Abstract
There is an urgent need for new treatments to prevent and ameliorate severe illness and death induced by SARS-CoV-2 infection in COVID-19 patients. The coronavirus mouse hepatitis virus (MHV)-1 causes pneumonitis in mice which shares many pathological characteristics with human SARS-CoV infection. Previous studies have shown that the amino acid gamma-aminobutyric acid (GABA) has anti-inflammatory effects. We tested whether oral treatment with GABA could modulate the MHV-1 induced pneumonitis in susceptible A/J mice. As expected, MHV-1-inoculated control mice became severely ill (as measured by weight loss, clinical score, and the ratio of lung weight to body weight) and >60% of them succumbed to the infection. In contrast, mice that received GABA immediately after MHV-1 inoculation became only mildly ill and all of them recovered. When GABA treatment was initiated after the appearance of illness (3 days post-MHV-1 infection), we again observed that GABA treatment significantly reduced the severity of illness and greatly increased the frequency of recovery. Therefore, the engagement of GABA receptors (GABA-Rs) prevented the MHV-1 infection-induced severe pneumonitis and death in mice. Given that GABA-R agonists, like GABA and homotaurine, are safe for human consumption, stable, inexpensive, and available worldwide, they are promising candidates to help prevent severe illness stemming from SARS-CoV-2 infection and other coronavirus strains.
While GABA is well known as a neurotransmitter that is commonly used in the central nervous system, it is becoming increasingly appreciated that many immune cells express GABA-Rs. The biological roles of GABA-Rs on immune cells are not yet well understood, but there is a growing body of evidence that the activation of these receptors generally has immunoregulatory actions. In the innate immune system, antigen-presenting cells (APCs) express GABA-A-type receptors (GABAA-Rs) which form a chloride channel and their activation reduces APC reactivity (1, 2). Neutrophils express GABA-B-type receptors (GABAB-Rs) which are G-protein coupled receptors that modulate their function (3). In the CNS, microglia express both GABAA-Rs and GABAB-Rs and their activation reduces microglia responsiveness to inflammatory stimuli (4). Alveolar macrophages express GABAA-Rs and application of a GABAA-R-specific agonist decreases the expression of many pro-inflammatory molecules in cultures of LPS-stimulated lung macrophages (5). In the adaptive immune system, we have shown that GABA-R activation promotes effector T cell cycle arrest without inducing apoptosis (6). In vivo, administration of GABA, or the GABAA-R-specific agonist homotaurine, inhibits autoreactive Th1 and Th17 cells while promoting CD4+ and CD8+ Treg responses (7–9). Taking advantage of these properties, we have demonstrated that administration of GABA or homotaurine inhibits disease progression in mouse models of type 1 diabetes (T1D), multiple sclerosis, and rheumatoid arthritis, and limits inflammation in a mouse model of type 2 diabetes (1, 6, 8–10).
There is an urgent need to develop new treatments to reduce severe illness and death in COVID-19 patients. Patients who develop severe illness appear to mount weaker and delayed innate immune responses to SARS-CoV-2 infection, which leads to excessive adaptive immune responses later that do not taper off appropriately (11–13). This can lead to “cytokine storms”, disseminated intravascular coagulation, multiple organ dysfunction syndrome (MODS), and death. Studies of anti-CD3-activated human PBMC have shown that GABA inhibits IL-6, CXCL10/IP-10, CCL4, CCL20, and MCP-3 production (14). Longitudinal studies of COVID-19 patients reveal that high levels of serum IL-6 and Th1, Th17, and Th2-secreted proteins are associated with progression to severe illness (11, 15). Many of these biomarkers of severe illness have been shown to be reduced by GABA-R agonists in the aforementioned in vitro studies of human PBMC and/or mouse models of autoimmune disease. Currently, however, there is no information on whether GABA treatment modulates the outcome of viral infections.
Like SARS-CoV and SARS-CoV-2, mouse hepatitis virus (MHV)-1 is a pneumotropic beta-coronavirus of the group 2 lineage and is widely used as a safe model of SARS-CoV infection (16–19). MHV-1 infection creates a lethal pneumonitis, similar to SARS-CoV-induced disease, in A/J mice. Intranasal inoculation with 5000 plaque-forming units (PFU) of MHV-1 in A/J mice induces an acute respiratory distress syndrome with a high lethality rate. The infected mice develop pathological features of SARS-CoV-2, including high levels of pulmonary cytokines/chemokines, pneumonitis, dense macrophage infiltrates, hyaline membranes, fibrin deposits, accompanied by loss of body weights and respiratory distress (16–19).
We studied whether oral GABA treatment beginning at the time of MHV-1 inoculation or starting three days post-inoculation (by which time signs of illness are apparent), could modulate the severity of the ensuing illness and the rate of death
Materials and methods
Mice.
Female A/J mice (7 weeks in age) were purchased from the Jackson Laboratory and maintained in microisolator cages and fed with a standard diet and water ad libitum. One week after arrival, they were inoculated with MHV-1. The mice were immediately randomized and treated (or not treated) with GABA, as described below. This study was carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols for all experiments using vertebrate animals were approved by the Animal Research Committee at UCLA.
Reagents.
GABA was purchased from Millipore-Sigma (stock # A2129, St. Louis, MO, USA).
Virus.
MHV-1, DBT cells, and HeLa-CECAM1 were generously provided by Dr. Stanley Perlman (University of Iowa). MHV-I virus was prepared and titered as previously described (16–19).
Viral infection and GABA treatment.
At 8 weeks in age, female A/J mice were anesthetized and inoculated intranasally with 5000 PFU MHV-1 in 50 μl cold Dulbecco’s modified Eagle’s medium (DMEM). The mice were immediately randomized and provided with plain water (controls) or water that contained GABA (20 mg/ml) for the entirety of the observation period. Another group of MHV-1 inoculated mice received plain water for three days, by which time they displayed signs of illness, and then were placed on GABA-containing water for the rest of the observation period. We monitored their body weights daily beginning on the day of infection and up to 14 days post-infection.
Illness scoring.
Individual mice were monitored for illness development and progression which were scored on the following scale: 0) no symptoms, 1) slightly ruffled fur and altered hind limb posture; 2 ruffled fur and mildly labored breathing; 3) ruffled fur, inactive, moderately labored breathing; 4) ruffled fur, obviously labored breathing and lethargy; 5) moribund and death.
The percent survival of each group of mice was determined longitudinally for each group. Mice with a disease score of 5 were weighed, euthanized, and their lungs removed and weighed for calculation of lung coefficient index (the ratio of lung weight to total body weight, which reflects the extent of edema and inflammation in the lungs). On day 14 post-infection, the surviving animals were weighed, euthanized, and their lungs were removed and weighed for determination of the lung coefficient index.
Results and Discussion
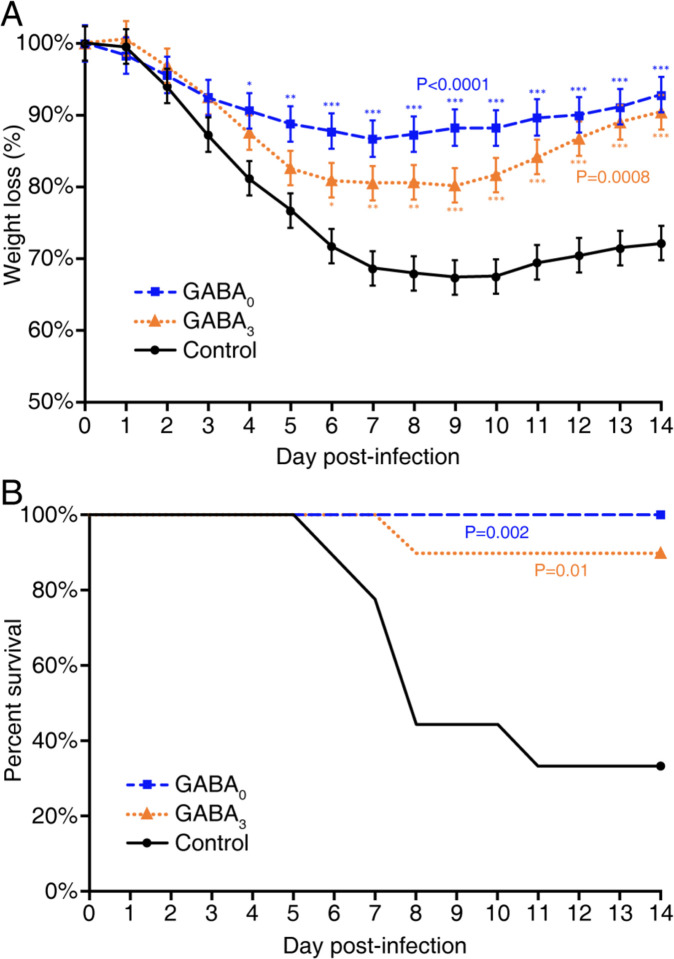
Following MHV-1 inoculation, the mice receiving plain water began to progressively lose body weight each day. By day 6, this control group had lost an average of 23% of their weights, as expected (16–19). At this time point, the mice that had been given GABA immediately after MHV-1 infection had lost an average of 11% of their body weights, and those given GABA three days after infection had lost an average of 17% of their body weights (Fig. 1A). After day 6, the mice in the control group began to succumb to their illness and only 3/9 mice survived to day 14 post-infection (Fig. 1B). In contrast, none of the mice given GABA starting immediately after MHV-1 inoculation died (Fig. 1B), and their body weight was on average only 7% below their starting weights 14 days post-infection (Fig. 1A). Of the mice that began GABA treatment 3 days post-infection, 1/9 mice died (on day 9), and their body weights at 14 days post-infection were 90% of their starting weight. The survival curves for each group are shown in Fig. 1B.
Figure 1. GABA treatment reduces body weight loss and death rate in MHV-1 infected mice.
Female A/J mice were inoculated with MHV-1 intranasally and immediately placed on plain water (control, solid black line) or water containing 20 mg/ml GABA (GABA0, dashed blue line) or given plain water for 3 days post-infection and then placed on water containing 20 mg/ml GABA (GABA3, orange dotted line) for the remaining observation period. A) Daily changes in % body weights post-infection (% of day 0), p<0.0001 and p=0.0008 for GABA0 and GABA3 (respectively) vs. control by repeated measure ANOVA. (GABA0 vs. GABA3,p=0.175.) B) Daily percent of surviving mice in each group, p=0.002 and p=0.01 for GABA0 and GABA3 vs. control, respectively by log-rank test. (p=0.32 for GABA0 vs. GABA3) N=9 mice in the control group, 10 mice in each GABA-treated group. Data shown are from two separate studies with 4–5 mice/group. *p<0.05, **p<0.01, ***p<0.001 vs. the control.
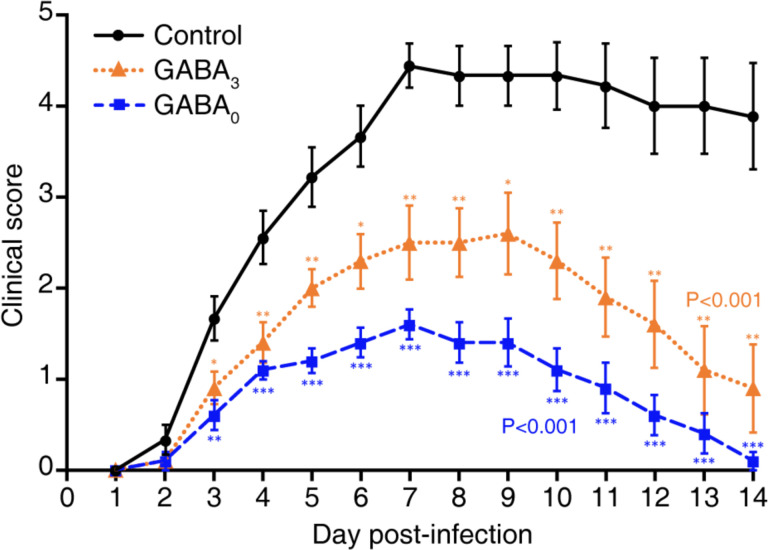
In terms of illness, MHV-1 infected control mice began to display signs of illness two days post-infection and rapidly became severely ill thereafter, with their illness peaking around day 7 post-infection. While most control mice died between days 6–11 post-infection, those that survived displayed only partial recovery from illness. In contrast, the mice receiving GABA immediately after MHV-1 inoculation developed only mild illness, with the highest average illness score of 1.6 on day 7 post-infection. Illness in the mice given GABA at 3 days post-infection was also significantly reduced compared to that in the control group, and their maximum mean illness score was 2.5. Thus, GABA treatment immediately after MHV-1 infection, or 3 days later when the clinical signs of the disease were apparent, reduced the subsequent severity of the disease and the death rate.
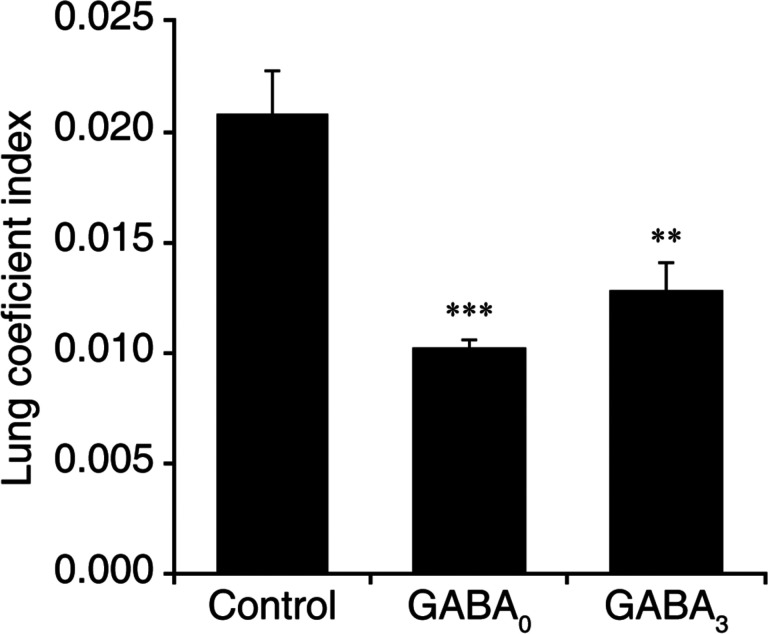
The lung coefficient index reflects the edema and inflammation in the lung. The lung coefficient index of mice that were given GABA immediately after MHV-1 infection was 49% of that of control mice (p<0.001). The mice receiving GABA treatment beginning 3 days post-infection had a lung coefficient index that was 62% of that in the control mice (p<0.01). This provides an independent measure indicating that GABA treatment limited the MHV-1 induced pulmonary edema and inflammation in A/J mice.
Together, the reduction in body weight loss, illness scores, death rate, and lung coefficient index indicate that GABA treatment can reduce illness severity and death rate following coronavirus infection, even when the treatment is initiated after symptoms appear.
Since weaker and delayed early immune responses to SARS-CoV-2 infection are associated with more severe illness in COVID-19 patients (11) and GABA has anti-inflammatory effects, we anticipated that treatment with GABA immediately after MHV-1 infection might be deleterious by limiting or delaying innate immune responses. We were surprised that early GABA treatment immediately after MHV-1 infection was very effective in preventing illness progression and death, suggesting a rapid effect of GABA on innate immune responses and/or the lung airway cells. The lung epithelial cells of mice and humans also express GABAA-Rs (20, 21). It is possible that the activation of these GABAA-Rs leads to Cl− efflux, which would act to limit Ca2+ influx in these epithelial cells. Because many viruses, including coronaviruses, elevate intracellular Ca2+ concentrations in order to enhance viral replication (22, 23), the activation of GABAA-Rs may have limited MHV-1 replication, a possibility that we are currently investigating.
Additionally, treatment with GABA, a GABAB-agonist, or GABAA-R positive allosteric modulators can reduce inflammation and improve alveolar fluid clearance and lung functional recovery in rodent models of acute lung injury (24–28). Whether these capabilities contributed to GABA’s ability to limit the progression of pneumonitis soon after MHV-1 infection is an open question. Thus, treatment with GABA-R agonists may have multiple beneficial actions for the treatment of COVID-19 patients.
GABA treatment was tested in hundreds of epilepsy patients for its ability to reduce seizures (29–31). While it had no clinical benefit (probably because it cannot cross the blood-brain barrier), it had no adverse effects in these long-term studies. A more recent phase Ib GABA oral dosing study also indicated that GABA is safe (32) and there are currently several ongoing clinical trials that are administering oral GABA to individuals with T1D (ClinicalTrials.gov Identifiers: NCT02002130, NCT03635437, NCT03721991, NCT04375020). In addition, the GABAA-R specific agonist homotaurine was tested in a large long-term phase III clinical trial for Alzheimer’s disease, and while it was not effective it had an excellent safety record (see (8, 9) for a discussion of homotaurine’s safety). Both GABA and homotaurine are inexpensive, stable at room temperature, and available worldwide making them excellent candidates for clinical testing as adjunctive treatments for COVID-19.
These studies indicate the potential usefulness of GABA and/or homotaurine as a treatment for COVID-19 and other coronavirus infections. However, until clinical trials are completed and GABA and/or homotaurine are approved for use in the treatment of COVID-19 by relevant governing bodies, GABA and homotaurine should not be consumed by COVID-19 patients as they may pose health risks, such as dampening beneficial immune or physiological responses.
Fig. 2. GABA treatment reduces illness scores in MHV-1 infected mice.
The animals described in Fig. 1 were scored daily for the severity of their illness as detailed in Methods. Data shown are the mean clinical scores +/− SEM of each group from two separate experiments. Overall p<0.001 for GABA0 and GABA3 vs. control. GABA0 vs. GABA3 p=0.042 using the Kruskal-Wallis test. *p<0.05, **p<0.01, ***p<0.001.
Figure 3. GABA treatment reduces the lung coefficient index in MHV-1 infected mice.
The lungs were harvested and weighed when an animal became moribund or at 14 days post-infection. Data shown are the mean lung coefficient index ± SEM for each group from two separate studies. ***p<0.001 and **p<0.01 for GABA0 and GABA3 (respectively) vs. control water treated group by Student’s t-test.
Acknowledgments.
We would like to thank Dr. Stanley Perlman for generously providing MHV-1, DBT cells, and HeLa-CECAM1 cells. We would also like Drs. Min Song for assistance, Cindy Chau for advice, and Jeffery Gornbein for statistical analysis. This work was supported by grants to DLK from the UCLA DGSOM-Broad Stem Cell Research Center COVID-19 Research Award (ORC #20-34), The National Institutes of Health (R21 DE029020), and the Department of Defense (CDMRP PR191176), as well as DLK’s unrestricted funds.
Footnotes
Disclosures. DLK and JT are inventors of GABA-related patents. DLK serves on the Scientific Advisory Board of Diamyd Medical. BM has no financial conflicts of interest.
References
- 1.Tian J, Yong J, Dang H, Kaufman DL. Oral GABA treatment downregulates inflammatory responses in a mouse model of rheumatoid arthritis. Autoimmunity. 2011;44:465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107(6):2580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rane MJ, Gozal D, Butt W, Gozal E, Pierce WM, Jr., Guo SZ, Wu R, Goldbart AD, Thongboonkerd V, McLeish KR, Klein JB. Gamma-amino butyric acid type B receptors stimulate neutrophil chemotaxis during ischemia-reperfusion. J Immunol. 2005;174(11):7242–9. [DOI] [PubMed] [Google Scholar]
- 4.Lee M, Schwab C, McGeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia. 2011;59(1):152–65. doi: 10.1002/glia.21087 [DOI] [PubMed] [Google Scholar]
- 5.Januzi L, Poirier JW, Maksoud MJE, Xiang YY, Veldhuizen RAW, Gill SE, Cregan SP, Zhang H, Dekaban GA, Lu WY. Autocrine GABA signaling distinctively regulates phenotypic activation of mouse pulmonary macrophages. Cell Immunol. 2018;332:7–23. Epub 2018/07/19. doi: 10.1016/j.cellimm.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Tian J, Lu Y, Zhang H, Chau CH, Dang HN, Kaufman DL. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173(8):5298–304. [DOI] [PubMed] [Google Scholar]
- 7.Tian J, Dang H, Nguyen AV, Chen Z, Kaufman DL. Combined therapy with GABA and proinsulin/alum acts synergistically to restore long-term normoglycemia by modulating T-cell autoimmunity and promoting beta-cell replication in newly diabetic NOD mice. Diabetes. 2014;63(9):3128–34. doi: 10.2337/db13-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian J, Dang H, O’Laco K, Song M, Tiu B-C, S G, Zakarian C, Kaufman D. Homotaurine treatment enhances CD4+ and CD8+ Treg responses and synergizes with low-dose anti-CD3 to enhance diabetes remission in type 1 diabetic mice. ImmuoHorizons. 2019:October 21;3(10):498–510. doi: 10.4049/immunohorizons.1900019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian J, Dang H, Wallner M, Olsen R, Kaufman DL. Homotaurine, a safe blood-brain barrier permeable GABAA-R-specific agonist, ameliorates disease in mouse models of multiple sclerosis. Sci Rep. 2018;8(1):16555 Epub 2018/11/10. doi: 10.1038/s41598-018-32733-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian J, Dang HN, Yong J, Chui WS, Dizon MP, Yaw CK, Kaufman DL. Oral treatment with gamma-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS One. 2011;6(9):e25338. doi: 10.1371/journal.pone.0025338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, Levantovsky R, Malle L, Moreira A, Park MD, Pia L, Risson E, Saffern M, Salome B, Esai Selvan M, Spindler MP, Tan J, van der Heide V, Gregory JK, Alexandropoulos K, Bhardwaj N, Brown BD, Greenbaum B, Gumus ZH, Homann D, Horowitz A, Kamphorst AO, Curotto de Lafaille MA, Mehandru S, Merad M, Samstein RM, Sinai Immunology Review P. Immunology of COVID-19: Current State of the Science. Immunity. 2020. Epub 2020/06/09. doi: 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181(5):1036–45 e9. Epub 2020/05/18. doi: 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadjad J, Yatim N, Barnabei L, et a. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. MedRxiv. 2020; 10.1101/2020.04.19.20068015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandage AK, Jin Z, Korol SV, Shen Q, Pei Y, Deng Q, Espes D, Carlsson PO, Kamali-Moghaddam M, Birnir B. GABA Regulates Release of Inflammatory Cytokines From Peripheral Blood Mononuclear Cells and CD4(+) T Cells and Is Immunosuppressive in Type 1 Diabetes. EBioMedicine. 2018;30:283–94. Epub 2018/04/09. doi: 10.1016/j.ebiom.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A, Yale IT, Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, Iwasaki A. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–9. Epub 2020/07/28. doi: 10.1038/s41586-020-2588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Albuquerque N, Baig E, Ma X, Zhang J, He W, Rowe A, Habal M, Liu M, Shalev I, Downey GP, Gorczynski R, Butany J, Leibowitz J, Weiss SR, McGilvray ID, Phillips MJ, Fish EN, Levy GA. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J Virol. 2006;80(21):10382–94. Epub 2006/10/17. doi: 10.1128/JVI.00747-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanolkar A, Hartwig SM, Haag BA, Meyerholz DK, Epping LL, Haring JS, Varga SM, Harty JT. Protective and pathologic roles of the immune response to mouse hepatitis virus type 1: implications for severe acute respiratory syndrome. J Virol. 2009;83(18):9258–72. Epub 2009/07/03. doi: 10.1128/JVI.00355-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanolkar A, Hartwig SM, Haag BA, Meyerholz DK, Harty JT, Varga SM. Toll-like receptor 4 deficiency increases disease and mortality after mouse hepatitis virus type 1 infection of susceptible C3H mice. J Virol. 2009;83(17):8946–56. Epub 2009/06/26. doi: 10.1128/JVI.01857-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanolkar A, Fulton RB, Epping LL, Pham NL, Tifrea D, Varga SM, Harty JT. T cell epitope specificity and pathogenesis of mouse hepatitis virus-1-induced disease in susceptible and resistant hosts. J Immunol. 2010;185(2):1132–41. Epub 2010/06/18. doi: 10.4049/jimmunol.0902749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin N, Kolliputi N, Gou D, Weng T, Liu L. A novel function of ionotropic gamma-aminobutyric acid receptors involving alveolar fluid homeostasis. J Biol Chem. 2006;281(47):36012–20. Epub 2006/09/28. doi: 10.1074/jbc.M606895200 [DOI] [PubMed] [Google Scholar]
- 21.Xiang YY, Chen X, Li J, Wang S, Faclier G, Macdonald JF, Hogg JC, Orser BA, Lu WY. Isoflurane regulates atypical type-A gamma-aminobutyric acid receptors in alveolar type II epithelial cells. Anesthesiology. 2013;118(5):1065–75. Epub 2013/03/15. doi: 10.1097/ALN.0b013e31828e180e [DOI] [PubMed] [Google Scholar]
- 22.Bai D, Fang L, Xia S, Ke W, Wang J, Wu X, Fang P, Xiao S. Porcine deltacoronavirus (PDCoV) modulates calcium influx to favor viral replication. Virology. 2020;539:38–48. Epub 2019/11/02. doi: 10.1016/j.virol.2019.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraeft SK, Chen DS, Li HP, Chen LB, Lai MM. Mouse hepatitis virus infection induces an early, transient calcium influx in mouse astrocytoma cells. Exp Cell Res. 1997;237(1):55–62. Epub 1998/01/07. doi: 10.1006/excr.1997.3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang T, Zhang Y, Wang C, Gao J. Propofol reduces acute lung injury by up-regulating gamma-aminobutyric acid type a receptors. Exp Mol Pathol. 2019;110:104295 Epub 2019/08/17. doi: 10.1016/j.yexmp.2019.104295 [DOI] [PubMed] [Google Scholar]
- 25.Kaynar G, Yurdakan G, Comert F, Yilmaz-Sipahi E. Effects of peripheral benzodiazepine receptor ligand Ro5–4864 in four animal models of acute lung injury. J Surg Res. 2013;182(2):277–84. Epub 2012/11/07. doi: 10.1016/j.jss.2012.10.023 [DOI] [PubMed] [Google Scholar]
- 26.Fortis S, Spieth PM, Lu WY, Parotto M, Haitsma JJ, Slutsky AS, Zhong N, Mazer CD, Zhang H. Effects of anesthetic regimes on inflammatory responses in a rat model of acute lung injury. Intensive Care Med. 2012;38(9):1548–55. Epub 2012/06/20. doi: 10.1007/s00134-012-2610-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chintagari NR, Liu L. GABA receptor ameliorates ventilator-induced lung injury in rats by improving alveolar fluid clearance. Crit Care. 2012;16(2):R55 Epub 2012/04/07. doi: 10.1186/cc11298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin S, Merchant ML, Ritzenthaler JD, McLeish KR, Lederer ED, Torres-Gonzalez E, Fraig M, Barati MT, Lentsch AB, Roman J, Klein JB, Rane MJ. Baclofen, a GABABR agonist, ameliorates immune-complex mediated acute lung injury by modulating pro-inflammatory mediators. PLoS One. 2015;10(4):e0121637. doi: 10.1371/journal.pone.0121637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otomo E, Araki G, Mori A, Kurihara M. Clinical evaluation of GABA in the treatment of cerebrovascular disorders. Multi-center double-blind study in comparison with pyrithioxine and placebo. Arzneimittelforschung. 1981;31(9):1511–23. [PubMed] [Google Scholar]
- 30.Loeb C, Benassi E, Bo GP, Cocito L, Maffini M, Scotto P. Preliminary evaluation of the effect of GABA and phosphatidylserine in epileptic patients. Epilepsy Res. 1987;1(3):209–12. [DOI] [PubMed] [Google Scholar]
- 31.Tower DB, Roberts E, editors. Inhibition in the Nervous System and GABA. New York: Pergamon Press; 1960. [Google Scholar]
- 32.Li J, Zhang Z, Liu X, Wang Y, Mao F, Mao J, Lu X, Jiang D, Wan Y, Lv JY, Cao G, Zhang J, Zhao N, Atkinson M, Greiner DL, Prud’homme GJ, Jiao Z, Li Y, Wang Q. Study of GABA in Healthy Volunteers: Pharmacokinetics and Pharmacodynamics. Front Pharmacol. 2015;6:260. doi: 10.3389/fphar.2015.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]