Summary
Azithromycin (AZM) is a synthetic macrolide antibiotic effective against a broad range of bacterial and mycobacterial infections. Due to an additional range of anti‐viral and anti‐inflammatory properties, it has been given to patients with the coronaviruses SARS‐CoV or MERS‐CoV. It is now being investigated as a potential candidate treatment for SARS‐CoV‐2 having been identified as a candidate therapeutic for this virus by both in vitro and in silico drug screens. To date there are no randomised trial data on its use in any novel coronavirus infection, although a large number of trials are currently in progress. In this review, we summarise data from in vitro, murine and human clinical studies on the anti‐viral and anti‐inflammatory properties of macrolides, particularly AZM. AZM reduces in vitro replication of several classes of viruses including rhinovirus, influenza A, Zika virus, Ebola, enteroviruses and coronaviruses, via several mechanisms. AZM enhances expression of anti‐viral pattern recognition receptors and induction of anti‐viral type I and III interferon responses. Of relevance to severe coronavirus‐19 disease (COVID‐19), which is characterised by an over‐exuberant innate inflammatory response, AZM also has anti‐inflammatory properties including suppression of IL‐1beta, IL‐2, TNF and GM‐CSF. AZM inhibits T cells by inhibiting calcineurin signalling, mammalian target of rapamycin activity and NFκB activation. AZM particularly targets granulocytes where it concentrates markedly in lysosomes, particularly affecting accumulation, adhesion, degranulation and apoptosis of neutrophils. Given its proven safety, affordability and global availability, tempered by significant concerns about antimicrobial stewardship, there is an urgent mandate to perform well‐designed and conducted randomised clinical trials.
Keywords: azithromycin, coronavirus, COVID‐19, macrolide, mechanism, review, SARS‐CoV‐2, virus
Abbreviations
- AZM
azithromycin
- CAP
community acquired pneumonia
- CCL
C‐C motif ligand
- CD
cluster of differentiation
- CF
cystic fibrosis
- COVID‐19
coronavirus‐19 disease
- COX
cyclooxygenase
- cPLA2
cytosolic phospholipase A2
- CRP
C‐reactive protein
- CXCL
C‐X‐C motif ligand
- DPB
diffuse panbronchiolitis
- ERK
extracellular signal‐regulated kinase
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor (CSF2)
- HCQ
hydroxychloroquine
- hPSC
human pluripotent stem cell
- ICU
intensive care unit
- IL
interleukin
- IFN
interferon
- IRF3
Interferon Regulatory Factor 3
- ISG
interferon‐stimulated gene
- IVM
ivermectin
- LMWH
low molecular weight heparin
- MAPK
mitogen‐activated protein kinase
- MCL1
myeloid cell leukaemia sequence 1
- MDA5
melanoma differentiation‐associated protein 5
- MxA
myxoma virus resistance A
- NALP3
NACHT, LRR, and PYD domains‐containing protein 3
- NCT
National Clinical Trial
- PBEC
primary bronchial epithelial cell
- PGE2
prostaglandin E2
- PRR
pattern recognition receptor
- RIG‐1
retinoic acid‐inducible gene 1
- RV
rhinovirus
- SARS
severe acute respiratory syndrome
- TBK1
TANK‐binding kinase 1
- TGF‐beta
transforming growth factor beta
- TLR
Toll‐like receptor
1. INTRODUCTION
Azithromycin (AZM) is a second‐generation, broad‐spectrum, synthetic macrolide antibiotic used since the early 1980s 1 , 2 to treat a wide range of bacterial and mycobacterial infections of respiratory and skin infections. It is therefore on the WHO list of essential medications, 3 and manufactured on a large scale globally. Its antibacterial activity derives from its ability to bind to the 50S ribosomal subunit, inhibiting protein synthesis. 4 It also has an intriguing range of anti‐viral and anti‐inflammatory properties, and is now being investigated as a potential candidate treatment for viruses including SARS‐CoV‐2, which causes coronavirus‐19 disease (COVID‐19). It has been used as a treatment in previous coronavirus diseases during the epidemics of severe acute respiratory syndrome (SARS) in 2003 and Middle East respiratory syndrome (MERS) 5 in 2012, but to date there are no randomised trial data on its use in any novel coronavirus infection. Its proven safety, affordability and global availability make it an attractive candidate for repurposing as a treatment for COVID‐19. Given the expected massive global impact of COVID‐19, particularly in low‐to‐middle income countries, it is important not only to develop therapies that treat the virus successfully, but also to ensure that these therapies are readily implementable at all levels of development and economy. 6 This review summarizes the current understanding of the anti‐viral and anti‐inflammatory effects of AZM, with a view to supporting our knowledge in the pursuit of a COVID‐19 therapy that can help tackle this virus globally.
2. MECHANISMS OF ANTI‐VIRAL EFFECTS
A range of human in vitro and in vivo studies provide evidence of anti‐viral activity of macrolides across a broad range of viral species and families (Table 1). Some studies suggest improved symptom resolution and reduction, 17 , 18 , 19 , 20 , 21 , 22 although not all studies have observed these effects. 23 , 24 , 25 , 26
TABLE 1.
Viral infections in which azithromycin has demonstrated anti‐viral effects
| Pathogen | Findings | Method | Study |
|---|---|---|---|
| Human rhinovirus (Picornavirus) | Enhanced viral‐induced type I and III IFN leading to reduced RV replication and release | In vitro study. PBEC. 10 μM, 50 μM | Gielen et al 7 |
| Reduced RV replication | In vitro study. PBEC from cystic fibrosis patients. 50 μM | Schogler et al 8 | |
| In vitro study. PBECs and BEAS‐2B cells 50 μM, 10 μM | Porter et al 9 | ||
| Increases RV PRR presentation | In vitro study. PBEC. 10 μM, 50 μM | Gielen et al 7 | |
| Induces anti‐viral ISGs viperin and MxA | In vitro study. PBECs and BEAS‐2B cells 50 μM, 10 μM | Porter et al 9 | |
| Coronaviruses (alpha and beta) | AZM associated with reduced viral load in children with coronaviruses | Clinical trial. Dose ≥20 mg/kg | Doan et al 10 |
| Zika (Flavivirus) | AZM markedly reduces viral proliferation and virus‐induced cytopathic effects | In vitro study. U87 glial cells and hPSC‐derived astrocytes, 0 μM to >100 μM | Retallack et al 11 |
| AZM upregulates type I and III interferon responses | In vitro study. HT‐29 human colon epithelial cell line and A549 lung epithelial cell line. 10 μM, 50 μM | Li et al 12 | |
| AZM upregulates viral pathogen recognition receptors MDA5 and RIG‐1 | In vitro study. A549 lung epithelial cell line. 10 μM, 50 μM | ||
| AZM increases levels of phosphorylated TBK1 and IRF3 | In vitro study. HT‐29 human colon epithelial cell line, 10 μM, 50 μM | ||
| Human primary fibroblasts, 5 μM, 20 μM. RAW264.7 macrophage cells 1.5 μM, 3 μM | |||
| Enteroviruses (Picornaviruses) | AZM improved survival and clinical symptom scores in murine model | In vivo study. Mice infected i.p. with EV‐A71‐MZ‐MA1. AZM dose 30 mg/kg/day | Zeng et al 13 |
| Ebola (Ebola viruses) | AZM demonstrates high in vitro anti‐viral potency and low cytotoxicity | In vitro study. HeLa cells (viral replication). HEK 293T cells (viral entry and cytotoxicity). 0.5 to 50 μM | Madrid et al 14 |
| SARS (Coronavirus) | AZM associated with improvement in 90 d survival rate and time to discontinuation of mechanical ventilation | Single‐centre, retrospective cohort evaluation of hospitalized patients with moderate or severe ARDS, using a propensity score analysis | Kawamura et al 15 |
| Influenza A (Orthomyxovirus) | Reduction in IL‐6, IL‐8, IL‐17, CXCL9, sTNF and CRP | Randomised, open‐label, multicentre trial of patients with severe influenza. 500 mg AZM od + 75 mg oseltamivir bd/75 mg oseltamivir bd. | Lee et al 16 |
Abbreviations: AZM, azithromycin; CRP, C‐reactive protein; CXCL, C‐X‐C motif ligand; hPSC, human pluripotent stem cell; IL, interleukin; IFN, interferon; IRF3, Interferon Regulatory Factor 3; ISG, interferon‐stimulated gene; MDA5, melanoma differentiation‐associated protein 5; MxA, myxoma virus resistance A; PBEC, primary bronchial epithelial cell; PRR, pattern recognition receptor; RIG‐1, retinoic acid‐inducible gene 1, RV, rhinovirus; SARS, severe acute respiratory syndrome; TBK1, TANK‐binding kinase 1.
2.1. Mechanisms of anti‐viral effects against rhinovirus
In several clinical trials, macrolides reduced exacerbations in airways diseases, particularly asthma. 27 , 28 , 29 , 30 As the majority of such exacerbations are triggered by viral infections, 31 most commonly rhinoviruses (RV), 32 the effects of macrolides have been studied most extensively against RV. AZM reduces RV replication and release during in vitro infection of primary human bronchial epithelial cells (PBEC). 7 This finding was replicated in PBEC from patients with cystic fibrosis or healthy controls, where AZM treatment again led to a sevenfold to ninefold reduction in viral shedding, respectively. 8 The use of AZM alone increased viral‐induced interferons (IFNs) and interferon‐stimulated gene (ISG) mRNA expression and hence production of these gene products. 7 , 8 In the latter study, while viral replication was suppressed, AZM did not suppress pro‐inflammatory responses.
In vivo data from the AMAZES study, the largest clinical trial of a long‐term macrolide in airways disease, showed a striking 40% reduction in asthma exacerbations with AZM. 29 The mechanism is unknown, and would be consistent with an anti‐viral effect, although metagenomic analyses suggest an antibacterial effect reducing Haemophilus influenzae 33 , 34 may be the predominant mechanism. The effect on viruses may relate to H. influenzae upregulation of ICAM‐1, a major receptor for both Haemophilus and rhinovirus (RV). 35
Other macrolides also have anti‐viral effects in RV infection including Mac5, an oleandomycin macrolide. Both AZM and Mac5 suppressed RV replication and enhanced RV‐induced type I and type III IFNs, as well as the ISGs viperin/MxA. 9 In this study, macrolides did not affect interleukin (IL)‐6 and ‐8, but secretion of IL‐1β, IL‐6 and IL‐8 were reduced by clarithromycin (another macrolide) in a separate study of RV, 36 alongside inhibition of viral replication and ICAM‐1. Macrolides such as AZM augment infection‐induced IFN responses. 9 This is of relevance to coronaviruses as type I IFN inhibit replication of both SARS‐CoV 37 and SARS‐CoV‐2 38 in vitro.
RV replication was also inhibited by the macrolides erythromycin 39 and bafilomycin 40 in PBEC. In both studies, macrolides reduced RV‐induced NFκB activation and decreased acidity of endosomes in epithelial cells. Bafilomycin inhibited cytokine production and ICAM‐1 expression.
2.2. Mechanism of effects in influenza A
In a randomised trial in patients with influenza A receiving oseltamivir, 5 days' adjunctive AZM 500 mg daily was associated with more rapid reductions in plasma concentrations of IL‐6, IL‐8, IL‐17, CXCL9, soluble tissue necrosis factor (TNF) and C‐reactive protein (CRP). 16 However, this was an open‐label study, with a small sample size (n = 50), and the effect was small, with no significant changes in viral clearance or time to symptom resolution. In a second, larger, open‐label, randomised controlled trial 2 days of clarithromycin 500 mg and naproxen 200 mg twice daily reduced 30 day mortality, high dependency unit admission and hospital stay in 217 elderly patients with H2N2 influenza. 18 The effect size was marked, although the study is limited by lack of blinding and by the likelihood that much of the effect might be attributable to the antibacterial properties of clarithromycin, as bacterial pneumonias are responsible for a high proportion of influenza deaths, particularly in the elderly.
Nonetheless in vitro clarithromycin reduced viral replication in the A549 human lung cell line. 41 Likewise clarithromycin reduced viral titres and supernatant cytokines on cultured human tracheal epithelial cells, associated with reduction in surface expression of the influenza A receptor Sα2, 6Gal, inhibition of NFκB and reduced acidification of the endosome required for intracellular release of viral RNA. 42 More recent data also showed a reduction in H1N1 viral replication in A549 cells with AZM with an IC50 of 68 μM, with an effect most apparent during viral particle internalisation. 43
Some murine studies have investigated macrolides in vivo. Erythromycin improved survival during severe H2N2 infection, 44 associated with reduced bronchoalveolar lavage (BAL) IFN‐γ, inflammatory cells and nitric‐oxide‐derived free radicals. Other macrolides leucomycin A3, spiramycin and a non‐antibacterial erythromycin derivative (EM900) each reduced weight loss, improved survival and reduced viral protein expression in H1N1 influenza. 45 In a short‐term H1N1 infection model, AZM reduced expression of viral proteins 2 days post infection. 43 However, the effect was not sustained, and not associated with a change in virus‐induced weight loss, a sensitive measurement of influenza pathology. Another study found AZM reduced lung viral titres at day 6 post infection, though the effects were not additive to that achieved with oseltamivir in terms of survival, viral titres or cytokine levels, 46 and so these data remain conflicting. 47 In a separate influenza study, AZM decreased total leukocyte accumulation in lung tissue and BAL, with the largest reduction being in neutrophils, and associated with decreased inflammatory mediators.
2.3. Mechanism of anti‐viral effect in Zika virus
In a drug screen of 2177 compounds against the flavivirus Zika, AZM reduced viral proliferation and virus‐induced cytopathic effects in glial cell lines and human astrocytes. 11 A further in vitro study found AZM to effectively suppress Zika infection by targeting a late stage in the viral life cycle. 12 AZM also upregulated expression of type I and III IFNs and several of their downstream ISGs, paralleling activities of AZM in RV. 7 , 8 Furthermore, AZM induced enhanced expression of the anti‐viral pattern recognition receptors (PRRs) MDA5 and RIG‐1, as well as the levels of phosphorylated TBK1 and IRF3.
2.4. Anti‐viral effects in Ebola
AZM was similarly evaluated in a drug screen for its efficacy as a therapy for Ebola. 14 While AZM demonstrated high in vitro potency (50% effective concentration [EC50] = 5.1 μM) and low toxicity, when tested in an in vivo mouse model it did not consistently improve survival in mice or guinea pigs.
2.5. Anti‐viral effects in enteroviruses
Enterovirus A71 (EV‐A71) causes hand, foot and mouth disease in young children. AZM and spiramycin (another macrolide) provided significant in vivo protection against EV‐A71 infection in mice. 13 Spiramycin impaired EV‐A71 viral RNA synthesis, and it is likely spiramycin and AZM work through a common mechanism, after viral entry, impairing viral RNA synthesis either directly or indirectly.
2.6. Anti‐viral effects of AZM in coronaviruses
Human coronaviruses are enveloped positive‐stranded RNA viruses of the Coronaviridae family in the Nidovirales order comprising four genera (Alpha‐, Beta‐, Gamma‐, Delta‐coronaviruses). 48 These viruses are endemic respiratory and gastrointestinal viruses and the Betacoronavirus genus includes the pandemic viruses MERS‐CoV, SARS‐CoV and SARS‐CoV‐2. AZM was used in a third of patients treated for MERS‐CoV, although without a clinical evidence base. 5 A retrospective cohort analysis of 349 patients across 14 sites in Saudi Arabia found no significant reduction in 90‐day mortality (odds ratio [OR] 0.84 95% confidence interval [CI] 0.47‐1.51) or improvement in MERS‐CoV RNA clearance (hazard ratio HR 0.88 [0.47‐1.64] with macrolide use). 5 However, this was a non‐randomised, retrospective observational study, in which it was unknown on what basis treatment allocation decisions were made, and randomised data are needed.
Interesting data have recently emerged from a mass eradication programme amongst preschool children in Niger. Children up to age 5 were cluster‐randomised by community to a single oral dose of AZM or placebo every 6 months and nasopharyngeal swabs were taken for viral RNA sequencing. After 24 months, AZM use was associated with an eightfold reduction in viral load of Alphacoronavirus and a 14‐fold reduction in Betacoronavirus viral load, though there was no difference in the prevalence of these viruses. 10
Since the outbreak of the current SARS‐CoV‐2 pandemic, several drug screens have investigated potential candidate drugs against this virus. A screen of 1520 approved and off‐patent drugs identified 90 drugs which inhibited SARS‐CoV‐2 viral replication at 10 μM. 48 These included ATPase proton pump inhibitors, protease inhibitors, viral protease inhibitors, drugs targeting the angiotensin pathway and AZM. AZM had an EC50 of 2.12 μM and EC90 8.65, and selectivity index >19, which is very comparable to the control compound remdesivir (EC50 = 1.65, EC90 = 2.52), the only anti‐viral with proven clinical efficacy against SARS‐CoV‐2 in clinical trials to date. 49 , 50 Likewise AZM was also identified as a target in a bioinformatic screening analysis of potentially relevant pathways with the potential for development into pharmaceutically acceptable forms, 51 in this case by inhibiting autophagy via inhibition of the vacuolar ATPase necessary for autophagosome‐lysosome fusion. 52 A focussed study on two candidate molecules, hydroxychloroquine and AZM, suggested a synergistic inhibition of SARS‐CoV‐2 replication in Vero cells at 5 and 10 μM concentrations, respectively. 53 This synergy has been proposed to allow effective use of hydroxychloroquine at less toxic concentrations, and is an approach tried in a small observational study which suggested enhanced virological clearance with hydroxychloroquine, particularly in combination with AZM. 54 However this study was very small, with AZM data from only six patients, and was open‐label and non‐randomised, allowing no useful conclusions to be drawn. Moreover, there are concerns that combination therapy may enhance cardiovascular side effects as both molecules individually can cause prolongation of the QT interval. 55 This combination has been tested in non‐human primates, where a significant anti‐viral effect was not seen in the five macaques which received AZM in addition to hydroxychloroquine. 56
3. ANTI‐INFLAMMATORY EFFECTS
Whilst viruses can cause tissue damage by direct cytopathic effects on the infected cells, morbidity and mortality in severe disease are typically attributable to the host inflammatory response, including in COVID‐19. 57 AZM and other macrolides have a number of immunomodulatory properties which have proven clinical efficacy in a broad range of respiratory diseases including asthma, 29 COPD, 58 post lung transplant obliterative bronchiolitis 59 and diffuse pan bronchiolitis (DPB). 60 , 61 , 62 , 63 In DPB, a dramatic increase in survival 60 , 62 , 63 has been attributed to the ability of AZM to inhibit dysregulated IL‐1β, IL‐2, TNF and GM‐CSF. 64 Therefore, the anti‐inflammatory properties of AZM (summarised in Table 2 and Figure 1) may be clinically important in the management of viral diseases.
TABLE 2.
Immunomodulatory and anti‐inflammatory properties of azithromycin
| Property | Effect | Specific Findings | Study |
|---|---|---|---|
| General anti‐inflammatory properties | |||
| Destabilisation of NALP3 mRNA levels | Decreased IL‐beta production | LPS‐stimulated THP‐1 monocytes. AZM reduced IL‐1beta, NALP3 protein and NFκB activity | Lendermon et al 65 |
| Inhibition of inflammatory cytokine release | Decreased CXCL8 (IL‐8), NFκB and AP‐1 from epithelial cells | Clinical trial in recurrent genital C. trachomatis infection. Decreased IL‐1beta, CXCL‐1, ‐5, ‐8, ‐9, CCL2, ‐5, MCL1, MAPK1 | Srivastava et al 66 |
| Airway epithelial cell lines. Decrease in CXCL8 mRNA, and NFκB and AP‐1 binding | Cigana et al 67 | ||
| Decreased CXCL8 (IL‐8), MAPK and 8‐isoprostane in airway smooth muscle cells | IL‐17‐stimulated primary human airway smooth muscle cells | Vanaudenaerde et al 68 | |
| Decreased PGE2 synthesis | Human polymorphonuclear and mononuclear leukocytes. Decreased LPS‐induced PGE2 by suppression of cPLA2, COX‐1, COX‐2 | Miyazaki et al 69 | |
| Decreased TNF from cystic fibrosis airway epithelial cells | Human CF and non‐CF cell lines. Decreased TNF mRNA and protein and NFκB and Sp1 binding | Cigana et al 70 | |
| Decreased GM‐CSF | Airway epithelial (A549) cell lines. Reduced TNF‐induced GM‐CSF mRNA and protein expression | Yamasawa et al 71 | |
| Reduction of cytokine‐induced endothelin 1 expression in epithelial cells | Human bronchial epithelial cells. Erythromycin and clarithromycin reduced enfothelin‐1 expression | Takizawa et al 72 | |
| Inhibition of endocytosis/induction of phospholipidosis | |||
| Decreases motility and fluidity of the plasma membrane | J774 macrophage cell line | Tyteca et al 73 | |
| Slows membrane trafficking towards lysosomes | J774 macrophage cell line | Tyteca et al 74 | |
| Inhibition of fluid phase endocytosis of macromolecules | J774 macrophage cell line | Tyteca et al 74 | |
| Down‐regulates and delays recycling of surface transferrin receptors | J774 macrophage cell line | Tyteca et al 74 | |
| Inhibition of pinocytosis of macromolecules and their transport from plasma membrane to endo/lysosomes | J774 macrophage cell line | Tyteca et al 74 | |
| Increase of lysosomal hydrolase activity in fibroblasts | Fibroblast homogenates. Increased activity of sulfatase A, phospholipase A1, cathepsin B | Gerbaux et al 75 | |
| Lysosomal enzyme depletion/extracellular secretion of lysosomal enzymes | Rat kidney cells. Redistribution of mannose 6‐phosphate receptor | Ikeda et al 76 | |
| Effects on airway inflammatory cells | |||
| Accumulation intracellularly within phagocytes | Prolonged macrolide retention intracellularly | Human in vivo 210 h T1/2 in neutrophils. Concentration in alveolar macrophages, in neutrophils, in phagocytic and epithelial cell lines | Wildfeuer et al, Capitano et al, Bosnar et al 77 , 78 , 79 |
| Prolonged AZM retention within neutrophils | Concentrations 2000 to 3000 times higher in neutrophils than plasma | Wilms et al 80 | |
| Accumulation of macrolides in alveolar macrophages | Human in vivo 500‐fold accumulation in alveolar macrophages | Lucchi et al, Capitano et al 77 , 81 | |
| Neutrophils | Inhibition of neutrophil chemotaxis | Murine pseudomonas model and human neutrophils. Reduced neutrophil chemotaxis via ERK‐1 and ERK‐2 | Tsai et al 82 |
| Down regulation of neutrophil chemokine production | Human blood. Decreased azurophilic granule enzyme activities | Culić et al, Tsai et al 83 , 84 | |
| Attenuation of neutrophil oxidative burst | Human blood neutrophils | Nozoe et al 85 | |
| Down regulation of MPO production | Human in vivo blood neutrophils. Decreased MPO concentration | Culić et al 84 | |
| Increased neutrophil apoptosis | Human in vivo blood neutrophils. Increased neutrophil apoptosis 28 days post dose | Culić et al 84 | |
| Inhibition of neutrophil elastase and MMP9 | Human in vivo. Clarithromycin reduced airway neutrophil elastase and MMP9 | Simpson et al 86 | |
| Macrophages | Increased phagocytosis | Human alveolar macrophages. Increased phagocytosis of apoptotic bronchial epithelial cells and neutrophils | Hodge et al, Yamaryo et al 87 , 88 |
| Macrophage lysosomes more resistant to oxidant challenge | Human alveolar macrophages ex vivo. Reduced oxidative lysosomal membrane permeabilisation | Persson et al 89 | |
| Polarization towards M2 phenotype | In vitro polarised J774 macrophage cell line. Increased M2 markers mannose receptor, CD23, arginase, decreased CCR7 | Murphy et al 90 | |
| Reduction in production of GM‐CSF and IL‐1beta | Murine LPS challenge. Decreased GM‐CSF, IL‐1beta, TNF, CCL2 | Bosnar et al 91 | |
| Suppression of IL‐12p40 by macrophages | LPS‐stimulated macrophage cell lines. Decreased IL‐12p40 induction by inhibited AP‐1, NFAT, ICSBP binding | Yamauchi et al 92 | |
| Increased mannose receptor expression | Human in vivo trial. Increased mannose receptor expression and phagocytosis | Hodge et al 93 | |
| Decreased CXCL8 (IL‐8) production | Human ex vivo blood and lung macrophages. CXCL8 inhibited at 400 mg/L | Kurdowska et al 94 | |
| Dendritic cells | Modulation towards a regulatory phenotype | Monocyte‐derived dendritic cells enhanced IL‐10 release and inhibited IL‐6, IL‐12p40, CXCL10, CXCL11 and CCL22 release | Polancec et al, Sugiyama et al 95 , 96 |
| CD40, CD86, and MHCII expression inhibited | Murine bone marrow derived DCs and murine histoincompatible bone marrow transplant model. Decreased CD40 and CD86 | Iwamoto et al 97 , 98 | |
| Natural Killer cells | Inhibition of cytotoxic function through down regulation of perforin expression | Human NK cells. Decreased CD69, perforin and cytotoxicity | Lin et al 99 |
| Effects on airway mucosal stromal cells | |||
| Smooth muscle cells | Antiproliferative effect | Rabbit tracheal smooth muscle cells. Reduced proliferation, increased autophagy | Stamatiou et al 100 |
| Relaxant effect | Rabbit tracheal smooth muscle cells. Smooth muscle relaxation | Daenas et al 101 | |
| Airway epithelium | |||
| Enhanced airway epithelial integrity | Increased transepithelial electrical resistance by altered processing of tight junction proteins | Asgrimsson et al, Halldorsson et al 102 , 103 | |
| Inhibition of inflammatory mucin release | Human cell lines and primary cells. Inhibition of MUC5AC production | Imamura et al, Ribeiro et al 104 , 105 | |
| Modulated CXCL8 (IL‐8) production | Human bronchial epithelial cells. Increased CXCL8 release | Shinkai et al 106 | |
| Reduced CXCL8 (IL‐8) | Human trial. Roxithromycin reduced CXCL8 in nasal lavage in chronic rhinosinusitis, with clinical improvement | Wallwork et al, Yamada et al 107 , 108 |
Abbreviations: CCL, C‐C motif ligand; CD, cluster of differentiation; CF, cystic fibrosis; CXCL, C‐X‐C motif ligand; DPB, diffuse panbronchiolitis; COX, cyclooxygenase; cPLA2, cytosolic phospholipase A2; ERK, extracellular signal‐regulated kinase; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor (CSF2); IL, interleukin; MAPK, mitogen‐activated protein kinase; MCL1, myeloid cell leukaemia sequence 1; NALP3, NACHT, LRR, and PYD domains‐containing protein 3; PGE2, Prostaglandin E2; TGF‐beta, transforming growth factor beta.
FIGURE 1.
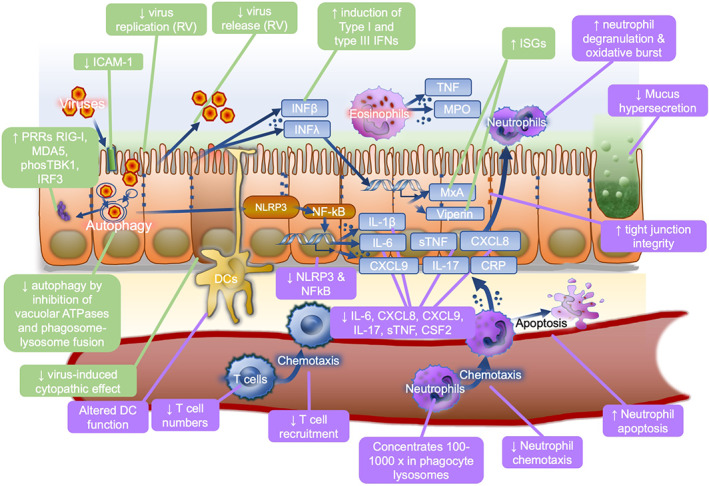
Anti‐viral and anti‐inflammatory effects of macrolides. Schematic showing major proposed mechanisms of azithromycin anti‐viral (green) and anti‐inflammatory or immunomodulatory (purple) activities. AZM, azithromycin; CRP, C‐reactive protein; CSF2, colony‐stimulating factor 2 (GM‐CSF); CXCL, C‐X‐C motif chemokine ligand; DC, dendritic cell; ICAM1, intracellular cell adhesion molecule 1; IFN, interferon; IL, interleukin; IRF3, Interferon Regulatory Factor 3; ISG, interferon‐stimulated gene; MDA5, melanoma differentiation‐associated protein 5; MPO, myeloperoxidase; MxA, myxoma resistance protein 1; NFκB, nuclear factor kappa B; NLRP3, nucleotide‐binding oligomerisation domain; phosTBK1, phosphorylated TANK‐binding kinase 1; PRR, pattern recognition receptor; RIG‐I, retinoic acid‐inducible gene I; RV, rhinovirus; Th17, type‐17 T‐helper; TLR, Toll‐like receptor; (s)TNF, (soluble) tissue necrosis factor
3.1. Immunomodulatory effects on phagocytes
AZM is rapidly absorbed after oral administration with a large volume of distribution 109 and a long serum half‐life of approximately 3 days, 110 leading to a high and sustained tissue concentration. A striking feature of macrolides is that they can accumulate in host cells including epithelial cells and most particularly in phagocytes where they may concentrate 100‐ to 3000‐fold in the lysosomes of phagocytes, 77 , 81 , 111 , 112 being subsequently retained intracellularly 78 , 80 , 81 and released when these cells die. Therefore typical AZM concentrations after one‐three 500 mg oral doses may be 0.29 μM (0.22 mg/L) in plasma, but 12 μM in lung tissue homogenate, 48 μM in bronchial washings and 260 μM in alveolar macrophages. 113 Several studies have observed initial stimulatory effects of AZM on immune and epithelial cells. Acutely, AZM stimulates neutrophil degranulation and phagocytosis‐associated oxidative burst, mediated via modulation of Erk1/2 signalling. 79 These initial stimulatory effects are followed by modulation of transcription factors activator protein (AP)‐1, nuclear factor kappa B (NFκB), inflammatory cytokine and mucin release, with overall anti‐inflammatory effects.
Many inflammatory cytokine levels are reduced by AZM, including IL‐6, IL‐8 (CXCL8), TNF 114 and GM‐CSF, as well as matrix metalloproteases MMP‐1, ‐2, ‐9, ‐10 and ‐13, and modulation of lipid metabolism and cell cycle pathways (Table 2). 70
One pathway for macrolide immunomodulation is through binding to macrophilin‐12 inhibiting calcineurin and thus T cell activation, via the same mechanism as tacrolimus, 29 with consequent downstream inhibition of many immune cells including eosinophils and basophils. 68 Macrolides also inhibit mammalian target of rapamycin (mTOR) activity, also important in T cell activation and granulocyte differentiation, 115 suppressing cell proliferation and CD4 + T cell cytokine secretion. 116 A third pathway modulated by macrolides is activity of the transcription factors NFκB and AP‐1. AZM suppresses p65, a component of NFκB 117 and attenuates NFκB activation in lung epithelial cells. 118 This inhibition reduces epithelial cell IL‐8 production, 67 , 118 stromal cell proliferation 66 and macrophage expression of IL‐12p40 119 and, indirectly, IL‐1β. 65 , 92
In macrophages, AZM has several effects including attenuation of lipopolysaccharide‐induced pro‐inflammatory cytokines through inhibition of AP‐1, and increasing phagocytosis, 120 enhancing the resistance of lysosomes to oxidant challenge 93 and promoting M2 polarization of macrophages. 89 , 90 , 119 Macrolides including AZM can also increase the phagocytosis of apoptotic epithelial cells 121 and neutrophils by macrophages, 87 which can ameliorate inflammation.
3.2. Effects on other cell types
In vitro AZM modulated differentiation and maturation of dendritic cells towards a regulatory phenotype with increased phagocytic capacity, 88 , 95 with inhibited expression of CD40, CD86, MHCII and IL‐12. 96 , 97 Likewise AZM inhibited the cytotoxic function of natural killer cells through down regulation of perforin. 98
AZM may have anti‐inflammatory effects directly on epithelial cells, such as suppression of GM‐CSF release, 99 TNF, 114 inhibition of IL‐8 production 118 and modulation of the anti‐viral PRRs RIG‐I and MDA5. 7 AZM inhibition of AP‐1 activation reduces production of MUCA5C responsible for inflammation‐induced changes in airway mucus. 71 , 122 Macrolides inhibit airway epithelial cell mucus secretion 123 and directly inhibit neutrophil elastase. 124 , 125 Another effect of macrolides on airway epithelial cells observed in vitro is increased epithelial barrier integrity by alterations in tight junction proteins, including claudins. 102 , 126
Overall, macrolides have a number of inhibitory effects on the production of pro‐inflammatory cytokines from innate and adaptive immune cells, and most markedly on the accumulation, adhesion and apoptosis of pulmonary neutrophils.
4. POTENTIAL CLINICAL UTILITY IN COVID‐19
Beyond its anti‐viral properties, the anti‐inflammatory effects of AZM may be clinically important in treating the cytokine storm which is a prominent feature of influenza A and of COVID‐19. An exuberant production of pro‐inflammatory cytokines including TNF, IL 1β, IL‐6, G‐CSF and IP‐10 are significantly increased in COVID‐19 disease, 103 and are associated with features of hemophagocytic lymphohistiocytosis 127 and interstitial mononuclear inflammatory infiltrates, dominated by lymphocytes, 128 and with poor clinical outcomes. 103 However, in contrast to influenza A, where this cytokine storm occurs early in disease, most COVID‐19 related deaths occur due to sudden, late respiratory failure, on average at day 14 after symptom onset, 129 by which point viral loads have markedly decreased. Severe COVID‐19 disease is associated with loss of alveolar macrophages 130 and an influx of pro‐inflammatory monocyte‐derived macrophages. 131
The importance of controlling this inflammation is demonstrated by the recent positive findings of the RECOVERY trial showing a significant mortality benefit with dexamethasone in patients with severe COVID‐19 disease and respiratory failure. 132 Interestingly, there was no benefit in those randomised at earlier disease stages, consistent with a lower degree of inflammation in these individuals, and suggesting other anti‐inflammatory approaches with fewer side effects might be valuable. The lag between symptom onset and severe disease provides a therapeutic window in which AZM anti‐inflammatory properties may reduce severe pulmonary inflammation, benefiting from the propensity of macrolides to accumulate in phagocytes, 60 , 111 which targets them specifically to the sites of pathology in COVID‐19.
It is understandable therefore, that more than 80 clinical trials have been designed to test AZM efficacy in COVID‐19 (Table S1). These differ significantly from each other according to dosing regime, duration of therapy, whether being used in combination with hydroxychloroquine and, critically, according to the population being studied. Those recruiting in primary care will tend to study the anti‐viral effects in early disease, whilst those recruiting in secondary care will be studying more the anti‐inflammatory effects important in late disease. The first trial to publish results compared standard care with hydroxychloroquine (HCQ) 400 mg twice daily or with HQC 400 mg twice daily and AZM 500 mg once daily for 7 days in hospitalised patients with a median duration of symptoms of 7 days prior to randomisation. 133 There was no reduction in symptoms or requirement for ventilation with either HCQ plus AZM compared with HCQ alone (odds ratio 0.82; 95% confidence interval 0.47‐1.43), but data from other populations, disease stages and without HCQ are urgently needed. If studies show clinical efficacy it will be essential to determine which populations benefit and what criteria to use as clinical indications for therapy. There is also a need for further human in vivo mechanistic studies to determine which of the manifold potential mechanisms are dominant in patients with disease.
AZM is generally well tolerated, the most common side effect being diarrhoea, 29 it is contraindicated in pregnancy and known hypersensitivity. Whilst there have been concerns about cardiovascular risk, huge epidemiological studies suggest these are very small effects (eg, 47 extra deaths/million prescriptions) or perhaps no effect when corrected for confounding, 134 and a Cochrane review of 183 trials found no evidence of an increase in cardiac disorders with macrolides (OR 0.87). 135 Concerns have been raised about the potential interactions between HCQ and AZM increasing risk of side effects. It should be used in caution in those receiving some other drugs including fluoroquinolones such as moxifloxacin and levofloxacin, and in patients with ongoing proarrhythmic conditions, and QT prolongation was more frequent in people with COVID‐19 receiving HCQ (14.6%) or the combination of HCQ and AZM (14.7%) than standard care (1.7%), an effect likely purely attributable to the HCQ. 133
Given the significant clinical utility of AZM as an antibiotic, the current rapid spread of antimicrobial resistance is of particular concern. Widespread use of AZM to treat viral infections runs an inevitable risk of increasing the development of drug‐resistant bacteria, and indeed there are good data that increasing rates of macrolide resistance in Streptococcus pneumoniae in the United States correlated closely with global sales of AZM, while in some regions such as China resistance rates approach 90% for Mycoplasma pneumoniae and nearly 100% for S. pneumoniae. 110 Resistance is a particularly high risk with macrolides due to several features including their long half‐life, the widespread use of the drug, and the high‐level macrolide, lincosamide and streptogramin (MLSB) resistance phenotype attributable to mutations in the erm gene and which are frequently associated with resistance to other classes of antibiotics on the same mobile genetic elements. 110 Therefore, it will be important to understand the potential anti‐viral and anti‐inflammatory properties of other novel macrolides which have been synthesised but do not have broad‐spectrum antibacterial properties and might therefore reduce development of resistance 136 and disruption to the natural microbiome. 137
5. CONCLUSIONS
As a therapeutic class, macrolides, and in particular AZM, with its long therapeutic half‐life, good safety profile and very strong evidence base in bacterial diseases are fascinating molecules. Macrolides undoubtedly have broad‐spectrum anti‐viral properties in vitro. AZM consistently emerges as a candidate molecule in anti‐viral drug screens against respiratory viruses, and there are tantalising hints of clinical efficacy in clinical studies to date. The additional anti‐inflammatory properties displayed by some macrolides, including AZM, may well prove to be clinically important in reducing immunopathology in some viral diseases, not least against the pandemic Betacoronaviruses in which activation of an over‐exuberant inflammatory cascade seems to be critical to mortality. However there is currently insufficient evidence to justify their use clinically, but rather, a clear mandate to perform well‐designed and conducted randomised trials in patients with chronic airways disorders and those with pandemic respiratory viruses including influenza A, SARS‐CoV‐2 and in future pandemics of novel coronaviruses which increasingly appear to be an inevitable prospect.
CONFLICT OF INTEREST
M.E.O. None. T.S.C.H. has received unrestricted research grants from the Wellcome Trust (211050/Z/18/z, 211050/Z/18/A) and from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), the University of Oxford COVID‐19 Research Response Fund and from Pfizer to support conduct of a trial of azithromycin in COVID‐19.
AUTHOR CONTRIBUTIONS
Madeleine E. Oliver and Timothy S. C. Hinks jointly conceived the article, conducted the literature review and drafted the manuscript. All authors approved the final manuscript.
Supporting information
Table S1 Current clinical trials of azithromycin in SARS‐CoV‐2
ACKNOWLEDGEMENTS
This work was supported by grants from the Wellcome Trust (211050/Z/18/z, 211050/Z/18/A) and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC) to T.S.C.H. The views expressed are those of the authors and not those of the NHS or NIHR. T.S.C.H. has received unrestricted research grants from the University of Oxford COVID‐19 Research Response Fund and from Pfizer to support conduct of a trial of azithromycin in COVID‐19.
Oliver ME, Hinks TSC. Azithromycin in viral infections. Rev Med Virol. 2021;31:e2163. 10.1002/rmv.2163
Funding information University of Oxford, Grant/Award Number: University of Oxford COVID‐19 Research Response Fund; Wellcome Trust, Grant/Award Numbers: 211050/Z/18/A, 211050/Z/18/z; Pfizer; National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC)
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Bright GM, Hauske JR. Azahomoerythromycin D derivative and intermediates therefor. USA1984.
- 2. Kobrehel G, Radobolja G, Tamburasev Z, Djokic S. 11‐Aza‐10‐deoxo‐10‐dihydroerythromycin A and derivatives thereof as well as a process for their preparation. USA1982.
- 3. World Health Organization . Model List of Essential Medicines. 21st ed. Geneva: World Health Organization; 2019. [Google Scholar]
- 4. Schönfeld W, Kirst HA. Effects of Macrolide Antibiotics on Ribosome Function. Basel: Birkhäuser Verlag; 2002. [Google Scholar]
- 5. Arabi YM, Deeb AM, Al‐Hameed F, et al. Macrolides in critically ill patients with Middle East respiratory syndrome. Int J Infect Dis. 2019;81:184‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. C‐CRCEa . Global coalition to accelerate COVID‐19 clinical research in resource‐limited settings. Lancet. 2020;395(10233):1322‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti‐viral responses in bronchial epithelial cells. Eur Respir J. 2010;36(3):646‐654. [DOI] [PubMed] [Google Scholar]
- 8. Schogler A, Kopf BS, Edwards MR, et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur Respir J. 2015;45(2):428‐439. [DOI] [PubMed] [Google Scholar]
- 9. Porter JD, Watson J, Roberts LR, et al. Identification of novel macrolides with antibacterial, anti‐inflammatory and type I and III IFN‐augmenting activity in airway epithelium. J Antimicrob Chemother. 2016;71(10):2767‐2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doan T, Hinterwirth A, Arzika AM, et al. Reduction of coronavirus burden with mass azithromycin distribution. Clin Infect Dis. 2020;ciaa606. doi: 10.1093/cid/ciaa606. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Retallack H, Di Lullo E, Arias C, et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A. 2016;113(50):14408‐14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li C, Zu S, Deng YQ, et al. Azithromycin protects against Zika virus infection by upregulating virus‐induced type I and III interferon responses. Antimicrob Agents Chemother. 2019;63(12):e00394–19. doi: 10.1128/AAC.00394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeng S, Meng X, Huang Q, et al. Spiramycin and azithromycin, safe for administration to children, exert antiviral activity against enterovirus A71 in vitro and in vivo. Int J Antimicrob Agents. 2019;53(4):362‐369. [DOI] [PubMed] [Google Scholar]
- 14. Madrid PB, Panchal RG, Warren TK, et al. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect Dis. 2015;1(7):317‐326. [DOI] [PubMed] [Google Scholar]
- 15. Kawamura K, Ichikado K, Takaki M, Eguchi Y, Anan K, Suga M. Adjunctive therapy with azithromycin for moderate and severe acute respiratory distress syndrome: a retrospective, propensity score‐matching analysis of prospectively collected data at a single center. Int J Antimicrob Agents. 2018;51(6):918‐924. [DOI] [PubMed] [Google Scholar]
- 16. Lee N, Wong CK, Chan MCW, et al. Anti‐inflammatory effects of adjunctive macrolide treatment in adults hospitalized with influenza: a randomized controlled trial. Antiviral Res. 2017;144:48‐56. [DOI] [PubMed] [Google Scholar]
- 17. Shinahara W, Takahashi E, Sawabuchi T, et al. Immunomodulator clarithromycin enhances mucosal and systemic immune responses and reduces re‐infection rate in pediatric patients with influenza treated with antiviral neuraminidase inhibitors: a retrospective analysis. PLoS One. 2013;8(7):e70060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung IFN, To KKW, JFW C, et al. Efficacy of clarithromycin‐naproxen‐oseltamivir combination in the treatment of patients hospitalized for influenza a(H3N2) infection: an open‐label randomized, controlled, phase IIb/III trial. Chest. 2017;151(5):1069‐1080. [DOI] [PubMed] [Google Scholar]
- 19. Higashi F, Kubo H, Yasuda H, Nukiwa T, Yamaya M. Additional treatment with clarithromycin reduces fever duration in patients with influenza. Respir Investig. 2014;52(5):302‐309. [DOI] [PubMed] [Google Scholar]
- 20. Walkey AJ, Wiener RS. Macrolide antibiotics and survival in patients with acute lung injury. Chest. 2012;141(5):1153‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tahan F, Ozcan A, Koc N. Clarithromycin in the treatment of RSV bronchiolitis: a double‐blind, randomised, placebo‐controlled trial. Eur Respir J. 2007;29(1):91‐97. [DOI] [PubMed] [Google Scholar]
- 22. Ninomiya K, Fukui T, Imai T, Matsui M, Matsuoka K. Effect of maclorides on duration and resolution of symptoms and complication of pneumonia in children with influenza. J Nippon Med Sch. 2002;69(1):53‐57. [DOI] [PubMed] [Google Scholar]
- 23. McCallum GB, Morris PS, Chatfield MD, et al. A single dose of azithromycin does not improve clinical outcomes of children hospitalised with bronchiolitis: a randomised, placebo‐controlled trial. PLoS One. 2013;8(9):e74316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin‐Loeches I, Bermejo‐Martin JF, Valles J, et al. Macrolide‐based regimens in absence of bacterial co‐infection in critically ill H1N1 patients with primary viral pneumonia. Intensive Care Med. 2013;39(4):693‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pinto LA, Pitrez PM, Luisi F, et al. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: a randomized, double‐blinded, and placebo‐controlled clinical trial. J Pediatr. 2012;161(6):1104‐1108. [DOI] [PubMed] [Google Scholar]
- 26. Kneyber MC, van Woensel JB, Uijtendaal E, Uiterwaal CS, Kimpen JL. Dutch antibiotics in RSVTRG. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatr Pulmonol. 2008;43(2):142‐149. [DOI] [PubMed] [Google Scholar]
- 27. Kew KM, Undela K, Kotortsi I, Ferrara G. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015;9:CD002997. [DOI] [PubMed] [Google Scholar]
- 28. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double‐blind placebo‐controlled trial. Thorax. 2013;68(4):322‐329. [DOI] [PubMed] [Google Scholar]
- 29. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2017;390(10095):659‐668. [DOI] [PubMed] [Google Scholar]
- 30. Johnston SL, Blasi F, Black PN, Martin RJ, Farrell DJ, Nieman RB. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354(15):1589‐1600. [DOI] [PubMed] [Google Scholar]
- 31. Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9‐11 year old children. BMJ. 1995;310(6989):1225‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kelly JT, Busse WW. Host immune responses to rhinovirus: mechanisms in asthma. J Allergy Clin Immunol. 2008;122(4):671‐682. quiz 83‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor SL, Leong LEX, Mobegi FM, et al. Long‐term azithromycin reduces Haemophilus influenzae and increases antibiotic resistance in severe asthma. Am J Respir Crit Care Med. 2019;200(3):309‐317. [DOI] [PubMed] [Google Scholar]
- 34. Taylor SL, Ivey KL, Gibson PG, Simpson JL, Rogers GB, Group ASR . Airway abundance of Haemophilus influenzae predicts response to azithromycin in adults with persistent uncontrolled asthma. Eur Respir J. 2020;2000194. doi: 10.1183/13993003.00194-2020. [DOI] [PubMed] [Google Scholar]
- 35. Sajjan US, Jia Y, Newcomb DC, et al. H. influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM‐1 and TLR3 expression. FASEB J. 2006;20(12):2121‐2123. [DOI] [PubMed] [Google Scholar]
- 36. Jang YJ, Kwon HJ, Lee BJ. Effect of clarithromycin on rhinovirus‐16 infection in A549 cells. Eur Respir J. 2006;27(1):12‐19. [DOI] [PubMed] [Google Scholar]
- 37. Stroher U, DiCaro A, Li Y, et al. Severe acute respiratory syndrome‐related coronavirus is inhibited by interferon‐alpha. J Infect Dis. 2004;189(7):1164‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lokugamage KG, Hage A, Schindewolf C, Rajsbaum R, Menachery VD. SARS‐CoV‐2 is sensitive to type I interferon pretreatment. bioRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 39. Suzuki T, Yamaya M, Sekizawa K, et al. Erythromycin inhibits rhinovirus infection in cultured human tracheal epithelial cells. Am J Respir Crit Care Med. 2002;165(8):1113‐1118. [DOI] [PubMed] [Google Scholar]
- 40. Suzuki T, Yamaya M, Sekizawa K, et al. Bafilomycin A(1) inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM‐1. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1115‐L1127. [DOI] [PubMed] [Google Scholar]
- 41. Miyamoto D, Hasegawa S, Sriwilaijaroen N, et al. Clarithromycin inhibits progeny virus production from human influenza virus‐infected host cells. Biol Pharm Bull. 2008;31(2):217‐222. [DOI] [PubMed] [Google Scholar]
- 42. Yamaya M, Shinya K, Hatachi Y, et al. Clarithromycin inhibits type a seasonal influenza virus infection in human airway epithelial cells. J Pharmacol Exp Ther. 2010;333(1):81‐90. [DOI] [PubMed] [Google Scholar]
- 43. Tran DH, Sugamata R, Hirose T, et al. Azithromycin, a 15‐membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J Antibiot (Tokyo). 2019;72(10):759‐768. [DOI] [PubMed] [Google Scholar]
- 44. Sato K, Suga M, Akaike T, et al. Therapeutic effect of erythromycin on influenza virus‐induced lung injury in mice. Am J Respir Crit Care Med. 1998;157(3 Pt 1):853‐857. [DOI] [PubMed] [Google Scholar]
- 45. Sugamata R, Sugawara A, Nagao T, et al. Leucomycin A3, a 16‐membered macrolide antibiotic, inhibits influenza A virus infection and disease progression. J Antibiot (Tokyo). 2014;67(3):213‐222. [DOI] [PubMed] [Google Scholar]
- 46. Fage C, Pizzorno A, Rheaume C, Abed Y, Boivin G. The combination of oseltamivir with azithromycin does not show additional benefits over oseltamivir monotherapy in mice infected with influenza A(H1N1)pdm2009 virus. J Med Virol. 2017;89(12):2239‐2243. [DOI] [PubMed] [Google Scholar]
- 47. Beigelman A, Mikols CL, Gunsten SP, Cannon CL, Brody SL, Walter MJ. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir Res. 2010;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—preliminary report. N Engl J Med. 2020;NEJMoa2007764. doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 50. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395(10236):1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nabirotchkin S, Peluffo AE, Bouaziz J, Cohen D. Focusing on the unfolded protein response and autophagy related pathways to reposition common approved drugs against COVID‐19. Preprints. 2020.doi: 10.20944/preprints202003.0302.v1. [DOI] [Google Scholar]
- 52. Renna M, Schaffner C, Brown K, et al. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest. 2011;121(9):3554‐3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Andreani J, Le Bideau M, Duflot I, et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS‐CoV‐2 shows synergistic effect. Microb Pathog. 2020;145:104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gautret P, Lagier J, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clincial trial. Int J of Antimicrobial Agents. 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55. Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;e201834. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maisonnasse P, Guedj J, Contreras V, et al. Hydroxychloroquine use against SARS‐CoV‐2 infection in non‐human primates. Nature. 2020.doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 57. Buonaguro FM, Ascierto PA, Morse GD, et al. Covid‐19: time for a paradigm change. Rev Med Virol. 2020;e2134. doi: 10.1002/rmv.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J. 2011;37(1):164‐172. [DOI] [PubMed] [Google Scholar]
- 60. Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. Immunomodulatory effects of macrolide antibiotics—part 1: biological mechanisms. Respiration. 2011;81(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 61. Hui D, Yan F, Chen RH. The effects of azithromycin on patients with diffuse panbronchiolitis: a retrospective study of 29 cases. J Thorac Dis. 2013;5(5):613‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low‐dose erythromycin. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1829‐1832. [DOI] [PubMed] [Google Scholar]
- 63. Nagai H, Shishido H, Yoneda R, Yamaguchi E, Tamura A, Kurashima A. Long‐term low‐dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration. 1991;58(3–4):145‐149. [DOI] [PubMed] [Google Scholar]
- 64. Weng D, Wu Q, Chen XQ, et al. Azithromycin treats diffuse panbronchiolitis by targeting T cells via inhibition of mTOR pathway. Biomed Pharmacother. 2019;110:440‐448. [DOI] [PubMed] [Google Scholar]
- 65. Bosnar M, Kragol G, Kostrun S, et al. N′‐substituted‐2′‐O,3′‐N‐carbonimidoyl bridged macrolides: novel anti‐inflammatory macrolides without antimicrobial activity. J Med Chem. 2012;55(13):6111‐6123. [DOI] [PubMed] [Google Scholar]
- 66. Miller SA, Selzman CH, Shames BD, Barton HA, Johnson SM, Harken AH. Chlamydia pneumoniae activates nuclear factor kappaB and activator protein 1 in human vascular smooth muscle and induces cellular proliferation. J Surg Res. 2000;90(1):76‐81. [DOI] [PubMed] [Google Scholar]
- 67. Srivastava P, Vardhan H, Bhengraj AR, et al. Azithromycin treatment modulates the extracellular signal‐regulated kinase mediated pathway and inhibits inflammatory cytokines and chemokines in epithelial cells from infertile women with recurrent Chlamydia trachomatis infection. DNA Cell Biol. 2011;30(8):545‐554. [DOI] [PubMed] [Google Scholar]
- 68. Rolfe FG, Valentine JE, Sewell WA. Cyclosporin A and FK506 reduce interleukin‐5 mRNA abundance by inhibiting gene transcription. Am J Respir Cell Mol Biol. 1997;17(2):243‐250. [DOI] [PubMed] [Google Scholar]
- 69. Miyazaki M, Zaitsu M, Honjo K, Ishii E, Hamasaki Y. Macrolide antibiotics inhibit prostaglandin E2 synthesis and mRNA expression of prostaglandin synthetic enzymes in human leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2003;69(4):229‐235. [DOI] [PubMed] [Google Scholar]
- 70. Vanaudenaerde BM, Wuyts WA, Geudens N, et al. Macrolides inhibit IL17‐induced IL8 and 8‐isoprostane release from human airway smooth muscle cells. Am J Transplant. 2007;7(1):76‐82. [DOI] [PubMed] [Google Scholar]
- 71. Nie YC, Wu H, Li PB, et al. Naringin attenuates EGF‐induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs‐AP‐1 and IKKs‐IkappaB‐NF‐kappaB signaling pathways. Eur J Pharmacol. 2012;690(1–3):207‐213. [DOI] [PubMed] [Google Scholar]
- 72. Takizawa H, Desaki M, Ohtoshi T, et al. Erythromycin and clarithromycin attenuate cytokine‐induced endothelin‐1 expression in human bronchial epithelial cells. Eur Respir J. 1998;12(1):57‐63. [DOI] [PubMed] [Google Scholar]
- 73. Tyteca D, Schanck A, Dufrene YF, et al. The macrolide antibiotic azithromycin interacts with lipids and affects membrane organization and fluidity: studies on Langmuir‐Blodgett monolayers, liposomes and J774 macrophages. J Membr Biol. 2003;192(3):203‐215. [DOI] [PubMed] [Google Scholar]
- 74. Tyteca D, Van Der Smissen P, Mettlen M, et al. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid‐phase and receptor‐mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp Cell Res. 2002;281(1):86‐100. [DOI] [PubMed] [Google Scholar]
- 75. Gerbaux C, Van Bambeke F, Montenez JP, Piret J, Morlighem G, Tulkens PM. Hyperactivity of cathepsin B and other lysosomal enzymes in fibroblasts exposed to azithromycin, a dicationic macrolide antibiotic with exceptional tissue accumulation. FEBS Lett. 1996;394(3):307‐310. [DOI] [PubMed] [Google Scholar]
- 76. Ikeda K, Hirayama M, Hirota Y, Asa E, Seki J, Tanaka Y. Drug‐induced phospholipidosis is caused by blockade of mannose 6‐phosphate receptor‐mediated targeting of lysosomal enzymes. Biochem Biophys Res Commun. 2008;377(1):268‐274. [DOI] [PubMed] [Google Scholar]
- 77. Wilms EB, Touw DJ, Heijerman HG. Pharmacokinetics of azithromycin in plasma, blood, polymorphonuclear neutrophils and sputum during long‐term therapy in patients with cystic fibrosis. Ther Drug Monit. 2006;28(2):219‐225. [DOI] [PubMed] [Google Scholar]
- 78. Wildfeuer A, Laufen H, Zimmermann T. Distribution of orally administered azithromycin in various blood compartments. Int J Clin Pharmacol Ther. 1994;32(7):356‐360. [PubMed] [Google Scholar]
- 79. Ishimoto H, Mukae H, Sakamoto N, et al. Different effects of telithromycin on MUC5AC production induced by human neutrophil peptide‐1 or lipopolysaccharide in NCI‐H292 cells compared with azithromycin and clarithromycin. J Antimicrob Chemother. 2009;63(1):109‐114. [DOI] [PubMed] [Google Scholar]
- 80. Bosnar M, Kelneric Z, Munic V, Erakovic V, Parnham MJ. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob Agents Chemother. 2005;49(6):2372‐2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Capitano B, Mattoes HM, Shore E, et al. Steady‐state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest. 2004;125(3):965‐973. [DOI] [PubMed] [Google Scholar]
- 82. Tsai WC, Rodriguez ML, Young KS, et al. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am J Respir Crit Care Med. 2004;170(12):1331‐1339. [DOI] [PubMed] [Google Scholar]
- 83. Culic O, Erakovic V, Cepelak I, et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol. 2002;450(3):277‐289. [DOI] [PubMed] [Google Scholar]
- 84. Tsai WC, Standiford TJ. Immunomodulatory effects of macrolides in the lung: lessons from in‐vitro and in‐vivo models. Curr Pharm des. 2004;10(25):3081‐3093. [DOI] [PubMed] [Google Scholar]
- 85. Nozoe K, Aida Y, Fukuda T, Sanui T, Nishimura F. Mechanisms of the macrolide‐induced inhibition of superoxide generation by neutrophils. Inflammation. 2016;39(3):1039‐1048. [DOI] [PubMed] [Google Scholar]
- 86. Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177(2):148‐155. [DOI] [PubMed] [Google Scholar]
- 87. Yamaryo T, Oishi K, Yoshimine H, Tsuchihashi Y, Matsushima K, Nagatake T. Fourteen‐member macrolides promote the phosphatidylserine receptor‐dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrob Agents Chemother. 2003;47(1):48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Polancec DS, Munic Kos V, Banjanac M, et al. Azithromycin drives in vitro GM‐CSF/IL‐4‐induced differentiation of human blood monocytes toward dendritic‐like cells with regulatory properties. J Leukoc Biol. 2012;91(2):229‐243. [DOI] [PubMed] [Google Scholar]
- 89. Murphy BS, Sundareshan V, Cory TJ, Hayes D Jr, Anstead MI, Feola DJ. Azithromycin alters macrophage phenotype. J Antimicrob Chemother. 2008;61(3):554‐560. [DOI] [PubMed] [Google Scholar]
- 90. Legssyer R, Huaux F, Lebacq J, et al. Azithromycin reduces spontaneous and induced inflammation in DeltaF508 cystic fibrosis mice. Respir Res. 2006;7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bosnar M, Bosnjak B, Cuzic S, et al. Azithromycin and clarithromycin inhibit lipopolysaccharide‐induced murine pulmonary neutrophilia mainly through effects on macrophage‐derived granulocyte‐macrophage colony‐stimulating factor and interleukin‐1beta. J Pharmacol Exp Ther. 2009;331(1):104‐113. [DOI] [PubMed] [Google Scholar]
- 92. Lendermon EA, Coon TA, Bednash JS, Weathington NM, McDyer JF, Mallampalli RK. Azithromycin decreases NALP3 mRNA stability in monocytes to limit inflammasome‐dependent inflammation. Respir Res. 2017;18(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Persson HL, Vainikka LK, Sege M, Wennerstrom U, Dam‐Larsen S, Persson J. Leaky lysosomes in lung transplant macrophages: azithromycin prevents oxidative damage. Respir Res. 2012;13:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kurdowska A, Noble JM, Griffith DE. The effect of azithromycin and clarithromycin on ex vivo interleukin‐8 (IL‐8) release from whole blood and IL‐8 production by human alveolar macrophages. J Antimicrob Chemother. 2001;47(6):867‐870. [DOI] [PubMed] [Google Scholar]
- 95. Sugiyama K, Shirai R, Mukae H, et al. Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells. Clin Exp Immunol. 2007;147(3):540‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Iwamoto S, Azuma E, Kumamoto T, et al. Efficacy of azithromycin in preventing lethal graft‐versus‐host disease. Clin Exp Immunol. 2013;171(3):338‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Iwamoto S, Kumamoto T, Azuma E, et al. The effect of azithromycin on the maturation and function of murine bone marrow‐derived dendritic cells. Clin Exp Immunol. 2011;166(3):385‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lin SJ, Yan DC, Lee WI, Kuo ML, Hsiao HS, Lee PY. Effect of azithromycin on natural killer cell function. Int Immunopharmacol. 2012;13(1):8‐14. [DOI] [PubMed] [Google Scholar]
- 99. Yamasawa H, Oshikawa K, Ohno S, Sugiyama Y. Macrolides inhibit epithelial cell‐mediated neutrophil survival by modulating granulocyte macrophage colony‐stimulating factor release. Am J Respir Cell Mol Biol. 2004;30(4):569‐575. [DOI] [PubMed] [Google Scholar]
- 100. Stamatiou R, Paraskeva E, Boukas K, Gourgoulianis KI, Molyvdas PA, Hatziefthimiou AA. Azithromycin has an antiproliferative and autophagic effect on airway smooth muscle cells. Eur Respir J. 2009;34(3):721‐730. [DOI] [PubMed] [Google Scholar]
- 101. Daenas C, Hatziefthimiou AA, Gourgoulianis KI, Molyvdas PA. Azithromycin has a direct relaxant effect on precontracted airway smooth muscle. Eur J Pharmacol. 2006;553(1–3):280‐287. [DOI] [PubMed] [Google Scholar]
- 102. Asgrimsson V, Gudjonsson T, Gudmundsson GH, Baldursson O. Novel effects of azithromycin on tight junction proteins in human airway epithelia. Antimicrob Agents Chemother. 2006;50(5):1805‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Imamura Y, Yanagihara K, Mizuta Y, et al. Azithromycin inhibits MUC5AC production induced by the Pseudomonas aeruginosa autoinducer N‐(3‐Oxododecanoyl) homoserine lactone in NCI‐H292 cells. Antimicrob Agents Chemother. 2004;48(9):3457‐3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ribeiro CM, Hurd H, Wu Y, et al. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well‐differentiated human airway epithelia. PLoS One. 2009;4(6):e5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shinkai M, Foster GH, Rubin BK. Macrolide antibiotics modulate ERK phosphorylation and IL‐8 and GM‐CSF production by human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L75‐L85. [DOI] [PubMed] [Google Scholar]
- 107. Wallwork B, Coman W, Mackay‐Sim A, Greiff L, Cervin A. A double‐blind, randomized, placebo‐controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. 2006;116(2):189‐193. [DOI] [PubMed] [Google Scholar]
- 108. Yamada T, Fujieda S, Mori S, Yamamoto H, Saito H. Macrolide treatment decreased the size of nasal polyps and IL‐8 levels in nasal lavage. Am J Rhinol. 2000;14(3):143‐148. [DOI] [PubMed] [Google Scholar]
- 109. Rapp RP. Pharmacokinetics and pharmacodynamics of intravenous and oral azithromycin: enhanced tissue activity and minimal drug interactions. Ann Pharmacother. 1998;32(7–8):785‐793. [DOI] [PubMed] [Google Scholar]
- 110. Serisier DJ. Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respir Med. 2013;1(3):262‐274. [DOI] [PubMed] [Google Scholar]
- 111. Parnham MJ, Erakovic Haber V, Giamarellos‐Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225‐245. [DOI] [PubMed] [Google Scholar]
- 112. Lucchi M, Damle B, Fang A, et al. Pharmacokinetics of azithromycin in serum, bronchial washings, alveolar macrophages and lung tissue following a single oral dose of extended or immediate release formulations of azithromycin. J Antimicrob Chemother. 2008;61(4):884‐891. [DOI] [PubMed] [Google Scholar]
- 113. Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID‐19. Clin Pharmacol Ther. 2020;108(2):201‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cigana C, Assael BM, Melotti P. Azithromycin selectively reduces tumor necrosis factor alpha levels in cystic fibrosis airway epithelial cells. Antimicrob Agents Chemother. 2007;51(3):975‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hua W, Liu H, Xia LX, et al. Rapamycin inhibition of eosinophil differentiation attenuates allergic airway inflammation in mice. Respirology. 2015;20(7):1055‐1065. [DOI] [PubMed] [Google Scholar]
- 116. Ratzinger F, Haslacher H, Poeppl W, et al. Azithromycin suppresses CD4(+) T‐cell activation by direct modulation of mTOR activity. Sci Rep. 2014;4:7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Morinaga Y, Yanagihara K, Miyashita N, et al. Azithromycin, clarithromycin and telithromycin inhibit MUC5AC induction by Chlamydophila pneumoniae in airway epithelial cells. Pulm Pharmacol Ther. 2009;22(6):580‐586. [DOI] [PubMed] [Google Scholar]
- 118. Cigana C, Nicolis E, Pasetto M, Assael BM, Melotti P. Anti‐inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. 2006;350(4):977‐982. [DOI] [PubMed] [Google Scholar]
- 119. Yamauchi K, Shibata Y, Kimura T, et al. Azithromycin suppresses interleukin‐12p40 expression in lipopolysaccharide and interferon‐gamma stimulated macrophages. Int J Biol Sci. 2009;5(7):667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hodge S, Hodge G, Jersmann H, et al. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(2):139‐148. [DOI] [PubMed] [Google Scholar]
- 121. Hodge S, Hodge G, Brozyna S, Jersmann H, Holmes M, Reynolds PN. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur Respir J. 2006;28(3):486‐495. [DOI] [PubMed] [Google Scholar]
- 122. Araki N, Yanagihara K, Morinaga Y, et al. Azithromycin inhibits nontypeable Haemophilus influenzae‐induced MUC5AC expression and secretion via inhibition of activator protein‐1 in human airway epithelial cells. Eur J Pharmacol. 2010;644(1–3):209‐214. [DOI] [PubMed] [Google Scholar]
- 123. Shimizu T, Shimizu S, Hattori R, Gabazza EC, Majima Y. In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells. Am J Respir Crit Care Med. 2003;168(5):581‐587. [DOI] [PubMed] [Google Scholar]
- 124. Shao MX, Nadel JA. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF‐alpha‐converting enzyme. J Immunol. 2005;175(6):4009‐4016. [DOI] [PubMed] [Google Scholar]
- 125. Park JA, He F, Martin LD, Li Y, Chorley BN, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from well‐differentiated human bronchial epithelial cells in vitro via a protein kinase C{delta}‐mediated mechanism. Am J Pathol. 2005;167(3):651‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Halldorsson S, Gudjonsson T, Gottfredsson M, Singh PK, Gudmundsson GH, Baldursson O. Azithromycin maintains airway epithelial integrity during Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 2010;42(1):62‐68. [DOI] [PubMed] [Google Scholar]
- 127. Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin‐1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44(2):275‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan. China Intens Care Med. 2020;46(5):846‐848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Liao M, Liu Y, Yuan J, et al. The landscape of lung bronchoalveolar immune cells in COVID‐19 revealed by single‐cell RNA sequencing. bioRxiv. 2020.
- 131. Zhou Y, Fu B, Zheng X, et al. Aberrant pathogenic GM‐CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv. 2020.
- 132. RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19—preliminary report. N Engl J Med. 2020;NEJMoa2021436. doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 133. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild‐to‐moderate Covid‐19. N Engl J Med. 2020;NEJMoa2019014. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hinks TSC, Barber VS, Black J, et al. A multi‐centre open‐label two‐arm randomised superiority clinical trial of azithromycin versus usual care in ambulatory COVID‐19: study protocol for the ATOMIC2 trial. Trials. 2020;21(1):718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hansen MP, Scott AM, McCullough A, et al. Adverse events in people taking macrolide antibiotics versus placebo for any indication. Cochrane Database Syst Rev. 2019;1:CD011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yanagihara K, Izumikawa K, Higa F, et al. Efficacy of azithromycin in the treatment of community‐acquired pneumonia, including patients with macrolide‐resistant Streptococcus pneumoniae infection. Intern Med. 2009;48(7):527‐535. [DOI] [PubMed] [Google Scholar]
- 137. Jakobsson HE, Jernberg C, Andersson AF, Sjolund‐Karlsson M, Jansson JK, Engstrand L. Short‐term antibiotic treatment has differing long‐term impacts on the human throat and gut microbiome. PLoS One. 2010;5(3):e9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Current clinical trials of azithromycin in SARS‐CoV‐2
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


