Summary
Severe acute respiratory syndrome coronavirus‐2 causes the clinical syndrome of coronavirus disease of 2019 (COVID‐19) which has become a global pandemic resulting in significant morbidity and mortality. While the virus primarily affects the respiratory system, it also causes a wide variety of complex cardiac manifestations such as acute myopericarditis, acute coronary syndrome, congested heart failure, cardiogenic shock and cardiac arrhythmias. There are numerous proposed mechanisms of cardiac injury, including direct cellular injury, pro‐inflammatory cytokine storm, myocardial oxygen‐demand mismatch, and systemic inflammation causing multi‐organ failure. Additionally, medications commonly used to treat COVID‐19 patients have various cardiovascular side effects. We aim to provide a succinct review about the pathophysiology and cardiac manifestations of COVID‐19, as well as treatment considerations and the various adaptations made to the current healthcare structure as a result of the pandemic.
Keywords: cardiovascular diseases, complications, COVID‐19
Abbreviations
- ACC
American College of Cardiology
- ACS
Acute coronary syndrome
- ACE 2
Angiotensin‐converting enzymes 2
- ACEIs
Angiotensin‐converting enzyme inhibitors
- ARBs
angiotensin II receptor blockers
- ARDS
acute respiratory distress syndrome
- AS
aortic stenosis
- ASD
atrial septal defect
- ASE
American Society of Echocardiography
- COVID‐19
coronavirus disease 2019
- CK‐MB
creatinine kinase myocardial band
- CRP
C‐reactive protein
- DMR
degenerative mitral regurgitation
- ECMO
extracorporeal membrane oxygenation
- FMR
functional mitral regurgitation
- G‐CSF
granulocyte‐colony stimulating factor
- GDMT
goal‐directed medical therapy
- HCQ
hydroxychloroquine
- HERG
Block Kv11.1
- ICU
intensive care unit
- LAAO
left atrial appendage closure
- MRI
magnetic resonance imaging
- NT‐proBNP
N‐terminal (NT)‐pro hormone BNP
- NSTEMI
non‐ST‐elevation cardiopulmonary resuscitation myocardial infarction
- PAPR
powered air purifying respirator
- PPE
personal protective equipment
- PFO
patent foramen ovale
- POCUS
focus point‐of‐care cardiac ultrasound
- RAS
renin‐angiotensin system
- SARS‐CoV‐2
severe acute respiratory coronavirus‐2
- SCAI
Society for Cardiovascular Angiography and Interventions
- STEMI
ST‐elevation myocardial infarction
- TAVR
trans‐catheter aortic valve replacement
- TEE
transesophageal echocardiogram
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) is the pathogen responsible for the clinical syndrome of coronavirus disease of 2019 (COVID‐19), a global pandemic that has affected over 26 million people worldwide as of 4 September 2020. 1 Even though the case fatality rate has remained low (estimated to be 1.4%) among young and healthy individuals, the rate increases significantly in patients age over 60 years and in those with pre‐existing medical comorbidities. 2 While the virus predominantly affects the respiratory tract, it can lead to a wide variety of cardiovascular manifestations. 2 , 3 , 4 , 5 , 6 Therefore, a thorough understanding of this complex interplay between SARS‐CoV‐2 and cardiovascular system is critical. We aim to provide a succinct overview of the cardiac implications of COVID‐19 with focused discussion on its pathophysiology, clinical manifestations, treatment considerations, and the various adaptations made to the current healthcare structure amid the pandemic.
2. PATHOGENETIC MECHANISMS OF CARDIAC INJURY
On a cellular level, the hallmark of COVID‐19 is a state of hyper‐inflammation mediated by the cytokine storm. Numerous key inflammatory markers have been found to be elevated in these patients with systemic illness, including IL‐6, IL‐2, IL‐7, TNF‐α, IFN‐γ, granulocyte‐colony stimulating factor (G‐CSF), C‐reactive protein (CRP), procalcitonin and ferritin. 2 Several mechanisms of cardiac injury have been proposed. First, it has been hypothesized that SARS‐CoV‐2 can cause direct myocardial injury by entering human cells via binding with the angiotensin‐converting enzymes 2 (ACE2) receptor on cell membrane. Subsequently, this can lead to acute myocardial injury by affecting the neurohumoral pathways of the cardiovascular system. 7 , 8 The virus may also increase myocardial metabolic demand in the setting of systemic infection and hypoxia, leading to oxygen supply‐demand mismatch and acute myocardial injury. Additionally, systemic inflammation caused by increased levels of pro‐inflammatory cytokines may also cause multi‐organ failure, including the heart. 9 , 10 Furthermore, severe illness caused by the virus can lead to significant electrolyte abnormalities, predisposing the patient to cardiac arrhythmia. 11 Increased coronary blood flow and systemic inflammation may also increase shear stress on the vascular endothelium, increasing the risk of plaque rupture and thrombosis, leading to acute myocardial infarction. 12 Finally, COVID‐19 is associated with a hypercoagulable state. Case reports have found positive anti‐cardiolipin IgA and anti–β2‐glycoprotein I IgA and IgG antibodies in patients tested positive for the infection who suffered multiple infarcts. 13 , 14 , 15
3. CARDIAC MARKERS AND PROGNOSIS
Elevation of cardiac markers are common among patients with COVID‐19, and several cardiac markers have been found to be helpful in predicting prognosis in these patients. A study conducted in Wuhan, China, including 273 COVID‐19 patients, found that increased serum levels of creatinine kinase myocardial band (CK‐MB), myosin, ultra‐TnI and N‐terminal (NT)‐pro hormone BNP (NT‐proBNP) correlated directly with increased disease severity and case‐fatality rate. 16 High‐sensitivity cardiac troponin (s‐cTn) level has also been found to be independently associated with mortality. In a cohort study of 191 patients with COVID‐19, the univariable odds ratio for mortality when s‐cTnI concentrations were above the 99th percentile upper reference limit was 80.1 (95% confidence interval [CI] 10.3–620.4, p < 0.0001). This odds ratio was higher than those of all other biomarkers tested, including D‐dimer. 9 Additionally, a separate study including 416 patients hospitalized for COVID‐19 found that patients with elevated troponin on presentation are more likely to require invasive or non‐invasive ventilation (22% vs. 4% and 46% vs. 4%), to develop acute respiratory distress syndrome (ARDS; 59% vs. 15%) or acute kidney injury (9% vs. 0%, p < 0.001 for all). The mortality was also 10‐fold higher in those with elevated markers indicating cardiac injury on presentation (51% vs. 5%), adjusted hazard ratio 3.41 (95% CI 1.62–7.16). 17 However, while the rise of cardiac enzymes has been associated with worse prognosis, it is worth noting that presence of cardiac markers is fairly non‐specific in COVID‐19 patients and is expected to be elevated in both non‐ischemic and ischemic myocardial injury. This has been identified as pathophysiologic basis of acute cardiac injury in COVID‐19 patients, with non‐ischemic injury (secondary to cytokine storm, stress cardiomyopathy, viral myocarditis, or hypoxia induced cardiac myocyte death) being the predominant mechanism. 18 Abnormal troponin, in particular, has been found in more than half of the patients diagnosed with COVID‐19. Thus, clinicians are only advised to measure troponin if acute myocardial infarction is suspected; abnormal troponin alone should not be considered evidence of acute myocardial infarction without other corroborating clinical evidence. 19
4. ACUTE MYOCARDITIS
Myocarditis/myopericarditis in COVID‐19 patients have been reported in case reports or case series. In a case series including 150 hospitalized patients in Wuhan, China, 7% of deaths were attributable to myocarditis and associated circulatory failure, while in 33% of these cases myocarditis were thought to play a role, if not directly causal for the patients' demise. 20 However, despite increasing number of case reports, the true prevalence of COVID‐19 myocarditis remains unknown. 21 Presenting symptoms are broad and non‐specific, including fatigue, chest discomfort, dyspnoea, heart failure as well as fulminant myocarditis with hemodynamic instability. 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 Patients are often found to have ST‐T wave changes on electrocardiogram or diffuse ST‐segment elevation mimicking ST‐elevation myocardial infarction (STEMI), as well as increased levels of cardiac enzymes, such as NT‐proBNP and high‐sensitivity troponin T. Echocardiogram may demonstrate left ventricular or biventricular systolic dysfunction, with or without regional wall motion abnormalities. Inversion recovery and T2‐mapping sequences on cardiac magnetic resonance imaging (MRI) can show marked biventricular myocardial interstitial edema, with diffuse late gadolinium enhancement involving the entire biventricular wall. 22 , 23 , 24 , 25 Autopsy studies have suggested pathological evidence of myocarditis in these patients. In the first autopsy series in the United States including four patients who expired from COVID‐19, cardiomegaly was found to be a salient feature. While sections of the myocardium did not show large area of necrosis, cardiac histopathology did show scattered individual cell myocyte necrosis in each heart examined. In rare areas, lymphocytes were found adjacent to the degenerating myocytes. The clinical significance of these pathologic findings is not immediately clear but may represent early manifestation of viral myocarditis. 34 Treatment regimen for patients with suspected acute myocarditis/pericarditis from COVID‐19 varied from case to case. Use of standard guideline‐directed heart failure regimen, inotropes, antiviral medications (lopinavir/ritonavir), steroids, chloroquine, or mechanical circulatory support devices have been reported. 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33
5. ACUTE CORONARY SYNDROME
Acute coronary syndrome (ACS) secondary to acute myocardial ischaemia must be distinguished from other forms of acute cardiac injury. Because cardiac manifestations of COVID‐19 are variable, mimickers of ACS like acute myocarditis/pericarditis, stress cardiomyopathy or coronary artery spasm can often add to the diagnostic dilemma. 7 The systemic inflammation and catecholamine surge caused by COVID‐19 also increases plaque vulnerability and rupture. 7 , 35 In a case series conducted by Bangalore et al., 18 patients with COVID‐19 with ST‐segment elevation on electrocardiogram indicating potential acute myocardial infarction were studied. 36 Among these patients, 10 had ST‐segment elevation on presentation, while the other 8 patients developed ST‐elevation during hospitalization. Nine patients underwent coronary angiography, of whom 6 had obstructive disease, and 5 underwent percutaneous coronary intervention (PCI). It is worth noting the high prevalence of non‐obstructive disease on coronary angiography, possibly secondary to coronary spasm, microthrombi, hypoxic injury, or direct endothelial injury. The prognosis among these patients were poor, with 13 patients having in‐hospital mortality. 36 There was also one case report of spontaneous coronary artery dissection in France in a patient with COVID‐19 presenting as ACS. 37 In light of the pandemic, cardiology societies of the United States and other countries have proposed guidelines regarding triage, management, and utilization of the cardiac catheterization laboratories, which will be explored further in the following sections.
6. CONGESTIVE HEART FAILURE AND CARDIOGENIC SHOCK
Development of new onset heart failure or cardiogenic shock have also been reported in patients with COVID‐19. In a cohort study including 191 hospitalized COVID‐19 patients in Wuhan, China, 44 (23%) developed new onset heart failure. 9 In a separate case series involving 21 patients admitted to the intensive care unit (ICU), cardiomyopathy developed in 7 (33%) of the patients, 38 and heart failure was more common among those who did not survive the hospitalization compared to those who survived (51.9% vs. 11.7%). Additionally, right‐sided heart failure can develop in the setting of concomitant lung disease as well as acute respiratory distress syndrome. 39 Cardiogenic shock secondary to COVID‐19 has been described in isolated case reports. For instance, there is a case report of a 68‐year‐old patient with confirmed COVID‐19 infection who presented with flu‐like illness then rapidly degenerated into ARDS and cardiogenic shock requiring venous‐arterial extracorporeal membrane oxygenation (ECMO) and mechanical ventilation. Endomyocardial biopsy in this patient demonstrated myocardial inflammation as well as viral particles. 40 Given that COVID‐19 patients commonly present with pneumonia and ARDS, it is sometimes difficult to differentiate pulmonary edema from cardiogenic shock from ground glass opacities as a result of ARDS. Clinical presentation, laboratory markers such as BNP, echocardiography, or right heart catheterization in selected cases to determine cardiac output and filling pressures may be helpful in distinguishing the two and guide clinical decision making. 41 , 42 Ascertaining whether the patient has a cardiogenic shock component is important, especially in selection of veno‐venous versus veno‐arterial ECMO cannulation for the critically ill patients. Regardless, the prognosis in these patients remain poor. In a case series of COVID‐19 patients with cardiogenic shock supported by ECMO, 83.3% (5/6) of the patients did not survive. 39 , 43 More data is needed regarding the role of ECMO in COVID‐19 patients.
7. CARDIAC ARRHYTHMIAS
Atrial and ventricular arrhythmia are common among patients with COVID‐19. In a study including 138 hospitalized patients with COVID‐19, cardiac arrhythmia was reported in 16.7% of hospitalized and 44.4% of ICU patients. 44 In a different study including 187 hospitalized patients with COVID‐19, ventricular tachycardia/ventricular fibrillation was found in 5.9% of patients (11/187). In addition, patients with elevated troponin levels developed more frequent malignant arrhythmias (6 patients [11.5%] vs. 7 patients [5.2%]), including ventricular tachycardia/ventricular fibrillation, compared to patients without elevated troponin. 45 Atrial and ventricular arrhythmia have also been reported in association with fulminant myocarditis with cardiogenic shock. 25 , 39 Furthermore, there had been anecdotal reports and experiences of patients developing cardiopulmonary arrest with pulseless electrical activity or ventricular fibrillation during recovery phase of acute pulmonary illness. Based on these findings, the Heart Rhythm Society, American College of Cardiology Electrophysiology Council and American Heart Association Electrocardiography and Arrhythmias Committee published a joint statement addressing the issues facing electrophysiologists during the pandemic. The statement recognized that improved understanding of this condition is important in guiding the need for additional arrhythmia monitoring, such as mobile cardiac telemetry post discharge and whether an implantable cardioverter defibrillator is warranted in those with impaired left ventricular systolic function secondary to COVID‐19. 46
8. IMPACT OF SOCIAL DISTANCING AND SELF‐QUARANTINE
Self‐quarantine and shelter‐at‐home orders in response to COVID‐19 pandemic have also played an adverse role in the cardiovascular outcomes of patients. Dramatic reduction in physical activity because of the shelter‐at‐home order is seen. The lock‐down has had effects not only on individuals who routinely performed recreational sports but also for individuals who commuted to work by walking or cycling. Fitbit data showed that the severity of decline in steps varied country by country. The United States had a 12% decline in steps count, and the European countries had decline in steps count ranging from 7% to 38%, during the week ending 22 March 2020. 47 The reduction in physical activity in turn can lead to development of insulin resistance, decreased muscle mass, bone loss, decreased aerobic capacity, worsening hypertension, and dyslipidaemia. Additionally, as weight gain has been reported during extensive leave‐periods in the past, it has been hypothesized as one of the adverse consequences of the current pandemic, driven by physical inactivity, unhealthy diet, and prolonged television viewing. Social isolation and depression can also amplify the burden on the cardiovascular system. 48 , 49 On a societal level, as most hospitals have suspended elective procedures and admissions, there has been a significant drop in access to emergency department (by 30% in Milan) and a up to 50% decrease in the incidence of non‐ST‐elevation myocardial infarction cases reported in some regions of the world. The emphasis on prevention and early recognition of chest pain symptoms might be lost in the pandemic, resulting in deleterious delay in care, and late presentations with more advanced disease process on arrival. 50 , 51 , 52
9. EFFECT ON THE RENIN‐ANGIOTENSIN SYSTEM
ACE2 plays an important role in the renin‐angiotensin system (RAS) and have been long established to play a critical role against heart failure with reduced ejection fraction, myocardial infarction, hypertension and diabetes. The ACE2 receptor is widely expressed in the human body, including the cardiovascular system, lungs, gastrointestinal tract, kidneys, central nervous system and adipose tissues. 53 More recently, controversy has arisen regarding the role of ACE2 receptor in patients infected with COVID‐19, as ACE2 also acts as the cellular receptor for SARS‐CoV‐2. Infection occurs when SARS‐CoV‐2 spike proteins bind to the ACE2 receptor. ACE2 receptors can be upregulated by the use of renin‐angiotensin‐aldosterone system inhibitors in different organs and tissues, including heart, kidney and aorta, in rodent models. 54 , 55 Therefore, it has been hypothesized that the use of angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers (ACEI/ARB) in COVID‐19 patients may lead to more severe infection and harmful effects. 5 , 56 , 57 , 58 However, this hypothesized harmful effect has not been corroborated in humans so far. In a retrospective, multi‐center study including 1128 adult hospitalized patients with hypertension diagnosed with COVID‐19 (188 patients taking ACEI/ARB and 940 patients without taking the medicine), all‐cause mortality was found to be lower in the ACEI/ARB group after adjusting for age, gender, co‐morbidities, and in‐hospital medications. 59 In a separate single‐center case series involving 1178 patients with COVID‐19, the percentage of severe and non‐severe infections (32.9% vs. 30.7%, p = 0.65), and the percentage of non‐survivors and survivors (23.7% vs. 33.0%, p = 0.34) did not differ significantly between the group of taking ACEI/ARB and those who were not on the medications, suggesting that ACEI/ARB are not associated with severity or mortality of COVID‐19 in such patients. 60 Thus, both the American and European Societies of Cardiology have published statements expressing that RAS inhibitors are safe and should be continued in patients with COVID‐19 infections according to established guidelines. 61 , 62
10. CATH LAB PROTOCOLS AND CONSIDERATIONS
Cardiac catheterization during the pandemic poses a unique challenge to healthcare professionals. Timely intervention must be balanced against adequate screening of patients for COVID‐19 and proper protection of healthcare personnel. Groups from different regions of the world have published protocols regarding the screening, triage, revascularization choice in patients with suspected or confirmed COVID‐19, as well as personnel protection, environmental engineering and administrative control measures. 63 , 64 , 65 Professional societies have provided guideline recommendations regarding patient selection for catheterization procedures as well as resource allocation and protection of healthcare workers (Figure 1). The guideline recommends that most elective procedures (such as coronary angiography for stable ischemic heart disease) should be deferred, though what is truly elective requires clinical judgment. While China has published a report on protocols using fibrinolytic therapy in patients with STEMI, 66 this remains a controversial subject in the United States, where PCI is the primary method of revascularization. Fibrinolysis can be considered in relatively stable STEMI patients with advanced COVID‐19. In patients with STEMI who requires PCI, proper personal protective equipment (PPE) (including gown, gloves, goggles, and N95 masks) must be worn. Powered air purifying respirator (PAPR) is reasonable in patients who require cardiopulmonary resuscitation (CPR), intubation or those who were vomiting. For most patients presenting with non‐ST‐elevation CPR myocardial infarction (NSTEMI), diagnostic testing for COVID‐19 should be performed prior to cardiac catheterization to allow a more informed decision regarding infection control. Unstable NSTEMI patients, however, should be considered under the STEMI umbrella. As for resource allocation, guideline recommends shift‐based allocation of staff/physicians, with separation of individuals with overlapping skillsets. Additionally, pulmonary artery catheter placement, pericardiocentesis, and intra‐aortic balloon pump insertion could be considered for bedside performance to limit infectious risk of transporting patient from wards to the catheterization lab. 67
FIGURE 1.
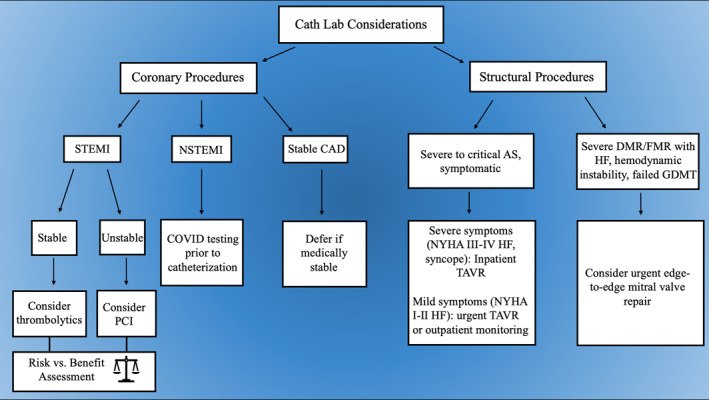
Summary of the major clinical decision branching points for triaging coronary and structural procedures during the coronavirus disease of 2019 pandemic. AS, aortic stenosis; CAD, coronary artery disease; DMR/FMR, degenerative/functional mitral regurgitation; GMDT, goal‐directed medical therapy; HF, heart failure; NSTEMI, non‐ST‐elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction; TAVR, transcatheter aortic valve replacement
11. REFERRAL FOR STRUCTURAL PROCEDURES
Challenges in caring for patients with structural heart disease in the usual fashion have ensued from the pandemic caused difficulties in effectively triaging these patients. As a result, the American College of Cardiology (ACC) and the Society for Cardiovascular Angiography and Interventions (SCAI) published a joint statement to provide a framework for triaging and intervention on patients with structural heart disease, especially those in need of trans‐catheter aortic valve replacement (TAVR) and percutaneous mitral valve repair (Figure 1). With regards to TAVR, the urgency of procedure is dictated by severity of symptoms associated with aortic stenosis (AS). The statement recommends that it is reasonable to schedule TAVR for inpatients with severe to critical symptomatic AS associated with class III or IV heart failure symptoms or syncope. For patients with minimally symptomatic (NYHA Class I–II CHF) severe‐to‐critical AS, it is reasonable to consider either urgent TAVR or close outpatient virtual monitoring by a valve coordinator. For patients with asymptomatic severe to critical AS, TAVR should be postponed until hospital operations return to normal. Additionally, the majority of TAVR procedures should be performed with moderate sedation, and most patients should not require ICU level care after the procedure. This is important in conserving critical care beds for the critically ill patients, especially in parts of the country most hard‐hit by the pandemic. With regards to mitral valve procedures, the committee determined that most edge‐to‐edge repair of mitral valve can be safely deferred. The procedure should be considered in inpatients with severe functional mitral regurgitation (FMR) or degenerative mitral regurgitation (DMR) who cannot be safely discharged despite goal‐directed medical therapy (GDMT) and those with congestive heart failure, outpatients with severe FMR or DMR with hospitalization within 30 days despite GDMT, or patients with severe DMR or FMR who are in low‐output, decompensated heart failure requiring ICU level care where edge to edge repair might improve the hemodynamics of the patient. On the other hand, transcatheter mitral valve in valve replacement and paravalvular leak closure procedures are resource‐intensive and should be deferred until the pandemic has resolved, provided that such patients can be safely managed with medical therapy in the meantime. Other commonly performed structural heart disease interventions, such as patent foramen ovale (PFO) closure, atrial septal defect (ASD) closure, left atrial appendage (LAA) closure and alcohol septal ablation for hypertrophic cardiomyopathy, are rarely urgent and should be deferred until the resolution of the pandemic when deemed medically safe. 68
12. ECHO LAB CONSIDERATIONS
In light of the COVID‐19 outbreak, the American Society of Echocardiography (ASE) has also published a statement to help address triaging and clinical decision making for COVID positive patients. On a bird's eye view, it is recommended that all echocardiographic (transthoracic echocardiograms, stress echocardiogram and transesophageal echocardiogram [TEE]) indications be reviewed to identify ‘non‐elective or urgent/emergent indications.’ All others should be deferred. Specifically, TEE carries a heightened risk for spread of SARS‐CoV‐2 because it provokes aerosolization of large amount of virus due to coughing or gagging during the procedure. Cautious consideration of benefit versus risks of exposure to healthcare personnel should be weighed for every TEE request. Alternative modalities (such as use of contrast enhanced computed tomography or MRI) should also be considered when appropriate, such as for evaluation of left atrial appendage thrombus prior to cardioversion. Similarly, treadmill or bicycle echocardiographic tests in patients with COVID‐19 also increase risk of exposure to healthcare personnel due to deep breathing and/or coughing during exercise, and therefore should generally be deferred. Alternatively, a pharmacologic stress echocardiogram could be performed if it will change clinical management. Location of the echocardiographic procedure should be carefully chosen. For example, imaging patients with suspected or confirmed COVID‐19 status in their isolation rooms might help prevent spread of disease to other parts of the hospital. For outpatient procedures, a separate room or separate machine maybe set aside for suspected or confirmed COVID‐19 patients. As for imaging protocols, a focus point‐of‐care cardiac ultrasound (POCUS) performed at bedside by clinicians who are already taking care of these patients might be effective for identifying important cardiac abnormalities and assessing cardiac contribution to overall disease process, all without exposing others or utilizing additional resources. POCUS images could also be saved and interpreted remotely by other practitioners if needed. Echocardiographic protocols should be as focused as possible to answer the clinical questions without need to return for further images, and scan time should be minimized by excluding students or novice practitioners from performing imaging. 69
13. TREATMENT CONSIDERATIONS
Many medications have been trialed in COVID‐19 positive patients with hope to improve outcomes. However, many of these medications have considerable cardiovascular side effects. Among these are the anti‐viral medications (ribavirin and lopinavir/ritonavir), whose potential cardiac side effects (with incidence >0.01%) include tachycardia, myocardial infarction, cardiomyopathy, arrhythmia, hypo‐ or hypertension, vasculitis, deep vein thrombosis, ischemic events, atrial ventricular block. Lopinavir/Ritonavir is also known to increase the concentration of anti‐arrhythmic medications such as amiodarone, dronedarone. Glucocorticoids have also been used to treat COVID‐19 positive patients. Glucocorticoids such as methylprednisolone can cause fluid retention, hypertension as well as electrolyte disturbances. Other medications studied, including biologics such as (eculizumab and tocilizumab) and immunosuppressive agents, have been reported to cause bradycardia, atrial ventricular block as well as hypertension. 50 Specifically, the use of hydroxychloroquine (HCQ) in COVID‐19 positive patients has garnered wide‐spread interest. HCQ is known to block Kv11.1 (HERG) and induce prolong QT, thus predisposing the patient to malignant arrhythmia such as torsade de points. 70 , 71 However, studies have found that these arrhythmic toxicities are mostly encountered in chronic use with multiple concomitant QT prolonging agents, metabolic abnormalities, renal insufficiency, as well as medication overdose. 72 , 73 Because the use of HCQ in COVID‐19 has relatively short duration, the risk of developing significant arrhythmia is relatively low. Special precautions should be considered, however, in patents with (1) known congenital long QT syndrome; (2) severe renal insufficiency; (3) concomitant QT prolonging medications; and (4) significant electrolyte imbalances (hypomagnesemia and hyper‐ or hypokalaemia). In patients with CrCl <10 mL/min, HCQ dose should be reduced by 50%, and any significant electrolyte abnormalities should be corrected prior to administration of the medication. Additionally, it is also reasonable to temporarily stop class III anti‐arrhythmic while the patient is on HCQ. Electrocardiogram monitoring should also be considered as an additional precautionary step. 46 , 74 Azithromycin, a macrolide and frequently used antibiotic, has pro‐arrhythmic properties, with epidemiologic studies estimating over 47 cardiovascular deaths presumed arrhythmic per 1 million completed courses. 75 , 76 The data evaluating the pro‐arrhythmic effect of chloroquine and azithromycin combination is limited. However, an in vivo study has not shown synergistic arrhythmic effect of azithromycin with or without chloroquine. 77 Cardiovascular side effect of the above‐mentioned medications are summarized in Table 1.
TABLE 1.
Summary of notable cardiovascular side effects of medications used to treat coronavirus disease of 2019
| Medications | Cardiovascular side effects |
|---|---|
| (A) Anti‐viral medications | |
| Ribavirin | Chest pain (5%–9%), flushing (3%–4%) |
| Lopinavir/Ritonavir | Vasodilatation (<3%), atrial fibrillation (<2%), second‐ and third‐degree AV block (<25), bradycardia (<2%) and deep vein thrombosis (<2%) |
| Remdesivir | Under‐investigation for coronavirus disease of 2019, safety and efficacy not yet established |
| (B) Glucocorticoids | |
| Methylprednisolone | Bradycardia, arrhythmias, heart failure, edema, hypertension, syncope, thromboembolism |
| (C) Anti‐malarial drugs | |
| Hydroxychloroquine | Cardiomyopathy, prolonged QT intervals, torsade de pointes, ventricular arrhythmias |
| (D) Macrolide antibiotics | |
| Azithromycin | Chest pain (<1%), palpitations (<1%), cardiac arrhythmias, QT prolongation, torsades de pointes, ventricular tachycardia |
| (E) Biological drugs | |
| Eculizumab, tocilizumab | Bradycardia, Atrioventricular block, hypertension |
Notes: Frequencies of the side effects, when known, are noted in the table. QT interval denotes the time period from the start of Q wave to the end of the T wave on an electrocardiogram.
14. CONCLUSION
In summary, the ongoing COVID‐19 pandemic poses an almost unprecedented challenge to the healthcare community. The infection can lead to a variety of complex cardiovascular complications and has caused significant morbidity and mortality to numerous patients. It is important for healthcare providers to be familiar with these cardiac manifestations, diagnostic criteria as well as management considerations in order to provide timely and adequate care to patients. However, much remains to be learned about this novel virus and its clinical presentations, as well as options in prevention and treatment. As the medical community gains more experience with COVID‐19, information and best‐practice experiences should be shared in a timely manner.
CONFLICT OF INTEREST
The authors declare no conflict of interest. No funding was received for this project.
AUTHORS CONTRIBUTIONS
Jing Liu: Conceived the idea of writing the manuscript with Umair Khalid, performed the literature search, created the table, and wrote the initial draft with co‐authors. Salim S. Virani: Helped in write up of first half of the initial draft with Jing Liu and Mahboob Alam. Mahboob Alam: Helped in write up of first half of the initial draft with Jing Liu and Salim S. Virani. Ali Denktas: Helped in write up of second‐half of the initial draft with Jing Liu and Ihab Hamzeh. Ihab Hamzeh: Helped in write up of second half of the initial draft with Jing Liu and Ali E. Denktas. Umair Khalid: Conceived the idea of writing the manuscript with Jing Liu, created the figure, helped with write up of initial draft and edited it further to the create the final draft of the manuscript that was submitted to RMV.
DATA AVAILABILITY STATEMENT
No data has been shared other than with the co‐authors or RMV editorial board.
References
References
- 1. Worldometer. COVID‐19 coronavirus pandemic; 2020. https://www.worldometers.info/coronavirus/#countries. Accessed September 4, 2020.
- 2. Akhmerov A, Marban E. COVID‐19 and the heart. Circ Res. 2020;126(10):1443–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID‐19. Circulation. 2020;141(23):1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrari R, Di Pasquale G, Rapezzi C. Commentary: what is the relationship between Covid‐19 and cardiovascular disease? Int J Cardiol. 2020;310:167–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hulot JS. COVID‐19 in patients with cardiovascular diseases. Arch Cardiovasc Dis. 2020;113(4):225‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long‐term implications. Eur Heart J. 2020;41(19):1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109(5):531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen D, Li X, Song Q, Hu C, Su F, Dai J. Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID‐19). medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bansal M. Cardiovascular disease and COVID‐19. Diabetes Metab Syndr. 2020;14(3):247‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with covid‐19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemostasis. 2020;(18):1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19: a Comment. J Thromb Haemostasis. 2020;18(8):2060–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han H, Xie L, Liu R, et al. Analysis of heart injury laboratory parameters in 273 COVID‐19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92(7):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mishra AK, Sahu KK, Lal A, Sargent J. Patterns of heart Injury in COVID ‐ 19 and relation to outcome. J Med Virol. 2020. 10.1002/jmv.25847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. James L, Januzzi J. Troponin and BNP use in COVID‐19. American College of cardiology; 2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/18/15/25/troponin-and-bnp-use-in-covid19. Updated March 18, 2020. Accessed April 30, 2020.
- 20. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID‐19‐related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463‐1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako‐Tsubo syndrome in a patient with SARS‐CoV‐2 respiratory infection. Eur Heart J. 2020;41(19):1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng JH, Liu YX, Yuan J, et al. First case of COVID‐19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020. 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paul JF, Charles P, Richaud C, Caussin C, Diakov C. Myocarditis revealing COVID‐19 infection in a young patient. Eur Heart J Cardiovasc Imaging. 2020;21(7):776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Besler MS, Arslan H. Acute myocarditis associated with COVID‐19 infection. Am J Emerg Med. 2020. 10.1016/j.ajem.2020.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rehman M, Gondal A, Rehman NU. Atypical manifestation of COVID‐19‐induced myocarditis. Cureus. 2020;12(6):e8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hussain H, Fadel A, Alwaeli H, Guardiola V. Coronavirus (COVID‐19) fulminant myopericarditis and acute respiratory distress syndrome (ARDS) in a middle‐aged male patient. Cureus. 2020;12(6):e8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coyle J, Igbinomwanhia E, Sanchez‐Nadales A, Danciu S, Chu C, Shah N. A recovered case of COVID‐19 myocarditis and ARDS treated with corticosteroids, tocilizumab, and experimental AT‐001. JACC Case Rep. 2020;2(9):1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonnet M, Craighero F, Harbaoui B. Acute myocarditis with ventricular noncompaction in a COVID‐19 patient. JACC Heart Fail. 2020;8(7):599‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID‐19: a cause of raised troponin and ECG changes. Lancet. 2020;395(10235):1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Irabien‐Ortiz A, Carreras‐Mora J, Sionis A, Pamies J, Montiel J, Tauron M. Fulminant myocarditis due to COVID‐19. Rev Esp Cardiol. 2020;73(6):503‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in covid‐19: the first autopsy series from new Orleans. medRxiv. 2020. 10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schoenhagen P, Tuzcu EM, Ellis SG. Plaque vulnerability, plaque rupture, and acute coronary syndromes: (multi)‐focal manifestation of a systemic disease process. Circulation. 2002;106(7):760‐762. [DOI] [PubMed] [Google Scholar]
- 36. Bangalore S, Sharma A, Slotwiner A, et al. ST‐segment elevation in patients with covid‐19 ‐ a case series. N Engl J Med. 2020;382:2478–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Courand P‐Y, Harbaoui B, Lantelme P. Spontaneous coronary artery dissection in a patient with COVID‐19. JACC Cardiovasc Interv. 2020;13(12):e107–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington state. J Am Med Assoc. 2020;323(16):1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID‐19) pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. J Am Med Assoc. 2012;307(23):2526‐2533. [DOI] [PubMed] [Google Scholar]
- 42. Karmpaliotis D, Kirtane AJ, Ruisi CP, et al. Diagnostic and prognostic utility of brain natriuretic Peptide in subjects admitted to the ICU with hypoxic respiratory failure due to noncardiogenic and cardiogenic pulmonary edema. Chest. 2007;131(4):964‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID‐19: the potential role of extracorporeal membrane oxygenation. J Am Med Assoc. 2020;323(13):1245–1246. [DOI] [PubMed] [Google Scholar]
- 44. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac Electrophysiology during the coronavirus (COVID‐19) pandemic from the heart Rhythm society COVID‐19 Task Force; Electrophysiology section of the American College of cardiology; and the Electrocardiography and arrhythmias committee of the Council on clinical cardiology, American heart association. Circulation. 2020;141(21):e823–e831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. The impact of coronavirus on global activity. Fitbit staff; 2020. https://blog.fitbit.com/covid-19-global-activity/. Accessed April 30, 2020.
- 48. Lippi G, Henry BM, Bovo C, Sanchis‐Gomar F. Health risks and potential remedies during prolonged lockdowns for coronavirus disease 2019 (COVID‐19). Diagnosis (Berl). 2020;7(2):85–90. [DOI] [PubMed] [Google Scholar]
- 49. Lippi G, Henry BM, Sanchis‐Gomar F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID‐19). Eur J Prev Cardiol. 2020;27(9):906–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gori T, Lelieveld J, Munzel T. Perspective: cardiovascular disease and the Covid‐19 pandemic. Basic Res Cardiol. 2020;115(3):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Villano A, Lanza GA, Crea F. Microvascular angina: prevalence, pathophysiology and therapy. J Cardiovasc Med. 2018;19(Suppl 1):e36‐e39. [DOI] [PubMed] [Google Scholar]
- 52. Naderi S. Microvascular coronary dysfunction‐an overview. Curr Atherosclerosis Rep. 2018;20(2):7. [DOI] [PubMed] [Google Scholar]
- 53. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system. Circ Res. 2020;126:1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ferrario CM, Varagic J. The ANG‐(1‐7)/ACE2/mas axis in the regulation of nephron function. Am J Physiol Ren Physiol. 2010;298(6):F1297‐F1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin‐(1‐7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289(3):H1013‐H1019. [DOI] [PubMed] [Google Scholar]
- 56. Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25(6):291‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rossi GP, Sanga V, Barton M. Potential harmful effects of discontinuing ACE‐inhibitors and ARBs in COVID‐19 patients. Elife. 2020;9:e57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA. Controversies of renin‐angiotensin system inhibition during the COVID‐19 pandemic. Nat Rev Nephrol. 2020;16:305–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bozkurt B, Kovacs R, Harrington B. HFSA/ACC/AHA statement addresses concerns Re: using RAAS antagonists in COVID‐19 Published. American College of Cardiology. 2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19. Updated March 17, 2020. Accessed April 30, 2020. [Google Scholar]
- 62. de Simone G. Position statement of the ESC Council on hypertension on ACE‐inhibitors and angiotensin receptor blockers. Eur Soc Cardiol. 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang. Accessed April 30, 2020. [Google Scholar]
- 63. Szerlip M, Anwaruddin S, Aronow HD, et al. Considerations for cardiac catheterization laboratory procedures during the COVID‐19 pandemic perspectives from the society for cardiovascular angiography and interventions emerging leader mentorship (SCAI ELM) members and graduates. Cathet Cardiovasc Interv. 2020;96(3):586–597. [DOI] [PubMed] [Google Scholar]
- 64. Han Y, Zeng H, Jiang H, et al. CSC expert consensus on principles of clinical management of patients with severe emergent cardiovascular diseases during the COVID‐19 epidemic. Circulation. 2020;141 e810–e816. [DOI] [PubMed] [Google Scholar]
- 65. Mahmud E, Dauerman HL, Welt FG, et al. Management of acute myocardial infarction during the COVID‐19 pandemic. Cathet Cardiovasc Interv. 2020;96(2):336–345. [DOI] [PubMed] [Google Scholar]
- 66. Zeng J, Huang J, Pan L. How to balance acute myocardial infarction and COVID‐19: the protocols from Sichuan Provincial People's Hospital. Intensive Care Med. 2020;46(6):1111–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Welt FGP, Shah PB, Aronow HD, et al. Catheterization laboratory considerations during the coronavirus (COVID‐19) pandemic: from ACC's interventional Council and SCAI. J Am Coll Cardiol. 2020;75(18):2372–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shah PB, Welt FGP, Mahmud E, et al. Triage considerations for patients referred for structural heart disease intervention during the coronavirus disease 2019 (COVID‐19) pandemic: an ACC/SCAI consensus statement. Catheter Cardiovasc Interv. 2020;96(3):659–663. [DOI] [PubMed] [Google Scholar]
- 69. Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. J Am Coll Cardiol. 2020;75(24):3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Traebert M, Dumotier B, Meister L, Hoffmann P, Dominguez‐Estevez M, Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 2004;484(1):41‐48. [DOI] [PubMed] [Google Scholar]
- 71. Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for drug interactions on QTc in exploratory COVID‐19 (coronavirus disease 2019) treatment. J Am Coll Cardiol. 2020;141:e906–e907. [DOI] [PubMed] [Google Scholar]
- 72. Demaziere J, Fourcade JM, Busseuil CT, Adeleine P, Meyer SM, Saissy JM. The hazards of chloroquine self prescription in west Africa. J Toxicol Clin Toxicol. 1995;33(4):369‐370. [DOI] [PubMed] [Google Scholar]
- 73. Cervera A, Espinosa G, Font J, Ingelmo M. Cardiac toxicity secondary to long term treatment with chloroquine. Ann Rheum Dis. 2001;60(3):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kapoor A, Pandurangi U, Arora V, et al. Cardiovascular risks of hydroxychloroquine in treatment and prophylaxis of COVID‐19 patients: a scientific statement from the Indian Heart Rhythm Society. Indian Pacing Electrophysiol J. 2020;20(3):117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. World Health Organization . The cardiotoxocity of antimalarials.World Health Organization; 2016. https://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report-session2.pdf. Updated April 30, 2020. Accessed. [Google Scholar]
- 77. Fossa AA, Wisialowski T, Duncan JN, Deng S, Dunne M. Azithromycin/chloroquine combination does not increase cardiac instability despite an increase in monophasic action potential duration in the anesthetized Guinea pig. Am J Trop Med Hyg. 2007;77(5):929‐938. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data has been shared other than with the co‐authors or RMV editorial board.


