Abstract
Background
Arkansas is a rural state of 3 million people. It is ranked fifth for poverty nationally. The first case of coronavirus disease 2019 (COVID‐19) in Arkansas occurred on 11 March 2020. Since then, approximately 8% of all Arkansans have tested positive. Given the resource limitations of Arkansas, COVID‐19 convalescent plasma (CCP) was explored as a potentially lifesaving, therapeutic option. Therefore, the Arkansas Initiative for Convalescent Plasma was developed to ensure that every Arkansan has access to this therapy.
Study Design and Method
This brief report describes the statewide collaborative response from hospitals, blood collectors, and the Arkansas Department of Health (ADH) to ensure that CCP was available in a resource‐limited state.
Results
Early contact tracing by ADH identified individuals who had come into contact with “patient zero” in early March. Within the first week, 32 patients tested positive for COVID‐19. The first set of CCP collections occurred on 9 April 2020. Donors had to be triaged carefully in the initial period, as many had recently resolved their symptoms. From our first collections, with appropriate resource and inventory management, we collected sufficient CCP to provide the requested number of units for every patient treated with CCP in Arkansas.
Conclusions
The Arkansas Initiative, a statewide effort to ensure CCP for every patient in a resource‐limited state, required careful coordination among key players. Collaboration and resource management was crucial to meet the demand of CCP products and potentially save lives.
Keywords: donors, FFP transfusion, transfusion service operations
Abbreviations
- ADH
Arkansas Department of Health
- AICP
Arkansas Initiative for Convalescent Plasma
- AR CCP EATP
Arkansas Expanded Access COVID‐19 Convalescent Plasma Treatment Program
- CCP
COVID‐19 convalescent plasma
- COVID‐19
coronavirus disease 2019
- EAP
expanded access protocol
- eIND
emergency use investigational new drug application
- FDA
US Food and Drug Administration
- UAMS
University of Arkansas for Medical Sciences
1. INTRODUCTION
Arkansas is a resource‐limited state, and almost half (44%) of Arkansans live in rural areas. 1 As a measure of context, only 19% of people live in rural areas in the United States. 1 There are only eight metropolitan areas with a population of greater than 50,000 in the state. 2 Arkansas also ranks fifth nationally for poverty. 1 , 3 Given the rurality and poverty of the state, there are high rates of comorbidities such as obesity, diabetes, and cardiovascular disease among Arkansans. 4 , 5 In addition, the residents in Arkansas have many barriers to health care access, including access to therapies and timely treatment of life‐threatening illnesses such as coronavirus disease 2019 (COVID‐19).
COVID‐19 is caused by severe acute respiratory syndrome coronavirus 2. It was first documented in the United States in Washington state on 21 January 2020. 6 The community spread of the virus impacted states such as Washington, California, and New York before the first COVID‐19 case in Arkansas. The first confirmed case of coronavirus in Arkansas was reported on 11 March 2020. 7 The number of confirmed cases has been increasing in this resource‐limited state from March in a steady manner (Figure 1, generated by the Arkansas Department of Health [ADH]).
FIGURE 1.
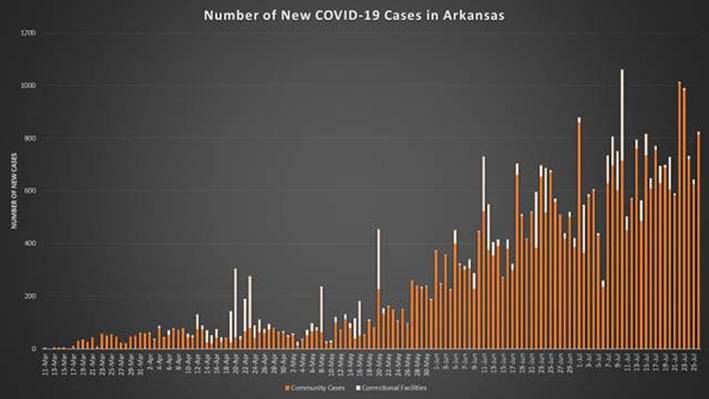
Number of new COVID‐19 Cases in Arkansas by date [Color figure can be viewed at wileyonlinelibrary.com]
Given the increase in coronavirus cases in the state, ADH proactively began implementing emergency preparedness lessons learned from states such as New York, which was the site of the country's largest outbreak. The lessons learned had to be applied to a resource‐constrained state where access to investigational drugs such as remdesivir was not as readily available as in other states in the country. Among them was the investigational use of convalescent plasma in patients with COVID‐19. Convalescent plasma, a passive antibody treatment, involves the transfer of antibodies against an infectious agent from a recovered person to an ill patient. This therapy was used successfully to treat diseases such as the 1918 influenza and H1N1 pandemics. 8 , 9 , 10 ADH, in collaboration with the University of Arkansas for Medical Sciences (UAMS), instituted operational activities that enabled the coordination of therapies, including COVID‐19 convalescent plasma (CCP), throughout the state.
This report describes the phased response (Figure 2) and challenges faced by the hospitals, the two major blood collectors, and ADH to coordinate the self‐sustaining collection and equitable delivery of CCP to patients with COVID‐19 in a resource‐limited state.
FIGURE 2.
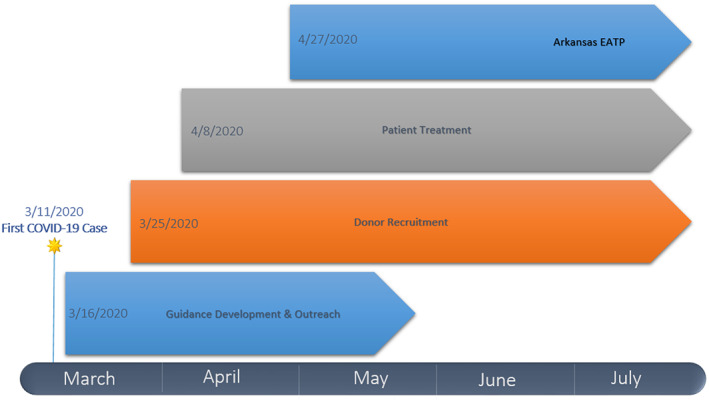
The Arkansas Initiative [Color figure can be viewed at wileyonlinelibrary.com]
2. MARCH 2020: STATEWIDE COLLABORATION FORMED THE ARKANSAS INITIATIVE FOR CONVALESCENT PLASMA
With the advent of the first case, ADH approached the state hospital, UAMS, to provide CCP for patients with COVID‐19. A lack of hospital‐based donor centers in the state was one of the barriers identified by UAMS, which also lacks a donor center. Thus, CCP provision would require the help of local blood collectors. There are four local blood collectors in the state, but only two cover a sizeable geographic footprint. The collectors were recruited to collect convalescent plasma units for every patient with COVID‐19 requiring this treatment.
CCP product inventory development was the initial challenge for local blood collectors. Thus, they quickly changed their focus from potential COVID‐19–related blood shortages to the collection of CCP, an investigational blood product. Given the distant locations of collection facilities in the state, donor and staff safety training and implementation protocols regarding CCP collections were instituted first at centers near COVID‐19 hot spots. One blood collector pursued the screening of all CCP units for isohemagglutinin (anti‐A, anti‐B) titers. The designation of ABO low‐titer units optimized patient access to CCP products in the setting where group O and A‐type CCP units were more abundant than group B or AB‐type CCP units. Because HLA testing is costly, the cost of CCP was kept low by phasing donor collections, with males and nonpregnant females as the first targeted donors, and later expanding to previously pregnant females. Additional effort was then required by the blood collectors to coordinate HLA testing before CCP collection from female donors.
The second challenge to patients with COVID‐19 in Arkansas involved the general lack of opportunities for hospitals in the state to participate in COVID‐19 clinical trials sponsored by either academic institutions or pharmaceutical companies. Many hospitals in Arkansas lack the infrastructure to support research, such as institutional review boards and research coordinators. However, even hospitals with adequate research infrastructure were unable to join clinical studies involving CCP or remdesivir because the number of hospitalized patients in Arkansas was deemed too low by sponsors. Another challenge was the lack of specialty‐trained physicians, such as transfusion medicine physicians, at many hospitals throughout the state. There are 85 hospitals in Arkansas; 56 have hospital blood banks. Given these challenges, UAMS and ADH reached out to all transfusion‐capable hospitals in the state through weekly meetings to provide guidance on caring for patients with COVID‐19 requiring convalescent plasma. Hospitals were educated on the two mechanisms that were available in March for the provision of CCP: the FDA emergency use investigational new drug application (eIND) and the Mayo Clinic–sponsored expanded access protocol (EAP). A guidance document for hospitals was also created by UAMS and ADH that described these CCP delivery mechanisms. Regardless of the mechanism chosen by the hospital, the role of UAMS and ADH was to ensure the equitable delivery of CCP to them. Conversations were held on the ethical provision of CCP in the likelihood of CCP shortages in the state. This overall initiative to provide at least two units of CCP for every COVID‐19 patient in the state was called the Arkansas Initiative for Convalescent Plasma (AICP).
3. APRIL 2020: STATEWIDE CCP PROVISION
Our first request for CCP for a patient with COVID‐19 was received on 8 April 2020. A major challenge during this phase was to coordinate the donation and availability of CCP for eligible patients. The eligibility criteria for patients to receive CCP are (a) laboratory‐confirmed COVID‐19 and (b) severe or immediately life‐threatening COVID‐19. The US Food and Drug Administration (FDA) defines severe disease as having one or more of the following: shortness of breath (dyspnea), respiratory frequency >30/min, blood oxygen saturation <93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300, and lung infiltrates >50% within 24 to 48 hours. 11 Immediately life‐threatening disease is defined as having one or more of the following: respiratory failure, septic shock, and multiple organ dysfunction or failure. Informed consent by either the patient or a health care proxy is required before CCP transfusion. 11
We collected CCP from our first set of donors on 9 April 2020. Given that our first COVID‐19 case was diagnosed on 11 March, following FDA guidelines, 8 April was our first opportunity to collect CCP. The first issue was recruiting an adequate number of Arkansans who had recovered from COVID‐19 as CCP donors. However, during the first week of April, only 0.03% of Arkansans had been diagnosed with COVID‐19. Toward solving these issues, we used contact‐tracing data from ADH to generate a registry of COVID‐19–tested patients who were specifically asked by the contact tracer whether they wanted to donate CCP. This registry and COVID‐19 patient lists managed by health networks in the state such as the Baptist Health System were used to recruit CCP donors. Several hospitals, in addition to the Baptist Health System, collaborated in this endeavor.
The donors were recruited using the FDA criteria for CCP donors: (a) evidence of COVID‐19 documented by a diagnostic test (eg, nasopharyngeal swab) at the time of illness; (b) complete resolution of symptoms at least 14 days before the donation; (c) male donors, or female donors who have not been pregnant, or female donors who have been tested since their most recent pregnancy and results interpreted as negative for HLA antibodies; and (d) negative diagnostic (eg, nasopharyngeal swab) results for COVID‐19 if donating after 14 days or before 28 days after symptom resolution. 11 CCP donors had to meet all general blood donor eligibility criteria, in addition to the requirement for donating CCP, and undergo blood donor testing to be acceptable. Repeat testing for COVID‐19 was performed at two large health institutions and ADH. The state blood collectors, the Arkansas Blood Institute and the Community Blood Center of the Ozarks, worked diligently to increase CCP inventory through media outreach and conversations with their hospital customers starting in April. Physicians from local hospitals around the state also conducted media outreach. Individuals who were previously positive for COVID‐19 were responsive to the media request to donate CCP to patients suffering from the illness.
The second donation‐related issue was that many potential CCP donors did not have convenient access to a donation center. To mitigate this issue, mobile drives were held by one of the blood collectors to ensure the collection of CCP from donor clusters who had access issues and were present in more isolated parts of the state. Prescreening of these donors and appointment‐driven mobile drives ensured successful CCP collections in a socially distanced yet cost‐effective manner.
Weekly calls with members of the AICP functioned to streamline and coordinate donor recruitment, CCP delivery to hospitals, and CCP inventory management. The local blood collectors also shared CCP products with each other when short‐term shortages occurred, and the outpouring of support from donors aided in building the CCP inventory in the state.
4. MAY 2020: ESTABLISHED ARKANSAS EXPANDED ACCESS COVID‐19 CONVALESCENT PLASMA TREATMENT PROGRAM
Hospital requests for CCP were met without difficulty using the AICP, and each eligible patient in Arkansas received at least one unit of CCP. There was no delay in the delivery of CCP to the patient. Most patients in Arkansas received at least two units of CCP (Figure 3). The patients who received one unit of plasma were those enrolled in the Mayo EAP, those who died before receiving their second unit, or as determined by their ordering physician. Reviewing the vein‐to‐vein processes and mechanisms for obtaining CCP, approximately 25 hospitals in Arkansas noted difficulties in completing the individual requests for the FDA eIND and the data collection forms for the Mayo Clinic EAP. Based on the hospitals’ input, UAMS prepared a new intermediate‐size patient population expanded‐access investigational new drug application for the FDA. We anticipated recruiting 1000 patients into the EAP from hospitals with high numbers of patients with COVID‐19. The resulting Arkansas Expanded Access COVID‐19 Convalescent Plasma Treatment Program (AR CCP EATP) was a statewide EAP sponsored by UAMS with participating sites throughout the state.
FIGURE 3.
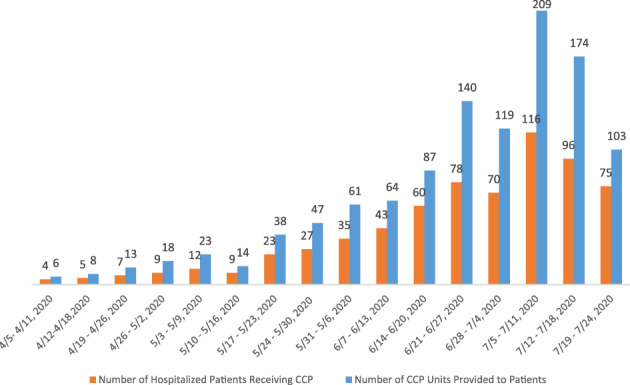
CCP units administered to hospitalized patients in Arkansas, April–July 2020 [Color figure can be viewed at wileyonlinelibrary.com]
While the idea of the AR CCP EATP was good, it was difficult to implement this program throughout this resource‐constrained state. Many hospitals interested in participating in the AR CCP EATP did not have resources such as in‐house coordinators, legal advisors, or institutional review boards to review the study documents or guide the principal investigator through the legal, regulatory, and on‐boarding processes for the study. While UAMS was supportive of serving as the sponsor site for Arkansas, its research infrastructure was stretched to onboard all interested hospitals in the state. The lack of federal or state funding also made this program difficult to implement throughout the state.
5. JUNE/JULY 2020: REVISED DONOR RECRUITMENT STRATEGIES TO MEET INCREASED CCP DEMAND
During June and July, following the Memorial Day holiday (25 May), the number of patients hospitalized with COVID‐19 increased dramatically in the state. A concomitant increase in CCP use occurred throughout the state—there was a 700% increase in requests for CCP from local hospitals. Efforts were made to rapidly scale up CCP collection by increasing donor recruitment via the same channels used initially (eg, physician and blood center media requests). While the response from CCP donors during June and July was not as robust as during March and April, CCP demands in the state were met. One potential explanation for the decrease in altruistic CCP donations was the recruitment of CCP donors by hyperimmune globulin manufacturers, which pay their donors. One of our blood collectors experienced a 75% decrease in CCP donors during this period. To counteract the loss of donors to plasma collection centers, more donors had to be recruited using the ADH registry. Eligible donors were called by ADH nurses requesting that they donate CCP while highlighting the importance of their donation. The deferral time of 28 days between CCP donations was decreased to 14 days by our blood collectors, allowing for more frequent CCP donations. Some blood collectors offered free antibody tests to whole blood donors. If the donor had a positive antibody test and met FDA eligibility criteria for CCP donation, these donors were recruited to donate CCP. The FDA also changed their CCP donor qualification criteria to allow donation within 14 days of symptom resolution. 11 Hospitals also encouraged patients who had recovered from COVID‐19 to consider donating CCP after their stay in the hospital, given that hospitalized patients potentially have higher titers of COVID‐19 antibodies than nonhospitalized positive individuals. Through the AICP, we met all the requests for CCP within 24 hours. Often, the blood collectors had to provide one unit of CCP at a time to ensure that all patients received the minimum of two units if two units were ordered by their physician.
6. CONCLUSION
The provision of CCP through the statewide collaborative network, the AICP, allowed for a self‐sustaining mechanism to provide this lifesaving therapy to patients with COVID‐19 in Arkansas. We were able to provide at least two units of CCP to every patient in Arkansas despite resource constraints, initial low state‐positivity rates, and increased demand for this treatment in June and July. The AR CCP EATP, which was well intentioned, was not able to accomplish the anticipated recruitment goals because of the lack of funding and internal and external institutional resources, such as research coordinators. This experience highlights the need for adaptability to an ever‐changing pandemic landscape. The hospitals, the blood collectors, and ADH were successful in navigating the resource constraints faced by the state and in ensuring the availability of CCP to Arkansans. The collaboration serves as a model that can be used by other resource‐constrained states to fulfill the demand of CCP for COVID‐19 patients.
CONFLICT OF INTEREST
T.L., B.Q., S.K., M.C., S.C., A.R., S.H., T.R., A.R., D.A., S.R., S.P., A.K., and N.P. declare no conflicts of interests. T.S.I. is a consultant and received research funding from Terumo BCT.
ACKNOWLEDGMENTS
The authors thank Jennifer Hunt, MD; Jennifer Laudadio, MD; Ericka Olgaard, DO; Shuk‐Mei Ho, PhD; and Laura P. James, MD, for their support of the Arkansas Expanded Access COVID‐19 Convalescent Plasma Treatment Program. We are grateful for the review of this article performed by Jay S. Raval, MD, and Evan Bloch, MD.
Ipe TS, Le T, Quinn B, et al. Provision of COVID‐19 Convalescent Plasma in a Resource‐Constrained State. Transfusion. 2020;60:2828–2833. 10.1111/trf.16118
REFERENCES
- 1. Miller W, Knapp T. Rural Profile of Arkansas 2019 [monograph on the internet]. 2019. https://www.uaex.edu/publications/pdf/MP551.pdf. Accessed July 15, 2020.
- 2. Wikipedia . Arkansas metropolitan areas [monograph on the Internet]. 2020. https://en.wikipedia.org/wiki/Arkansas_metropolitan_areas. Accessed July 15, 2020.
- 3. US Census Bureau . Quick facts Arkansas [monograph on the Internet]. 2020. https://www.census.gov/quickfacts/fact/table/AR/PST045219. Accessed July 15, 2020.
- 4. Robert Wood Johnson Foundation . New report finds progress to prevent obesity at risk [monograph on the Internet]. 2017. https://www.rwjf.org/en/library/articles‐and‐news/2017/08/new‐report‐finds‐progress‐to‐prevent‐obesity‐at‐risk.html. Accessed July 15, 2020.
- 5. American Heart Association . Arkansas state fact sheet [monograph on the Internet]. 2017. https://cpr.heart.org/‐/media/files/about‐us/policy‐research/fact‐sheets/quality‐systems‐of‐care/quality‐systems‐of‐care‐arkansas.pdf?la=en. Accessed July 15, 2020.
- 6. Washington State Department of Health . Novel coronavirus outbreak 2020 [monograph on the Internet]. 2020. https://www.doh.wa.gov/Emergencies/Coronavirus. Accessed July 15, 2020.
- 7. Governor Hutchinson confirms state's first presumptive positive COVID‐19 case [monograph on the Internet]. 2020. https://web.archive.org/web/20200313193516/https:/governor.arkansas.gov/news‐media/press‐releases/governor‐hutchinson‐confirms‐states‐first‐presumptive‐positive‐covid‐19‐cas. Accessed July 15, 2020.
- 8. Marano G, Vaglio S, Pupella S, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta‐analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. [DOI] [PubMed] [Google Scholar]
- 10. Hung IF, To KK , Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. US Food and Drug Administration . Recommendations for Investigational COVID‐19 Convalescent Plasma. FDA Vaccine, Blood, Biologics ‐ Investigational New Drug (IND) or Device Exemption (IDE) Process (CBER). 2020. www.fda.gov/vaccines‐blood‐biologics/investigational‐new‐drug‐ind‐or‐device‐exemption‐ide‐process‐cber/revised‐information‐investigational‐covid‐19‐convalescent‐plasma#foot. Accessed April 14, 2020. [Google Scholar]


