Dear Editor,
The novel coronavirus disease 2019 (COVID‐19) occurring in many countries worldwide has shown to be associated with hematological disorders. 1 , 2 Moderate thrombocytopenia has been found in nearly 36% of COVID‐19 patients. 2 However, deep thrombocytopenia is rare and is associated with disease severity. 3 Thrombocytopenia pathogenesis during COVID‐19 seems to be complex and multifactorial. 4 , 5 Immuno‐hematological diseases have been described in patients with COVID‐19, such as immune thrombocytopenic purpura (ITP), 6 , 7 autoimmune hemolytic anemia (AIHA), 8 and antiphospholipid syndrome. 9 Here, we wish to report a case of new‐onset ITP occurring a month after first COVID‐19 symptoms.
A 63‐year‐old woman with autoimmune hypothyroidism and stroke medical history presented to the emergency department for a 7‐day history of asthenia, fever, dry cough, and headaches. At admission, she was afebrile and her vitals were unremarkable. She was able to breathe normally without oxygen. Chest examination found bilateral crackles of lung bases. Blood tests showed lymphocytopenia (600/mm3), with normal platelets (197 000/mm3) and hemoglobin (12.4 g/dL) levels. C‐reactive protein (CRP) was 38 mg/L, and creatinine was in the normal range. She was tested negative for the nasal SARS‐CoV‐2 real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) but her chest CT scan found bilateral and subpleural frosted glass beaches, predominant in the two lower lung lobes (10% to 25% of all lung volume), evocative of moderate COVID‐19 pneumonia (Figure 1A). Her husband was tested positive for the nasal SARS‐CoV‐2 RT‐PCR the same day. Because of normal vital parameters, she was able to go back home. She started a self‐medication with azithromycin 500 mg per day and hydroxychloroquine 600 mg per day for 6 and 12 days, respectively.
Figure 1.
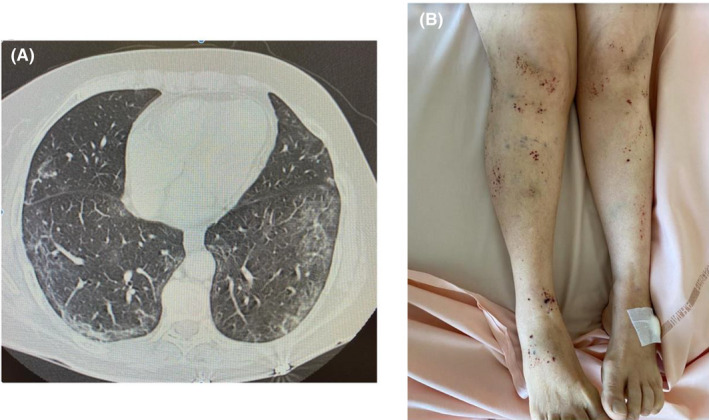
A, Chest CT scan showing bilateral and subpleural frosted glass beaches evocative of COVID‐19 pneumonia. B, Lower limb purpura and bruises
On day 26 after first symptoms, she presented again to the emergency department because of a lower limb purpura (Figure 1B). She was afebrile, without cough and headaches since a week. She did not report digestive bleeding nor hematuria. Physical examination found bruises of both arms and legs, and there was no neurologic abnormality. Laboratory tests found severe thrombocytopenia (3000/mm3) with persistent lymphocytopenia (900/mm3). Hemoglobin and neutrophil counts were in the normal range. She did not report any new medication than her usual treatment and the recent association of azithromycin and hydroxychloroquine. The prothrombin, activated partial thromboplastin times, and fibrinogen levels were normal. CRP level was 4.0 mg/L. There was no hemolysis, and the peripheral blood smear did not show schistocytes. Antinuclear antibodies were found with a nucleolar coloration (titer 1/160, no antibody specificity). Direct antiglobulin test and antiphospholipid antibodies were negative. Bone marrow aspiration showed a slight dyserythropoiesis with normal pleomorphic megakaryocytes, erythroblasts, and granulocytes. There was no evidence of hemophagocytosis. The SARS‐CoV‐2 qualitative serology was positive (IgG alone). The abdominal CT scan did not find any lymph nodes, hepatomegaly, nor splenomegaly. Intravenous immune globulin (IvIg) was administered at a rate of 1 g/kg of body weight. A novel 1 g/kg of body weight IvIg infusion was administered on day 28 because of persistent thrombocytopenia. Platelet levels progressively increased to 38 000/mm3, 95 000/mm3, and 145 000/mm3 on days 29, 31, and 33. Purpura of lower limbs and bruises totally disappeared. The patient was discharged from the hospital on day 33.
In our observation, COVID‐19 diagnosis was made retrospectively because of (1) evocative viral symptoms and chest CT scan, (2) husband positive nasal SARS‐CoV‐2 RT‐PCR during the symptomatic phase and (3) patient positive serology a month after first symptoms. False‐negative SARS‐CoV‐2 RT‐PCR is well known and can reach more than 50% of patients with effective infection. Kucirka et al 10 reported, in patients with symptomatic COVID‐19, a false‐negative RT‐PCR rate ranging from 20% to 66%.
The causes of secondary ITP in adults include viral infections such as hepatitis C virus, Epstein‐Barr virus, human immunodeficiency virus, or SARS‐CoV‐1. 11 , 12 Immune cytopenia associated with SARS‐CoV‐2 infection has been first reported by Zulfiqar et al 6 with the case of a 65‐year‐old woman presenting with ITP in the early course of COVID‐19. Lazarian et al 8 reported a case series of 7 patients presenting with AIHA occurring during the inflammatory state of COVID‐19. Authors then supposed that COVID‐19 immune cytopenia occurred within a timeframe compatible with the cytokine storm. In our observation, ITP was diagnosed after viral symptom resolution and off the inflammatory timeframe of infection. It is supported by the fact that inflammatory blood markers already dropped off at the time of ITP diagnosis. Recently, Mahevas et al 7 reported a case series of 14 new‐onset ITP after COVID‐19 and found that deep thrombocytopenia occurs from 2 to 30 days after first COVID‐19 symptoms. Even if cause and effect between ITP and COVID‐19 cannot be confirmed in our patient, these observations support that a close follow‐up after COVID‐19 is needed for such patients.
Moreover, drug‐induced ITP has also been reported. While hydroxychloroquine‐induced thrombocytopenia has never been reported, macrolide‐induced severe thrombocytopenia has been described including azithromycin. 13 This exceptional drug‐induced side effect always appears during the course of azithromycin treatment. In our observation, ITP diagnosis has been made 13 days after drug discontinuation. Therefore, it did not support drug‐induced thrombocytopenia.
Therapeutic management of ITP during COVID‐19 pandemic is difficult, and patricians need to consider bleeding, thrombotic and septic risks of each patient to lead ITP treatment. 5 However, IvIg permitted to quickly increase platelet counts in our patient, without side effect. In the case series of Mahevas et al, 7 IvIg was used in 9/14 cases and all patients completely recovered from ITP and COVID‐19 without thrombotic complication. It suggests that IvIg can be used in such patients, without severe side effects.
CONFLICT OF INTEREST
Levraut M, Ottavi M, Lechtman S, Mondain V, and Jeandel PY have no competing interests.
AUTHOR CONTRIBUTIONS
Michael Levraut played a major role in the writing of the manuscript. Marie Ottavi played a major role in taking care of the patient and the writing of the manuscript. Sarah Lechtman played a major role in taking care of the patient and revised the manuscript. Véronique Mondain played a role in taking care of the patient and revised the manuscript. Pierre‐Yves Jeandel played a major role in taking care of the patient, the writing, and revising the manuscript.
REFERENCES
- 1. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95(6):E131‐E134 [DOI] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost. 2020;120(6):949‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pavord S, Thachil J, Hunt BJ, et al. Practical guidance for the management of adults with immune thrombocytopenia during the COVID‐19 pandemic. Br J Haematol. 2020;189(6):1038‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zulfiqar A‐A, Lorenzo‐Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient With Covid‐19. N Engl J Med. 2020;382(18):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahevas M, Moulis G, Andres E, et al. Clinical characteristics, management and outcome of COVID‐19‐associated immune thrombocytopenia: a French multicentre series. Br J Haematol. 2020;190(4):e17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lazarian G, Quinquenel A, Bellal M, et al. Autoimmune haemolytic anaemia associated with COVID‐19 infection. British J Haematol. 2020;190(1):29‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false‐negative rate of reverse transcriptase polymerase chain reaction‐based SARS‐CoV‐2 Tests by time since exposure. Ann Intern Med. 2020;173(4):262‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moulis G, Palmaro A, Montastruc J‐L, Godeau B, Lapeyre‐Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia : a nationwide population‐based study in France. Blood. 2014;124(22):3308‐3315. [DOI] [PubMed] [Google Scholar]
- 12. Yang M, Ng MH, Li CK. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology. 2005;10(2):101‐105. [DOI] [PubMed] [Google Scholar]
- 13. Butt MU, Jabri A, Elayi SC. Azithromycin‐induced thrombocytopenia: a rare etiology of drug‐induced immune thrombocytopenia. Case Rep Med. 2019;2019:e6109831. [DOI] [PMC free article] [PubMed] [Google Scholar]


