Abstract
SARS‐CoV‐2, the causative agent of COVID‐19 pandemic caught the world unawares by its sudden onset in early 2020. Memories of the 1918 Spanish Flu were rekindled raising extreme fear for the virus, but in essence, it was the host and not the virus, which was deciding the outcome of the infection. Age, gender, and preexisting conditions played critical roles in shaping COVID‐19 outcome. People of lower socioeconomic strata were disproportionately affected in industrialized countries such as the United States. India, a developing country with more than 1.3 billion population, a large proportion of it being underprivileged and with substandard public health provider infrastructure, feared for the worst outcome given the sheer size and density of its population. Six months into the pandemic, a comparison of COVID‐19 morbidity and mortality data between India, the United States, and several European countries, reveal interesting trends. While most developed countries show curves expected for a fast‐spreading respiratory virus, India seems to have a slower trajectory. As a consequence, India may have gained on two fronts: the spread of the infection is unusually prolonged, thus leading to a curve that is “naturally flattened”; concomitantly the mortality rate, which is a reflection of the severity of the disease has been relatively low. I hypothesize that trained innate immunity, a new concept in immunology, may be the phenomenon behind this. Biocultural, socioecological, and socioeconomic determinants seem to be influencing the outcome of COVID‐19 in different regions/countries of the world.
1. INTRODUCTION
1.1. COVID‐19 and Spanish Flu
SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus‐2) that causes coronavirus disease 2019 (COVID‐19) engulfed the world in a short span of time and created worldwide panic and paranoia (Banerjee, 2020; Ho, Chee, & Ho, 2020; Larson, 2020; Park, 2020). The last pandemic of similar magnitude that the world witnessed in terms of a worldwide reach and impact should be the Spanish Flu of 1918 (Spinney, 2017). Both Spanish Flu and COVID‐19 involve fast‐spreading respiratory viruses; the Spanish Flu, caused by an influenza virus ravaged for more than a year and killed 40 to 50 million or more people across the globe (Taubenberger & Morens, 2006), while COVID‐19 has so far killed over 640 000 people. Three more Flu pandemics happened in between in 1957 (Asian Flu), 1968 (Hong Kong Flu), and 2009 (Swine Flu) that had much lower deaths than the 1918 pandemic (CDC, 2020); even though the 1957 Flu and 1968 Flu caused around 1 million deaths or more each, intriguingly, the global response to these two pandemics was negligible, almost nonexistent in comparison to the one shown to COVID‐19 (Honigsbaum, 2020). COVID‐19 has caused significantly lesser mortality than the 1918 pandemic but is predicted to impact the world economy more severely than the Spanish Flu did a century ago (Smith, 2020). The world has changed enormously between 1918 and 2020. Apart from industrialization and globalization, two technological advances are notable in the context of the current pandemic: one, information technology has connected the world like never before and the other genomics revolution has helped the research community in an unprecedented manner to understand the disease and the virus better and seek remedies quicker (Ellinghaus et al., 2019; van Dorp et al., 2020; Zhu et al., 2020).
1.2. India and response to COVID‐19
India, the second‐most populous country in the world tested its first COVID‐19 case on Jan 30, 2020 (Prasad, Potdar, Abraham, & Basu, 2020). However, India did not seem to react strongly to the pandemic until mid‐March; only after Italy and Spain were reporting an increasing number of daily deaths due to COVID‐19 did India begin to react rather aggressively to the emerging situation (Bhatia & Abraham, 2020). By the last week of March, India was in a grip of anxiety about COVID‐19 and the possible impact it could have on its population. With a constant barrage of information (and misinformation) from television channels and social media outlets, the anxiety turned into fear and paranoia (Iqbal & Dar, 2020; Kadam & Atre, 2020). India is a highly structured and heterogeneous society in several parameters such as socioeconomic status, religion, culture, language, ethnicity, etc.; a majority of its populace lack a basic level of formal education. A vast majority (close to 70%) of Indians live in rural areas, while the major cities (around 10) have high population density. Poverty and lack of access to quality education continue to be a major and pervasive problem across the country affecting the majority of Indians, while an increasing subset of Indians also has moved from the lower to the upper‐middle class in the last three decades, thanks to economic liberalization policies adopted by successive governments.
A combination of factors such as an exaggerated administrative response to COVID‐19 that was not necessarily data‐driven (Patel, 2020a), a lack of understanding of local disease dynamics by the experts (Thomas, 2020), a failure to project India‐specific disease models by epidemiologists (Bhatia & Abraham, 2020; Kanwal, 2020), non‐pharmaceutical prescriptions that were unreasonable and most of the times impractical for a complex society such as India's (Chetterjee, 2020; Dore, 2020) and ready access to cheap internet‐phone technology successfully transformed SARS‐CoV‐2 into a virtual monster, even before it got a foothold in the country. A highly disproportionate share of attention and resources were diverted towards managing COVID‐19 without any sound rationale (Krishna, 2020); for a country that faces even bigger problems (issues such as overgrown population, poverty, huge social and economic inequalities, confrontation with neighboring countries on a constant basis, and natural calamities) including a huge burden of other communicable and non‐communicable diseases (Patel, 2020b), the administration surprisingly thought it prudent to impose an unprecedented measure in the form of a country‐wide lockdown for 55 days (initially planned only for 21 days) (Patel, 2020c) with a hope that the virus transmission chain would be broken. Obviously, India's policy was guided by similar measures undertaken by the developed countries of the west and more so by the presumption that the virus and the disease were non‐discriminatory in nature (Patel, 2020a; Thomas, 2020).
Measures such as social distancing, which may work well for a western society are almost impractical for a country like India (Chetterjee, 2020), especially in densely populated urban slums. A lockdown measure while sounded extremely logical for the educated class, the poor who live on daily wages to run their families felt it like a catastrophe; while the affluent class could afford the expensive private hospitals that could provide them with standard‐of‐care treatment, the less fortunate preferred non‐cooperation with the administration toward testing and contact tracing (Bhatia & Abraham, 2020; Dwary, 2020) understandably due to the fear of being quarantined in dingy, unclean, understaffed public COVID‐19 isolation centers. The fear of dying from the disease while being away from their families in such isolation centers also may have led to this behavior. The educated middle and higher class were religiously following the measures such as “stay‐at‐home orders” prescribed by the administration, while the lower middle class and the poor suffered in silence and confusion.
India, as a consequence of its neoliberal economic policies (Raveendran, 2020), has a large populace of migrant workers who have left their homes in rural India to work on daily wages in far‐off cities mostly in different states from where they come from. Once the panic about the pandemic set in and a lockdown was imposed, these workers in urban centers felt a complete lack of trust either on their employers or the administration to save them from an impending “death warrant” and took it upon themselves to walk thousands of kilometers along with their families on foot to their native places as all forms of transport were shut simultaneously (Mukhra, Krishan, & Kanchan, 2020). The migrant exodus was a humanitarian crisis unfolding on the one hand, on the other, there was an apprehension that the migrants were carrying the virus with them and seeding it across the country (Bhatia & Abraham, 2020). The government possibly went by the advice that: the virus can be curtailed by a short three‐week nation‐wide lockdown (a presumption that eventually turned out to be naïve and preposterous), and that if the migrants are allowed to “reverse‐migrate”, they may increase the spread of infection; considering these, a 21‐day “complete” lockdown was announced. India had the highest death toll (more than 13 million) among all nations during the 1918 Spanish Flu pandemic (Chandra, Kuljanin, & Wray, 2012). The government could have heavily weighed its decision on this critical statistic to impose a country‐wide lockdown in order to avert a “disaster” of a similar kind, or worse. However, India had around 500 confirmed cases and only 10 deaths due to COVID‐19 by Mar 24, 2020 when the lockdown was imposed; there was no evidence other than speculation about how much the virus had spread or not spread in the community, and therefore, even though the intentions were good, it was an exaggerated and mistimed response especially considering the migrant worker crisis that ensued (Pandey, 2020; Raveendran, 2020).
It was obvious that a one‐size‐fits‐all approach to tackle COVID‐19 in India was going to fail but was still pursued (Muliyil, 2020; Patel, 2020a, 2020b, 2020c; Thomas, 2020). It is a fact that India has not developed a biocultural or “cultural‐biological research” (Goodman, 2013; Hoke & Schell, 2020) framework of human biology within the realm of medical sciences in order to better understand the diseases that affect its population. A discipline like medical anthropology, which is most relevant to a country like India (Joshi, 2016) has not been given any impetus as a sustainable research area for many decades. The medical curriculum in India, as is true elsewhere, has no emphasis on human biology; therefore, the concepts of how environment, culture, and socioeconomic factors can and could influence human health and disease are hardly discussed and researched (Dufour, 2006; Mishra, 2007). Increasing privatization of medical education‐care in the country is not helping the cause either, as profit is prioritized over addressing real and important issues. As a result, COVID‐19 was viewed purely as a medical problem and the possibility that COVID‐19 could be different in India due to its inherent differences in environment, culture, prevailing diseases were not even explored; such thoughts are even derided in medical institutions (Mishra, 2007).
While the success of the stringent measures undertaken to tackle COVID‐19 the world over including India is debatable (Melnick & Loannadis, 2020), we cannot deny the fact that the conservative reductionist approach that has well‐guarded and nurtured medicine and biology throughout history, failed to address in a measured way the most important public health crisis of our times (Klement, 2020); yes, some countries have done well in managing the disease but the majority, most of which are highly developed were found wanting in dealing with the emergency, especially in keeping the mortality rate low. It is said that the Spanish Flu revived the field of virology and inspired many successful careers in it (Horton, 2020; Taubenberger, Hutlin, & Morens, 2007). However, a knowledge on viruses that spans a century or more has not helped us to fully comprehend SARS‐CoV‐2, at least thus far. One of the unique features of SARS‐CoV‐2 has been that it has not behaved like a typical respiratory virus, at least when compared to the influenza viruses that have caused past pandemics. It is affecting multiple organs (Gupta et al., 2020) with a range of symptoms in different age groups (Liu et al., 2020), surprisingly sparing children (Fischer, 2020) and selectively targeting people with comorbidities (Guan et al., 2020). Dr. Antony Fauci, of the NIAID, has famously said that he has not seen a virus like SARS‐CoV‐2 where up to 40% people with the infection show no symptoms (Fottrell, 2020; Kronbichler et al., 2020). An integrated narrative between virologists, epidemiologists, clinicians, and infectious disease experts to explain real‐world experiences during the course of the pandemic was lacking (Guigliano, 2020; Yong, 2020), further pointing toward the limitations of reductionist approaches to understand and address human diseases at the population level. If this was the situation in the most scientifically advanced country like the United States, it was even bad if not worse in a developing country like India (Saraya, 2020), which spends less than 2% of its GDP on health and research.
COVID‐19, no doubt laid bare the ground realities of our deficiencies in understanding of diseases in general and viral diseases in particular at the population level. For instance, COVID‐19 has shown us that there are deeper connections than we thought, between lifestyle disorders and the immune system. Our direct knowledge about host‐virus interactions regarding human viruses comes from two sources: the major one, from the experimental interventions we carry out on virus‐infected cell lines and animal models; and a minor one, comes from our observational studies in the affected human population. Therefore, our knowledge of host‐virus interactions is mostly based on our studies on model systems carried out in the confines of a biosafety‐compliant laboratory. However, in the real‐world, the virus is encountering completely different hosts and environments, which are interacting with each other in complex ways. Overlooking this fact, in a deterministic mindset, we try to extrapolate our model system‐based knowledge on to the real‐world, which obviously is a flawed approach to the understanding of human health and disease. For example, we did not incorporate parameters such as socioeconomic, ecological, cultural, and behavioral factors into our mathematical models in predicting the course of COVID‐19, resulting in grossly inaccurate estimates (Nadella, Swaminatahn, & Subramanian, 2020). If we do not acknowledge the limitations of our current approaches to understanding and handling human diseases and act fast, we are bound to see more COVID‐19‐like situations in the future, where we will see a similar disconnect between our model systems‐based knowledge and the real‐world situation. One potential solution to this long‐standing problem could be to begin to reorient ourselves toward a medical/biocultural anthropological‐centric approach to research on human health and diseases (Hoke & Schell, 2020). It is a different matter that India's response to COVID‐19 could have been better if it had not neglected its huge infectious disease burden over the years and built a robust public health surveillance, monitoring, and management system besides addressing its lacunae of dialectical thinking in health sciences.
1.3. Host decides the outcome of a host‐virus interaction
Whenever a new human virus that has an epidemic potential emerges, the fear related to past pandemics gets the better of us and in an attempt to be precautionary, we tend to overlook the host and its defenses and end up projecting the virus in a “larger‐than‐life” image. A cardinal feature of any viral infection in a verebrate host is the host‐virus interaction that takes place at multiple levels which shall ultimately decide the outcome. Each host‐virus interaction is unique, and the outcome could be in favor of either player. But first, let us understand the strengths and weaknesses of the two players involved. The virus is a simple macromolecular entity with a nucleic acid genome wrapped around by proteins and lipids; the human host is a far more advanced biological system with complexity built in several layers; defense against foreign invaders is one of the defining features of the host. The most stable state for a virus is inside a host cell since it is a non‐living entity on its own, while the host has multiple layers of sophisticated defense mechanisms to prevent the virus from infecting and causing harm to it. Moreover, the host has the advantage of millions of years of evolution behind it that has made its defense against invading microbes strong and efficient. Unless there is a compromise in the host defense, it is a very uphill task for the virus to gain entry and/or flourish inside it; the virus on the other hand being a microbe has the advantage of stealth and being a parasite, it relentlessly looks to find a breach in the host defense. The complex nature of the host that makes it a stronger player in the interaction also makes it a vulnerable player under certain conditions, which compromise its defenses; the conditions may or may not be directly affecting the defense system, but even those that affect other physiological processes can also have an impact. The virus, which is in low numbers to begin with, in a natural infection setting, will use the breach in host defense to establish itself and multiply to large numbers to the extent that it overwhelms the host response and can now dictate the outcome of the infection. Understanding the conditions in the host that allowed a successful viral infection and the pathophysiological causes responsible for these conditions is the key to understanding host‐virus interactions. Fortunately, there will always be resistant hosts in the population that will help us to learn what difference in conditions led to a successful infection in the susceptible hosts. This information, which obviously will be different for different viral diseases, should be the guiding principle in designing the right preventive and therapeutic strategy. To accomplish this, we will need sustained efforts in research on the biology of the virus, the host and their interactions; importantly, COVID‐19 has made us realize that these efforts should be centered on a “transdisciplinary” systems medicine approach rather than reductionist science (Klement, 2020).
1.4. Host immune response to SARS‐CoV‐2
It is important to understand the host immune response to a viral infection like COVID‐19. SARS‐CoV‐2 is an RNA virus, and when it infects the host cell by binding to the host cell membrane receptor (ACE2, Angiotensin‐converting enzyme), it releases its RNA genome into the cytoplasm, and since the RNA is positive‐sense (like cellular mRNA), it is recognized by the host translational machinery and is translated into numerous viral proteins (Jiang, Hillyer, & Du, 2020). The viral proteins now begin to make more copies of the RNA, which will eventually make more copies of the protein, and the cycle continues. New viruses are assembled using the newly made RNA and viral structural proteins; at a point of time when sufficient new viral particles are made, the cell collapses allowing the virus to find new cells. However, this outcome, which happens to be in favor of the virus is not always possible. The host cell has an in‐built defense mechanism called the “innate immunity” that is capable of sensing and reacting to a foreign entity inside it. Innate immunity and adaptive (also called acquired) immunity are the two proverbial arms of vertebrate host immunity.
Innate immunity arm is the remnant of an ancient defense system seen in lower organisms including plants. It is functional both at the individual cell level and at a higher level where several innate immune cells participate in recognizing and countering the foreign organism (Figure 1) (Frieman, Heise, & Baric, 2008). The innate immune arm is nonspecific, due to which it is less robust but is the first to see and counter the infection. While it tries to limit the infection, it also helps to prime the adaptive immunity arm to prepare for a more specific and robust assault on the pathogen. The innate immune mechanism at the cellular level is mediated by pattern recognition receptors (PRR) that recognize specific features in a pathogen, in this case, specific structural features in the SARS‐CoV‐2 RNA. These features are mostly unique to viral RNA and not present in the host cellular RNA, hence are pathogen‐specific (pathogen‐associated molecular patterns, PAMP) (Gasteiger et al., 2017). PRRs like RLRs (RIG‐I like receptors) and TLRs (Toll‐like receptors) are known to recognize viral RNA, and they will initiate a signaling cascade that will lead to the production of type I and III interferons (IFN). IFNs are potent antiviral cytokines that are usually secreted out of the cells so that they bind to membrane receptors of neighboring cells to raise an alarm about the foreign invader present in the vicinity. Type I IFNs are secreted by most nucleated cells; type III IFNs are secreted by cells of the epithelium, hepatocytes, and some immune cells; type II IFN is secreted exclusively by immune cells (Chinnaswamy, 2016). IFNs signal to the cells and lead to the expression of several hundred antiviral genes called IFN‐stimulated genes (ISG). Some ISGs have direct antiviral effects (e.g., RNA degrading enzymes), others help to shape the subsequent adaptive immune response, but for a large number of others we do not yet know their exact functions during viral infections (Crosse, Monson, Beard, & Helbig, 2018). The innate immune arm is highly efficient in raising the initial signals and since it is the first link between the pathogen and adaptive immunity, it assumes a lot of significance in the overall immune response of the host to the virus. In many instances of chronic viral infections, it is the innate immune arm that is found to be defective, possibly because it failed to give the correct and optimal signals to the adaptive immunity arm to develop and act efficiently (Altfeld & Gale Jr, 2015; Heim, 2013). The innate immune arm sometimes on its own can control pathogen infections without the need for an adaptive immune arm help (Sturdevant & Caldwell, 2014). All these underlie the significance of the innate immunity system in combating pathogens.
FIGURE 1.
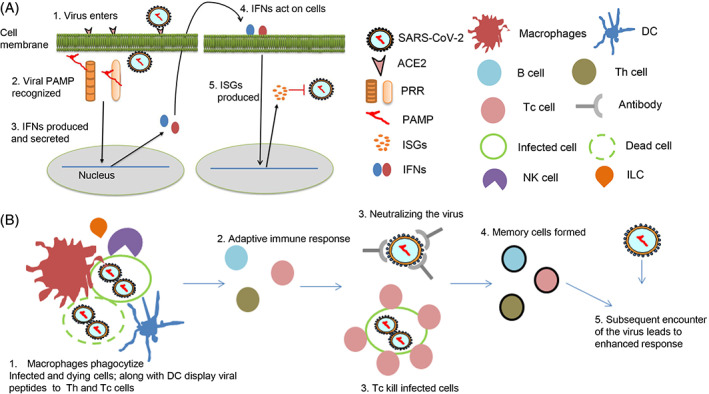
Innate and adaptive immunity against a virus explained. A, Innate immunity comprises of mechanisms to recognize viral intermediates and respond by secreting IFNs. B, Innate immune cells like macrophages and DCs are also the link to the adaptive immunity that ultimately is responsible for clearing the virus infection. NK cells monitor for virus‐infected cells and secrete type II IFN, IFN‐γ besides actively killing the infected cells (Brandstadter & Yang, 2011)
The innate immune arm at the higher level comprises the innate immune cells, which cooperatively act in order to suppress the pathogen. These cells include macrophages, neutrophils, natural killer (NK) cells, dendritic cells (DC), innate lymphoid cells (ILC), and others. Macrophages are one of the first to recognize a virally infected cell, and getting cues from the infected cells and the surrounding ILCs efficiently engulf the infected cell and clear the infection locally. Macrophages and DCs also carry small peptides derived from the viral proteins and display them to activate specific T helper (Th) cells, which then orchestrate the development of specific T cytotoxic (Tc) cells that will eventually kill the virus‐infected cells on a large scale (Figure 1B). Depending on the type of pathogen (extracellular or intracellular), the Th cells secrete specific cytokines that fall under two broad classes: Th1 and Th2. Th1 immunity is required to clear intracellular pathogens like viruses, protozoan parasites, and bacteria, while Th2 immunity protects the hosts from extracellular pathogens, mainly nematode parasites (Berger, 2000). A combination of Th1, Th2 cells, and also others like Th17, Tregs coexist during any infection; they secrete potent factors called cytokines, which mediate the intended effects on different cells of the immune system. Antibodies, key components of an adaptive immune response, are raised by B cells against exposed pathogen proteins with help from Th cells. Hence a cooperative and an orchestrated immune response both at the individual infected cellular level and at a higher systems level are required to combat pathogens. While the adaptive immune arm being more specific and robust, also has a built‐in memory: the T (both Th and Tc) and B cells once they successfully clear the infection can become quiescent for long duration of time and can get activated upon encounter of the same pathogen subsequently leading to an even better response (Clark & Kupper, 2005). This is the basis of vaccines, where instead of a live pathogen an inactivated one or components of it are formulated and injected into the host to gain a primary memory response that can get activated when the host encounters a real pathogen in the future.
1.5. Host variation in immune response
As we would imagine, several genes and pathways are involved in the above‐described immune response to ensure the successful elimination of a pathogen. Therefore, a certain level of variation is expected between different hosts in the responses they raise against viral infection. The variation can be influenced by genetics and the environment. The environment here is used in a broader sense to include such factors as ecological, cultural, and socioeconomic and any other variable other than genetic that could influence a phenotype by nongenetic and epigenetic mechanisms (Danchin, 2013). Variation in genes in the form of single nucleotide polymorphisms (SNP) is found abundantly on the human genome, which now, thanks to the genomics revolution, can be identified quickly and easily from a large number of different hosts to understand if they are responsible for the variation in the immune response. There are now methods, thanks again to the genomics revolution, which can also identify with high resolution the epigenetic changes that control the gene expression in the host and examine its influence on the host immune response. The macro‐environment present on the outside of the host (e.g., nutrition, lifestyle, hygiene) can influence its inside by modifying the epigenetics of the host (Feil & Fraga, 2011). Further, age and gender‐related changes from within the host can also contribute to epigenetic variation (Horvath, 2013; Kuroki & Tachibana, 2018). Therefore, while it is easy to understand how genetic variation may or may not play a role in the outcome of the SARS‐CoV‐2 infection, the influence of the epigenetic variation is more complex to analyze because it is directly influenced by the macro environment, which is not a simple variable to incorporate into models. The host immune response can therefore be significantly influenced by any factor, including age, gender, nutrition, and living conditions of the host that will alter its epigenetics. More importantly, the hereditary contribution of nongenetic and epigenetic factors to health and disease is not as well understood as the genetic factors, even though its significance is being increasingly recognized (Danchin, 2013; Danchin, Pocheville, & Huneman, 2018).
A typical viral infection, therefore, can have a range of fates in the host depending on the immune response dictated by genetics and the environment. Resistant hosts efficiently clear the virus in quick time with a robust innate and adaptive immune response while the vulnerable hosts cannot do this and allow the virus to flourish and multiply to large numbers. Adaptive immunity, which was not until long ago synonymous with immunology, has been studied extensively with the help of mice and other animal models. Innate immunity is the newer and lesser‐studied aspect of immunology. I will explain the newest concept within innate immunity called “Trained Immunity” in a later section of this article. In summary, we can imagine that a virus after infecting a host would end up causing an asymptomatic disease or a disease with mild or severe symptoms depending on the host immune response. More resistant hosts are likely to have asymptomatic or mild disease, while the vulnerable hosts will end up with severe disease, and a subset among them will also succumb to it. The asymptomatic and mild symptomatic hosts may actually be helping the spread of the virus in the community by acting as carriers to infect the most vulnerable hosts where the virus can replicate to high levels (Yu et al., 2020; Zimmerman & Curtis, 2020).
Viruses that cause acute infections like COVID‐19 rely heavily on the availability of susceptible hosts around to cause wide‐spread morbidity and mortality. After infection, if the virus encounters more resistant hosts around, it will fail to cause wide‐spread infection due to what is called the “herd effect” (Joh & Samuel, 2000). In COVID‐19, it is known that viral load is proportional to the severity of symptoms (Yu et al., 2020). Therefore, the total viral load in a community will be the highest when the virus has reached the maximum possible vulnerable hosts, that is, those who will show severe symptoms; this maximum viral load in the community can be referred to as the “critical mass” of the virus. Critical mass will decide how many hosts are likely to succumb from the infection. A virus is less likely to reach its critical mass in a community when it encounters more number of resistant hosts due to the herd effect; this should be true even if the resistant hosts are asymptomatic or mild symptomatic carriers since viral load that can facilitate the spread will be, less in them (Guallar et al., 2020; Yu et al., 2020). The herd effect can be got by vaccination or from adaptive immunity arising out of a natural infection. On the other hand, if the virus encounters more vulnerable hosts around, then a “reverse herd effect” will help it reach the maximum number of them much faster and shall attain the critical mass more easily. It is important, therefore, to look at the COVID‐19 morbidity and mortality in different countries of the world since the hosts would be different in them and the immune responses would also be expectedly different. In the next section, I look at data that is available publicly about the number of cases and deaths that have occurred in different countries of the world since the beginning of the COVID‐19 pandemic. I have chosen eight European countries where the COVID‐19 effect has been maximal and also the United States, which has recorded the largest number of deaths so far due to COVID‐19.
2. INDIA SHOWS A DIFFERENT COVID‐19 TREND IN COMPARISON TO DEVELOPED COUNTRIES
SARS‐CoV‐2 was first detected in Wuhan city of China as a causative factor of unexplained pneumonia cases that were spreading from human‐to‐human (Zhu et al., 2020). It is believed that by the first week of Mar 2020, the rest of the world got infected with the virus possibly through international travelers initially arriving from China. By the first and second week of Mar 2020 most affected countries started to record deaths associated with COVID‐19. India had its first death due to COVID‐19 on Mar 12, 2020 (Table 1). Figure 2A shows the daily number of cases plotted against the daily deaths (7‐day moving averages) due to COVID‐19 beginning from the day the first case was reported until July 24, 2020 for each country. The time period of the data shown has a range of 143 to 185 days for the 10 countries. This data shows that (1) both the morbidity (number of cases) and mortality (number of deaths) curves for all the countries except India have initial steeper slopes and, (2) all of the countries have gone past their peaks in the number of daily deaths ranging from 93 days (Sweden) to 113 (Italy) days while India has not yet past its peak (0 days).
TABLE 1.
Deaths due to COVID‐19 in the United States, India, and prominent European countries
| Country | First death reported on (2020) | Total number of deaths | Deaths/100 k population a | 2019 Population size (millions) |
|---|---|---|---|---|
| Germany | Mar 9th | 9201 | 10.99 | 83.0 |
| Belgium | Mar 11th | 9817 | 85.90 | 11.5 |
| Italy | Feb 21st | 35 097 | 58.07 | 60.4 |
| UK | Mar 5th | 45 677 | 68.64 | 66.6 |
| Spain | Mar 3rd | 28 432 | 60.84 | 46.9 |
| France | Feb 15th | 30 192 | 45.06 | 67 |
| Netherlands | Mar 6th | 6139 | 35.74 | 17.3 |
| Sweden | Mar 11th | 5697 | 55.74 | 10.2 |
| Switzerland | Mar 5th | 1977 | 23.19 | 8.57 |
| Ireland | Mar 11th | 1763 | 36.32 | 4.9 |
| USA | Feb 29th | 148 490 | 44.11 | 328.2 |
| India | Mar 12th | 31 425 | 2.26 | 1369 |
Note: The data were obtained from worldometers.info on July 25, 2020.
Obtained from Johns Hopkins University on 25th July 2020.
FIGURE 2.
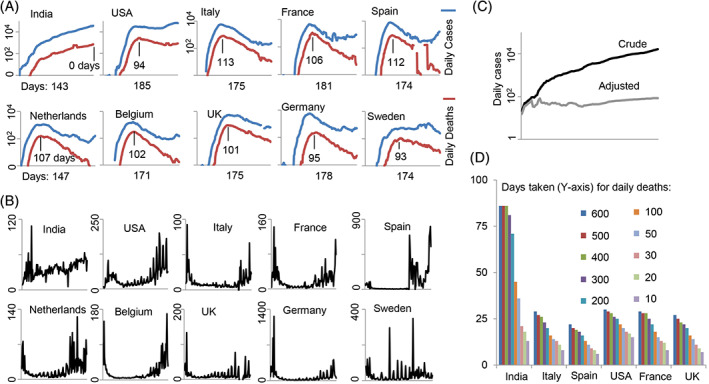
A comparison of COVID‐19 morbidity and mortality data for India and nine other developed countries. A, Number of daily cases (blue line; 7‐day moving average) is plotted along with the daily deaths (red line; 7‐day moving average) reported due to COVID‐19. The day of the first reported case of COVID‐19 in each country was selected as the initial data point and deaths and cases until Jul 24, 2020 are shown. The number of days shown below the X‐axis indicates the total number of days past since the first recorded case. The number of days shown above the X‐axis indicates the number of days past the peak in daily deaths based on a 7‐day moving average. B, Number of daily cases (7‐day moving average) was divided by the number of daily deaths and plotted. The initial part of the curve for almost all countries except India shows a “head” and the later part a “tail”. C, The actual daily cases (dark line) show an increasing trend but when it was normalized for the number of tests done during this period (Mar 20, 2020‐Jul 18, 2020) the curve loses its slope (gray line); note: the adjusted curve may not show actual daily death numbers, but is only shown to compare the slopes of the curves. D, Time in days each country took to reach the first 10, 20, etc. deaths. The data on daily testing, daily cases, and daily deaths were obtained from ourworldindata.org
Figure 2B shows a curve that is a ratio of daily cases to daily deaths due to COVID‐19 for all the countries (ie, the number of cases required for each daily death). The data begins with the day the first death was recorded due to COVID‐19 for each country and ends on July 24, 2020. This presentation of data shows that most of the countries display a curve that has a “head”, a “body”, and a “tail”. The head shows that a relatively lesser number of cases died at the beginning of the pandemic, the body is represented by a flat line showing that more number of cases died during the peak of the pandemic, while the tail that is represented by a rising curve toward the end of the pandemic is informing of a likely recovery phase. For most countries, the tail is raised toward the end suggesting an improving situation due to fewer deaths for a given number of cases. The Indian curve looks different even here. The curve seems to have only the tail, but not the head or the body, and the tail seems to be rising with a shallow slope. Does it suggest that India may have had the COVID‐19 even before it started testing, and what we are seeing is actually a stabilized infection rather than a spreading infection seen typically in the rest of the countries in the first 2 to 3 months after the onset? The tail in the Indian mortality curve may also be representative of a continuously improving recovery phase in the infection; that is, more number of Indians with COVID‐19 was recovering from the infection than those that were succumbing to it, throughout the study period. The curve in Figure 2A for India does show an increasing trend giving the impression that the morbidity and mortality may be continuously and steadily increasing. However, when we normalize the daily case data by adjusting for the increase in the number of tests done during the study period, it clearly does not show a similar increase (Figure 2C). When we look at how many days it took for each country to take its daily deaths from 10 to 600 per day, it shows that India is taking a longer time in reaching this number of daily deaths when compared to other countries (Figure 2D). In the last few weeks, however, this trend has changed, and the increase in deaths is taking lesser time than before; in spite of this late surge in deaths, India has taken a longer time overall to increase its COVID‐19 mortality relative to other countries. This could be because of two reasons: (1) a slow increase in testing frequency at the beginning of the pandemic that is specific to India may be causing this pattern or, (2) the virus is encountering more and more resistant hosts in the population in India.
When we look at the deaths happening in different countries due to COVID‐19, normalized to their population size (deaths/100 k population), India has a much lower number of deaths in comparison to other countries (Table 1). Several recent reports have also concluded that COVID‐19 has been mild in India resulting in lower mortality (Chakrabarti et al., 2020; Gupta & Misra, 2020; Ram, Babu, & Prabhakaran, 2020; Reddy & Pandey, 2020). In the early stages of the pandemic, critics disbelieved the official data (Radhakrishnan, Sen, & Singaravelu, 2020) leading to the interpretation that the lower death rate in India is actually due to lower testing and underreporting rather than milder disease. However, a study shows that the percent positivity rate (glossary) has remained stable or even declined suggesting no signs of drastically increasing infection rate in the country (Abraham et al., 2020). In absence of adequate testing for the disease, which is a limitation not only in India but other larger countries like the United States, a reliable indicator could be the deaths occurring due to any kind of illnesses including those due to severe acute respiratory illnesses (SARI) or influenza‐like illnesses (ILI). Even though the first confirmed case in India happened at the end of Jan 2020, the reports coming from various sources like local hospitals, print as well as electronic media was that there was no exaggerated increase in any form of deaths either due to SARI/ILI or due to other non‐COVID‐19‐related issues (Biswas, 2020; Ulmer & Khanna, 2020) up till the end of May. Others have also observed this (Ram et al., 2020). On the contrary, the deaths due to all causes actually decreased during most part of the lockdown period (Ulmer & Khanna, 2020) likely because of decreased accidents and pollution‐related illnesses. However, the health care system and local crematoriums in big cities did start feeling the pressure of increased load toward mid‐June (Jain & Dasgupta, 2020; Parth, 2020), which was also reflected accurately by official data on increasing COVID‐19 cases and deaths during this period (worldometers.info). There was confusion, especially in the cities of Mumbai, Chennai, and Delhi, on classifying deaths due to COVID‐19 “only” and those that were due to accompanying comorbidities and “coincidental” COVID‐19. This was resolved and all deaths due to COVID‐19 irrespective of comorbidities were reported later as a backlog leading to a sudden spike in deaths on some days (TNN, 2020; Radhakrishnan, 2020; worldometer.info). Otherwise, the deaths per day have seen a slow increase reaching the 500 mark in 5 months after the first confirmed case, with a total death count of 31 425 in 6 months, which is in no way a sign of a rampaging disease for a 1.37 billion populous country especially considering the fact that a lot of deaths are comorbidity‐related. It was also felt by some that the disease has been missed in rural India where the majority of the Indian populace (~66%) resides (Sharma, 2020), however, no evidence for a disproportionate presence of the disease in rural India was reported and indeed the majority of cases and deaths in the country have so far come from very few major cities (Awasthi & Mavlankar, 2020; Saxena, 2020), as expected.
The population in urban slums in India is very dense; up to 10 people share a small apartment of 100 sq ft or less and not to mention the hazards of using common toilets between several such apartments. The Dharavi slum of Mumbai with a radius of 2.5 km is home to 750 000‐800 000 people and has mostly underprivileged people with scant facilities, living in substandard conditions mostly catering to the menial jobs that run the city of Mumbai. Dharavi could be an important case study on COVID‐19 since it represents a major subset of the Indian population living in poverty with limited access to healthcare. There were ample opportunities for SARS‐CoV‐2 to cause a massive spread and mortality if it were to infect the hosts indiscriminately (Adiga et al., 2020). It was indeed predicted on Apr 4, 2020 when the Dharavi slum recorded its first COVID‐19 death that India was on for an “onslaught” (Sud, Regan, & Mitra, 2020). The slum was a major concern for the state government and was under strict screening and surveillance throughout the pandemic period (Shelar & Mahale, 2020). However, till the end of June, there were only 81 deaths that includes zero deaths reported for the month of June in this slum, which has the dubious reputation of being the largest slum in Asia; the daily case count also has reduced to single digits (Sarkar, 2020). These are surprising figures, considering the Reproductive number (Ro) of SARS‐CoV‐2 that ranges from 2.24 to 3.58 according to some estimates (Zhao et al., 2020). The Dharavi COVID‐19 example is a complete contradiction of expectations and expert opinions on how the pandemic was supposed to behave. The WHO has cited Dharavi along with Spain, Italy, and S. Korea as examples where COVID‐19 has been adequately managed (Eeshanpriya, 2020), even though the success was ascribed to the mitigation measures that were apparently carried out. The living conditions in Dharavi make the diligent implementation of non‐pharmaceutical interventions almost impractical, yet we see that the virus has not caused any serious problem in this slum, clearly suggesting that it does not prefer hosts in this setting. This will also suggest that COVID‐19 is fundamentally different from other pandemic diseases that have occurred before (Spanish Flu, Asian Flu, Hong Kong Flu, and Bubonic Plague) where apparently the poor and underprivileged were the main targets. Dharavi should become a part of a research program with a biocultural anthropological perspective on COVID‐19; this effort could reveal important insights and interpretations on how the disease has manifested at the population level, not only in India but the world over.
From the above observations, it appears that SARS‐CoV‐2 has been a mild disease in India, at least so far, in terms of mortality rates, in contrast to observations in the United States and European countries. This conclusion is valid in light of the fact that a large majority of India does not have access to a good health care system; a lack of proper health care in severe COVID‐19 patients could have easily amplified the death toll manifold; however, current mortality data and reports from various sources does not support such a trend. Does this mean that Indians in general are more resistant to a severe form of the infection? Does the average Indian host have a superior immune response to SARS‐CoV‐2?
One argument for such low deaths occurring in India due to COVID‐19 is that the country went into an early lockdown that could have curtailed the spread of the infection (Hollingsworth, 2020; Mahajan & Koushal, 2020). However, all other countries under comparison (except Sweden) also went into lockdown since the beginning of the pandemic when initial few cases or deaths were recorded, but could not prevent high mortality rates. A study has shown that substantial transmission of SARS‐CoV‐2 in the community would have already happened even before symptoms appear (He et al., 2020). This means control measures such as a lockdown will not be effective once symptoms start to show up in the community (He et al., 2020). Therefore, the interpretation that lockdown measures implemented in India have resulted in lower deaths due to COVID‐19 may be erroneous (Mahajan & Koushal, 2020). This is apart from the fact that the strict implementation of the measures in India was questionable (Bhattacharryya, 2020). Most importantly, a study has shown that over 44% of the positive cases could not be contact traced suggesting community transmission was ongoing during the lockdown period in India (Abraham et al., 2020).
3. TRAINED INNATE IMMUNITY COULD BE AN IMPORTANT PROTECTIVE FACTOR IN PANDEMICS
Trained immunity or trained innate immunity is a recent paradigm in immunology that is fast catching up to reveal its enormity in the impact it may have on human health and disease (Netea et al., 2020a). It is now recognized that the innate immune system like the adaptive immune arm can also have some memory so that its response to a subsequent encounter of not only the same but related stimulus is more pronounced. However, unlike the adaptive immune system whose memory derives from genetically reorganized clones of T and B cells that remain quiescent for long periods of time under cytokine influence, the innate immune cells depend on epigenetic reprogramming to retain a memory of an initial encounter‐response to a stimulus. Apparently, the innate immune cells like macrophages, NK, and even DCs get trained because of an initial encounter of the stimulus and can retain the training as a memory imprinted in their epigenetic landscape. The memory is not only in the cells that went through this training but even the hematopoietic stem cell precursors in the bone marrow are also reprogrammed by the stimulus so that a long‐lasting memory is perpetuated. Furthermore, other types of cells like epidermal and stromal stem cells also are known to be trainable suggesting far‐reaching implications into our understanding of vertebrate biology as this area gets more attention. Trained immunity is a common phenomenon in plants and invertebrates while it showed up in different observations in vertebrate biology too. For example, it was known that the BCG vaccine protected children against other respiratory diseases and decreased mortality (Garly et al., 2003). The phenomenon was defined first by Netea and colleagues (Netea, Quintin, & van der Meer, 2011). They made an observation that the β‐glucan component of the pathogen C. albicans can induce memory in mice that could protect them from other infections like S. aureus (Quintin et al., 2012). BCG vaccine against Tuberculosis has known to have nonspecific protective effects against other infections and also against tumors; this is also attributed to trained immunity (Netea et al., 2020b).
Genomics studies have taught us that the observed variation in a polygenic trait such as an immune response is controlled by more nongenetic and epigenetic factors than genetic factors (Brodin et al., 2015; Brodin & Davis, 2017; Liston, Carr, & Linterman, 2016). Any given population will have a mix of hosts that will have a range of responses against an infection dictated by all those factors. Figure 3A shows a typical mortality curve in an epidemic/pandemic infection; it is a simplistic model based on the observations made on COVID‐19 regarding host susceptibility and presymptomatic transmission (He et al., 2020; Huang et al., 2020). SARS‐CoV‐2 has the serial interval smaller than the incubation period (glossary); hence, the substantial transmission would have happened before the community realizes that an infection is ongoing. When a new fast‐spreading virus enters the community, depending on its Ro it may take a certain amount of time to reach a critical mass. Measures to counter the spread of the infection after this critical mass is reached would be of little help in reducing the casualties significantly, as it would have reached the maximum number of vulnerable hosts (He et al., 2020).
FIGURE 3.
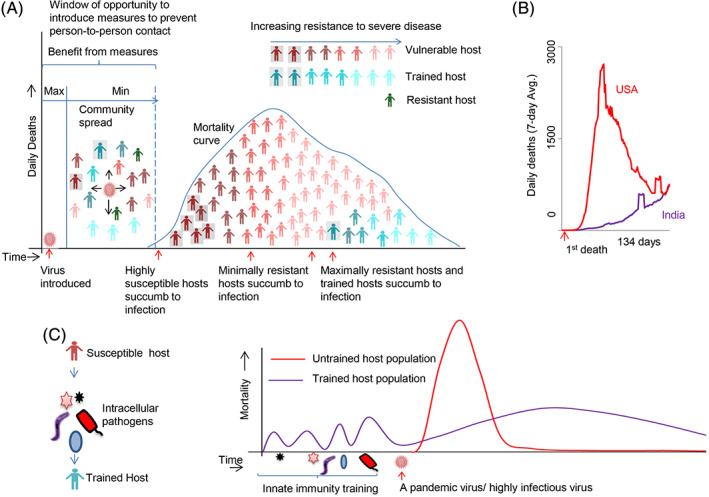
India shows a less severe COVID‐19 likely because it has more trained hosts due to endemic exposure to intracellular pathogens. A, A simplistic mortality curve model based on data for COVID‐19 considering the nature of the hosts that are succumbing to infections (Huang et al., 2020) is drawn. Based on the data from He et al. (2020), a presymptomatic transmission is assumed (a 100% transmission is an assumption in the model, even though He et al. (2020) have shown it as 44%). The resistance of the hosts increases over time in the mortality curve as shown. B, The curve of daily deaths for the United States and India. The data for each country begins from the day it had its first death and goes till the next 134 days. The 134 days was chosen since India had passed that many days from the day of its first death due to COVID‐19, till Jul 24, 2020. C, Trained immunity could also be due to exposure to endemic intracellular pathogens. The Indian hosts get a constant exposure to infections from M. tuberculosis, arthropod‐borne RNA viruses, and protozoan parasites and develops a trained immune system that protects them from a severe disease from a new but related pathogen like SARS‐CoV‐2
Any population would have a mix of hosts with varying levels of immune responses. The most vulnerable will appear in the first part of the mortality curve as they have the least chance of fighting the virus, for example, older individuals with frail immune systems (Figure 3A). The next part is occupied by the slightly resistant hosts, but their resistance is limited and handicapped by their comorbid conditions. This group would comprise of individuals with several preexisting conditions like heart, lung, kidney, and metabolic ailments (Zadori, Vansca, Farkas, Hegyi, & Eross, 2020). These hosts are resistant enough but due to their comorbidities, their successful response to the virus is compromised. Naturally, a large proportion of the population in urban areas with lifestyle disorders would populate the middle part of the curve. The most resistant hosts for the infection including trained hosts would fall on the latter part of the curve. According to this model the mortality curve can be flattened by 1) decreasing the Ro of the virus either naturally by having communities with lower population density or artificially by implementing social distancing measures before a critical mass of the virus is attained, and 2) having more resistant hosts including trained hosts in the community.
The data on daily deaths for 134 consecutive days for the United States and India are shown in Figure 3B. The United States has a very steep forward slope followed by a similar slope in the backward direction in a short period of time, but the curve has not reached the bottom yet. India, in comparison, seems to be having a flat curve all along with a slow increase in death toll toward the end. Based on the model in Figure 3A, India could not have achieved this flatness in the curve through population density; neither is there convincing evidence to say that the lockdown measure was successful. Flattening or bending of the mortality curve during the course of COVID‐19 is something that every country thrived for, but India seems to have achieved this feat, somewhat, naturally. Is it possible that India has a large number of resistant hosts? COVID‐19 is causing severe infections in aged and people with comorbidities in the developed countries (Guan et al., 2020; Liu, Chen, Lin, & Han, 2020); more people from the socioeconomically deprived background are dying due to the disease (Fielding‐Miller, Sundaram, & Brouwer, 2020; van Dorn, Cooney, & Sabin, 2020). India has a relatively younger population compared to the other countries, but certainly has a large proportion of people with comorbidities like diabetes, heart diseases, and recently even cancer (Chandramohan & Thomas, 2018; Corsi & Subramanian, 2019). These diseases have now reached epidemic proportions in the country (Kaveeshwar & Cornwall, 2014; Prabhakaran, Jeemon, & Roy, 2016). So, why are we not seeing (or not seen so far) a similar steep mortality curve in India like the other developed countries? Is it then possible that India has more trained hosts?
4. INDIA COULD HAVE GAINED FROM ITS ENDEMIC PATHOGENS
India does have a BCG vaccination program for children in place. However, India is also endemic to several RNA viruses like DENV (Dengue), JEV (Japanese encephalitis virus), H1N1 (Influenza), CHIKV (Chikungunya), and others (Ganeshkumar et al., 2018; Ravi, 2006; Suri & Sen, 2011; Tiwari, Singh, Tiwari, & Dhole, 2012). Recent emerging RNA viruses like Zika and Nipah also have found a foothold in the country (Rolph & Mahalingam, 2019; Thomas et al., 2009). Another RNA virus, Coxsackievirus (CV‐16) that causes hand‐foot‐and‐mouth disease in children was also reported in the last few years in India (Rao, Naidu, Maiya, Babu, & Bailly, 2017). Therefore, the Indian population is constantly exposed to RNA viruses either endemically or epidemically or as emergent infections. The susceptible hosts vary in each of the above diseases, but a large proportion of the population remains resistant to infections or gets infected with mild symptoms and recovers. For instance, children are the most susceptible hosts in CV‐16 and JEV infections, while adults are affected more with DENV and CHIKV. Not only RNA viruses, but India is endemic to Tuberculosis, Leishmania, and Malaria infections (Gutierrez et al., 2006; Kumar, Valecha, Jain, & Dash, 2007; Singh, Hasker, Boelaert, & Sundar, 2016).
A large number of studies have now reported that BCG vaccination may have a role in offering protection against the ongoing COVID‐19 (Netea et al., 2020b; Escobar, Molina‐Cruz, & Barillas‐Mury, 2020; Gursel & Gursel, 2020; Ozdemir, Kucuksezer, & Tamay, 2020; Covian, Retamal‐Diaz, Bueno, & Kalergis, 2020; Madan et al., 2020; Weng et al., 2020; Meena, Yadav, & Kumar, 2020). If this were to be true then India, which not only has a BCG vaccination program for children, could benefit from the fact that Tuberculosis is endemic to the region. Similar to the RNA viruses M. tuberculosis, the causative agent of Tuberculosis may also be constantly stirring up the immune responses of an average Indian on a regular basis. The fact that a large proportion of the Indian population is latent carriers of M. tuberculosis, without frank disease is evidence of this (Houben & Dodd, 2016). RNA viruses like DENV, CHIKV, JEV, and parasites that cause Malaria and Leishmania are transmitted by arthropod vectors that are difficult to control due to large population size, poverty, and lack of sound infrastructure in the country. The population density especially in urban centers also makes the transmission of respiratory diseases like Tuberculosis and H1N1 Influenza much easier since they get transmitted by the aerial route. Therefore, the Indian host has to constantly encounter these pathogens on a daily basis (e.g., M. tuberculosis), some every season (e.g., DENV, H1N1).
A Th1 adaptive immune response is the one that ultimately gets rid of intracellular pathogens like viruses, bacteria, or protozoan parasites (Ley, 2017). Macrophages and possibly DCs and other immune cells also have polarized states that reflect the nature of the pathogen that they have encountered. For example, M1 macrophages are more proinflammatory and eliminate M. tuberculosis and Leishmania in resistant hosts (Atri, Guerfali, & Laouini, 2018). Since innate immunity is non‐specific, several PRRs that recognize different PAMPs may stimulate similar endpoint responses. For example, Lipopolysaccharide a TLR‐4 ligand and poly(I:C) a TLR‐3 ligand both lead to the stimulation of common pathways inside the cell (Reimer, Brcic, Schweizer, & Jungi, 2008). Therefore, a common overlapping epigenetic landscape may be defining the responses to different intracellular pathogens dealt by innate immune cells. Exposure to one intracellular pathogen would imply that trained immunity would ensure an augmented response to another related pathogen. It is known that SARS‐CoV‐2 does not stimulate a robust IFN response in infected cells (O'Brien et al., 2020). Therefore, a trained cell would be in a far more advantageous position to raise a response against SARS‐CoV‐2 compared to a non‐trained cell since the threshold for stimulation would be lower in the trained cell (Figure 3C).
The important conclusion we can draw from India's COVID‐19 mortality curve is that the infection is unusually prolonged for a novel virus‐like SARS‐CoV‐2 (Figures 2A and 3B) (Banerjee, 2020a). This would also mean, contrary to the model shown in Figure 3A, the community spread is still ongoing in India when compared to other European countries (Banerjee, 2020b). The most likely explanation for this is that there is a natural resistance in the Indian population that is halting the rapid spread of the virus in the community. This is in contrast to the European countries that have seen an almost complete decline in morbidity and mortality within ~3 months of the first infection in their respective communities (Figure 2A); interestingly these countries have not seen a reemergence of the virus even after lifting their lockdown measures (Bhattacharryya, 2020). Have these communities therefore reached their herd immunity threshold? (Banerjee, 2020a; Banerjee, 2020b). A minimum of 50% of the population should get infected and recover from COVID‐19 in order to achieve a herd immunity threshold for a conservative estimate of Ro = 2.0 (Petersen et al., 2020); however, recent seroprevalence studies have shown a prevalence rate of only 5% in Spain (with lockdown) and around 7.3% in Sweden (without lockdown) (Griffin, 2020). There could be two possibilities to explain this discrepancy: either the estimate of the spread of the infection in the community is grossly inaccurate (due to less sensitive tests) or there is some form of preexisting immunity even in these communities (Lourenco, Pinotti, Thompson, & Gupta, 2020), maybe to a lesser extent than that prevalent in India. Neuroscientist Karl Friston calls this latter possibility as the “immunological dark matter” in COVID‐19 (Spinney, 2020). Since SARS‐CoV‐2 is a supposedly novel virus, and unless the cross‐protection to SARS‐CoV‐2 from preexisting immunity to other coronaviruses is unequivocally proved (Lourenco et al., 2020), adaptive immunity is less likely to be the protective factor (Banerjee, 2020a, Banerjee, 2020b). In an elegant analysis, Banerjee (2020a) has pointed out that the protection seen in India from COVID‐19 could be because of an innate immunity factor. Therefore, the Indian COVID‐19 mortality data allows us to hypothesize that the presence of hosts in the population who have some form of resistance (not necessarily adaptive immunity‐mediated herd immunity) can help prolong the spread of the infection to the vulnerable hosts and significantly flatten the curve (Figure 4). If the hypothesis is true, then as a natural consequence, such a population would end up with lesser total mortality compared to those populations without a significant number of resistant hosts due to the fact that pandemics do not last for a long time (Singh, 2020). In support of this claim, a modeling study has shown that a heterogeneous host population in terms of susceptibility to disease can lower the threshold for herd immunity (Gomes et al., 2020). Another modeling study suggests that SARS‐CoV‐2 infection will linger on until a “hub” is infected which leads to a sudden rise in infections leading to a fast progression toward a peak (Herrmann & Schwartz, 2020). Since the Indian COVID‐19 mortality curve has still not seen its peak even after 6 months, it is an indication that the virus is struggling to reach a certain critical number of vulnerable hosts, possibly because it is encountering resistant hosts frequently. In line with this, the Indian health ministry has consistently denied that a community spread of COVID‐19 has happened in India (Kaur, 2020).
FIGURE 4.
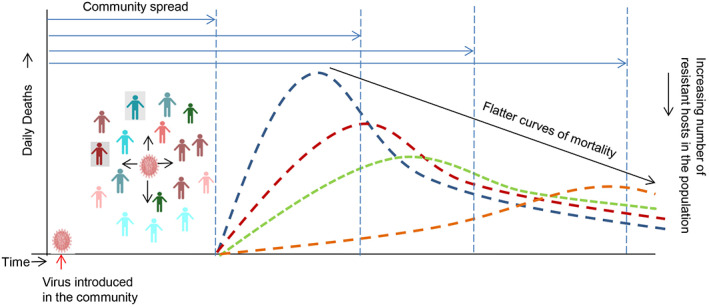
The COVID‐19 curve can be flattened by having more resistant hosts in the population that can halt the virus from reaching its critical mass rapidly. Populations that have more resistant hosts will prolong the community spread time and lead to a natural flattening of the curve. The gain from such naturally flattened curves is that the burden on public health care provider system will be less; however, the loss is that such populations may become endemic to such infections (Banerjee, 2020b)
India has an average death rate of ~27 000/day due to all causes (www.TheGlobalEconomy.com; data for 2018). Around 1200 deaths occur every day due to infectious diseases, more than 98% contributed by Tuberculosis alone (Table 2; www.ncdc.gov.in). India has so far 31 425 deaths due to COVID‐19 in a span of around 6 months with a flattened curve that is yet to peak. The deaths per day due to COVID‐19 touched the 500 mark after 5 months since the first confirmed SARS‐CoV‐2 infection in India (worldometers.info); the shallow slope of the Indian curve would mean it would take a considerable amount of time to reach the death toll similar to that of Tuberculosis in India (Table 2), provided the infection persists that long. This will suggest that COVID‐19 has not been a bigger public health problem than Tuberculosis in the Indian population. Any assessment of a public health crisis in India should involve and account for the vast majority of its population who live near or below the poverty line; but so far there have been no signs that this large stakeholder group is adversely affected by COVID‐19 (Dharavi, for example). Yes, the urban hospitals appear to be crowded in recent weeks, but even here, the situation is not as bad as it was expected and cities like Delhi have started to show signs of recovery (Rukmini & Rampal, 2020). It is also true that the exaggerated attention the virus has got, mainly due to improper communication through media channels, has led to an increase in hospital traffic. For example, instead of focusing on percent positivity rates, most media outlets harp on increasing cases, despite the fact that this measure is confounded by testing frequency (ourworldindata.org; Figure 2C). In such situations, people who can afford private hospitals, out of fear from the constant information of increasing cases around them, would like to get themselves tested and treated for the disease even if they have mild symptoms, and hence could lead to an increase in demand for hospital beds. On the contrary, the poor who face other pertinent problems than COVID‐19 in their lives, are seen to be moving on with their daily activity as usual, since the time the lockdown was lifted. In summary, the Indian mortality rates due to COVID‐19 have not reached alarming rates and are likely to remain so given the naturally flattened nature of its mortality curve. The same, may not be said about the morbidity curve, which is seeing a constant increase (now reaching >45 000 cases/day; worldometers.info). After adjusting for age‐dependent mortality, India had ~12 times less mortality due to COVID‐19 in the age group of >65 y, relative to the United States by the end of June 2020. This figure is highly deflated as it does not account for the population density differences between the two countries, which will directly affect the Ro of the virus. A modeling study had predicted 1.56 million deaths with a peak by mid‐July due to an uninterrupted COVID‐19 pandemic in India (Chatterjee, Chatterjee, Kumar, & Shankar, 2020). Therefore, the deaths due to COVID‐19 in India so far have defied all projections arguing for alternate explanations to understand this phenomenon.
TABLE 2.
Number of yearly cases and deaths from infectious diseases in India
| Disease | 2016 cases, deaths | 2017 cases, deaths | 2018 cases, deaths | 2019 cases, deaths |
|---|---|---|---|---|
| H1N1 a |
1786 263 |
38 811 2270 |
15 266 1128 |
28 798 1218 |
| Dengue a |
129 166 245 |
188 401 325 |
101 192 172 |
136 422 132 |
| AES a |
11 651 1301 |
13 672 1097 |
11 388 636 |
14 995 710 |
| Malaria a |
8,44 558 194 |
4,29 928 96 |
3,34 693 50 |
19 980 2 |
| Tuberculosis b |
2 790 000 435 000 |
2 740 000 421 000 |
2 690 000 450 000 |
NA |
Abbreviations: AES, acute encephalitis syndrome, NA, not available.
Obtained from www.ncdc.gov.in.
Global tuberculosis reports, WHO.
The rising number of COVID‐19 cases in India (from more than 30 000/day in last two weeks) is concerning at present; however, India is constantly increasing its testing capacity (Figure 2C), and therefore, an increase in caseload may be irrelevant as long as there is no drastic increase in percent positivity rates. The deaths/day are currently hovering around 750 to 800/day, which may not be a cause for concern as most deaths are due to preexisting conditions where SARS‐CoV‐2 may not be a direct cause of death. The daily deaths seemed to increase in Delhi and Mumbai in the middle of June (Jain & Dasgupta, 2020; Parth, 2020), but the cases are now (end of July) showing a downward trend in big cities (Rukmini & Rampal, 2020), except maybe in southern India, which is showing a late surge. The real impact of SARS‐CoV‐2 infection will become clear only when we compare season‐matched pre‐COVID‐19 mortality rates in India due to non‐communicable diseases with that of the mortality rates in these diseases after COVID‐19 testing became possible, in order to ascertain the “extra” death toll in these diseases accountable to the virus. Many state governments have set up “COVID‐19 mortality audit committees” comprising of physicians and pathologists to correctly assess the cause of a death where SARS‐CoV‐2 was associated with; such an exercise will anyway be highly challenging even for the best‐trained doctors. Therefore, it remains to be determined if COVID‐19 has significantly exaggerated the mortality in people with non‐communicable diseases in India; it will be an important statistic as most of the deaths are occurring in the urban population, which have a high prevalence of lifestyle diseases.
The question is: where does India currently stand in COVID‐19, and where is it likely headed? If we presume that the pandemic had a similar effect in India as it had on other developed countries, then, thanks to inadequate testing, India may have grossly underreported COVID‐19 mortality (Figure 5A, model A). However, this appears to be least likely as concluded by several recent reports (Chakrabarti et al., 2020; Gupta & Misra, 2020; Ram et al., 2020; Reddy & Pandey, 2020). The other likely scenario is that COVID‐19 in India had a peak of infections and deaths sometime in the past (Figure 2B), but the severity was so low that it went unnoticed in the background of other deaths from other diseases, especially those deaths that have occurred due to metabolic and cardiovascular diseases (model B, Figure 5A). If this model is correct than the increase happening currently in infection rate is only because of an increase in the rate of testing (Figure 2C); in support of this, a study has shown that the percent positivity rate has shown a small range of 3% to 6% from Mar to Apr 2020 (Abraham et al., 2020). Model B, which premises that infection peak happened in the past could be wrong if the infection indeed began recently, but the rate of spread was exactly the same as the increase in testing rate, which will be highly coincidental, and therefore, less likely. If Model B is valid, then the observed COVID‐19 death numbers in India would continue to increase until they catch up with the actual COVID‐19 mortality curves (model B, Figure 5A). The evidence against this models is that the health care system and crematorium records in cities like Mumbai and Delhi show a likely peak happening only from mid‐Jun (Jain & Dasgupta, 2020; Parth, 2020); if true, this means that COVID‐19 in India is currently at its peak or is yet to reach there (model C, Figure 5A). It is also possible that COVID‐19 may become endemic in India with regular smaller peaks or even a plateau for a long period of time until the critical mass is reached (Endemicity, Figure 5A).
FIGURE 5.
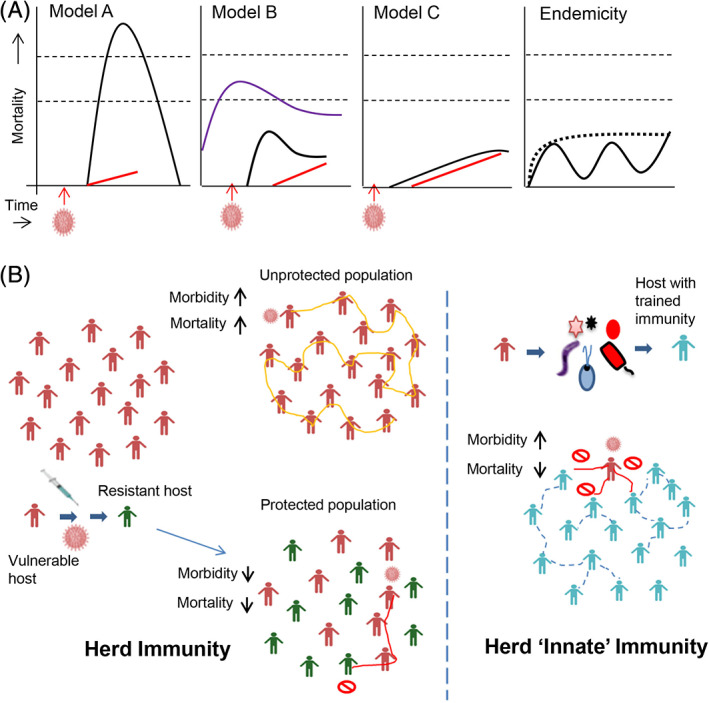
India may have escaped a severe COVID‐19 disease due to trained innate “herd” immunity. A, Presumptive models to explain COVID‐19 deaths in India. Model A presumes high mortality similar to the one seen in developed countries, but inadequate testing for SARS‐CoV‐2 in India has not captured a significant part of the mortality. A country with daily deaths of >27 000 due to all causes (upper thin dashed line) and >1200 due to other infectious diseases (lower thin dashed line), will have to have a large increase in death rate above and over these figures to be detected as a genuine signal from new causes. Model B presumes that COVID‐19 was present sometime before the testing began in India and had its peak some time ago, but went undetected in the background of other deaths involving comorbidities and infections. Model C presumes that SARS‐CoV‐2 has not yet reached its critical mass in the Indian population and mortality is currently showing its peak or the peak is yet to come. (Black solid curve‐actual daily mortality due to COVID‐19; Blue curve‐daily mortality due to other infections; Red solid line‐COVID‐19 daily mortality rate confirmed by diagnostic testing). The endemicity model presumes that COVID‐19 may become a perpetual infection of a low grade similar to other viral infections like Dengue in the Indian population; there may be several smaller peaks (solid line) or a plateau (thick dashed line). B, Innate 'herd' immunity may be an important protective factor in pandemics. Trained immunity so far has only considered the BCG vaccine as a likely primer for boosting immunity against SARS‐CoV‐2. Here, I propose that endemic pathogens like other RNA viruses, M. tuberculosis (as latent infections) and others may have boosted the innate immunity of the Indian population that may be offering protection to a large majority of people from severe COVID‐19. Whether an innate 'herd' immunity factor is functioning similar to the concept of the classical herd (immunity) effect, needs further investigation
The age break‐up for COVID‐19 deaths in India is: 0.5% in <15 years; 2.5% in 15 to 30 years; 11.4% in 30 to 45 years; 35.1% in 45 to 60, and 50.5% in >60 years of age. Around 73% of COVID‐19 deaths have occurred in patients with comorbidities, and men account for 64% deaths (Ministry of Health, Govt. of India, Press release May 5; Mudur, 2020). However, two observations that stand out from India are: COVID‐19 deaths are about eight‐fold higher in India in the age below 60 years compared to a similar age group in Italy (Mukhopadhyay, 2020); the other is that the disease has been more severe in women than men in India (Joe, Kumar, Rajpal, Mishra, & Subramanian, 2020). This is in contrast to observations in the developed countries (Wenham, Smith, & Morgan, 2020), perhaps also a reflection of how the disease has manifested differently in the Indian population. The state of Kerala, which has the highest percentage of aged people in the country is commended for its management of the disease with just 24 deaths at the end of June even though the first case in the country was detected here; this is despite the fact that Kerala has a large tourist population and natives living abroad who frequently travel (Gulia & Kumar, 2020). Over 50% of total COVID‐19 deaths in India have occurred in five major cities (Awasthi & Mavlankar, 2020). This may suggest that rural India will see the spread much later after the urban centers are through with their peaks. India may end up showing huge morbidity and even end up becoming endemic to COVID‐19 (Banerjee, 2020b), but if the current trend holds up in mortality, it would see lesser relative mortality in comparison to the other developed countries (Reddy & Pandey, 2020). This could be significant considering the fact that India is a developing country with a large section of the populace living in poverty. This would mean that COVID‐19 in India would leave a negligible impact on its impoverished population compared to its top killer disease, Tuberculosis. The irony is that this same killer disease along with other intracellular pathogens and RNA viruses may have protected a large majority of the Indian population from a severe COVID‐19 through trained “herd” immunity (Figure 5B). While COVID‐19 seems to have spared the poor in India so far, what damage it may cause to the urban middle and upper‐middle class is still not clear, as the disease is still ongoing. The other irony here is that the poorer sections of the community, who usually are at the mercy of those in the higher layers of the power pyramid, may have inadvertently protected the latter by acting as “speed breakers” in the spread of SARS‐CoV‐2 and may have minimized the damage due to severe COVID‐19 in urban centers of India. Certainly, the Indian COVID‐19 experience would remain interesting for future research on viral epidemiology and also trained immunity.
5. SOCIOECOLOGICAL AND SOCIOECONOMIC FACTORS AS POSSIBLE DETERMINANTS OF COVID‐19
The problem with comparing COVID‐19 morbidity and mortality rates between different countries and regions of the world is that there may be several factors like inadequate testing, differing specificity and sensitivity of tests, non‐uniform testing methods and phenotype classification, demographics, socioecological, socio‐economical, and others that may be confounders (O'Neill & Netea, 2020). With this caveat in mind, such a comparison of COVID‐19 mortality indeed shows interesting trends (Figure 6). We see that Africa and Asia (including India) have a trend similar to that of India in that the disease seems to be prolonged and mild. Oceania has a distinct trend with the least severe disease among all continents. Europe saw the earliest transmission (of course, after China where the disease is mild/controlled) followed by North America. South America seems to be also delayed in its transmission similar to Asia and Africa, but the mortality seems higher (Figure 6A). Brazil is consistently seeing more than 1000 deaths/per day on an average in the last 60 days (worldometer.info). Interestingly, the eastern states of the United States had a peak closely following that of Europe, but the southern and western states seem to be following the trend of the South American countries with a late surge in infections. There is a temporal matching in the mortality curves of Asia, South America, and the late surge curve seen in North America (Figure 6A), but the mortality is less in Asia compared to the latter two continents. When we classify the countries based on income, we see a clear trend where income is directly proportional to the daily death rate due to COVID‐19 (Figure 6B). This may be a reasonably correct interpretation since the COVID‐19 cases do not seem to correlate with income (Figure 6C). When we look at the number of cases needed for each death, there is a clear inverse correlation with income (Figure 6D). Brazil and India, although have a similar slower trajectory of COVID‐19 mortality curves compared to the European countries, they fall into two different groups economically (India is LMI while Brazil is UMI, Figure 6B‐D), offering a potential explanation to their different mortality rates due to the pandemic (India has 32 096 deaths while Brazil has 86 496 deaths on July 26, 2020; worldometers.info). Overall, while the data in Figure 6B‐D may say that lower socioeconomic status on a global level may correlate with a less severe COVID‐19, paradoxically lower socioeconomic status is a risk factor for severe COVID‐19 in advanced countries like the United States and UK (Bentley, 2020).
FIGURE 6.
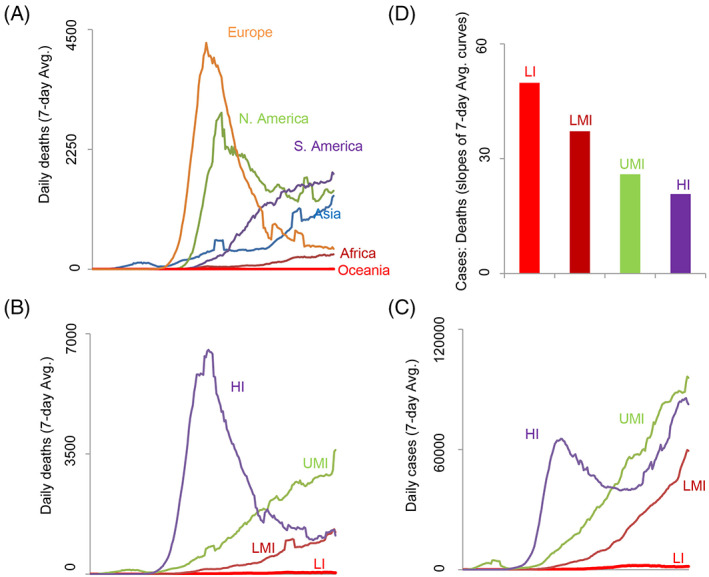
Geographical, socioecological, and socioeconomic factors may be influencing COVID‐19 outcome in different regions/countries of the world. A, Daily death curves showing different trajectories in different continents of the world. B, The daily death curves show striking differences in countries grouped according to their income status showing a negative correlation with the slope of the curves. C, The daily case curves, however, do not show a similar negative correlation with income. D, Lesser number of deaths occurs for a given number of cases in countries with lower incomes compared to the ones with higher incomes. Slopes were calculated for the daily case curves and death curves and ratios are plotted for different groups of countries (LI, low income; LMI, lower middle income; HMI, higher middle income; HI, high income). All the data shown in this figure was from Dec 31, 2019 to Jul 24, 2020. All data were obtained from ourworldindata.org
6. POSSIBLE FACTORS EXPLAINING LESS SEVERE COVID‐19 IN SOME REGIONS/COUNTRIES
The main argument I have for the observed mild COVID‐19 trend in India is that of trained innate immunity due to endemic pathogens, but there could be other reasons to explain this phenomenon not only in India but also in other regions where we see similar trends (Figure 6). Preexisting adaptive immunity to other viruses and cross‐reactivity with SARS‐CoV‐2 may be one factor (Grifoni et al., 2020; Weiskopf et al., 2020). The demographic difference is another important factor; many countries in Africa and Asia are more youthful than the western countries and since the disease has affected mostly the older people, this variable could be an important confounder (Njenga et al., 2020). Modeling studies have shown that the presence of a more heterogeneous population in terms of age can lower the herd immunity threshold in a population (Britton, Ball, & Trapman, 2020). Socioecological factors, including population density, population mobility, and social behavior may have played a role in Africa (Cabore et al., 2020). Ambient temperature and humidity (Yuan, Jiang, & Li, 2020), vitamin D, food habits, and water sources seem to be an explanation for different COVID‐19 trends in different European countries (Bornstein et al., 2020; Bousquet et al., 2020; Rhodes, Subramanian, Laird, Griffin, & Kenny, 2020). Above all, differences in testing methods (specificity and sensitivity), testing frequency, classification criteria for deaths from COVID‐19 may be confounding the analysis presented here as well as in other similar reports (O'Neill & Netea, 2020; Undela & Gudi, 2020). This is a major limitation of ecological analysis of COVID‐19 data (Li & Hua, 2020).
7. CONCLUDING THOUGHTS
In this article, I propose that innate immunity training that occurs due to constant exposure to microbial stimuli may be protecting a majority of the Indian population from a severe form of SARS‐CoV‐2 infection. Others have opined that this phenomenon may be related to the hygiene hypothesis (Sehrawat & Rouse, 2020). While epidemiological and experimental evidence is awaited to unequivocally refute this viewpoint, it certainly is suggestive of positive deviance (Marsh, Schroeder, Dearden, Sternin, & Sternin, 2004) in COVID‐19.
Human biology is thought to “emerge from a complex dance” of genes and the environment (Goodman, 2006). COVID‐19 can be a great opportunity to understand this phenomenon in depth (Pearl, 1930). Genetics may have had a significant role in the protection from severe COVID‐19 in some regions of the world like the Southeast Asian (Sohn, Phanuphak, Baral, & Kamarulzaman, 2020) and East Asian countries; Japan, despite having the highest percentage of older individuals, has not been severely affected by COVID‐19. East Asians are known to have some distinct polymorphisms related to genes in immunity (Oliveira et al., 2018; Prokunina‐Olsson et al., 2013). For example, the dinucleotide variant that gives rise to the novel human IFN‐L4 gene and other type III IFN polymorphisms are under strong positive selection pressure in East Asians (Key et al., 2014; Manry et al., 2011). On the other hand, the environment could have played a significant role in explaining the COVID‐19 trend in South Asia, including India and Africa. Even though ecological analysis of COVID‐19 is problematic, it is hard to miss the inverse correlation of income and severity of COVID‐19 in different countries of the world (Figure 6B‐D; Cash & Patel, 2020; Schellekens & Sourrouille, 2020). It is also interesting to note that COVID‐19 has been relatively severe in more organized societies than less organized ones. In addition, COVID‐19 has not affected children, unlike past Flu pandemics, possibly because SARS‐CoV‐2 likes to target phenotypes that have certain acquired characteristics found in adults (e.g., life‐style disorders), despite the fact that the latter have more stronger immunity. These observations point to a strong effect of environment in the biology of COVID‐19; understanding this effect may hold the key to what Karl Friston refers to as the “immunological dark matter” (Spinney, 2020).
AUTHOR CONTRIBUTIONS
Sreedhar Chinnaswamy: Conceptualization; formal analysis; investigation; resources; writing‐original draft; writing‐review and editing.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
GLOSSARY
Ro: a measure of how rapidly the virus spreads, which essentially indicates the average number of people the virus can spread to; it is calculated based on several parameters, including the closeness of contacts termed the contact rate.
Serial Interval: time interval in a transmission chain between successive symptomatic cases.
Incubation period: time between infection and symptoms.
Vulnerable hosts: are those hosts who show severe symptoms and will harbor more viral load during the infection and are also more likely to die.
Resistant hosts: are those hosts who may get infected by the virus, but viral loads are not very high and will recover with no or mild symptoms.
Critical mass: the maximum viral load that a virus can develop collectively in a population due to its infection of all the vulnerable hosts; the critical mass is proportional to the number of vulnerable hosts in the population.
Percent positivity rate: number of positive cases divided by the number of tests done.
Herd effect or Herd Immunity: a phenomenon related to acquired immunity that offers protection to some fraction of the susceptible individuals in a population because a larger majority of the population has developed immunity either naturally after the infection or due to vaccination.
Morbidity: a measure of how much a disease is present, for example, COVID‐19 cases in a population.
Mortality: a measure of deaths that have occurred as a result of a disease.
Epigenetics: A subject that deals with the changes that happen during the expression of genes in the DNA genome through several mechanisms like methylation of DNA and chromatin; the changes could be inheritable.
Comorbidities: diseases that are usually present already in a patient in the context of another disease that is present simultaneously.
ACKNOWLEDGMENTS
The author is an Intermediate Fellow of the DBT‐Wellcome trust India Alliance (grant no.170 IA/I/17/1/503122). The author thanks the Director and colleagues at the National Institute of Biomedical Genomics for helpful discussions.
Chinnaswamy S. SARS‐CoV‐2 infection in India bucks the trend: Trained innate immunity? Am J Hum Biol. 2021;33:e23504. 10.1002/ajhb.23504
Funding information Wellcome DBT India Alliance, Grant/Award Number: 170 IA/I/17/1/503122
REFERENCES
- Abraham, P. , Aggarwal, N. , Babu, G. R. , Barani, S. , Bhargava, B. , Bhatnagar, T. , … Yadav, N. (2020). Laboratory surveillance for SARS‐CoV‐2 in India: Performance of testing & descriptive epidemiology of detected COVID‐19, January 22–April 30, 2020. Indian Journal of Medical Research, 121, 424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adiga, A. , Chu, S. , Eubank, S. , Kuhlman, C. J. , Lewis, B. , Marathe, A. , … Wilson, M. L. (2020). Disparities in spread and control of influenza in slums of Delhi: Findings from an agent‐based modelling study. BMJ Open, 8, e017353. 10.1136/bmjopen-2017-017353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altfeld, M. , & Gale, M., Jr. (2015). Innate immunity against HIV‐1 infection. Nature Immunology, 16, 554–562. [DOI] [PubMed] [Google Scholar]
- Atri, C. , Guerfali, F. Z. , & Laouini, D. (2018). Role of human macrophage polarization in inflammation during infectious diseases. International Journal of Molecular Sciences, 19(6), 1801. 10.3390/ijms19061801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi, A. , & Mavlankar, D. (2020). COVID‐19 in India: An epidemic in congested cities. BMJ Journals‐COVID‐19 Blog. https://blogs.bmj.com/covid-19/2020/06/01/covid-19-in-india-an-epidemic-in-congested-cities/ [Google Scholar]
- Banerjee, D. (2020). How COVID‐19 is overwhelming our mental health. Nature India. https://www.natureasia.com/en/nindia/article/10.1038/nindia.2020.46 [Google Scholar]
- Banerjee, S. (2020a). Can innate immunity flatten the curve? Covid‐19—A case study. Preprints 2020, 2020040353. 10.20944/preprints202004.0353.v1 [DOI] [Google Scholar]
- Banerjee, S. (2020b). Is novel coronavirus novel: Covid‐19, a pandemic or an endemic? Preprints 2020, 2020050515. 10.20944/preprints202005.0515.v1 [DOI] [Google Scholar]
- Bentley, G. R. (2020). Don't blame the BAME: Ethnic and structural inequalities in susceptibilities to COVID −19. American Journal of Human Biology. 10.1002/ajhb.23478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, A. (2000). Th1 and Th2 responses: What are they? BMJ, 321(7258), 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia, R. , & Abraham, P. (2020). Lessons learnt during the first 100 days of COVID‐19 pandemic in India. Indian Journal of Medical Research, 151, 387–391. 10.4103/ijmr.IJMR_1925_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharryya, I. (2020). Why India's lockdown has been a spectacular failure. The Wire. Retrieved from https://thewire.in/government/india-covid-19-lockdown-failure.
- Biswas, S. (2020). India coronavirus: The 'mystery' of low Covid‐19 death rates. BBC. Retrieved from https://www.bbc.com/news/world-asia-india-52435463.
- Bornstein, S. R. , Voit‐Bak, K. , Scmidt, D. , Morawietz, H. , Bornstein, A. B. , Balanzew, W. , … Straube, R. (2020). Is there a role for environmental and metabolic factors predisposing to severe COVID‐19? Hormone and Metabolic Research, 52, 540–546. 10.1055/a-1182-2016 [DOI] [PubMed] [Google Scholar]
- Bousquet, J. , Anto, J. M. , Laccarino, G. , Czarlewski, W. , Haahtela, T. , Anto, A. , … Zuberbier, T. (2020). Is diet partly responsible for differences in COVID‐19 death rates between and within countries? Clinical and Translational Allergy, 10(16), 16. 10.1186/s13601-020-00323-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstadter, J. D. , & Yang, Y. (2011). Natural killer cell responses to viral infection. Journal of Innate Immunity, 3(3), 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton, T. , Ball, F. , & Trapman, P. (2020). A mathematical model reveals the influence of population heterogeneity on her immunity to SARS‐CoV‐2. Science, 369, 846–849. 10.1126/science.abc6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin, P. , & Davis, M. M. (2017). Human immune system variation. Nature Reviews Immunology, 17(1), 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin, P. , Jojic, V. , Gao, T. , Bhattacharyya, S. , Angel, C. J. L. , Furman, D. , … Davis, M. M. (2015). Variation in the human immune system is largely driven by non‐heritable influences. Cell, 160, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabore, J. W. , Karamagi, H. C. , Kipruto, H. , Asamani, J. A. , Droti, B. , Seydi, A. B. W. , … Moeti, M. R. (2020). The potential effects of widespread community transmission of SARS‐CoV‐ 2 infection in the World Health Organization African region: A predictive model. BMJ Global Health, 5, e002647. 10.1136/bmjgh-2020-002647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash, R. , & Patel, V. (2020). The art of medicine. Has COVID‐19 subverted global health? The Lancet, 30, 1687–1688. 10.1016/S0140-6736(20)31089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2020). Past pandemics. Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. Retrieved from https://www.cdc.gov/flu/pandemic-resources/basics/past-pandemics.html.
- Chakrabarti, S. S. , Kaur, U. , Banerjee, A. , Ganguly, U. , Banerjee, T. , Saha, S. , … Chakrabarti, S. (2020). COVID‐19 in India: Are biological and environmental factors helping to stem the incidence and severity? Ageing and Disease, 11(3), 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra, S. , Kuljanin, G. , & Wray, J. (2012). Mortality from the influenza pandemic of 1918‐1919: The case of India. Demography, 49(3), 857–865. [DOI] [PubMed] [Google Scholar]
- Chandramohan, K. , & Thomas, B. (2018). Cancer trends and burden in India. The Lancet Oncology, 19, e663. [DOI] [PubMed] [Google Scholar]
- Chatterjee, K. , Chatterjee, K. , Kumar, A. , & Shankar, S. (2020). Healthcare impact of COVID‐19 epidemic in India: A stochastic mathematical model. Medical Journal Armed Forces India, 76(2), 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetterjee, P. (2020). Gaps in India's preparedness for COVID‐19 control. The Lancet, 20, 544. 10.1016/S1473-3099(20)30300-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaswamy, S. (2016). Gene‐disease association with human IFNL locus polymorphisms extends beyond hepatitis C virus infections. Genes and Immunity, 17, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, R. , & Kupper, T. (2005). Old meets new: The interaction between innate and adaptive immunity. Journal of Investigative. Dermatology, 125, 629–637. [DOI] [PubMed] [Google Scholar]
- Corsi, D. J. , & Subramanian, S. V. (2019). Socioeconomic gradients and distribution of diabetes, hypertension, and obesity in India. JAMA Network Open, 2(4), e190411. 10.1001/jamanetworkopen.2019.0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covian, C. , Retamal‐Diaz, A. , Bueno, S. M. , & Kalergis, A. M. (2020). Could BCG vaccination induce protective trained immunity for SARS‐CoV‐2? Frontiers in Immunology, 11(970), 1–7. 10.3389/fimmu.2020.00970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosse, K. M. , Monson, E. A. , Beard, M. R. , & Helbig, K. J. (2018). Interferon‐stimulated genes as enhancers of antiviral innate immune signaling. Journal of Innate Immunity, 10, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin, E. (2013). Avatars of information: Towards an inclusive evolutionary synthesis. Trends in Ecology and Evolution, 28(6), 351–358. [DOI] [PubMed] [Google Scholar]
- Danchin, E. , Pocheville, A. , & Huneman, P. (2018). Early in life effects and heredity: Reconciling neo‐Darwinism with neo‐Lamarckism under the banner of the inclusive evolutionary synthesis. Philosophical Transactions of the Royal Society B, 374(20180113), 20180113. 10.1098/rstb.2018.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore, B. (2020). Covid‐19: Collateral damage of lockdown in India. BMJ, 369, m1711. 10.1136/bmj.m1711 [DOI] [PubMed] [Google Scholar]
- Dufour, D. L. (2006). Biocultural approaches in human biology. American Journal of Human Biology, 18, 1–9. [DOI] [PubMed] [Google Scholar]
- Dwary, A. (2020). On camera, COVID‐19 health staff attacked, chased away in Madhya Pradesh's indore. NDTV.com. Retrieved from https://www.ndtv.com/india-news/coronavirus-lockdown-india-on-camera-health-workers-attacked-in-madhya-pradeshs-indore-2-doctors-inj-2204649.
- Eeshanpriya, M. S. (2020). WHO praises Dharavi's Covid fight. Hindustan Times. Retrieved from https://www.hindustantimes.com/mumbai‐news/who‐praises‐dharavi‐s‐covid‐fight/story‐quFz7j1kyguZztFCwN8ZtK.html.
- Ellinghaus, D. , Degenhardt, F. , Bujanda, L. , Buti, M. , Albillos, A. , Invernizzi, P. , … Karlsen, T. H. (2019). Genomewide association study of severe Covid‐19 with respiratory failure. New England Journal of Medicine. 10.1056/NEJMoa2020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar, L. E. , Molina‐Cruz, A. , & Barillas‐Mury, C. (2020). BCG vaccine protection from severe coronavirus disease 2019 (COVID‐19). Proceedings of the National Academy of Sciences, 117(30), 17720–17726. 10.1073/pnas.2008410117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil, R. , & Fraga, M. F. (2011). Epigenetics and the environment: Emerging patterns and implications. Nature Review Genetics, 13, 97–109. [DOI] [PubMed] [Google Scholar]
- Fielding‐Miller, R. , Sundaram, M. , & Brouwer, K. (2020). Social determinants of COVID‐19 mortality at the county level. medRxiv. 10.1101/2020.05.03.20089698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, A. (2020). Resistance of children to Covid‐19. How? Mucosal Immunology, 13, 563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fottrell, Q. (2020). Fauci says in 40 years of dealing with viral outbreaks, he's never seen anything like COVID‐19. MarketWatch.com. Retrieved from https://www.marketwatch.com/story/fauci‐says‐covid‐19‐has‐one‐characteristic‐hes‐never‐seen‐before‐ive‐been‐dealing‐with‐viral‐outbreaks‐for‐the‐last‐40‐years‐2020‐06‐23.
- Frieman, M. , Heise, M. , & Baric, R. (2008). SARS coronavirus and innate immunity. Virus Research, 133, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshkumar, P. , Murhekar, M. V. , Poornima, V. , Saravanakumar, V. , Sukumaran, K. , Anandaselvasankar, A. , … Mendale, S. M. (2018). Dengue infection in India: A systematic review and meta‐analysis. PLoS Neglected Tropical Diseases, 12(7), e0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garly, M. L. , Martins, C. L. , Bale, C. , Balde, M. A. , Hedegaard, K. L. , Gustafson, P. , … Aaby, P. (2003). BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non‐specific beneficial effect of BCG? Vaccine, 21, 2782–2790. [DOI] [PubMed] [Google Scholar]
- Gasteiger, G. , D'osualdo, A. , Schubert, D. A. , Weber, A. , Bruscia, E. M. , & Hartl, D. (2017). Cellular innate immunity: An old game with new players. Journal of Innate Immunity, 9, 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, M. G. M. , Corder, R. M. , King, J. G. , Langwig, K. E. , Souto‐Maior, C. , Carneiro, J. , …, & Aguas, R. (2020). medRxiv preprint. 10.1101/2020.04.27.20081893. [DOI]
- Goodman, A. (2006). Seeing culture in biology. In Ellison G. T. H. & Goodman A. H. (Eds.), The nature of difference: Science, society, and human biology (pp. 225–241). New York: Taylor and Francis. [Google Scholar]
- Goodman, A. H. (2013). Bringing culture into human biology and biology back into anthropology. American Anthropologist, 115(3), 359–373. [Google Scholar]
- Griffin, S. (2020). Covid‐19: Herd immunity is “unethical and unachievable,” say experts after report of 5% seroprevalence in Spain. BMJ, 370, 10.1136/bmj.m2728 [DOI] [PubMed] [Google Scholar]
- Grifoni, A. , Weiskopf, D. , Ramirez, S. I. , Mateus, J. , Dan, J. M. , Rydzynski, C. , … Sette, A. (2020). Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell, 181, 1489–1501.e15. 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guallar, M. P. , Merino, R. , Donat‐Vargas, C. , Corral, O. , Jouve, N. , & Soriano, V. (2020). Inoculum at the time of SARS‐CoV‐2 exposure and risk of disease severity. International Journal of Infectious Diseases, 97, 290–292. 10.1016/j.ijid.2020.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W.‐j. , Liang, W.‐h. , Zhao, Y. , Liang, H.‐r. , Chen, Z.‐s. , Li, Y.‐M. , … He, J.‐X. (2020). Comorbidity and its impact on 1590 patients with Covid‐19 in China: A Nationwide analysis. European Respiratory Journal, 55(5), 2000547. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W.‐j. , Ni, Z.‐y. , Hu, Y. , Liang, W.‐h. , Ou, C.‐q. , He, J.‐x. , … Li, L.‐J. (2020). Clinical characteristics of coronsvirus disease 2019 in China. New England Journal of Medicine, 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigliano, F. (2020). COVID pandemic has reminded us there are limits to our understanding of science. The Print. Retrieved from https://theprint.in/opinion/covid-pandemic-has-reminded-us-there-are-limits-to-our-understanding-of-science/428844/.
- Gulia, K. K. , & Kumar, V. M. (2020). Reverse quarantine: Management of COVID‐19 by Kerala with its higher number of aged population. Pshychogeriatrics. 10.1111/psyg.12582 [DOI] [Google Scholar]
- Gupta, A. , Madhavan, M. V. , Sehgal, K. , Nair, N. , Mahajan, S. , Sehrawat, T. S. , … Landry, D. W. (2020). Extrapulmonary manifestations of COVID‐19. Nature Medicine, 26, 1017–1032. 10.1038/s41591-020-0968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, R. , & Misra, A. (2020). COVID19 in south Asians/Asian Indians: Heterogeneity of data and implications for pathophysiology and research. Diabetes Research and Clinical Practice, 165, 108267. 10.1016/j.diabres.2020.108267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursel, M. , & Gursel, I. (2020). Is global BCG vaccination‐induced trained immunity relevant to the progression of SARS‐CoV‐2 pandemic? Allergy, 75, 1815–1819. 10.1111/all.14345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, M. C. , Ahmed, N. , Willery, E. , Narayanan, S. , Hasnain, S. E. , Chauhan, D. S. , … Supply, P. (2006). Predominance of ancestral lineages of Mycobacterium tuberculosis in India. Emerging Infectious Diseases, 12(9), 1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Lau, E. H. Y. , Wu, P. , Deng, X. , Wang, J. , Hao, X. , & Leung, G. M. (2020). Tempioral dynamics in viral shedding and transmissibility of COVID‐19. Nature Medicine, 26, 672–675. [DOI] [PubMed] [Google Scholar]
- Heim, M. H. (2013). Innate immunity and HCV. Journal of Hepatology, 58, 564–574. [DOI] [PubMed] [Google Scholar]
- Herrmann, H. A. , & Schwartz, J.‐M. (2020). Why COVID‐19 models should incorporate the network of social interactions. Physical Biology. 10.1088/1478-3975/aba8ec [DOI] [PubMed] [Google Scholar]
- Ho, C. S. , Chee, C. Y. , & Ho, R. C. (2020). Mental health strategies to combat the psychological impact of COVID‐19 beyond paranoia and panic. Annals Academy of Medicine Singapore, 49, 155–160. [PubMed] [Google Scholar]
- Hoke, M. K. , & Schell, L. M. (2020). Doing biocultural anthropology: Continuity and change. American Journal of Human Biology, 32. 10.1002/ajhb.23471 [DOI] [PubMed] [Google Scholar]
- Hollingsworth, J. (2020). How does India, a country of 1.3 billion people, have around 1,000 coronavirus deaths? CNN. https://edition.cnn.com/2020/04/28/india/india-coronavirus-outbreak-explained-intl-hnk/index.html [Google Scholar]
- Honigsbaum, M. (2020). Revisiting the 1957 and 1968 influenza pandemics. The Lancet, 395, 1824–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, R. (2020). COVID‐19—What we can expect to come. The Lancet, 395, 1821. 10.1016/S0140-6736(20)31355-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, S. (2013). DNA methylation age of human tissues and cell types. Genome Biology, 14, 3156. 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben, R. M. , & Dodd, P. J. (2016). The global burden of latent tuberculosis infection: A re‐estimation using mathematical modelling. PLoS Medicine, 25(13), 10, e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal, N. , & Dar, K. A. (2020). Coronavirus disease (COVID‐19) pandemic: Furnishing experiences from India. Psychological Trauma: Theory, Research, Practice, and Policy, 12, S33–S34. 10.1037/tra0000770 [DOI] [PubMed] [Google Scholar]
- Jain, P. , & Dasgupta, S. (2020). Endless queues & funerals, scared staff—Delhi's Covid crisis unravels in chaos at crematoria. The Print. Retrieved from https://theprint.in/india/endless‐queues‐funerals‐scared‐staff‐delhis‐covid‐crisis‐unravels‐in‐chaos‐at‐crematoria/437088/.
- Jiang, S. , Hillyer, C. , & Du, L. (2020). Neutralizing antibodies against SARS‐CoV‐2 and other human coronaviruses. Trends in Immunology, 41, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe, W. , Kumar, A. , Rajpal, S. , Mishra, U. S. , & Subramanian, S. V. (2020). Equal risk, unequal burden? Gender differentials in COVID‐19 mortality in India. Journal of Global Health Science, 2(1), e17. 10.35500/jghs.2020.2.e17 [DOI] [Google Scholar]
- Joh, T. J. , & Samuel, R. (2000). Herd immunity and herd effect: New insights and definitions. European Journal of Epidemiology, 16, 601–606. [DOI] [PubMed] [Google Scholar]
- Joshi, P. C. (2016). Emergence of medical anthropology in India. The Eastern Anthropologist, 69(1), 1–11. [Google Scholar]
- Kadam, A. B. , & Atre, S. R. (2020). Negative impact of social media panic during the COVID‐19 outbreak in India. Journal of Travel Medicine, 27(3), 1–2. 10.1093/jtm/taaa057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal, R. (2020). Coronavirus: How many cases will India see? Here's one expert's best‐case prediction. IndiaToday, Retrieved from https://www.indiatoday.in/india/story/coronavirus-cases-india-covid-19-ramanan-laxminarayan-interview-1658087-2020-03-21.
- Kaur, G. (2020). COVID‐19 Cases Cross 12 Lakhs in India as Government Reconfirms “No Community Spread”. Grainmart.in. Retrieved from https://www.grainmart.in/news/covid‐19‐cases‐cross‐12‐lakhs‐in‐india‐as‐government‐reconfirmsno‐community‐spread/.
- Kaveeshwar, S. A. , & Cornwall, J. (2014). The current state of diabetes mellitus in India. Australasian Medical Journal, 7(1), 45–48. 10.4066/AMJ.2014.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key, F. M. , Peter, B. , Dennis, M. Y. , Huerta‐Sanchez, E. , Tang, W. , Prokunina‐Olsson, L. , … Andrés, A. M. (2014). Selection on a variant associated with improved viral clearance drives local, adaptive pseudogenization of interferon lambda 4 (IFNL4) . PLoS Genetics, 10, e1004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement, R. J. (2020). The SARS‐CoV‐2 crisis: A crisis of reductionism? Public Health, 185, 70–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna, S. (2020). The Covid‐19 panic and the global north‐south divide—A perspective. Indian Journal of Medical Ethics, 05, 167–168. 10.20529/IJME.2020.048 [DOI] [PubMed] [Google Scholar]
- Kronbichler, A. , Kresse, D. , Yoon, S. , Lee, K. H. , Effenberger, M. , & Shin, J. (2020). Asymptomatic patients as a source of COVID‐19 infections: A systematic review and meta‐analysis. In International journal of infectious diseases (Vol. 98, pp. 180–186). 10.1016/j.ijid.2020.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. , Valecha, N. , Jain, T. , & Dash, A. P. (2007). Burden of malaria in India: Retrospective and prospective view. American Journal of Tropical Medicine and Hygiene, 77, 69–78. [PubMed] [Google Scholar]
- Kuroki, S. , & Tachibana, M. (2018). Epigenetic regulation of mammalian sex determination. Molecular and Cellular Endocrinology, 468, 31–38. [DOI] [PubMed] [Google Scholar]
- Larson, H. J. (2020). Blocking information on COVID‐19 can fuel the spread of misinformation. Nature, 580, 306. [DOI] [PubMed] [Google Scholar]
- Ley, K. (2017). M1 means kill; M2 means heal. Journal of Immunology, 199, 2191–2193. [DOI] [PubMed] [Google Scholar]
- Li, S. , & Hua, X. (2020). The closer to the Europe Union headquarters, the higher risk of COVID‐19? Cautions regarding ecological studies of COVID‐19. medRxiv preprint. 10.1101/2020.04.23.20077008. [DOI]
- Liston, A. , Carr, E. J. , & Linterman, M. A. (2016). Shaping variation in the human immune system. Trends in Immunology, 37(10), 637–646. [DOI] [PubMed] [Google Scholar]
- Liu, K. , Chen, Y. , Lin, R. , & Han, K. (2020). Clinical features of COVID‐19 in elderly patients: A comparison with young and middle‐aged patients. Journal of Infection, 80(6), e14–e18. 10.1016/j.jinf.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Mao, B. , Liang, S. , Yang, J.‐W. , Lu, H.‐W. , Chai, Y.‐H. , & Xu, J.‐F. (2020). Association between age and clinical characteristics and outcomes of COVID‐19. European Respiratory Journal, 55(5), 2001112. 10.1183/13993003.01112-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco, J. , Pinotti, F. , Thompson, C. , & Gupta, S. (2020). The impact of host resistance on cumulative mortality and the threshold of herd immunity for SARS‐CoV‐2. medRxiv Preprint. 10.1101/2020.07.15.20154294. [DOI]
- Madan, M. , Pahuja, S. , Mohan, A. , Pandey, R. M. , Madan, K. , Tiwari, P. , … Mittal, S. (2020). TB infection and BCG vaccination: Are we protected from COVID‐19? Public Health, 185, 91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan, P. , & Koushal, J. (2020). Epidemic trend of COVID‐19 transmission in India during lockdown‐1Phase. Journal of Community Health. 10.1007/s10900-020-00863-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manry, J. R. M. , Laval, G. , Patin, E. , Fornarino, S. , Itan, Y. , Fumagalli, M. , … Casanova, J.‐L. (2011). Evolutionary genetic dissection of human interferons. Journal of Experimental Medicine, 208, 2747–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, D. R. , Schroeder, D. G. , Dearden, K. A. , Sternin, J. , & Sternin, M. (2004). The power of positive deviance. BMJ, 329(7475), 1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena, J. , Yadav, A. , & Kumar, J. (2020). BCG vaccination policy and protection against COVID‐19. The Indian Journal of Pediatrics, 87(749). 10.1007/s12098-020-03371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick, E. R. , & Loannadis, J. P. (2020). Should governments continue lockdown to slow the spread of covid‐19? BMJ. BMJ, 369, m1924. 10.1136/bmj.m1924 [DOI] [PubMed] [Google Scholar]
- Mishra, A. (2007). Teaching medical anthropology in India. Anthropology Newsletter, 48(3), 28–29. 10.1525/an.2007.48.3.28 [DOI] [Google Scholar]
- Mudur, G. S. (2020). Age break‐up of Covid‐19 toll. The Telegraph. Retrieved from https://www.telegraphindia.com/india/age-break-up-of-covid-19-toll/cid/1774891.
- Mukhopadhyay, P. (2020). Is India's Covid‐19 death rate higher than Italy's? Hindustan Times. Retrieved from https://www.hindustantimes.com/opinion/is‐india‐s‐covid‐19‐death‐rate‐higher‐than‐italy‐s/story‐F73TUEHkNkDrgBT6WAeDEM.html.
- Mukhra, R. , Krishan, K. , & Kanchan, T. (2020). COVID‐19 sets off mass migration in India. Archives of Medical Research. 10.1016/j.arcmed.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muliyil, J. P. (2020). A science‐based response to COVID‐19. Indian Journal of Public Health, 64, S90. 10.4103/ijph.IJPH_530_20 [DOI] [PubMed] [Google Scholar]
- Nadella, P. , Swaminatahn, A. , & Subramanian, S. V. (2020). Forecasting efforts from prior epidemics and COVID‐19 predictions. European Journal of Epidemiology. 10.1007/s10654-020-00661-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea, M. G. , Dominguez‐Andres, J. , Barreiro, L. B. , Chavakis, T. , Divangahi, M. , Fuchs, E. , … Latz, E. (2020a). Defining trained immunity and its role in health and disease. Nature Reviews Immunology, 20, 375–378. 10.1038/s41577-020-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea, M. G. , Giamarellos‐Bourboulis, E. J. , Dominguez‐Andres, J. , Curtis, N. , van Crevel, R. , van de Veerdonk, F. L. , & Bonten, M. (2020b). Trained immunity: A tool for reducing susceptibility and severity of SARS‐CoV‐2 infection. Cell, 181, 969–977. 10.1016/j.cell.2020.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea, M. G. , Quintin, J. , & van der Meer, J. W. (2011). Trained immunity: A memory for innate host defense. Cell Host & Microbe, 9, 355–361. [DOI] [PubMed] [Google Scholar]
- Njenga, M. K. , Dawa, J. , Nanyingi, M. , Gachohi, J. , Ngere, I. , Letko, M. , … Osoro, E. (2020). Why is there low morbidity and mortality of COVID‐19 in Africa? The American Journal of Tropical Medicine and Hygiene, 103, 564–569. 10.4269/ajtmh.20-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, T. R. , Thomas, D. L. , Jackson, S. S. , Prokunina‐Olsson, L. , Donnelly, R. P. , & Hartmann, R. (2020). Weak induction of interferon expression by SARS‐CoV‐2 supports clinical trials of interferon lambda to treat early COVID‐19. Clinical Infectious Diseases, ciaa453. 10.1093/cid/ciaa453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, L. A. J. , & Netea, M. G. (2020). BCG‐induced trained immunity: Can it offer protection against COVID‐19? Nature Reviews Immunology, 20, 35–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, M. , Lert‐itthiporn, W. , Cavadas, B. , Fernandes, V. , Chuansumrit, A. , Anunciação, O. , … Sakuntabhai, A. (2018). Joint ancestry and association test indicate two distinct pathogenic pathways involved in classical dengue fever and dengue shock syndrome. PLoS Neglected Tropical Diseases, 12(2), e0006202. 10.1371/journal.pntd.0006202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourworldindata .org (n.d.). COVID‐19 coronavirus pandemic. Retrieved from https://ourworldindata.org/coronavirus/.
- Ozdemir, C. , Kucuksezer, U. C. , & Tamay, Z. U. (2020). Is BCG vaccination affecting the spread and severity of COVID‐19? Allergy, 75, 1824–1827. 10.1111/all.14344 [DOI] [PubMed] [Google Scholar]
- Pandey, V. (2020). Coronavirus lockdown: The Indian migrants dying to get home. BBC. https://www.bbc.com/news/world-asia-india-52672764 [Google Scholar]
- Park, S. E. (2020). Epidemiology, virology, and clinical features of severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2; coronavirus Disease‐19). Clinical and Experimental Pediatrics, 63, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parth, M. N. (2020). In Mumbai's crematoriums, exhausted workers pull on to earn a living despite psychological stress, fear of COVID‐19. First Post. Retrieved from https://www.firstpost.com/india/in-mumbais-crematoriums-exhausted-workers-pull-on-to-earn-a-living-despite-psychological-stress-fear-of-covid-19-8492921.html.
- Patel, V. (2020a). Data must be applied to context and societal response must be proportionate. The Indian Express. Retrieved from https://indianexpress.com/article/opinion/columns/coronavirus‐india‐lockdown‐medical‐facilities‐covid‐19‐vaccine‐cure‐testing‐india‐deaths‐6331716/.
- Patel, V . (2020b). COVID‐19 and TB: A tale of two bugs. The Indian Express. Retrieved from https://indianexpress.com/article/opinion/columns/a‐tale‐of‐two‐bugs‐coronavirus‐india‐tb‐6313305/.
- Patel, V. (2020c). India's tryst with Covid‐19. The India Forum. Retrieved from https://www.theindiaforum.in/article/indi-s-tryst-covid-19.
- Pearl, R. (1930). Some aspects of the biology of human populations. In Cowdry E. V. (Ed.), Human biology and racial welfare (pp. 515–552). New York: Paul B. Hoeber, Inc.. [Google Scholar]
- Petersen, E. , Koopmans, M. , Go, U. , Hamer, D. H. , Petrosillo, N. , Castelli, F. , … Simonsen, L. (2020). Comparing SARS‐CoV‐2 with SARS‐CoV and influenza pandemics. The Lancet Infectious Diseases, 20(9), e238–e234. 10.1016/S1473-3099(20)30484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran, D. , Jeemon, P. , & Roy, A. (2016). Cardiovascular diseases in India: Current epidemiology and future directions. Circulation, 133, 1605–1620. [DOI] [PubMed] [Google Scholar]
- Prasad, S. , Potdar, V. , Abraham, P. , & Basu, A. (2020). Transmission electron microscopy imaging of SARS‐CoV‐2. Indian Journal of Medical Research, 151, 241–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokunina‐Olsson, L. , Muchmore, B. , Tang, W. , Pfeiffer, R. M. , Park, H. , Dickensheets, H. , … O'Brien, T. (2013). A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nature Genetics, 45, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin, J. , Saeed, S. , Martens, J. H. , Giamarellos‐Bourboulis, E. J. , Ifrim, D. C. , Logie, C. , … Netea, M. G. (2012). Albicans infection affords protection against reinfection via functional reprogramming of Candida monocytes. Cell Host & Microbe, 12, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan, R. K. (2020). Tamil Nadu under‐reported COVID deaths by as many as 444 for the period from March 1 to June 10. The Hindu (Frontline). Retrieved from https://frontline.thehindu.com/dispatches/tamil‐nadu‐under‐reported‐covid‐deaths‐by‐as‐many‐as‐444‐for‐the‐period‐from‐march‐1‐to‐june‐10/article32164867.ece.
- Radhakrishnan, V. , Sen, S. , & Singaravelu, N. (2020). Is India undercounting its COVID‐19 deaths? The Hindu. Retrieved from https://www.thehindu.com/data/data-is-india-undercounting-its-covid-19-deaths/article31628155.ece.
- Ram, C. V. S. , Babu, G. R. , & Prabhakaran, D. (2020). COVID‐19 Pandemic in India. European Heart Journal, 00, 1–3. 10.1093/eurheartj/ehaa493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, D. C. , Naidu, J. R. , Maiya, P. P. , Babu, A. , & Bailly, J. L. (2017). Large‐scale HFMD epidemics caused by Coxsackievirus A16 in Bangalore, India during 2013 and 2015. Infection Genetics and Evolution, 55, 228–235. [DOI] [PubMed] [Google Scholar]
- Raveendran, R. (2020). India's migrant workers crisis not just about economic inequality, But Social Too. Outlook. Retrieved from https://www.outlookindia.com/website/story/opinion‐indias‐migrant‐workers‐conundrum‐is‐not‐just‐about‐economic‐inequality‐but‐social‐too/353682.
- Ravi, V. (2006). Re‐emergence of chikungunya virus in India. Indian Journal of Medical Microbiology, 24, 83–84. [DOI] [PubMed] [Google Scholar]
- Reddy, S. , & Pandey, S. (2020). How India fared in containing Covid deaths—Analysis. Financial Express. Retrieved from https://www.financialexpress.com/opinion/how‐india‐fared‐in‐containing‐covid‐deaths‐analysis/1955598/.
- Reimer, T. , Brcic, M. , Schweizer, M. , & Jungi, T. W. (2008). Poly(I:C) and LPS induce distinct IRF3 and NF‐κB signaling during type‐I IFN and TNF responses in human macrophages. Journal of Leukocyte Biology, 83, 1249–1257. [DOI] [PubMed] [Google Scholar]
- Rhodes, J. M. , Subramanian, S. , Laird, E. , Griffin, G. , & Kenny, R. A. (2020). Vitamin D deficiency and COVID‐19 severity—Plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2, and thrombosis. Journal of Internal Medicine. 10.1111/joim.13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolph, M. S. , & Mahalingam, S. (2019). Zika's passage to India. The Lancet Infectious Diseases, 19, 469–470. [DOI] [PubMed] [Google Scholar]
- Rukmini, S. , & Rampal, N. (2020). A tale of two cities: How Chennai, Delhi improved Covid figures, with and without lockdown. IndiaToday. Retrieved from https://www.indiatoday.in/diu/story/how‐chennai‐delhi‐improved‐covid‐coronavirus‐figures‐with‐and‐without‐lockdown‐1700583‐2020‐07‐14.
- Saraya, A. (2020). Indian response to COVID‐19: Expertise and transparency. Indian Journal Public Health, 64, S243–S244. 10.4103/ijph.IJPH_504_20 [DOI] [PubMed] [Google Scholar]
- Sarkar, S. (2020). Covid‐19 count in Mumbai's Dharavi rises to 2,218, eight new cases emerge. Hindustan Times. Retrieved from https://www.hindustantimes.com/india‐news/covid‐19‐count‐in‐mumbai‐s‐dharavi‐rises‐to‐2‐218‐eight‐new‐cases‐emerge/story‐4pmR4e6tatigDmU5w20aaK.html.
- Saxena, S. (2020). Top 5 coronavirus‐hit cities account for nearly 50% of India's Covid‐19 cases. Hindustan Times. Retrieved from https://www.hindustantimes.com/india‐news/top‐5‐coronavirus‐hit‐cities‐account‐for‐nearly‐50‐of‐india‐s‐covid‐19‐cases/story‐mdwaBt07cOKozkv7y6NE3J.html.
- Schellekens, P. , & Sourrouille, D. (2020). COVID‐19 mortality in rich and poor countries: A tale of two pandemics? Policy research working paper 9260, Washington, DC: World Bank. http://documents1.worldbank.org/curated/en/559181590712052524/pdf/COVID‐19‐Mortality‐in‐Rich‐and‐Poor‐Countries‐A‐Tale‐of‐Two‐Pandemics.pdf [Google Scholar]
- Sehrawat, S. , & Rouse, B. T. (2020). Does the hygiene hypothesis apply to COVID‐19 susceptibility? Microbes and Infection. 10.1016/j.micinf.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. (2020). What explains India's low COVID‐19 death rate? The Quint. Retrieved from https://fit.thequint.com/coronavirus/explained-indias-low-covid-19-fatality-rate.
- Shelar, J. , & Mahale, A. (2020). Coronavirus in Dharavi | when a virus finds space in India's largest slum. The Hindu. Retrieved from https://www.thehindu.com/news/cities/mumbai/when‐a‐virus‐finds‐space‐in‐indias‐largest‐slum/article31537623.ece.
- Singh, O. P. , Hasker, E. , Boelaert, M. , & Sundar, S. (2016). Elimination of visceral leishmaniasis in the Indian subcontinent. The Lancet Infectious Diseases, 16(12), e304–e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S. K. (2020). COVID‐19: A master stroke of nature. AIMS Public Health, 7(2), 393–402. 10.3934/publichealth.2020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, N. (2020). Why coronavirus is pushing the economy more than Spanish flu. The Print. Retrieved from https://theprint.in/opinion/why‐coronavirus‐is‐punishing‐the‐economy‐more‐than‐spanish‐flu/415898/.
- Sohn, A. H. , Phanuphak, N. , Baral, S. , & Kamarulzaman, A. (2020). Know your epidemic, know your response: Understanding and responding to the heterogeneity of the COVID‐19 epidemics across Southeast Asia. Journal of the International AIDS Society, 23, e25557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinney, L. (2017). Pale rider: The Spanish Flu of 1918 and how it changed the world (First US Edition). New York: Public Affairs.
- Spinney, L. (2020). Covid‐19 expert Karl Friston: 'Germany may have more immunological “dark matter”'. The Guardian. Retrieved from https://www.theguardian.com/world/2020/may/31/covid‐19‐expert‐karl‐friston‐germany‐may‐have‐more‐immunological‐dark‐matter.
- Sturdevant, G. L. , & Caldwell, H. D. (2014). Innate immunity is sufficient for the clearance of chlamydia trachomatis from the female mouse genital tract. Pathogens and Disease, 72, 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud, V. , Regan, H. , & Mitra, E. (2020). Doctors say India must prepare for an 'onslaught' as one of Asia's biggest slums reports first coronavirus death. CNN. https://edition.cnn.com/2020/04/03/asia/india-doctors-coronavirus-intl-hnk/index.html [Google Scholar]
- Suri, J. C. , & Sen, M. K. (2011). Pandemic influenza—Indian experience. Lung India, 28, 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger, J. K. , Hutlin, J. V. , & Morens, D. M. (2007). Discovery and characterization of the 1918 pandemic influenza virus in historical context. Antiviral Therapy, 12, 581–591. [PMC free article] [PubMed] [Google Scholar]
- Taubenberger, J. K. , & Morens, D. M. (2006). 1918 influenza: The mother of all pandemics. Emerging Infectious Diseases, 12, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, B. , Chandran, P. , Lilabi, M. P. , George, B. , Sivakumar, C. P. , Jayadev, V. K. , … Hafeez, N. (2009). Nipah virus infection in Kozhikode, Kerala, South India, in 2018: Epidemiology of an outbreak of an emerging disease. Indian Journal of Community Medicine, 44, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, G. (2020). Death in the time of coronavirus. Indian Journal of Medical Ethics, 5(2), 98–99. 10.20529/IJME.2020.036 [DOI] [PubMed] [Google Scholar]
- Tiwari, S. , Singh, R. K. , Tiwari, R. , & Dhole, T. N. (2012). Japanese encephalitis: A review of the Indian perspective. Brazilian Journal of Infectious Diseases, 16, 564–573. [DOI] [PubMed] [Google Scholar]
- TNN . (2020). 2k more Covid deaths, cases rise to 3.5 lakh. The Times of India. Retrieved from https://timesofindia.indiatimes.com/india/2k-more-covid-deaths-cases-rise-to-3-5-lakh/articleshow/76415524.cms.
- Ulmer, A. , & Khanna, S. (2020). Mystery of India's lower death rates seems to defy coronavirus trend. MSN, Retrieved from https://www.msn.com/en-in/news/newsindia/mystery-of-indias-lower-death-rates-seems-to-defy-coronavirus-trend/ar-BB138Suq.
- Undela, K. , & Gudi, S. K. (2020). Assumptions for disparities in case‐fatality rates of coronavirus disease (COVID‐19) across the globe. European Review for Medical and Pharmacological Sciences, 24, 5180–5182. [DOI] [PubMed] [Google Scholar]
- van Dorn, A. , Cooney, R. E. , & Sabin, M. L. (2020). COVID‐19 exacerbating inequalities in the US. The Lancet, 395, 243–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp, L. , Acman, M. , Richar, D. , Shaw, L. P. , Ford, C. E. , Ormond, L. , … Balloux, F. (2020). Emergence of genomic diversity and recurrent mutations in SARS‐CoV‐2. Infection Genetics and Evolution, 83, 104351. 10.1016/j.meegid.2020.104351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf, D. , Schmitz, K. S. , Raadsen, M. P. , Grifoni, A. , Okba, N. M. A. , Endeman, H. , … de Vries, R. D. (2020). Phenotype and kinetics of SARS‐CoV‐2‐specific T cells in COVID‐19 patients with acute respiratory distress syndrome. Science Immunology, 5, eabd2071. 10.1126/sciimmunol.abd2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, C.‐H. , Saal, A. , Butt, W.‐W. , Bica, N. , Fisher, J. Q. , Tao, J. , & Chan, P. A. (2020). Bacillus Calmette–Guérin vaccination and clinical characteristics and outcomes of COVID‐19 in Rhode Island, United States: A cohort study. Epidemiology and Infection, 148, e140. 10.1017/S0950268820001569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenham, C. , Smith, J. , & Morgan, R. (2020). Gender and COVID‐19 working group. COVID‐19: The gendered impacts of the outbreak. The Lancet, 395(10227), 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worldometers .info (n.d.). COVID‐19 coronavirus pandemic. Retrieved from https://www.worldometers.info/coronavirus/.
- Yong, E. (2020). Why the coronavirus is so confusing. The Atlantic. Retrieved from https://www.theatlantic.com/health/archive/2020/04/pandemic-confusing-uncertainty/610819/
- Yu, X. , Sun, S. , Shi, Y. , Wang, H. , Zhao, R. , & Sheng, J. (2020). SARS‐CoV‐2 viral load in sputum correlates with risk of COVID‐19 progression. Critical Care, 24, 170. 10.1186/s13054-020-02893-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, S. , Jiang, S.‐C. , & Li, Z.‐L. (2020). Do temperature and humidly impact the spread of the novel corona virus? Frontiers in Public Health, 8(240), 1–4. 10.3389/fpubh.2020.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadori, N. , Vansca, S. , Farkas, N. , Hegyi, P. , & Eross, B. (2020). The negative impact of comorbidities on the disease course of COVID‐19. Intensive Care Medicine. 10.1007/s00134-020-06161-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S. , Lin, Q. , Ran, J. , Musa, S. S. , Yang, G. , Wang, W. , … Wang, M. H. (2020). Preliminary estimation of the basic reproduction number of novel coronavirus (2019‐nCoV) in China, from 2019 to 2020: A data‐driven analysis in the early phase of the outbreak. International Journal of Infectious Diseases, 92, 214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N. , Zhang, D. , Wang, W. , Li, X. , Yang, B. , Song, J. , … Zhan, F. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 382, 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, P. , & Curtis, N. (2020). Coronavirus infections in children including COVID‐19: An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. The Pediatric Infectious Disease Journal, 39, 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]


