Abstract
The emergence of novel coronavirus (SARS‐CoV‐2) in 2019 in China marked the third outbreak of a highly pathogenic coronavirus infecting humans. The novel coronavirus disease (COVID‐19) spread worldwide, becoming an emergency of major international concern. However, even after a decade of coronavirus research, there are still no licensed vaccines or therapeutic agents to treat the coronavirus infection. In this context, apitherapy presents as a promising source of pharmacological and nutraceutical agents for the treatment and/or prophylaxis of COVID‐19. For instance, several honeybee products, such as honey, pollen, propolis, royal jelly, beeswax, and bee venom, have shown potent antiviral activity against pathogens that cause severe respiratory syndromes, including those caused by human coronaviruses. In addition, the benefits of these natural products to the immune system are remarkable, and many of them are involved in the induction of antibody production, maturation of immune cells, and stimulation of the innate and adaptive immune responses. Thus, in the absence of specific antivirals against SARS‐CoV‐2, apitherapy could offer one hope toward mitigating some of the risks associated with COVID‐19.
Keywords: antiviral, bee venom, beeswax, COVID‐19, honey, honeybees, pollen, propolis, royal jelly
1. INTRODUCTION
Coronaviruses are large enveloped RNA viruses that cause a wide range of respiratory diseases in humans, including self‐limited upper airway infections and severe pneumonia. Seven types of coronavirus that infect humans, called human coronavirus (CoVh), have been identified. Of these, two are classified as alpha‐coronavirus (229E and NL63) and five as betacoronavirus (OC43, HKU1, SARS‐CoV, SARS‐CoV‐2, and MERS‐CoV) (Centers for Disease Control and Prevention, 2020; Lim, Ng, Tam, & Liu, 2016). Three outbreaks caused by these CoVh have been reported: the acute and severe respiratory syndrome (SARS‐CoV) started in 2002 in China; the Middle East respiratory syndrome (MERS‐CoV) started in 2012 in Saudi Arabia; and the COVID‐19 pandemic (SARS‐CoV‐2) which was first reported in December 2019 (Lim et al., 2016; Park, 2020). This most recent outbreak started as a set of pneumonia cases in the city of Wuhan (Hubei province, China), which were associated with a betacoronavirus named the novel coronavirus of 2019 (2019‐nCov) (Zhu et al., 2020a). Thereafter, the 2019‐nCov genome sequence revealed 89% similarity and 80% identity with SARS‐CoV, and the International Virus Taxonomy Committee renamed the 2019‐nCov as SARS‐CoV‐2 (Lee et al., 2014).
COVID‐19 is an infectious disease caused by SARS‐CoV‐2 that affects the lower respiratory tract and hematological system, in which its main clinical signs, such as fever, cough, and shortness of breath, are similar to other viral pneumonia (Deng et al., 2020; Zhai et al., 2020). The transmission of COVID‐19 occurs mainly from person to person through droplets produced by coughing or sneezing or through direct contact with an infected individual (Zhai et al., 2020). Several preventive measures, such as social distancing and lockdown of cities, have been adopted to control the COVID‐19 pandemic. However, these stringent measures have not been sufficient to control the high transmissibility of SARS‐CoV‐2 in some regions, which has become a public health threat worldwide (Park, 2020). SARS‐CoV‐2 has already infected more than 20 million people and caused approximately 800,000 deaths in virtually all countries around the world (Coronavirus Cases, 2020).
Due to the public health crisis created by COVID‐19, researchers have focused on the identification of drugs with therapeutic or prophylactic potential for the treatment and control of this viral infection. In this context, several studies have highlighted the advantages of the drug repurposing strategy, which aims to identify new uses for FDA‐approved drugs. For instance, some research centers have reported the promising therapeutic effects of hydroxychloroquine/chloroquine, remdesivir, lopinavir/ritonavir, ivermectin, and dexamethasone against severe cases of COVID‐19 (Ahn et al., 2020; Zhai et al., 2020). In addition, recent advances in immunotherapy have focused on mRNA SARS‐CoV‐2, and adenovirus type‐5 vectored vaccines with promising preliminary data of safety, tolerability, and immunogenicity against COVID‐19 (Ahn et al., 2020; Zhu et al., 2020b). However, to date, no specific antiviral agent or licensed vaccine is available to treat or prevent COVID‐19, and the control of the disease is still restricted to social distancing and isolation (Zhai et al., 2020).
Apitherapy is a promising source of pharmacological and nutraceutical agents for the treatment and prophylaxis of COVID‐19. Several honeybee products, such as honey, pollen, propolis, royal jelly, beeswax, and bee venom, have shown potent antiviral activity against pathogens that cause severe respiratory syndromes, including those caused by CoVh (Brown, Roberts, Cooper, & Jenkins, 2016; Hashem, 2020). In addition, the benefits of these natural products to the immune system are remarkable, and many of them are involved in the induction of antibody production, maturation of immune cells, and stimulation of the innate and adaptive immune responses (Babaei, Rahimi, Karimi, Tahmasebi, & Khaleghi Miran, 2016) (Figure 1). Therefore, in this mini‐review, we aim to present the therapeutic effects associated with several bee products that might be useful in the fight against the pandemic of the novel coronavirus (SARS‐CoV‐2).
FIGURE 1.
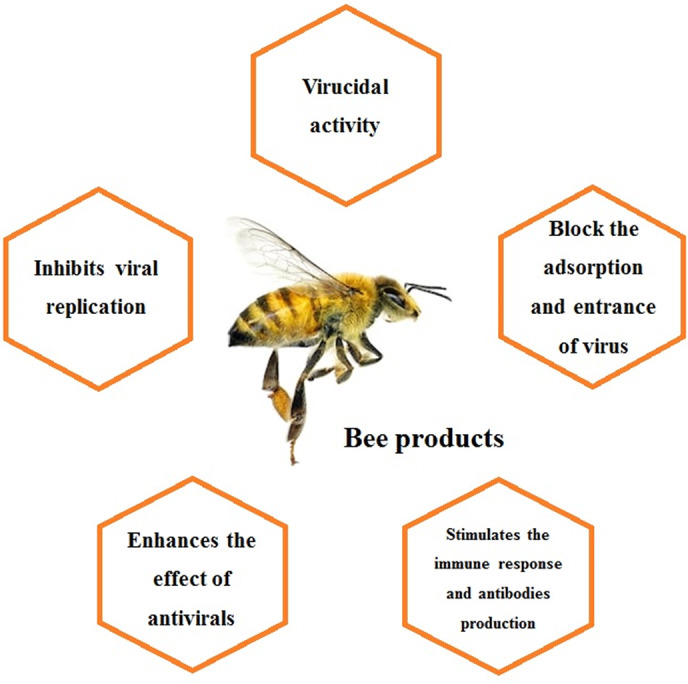
Schematic representation of the main effects of bee products that can be exploited against the novel coronavirus (SARS‐CoV‐2) [Colour figure can be viewed at wileyonlinelibrary.com]
1.1. Honey
Honey is a versatile and complex natural product formed from nectar, aphids excretion, or plant and fruit exudates. This honeybee product is composed of several bioactive chemicals that vary with the type of bee and environmental conditions (Meo, Al‐Asiri, Mahesar, & Ansari, 2017; Samarghandian, Farkhondeh, & Samini, 2017). The use of honey to treat microbial infections by ancient civilizations, such as Greeks, Chinese, Egyptians, Romans, Mayans, and Babylonians, dates back more than 5500 years (Meo et al., 2017). For instance, honey was used in traditional medicine to treat several viral respiratory diseases, such as bronchitis, throat infections, pneumonia, and flu (Samarghandian et al., 2017). Watanabe, Rahmasari, Matsunaga, Haruyama, and Kobayashi (2014) showed that the Manuka honey from Australia and New Zealand inhibited the viral replication of the influenza virus in vitro (IC50 3.6 ± 1.2 mg/mL) due to its virucidal effects and its synergistic interaction with the antivirals zanamivir or oseltamivir. Thus, this study corroborates to better understand the antiviral effect of honey against respiratory viruses (Watanabe et al., 2014).
The antiviral activity of some types of honey has been attributed to several properties, including its osmotic effect, naturally low pH, and the presence of several natural compounds (e.g., hydrogen peroxide, phenolic acids, flavonoids, and lysozyme) (Samarghandian et al., 2017). Flavonoids, such as quercetin and its derivatives (e.g., rutin, isorhamnetin, quercitrin, and isoquercetin), are often found in propolis and honey samples, and have presented antiviral activity against influenza virus (Choi, Song, & Kwon, 2011; Dayem, Choi, Kim, & Cho, 2015), human respiratory syncytial (Kaul, Middleton, & Ogra, 1985), human rhinovirus (Ganesan et al., 2012; Song, Park, Kwon, & Choi, 2013), human metapneumovirus (Komaravelli et al., 2015), parainfluenza (Kaul et al., 1985), and betacoronavirus (SARS‐CoV) (Yi et al., 2004). It is suggested that these compounds inhibit the 3‐chymotrypsin‐like cysteine protease (3C‐likepro) of hCoV (SARS‐CoV). This enzyme is one of the most promising targets for drug discovery against coronaviruses due to its critical role in the viral life cycle (Chen et al., 2006). However, the antiviral effect of honey against the novel coronavirus (SARS‐CoV‐2) has not been confirmed yet. A recent analysis of computational chemistry suggested the potential action of honey against SARS‐CoV‐2. Hashem (2020), through the molecular modeling approach, showed that six honeybee and propolis compounds (3‐phenyllactic acid, caffeic acid phenethyl ester [CAPE], lumichrome, galangin, chrysin, and caffeic acid) are potential inhibitors of SARS‐CoV‐2 3C‐likepro (Hashem, 2020).
Besides its promising antimicrobial activity, honey also stimulates the immune system and improves immunocompetence, which might reduce the severity of the novel coronavirus pneumonia. For instance, the addition of honey to drinking water (22 g/L ad libitum) increased the production of antibodies against the avian influenza virus H9N2, and reduced the heterophil/lymphocyte ratio in Japanese quails, confirming that honey improves the immune response against this virus (Babaei et al., 2016).
Due to its promising antiviral activity, clinical trials have been conducted to confirm the effect of honey against SARS‐CoV‐2. A phase 3 randomized, multicenter, and controlled study is analyzing the therapeutic benefits of honey supplementation (1 g/kg/day divided into 2–3 doses for consecutive 14 days) in patients with COVID‐19 (National Clinical Trial Number: NCT04323345) (Table 1). In another clinical trial, a group from Pakistan aims to evaluate the effectiveness of Nigella sativa/Black Cummins (1 mg orally) and honey (30 mL at 12/12 hours) in clearing the SARS‐CoV‐2, lowering the severity of COVID‐19 symptoms and decreasing the mortality rate (National Clinical Trial Number: NCT04347382). The results of these clinical trials, as well as other preclinical studies, will indicate the benefit of honey in the pharmacotherapy of COVID‐19 (Table 1).
TABLE 1.
Ongoing randomized, double‐blind clinical trials on the therapeutic and prophylactic use of bee products against patients with COVID‐19 patients
| NCT a number | Country | Number enrolled | Bee product | Intervention | Study phase | Study status | Category/setting |
|---|---|---|---|---|---|---|---|
| NCT04323345 | Egypt | 1000 | Honey | Supplementation of 1 mg/kg/day divided into 2–3 doses for 14 days with standard care b | III | Recruiting | Treatment/multicenter |
| NCT04347382 | Pakistan | 30 | Honey | 30 mL of honey orally twice a day for 14 days and Nigella sativa seed powder (1 mg) twice daily in capsule for a maximum of 14 days with standard care b | III | Recruiting | Treatment/single center |
| NCT04480593 | Brazil | 120 | Brazilian green Propolis extract | 400 or 800 mg/day orally or via nasoenteral tube with standard care b | II | Recruiting | Treatment/single center |
Note: The data were obtained from a manual search in clinicaltrial.gov.
NTC: National Clinical Trial.
Standard care: Includes standard symptomatic care along with use of antibacterial or antiviral (if advised by pulmonologist or infectious disease specialist). Current antibacterial or antiviral treatment including lopinavir/ritonavir tablets or arbidol or chloroquine phosphate or hydroxychloroquine or oseltamivir with or without azithromycin.
However, several clinical and preclinical studies (Table 2) have not indicated the honey origin and whether it is a unifloral, multifloral, or melate, which is a limitation of these studies. The antimicrobial activity of honey depends on its chemical composition, which varies depending on botanical sources, honeybee metabolism, and environmental, seasonal, and climatic conditions (De‐Melo, de Almeida‐Muradian, Sancho, & Pascual‐Maté, 2018). For instance, phenolic compounds are the main honey component associated with antiviral activity and present significant variations based on their botanic origin. These variations decisively affect the therapeutic property of honey. The presence of phenolic phytochemicals in honey can be used as a tool for classification and authentication, especially in the case of unifloral varieties (Cianciosi et al., 2018). Thus, the botanical and geographical origin of honey is essential factors to guarantee comparability between different studies and should be determined whenever possible.
TABLE 2.
Summary of the level of evidence currently available on the antiviral effect of bee products, with focus on activity against SARS‐CoV‐2
| Type of evidence | Honey | Propolis | Royal jelly | Beeswax | Pollen | Bee venom |
|---|---|---|---|---|---|---|
| Pre‐clinical (in vitro or in vivo) evidence of activity against SARS‐CoV‐2 | ||||||
| Clinical evidence of activity against SARS‐CoV‐2 | ||||||
| The clinical trial has been currently conducted against SARS‐CoV‐2 | ✔ | ✔ | ||||
| Pre‐clinical (in vitro or in vivo) evidence of antiviral activity (except against SARS‐CoV‐2) | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Clinical evidence of antiviral activity (except against SARS‐CoV‐2)—prophylaxis | ✔ | ✔ | ✔ | ✔ | ||
| Clinical evidence of antiviral activity (except against SARS‐CoV‐2)—Treatment | ✔ | ✔ |
1.2. Propolis
Propolis (bee glue) is a resinous substance produced by honeybees from substances collected from different parts of plants, such as leaves, flowers, buds, and exudates. Due to its properties, honeybees use propolis in the construction and adaptation of their hives (Anjum et al., 2019). In general, propolis is composed of approximately 50% resins, 30% waxes, 10% essential oils, 5% pollen, and 5% various organic compounds, including polyphenols, flavonoids, amino acids, minerals, ethanol, vitamin A, vitamin B complex, and vitamin E (Anjum et al., 2019; Siheri, Alenezi, Tusiimire, & Watson, 2017). Because propolis is commonly used as an antimicrobial agent against bacteria, viruses, and other pathogenic micro‐organisms that attack the hives (Anjum et al., 2019), several studies have aimed to investigate its activity against relevant human pathogens (Siheri et al., 2017). In this context, propolis showed promising antiviral effects in vitro and in vivo against influenza virus (Shimizu et al., 2008), human respiratory syncytial (Tomomi Takeshita et al., 2013), and human rhinovirus (Kwon, Shin, Perumalsamy, Wang, & Ahn, 2019).
The antiviral activity of propolis is associated with the presence of phenolic compounds (e.g., galangin, chrysin, p‐coumaric acid, kaempferol, and quercetin), which block or reduce the adsorption and entrance of the virus into the host cells (Kwon et al., 2019; Schnitzler et al., 2010). Since these are considered early steps of the viral cycle, the use of propolis may be more suitable for chemoprophylaxis. Furthermore, similar to honey, propolis is known to stimulate the adaptive immune response, which reinforces its prophylactic antiviral effect (Babaei et al., 2016).
However, preclinical studies should be conducted to evaluate the protective effect of propolis against lung infections caused by CoVhs, such as SARS‐CoV‐2 (Table 2). In addition, it is important to conduct randomized and controlled clinical trials to assess the therapeutic potential of propolis against COVID‐19. In this regard, currently, a randomized study of phase 2 has been conducted in Brazil to assess the impact of the use of Brazilian green propolis extract (400 or 800 mg/day orally or via nasoenteral tube) on oxygen therapy dependency time (in days) and hospitalization time (in days) of patients with COVID‐19 (National Clinical Trial Number: NCT04480593) (Table 1).
1.3. Royal jelly
Royal jelly is a hypo‐pharyngeal glandular white‐yellowish excretion, gelatinous–viscous sour taste and, a slight smell of phenol. It is produced by young worker honeybees and is used in the nutrition of larvae and adult queens. Royal jelly is an acid colloid (3.6–4.2 pH) composed of water (60%–70%), sugar (12%–15%), proteins (12%), lipids (5%–6%), and low amounts of vitamins and mineral salts (Fratini, Cilia, Mancini, & Felicioli, 2016a).
Some studies have shown that raw or purified royal jelly has notable antimicrobial activity. The use of royal jelly alone or in combination with propolis reduced the in vitro viral load of cells infected with the influenza virus A2 (Filipic & Likar, 1976a). In addition, clinical evidence shows that the combination of royal jelly with other bee products prevents infection during influenza outbreaks. For instance, the frequency of flu was significantly lower (3.7% incidence) among patients who used an apicomplex composed of honey, royal jelly (2%), pollen (3%), and propolis (1%) in comparison with untreated patients (38% incidence) (Filipic & Likar, 1976aa). However, the authors do not make clear the therapeutic scheme employed (route of administration, frequency, and time of the treatment). It limits the understanding of the impact of royal jelly in combination with other bee products on the prophylaxis of viral respiratory diseases. Moreover, this is the only clinical study on the benefits of royal jelly in the prophylaxis of viral diseases of the airways, which evidences the neglect of this subject in the biomedical literature (Table 2).
Besides the antiviral effect exhibited by royal jelly, it can also be used as a prophylactic agent due to its benefits to the immune tone. A study with mice revealed a higher production of antibodies and immunocompetent cell proliferation among animals that received the royal jelly supplementation (0.1 mL of royal jelly s.c. by 7 days) (Šver et al., 1996). These results highlight the therapeutic and prophylactic potential of royal jelly and its components against human respiratory syndromes, such as the causative agent of the current pandemic, that is, SARS‐CoV‐2.
Importantly, some peptides isolated from royal jellies, such as jelleines (Jelleine I–IV), are potent antibacterial and antifungal agents, which could be useful to avoid co‐infections in patients with COVID‐19. A systematic review that evaluated the burden of co‐infections in patients with confirmed SARS‐CoV‐2 infection indicated that overall, 7% of hospitalized patients with COVID‐19 experienced a bacterial co‐infection, increasing to 14% in studies that only included ICU patients (Lansbury, Lim, Baskaran, & Lim, 2020). The specie of bacteria most commonly associated with COVID‐19 is Mycoplasma pneumoniae, followed by Pseudomonas aeruginosa, Haemophilus influenzae, and Klebsiella pneumoniae. Furthermore, some fungal pathogens, such as Candida albicans, Aspergillus flavus, Aspergillus fumigatus, and Candida glabrata, have also been identified in patients infected with SARS‐CoV‐2 (Lansbury et al., 2020). In this regard, jelleines has shown high activity against K. pneumoniae (Jelleine‐I: 10 μg/mL; Jelleine‐II: 15 μg/mL), P. aeruginosa (Jelleine‐I: 10 μg/mL; Jelleine‐II: 15 μg/mL; Jelleine‐II: 30 μg/mL), and C. albicans (Jelleine‐I: 2.5 μg/mL; Jelleine‐II: 2.5 μg/mL) in vitro (Fontana et al., 2004). As shown by Lansbury et al. (2020), those are species frequently recovered from patients hospitalized with COVID‐19, thus suggesting the benefits of royal jelly in preventing co‐infections.
1.4. Beeswax
Beeswax is a complex lipid‐based organic compound secreted in a liquid form by specialized wax glands of younger worker bees. It is formed in scales and solidifies in contact with air (Fratini, Cilia, Turchi, & Felicioli, 2016b; Svečnjak et al., 2019). Beeswax is a mixture of more than 300 compounds, including fatty acid esters (~67%), hydrocarbons (12%–16%), free fatty acids (12%–14%), fatty alcohol (~1%), diesters, and exogenous substances, such as residues of propolis, pollen, and small pieces of floral component factors (Fratini, Cilia, Mancini, & Felicioli, 2016a).
In recent years, the effect of the crude extract of beeswax and its methanol, acetone, and ethanol extracts against pathogenic bacteria and fungi have been studied (Fratini, Cilia, Turchi, & Felicioli, 2016b). Regarding its antiviral activity, only one study has been identified. Mohamed, Hassan, Hammad, Amer, and Riad (2015) showed the antiviral effect of four beeswax extracts (i.e., ethanol white beeswax (EWBW), ethanol black beeswax (EBBW), acetone white beeswax (AWBW), and acetone black beeswax (ABBW)) against DNA (adenovirus‐7) and RNA (Rift Valley fever virus) viruses (Mohamed et al., 2015). In this study, the treatment of Vero cells infected by human adenovirus serotype 7 (adenovirus‐7) with ABBW and EBBW (100 μg/mL) reduced the viral load in 55.33% and 16.50% compared to untreated cells, respectively. On the other hand, the effect of beeswax against Rift Valley fever virus (RVFV) was less pronounced. All extracts decreased the viral title by less than 10% (ABBW and EWBW: 5.16% and AWBW and EBBW: 7.69%) when compared to infected cells (Mohamed et al., 2015). These results show that beeswax acts mainly against DNA viruses, and its antiviral effect is dependent on the conditions of extraction employed and botanical origin of beewaxs. SARS‐CoV‐2, similar to RVFV, is an enveloped and single‐stranded RNA virus that can be sensitive to the antiviral effects of beeswax extracts. However, no preclinical evidence has been found to support this hypothesis since the antiviral effect of beeswax has been poorly explored (Table 2). We encourage more studies to assess the potential of beeswax supplementation as a strategy to fight viral infections, especially those caused by respiratory viruses such as influenza virus and SARS‐CoV‐2.
1.5. Pollen
Pollen is a fine powder produced by flowering plants and collected by bees. When mixed with nectar (and/or honey) and salivary substances, it is used as the main food reserve for the hive (Denisow & Denisow‐Pietrzyk, 2016; Thakur & Nanda, 2020). Bee pollen is composed of proteins (5%–60%), essential amino acids, reducing sugars (13%–55%), lipids (4%–7%), nucleic acids (especially RNA), crude fiber (0.3%–20%), and minor components, such as minerals (Ca, Mg, Fe, Zn, Cu, K, and Na), vitamins (provitamin A [β‐carotene], vitamin E [tocopherol], niacin, thiamine, biotin, and folic acid), enzymes, phytosterols (β‐sitosterol, P‐sitosterol, and terpenes), flavonoids (catechins, kaempferol, quercetin, and isorhamnetin), and organic carotenoid pigments (lycopene and zeaxanthin) (Thakur & Nanda, 2020).
Bee pollen has been used in chemoprophylaxis of several infectious diseases as it has been shown to enhance the immunity of birds (Babaei et al., 2016), mice (Küpeli Akkol, Orhan, Gürubüz, & Yesilada, 2010), and humans (Filipic & Likar, 1976b). Although some studies have reported the antibacterial activity of extracts obtained from bee pollen against Gram‐positive and Gram‐negative bacteria, none of them has revealed its antiviral properties (Fatrcová‐Šramková et al., 2013; Mărgăoan et al., 2015).
However, many of the compounds present in bee pollen, especially polyphenols, have promising activity against CoVhs, such as SARS‐CoV and MERS (Chen et al., 2006; Yi et al., 2004). Eight different types of phenolic compounds have been identified in bee pollen by high‐performance liquid chromatography analysis. These compounds can be divided into two groups: flavonoids, represented by flavonols, flavones, flavanols, flavanones, anthocyanidin, chalcones, and isoflavones; and non‐flavonoids compounds, such as phenolic acids (Denisow & Denisow‐Pietrzyk, 2016; Thakur & Nanda, 2020). The phenolic phytochemicals with higher concentrations in bee pollen are quercetin and kaempherol, as well as their glycosides derivates (Rzepecka‐Stojko et al., 2015). According to Yi et al. (2004), quercetin inhibited the entry of SARS‐CoV into Vero E6 cells in vitro. The concentration required to inhibit 50% of SARS‐CoV (EC50) was 83.4 μM, which is a relatively low value considering the high concentration of this compound in the bee pollen (~60 μmol/g of quercetin in pollen of Apis mellifera L.) (Carpes et al., 2013). Additionally, quercetin‐3β‐galactoside, a glycoside derived from quercetin frequently found in bee pollen (Rzepecka‐Stojko et al., 2015), binds to SARS‐CoV 3CLpro and inhibits its proteolytic activity with an IC50 of 42.79 ± 4.95 μM. This inhibitory effect of SARS‐CoV 3CLpro depends on the hydroxyl group of quercetin, which forms a hydrogen bond with the Gln189 residue present on the active site of 3CLpro (Chen et al., 2006). Because SARS‐CoV‐2 3CLpro maintains the same residue Gln189 in its active site, it is suggested that the main protease of the novel coronavirus may also be sensitive to the inhibitory action of quercetin and its derivatives. Importantly, a recent computational study revealed that quercetin is predicted to form a hydrogen bond with Gln186 on active site of SARS‐CoV‐2 3CLpro, which results in low binding energy (−7.5 kcal/mol) and consequent high binding affinity (3.2 × 105 M−1) (Rehman, AlAjmi, & Hussain, 2020).
Kaempferol and its glycoside analogs inhibit the 3a channel protein of coronavirus. The 3a protein forms a cation‐selective channel that becomes expressed in the infected cell and is involved in the mechanism of virus release. Drugs that inhibit the ion channel can, therefore, inhibit virus release, becoming a source for the development of novel therapeutic antiviral agents against SARS‐CoV‐2 (Brockway & Denison, 2004; Li et al., 2020). Schwarz et al. (2014) showed that in oocytes from Xenopus laevis (African clawed frog) with expressed SARS‐CoV 3a protein, Ba2+‐sensitive current is weakly inhibited by Kaempeferol at 40 μM. In contrast to aglicone, the tested kaempferol glycosides hardly affected Ba2+‐sensitive endogenous current. Juglanin is the most potent kaempferol glycoside against SARS‐CoV 3a protein (IC50 2.3 μM), followed by tiliroside, afzelin, and kaempferol‐3‐O‐α‐rhamnopyranosyl (1 → 2)[α‐rhamnopyranosyl(1 → 6)]‐β‐glucopyranoside (Schwarz et al., 2014). Moreover, a molecular docking study revealed that Kaempferol might also act on SARS‐CoV‐2 3CLpro, showing binding energy and binding affinity of −7.8 kcal/mol and 5.2 × 105 M−1, respectively. It forms two hydrogen bonds, in which the one with the Gln189 residue described previously is critical to the catalytic activity of 3CLpro (Rehman et al., 2020).
Furthermore, quercetin and kaempferol exhibit inhibitory effects on inflammatory (IL‐6, IL‐8, IFN‐γ, iNOS, COX‐2, NF‐κB, and TNF‐α) (Pan, Lai, & Ho, 2010) and oxidative stress (LOX‐1, RNS, and ROS) (Banjarnahor & Artanti, 2014) mediators. It suggests that they could also prevent immunologic complications associated with COVID‐19, such as the macrophage activation syndrome (also known as secondary hemophagocytic lymphohistiocytosis), which results in a potentially fatal “cytokine storm” and acute respiratory distress syndrome (Biancatelli, Berrill, Catravas, & Marik, 2020). Thus, the anti‐inflammatory and antiviral effect of pollen is extremely relevant since when immunologic complications like cytokine storm occur, the antiviral treatment alone is not enough and should be combined with appropriate anti‐inflammatory treatment (Biancatelli et al., 2020; Zhai et al., 2020). Therefore, the bee pollen, which is the bee product with high amounts of quercetin, kaempferol, and its derivates (Rzepecka‐Stojko et al., 2015), can be a promising alternative to fight against COVID‐19 (Table 2).
1.6. Bee venom
Bee venom is produced by female worker bees and is composed of several active components, including enzymes (e.g., phospholipase A2 and hyaluronidase), peptides (e.g., melittin, apamin, mast cell degranulating peptide, and adolapin), amino acids, phospholipids, sugars, biogenic amines, volatile compounds, pheromones, and water (>80%) (Wehbe et al., 2019). At high concentrations, bee venom can induce pain, inflammation, and allergic reactions in humans. Furthermore, it can cause an excessive stress response of the immune system, which often leads to death. On the other hand, low concentrations (up to 5 μg/mL) of this bee product has shown several pharmacological effects in animal models, such as anti‐inflammatory, nociceptive, antimicrobial, and antitumoral effects (Memariani et al., 2019; Perumal Samy, Stiles, Franco, Sethi, & Lim, 2017). Interestingly, the co‐incubation of noncytotoxic concentrations of bee venom or melittin, the main component of bee venom, significantly inhibited the replication of the Influenza A virus (PR8) and the respiratory syncytial virus (RSV) (Uddin et al., 2016). In addition, bee venom significantly enhanced the differentiation of FOXP3‐expressing cells in CD4 T cells and mature CD4 thymocytes. It suggests that bee venom stimulates the differentiation of human regulatory T cells, which plays an important role in the immune response against SARS‐CoV infection (Caramalho et al., 2015).
Thus, future in vitro and in vivo studies may elucidate whether the bee venom and its components can be used as an antiviral agent against SARS‐CoV‐2 or as an immune system activator against COVID‐19 (Table 2). In addition, the toxicity of melittin can be reduced by chemical strategies, such as conjugation with polyethylene glycol (PEGylation), truncation, and synthesis of the peptide with dextrogenous (d‐)amino acids (Memariani et al., 2019). These approaches would minimize the toxicity of bee venom and allow the use of higher concentrations of this bee product against viral infections.
2. CONCLUSION
Bee products are known for their several medicinal and pharmaceutical properties, which have been explored since the beginning of society. Of these, the antiviral effect and the ability to stimulate the immune system using bee products stand out as potentially promising alternative in the therapy of severe viral respiratory infections, such as COVID‐19. Moreover, except for bee venom, apicomplexes are generally well tolerated and readily available, which can contribute to wide access in times of pandemic. The immunomodulatory, antiviral, anti‐inflammatory, antioxidant, and pro‐resolving effects of different bee products and their chemical components can be useful in prophylaxis, specific treatment, and even symptomatic treatment of COVID‐19. However, the number of current evidence is overwhelming, and large randomized and controlled clinical trials should be conducted to assess the real benefit of apitherapy against SARS‐CoV‐2. In this direction, here, we present the numerous possibilities of apitherapy in combating COVID‐19 and highlight the importance of bee products as a promising source of therapeutic and prophylactic strategies against this emergent public health crisis.
CONFLICT OF INTEREST
The authors declare that they have no financial interests or personal relationships that may appear to influence the work reported in this article.
ACKNOWLEDGMENTS
William G. Lima is grateful to Coordenação de Aperfeiçoamento de Pessoal do Nível Superior (CAPES) for a PhD fellowship.
Lima WG, Brito JCM, da Cruz Nizer WS. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID‐19 (SARS‐CoV‐2). Phytotherapy Research. 2021;35:743–750. 10.1002/ptr.6872
REFERENCES
- Ahn, D. G. , Shin, H. J. , Kim, M. H. , Lee, S. , Kim, H. S. , Myoung, J. , … Kim, S. J. (2020). Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID‐19). Journal of Microbiology and Biotechnology, 30(3), 313–324. 10.4014/jmb.2003.03011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum, S. I. , Ullah, A. , Khan, K. A. , Attaullah, M. , Khan, H. , Ali, H. , … Dash, C. K. (2019). Composition and functional properties of propolis (bee glue): A review. Saudi Journal of Biological Sciences, 26(7), 1695–1703. 10.1016/j.sjbs.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei, S. , Rahimi, S. , Karimi, T. M. A. , Tahmasebi, G. , & Khaleghi Miran, S. N. (2016). Effects of propolis, royal jelly, honey and bee pollen on growth performance and immune system of Japanese quails. Veterinary Research Forum: An International Quarterly Journal, 7(1), 13–20. [PMC free article] [PubMed] [Google Scholar]
- Banjarnahor, S. D. S. , & Artanti, N. (2014). Antioxidant properties of flavonoids. Medical Journal of Indonesia, 23(4), 239–244. 10.13181/mji.v23i4.1015 [DOI] [Google Scholar]
- Biancatelli, R. M. L. C. , Berrill, M. , Catravas, J. D. , & Marik, P. E. (2020). Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS‐CoV‐2 related disease (COVID‐19). Frontiers in Immunology, 11, 1451–1465. 10.3389/fimmu.2020.01451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockway, S. M. , & Denison, M. R. (2004). Molecular targets for the rational design of drugs to inhibit SARS coronavirus. Drug Discovery Today: Disease Mechanisms, 1(2), 205–209. 10.1016/j.ddmec.2004.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, H. L. , Roberts, A. E. L. , Cooper, R. , & Jenkins, R. E. (2016). A review of selected bee products as potential antibacterial, antifungal, and antiviral agents. Medical Research Archives, 4(7), 1–12. 10.18103/mra.v4i8.887 [DOI] [Google Scholar]
- Caramalho, I. , Melo, A. , Pedro, E. , Barbosa, M. M. P. , Victorino, R. M. M. , Pereira Santos, M. C. , & Sousa, A. E. (2015). Bee venom enhances the differentiation of human regulatory T cells. Allergy, 70(10), 1340–1345. 10.1111/all.12691 [DOI] [PubMed] [Google Scholar]
- Carpes, S. T. , de Alencar, S. M. , Cabral, I. S. R. , Oldoni, T. L. C. , Mourão, G. B. , Haminiuk, C. W. I. , … Masson, M. L. (2013). Polyphenols and palynological origin of bee pollen of Apis mellifera L. from Brazil. Characterization of polyphenols of bee pollen. CyTA Journal of Food, 11(2), 150–161. 10.1080/19476337.2012.711776 [DOI] [Google Scholar]
- Centers for Disease Control and Prevention . (2020). Human coronavirus types. Retrieved from https://www.cdc.gov/coronavirus/types.html.
- Chen, L. , Li, J. , Luo, C. , Liu, H. , Xu, W. , Chen, G. , … Jiang, H. (2006). Binding interaction of quercetin‐3‐β‐galactoside and its synthetic derivatives with SARS‐CoV 3CLpro: Structure‐activity relationship studies reveal salient pharmacophore features. Bioorganic and Medicinal Chemistry, 14(24), 8295–8306. 10.1016/j.bmc.2006.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, H. J. , Song, J. H. , & Kwon, D. H. (2011). Quercetin 3‐rhamnoside exerts antiinfluenza A virus activity in mice. Phytotherapy Research, 26(3), 1–12. 10.1002/ptr.3529 [DOI] [PubMed] [Google Scholar]
- Cianciosi, D. , Forbes‐Hernández, T. Y. , Afrin, S. , Gasparrini, M. , Reboredo‐Rodriguez, P. , … Battino, M. (2018). Phenolic compounds in honey and their associated health benefits: A review. Molecules, 23(9), 1–15. 10.3390/molecules23092322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus Cases . (2020). Worldometer. Retrieved from https://www.worldometers.info/coronavirus/.
- Dayem, A. A. , Choi, H. Y. , Kim, Y. B. , & Cho, S.‐G. (2015). Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS One, 10(3), e0121610. 10.1371/journal.pone.0121610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De‐Melo, A. A. M. , de Almeida‐Muradian, L. B. , Sancho, M. T. , & Pascual‐Maté, A. (2018). Composición y propiedades de la miel de Apis mellifera: una revisión. Journal of Apicultural Research , 57(1), 5–37. 10.1080/00218839.2017.1338444 [DOI] [Google Scholar]
- Deng, Y. , Liu, W. , Liu, K. , Fang, Y. Y. , Shang, J. , zhou, L. , … Liu, H. G. (2020). Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID‐19) in Wuhan, China. Chinese Medical Journal, 1, 1–15. 10.1097/cm9.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisow, B. , & Denisow‐Pietrzyk, M. (2016). Biological and therapeutic properties of bee pollen: A review. Journal of the Science of Food and Agriculture, 96(13), 4303–4309. 10.1002/jsfa.7729 [DOI] [PubMed] [Google Scholar]
- Fatrcová‐Šramková, K. , Nôžková, J. , Kačániová, M. , Máriássyová, M. , Rovná, K. , & Stričík, M. (2013). Antioxidant and antimicrobial properties of monofloral bee pollen. Journal of Environmental Science and Health ‐ Part B Pesticides, Food Contaminants, and Agricultural Wastes, 48(2), 133–138. 10.1080/03601234.2013.727664 [DOI] [PubMed] [Google Scholar]
- Filipic, B. , & Likar, M. (1976a). Inhibitory effect of propolis and royal jelly on some viruses. In Filipic B. (Ed.), Interferon scientific memoranda (pp. 13–16). Bufallo, NY: Calspan Corporation. [Google Scholar]
- Filipic, B. , & Likar, M. (1976b). Clinical value of royal jelly and propolis against viral infections. In Filipic B. (Ed.), Interferon scientific memoranda (pp. 18–32). Bufallo, NY: Calspan Corporation. [Google Scholar]
- Fontana, R. , Mendes, M. A. , de Souza, B. M. , Konno, K. , César, L. M. M. , Malaspina, O. , & Palma, M. S. (2004). Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides, 25(6), 919–928. 10.1016/j.peptides.2004.03.016 [DOI] [PubMed] [Google Scholar]
- Fratini, F. , Cilia, G. , Mancini, S. , & Felicioli, A. (2016a). Royal jelly: An ancient remedy with remarkable antibacterial properties. Microbiological Research, 192, 130–141. 10.1016/j.micres.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Fratini, F. , Cilia, G. , Turchi, B. , & Felicioli, A. (2016b). Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pacific Journal of Tropical Medicine, 9(9), 839–843. 10.1016/j.apjtm.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Ganesan, S. , Faris, A. N. , Comstock, A. T. , Wang, Q. , Nanua, S. , Hershenson, M. B. , & Sajjan, U. S. (2012). Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Research, 94(3), 258–271. 10.1016/j.antiviral.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem, H . (2020). In Silico Approach of Some Selected Honey Constituents as SARS‐CoV‐2 Main Protease (COVID‐19) Inhibitors. ChemRxiv. 10.26434/CHEMRXIV.12115359.V2 [DOI]
- Kaul, T. N. , Middleton, E. , & Ogra, P. L. (1985). Antiviral effect of flavonoids on human viruses. Journal of Medical Virology, 15(1), 71–79. 10.1002/jmv.1890150110 [DOI] [PubMed] [Google Scholar]
- Komaravelli, N. , Kelley, J. P. , Garofalo, M. P. , Wu, H. , Casola, A. , & Kolli, D. (2015). Role of dietary antioxidants in human metapneumovirus infection. Virus Research, 200(C), 19–23. 10.1016/j.virusres.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpeli Akkol, E. , Orhan, D. D. , Gürubüz, I. , & Yesilada, E. (2010). In vivo activity assessment of a “honey‐bee pollen mix” formulation. Pharmaceutical Biology, 48(3), 253–259. 10.3109/13880200903085482 [DOI] [PubMed] [Google Scholar]
- Kwon, M. J. , Shin, H. M. , Perumalsamy, H. , Wang, X. , & Ahn, Y. J. (2019). Antiviral effects and possible mechanisms of action of constituents from Brazilian propolis and related compounds. Journal of Apicultural Research, 59, 413–425. 10.1080/00218839.2019.1695715 [DOI] [Google Scholar]
- Lansbury, L. , Lim, B. , Baskaran, V. , & Lim, W. S. (2020). Co‐infections in people with COVID‐19: A systematic review and meta‐analysis. Journal of Infection, 81(2), 266–275. 10.1016/j.jinf.2020.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. Y. , Shin, J. H. , Park, K. H. , Kim, J. H. , Shin, M. G. , Suh, S. P. , … Kim, S. H. (2014). Identification, genotypic relation, and clinical features of colistin‐resistant isolates of acinetobacter genomic species 13BJ/14TU from bloodstreams of patients in a university hospital. Journal of Clinical Microbiology, 52(3), 931–939. 10.1128/JCM.02868-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Zhang, J. , Wang, N. , Li, H. , Shi, Y. , Guo, G. , … Zou, Q. (2020). Therapeutic drugs targeting 2019‐nCoV main protease by high‐throughput screening. BioRxiv, 1, 1–12. 10.1101/2020.01.28.922922 [DOI] [Google Scholar]
- Lim, Y. , Ng, Y. , Tam, J. , & Liu, D. (2016). Human coronaviruses: A review of virus–host interactions. Diseases, 4(4), 26. 10.3390/diseases4030026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mărgăoan, R. , Mărghitaş, L. A. , Dezmirean, D. S. , Gherman, B. , Chirilă, F. , Zacharias, I. , & Bobiş, O. (2015). Antimicrobial activity of bee pollen ethanolic and methanolic extracts on Staphylococcus aureus bacterial strain. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj‐Napoca. Animal Science and Biotechnologies, 72(1), 1–17. 10.15835/buasvmcn-asb:10791 [DOI] [Google Scholar]
- Memariani, H. , Memariani, M. , Shahidi‐Dadras, M. , Nasiri, S. , Akhavan, M. M. , & Moravvej, H. (2019). Melittin: From honeybees to superbugs. Applied Microbiology and Biotechnology, 103(8), 3265–3276. 10.1007/s00253-019-09698-y [DOI] [PubMed] [Google Scholar]
- Meo, S. A. , Al‐Asiri, S. A. , Mahesar, A. L. , & Ansari, M. J. (2017). Role of honey in modern medicine. Saudi Journal of Biological Sciences, 24(5), 975–978. 10.1016/j.sjbs.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, A. F. , Hassan, M. , Hammad, K. M. , Amer, M. A. , & Riad, S. A. (2015). Monitoring of the antiviral potential of bee venom and wax extracts against adeno ‐ 7 (DNA) and Rift Valley fever virus (RNA) viruses models. Journal of the Egyptian Society of Parasitology, 45(1), 193–198. 10.12816/0010865 [DOI] [PubMed] [Google Scholar]
- Pan, M. H. , Lai, C. S. , & Ho, C. T. (2010). Anti‐inflammatory activity of natural dietary flavonoids. Food & Function, 1(1), 15–31. 10.1039/c0fo00103a [DOI] [PubMed] [Google Scholar]
- Park, S. E. (2020). Epidemiology, virology, and clinical features of severe acute respiratory syndrome ‐coronavirus‐2 (SARS‐CoV‐2; coronavirus Disease‐19). Clinical and Experimental Pediatrics, 63, 119–124. 10.3345/cep.2020.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal Samy, R. , Stiles, B. G. , Franco, O. L. , Sethi, G. , & Lim, L. H. K. (2017). Animal venoms as antimicrobial agents. Biochemical Pharmacology, 134, 127–138. 10.1016/j.bcp.2017.03.005 [DOI] [PubMed] [Google Scholar]
- Rehman, M. T. , AlAjmi, M. F. , & Hussain, A. (2020). Natural compounds as inhibitors of SARS‐CoV‐2 main protease (3CLpro): A molecular docking and simulation approach to combat COVID‐19. ChemRxiv, 10.26434/CHEMRXIV.12362333.V2 [DOI] [PubMed]
- Rzepecka‐Stojko, A. , Stojko, J. , Kurek‐Górecka, A. , Górecki, M. , Kabała‐Dzik, A. , Kubina, R. , … Iriti, M. (2015). Polyphenols from bee pollen: Structure, absorption, metabolism and biological activity. Molecules, 20(12), 21732–21749. 10.3390/molecules201219800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian, S. , Farkhondeh, T. , & Samini, F. (2017). Honey and health: A review of recent clinical research. Pharmacognosy Research, 9(2), 121–127. 10.4103/0974-8490.204647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler, P. , Neuner, A. , Nolkemper, S. , Zundel, C. , Nowack, H. , Sensch, K. H. , & Reichling, J. (2010). Antiviral activity and mode of action of propolis extracts and selected compounds. Phytotherapy Research, 24(S1), S20–S28. 10.1002/ptr.2868 [DOI] [PubMed] [Google Scholar]
- Schwarz, S. , Sauter, D. , Wang, K. , Zhang, R. , Sun, B. , Karioti, A. , … Schwarz, W. (2014). Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Medica, 80(2–3), 177–182. 10.1055/s-0033-1360277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, T. , Hino, A. , Tsutsumi, A. , Park, Y. K. , Watanabe, W. , & Kurokawa, M. (2008). Anti‐influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice. Antiviral Chemistry & Chemotherapy, 19(1), 7–13. 10.1177/095632020801900102 [DOI] [PubMed] [Google Scholar]
- Siheri, W. , Alenezi, S. , Tusiimire, J. , & Watson, D. G. (2017). The chemical and biological properties of propolis. Bee Products ‐ Chemical and Biological Properties, 42, 137–178. 10.1007/978-3-319-59689-1_7 [DOI] [Google Scholar]
- Song, J. H. , Park, K. S. , Kwon, D. H. , & Choi, H. J. (2013). Anti–human rhinovirus 2 activity and mode of action of quercetin‐7‐glucoside from Lagerstroemia speciosa . Journal of Medicinal Food, 16(4), 274–279. 10.1089/jmf.2012.2290 [DOI] [PubMed] [Google Scholar]
- Svečnjak, L. , Chesson, L. A. , Gallina, A. , Maia, M. , Martinello, M. , Mutinelli, F. , … Waters, T. A. (2019). Standard methods for Apis mellifera beeswax research. Journal of Apicultural Research, 58(2), 1–18. 10.1080/00218839.2019.1571556 [DOI] [Google Scholar]
- Šver, L. , Oršolić, N. , Tadić, Z. , Njari, B. , Valpotić, I. , & Bašić, I. (1996). A royal jelly as a new potential immunomodulator in rats and mice. Comparative Immunology, Microbiology and Infectious Diseases, 19(1), 31–38. 10.1016/0147-9571(95)00020-8 [DOI] [PubMed] [Google Scholar]
- Takeshita, T. , Watanabe, W. , Toyama, S. , Hayashi, Y. , Honda, S. , Sakamoto, S. , … Kurokawa, M. (2013). Effect of Brazilian propolis on exacerbation of respiratory syncytial virus infection in mice exposed to tetrabromobisphenol A, a brominated flame retardant. Evidence‐Based Complementary and Alternative Medicine, 2013, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur, M. , & Nanda, V. (2020). Composition and functionality of bee pollen: A review. Trends in Food Science and Technology, 98, 82–106. 10.1016/j.tifs.2020.02.001 [DOI] [Google Scholar]
- Uddin, M. B. , Lee, B. H. , Nikapitiya, C. , Kim, J. H. , Kim, T. H. , Lee, H. C. , … Kim, C. J. (2016). Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. Journal of Microbiology, 54(12), 853–866. 10.1007/s12275-016-6376-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, K. , Rahmasari, R. , Matsunaga, A. , Haruyama, T. , & Kobayashi, N. (2014). Anti‐influenza viral effects of honey in vitro: Potent high activity of Manuka honey. Archives of Medical Research, 45(5), 359–365. 10.1016/j.arcmed.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Wehbe, R. , Frangieh, J. , Rima, M. , El Obeid, D. , Sabatier, J. M. , & Fajloun, Z. (2019). Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules, 24(16), 1–12. 10.3390/molecules24162997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, L. , Li, Z. , Yuan, K. , Qu, X. , Chen, J. , Wang, G. , … Xu, X. (2004). Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. Journal of Virology, 78(20), 11334–11339. 10.1128/jvi.78.20.11334-11339.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, P. , Ding, Y. , Wu, X. , Long, J. , Zhong, Y. , & Li, Y. (2020). The epidemiology, diagnosis and treatment of COVID‐19. International Journal of Antimicrobial Agents, 55(5), 105955. 10.1016/j.ijantimicag.2020.105955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, F. C. , Li, Y. H. , Guan, X. H. , Hou, L. H. , Wang, W. J. , Li, J. X. , … Chen, W. (2020a). Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: A dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. The Lancet, 395(10240), 1845–1854. 10.1016/S0140-6736(20)31208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N. , Zhang, D. , Wang, W. , Li, X. , Yang, B. , Song, J. , … Tan, W. (2020b). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 382(8), 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]


