Abstract
Introduction
The situation generated in the health system by the COVID‐19 pandemic has provoked a crisis involving the necessity to cancel non‐urgent and oncologic activity in the operating room and in day‐to‐day practice. As the situation continues, the need to reinstate attention for patients with chronic pain grows. The restoration of this activity has to begin with on‐site appointments and possible surgical procedures. On‐site clinical activity has to guarantee the safety of patients and health workers.
Objectives
The objective of this review was to evaluate how to manage activity in pain units, considering the scenario generated by the pandemic and the implications of chronic pain on the immune system and proposed pharmacological and interventional therapies.
Methods
Besides the established general recommendations (physical distance, surgical masks, gloves, etc.), we established specific recommendations that will allow patient treatment and relieve the disruption of the immune response. It is important to highlight the use of opioids with the least influence in the immune system. Further, individualized corticoid use, risk assessment, reduced immune suppression, and dose adjustment should take patient needs into account. In this scenario, we highlight the use of radiofrequency and neuromodulation therapies, techniques that do not interfere with the immune response.
Conclusions
We describe procedures to implement these recommendations for individual clinical situations, the therapeutic possibilities and safety guidelines for each center, and government recommendations during the COVID‐19 pandemic.
Keywords: COVID‐19, chronic pain, pain units, opioids, immunology, interventional pain management
Introduction
The actual COVID 19 pandemic has generated an important burden on the healthcare system, creating a dilemma for healthcare providers. To decrease the risk for exposure, most hospitals have postponed elective surgical procedures and have cancelled, or reduced, face‐to‐face interactions in different medical specialties. 1
On December 31, 2019, the World Health Organization (WHO) first reported cases of low respiratory airway infections in Wuhan (China). Initially, the clinical presentation was considered a pneumonia of unknown etiology. It took some time to link a virus of the coronavirus family as the etiologic agent. On February 11, 2020, the WHO announced that the disease caused by this novel virus was designated “COVID‐19,” an acronym for coronavirus disease 2019. As of October 4th, 2020, there are more than 34,986,502 cases of COVID‐19 with over 1,034,240 deaths worldwide. More up‐to‐date disease background information is available online from the European Centre for Disease Prevention and Control 2 and the WHO. 3
Currently, the mean lethality in Europe and the United Kingdom is 1.5% of the total case count and 11% of the hospitalized cases; however, we must also take into account that this estimate is subject to different bias of notification, and to the different policies for performing diagnostic tests. Up till now, in Spain there have been 32,086 deaths and 789,932 cases diagnosed by polymerase chain reaction (PCR) and antibody detection. The absolute number of deaths was greater in patients over 65 years of age, with those 65 to 79 years of age representing 44% of all deaths, and those over 80 years representing 46%. In general, by gender, deaths occur more commonly in men than in women, in all age groups. 2
Besides age and sex, other risk factors that increase the morbidity/mortality rates include smoking and preexisting medical conditions, such as asthma, obstructive pulmonary disease, congestive heart failure, diabetes, and immunocompromised states, including acquired immunodeficiency syndrome. 1
Analysis of the related data on severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) propagation in China seems to indicate that close contact between individuals is required for transmission. The propagation, in fact, is limited primarily to family members, healthcare providers, and other close contacts. 4 For these reasons, hospitals and other clinical settings have modified certain operating procedures. Furthermore, disease control and prevention centers have provided distancing recommendations depending on the phase of the emergency state. 5 During confinement, the decision made is generally directed to cancel all non‐urgent procedures. 6
The current crisis generated by the COVID‐19 pandemic has forced the reorganization of the health systems and the cancellation of surgical procedures to facilitate the treatment of patients in the critical units. 7 , 8 In Spain, the measures taken, similar to those taken in other E.U. nations, have included the incorporation of telemedicine options in hospitals, and limiting physical access to the hospital facilities to only urgent cases or patients with oncologic diseases receiving treatment. Moreover, within hospital facilities, designated COVID‐19 areas have been created, as have specific areas where COVID‐19 patients are not permitted to enter, to reduce intra‐hospital transmission. Following the established proper hygienic practices also allows for the care of patients, while preventing direct contact between the patient and healthcare personnel. 9
The primary aims of this article were to analyze how the reestablishment of the pain unit, specifically, should be performed, taking into account how the security of the patients and professionals might be affected; the actions we should take; how our daily practice must change to minimize the contagion rate; and how we may continue providing services to our patients, who, despite the current crisis, continue to suffer from chronic pain. Specifically, this article focuses on analysis of various treatment options for chronic pain and the impact that they have on the immune system. Meanwhile, we also seek to provide an orientation for pain physicians on how to effectively reestablish the activity in pain units, from restarting on‐site appointments to more advanced treatments, with the aim of improving the attention dedicated to people affected by chronic disease and pain, while minimizing the risk for COVID‐19 exposure.
Chronic Patients and Immunology
The current pandemic has not only have serious health impact but has also paralysed the global economy 10 with socioeconomic and sanitary consequences hard to estimate. Patients with chronic pathologies, such as patients with chronic pain, are usually more affected by this kind of problems. Chronic pain is usually paired with psychological problems like chronic depression and anxiety. 11 Being confined also contributes to an increase in depression and anxiety, 12 and depression contributes to a decrease in the immune system that may favor the infection. Furthermore, it is also known that patients with chronic pain—due to the effect of chronic pain on immunity as well as the different treatments that are used to alleviate pain—have a major immune system problem compared to other patients without these problems. 13
Pain is the herald and hallmark of many infections. Increasingly, infections are appreciated as triggers of chronic pain and downstream remodelling of the nervous system. Pain during infection was previously considered as the by‐product of immune responses to pathogens. However, recent studies have demonstrated that nociceptors can directly sense microorganisms and their products. 14 Thus, infectious pathogens produce pain by directly activating sensory neurons that modulate inflammation, an unsuspected role for the nervous system in host–pathogen interactions. 15
Pain can also occur as a result of direct immunoglobulin G (IgG)‐induced injury of nociceptive fibers via molecular mimicry, either postinfection (eg, pain with Guillain‐Barré syndrome from Campylobacter jejuni infection), or with sterile antigen exposure, which may break immunological tolerance (eg, aerosolized porcine neural tissue exposure and cancers in paraneoplastic syndromes). 16 Recent work in the field of immunology has also led to the recognition of autoimmune pain disorders caused by specific IgGs directed against antigens in the somatosensory pathway. Among all chronic pain disorders, patients with autoimmune pain are rare and typically have other neurological findings beyond nociception, as occasionally seen with certain paraneoplastic syndromes. 16 , 17
The immune system does not seem to have an active role in acute responses to painful stimuli (nociception); however, it is an active player in persistent pain states. Moreover, increasing evidence suggests a role for neuro‐immune interactions in the initiation, propagation, and chronification of pain associated with inflammatory and infectious diseases in diverse tissues. Modulating these interactions may offer novel ways of targeting both pain and the underlying disease. For example, inhibiting proinflammatory cytokines that drive chronic pain sensitization, or stimulation of antinociceptive cytokine signalling, could be exploited to block pain. However, despite the association of several immune mediators with nociceptor sensitization and pain responses in preclinical studies, little of this knowledge has been translated into pain‐relieving therapies. 14
Clearly, synergy between the adaptive and innate immune systems can lead to immunopathological disorders, including autoimmune diseases, immunodeficiencies, and hypersensitivity reactions. Therefore, a new approach to mitigate pain 18 should integrate this new understanding of neuro‐immune system involvement in nociceptive processing to develop novel analgesic strategies without interfering with the fundamental beneficial effects of the immune response. Moreover, as previously mentioned, the immune system is affected by chronic pain, as well as by diseases related to it, such as depression; hence, when activities for these patients are resumed, it is imperative that additional care be taken in ensuring the safety of the patients and healthcare workers.
Opioid Use In Patients With Chronic Pain and Immunology
The relationship between chronic opioid use and altered immune system activity has been widely studied since it was first proposed at the end of the nineteenth century in oncologic patients. Initial concern regarding the immunosuppressive effects of chronic opioid administration dates back to the 1890s. 19 , 20 The use of opioids affects both innate and adaptive immunity. 21 , 22 Opioid receptors (OP) include the mu (MOP), delta (DOP), kappa, (KOP), and non‐classic nociceptin/orphanin FQ (FOP) receptors, and all of them have analgesic effects. 23 The most studied receptor is the MOP, the activation of which modulates both innate and adaptive immunologic systems and decreases the capacity to eliminate pathogens. 21
Thus, opioids lead to changes in immune cell function, including proliferation, chemotaxis, cytotoxicity, cytokine synthesis, and secretion, as well as cytokine and chemokine receptor expression. 24 , 25
T lymphocytes are the main adaptive immune cells, and they regulate other lymphocytes, monocytes, and NK cells through neuroendocrine mechanisms or cytokine secretion. Importantly, T lymphocytes express the 3 ordinary opioid receptor types (MOP, KOP, and DOP). Continued opioid use induces T lymphocyte differentiation toward the Th2 subset while promoting Th1 apoptosis, thereby decreasing the Th1/Th2 ratio. 21 , 22 This is relevant because the cytokine release syndrome that occurs during COVID‐19 infection can be attributed to Th1 cell activation. One could expect that this activation would be decreased in patients with a smaller Th1 population, resulting in a less intense immune response and less severe clinical presentation. However, we also have to consider that a smaller Th1 population involves increased vulnerability to viral infections. 26 A decreased Th1/Th2 ratio also causes increased interleukin‐6 (IL‐6) levels concurrent with an increased Th2 population, 27 and IL‐6 is a principal factor in the exaggerated activation of the immune system during COVID‐19 infection. 28 B‐lymphocytes are largely involved with humoral immunity, primarily via antibody and memory cell production. Opioid effects on these cells are not well understood; however, they have been reported to include decreased antibody production, increased anti‐inflammatory cytokines, such as growth factor β, and secretion of nuclear factor κB, which causes Th1 apoptosis. 22
Although there is abundant evidence regarding the immunomodulatory effects of opioids, the clinical implications of these immunomodulatory effects for patients with pain on opioids remain unclear. In our usual practice, the use of opioids in patients with non‐cancer pain is restricted to severe refractory pain, adjusting the dose to the recommendations with low doses up to 40 morphine milligram equivalents (MME). 29 Taking into account that there are patients in chronic opioid therapy, we must continue this therapy during the pandemic using opioids with good safety profiles and low immunosuppressive activity, which could be optimal for patients with a high infection risk or receiving oncological treatment.
The use of opioids with little immunomodulatory activity, such as tramadol and buprenorphine, seems to be the most appropriate in this period, not using immediate‐release opioids in nononcological patients, and adjusting the dose very precisely, although we insist that it must be adjusted always when we use opioids, not only during the COVID‐19 pandemic. 30 , 31
Interventional Treatment Use in Patients With Pain and Immunology
Interventional pain management techniques are of value in the management of patients with chronic pain; thus, the reestablishment of this therapeutic modality is vital to the health of our patients.
Corticoids are efficient anti‐inflammatories and are frequently used for infiltration techniques. 32 The major problem with corticoids is their effects on the immune system. Corticoids have an immunosuppressive effect that depends on various factors, 33 including the administration route. The systemic route has the highest risk for facilitating immunosuppression. Alternatively, interventional techniques carry lower risk as the corticoids are administered locally. The dosage used also influences the immunosuppressive capacity, as the immunosuppressive effect is dose dependent. This effect can increase the risk for common infections 34 caused by viruses (especially herpes viruses), bacteria, or fungal agents (especially candidiasis). The duration of the immunosuppressive effect could be reduced by certain corticoids, including dexamethasone and betamethasone. 35 Finally, individual risk factors, such as age, subjacent disease, and the concomitant use of other immunosuppressors, must also be considered.
Radiofrequency
Radiofrequency (RF) is a pain‐relieving technique that uses oscillating electrical currents to produce a therapeutic biological effect with an electrical field. Conventional RF, also known as continuous radiofrequency, uses a continuous voltage that increases tissue temperature above the threshold for tissue injury (40ºC to 50ºC) to produce a disruption in the nerves that carry pain signals. 36 This type of RF has fewer immunological implications because its effect is achieved through direct interruption of pain transmission pathways. Pulsed radiofrequency (PRF) uses discontinuous voltage in short, high‐intensity pulses so that tissue temperature stays under the injury threshold.
Electric fields used in PRF modify the dynamic free radical equilibrium, even decreasing their generation. According to Brasil et al., 37 inflammatory mediators such as tumor necrosis factor‐ ⍺, IL‐1β, superoxide dismutase, and lipoperoxidation increase, while catalase enzyme decreases. In this study, these targets reverted to basal levels after PRF use. Therefore, we can infer that PRF is a safe technique for patients during the COVID‐19 pandemic.
Epiduroscopy
Epiduroscopy (interventional endoscopy spinal surgery) is a minimally invasive procedure that allows direct visualization of the epidural space with diagnostic and therapeutic options. 38 Recent advances allow the use of different therapeutic tools, such as a modified Fogarty catheter, for epidural use or an ablation system that uses quantum molecular radiofrequency technology.
Specific Recommendations
During the COVID‐19 pandemic the precautions to avoid complications must be maximized. To guarantee this, the most experienced clinician in the pain unit should perform the procedure to limit surgical duration, 39 and we further propose avoiding the use of general anesthesia. Although our routine clinical practice includes the use of corticosteroids, considering current recommendations to limit their use, we also suggest avoiding their use when performing an epiduroscopy. 1 , 40 , 41
Neuromodulation
Neuromodulation includes implantable and nonimplantable devices that use electric, chemical, or other agents to reversibly modify cerebral and neuronal activity. Based on this definition, we can separate treatment into 2 primary groups: (1) neurostimulation itself and variants, such as profound cerebral stimulation, peripheral nerve stimulation, or subcutaneous stimulation; and (2) intrathecal drug infusion used to treat oncological pain, chronic nononcological pain, or as a treatment of spasticity.
Neurostimulation
Generally, spinal cord stimulation (SCS) is a safe procedure since it is minimally invasive and reversible. Unlike long‐term or high‐dose opioids, SCS is not associated with hormonal or immunological dysfunction. 42 Complications are reported in 30% to 40% of patients with implanted devices, 43 , 44 while long‐term morbidity and mortality are rare. Most complications are minor and reversible. Complications can be divided into biological complications and hardware‐related complications. The first type, although less prevalent, currently is the most relevant during the COVID‐19 outbreak due to the possibility of increased infection risks.
Specific Recommendations
The primary recommendations for safely reinitiating these procedures will be the careful selection of patients, in which their misuse or overuse is avoided, while also avoiding cases that are not supported by firm evidence or approved by international healthcare systems. 45 Moreover, as in the case of epiduroscopy, to avoid lengthening surgical times and complications, it is recommended that initially these types of techniques be carried out by the most experienced professionals in the unit. 39
An important aspect of SCS is the question of performing a trial phase, and the duration of this stage, which are critical matters to decrease infection. Though all SCS literature advises a trial phase, there is no solid evidence supporting this recommendation. 46 , 47 The trial phase involves increased infection rates with unclear long‐term benefit. The recommendations are based on expert opinions rather than actual scientific evidence. Our recommendation in the context of this sanitary crisis is a stricter than usual method of patient selection, if possible. Strict clinical and psychological criteria will be applied. We recommend, in the context of this health crisis, adjusting the trial phase to patients, performing it in those in whom a short intraoperative test phase is considered possible to reduce the number of hospital visits and a second surgical intervention, 48 , 49 reducing the possibility of contagion. In cases where it is considered necessary, the standard trial phase must be carried out.
Intrathecal Drug Delivery Systems
Intrathecal drug delivery systems (IDDS) have been used for several indications since the 1980s. In 1991, the Food and Drug Administration (FDA) approved a programmable implantable infusion pump. In general, IDDS should be considered when pain remains uncontrolled after attempting more conservative options (ie, injections, oral opioids, or transdermal opioids), or when side effects limit appropriate medication titration.
The FDA‐approved intrathecal medications include morphine, baclofen, and ziconotide, 50 even though off‐label use of other medications, including hydromorphone, fentanyl, and bupivacaine, is quite common. Given the COVID‐19 pandemic, physicians must consider the immunologic sequelae of the infused medications, particularly opioids. 51
Specific Recommendations
IDDS implantation may require additional screening tools to optimize patient and healthcare personnel safety. Due to aerosolization, intubation and extubation are considered high‐risk activities. Thus, the use of general anesthesia should be avoided when possible. 52 , 53
It is well documented that long‐term opioid use can have detrimental effects on the immune system. The clinical consequences of these changes have not been established, and precautions should be observed to limit the risk for COVID‐19 exposure among patients and healthcare personnel regardless of opioid status. By integrating the above safety measures into practice, pain physicians can minimize risk while continuing to care for IDDS patients.
Ideas for Starting Therapy
A current goal of medical teams is the restoration of daily practice with medical appointments and surgical procedures. The restoration of this activity begins with presence‐based activity in the outpatient clinic that subsequently advances to surgical procedures. Importantly, restarting on‐site activity in the medical office must guarantee patient and employee safety. The most effective way to develop strategies to ensure this is to implement strategies that have worked for similar diseases with similar modes of transmission.
Although the SARS‐CoV‐2 virus is primarily transmitted by air droplets, transmission is also possible by exposure in areas in closed spaces with high aerosol concentration, or less frequently by direct contact. Hence, reinitiating activity must be carried out gradually, interspersing patients who are seen in person with others who participate in video calls. However, face‐to‐face visits must be prioritized for urgent patients with severe intractable chronic pain, as well as those with oncological pathology, to begin surgical activity in a second stage. 8 Subsequently, face‐to‐face communication must be initiated according to the established medical criteria (Table 1).
Table 1.
Patients Excluded From In‐person Visits
| >70 years old |
| Uncontrolled arterial hypertension |
| Uncontrolled diabetes |
| Severe respiratory disorders |
| Patients with clinical history of being admitted into the intensive care unit |
In all cases of face‐to‐face visits, the minimum security distance will be 2 m, both in the office and in admission areas and waiting rooms. This will be the limiting factor for scheduled in‐person appointments, which should extend the intervals between physical patients to integrate videoconferences or phone calls. The proposed rules for restoring outpatient clinic activity and common area maintenance are detailed in Table 2.
Table 2.
Common Area Parameters
| Maintain ≥2 m between patients |
| Arrive at the appointment on time |
| Limit the number of people using elevators (each trip) |
| Limit the number of seats in the waiting areas |
| Exhaustive cleaning: cleaning the office between each patient |
| Use hydroalcoholic dispensers, gloves, and surgical masks |
|
Perform the COVID‐19 checklist: Fever Cough Breathing difficulty Gastrointestinal disturbances Ocular disturbances Contact with other people infected by the virus |
Specific Recommendations for Ambulatory Procedures
Several measures have been employed in patients who undergo ambulatory procedures in our center. For instance, additional preoperative tests may include PCR testing, serology testing, and computed tomography (CT) imaging. PCR testing involves a throat or nasal swab to check for viral RNA, which is only present if the individual is actively infected. Unfortunately, PCR testing can have a high false‐negative rate due to inadequate sample collection and test performance. 54 Alternatively, serology testing involves collecting blood samples to screen for the presence of antibodies. PCR testing for SARS‐CoV‐2 should be performed 48 hours before the procedure, and a questionnaire including clinical data and potential contact with COVID‐19‐suspected or ‐positive cases should be administered. In our center, the specific recommendations for patients admitted for ambulatory procedures include a clinical and epidemiological telephone screening 24 hours before the intervention (Annex 1). If the screening is positive, the procedure is cancelled. If the screening is negative, the patient is advised to limit contact with other people and remain in isolation until the day of the surgery. The results of the PCR‐Cov‐2 test that was performed 24 hours prior are also verified. In the preoperative area, the patient comes protected with a mask and gloves, the previous steps are verified, and a second clinical feature questionnaire is completed (see Annex 1). The patient's temperature is recorded, and if there is suspicion of infection, the clinician in charge is alerted, and cancellation of the procedure is advisable. However, if all test results are negative, the intervention is performed according to the COVID‐19 protocol (Figure 1).
Figure 1.
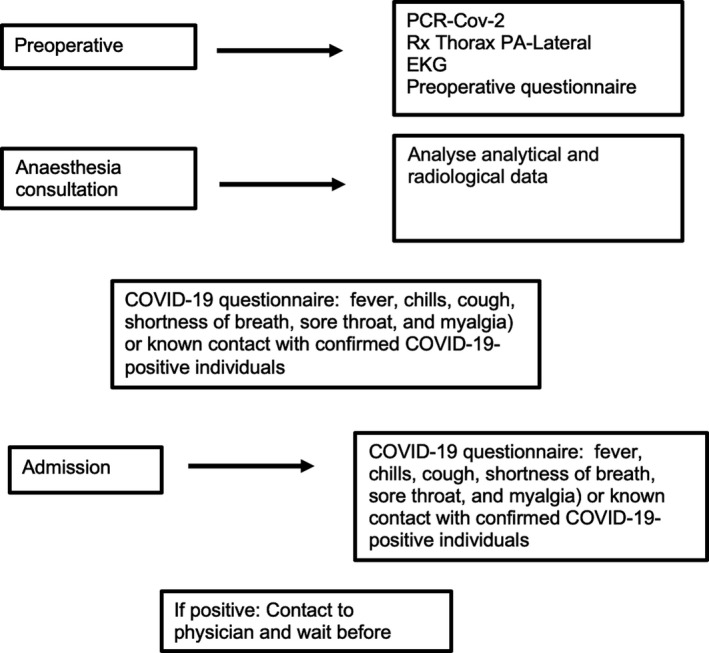
Intervention screening protocol.
Lastly, CT scanning is a very sensitive screening measure for COVID‐19, and although it has been used as the primary screening tool in some areas, 54 , 55 we have included anterior‐posterior and lateral chest X‐ray in our protocol because it is easier to perform in our area for ambulatory patients.
Conclusions
In the crisis generated by the COVID‐19 pandemic, we must agree on the recommendations that guarantee the safety and that maintain health care for patients with chronic pain. Thus, we must fulfil the general recommendations to avoid intra‐hospital horizontal transmission, avoiding increased COVID‐19 transmission, especially in populations with higher risk for complications from SARS‐CoV‐2 infection. Due to the close relationship between chronic pain, treatment, and the immune system, we must prescribe treatments in a careful and individualized manner.
We have selected treatments that generate the least amount of interference with the immune system, at the pharmacological and interventional level. Thus, the opioid dose should be adjusted and those with the least influence on the immune system should be chosen, such as tramadol and buprenorphine. The dose of corticosteroids should also be adjusted, and they should be avoided as far as possible, although if necessary, the least immunosuppressive drugs, such as dexamethasone and betamethasone, are to be used. Lastly, certain techniques have no impact on the immunity of patients, such as radiofrequency and neurostimulation, which would be highly recommended, and which may be considered earlier in the treatment algorithm.
Conflicts of Interest
David Abejón is a consultant for Boston Scientific, PRIM, Cardiva, Abbott, and Medtronic. Eva Monzón is a speaker for Cardiva. Dr. Deer is a consultant for Abbott, Axonics, Flowonix, Saluda Medical, Vertos, SpineThera, Nalu, Medtronic, Nevro, SI Bone, Stimgenics, SPR Therapeutics, Cornerloc Boston Scientific, and Vertiflex. Dr. Deer is a member of the advisory board for Abbott, Vertos, Flowonix, Nalu, SPR Therapeutics, and Vertiflex. Dr. Deer has equity options in Bioness, Vertiflex, Axonic, Vertos, SpineThera, Saluda Medical, Nalu, Cornerloc, and SPR Therapeutics. He is a research consultant for Abbott, Vertos, Mainstay Medical, Saluda, SPR Therapeutics, Boston Scientific, and Vertiflex. Dr. Deer has a patent pending for the DRG paddle lead with Abbott. Ricard Araujo was working for the Menarini in Enantyum project. Jonathan M. Hagedorn, Cristina Abad, Alberto Rios, Alejandro Zamora, and Ricardo Vallejo have no conflicts of interest to declare.
Annex 1. Clinical and Epidemiological Screening
| Yes | No | |
|---|---|---|
| “Have you had FEVER in the last 15 days?” | ||
| “Have you had dry coughing in the last 15 days?” | ||
| “Have you noticed the loss of taste or smell in the last 15 days?” | ||
| “Do you live with someone diagnosed with COVID 19?” |
References
- 1. Cohen SP, Baber ZB, Buvanendran A, et al. Pain management best practices from multispecialty organizations during the COVID‐19 pandemic and public health crises. Pain Med. 2020;21:1331–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Centre for Disease Prevention and Control . COVID‐19. https://www.ecdc.europa.eu/en/covid‐19‐pandemic (accessed October 4, 2020).
- 3. World Health Organization . Coronavirus disease (COVID‐19) outbreak. 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019 (accessed October 4, 2020).
- 4. Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation and treatment coronavirus (COVID‐19) [Updated April 6, 2020]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . Strategies for optimizing the supply of facemasks. Topics: Coronavirus [CoV] Publication. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/ppe‐strategy/face‐masks.html . Last updated March 19, 2020.
- 6. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID‐19 pandemic. JAMA. 2020;323:1912. [DOI] [PubMed] [Google Scholar]
- 7. Spinelli A, Pellino G. COVID‐19 pandemic: perspectives on an unfolding crisis. Br J Surg. 2020;107:785–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Marzo F, Sartelli M, Cennamo R, et al. Recommendations for general surgery activities in a pandemic scenario (SARS‐CoV‐2). Br J Surg. 2020;107:1104–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO Global Infection Prevention and Control Network . Infection prevention and control during health care when COVID‐19 is suspected. WHO. 2020;(i):1–5. https://apps.who.int/iris/rest/bitstreams/1272420/retrieve
- 10. Jones L, Palumbo D, Brown D. Coronavirus: a visual guide to the economic impact. BBC News. April 30, 2020. https://www.bbc.com/news/business‐51706225
- 11. de Heer EW, Gerrits MMJG, Beekman ATF, et al. The Association of depression and anxiety with pain: a study from NESDA. PLoS One. 2014;9:e106907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hao F, Tan W, Jiang L, et al. Do psychiatric patients experience more psychiatric symptoms during COVID‐19 pandemic and lockdown? A case‐control study with service and research implications for immunopsychiatry. Brain Behav Immun. 2020;87:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105:838–847. [DOI] [PubMed] [Google Scholar]
- 14. Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol. 2019;19:433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chiu IM, Heesters BA, Ghasemlou N, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu M, Bennett DLH, Querol LA, et al. Pain and the immune system: emerging concepts of IgG‐mediated autoimmune pain and immunotherapies. J Neurol Neurosurg Psychiatry. 2020;91:177–188. [DOI] [PubMed] [Google Scholar]
- 17. Klein CJ. Autoimmune‐mediated peripheral neuropathies and autoimmune pain. Handbook Clin Neurol. 2016;417–446. [DOI] [PubMed] [Google Scholar]
- 18. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. [DOI] [PubMed] [Google Scholar]
- 19. Cantacuzene J. Nouvelles recherches sur le mode de destruction des vibrions dans l’organisme. Ann Inst Pasteur. 1898;12:274–300. [Google Scholar]
- 20. Vallejo R, de Leon‐Casasola O, Benyamin R. Opioid therapy and immunosuppression. Am J Ther. 2004;11:354–365. [DOI] [PubMed] [Google Scholar]
- 21. Plein LM, Rittner HL. Opioids and the immune system—friend or foe. Br J Pharmacol. 2018;175:2717–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kosciuczuk U, Knapp P, Lotowska‐Cwiklewska AM. Opioid‐induced immunosuppression and carcinogenesis promotion theories create the newest trend in acute and chronic pain pharmacotherapy. Clinics (Sao Paulo). 2020;75:e1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Machelska H, Celik MÖ. Opioid receptors in immune and glial cells—implications for pain control. Front Immunol. 2020;11:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stein C, Machelska H. Modulation of peripheral sensory neurons by the immune system: implications for pain therapy. Pharmacol Rev. 2011;63:860–881. [DOI] [PubMed] [Google Scholar]
- 25. Flores LR, Wahl SM, Bayer BM. Mechanisms of morphine‐induced immunosuppression: effect of acute morphine administration on lymphocyte trafficking. J Pharmacol Exp Ther. 1995;272:1246–1251. [PubMed] [Google Scholar]
- 26. Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus disease 2019 (COVID‐19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wodehouse T, Demopoulos M, Petty R, et al. A randomized pilot study to investigate the effect of opioids on immunomarkers using gene expression profiling during surgery. Pain. 2019;160:2691–2698. [DOI] [PubMed] [Google Scholar]
- 28. Vardhana SA, Wolchok JD. The many faces of the anti‐COVID immune response. J Exp Med. 2020;217:e20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manchikanti L, Kaye AM, Knezevic NN, et al. Responsible, safe, and effective prescription of opioids for chronic non‐cancer pain: American society of interventional pain physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3–S92. [PubMed] [Google Scholar]
- 30. Sacerdote P, Manfredi B, Mantegazza P, Panerai AE. Antinociceptive and immunosuppressive effects of opiate drugs: a structure‐related activity study. Br J Pharmacol. 1997;121:834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franchi S, Moschetti G, Amodeo G, Sacerdote P. Do all opioid drugs share the same immunomodulatory properties? A review from animal and human studies. Front Immunol. 2019;12:2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Boxem K, Rijsdijk M, Hans G, et al. Safe use of epidural corticosteroid injections: recommendations of the WIP Benelux Work Group. Pain Pract. 2019;19:61–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strangfeld A, Eveslage M, Schneider M, et al. Treatment benefit or survival of the fittest: what drives the time‐dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann Rheum Dis. 2011;70:1914–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pappas DA, Hooper MM, Kremer JM, et al. Herpes zoster reactivation in patients with rheumatoid arthritis: analysis of disease characteristics and disease‐modifying antirheumatic drugs. Arthritis Care Res. 2015;67:1671–1678. [DOI] [PubMed] [Google Scholar]
- 35. Friedly JL, Comstock BA, Heagerty PJ, et al. Systemic effects of epidural steroid injections for spinal stenosis. Pain. 2018;159:876–883. [DOI] [PubMed] [Google Scholar]
- 36. Shealy CN. Percutaneous radiofrequency denervation of spinal facets. J Neurosurg. 1975;43:448–451. [DOI] [PubMed] [Google Scholar]
- 37. Brasil LJ, Marroni N, Schemitt E, Colares J. Effects of pulsed radiofrequency on a standard model of muscle injury in rats. Anesthesiol Pain Med. 2020;10:e97372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saberski LR, Kitahata LM. Direct visualization of the lumbosacral through the sacral hiatus. Anesth Analg. 1995;80:839–840. [DOI] [PubMed] [Google Scholar]
- 39. Shanthanna H, Cohen SP, Strand N, Lobo CA, Eldabe S, Bhatia ANS.Recommendations on chronic pain practice during the COVID‐19 pandemic. www.asra.com/covid‐19/cpguidance. Accessed March 8, 2020. [DOI] [PMC free article] [PubMed]
- 40. Sociedad Española del Dolor . Recomendaciones asistenciales de la Sociedad Española del Dolor (SED) ante la normalización progresiva de la actividad asistencial en la pandemia por COVID‐19 versión 1.0. https://www.sedolor.es/download/recomendaciones‐sed‐desescalada‐covid‐19/. Accessed March 8, 2020.
- 41. Angst MS, Clark JD. Opioid‐induced hyperalgesia. Anesthesiology. 2006;104:570–587. [DOI] [PubMed] [Google Scholar]
- 42. Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med. 2016;17:325–336. [DOI] [PubMed] [Google Scholar]
- 43. Bendersky D, Yampolsky C. Is spinal cord stimulation safe? A review of its complications. World Neurosurg. 2014;82:1359–1368. [DOI] [PubMed] [Google Scholar]
- 44. Thomson S, Huygen F, Prangnell S, et al. Appropriate referral and selection of patients with chronic pain for spinal cord stimulation: European consensus recommendations and e‐health tool. Eur J Pain. 2020;24:1169–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gong Y, Cao X, Mei W, et al. Anesthesia considerations and infection precautions for trauma and acute care cases during the COVID‐19 Pandemic. Anesth Analg. 2020;131:326–334. http://dx.doi.org/ 10.1213/ANE.0000000000004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. NICE Technology Appraisal Guidance 159: Spinal Cord Stimulation for Chronic Pain of Neuropathic or Ischaemic Origin. London: National Institute for Health and Care Excellence. 2008. www.nice.org.uk/guidance/ta159. Accessed March 11, 2020. [Google Scholar]
- 47. Deer TR, Lamer TJ, Pope JE, et al. The Neurostimulation Appropriateness Consensus Committee (NACC) safety guidelines for the reduction of severe neurological injury. Neuromodul Technol Neural Interface. 2017;20:15–30. [DOI] [PubMed] [Google Scholar]
- 48. Moriyama K, Murakawa K, Uno T, et al. A Prospective, open‐label, multicenter study to assess the efficacy of spinal cord stimulation and identify patients who would benefit. Neuromodul Technol Neural Interface. 2012;15:7–12. [DOI] [PubMed] [Google Scholar]
- 49. Eldabe S, Gulve A, Thomson S, et al. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost‐effectiveness? (TRIAL‐STIM Study): study protocol for a randomised controlled trial. Trials. 2018;19:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): recommendations for intrathecal drug delivery: guidance for improving safety and mitigating risks. Neuromodulation. 2017;20:155–176. [DOI] [PubMed] [Google Scholar]
- 51. Campana G, Sarti D, Spampinato S, Raffaeli W. Long‐term intrathecal morphine and bupivacaine upregulate MOR gene expression in lymphocytes. Int Immunopharmacol. 2010;10:1149–1152. [DOI] [PubMed] [Google Scholar]
- 52. Rowlands J, Yeager MP, Beach M, Patel HM, Huysman BC, Loftus RW. Video observation to map hand contact and bacterial transmission in operating rooms. Am J Infect Control. 2014;42:698–701. [DOI] [PubMed] [Google Scholar]
- 53. Loftus RW, Koff MD, Birnbach DJ. The dynamics and implications of bacterial transmission events arising from the anesthesia work area. Anesth Analg. 2015;120:853–860. [DOI] [PubMed] [Google Scholar]
- 54. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Greenland JR, Michelow MD, Wang L, London MJ. COVID‐19 Infection. Anesthesiology. 2020;132:1346–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]


