Dear Editor,
Hidradenitis suppurativa (HS) is a chronic disabling inflammatory skin disease that, in moderate to severe cases, usually requires the use of systemic antibiotics, retinoids, and immunosuppressants/immunomodulants including adalimumab, the first‐approved anti‐tumor necrosis factor‐alpha (TNF)‐α agent. Nevertheless, the presence of autoimmune conditions, particularly systemic lupus erythematosus (SLE), may represent a contraindication to the use of TNF‐α inhibitors due to the risk of drug‐induced lupus. 1 Thus, the unique association of HS with SLE may require the off‐label use of immune‐targeted therapies that is cautiously monitored during the current COVID‐19 pandemic. We report one patient with HS and SLE who developed COVID‐19 infection during treatment with off‐label secukinumab associated with hydroxychloroquine. Since COVID‐19 infection was diagnosed, secukinumab was interrupted, and azithromycin was combined with hydroxychloroquine for 2 weeks. When COVID‐19‐related symptoms completely resolved, nasopharyngeal swab results were negative and secukinumab therapy was reintroduced after 8 weeks from the last administration.
A 28‐year‐old woman presented with SLE since the age of 13, with renal involvement (class III nephritis), myocarditis, and antiphospholipid antibody syndrome that was previously treated with hydroxychloroquine and salicylic acid. Medical history also included autoimmune thyroiditis, fibromyalgia, and osteoporosis. First manifestations of HS were reported at the age of 20 years. Frequent flares (>2 episodes/months) occurred, and minimal clinical and ultrasonographic improvements were obtained with previous HS‐specific treatments. At our observation (Fig. 1a), the patient reported a worsening of disease associated with a relevant disease burden (Hurley II; IHS4: 12; pain‐Numeric Rating Scale [NRS]: 7/10; itch‐NRS: 3/10; HidraDisk: 62; Dermatology Life Quality Index [DLQI]: 15). Concomitant SLE showed low‐disease activity with current maintenance therapy consisting of hydroxychloroquine (400 mg/day), oral steroids, acetylsalicylic acid, and pregabalin. Because adalimumab was considered contraindicated, off‐label subcutaneous secukinumab injections at the dosage of 300 mg at weeks 0, 1, 2, 3, and 4, followed by monthly maintenance dosing, were prescribed.
Figure 1.
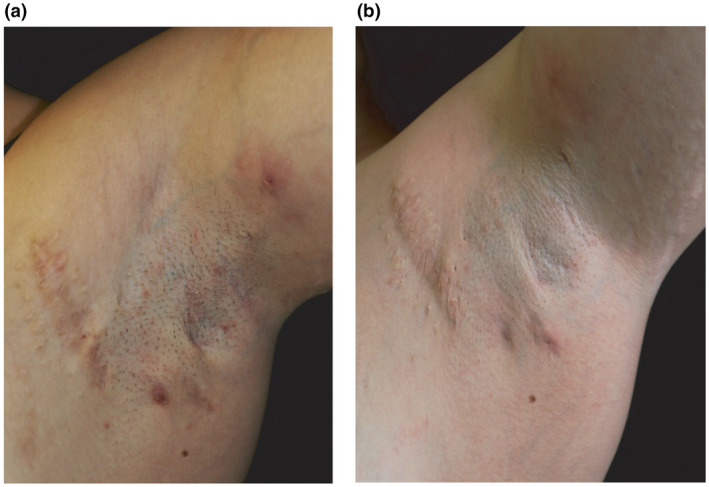
Clinical response to secukinumab therapy. Manifestations at the left axilla consisted of inflammatory HS nodules and fistulas prior to secukinumab therapy (a) and after 20‐week treatment (b).
After 20 weeks of secukinumab therapy (Fig. 1b), improvement of disease severity was observed (IHS4: 8, pain‐NRS: 0/10, and itch‐NRS: 3/10; HidraDisk: 13; DLQI:5; HiSCR: positive). After 24weeks of secukinumab therapy, the patient referred a flu‐like episode initially characterized by diarrhea and then followed by ageusia, anosmia, sinusitis, fever (body temperature >38 °C), asthenia, myalgia, and arthralgia, without cough or dyspnea. Nasopharyngeal swab was performed, as per COVID‐19 surveillance guidelines, resulting positive for SARS‐CoV‐2 on polymerase chain reaction (PCR) assay. Secukinumab was interrupted, and azithromycin was combined with hydroxychloroquine for 2 weeks. COVID‐19‐related symptoms completely resolved after 3 weeks with no worsening of HS manifestations. Two nasopharyngeal swabs with a 48‐hour interval were performed after complete resolution of COVID‐19 symptoms and resulted negative. Thus, secukinumab therapy was reintroduced after 8 weeks from the last administration.
Management of moderate to severe HS is challenging as no single drug is universally effective, thus requiring a personalized, patient‐by‐patient approach in most cases. We reported an uncommon association of HS and SLE treated with secukinumab therapy during the COVID‐19 pandemic. Previous publications reported clinical improvements obtained by IL‐17A inhibition in treating either HS or SLE. 2 , 3 Secukinumab was successfully tested in an open‐label trial at two different doses that showed the majority of HS patients achieving HiSCR, suggesting that secukinumab could be as effective as adalimumab. 3 Successful treatment of refractory lupus nephritis with secukinumab in a patient complicated with psoriasis vulgaris was described, highlighting that clinical benefits were associated with reduction of activated T helper 17 cells in peripheral blood and reduced infiltration of IL‐17‐positive lymphocytes in renal interstitium. 4 Thus, we considered secukinumab as a treatment option that resulted effective in treating HS. Notwithstanding the overall effectiveness on HS and no detrimental effects on SLE, secukinumab was interrupted when SARS‐Cov‐2 was diagnosed in accordance with established psoriasis treatment guidelines and the latest recommendations issued by national and international scientific societies on the management of COVID‐19 infection. Notably, our case developed SARS‐Cov‐2 infection during hydroxychloroquine therapy, which is under evaluation for the current management of the COVID‐19 infection. 5
Conflict of interest: Dr. Chiricozzi served as advisory board member and consultant, and has received fees and speaker's honoraria or has participated in clinical trials for Abbvie, Biogen, Fresenius Kabi, Leo Pharma, Lilly, Janssen, Novartis, Sanofi Genzyme, and UCB‐Pharma. Ketty Peris reports grants and personal fees for advisory Board meeting from Almirall, AbbVie, Biogen, Lilly, Celgene, Galderma, Leo Pharma, Novartis, Pierre Fabre, Sanofi, Sandoz, Sun Pharma, and Janssen, outside of the submitted work.
Funding source: None.
References
- 1. Almoallim H, Al‐Ghamdi Y, Almaghrabi H, et al. Anti‐tumor necrosis factor‐α induced systemic lupus erythematosus. Open Rheumatol J 2012; 6: 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prussick L, Rothstein B, Joshipura D, et al. Open‐label, investigator‐initiated, single‐site exploratory trial evaluating secukinumab, an anti‐interleukin‐17A monoclonal antibody, for patients with moderate‐to‐severe hidradenitis suppurativa. Br J Dermatol 2019; 181: 609–611. [DOI] [PubMed] [Google Scholar]
- 3. Casseres RG, Prussick L, Zancanaro P, et al. Secukinumab in the treatment of moderate to severe hidradenitis suppurativa: results of an open‐label trial. J Am Acad Dermatol 2020; 82: 1524–1526. [DOI] [PubMed] [Google Scholar]
- 4. Satoh Y, Nakano K, Yoshinari H, et al. A case of refractory lupus nephritis complicated by psoriasis vulgaris that was controlled with secukinumab. Lupus 2018; 27: 1202–1206. [DOI] [PubMed] [Google Scholar]
- 5. Gautret P, Lagier JC, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a six‐day follow up: a pilot observational study. Travel Med Infect Dis. 2020; 34: 101663. [DOI] [PMC free article] [PubMed] [Google Scholar]


