To the Editor,
It is widely reported in the literature that CD4, CD8, and total T cell count are significantly reduced in critically ill patients with coronavirus disease 2019 (COVID‐19). 1 , 2 , 3 , 4 Pallotto et al. 5 analyzed CD4/CD8 ratio in 38 hospitalized patients with COVID‐19 (reff). The authors propose an elevated CD4/CD8 ratio as a useful early predictive biomarker for development of critical illness in patients with COVID‐19. 5 Few weeks ago, we suggested natural killer (NK) cell count as a marker of severity in 34 hospitalized patients with COVID‐19 but we did not observe any significant alterations in CD4‐ and CD8‐lymphocyte counts or CD4/CD8 ratio. 4 On this topic, our research group firstly described a novel potential COVID‐19 severity marker, Krebs von den Lungen‐6 (KL‐6), which is a high molecular weight glycoprotein expressed by Type 2 pneumocytes and released in the bloodstream after epithelial damage and reparative proliferation processes. 6 , 7 , 8 In particular, the authors observed significantly higher serum KL‐6 concentrations in patients with severe COVID‐19 than those with milder disease. 7
This study aimed to investigate how a combination of COVID‐19 severity markers could be helpful in the clinical management of these patients.
We retrospectively enrolled 54 patients (median age, interquartile range [IQR], 64 [58–74] years; 61% males), hospitalized at COVID Unit of Siena University Hospital from March to May 2020. Hospitalization criteria included diagnosis of COVID‐19, vital organ involvement, and nasopharyngeal swabs positive for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) nucleic acid by reverse‐transcription polymerase chain reaction.
The study was conducted in compliance with the principles of the Declaration of Helsinki.
Peripheral blood samples were obtained on admission before starting specific pharmacological treatment for COVID‐19, and were processed by flow cytometry to lymphocyte immunophenotyping and chemiluminescence assay to KL‐6 detection. According to the need for intensive care unit (ICU) admission, mechanical ventilation, or high‐flow oxygen therapy, patients were divided into two groups: severe (n = 14) and nonsevere (n = 40).
The main characteristics of our COVID‐19 population, including lymphocyte subset results and KL‐6 concentrations, are reported in Table 1.
Table 1.
The main characteristics of population including, age (median, IQR), gender (%) and lymphocyte subsets at the hospital admission
| Parameters | Severe cases (n = 14) | Nonsevere cases (n = 40) | p value |
|---|---|---|---|
| Age (median, IQR) | 65 (59–71) | 64 (58–72) | NS |
| Gender, M/F | 12/2 | 21/19 | NS |
| Lymphocyte subsets (median, IQR) | |||
| CD45 (cells/µl) | 794 (537–1203) | 1341 (798–2071) | .0110 |
| CD3% | 72 (63–85) | 72 (66–75) | NS |
| CD3 (cells/µl) | 506 (389–943) | 782(445–1483) | .0372 |
| CD4% | 45 (37–52) | 43 (37–54) | NS |
| CD4 (cells/µl) | 340 (232–556) | 492 (269–753) | NS |
| CD8% | 21 (15–33) | 23 (18–32) | NS |
| CD8 (cells/µl) | 132 (103–344) | 289 (143–537) | .0471 |
| CD19% | 12 (7–27) | 13 (10–19) | NS |
| CD19 (cells/µl) | 99 (58–196) | 140 (66–279) | NS |
| NK cells % | 7.8 (3.9–13.5) | 12.6 (8.4–19.6) | .0233 |
| NK (cells/µl) | 69 (25–109) | 139 (101–211) | .0009 |
| CD4/CD8 | 2.3 (1.2–3.3) | 1.9 (1.2–2.8) | NS |
| KL‐6 (U/ml) | 1125 (495–2034) | 316 (210–398) | <.0001 |
Abbreviation: IQR, interquartile range.
Statistical analysis was performed using GraphPad Prism 8.0 software. Nonparametric one‐way analysis of variance test (Kruskal–Wallis test) and Dunn posttests were used for multiple comparisons. The Mann–Whitney test was used to compare pairs of variables. The χ 2 test was used for categorical variables as appropriate. We also performed a logistic regression, using the severe group as dependent variable against nonsevere patients, to assess the potential of serum markers in discriminating the two groups. Sensitivity, specificity, and positive and negative predicted values (PPV and NPV, respectively) were calculated for the cut‐off of the different variables.
The total number of lymphocytes (CD45+) was significantly lower in the severe than in the nonsevere group (median IQR, 794 [537–1203] vs. 1341 [798–2071], p = .0110). CD3 lymphocyte count was lower in the severe group (median IQR, 506 [389–943] vs. 782 [445–1483] cells/μl, p = .0372); likewise, CD8 count was depleted in the severe group (median IQR, 132 [103–344] vs. 289 [143–537] cells/μl, p = .0471). NK cells concentration was also significantly lower in severe than in nonsevere patients (median IQR, 69 [25–109] vs. 139 [101–211], p = .0009) and NK cell percentages showed the same pattern (median IQR, 7.8 [3.9–13.5] vs. 12.6 [8.4–19.6], p = .0233).
Serum KL‐6 concentrations were more elevated in the severe group than nonsevere group (median IQR, 1125 [495–2034] vs. 316 [210–398], p < .0001).
Testing the severe group as a dependent variable by logistic regression, with CD45‐, CD3‐, CD8‐, NK‐cells counts, and percentages and KL‐6 concentrations as independent variables, we obtained areas under the ROC curve of 87.9% (95% confidence interval, 73–100, NPV 71.4%, and PPV 84.6%, p = .0063; Figure 1).
Figure 1.
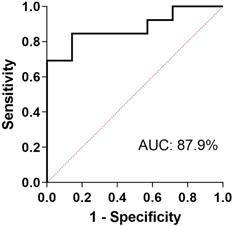
Severe group were tested as dependent variable and CD45‐, CD3‐, CD8‐, NK‐cells counts, and percentages and KL‐6 concentrations as independent variables. AUC was 87.9% (95% CI, 73–100, NPV 71.4%, and PPV 84.6%, p = .0063). AUC, area under the curve; CI, confidence interval; NK, natural killer; NPV, negative predicted value; PPV, positive predicted value
Thus, the present study confirmed that both NK cells and KL‐6 are associated with more severe COVID‐19. In fact, we observed significantly higher serum KL‐6 values, lower CD3‐, CD8‐ and CD45‐lymphocyte counts, and NK cells in severe than in nonsevere patients, in line with previous reports. 4 , 7 Our findings support the hypothesis that NK population plays an important role as first‐line defense with cytotoxic immune activity against SARS‐Cov2 infection. Since it was depleted in severe patients with lung respiratory involvement requiring mechanical ventilation, larger, and prospective studies would be worthwhile to confirm our results. Furthermore, the evidence of a significant increase of serum KL‐6 in more critical patients is intriguing and suggests the potential prognostic value of this biomarker on this field. The combination of these validated, reproducible, and nonexpensive bioindicators showed good accuracy in discriminating between severe and nonsevere patients, suggesting a promising value of this approach in the early prediction of a more aggressive disease phenotype.
Despite its monocentric design, our study confirms the reliable dysregulation of innate immune responses, particularly involving NK cells, and suggests that surveillance of a peripheral biomarkers’ panel, including lymphocyte cell counts and KL‐6, may be useful in the clinical management of patients with severe COVID‐19.
CONFLICT OF INTERESTS
All the authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Miriana d'Alessandro conceived the study and supervised all aspects of study. Miriana d'Alessandro, Laura Bergantini, Paolo Cameli, Lorenzo Remediani, Elena Bargagli, and Giuseppe Curatola collection of data and built database. Miriana d'Alessandro, Paolo Cameli, Laura Bergantini, Elena Bargagli, and Piersante Sestini data analysis and interpretation of results. All authors drafted and revised the papers.
ACKNOWLEDGMENT
The study was performed at Siena University: no funding or sponsors to declare.
#Siena COVID Unit
David Bennett, Francesco Bianchi, Felice Perillo, Nicola Lanzarone, Francesca Montagnani, Anna Perrone, Federico Franchi, Sabino Scolletta, Maria Antonietta Mazzei, Luca Volterrani, Serafina Valente, Giacomo Zanelli, Lucia Migliorini, Barbara Rossetti, Massimiliano Fabbiani, Cecilia Vagaggini, Pier Leopoldo Capecchi, Maria Grazia Cusi, Bruno Frediani, Lucia Cubattoli, Egidio Mastrocinque, Matteo Cameli, Matteo Nardi, Giovanni Bova, Fabrizio Mezzasalma, Susanna Guerrini, Amato Santoro, Giovanni Antonelli, Elisa Giacomin, Rodolfo Gentilini, Anna Sansoni, Raffaella Corbisiero, Maria Mencarelli, Francesco Pippi, Daniele Marri, Alessandro Lanari, Melissa Masini, Gulia Stella, Lorenzo Paglicci, Chiara Cassol, Roberto Valenti, Carla Caffarelli, Stefano Gonnelli, Andrea Lapi, Severino Gallo, Giovanni Donati, Elena Ceccarelli, Chiara Mattaliano, Irene Sellerio, Edoardo Conticini, Luca Cantarini, and Adriana Marinetti
REFERENCES
- 1. Alpaydin AO, Gezer NS, Simsek GO, et al. Clinical and radiological diagnosis of non‐SARS‐CoV‐2 viruses in the era of COVID‐19 pandemic [published online ahead of print August 8, 2020]. J Med Virol. 2020. 10.1002/jmv.26410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ok F, Erdogan O, Durmus E, Carkci S, Canik A. Predictive values of blood urea nitrogen/creatinine ratio and other routine blood parameters on disease severity and survival of COVID‐19 patients [published online ahead of print July 14, 2020]. J Med Virol. 2020. 10.1002/jmv.26300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hou W, Zhang W, Jin R, Liang L, Xu B, Hu Z. Risk factors for disease progression in hospitalized patients with COVID‐19: a retrospective cohort study. Infect Dis. 2020;52(7):498‐505. 10.1080/23744235.2020.1759817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'alessandro M, Bennett D, Montagnani F, et al. Peripheral lymphocyte subset monitoring in COVID19 patients: a prospective Italian real‐life case series [published online ahead of print May 14, 2020]. Minerva Med. 2020. 10.23736/S0026-4806.20.06638-0 [DOI] [Google Scholar]
- 5. Pallotto C, Suardi LR, Esperti S, et al. Increased CD4/CD8 ratio as a risk factor for critical illness in coronavirus disease 2019 (COVID‐19): a retrospective multicentre study. Infect Dis. 2020;52(9):675‐677. [DOI] [PubMed] [Google Scholar]
- 6. d'Alessandro M, Bergantini L, Cameli P, et al. Krebs von den Lungen‐6 as a biomarker for disease severity assessment in interstitial lung disease: a comprehensive review. Biomark Med. 2020;14(8):665‐674. 10.2217/bmm-2019-0545 [DOI] [PubMed] [Google Scholar]
- 7. d'Alessandro M, Cameli P, Refini RM, et al. Serum KL‐6 concentrations as a novel biomarker of severe COVID‐19 [published online ahead of print May 29, 2020]. J Med Virol. 2020;92:2216‐2220. 10.1002/jmv.26087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. d'Alessandro M, Cameli P, Bergantini L, Franchi F, Scolletta S, Bargagli E. Serum concentrations of Krebs von den Lungen‐6 in different COVID‐19 phenotypes [published online ahead of print August 14, 2020]. J Med Virol. 2020. 10.1002/jmv.26431 [DOI] [PMC free article] [PubMed] [Google Scholar]


