To the Editor:
December 2019 marked the emergence of the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 8 , 9 Several treatment approaches are under study. The antiviral remdesivir 10 has been shown to improve overall mortality in patients treated for COVID‐19, 1 and was approved by the United States Food and Drug Administration (FDA) for hospitalized patients with severe disease. 2 Tocilizumab, a humanized antiinterleukin‐6 receptor 11 antibody, can hasten COVID‐19‐related cytokine release syndrome recovery by 75%. 3 Implementation of COVID‐19 convalescent plasma (CCP) in the treatment of COVID‐19 infection was also suggested by the FDA. 4
An 8‐year‐old African‐American male with immune‐dysregulation polyendocrinopathy X‐linked (IPEX) syndrome underwent haploidentical, related bone marrow hematopoietic stem cell transplant (HSCT), and contracted SARS‐CoV‐2 during the periengraftment period, subsequently developing primary graft failure. The conditioning regimen included busulfan, fludarabine, rabbit antithymoglobulin, and posttransplant cyclophosphamide; graft versus host disease (GVHD) prophylaxis consisted of mycophenolate mofetil and cyclosporine.
Lack of engraftment and fever were noted on Day + 21 posttransplant. A sedated bone marrow aspiration was planned; prior to sedation he tested positive for COVID‐19 via nucleic acid amplification test. Development of respiratory distress prompted a chest CT that showed “bilateral ground‐glass opacities” (Figure 1); noninvasive ventilation was initiated. A 10‐day‐course treatment with remdesivir began on Day + 26.
FIGURE 1.
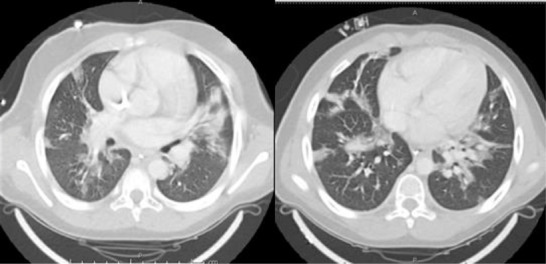
Chest computer tomography showing “ground‐glass” opacities consistent with COVID‐19 infection
We trended inflammatory parameters daily (Table 1), and based our treatment decision on a calculated H‐score 5 of 209, which correlated with a 92.8% risk probability of cytokine release syndrome. Two doses of tocilizumab and one unit of CCP 12 were given. On Day + 32, severe hypotension, acute hypoxemia, and mildly increased right ventricle systolic pressure ensued, requiring mechanical ventilation and nitric oxide. A comprehensive evaluation for superimposed infections was remarkable for a repeated positive SARS‐CoV‐2 test, Staphylococcus epidermidis and Candida parapsilosis infections, and BK and cytomegalovirus viremias.
TABLE 1.
Inflammatory markers trend after starting treatment for COVID‐19 infection
| Inflammatory markers | ||||||
|---|---|---|---|---|---|---|
| COVID treatment day | Days posttransplant | Ferritin (ng/mL) | Procalcitonin (ng/mL) | LDH (U/L) | CRP (mg/dL) | D‐dimer (ug/mL FEU) |
| Day −2 | 24 | 7790 | 0.5 | 432 | 9.87 | 13.4 |
| Day −1 | 25 | 14 167 | 0.68 | 626 | 10.8 | 18.31 |
| Start of treatment [Link] , [Link] | 26 | 14 272 | 1.04 | 780 | 11.8 | 17.11 |
| Day 2 c | 27 | 13 619 | 2.39 | 1019 | 16.2 | 16.5 |
| Day 3 d | 28 | 13 871 | 1.65 | 1092 | 8.9 | 13.6 |
| Day 4 | 29 | 10 532 | 1.18 | 1021 | 4.2 | 11.62 |
| Day 5 | 30 | 8797 | 0.66 | 962 | 2.3 | 9.67 |
| Day 6 | 31 | 8496 | 0.51 | 1018 | 1.7 | 10.62 |
| Day 7 | 32 | 6594 | 0.33 | 827 | 1.1 | 8.9 |
| Day 8 | 33 | 6533 | 0.18 | 857 | 0.7 | 8.45 |
| Day 9 | 34 | 5153 | 0.45 | 719 | 1.1 | 12.99 |
| Day 10 e | 35 | 4971 | 0.7 | 629 | 4.5 | 10.55 |
| Day 11 | 36 | 6066 | 0.57 | 645 | 4 | 5.96 |
| Day 12 | 37 | 5145 | 0.64 | 801 | 5.2 | 8.13 |
| Day 13 | 38 | 4648 | 0.69 | 945 | 5 | 8.39 |
| Day 14 | 39 | 6588 | 0.85 | 1208 | 4.2 | 7.92 |
| Day 15 | 40 | 8727 | 0.81 | 1140 | 3 | 7.09 |
| Day 16 f,g | 41 | 10 294 | 1.5 | 1136 | 2.3 | 8.01 |
| Day 17 | 42 | 28 884 | 2.99 | 1093 | 2.2 | 9.33 |
Note. Highest values are highlighted for each inflammatory marker.
a First dose of remdesivir.
bFirst dose of tocilizumab.
c Second dose of tocilizumab.
d First convalescent plasma transfusion.
e Treatment with remdesivir completed.
fThird dose of tocilizumab.
gSecond convalescent plasma transfusion.
Bone marrow aplasia, lack of donor marrow CD33+ cells, absence of donor‐specific antibodies, and compatible forward and backward flow cytometric crossmatches confirmed primary graft failure and immune rejection, commonly seen in patients with IPEX syndrome. In preparation for a second haploidentical related CD34+ selected peripheral hematopoietic stem cell infusion, conditioning with fludarabine for 3 days began on Day + 39 posttransplant. Salvage therapy with a second unit of CCP and a third dose of tocilizumab was given on Day + 41. However, despite all efforts, he died on Day + 42 posttransplant.
Compared to their immunocompetent counterparts, immunocompromised patients with COVID‐19 are at increased risk for secondary infections and progression to severe disease, as well as a different response to supportive care measures. 6 Studies have shown that SARS‐CoV‐2 acts mainly on T‐lymphocytes; hence, a severely immunocompromised patient experiences a poorer outcome. A case report depicted two adult posttransplant patients with adequate graft function, on immunosuppressive therapy, that eventually died after developing multiorgan failure. 7
Our patient was treated aggressively, and we attributed the first decreasing trend in inflammatory markers (Table 1) to achieving disease control. However, the combination of graft failure, COVID‐19 infection with multiorgan failure, and opportunistic infections contributed to his death. We hope that new treatments continue to stem from ongoing research, to achieve a different outcome in our patient population.
REFERENCES
- 1. Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter initial guidance on use of antivirals for children with COVID‐19/SARS‐CoV‐2. J Pediatric Infect Dis Soc. 2020. https://doi-org.ezproxy.lsuhsc.edu/10.1093/jpids/piaa045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. FDA. Coronavirus (COVID‐19) Update: FDA Issues Emergency Use Authorization for Potential COVID‐19 Treatment . U.S. Food and Drug Administration; May 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed May 30, 2020.
- 3. Alzghari SK, Acuña VS. Supportive treatment with tocilizumab for COVID‐19: a systematic review. J Clin Virol. 2020;127:104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeng Q‐L, Yu Zu‐J, Gou J‐J, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID‐19 patients. J Infect Dis. 2020;222(1):38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ardura MI, Hartley DM, Dandoy C, Lehmann L, Jaglowski S, JAuletta J. Addressing the impact of the coronavirus disease (COVID‐19) pandemic on hematopoietic cell transplantation: learning networks as a means for sharing best practices. Biol Blood Marrow Transplant. 2020;26(7):e147‐e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang J, Lin H, Wu Y, et al. COVID‐19 in posttransplant patients—report of 2 cases. Am J Transplant. 2020;20(7):1879‐1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Coronavirus Disease (COVID‐19 Outbreak Situation . World Health Organization; 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed May 25, 2020.
- 9.CDC. Coronavirus Disease 2019 (COVID‐19) Cases in the US . Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/coronavirus/2019‐ncov/cases-updates/cases‐in‐us.html. Accessed May 25, 2020.
- 10. Shio‐Shin J, Ping‐Ing L, Hsueh Po‐R. Treatment options for COVID‐19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajendran K, Narayanasamy K, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID‐19: systematic review. J Med Virol. 2020. https://doi-org.ezproxy.lsuhsc.edu/10.1002/jmv.25961 [DOI] [PMC free article] [PubMed] [Google Scholar]


