terms along with 90-day survival stratified by combined volume group allowed us to clearly discern and meaningfully describe the differential effects of HV and SV.
In summary, in patients undergoing RC captured by the NCDB, we demonstrated that increased surgical volume is associated with decreased short-term mortality. This effect largely appears to be driven by hospital and not individual surgeon volume. Volume-outcome effects at the surgeon level are best demonstrated within high volume hospitals, although these effects are small. We believe these findings are informative to contemporary efforts to regionalize complex oncologic surgical care.
Supplementary Material
Supplemental Table 1: Unweighted and propensity score weighted covariates
Supplemental Table 2: Case frequency by hospital type (a. Surgeon Volume, b. Hospital Volume)
Figure 2:
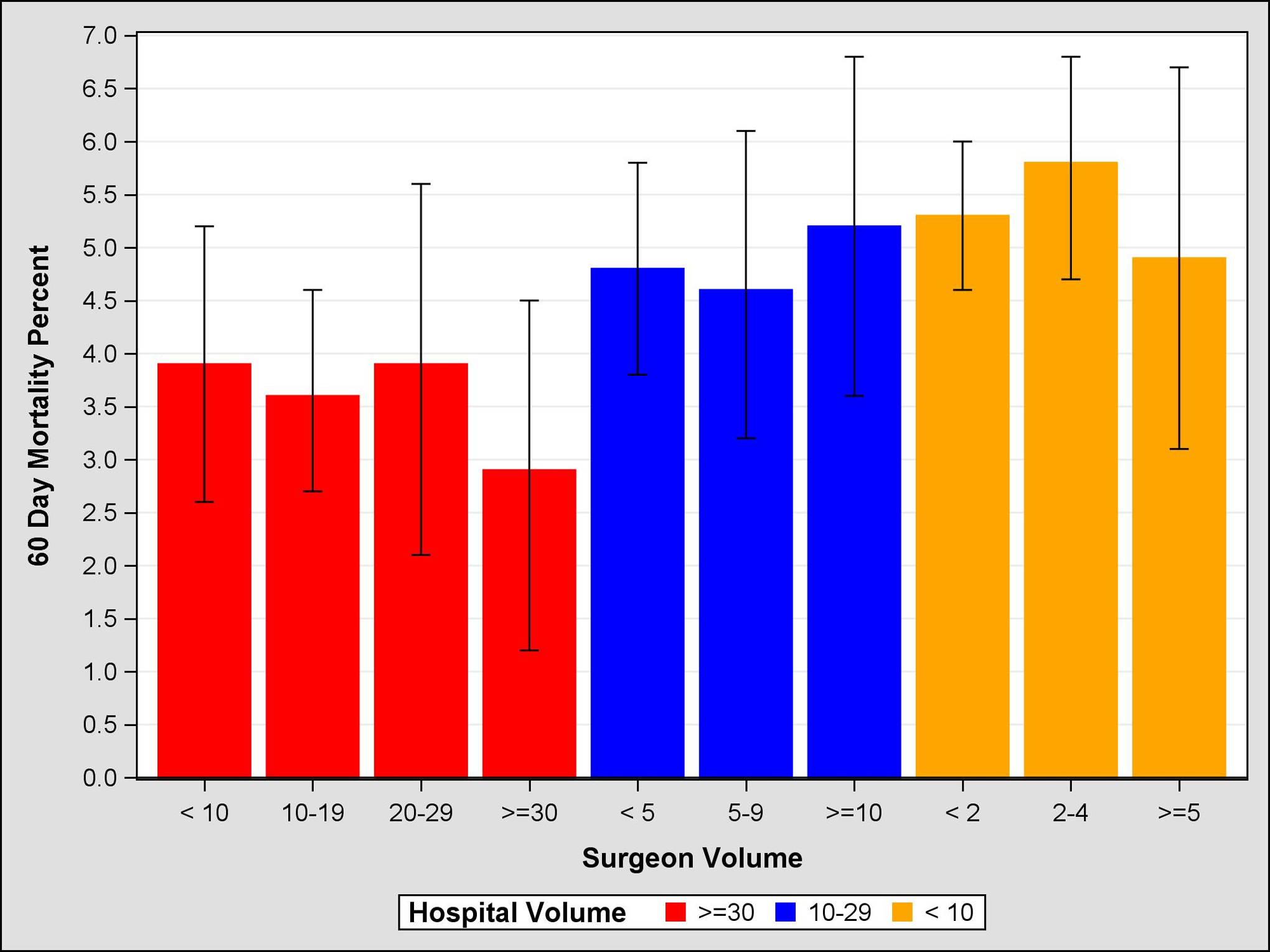
Propensity Score Weighted 60 Day Mortality, 95% Confidence Intervals, by Surgeon and Hospital Volume
Abbreviations:
- RC
Radical Cystectomy
- NCDB
National Cancer Database
- SV
Surgeon Volume
- HV
Hospital Volume
- IQR
Interquartile Range
- CI
Confidence Interval
- HR
Hazard Ratio
- NPI
National Provider Identification
- WHO
World Health Organization
Footnotes
Conflicts of Interest: None (for all authors)
References
- 1.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–37. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–27. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri CE, Lee B, Cookson MS, Bingham J, Clark PE, Smith JA Jr., et al. Association of procedure volume with radical cystectomy outcomes in a nationwide database. J Urol. 2007;178(4 Pt 1):1418–21; discussion 21–2. [DOI] [PubMed] [Google Scholar]
- 4.Goossens-Laan CA, Visser O, Hulshof MC, Wouters MW, Bosch JL, Coebergh JW, et al. Survival after treatment for carcinoma invading bladder muscle: a Dutch population-based study on the impact of hospital volume. BJU Int. 2012;110(2):226–32. [DOI] [PubMed] [Google Scholar]
- 5.Hollenbeck BK, Daignault S, Dunn RL, Gilbert S, Weizer AZ, Miller DC. Getting under the hood of the volume-outcome relationship for radical cystectomy. J Urol. 2007;177(6):2095–9; discussion 9. [DOI] [PubMed] [Google Scholar]
- 6.Marshall SJ, Hayn MH, Stegemann AP, Agarwal PK, Badani KK, Balbay MD, et al. Impact of surgeon and volume on extended lymphadenectomy at the time of robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium (IRCC). BJU Int. 2013;111(7):1075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer EK, Purkayastha S, Athanasiou T, Darzi A, Vale JA. Assessing the quality of the volume-outcome relationship in uro-oncology. BJU Int. 2009;103(3):341–9. [DOI] [PubMed] [Google Scholar]
- 8.McCabe JE, Jibawi A, Javle PM. Radical cystectomy: defining the threshold for a surgeon to achieve optimum outcomes. Postgrad Med J. 2007;83(982):556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan H, Atoria CL, Bach PB, Elkin EB. Hospital volume, complications, and cost of cancer surgery in the elderly. J Clin Oncol. 2015;33(1):107–14. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen ME, Mallin K, Weaver MA, Palis B, Stewart A, Winchester DP, et al. Association of hospital volume with conditional 90-day mortality after cystectomy: an analysis of the National Cancer Data Base. BJU Int. 2014;114(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun M, Ravi P, Karakiewicz PI, Sukumar S, Sammon J, Bianchi M, et al. Is there a relationship between leapfrog volume thresholds and perioperative outcomes after radical cystectomy? Urol Oncol. 2014;32(1):27 e7–13. [DOI] [PubMed] [Google Scholar]
- 12.Mayer EK, Bottle A, Aylin P, Darzi AW, Athanasiou T, Vale JA. The volume-outcome relationship for radical cystectomy in England: an analysis of outcomes other than mortality. BJU Int. 2011;108(8 Pt 2):E258–65. [DOI] [PubMed] [Google Scholar]
- 13.Leow JJ, Reese S, Trinh QD, Bellmunt J, Chung BI, Kibel AS, et al. Impact of surgeon volume on the morbidity and costs of radical cystectomy in the USA: a contemporary population-based analysis. BJU Int. 2015;115(5):713–21. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Higher surgeon and hospital volume improves long-term survival after radical cystectomy. Cancer. 2013;119(19):3546–54. [DOI] [PubMed] [Google Scholar]
- 15.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69(5):871–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs BL, Miller DC. The volume outcome relationship in urology: moving the field forward. J Urol. 2012;188(6):2037–8. [DOI] [PubMed] [Google Scholar]
- 17.Goossens-Laan CA, Gooiker GA, van Gijn W, Post PN, Bosch JL, Kil PJ, et al. A systematic review and meta-analysis of the relationship between hospital/surgeon volume and outcome for radical cystectomy: an update for the ongoing debate. Eur Urol. 2011;59(5):775–83. [DOI] [PubMed] [Google Scholar]
- 18.Werner RM, Asch DA. The unintended consequences of publicly reporting quality information. JAMA. 2005;293(10):1239–44. [DOI] [PubMed] [Google Scholar]
- 19.Tevis SE, Kennedy GD. Patient satisfaction: does surgical volume matter? J Surg Res. 2015;196(1):124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbach DR, Baxter NN. Does it matter what a hospital is “high volume” for? Specificity of hospital volume-outcome associations for surgical procedures: analysis of administrative data. BMJ. 2004;328(7442):737–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaassen Z, Shay R, Moses KA, Terris MK. Words of wisdom. Re: Volume outcomes of cystectomy--is it the surgeon or the setting? Eur Urol. 2014;65(4):846. [DOI] [PubMed] [Google Scholar]
- 22.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30(7):615–29. [DOI] [PubMed] [Google Scholar]
- 23.Trinh VQ, Trinh QD, Tian Z, Hu JC, Shariat SF, Perrotte P, et al. In-hospital mortality and failure-to-rescue rates after radical cystectomy. BJU Int. 2013;112(2):E20–7. [DOI] [PubMed] [Google Scholar]
- 24.Lerro CC, Robbins AS, Phillips JL, Stewart AK. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20(6):1759–65. [DOI] [PubMed] [Google Scholar]
- 25.Raghunathan TE LJ, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27(1):85–95. [Google Scholar]
- 26.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937–60. [DOI] [PubMed] [Google Scholar]
- 27.Cole SR HM. Adjusted Survival Curves with Inverse Probability Weights. Computer Methods and Programs in Biomedicine. 2004;75:45–9. [DOI] [PubMed] [Google Scholar]
- 28.Luft HS, Hunt SS, Maerki SC. The volume-outcome relationship: practice-makes-perfect or selective-referral patterns? Health Serv Res. 1987;22(2):157–82. [PMC free article] [PubMed] [Google Scholar]
- 29.Abboudi H, Khan MS, Guru KA, Froghi S, de Win G, Van Poppel H, et al. Learning curves for urological procedures: a systematic review. BJU Int. 2014;114(4):617–29. [DOI] [PubMed] [Google Scholar]
- 30.Dranove D A comment on “Does practice make perfect?”. Med Care. 1984;22(10):966. [PubMed] [Google Scholar]
- 31.Donabedian A The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–8. [DOI] [PubMed] [Google Scholar]
- 32.Siemens DR, Mackillop WJ, Peng Y, Berman D, Elharram A, Rhee J, et al. Processes of care and the impact of surgical volumes on cancer-specific survival: a population-based study in bladder cancer. Urology. 2014;84(5):1049–57. [DOI] [PubMed] [Google Scholar]
- 33.Konety BR, Dhawan V, Allareddy V, Joslyn SA. Impact of hospital and surgeon volume on in-hospital mortality from radical cystectomy: data from the health care utilization project. J Urol. 2005;173(5):1695–700. [DOI] [PubMed] [Google Scholar]
- 34.Morgan TM, Barocas DA, Keegan KA, Cookson MS, Chang SS, Ni S, et al. Volume outcomes of cystectomy--is it the surgeon or the setting? J Urol. 2012;188(6):2139–44. [DOI] [PubMed] [Google Scholar]
- 35.Smaldone MC, Simhan J, Kutikov A, Canter DJ, Starkey R, Zhu F, et al. Trends in regionalization of radical cystectomy in three large northeastern states from 1996 to 2009. Urol Oncol. 2013;31(8):1663–9. [DOI] [PubMed] [Google Scholar]
- 36.Konety BR, Allareddy V, Carroll PR. Factors affecting outcomes after radical cystectomy in African Americans. Cancer. 2007;109(3):542–8. [DOI] [PubMed] [Google Scholar]
- 37.Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250(3):363–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Unweighted and propensity score weighted covariates
Supplemental Table 2: Case frequency by hospital type (a. Surgeon Volume, b. Hospital Volume)


