Abstract
Exosomes isolated from plasma of lung transplant recipients with allograft injury contain donor‐derived lung self‐antigens (collagen V and Kα1 tubulin) and human leukocyte antigen (HLA) molecules. We present a case of a 76‐year‐old, female lung transplant recipient treated for acute cellular rejection with methylprednisolone and anti‐thymocyte globulin, who subsequently contracted SARS‐CoV‐2 and developed a sharp increase in the mean fluorescent intensity of anti‐HLA antibodies. Analysis of circulating exosomes during rejection, but before SARS‐CoV‐2 infection, revealed the presence of lung self‐antigens and HLA class II molecules. After the patient contracted SARS‐CoV‐2, exosomes with the SARS‐CoV‐2 spike protein were also found. After resolution of infectious symptoms, exosomes with SARS‐CoV‐2 spike protein were no longer detected; however, exosomes with lung self‐antigens and HLA class II molecules persisted, which coincided with a progressive decline in spirometric flows, suggesting chronic lung allograft dysfunction. We propose that the analysis of circulating exosomes may be used to detect allograft injury mediated by both rejection and infection. Furthermore, the detection of exosomes containing viral proteins may be helpful in identifying allograft injury driven by viral pathogens.
Keywords: allograft dysfunction, COVID‐19, exosomes, lung self antigens, lung transplant, rejection
Abbreviations
- ACR
acute cellular rejection
- AMR
antibody‐mediated rejection
- ATG
anti‐thymocyte globulin
- BID
twice daily
- CARV
community‐acquired respiratory virus
- CLAD
chronic lung allograft dysfunction
- COVID‐19
coronavirus disease 2019
- CT
computed tomography
- DSA
donor‐specific antibody
- HLA
human leukocyte antigen
- MFI
mean fluorescent intensity
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
In addition to causing viral pneumonitis, community‐acquired respiratory virus (CARV) infections may be a risk factor for acute and chronic rejection, the latter manifesting as bronchiolitis obliterans syndrome or restrictive allograft syndrome, both of which fall under the umbrella term of chronic lung allograft dysfunction (CLAD). 1 Long‐term survival after lung transplant is limited by CLAD, and treatment options for patients with CLAD are limited. The role of exosomes in allorecognition and CLAD is increasingly recognized within the transplant community; however, their role in lung injury from acute rejection or CARV infection remains unknown. Exosomes are normally involved in important intercellular communication pathways within the body and carry a diverse cargo of nucleic acids, lipids, proteins, and other metabolites. 2 , 3 We have shown that circulating exosomes isolated from lung transplant recipients with CLAD contain lung self‐antigens and donor‐derived human leukocyte antigen (HLA) class I and class II molecules. 4 Circulating exosomes isolated from lung transplant recipients diagnosed with CARV infections also carry viral antigens. 5 In addition to being biomarkers of allograft injury, circulating exosomes may play a role in propagating allograft dysfunction. 5
We present a case of a lung transplant recipient with acute cellular rejection (ACR) requiring augmented immunosuppression with methylprednisolone and anti‐thymocyte globulin (ATG). The patient subsequently contracted severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) resulting in coronavirus disease 2019 (COVID‐19) and then developed a steep rise in anti‐HLA antibodies with a progressive decline in lung function, concerning for CLAD driven by antibody‐mediated rejection (AMR). We will describe her clinical presentation, course of illness, and exosome profile, first in the setting of ACR, later in the setting of COVID‐19, and then in the setting of AMR‐mediated CLAD.
2. CASE DESCRIPTION
2.1. Clinical course
The patient is a 76‐year‐old woman, 9 years post bilateral lung transplant for chronic obstructive lung disease, whose post‐transplant course was complicated by osteoporosis, chronic renal insufficiency, and recurrent diverticulitis. Her maintenance immunosuppression consisted of tacrolimus (trough 6‐8 ng/mL), mycophenolic acid 360 mg BID, and prednisone 10 mg daily. Her spirometric flows showed an FEV1 of 2.51 L (108% predicted) and FVC of 3.14 L (104% predicted). During an admission for diverticulitis (January 2, 2020‐January 8, 2020), the patient had computed tomography (CT) of the abdomen and pelvis, which identified tiny pulmonary nodules within the lung bases. She subsequently had CT of the chest, a bronchoscopy with trans‐bronchial biopsy and bronchoalveolar lavage, and a serologic screen for anti‐HLA antibodies. The transbronchial biopsy revealed ACR and lymphocytic bronchiolitis (A2B1R), and serology results were notable for de‐novo anti‐HLA antibodies (DQA1 03:02, 1428 mean fluorescent intensity [MFI]; DQA1 05:05, 1479 MFI; DQ 05:03, 909 MFI; DQA1 06:01, 1042 MFI). The donor's HLA profile was unavailable, so whether these anti‐HLA antibodies were donor‐specific could not be determined. The patient was thus treated with a 3‐day course of methylprednisolone; however, her FEV1 continued to decline (880 mL) from baseline, prompting admission and treatment with another 3‐day course of methylprednisolone, plasmapheresis, intravenous immunoglobulin, and a 3‐day course of ATG (February 26, 2020‐March 10, 2020; Figure 1). The patient tolerated the treatment well and was discharged home with a lymphocyte count of 0.1 × 103/µL.
FIGURE 1.
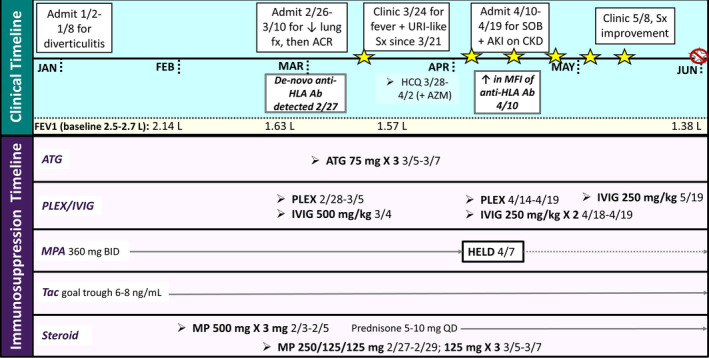
Clinical and immunosuppression timeline of casepatient. Stars represent positive SARS‐CoV‐2 nucleic acid testing. Ab, antibodies; ACR, acute cellular rejection; AKI, acute kidney injury; ATG, anti‐thymocyte globulin; AZM, azithromycin; BID, twice daily; CKD, chronic kidney disease; fx, function; HCQ, hydroxychloroquine; HLA, human leukocyte antigen; IVIG, intravenous immune globulin; MFI, mean fluorescent intensity; MP, methylprednisolone; PLEX, plasma exchange; Sx, symptoms; SOB, shortness of breath; Tac, tacrolimus; QD, daily; URI, upper respiratory infection
Eleven days after discharge (March 21, 2020), the patient reported malaise, lethargy, myalgias, diarrhea, and a temperature of 99.6 °F, but no respiratory symptoms. During a clinic visit on March 24, 2020, she had a chest X‐ray, a laboratory evaluation, and a nasal wash to screen for respiratory viruses, including SARS‐CoV‐2. Testing revealed SARS‐CoV‐2 and acute‐on‐chronic renal failure with a serum creatinine of 2.35 mg/dL (baseline 1.7 mg/dL), LDH of 306 Units/L, CRP of 8.1 mg/L, and a total white blood cell count of 3.7 × 103/µL with a lymphocyte count of 0.1 × 103/µL. Her chest X‐ray was unremarkable. She was started on a 5‐day course of hydroxychloroquine and continued azithromycin therapy. Seventeen days after symptom onset, she reported continued malaise, with new loss of smell and taste. Her oxygen saturation remained normal. Labs drawn during a follow‐up visit on April 7, 2020 revealed further declining renal function with a serum creatinine of 3.9 mg/dL, and the patient reported worsening shortness of breath, prompting hospital admission (April 10, 2020‐April 19, 2020).
Upon admission, CT of the chest revealed mild patchy ground glass opacities, and notable laboratory abnormalities included a D‐dimer of 2079 ng/mL, LDH of 266 Units/L, ferritin of 1178.04 ng/mL, CRP of 44.2 mg/L, and procalcitonin of 0.22 ng/mL. Supplemental oxygen was not required. Repeat nasal SARS‐CoV‐2 testing was positive. Anti‐HLA analysis revealed a marked rise in the previously detected anti‐HLA antibodies (DQA1 03:02, 6681 MFI; DQA1 05:05, 7632 MFI; DQ 05:03, 5029 MFI; DQA1 06:01, 4902 MFI), prompting a repeat course of plasmapheresis and intravenous immunoglobulin. The patient developed anti‐SARS‐CoV‐2 IgG; however, these antibodies were no longer detected after plasmapheresis. Her serum creatinine improved to baseline following receipt of intravenous fluids. Although the patient‐reported symptom improvement post‐discharge, SARS‐CoV‐2 continued to be detected by PCR from multiple nasal washings until June 3, 2020. Despite aggressive immunosuppression, the patient's lung function continued to decline even after the resolution of COVID‐19, consistent with the development of CLAD (bronchiolitis obliterans phenotype).
2.2. Exosome analysis
Circulating exosomes isolated from the patient's plasma samples dated January 10, 2020 and February 28, 2020 (when there was no evidence of SARS‐CoV‐2 infection) contained elevated levels of lung self‐antigens (collagen V and Kα1 tubulin; Figure 2), and the SARS‐CoV‐2 spike protein was not detected. However, after the patient developed COVID‐19 (April 10, 2020), there was a statistically significant increase in Kα1 tubulin (P < .02), and the SARS‐CoV‐2 spike protein appeared. This rise in Kα1 tubulin may have been triggered by viral pneumonitis, a heterologous immune response phenomenon.
FIGURE 2.
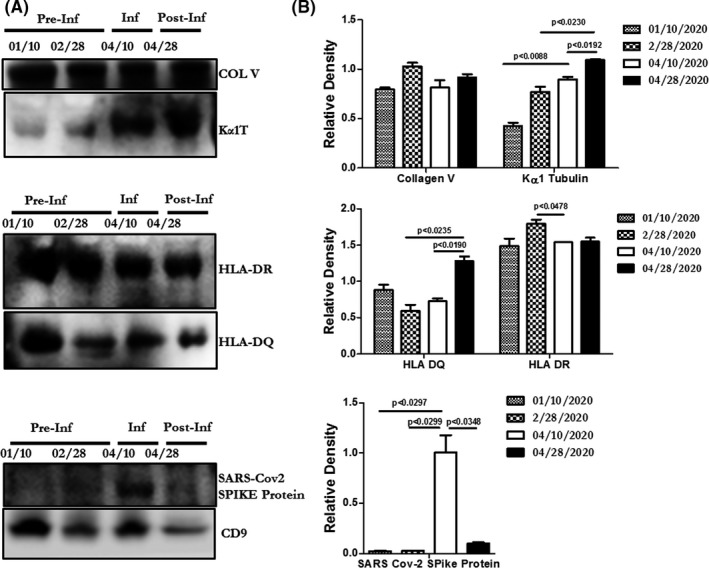
Exosome findings and densitometry analysis from patient plasma at select time points. A, Western blot of exosomes from patient plasma pre‐SARS‐CoV‐2 infection, during infection, and after symptom resolution. Exosomes carry lung self‐antigens (collagen V [COL V] and Kα1 tubulin [Kα1T]), HLA‐DQ and HLA‐DR, and SARS‐CoV‐2 spike protein. B, Densitometry analysis of exosomes from patient plasma for lung self‐antigens (COL V and Kα1 tubulin), HLA‐DQ and HLA‐DR, and SARS‐CoV‐2 spike protein. Circulating exosomes were isolated from 250 µL of plasma using the Total Exosome Isolation Kit (Invitrogen). Size distribution was determined using NanoSight (Malvern Panalytical); exosomes used in this report ranged in size from 50 to 200 nm. Exosomes were lysed and 20 µg proteins were resolved via polyacrylamide gel electrophoresis and transferred into a polyvinylidene difluoride membrane as previously described. 5 Antibodies used in the study are against Collagen V (COL V) (sc133162 anti‐mouse; Santa Cruz Biotechnology), Kα1 tubulin (sc8035 anti‐mouse), HLADQ (ab20173, anti‐mouse; Abcam Cambridge), HLA‐DR (ab136320, anti‐mouse; Abcam), SARS‐CoV‐2 spike protein (GTX 632604, anti‐mouse; Genetex) and CD9 (312112, anti‐mouse; BioLegend). CD9 was used as the internal control for exosomes and all the values were normalized with CD9. The blots were washed with PBS‐Tween (Thermo Fisher Scientific), developed using chemiluminescent horseradish peroxidase substrate (Amersham ECL Western Blotting Detection Kit; GE Healthcare UK, Buckinghamshire) and exposed using an Odyssey CLx Imaging System (LI‐COR Biosciences). Densitometry of gels was performed using ImageJ software (https://imagej.nih.gov/ij/) and statistical analysis was done using Prism software (Graph Pad). HLA, human leukocyte antigen; Inf, infection; Post‐Inf, post‐infection; Pre‐Inf, pre‐infection
Exosomes with lung self‐antigens and HLA‐DQ and HLA‐DR antigens were detected in January and February, at the time of known ACR. They persisted during SARS‐CoV‐2 infection in April and remained elevated when the MFI of anti‐HLA antibodies increased in April. The persistence of lung self‐antigens and HLA‐DQ and HLA‐DR antigens is suggestive of ongoing allograft injury and coincides with a progressive decline in lung function consistent with CLAD.
3. DISCUSSION
The emergence of SARS‐CoV‐2 and the resultant pandemic have changed the landscape of solid organ transplantation in terms of both donor allocation and risk to the recipient population. Our patient's clinical course has been particularly challenging as she developed ACR, which prompted aggressive immunosuppression with methylprednisolone and ATG. This may have increased her risk of contracting SARS‐CoV‐2 and delayed viral clearance. Despite her age and advanced degree of immunosuppression, symptoms of COVID‐19 were relatively mild and she never developed a cytokine storm, severe lung injury, or hypoxemia.
Notably, despite recent treatment with ATG and high‐dose corticosteroids, she developed a marked increase in the MFI of antibodies to HLAs in the weeks following SARS‐CoV‐2 infection. This type of anti‐HLA response to CARV was described in an abstract by Ainge‐Allen et al 6 prior to the emergence of SARS‐CoV‐2. In this single‐center retrospective analysis of 66 lung transplant recipients with CARV infection, 25 had detectable donor‐specific anti‐HLA antibodies (DSAs) after CARV infection, seven of whom developed de‐novo DSAs. Of these seven patients, two had HLA class I DSAs and six had HLA class II DSAs detected. Whether the development of de‐novo DSAs following CARV infection leads to AMR and allograft injury is yet to be determined.
Exosome‐mediated allorecognition pathways are known to help drive rejection following lung transplantation. For example, we have identified significantly increased levels of circulating exosomes with lung self‐antigens in lung transplant recipients with CLAD, but not in stable lung transplant recipients without CLAD. 4 These circulating exosomes contain not only lung self‐antigens, but also mismatched donor HLAs. The western blot of the patient's exosome content showed a rise in the lung self‐antigen Kα1 tubulin during COVID‐19 suggesting that SARS‐CoV‐2 drove immune responses to lung self‐antigens, a phenomenon known as a heterologous immune response. This immune response can lead to lung injury and further induction of circulating exosomes containing lung self‐antigens. Notably, donor‐derived HLA antigens (DQ and DR) were also detected in circulating exosomes before COVID‐19, during the infection, and after symptom resolution when the MFI of anti‐HLAs rose dramatically and spirometric flows continued to drop. This suggests ongoing allograft injury and coincides with the patient's development of CLAD. At this time, whether the exosomes are a biomarker of this patient's lung injury and/or they can propagate the injury is uncertain. However, we have demonstrated in mice that exosomes containing lung self‐antigens can be immunogenic, resulting in antibodies to lung self‐antigens leading to lung injury. 4
Collecting nasopharyngeal swabs for SARS‐CoV‐2 nucleic acid amplification testing is a guideline‐recommended method for COVID‐19 diagnosis in symptomatic patients; however, it is associated with an increased risk for false‐negative results compared to testing lower respiratory tract samples. 7 Although testing lower respiratory tract specimens may reduce the number of missed SARS‐CoV‐2 infections, bronchoalveolar lavage fluid is difficult to obtain from the most severely ill patients, and sample procurement poses an additional risk for SARS‐CoV‐2 aerosolization and transmission. Therefore, the ability to diagnose COVID‐19 via a peripheral blood draw would spare patients from bronchoscopy and protect healthcare workers from this higher‐risk procedure. We were able to identify the SARS‐CoV‐2 spike protein in circulating exosomes in our patient. Notably, we stopped detecting the circulating protein even though viral RNA continued to be isolated from a nasal swab. The discordance between the two results may be due to 1) resolution of viral pneumonitis and resultant resolution of SARS‐CoV‐2 spike protein‐containing exosomes, but persistent colonization of the nasopharynx from either viable or non‐viable virus or 2) reduced sensitivity of exosome analysis compared with amplification by PCR. Additional research is needed to further explore the role of exosome testing among patients with COVID‐19.
This case report has limitations. The patient's donor was never typed at DQA1, DPA1, or DPB1; therefore, we do not know whether anti‐HLAs are donor specific. In addition, we did not have access to plasma samples obtained while the patient was clinically well; therefore, it is possible that exosomes with lung self‐antigens and HLAs (DQ and DR) could have been in circulation even before lung injury.
We describe a case of COVID‐19 in a lung transplant recipient recently treated with methylprednisolone and ATG for ACR. Analysis of circulating exosomes revealed the presence of lung self‐antigens along with upregulation of donor‐derived HLA‐DR and HLA‐DQ antigens during ACR, COVID‐19, and AMR‐mediated CLAD. This suggests that these antigens, when identified in circulating exosomes, are markers of allograft injury. In addition, once the patient developed COVID‐19, the SARS‐CoV‐2 spike protein was detected in circulating exosomes, which coincided with a significant rise in Kα1 tubulin, suggesting a heterologous immune response with augmented lung injury. Further studies are needed to determine whether exosomes containing lung self‐antigens with and without viral antigens can help differentiate between infectious and immune‐mediated allograft injury. In addition, the detection of SARS‐CoV‐2 antigens within circulating exosomes may be an additional tool to aid in the diagnosis of COVID‐19.
DISCLOSURES
The authors of this manuscript have no disclosures to report.
AUTHOR CONTRIBUTIONS
Contributions of each author can be summarized as follows: KJG, TM, SB, AA, RW, and ST involved in the conceptual development; SB and MK involved in the exosome analysis; KJG, SB, AA, MDN, BB, HA, HM, AO, RW, TM, ST involved in writing and editing the paper.
Goodlet KJ, Bansal S, Arjuna A, et al. COVID‐19 in a lung transplant recipient: Exploring the diagnostic role of circulating exosomes and the clinical impact of advanced immunosuppression. Transpl Infect Dis.2021;23:e13480. 10.1111/tid.13480
REFERENCES
- 1. Mitchell AB, Glanville AR. Coronavirus and chronic lung allograft dysfunction: hiding in plain sight? Transplantation direct. 2018;4(8):e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654‐659. [DOI] [PubMed] [Google Scholar]
- 4. Gunasekaran M, Sharma M, Hachem R, Bremner R, Smith MA, Mohanakumar T. Circulating exosomes with distinct properties during chronic lung allograft rejection. J Immunol. 2018;200(8):2535‐2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunasekaran M, Bansal S, Ravichandran R, et al. Respiratory viral infection in lung transplantation induces exosomes that trigger chronic rejection. J Heart Lung Transplant. 2020;39(4):379‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ainge‐Allen HW, Benzimra M, Havryk AP, et al. The development of de novo donor specific antibodies following community acquired respiratory virus infection after lung transplantation: a novel association. J Heart Lung Transplant. 2014;32:S159‐160. [Google Scholar]
- 7. Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID‐19 Published 5/6/2020. Retrieved from https://www.idsociety.org/practice‐guideline/covid‐19‐guideline‐diagnostics/. Accessed July 13, 2020. [Google Scholar]


