Abstract
Background
The 2019 coronavirus disease (COVID‐19) has become a global pandemic and the published literature describing the virus has grown exponentially.
Methods
We conducted a systematic review of the literature to identify the symptoms, comorbidities present, radiological features and outcomes for adults testing positive for COVID‐19 admitted to hospital. The results across multiple studies were numerically pooled to yield total estimated.
Results
A total of 45 studies were included in this review with 14 358 adult participants (average age 51 years, male 51%). The pooled findings suggest that the most common symptom among patients was fever (81.2%) followed by cough (62.9%), fatigue (38.0%) and anorexia/loss of appetite (33.7%). The comorbidities that were most prevalent among patients with the virus were hypertension (19.1%), cardiovascular disease (17.9%), endocrine disorder (9.3%) and diabetes (9.2%). Abnormal chest X‐ray findings were present in 27.7% of patients and ground‐glass opacity was demonstrated on chest computerized tomography in 63.0% of patients. The most frequent adverse outcomes were acute respiratory distress syndrome (27.4%), acute cardiac injury (16.2%) and acute kidney injury (12.6%). Death occurred in 8.2% of patients and 16.3% required intensive care admission and 11.7% had mechanical ventilation. Bacterial or secondary infections affected 8.5% of patients and 6.9% developed shock.
Conclusions
COVID‐19 most commonly presents with fever, cough, fatigue and anorexia and among patients with existing hypertension and cardiovascular disease. It is important as serious adverse outcomes can develop such as acute respiratory distress syndrome, acute cardiac injury, acute kidney injury and death.
What’s known
First identified in December 2019 as a cause of pneumonia in Wuhan China, the coronavirus disease 2019 (COVID‐19) has become a global pandemic.
As this is a new disease, literature which scientists, clinicians and politicians could rely on to determine how best to control the spread of the virus and manage infected patients was limited and largely based on experience drawn from managing or other respiratory virus pandemics.
What’ new
Our review of 45 studies shows that majority of the literature is currently dominated by reports from China and whether the results are generalisable to other countries is uncertain.
The symptoms of patients with COVID‐19 are very heterogeneous as there are population with common features like fever and cough as well as those which are asymptomatic or have atypical symptoms such as loss of taste/smell.
Death occurred in 8.2% of hospitalised patients but many suffered from acute respiratory distress syndrome, acute cardiac injury and acute kidney injury and required intensive care admission and mechanical ventilation.
1. INTRODUCTION
First identified in December 2019 as a cause of pneumonia in Wuhan China, the coronavirus disease 2019 (COVID‐19) has become a global pandemic. 1 As of June 2020 there are over 9 million cases worldwide and it is responsible for nearly 470 000 deaths. 2 As this is a new disease, literature which scientists, clinicians and politicians could rely on to determine how best to control the spread of the virus and manage infected patients was limited and largely based on experience drawn from managing or other respiratory virus pandemics.
In the efforts to quickly understand this virus there has been an exponential growth of literature on COVID‐19 over a relatively short time span. 3 , 4 , 5 , 6 , 7 At the same time, there have been numerous reports from experts within their respective clinical disciplines providing opinions based on the interpretation of the limited published literature. 8 , 9 The few reviews published have limitations including not being systematic in nature, 10 including a small number of studies 11 or including studies with low patient number including case reports and small patient case series. 12
In view of the urgent need to understand the literature and inform practice, we aimed to determine systematically the evidence from studies of more than 100 adult patients that reported clinical features and outcomes of those affected by COVID‐19. As this virus affects patients from all settings such as community and hospitals, we pooled the findings of the individual studies to gain an estimate of how it affects the population as a whole.
2. METHODS
We conducted a systematic review of the literature to identify the symptoms, comorbidities present, radiological features and outcomes for adults testing positive for COVID‐19 admitted to hospital.
2.1. Inclusion criteria
We included studies that evaluated adults with a laboratory confirmed diagnosis of COVID‐19. The studies also had to report information on one or more of the following: clinical features of patients, comorbidities of patients, radiological findings for patients and outcomes for patients. In addition, we required that the sample size of the studies be greater than 100 patients so that less common symptoms would be captured and there would be sufficient sample size for calculating adverse event rates. There was no restriction based on language of study and Google Translate was used to convert studies from Chinese journals to English.
2.2. Search strategy
We searched MEDLINE and EMBASE using OVID on 26 April 2020. We used the following broad search terms in our search strategy: (“COVID‐19” OR “2019‐nCoV” OR “SARS‐COV‐2” OR “Wuhan coronavirus” OR “novel coronavirus” OR “new coronavirus”) AND (“clinical features” OR “presentation” OR “symptoms” OR “clinical course” OR “clinical characteristics” OR “outcomes” OR “complications” OR “ventilation” OR “intubation” OR “recovery” OR “death” OR “mortality” OR “survival”). We limited the search results to studies published in 2019 or 2020.
2.3. Study selection and data extraction
Because of the initially large number of studies the search terms returned, we screened study titles and abstracts in independent pairs (SB and JM, JT and CSK and DD and CWW) to assess the potential for each study to meet the inclusion criteria. Full articles of potentially relevant studies were retrieved and reviewed for inclusion. Studies where there were discrepancies regarding inclusion were reviewed in detail and decisions about inclusion were made by consensus. Data were extracted by SB and JT and checked by CSK. The data were collected on study design, country, year when it took place, number of participants, mean/median age of participants, % male, patient inclusion criteria, symptoms, comorbidities, radiological findings, follow‐up and adverse outcomes. We further collected data on admission criteria, criteria for starting oxygen and renal disease (acute kidney injury, end‐stage renal failure and dialysis). Risk of bias was performed based on the Ottawa‐Newcastle scale 13 with studies being assessed out of a maximum of 7 stars over three different domains: selection, comparability and outcome.
2.4. Data analysis
Data were extracted and presented in Tables. Statistical pooling according to methods by Kwok et al 14 Pooled results were presented in Figures for patient symptoms, comorbidities, radiological findings and outcomes along with the number of studies and number of patients that were pooled.
3. RESULTS
A total of 45 studies 3 , 4 , 5 , 6 , 7 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 were included in this review (Figure 1).
FIGURE 1.
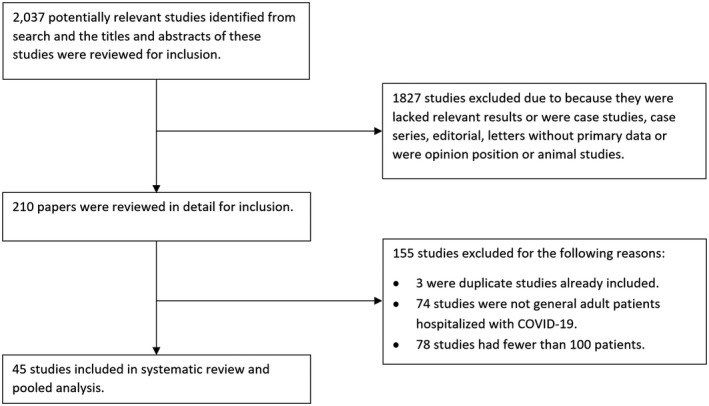
Flow diagram of study selection
The descriptions of the study design and patient characteristics are shown in Table 1. There were 40 retrospective cohort studies, 2 prospective cohort studies, 1 cross‐sectional study and 2 cohort studies of unclear design. All of the studies originated from China except for two studies from the United States and one multicentre European study which took place in Belgium, France, Italy and Spain. The studies included a cumulative total of 14 358 patients which ranged from 101 to 1590 from each individual study. Among the studies that reported mean age and sex of the participants, the average across the studies was 51 years and the proportion of male patients was 51%. All patients had laboratory confirmed COVID‐19 infection. Majority of studies did not report the exact criteria for admissions to hospital and the criteria for starting oxygen therapy (Table S1).
TABLE 1.
Study design and patient inclusion criteria
| Study ID | Study design; date | Location | Number of participants | Mean age | % Male | Inclusion criteria |
|---|---|---|---|---|---|---|
| Cai 2020 | Retrospective cohort study; Jan‐Mar 2020 | Shenzhen City, China | 298 | Median 47 | 49 | Patients with COVID‐19 at Third People’s Hospital |
| Cao 2020 | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 102 | Median 54 | 52 | Patients with COVID‐19 at Wuhan University Zhongnan Hospital |
| Chen 2020a | Retrospective cohort study; Jan‐Feb 2020 | Shanghai, China | 249 | Median 51 | 51 | Patients with COVID‐19 at Shanghai Public Health Clinical Centre |
| Chen 2020b | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 274 | Median 68 | 62 | Patients with COVID‐19 at Tongji Hospital |
| Deng 2020a | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 225 | Recovered group: 40, Death group median: 69 | Recovered group: 51, Death group: 73 | Patients with COVID‐19 at Tongji Medical College, Huazhong University of Science & Technology and Hankou branch of Central Hospital of Wuhan |
| Deng 2020b | Retrospective cohort study; Jan‐Mar 2020 | Wuhan, China | 112 | Median 65 | 51 | Patients with COVID‐19 at Renmin Hospital of Wuhan University |
| Du 2020 | Prospective cohort study; Dec 2019‐Feb 2020 | Wuhan, China | 179 | 58 | 54 | Patients with COVID‐19 (116/179) or clinically diagnosed (43/179) of COVID‐19 at Wuhan Pulmonary Hospital |
| Fan 2020 | Retrospective cohort study; Jan‐Feb 2020 | Shanghai, China | 148 | Median 50 | 49 | Patients with COVID‐19 at Shanghai Public Health Clinical Centre |
| Feng 2020 | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, Shanghai and Tongling, China | 476 | Median 53 | 57 | Patients with COVID‐19 at Jinyintan hospital in Wuhan, Shanghai Public Health Clinical Centre, Shanghai and Tongling People’s hospital |
| Garg 2020 | Retrospective cohort study; Mar 2020 | United States | 1482 | 74.5% >50 years of age | 54 | Patients with COVID‐19 in hospital across California, Colorado, Connecticut, Georgia, Iowa, Maryland, Michigan, Minnesota, New Mexico, New York, Ohio, Oregon, Tennessee, and Utah |
| Guan 2020a | Retrospective cohort study; Dec 2019‐Jan 2020 | China | 1099 | Median 47 | 58 | Patients with COVID‐19 at 552 hospitals across mainland China |
| Guan 2020b | Retrospective cohort study; Dec 2019‐Jan 2020 | China | 1590 | 49 | 53 | Patients with COVID‐19 at 575 hospitals across mainland China |
| Guo 2020 | Retrospective cohort study; Feb 2020 | Wuhan, China | 174 | Median 59 | 44 | Patients with COVID‐19 at to Wuhan Union hospital |
| Han 2020a | Retrospective matched cohort study; Feb 2020 | Wuhan, China | 206 | 63 | 44 | Patients with COVID‐19 at Union Hospital |
| Han 2020b | Retrospective cohort study; Jan‐March 2020 | Wuhan, China | 273 | Mild: 59. Severe: 59. Critical: 57 | 36 | Patients with COVID‐19 at Renmin Hospital of Wuhan University |
| Lechien 2020 | Prospective cohort study; March 2020 | Europe | 417 | 37 | 37 | Patients with COVID‐19 at 12 hospitals within Spain, Belgium, France and Italy |
| Li 2020a | Cohort study; Jan‐Feb 2020 | Hanchaun city, China | 225 | Average 50 | 53 | Patients with COVID‐19 at Hanchuan City People's Hospital |
| Li 2020b | Retrospective cohort study; Jan‐Mar 2020 | Wuhan, China | 548 | Median 60 | 51 | Patients with COVID‐19 at Sino‐French New Branch of Tongji Hospital, Huazhong University of Science and Technology |
| Liu 2020a | Retrospective cohort study; Dec 2019‐Jan 2020 | Wuhan, China | 137 | Median 57 | 46 | Patients with COVID‐19 at nine tertiary hospitals, Wuhan, China |
| Liu 2020b | Retrospective cohort study; Dec 2019‐Jan 2020 | Wuhan, China | 245 | 54 | 47 | Patients with COVID‐19 at Zhongnan Hospital |
| Lu 2020 | Retrospective cohort study; Jan‐Feb 2020 | Chongqing city, China | 304 | Median 44 | 60 | Patients with COVID‐19 at 42 officially designed hospitals in Chongqing city |
| Mao 2020 | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 214 | 52 | 41 | Patients with COVID‐19 at 3 centres (Main district, West branch and Tumor centre) of Union Hospital of Huazhong University of Science and Technology |
| Mo 2020 | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 155 | Median 54 | 56 | Patients with COVID‐19 in Zhongnan Hospital |
| Pan 2020 | Cross‐sectional study; Jan‐Mar 2020 | Wuhan, China | 204 | 52 | 52 | Patients with COVID‐19 who had chest CT and complete panel of laboratory tests from 3 hospitals (Wuhan Hanan Hospital, Wuhan Union Hospital and Huanggang Central Hospital) |
| Qin 2020 | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 452 | Median 58 | 52 | Patients with COVID‐19 in Tongji hospital |
| Tian 2020 | Retrospective cohort study; Jan‐Feb 2020 | Beijing, China | 262 | Median 47 | 49 | Patients with COVID‐19 in the designated hospitals in Beijing for special treatment of infectious diseases |
| Wan 2020 | Retrospective cohort study; Jan‐Feb 2020 | Chongqing, China | 135 | 47 | 53 | Patients with COVID‐19 in Chongqing University Three Gorges Hospital |
| Wang 2020a | Retrospective cohort study; Jan‐Feb 2020 | Wenzhou, China | 149 | 45 | 54 | Patients with COVID‐19 at Wenzhou municipal Centre for Disease Prevention and Control |
| Wang 2020b | Retrospective cohort study; Feb 2020 | Wuhan, China | 1012 | Median 50 | 52 | Patients with COVID‐19, age >16 years with ability to self‐care, respiratory rate <30, blood oxygen saturations >93% and a negative result for influenza virus at Dongxihu Fangcang Hospital |
| Wang 2020c | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 339 | 71 | 49 | Patients with COVID‐19 and age >60 years in the isolation ward of Renmin Hospital |
| Wang 2020d | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 116 | Median 54 | 58 | Patients with COVID‐19 at Renmin Hospital |
| Wang 2020e | Retrospective cohort study; Jan‐Feb 2020 | Xiaogan, China | 114 | Median 53 | 51 | Patients COVID‐19 and chest CT examination at Xiaogan Hospital |
| Wang 2020f | Cohort study; Jan‐Feb 2020 | Fuyang, China | 125 | 39 | 57 | Patients with COVID‐19 at People’s Hospital of Fuyang City |
| Wang 2020g | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 138 | Median 56 | 54 | Patients with COVID‐19 in the Critical Care Medicine of Zhongnan Hospital of Wuhan University |
| Xu 2020 | Retrospective cohort study; Dec 2019‐Mar 2020 | Wuhan, China | 187 | Median 62 | 55 | Patients with COVID‐19 at Hubei Provincial Hospital |
| Yan 2020 | Retrospective cohort study; Mar‐Apr 2020 | San Diego, United States | 169 | Median inpatient: 54, outpatient: 43 | Admitted inpatient: 9, outpatient: 52 | Patients with COVID‐19 at Jacobs and Hillcrest Medical Centres |
| Yao 2020 | Retrospective cohort study; Jan‐Mar 2020 | Huanggang city, China | 108 | Median 52 | 40 | Patients with COVID‐19 at Dabieshan Medical Centre |
| Zhang 2020a | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 140 | Median 57 | 51 | Patients with COVID‐19 at No. 7 Hospital of Wuhan |
| Zhang 2020b | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 663 | Median 56 | 48 | Patients with COVID‐19 at Renmin Hospital of Wuhan University |
| Zhang 2020c | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 120 | 45 | 36 | Patients with COVID‐19 at Renmin Hospital of Wuhan University |
| Zhang 2020d | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 221 | 55 | 49 | Patients with COVID‐19 at Zhongnan Hospital of Wuhan University |
| Zhao 2020a | Retrospective cohort study; Feb 2020 | Hunan, China | 101 | 54 | 45 | Patients with COVID‐19 at hospitals in four Hunan cities (Changsha, YueYang, ChangDe and Xiang‐Tan) |
| Zhao 2020b | Retrospective cohort study; Jan‐Feb 2020 | Hunan, China | 118 | 44 | 51 | Patients with laboratory‐confirmed diagnosis of COVID‐19 at The Second Hospital Xiangya Hospital |
| Zheng 2020 | Retrospective cohort study; Jan‐Feb 2020. | Changsha, China | 161 | 45 | 80 | Patients with laboratory‐confirmed diagnosis of COVID‐19 at First Hospital of Changsha |
| Zhou 2020 | Retrospective cohort study; Jan‐Feb 2020 | Wuhan, China | 191 | 72 | 62 | Patients with laboratory‐confirmed diagnosis of COVID‐19 at Jinyintan Hospital and Wuhan Pulmonary Hospital |
The quality of the included studies is shown in Table S2. All included studies were graded to be 4 to 6 stars out of a maximum of 7.
The symptoms reported by patients with COVID‐19 are described for each study in Table S3. Collectively pooled, the most common symptom among patients was fever (81.2%) followed by cough (62.9%), fatigue (38.0%) and anorexia/loss of appetite (33.7%) (Figure 2). More than one in five patients had shortness of breath (26.9%), anosmia/loss of taste (25.4%) and sputum/expectoration (24.2%). Only 2.8% of patients were asymptomatic. Other symptoms commonly reported but only from single studies are shown in Figure 3 and prevalent symptoms included weakness (85.0%), facial pain/heaviness (36.9%) and ear pain (14.6%).
FIGURE 2.
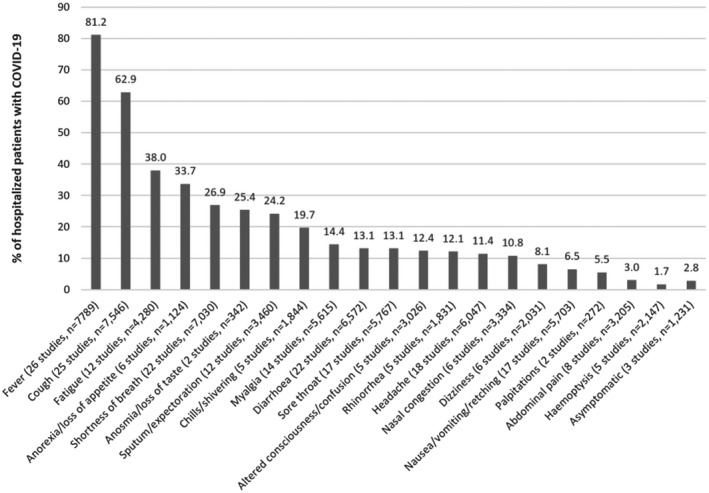
Symptoms reported from multiple studies of adults hospitalized with COVID‐19
FIGURE 3.
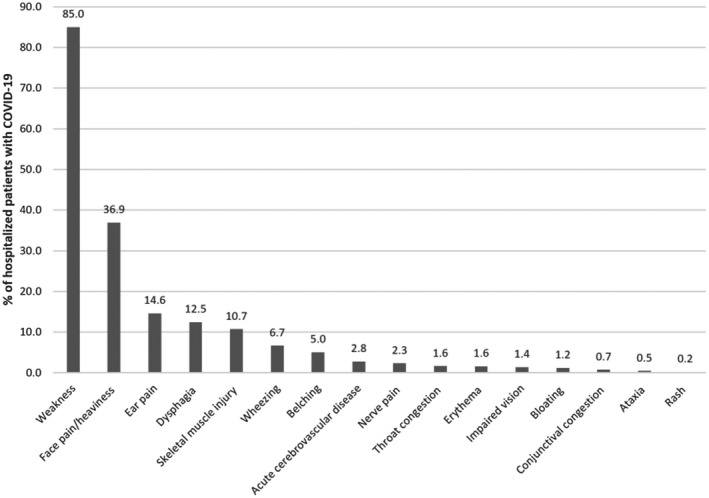
Symptoms reported from single studies of adults hospitalized with COVID‐19
The comorbidities of patients with COVID‐19 are shown for each study in Table S4. The most common comorbidities among these patients after pooling the studies was hypertension (19.1%), cardiovascular disease (17.9%), endocrine disease (9.3%) and diabetes (9.2%) (Figure 4). Patients with a smoking history represented 8.3% of the pooled cohort. The descriptions of acute kidney injury, end‐stage renal failure or dialysis use is shown in Table S5.
FIGURE 4.
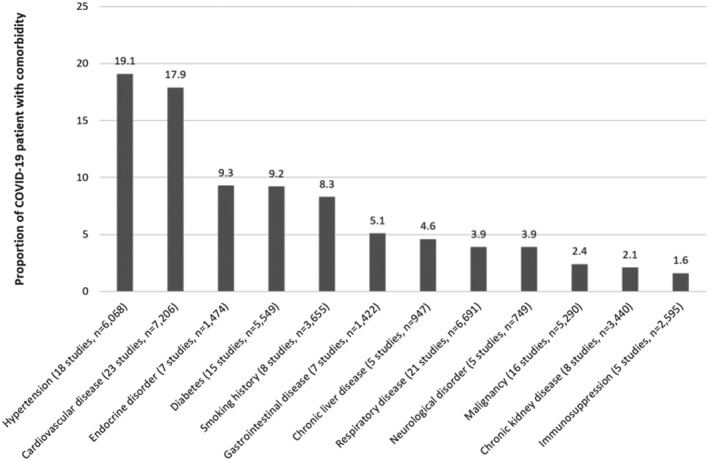
Comorbidities reported from multiple studies of adults hospitalized with COVID‐19
The findings from chest X‐ray and computerized tomography (CT) scans are reported for each study in Table S6. The pooled results suggest that 27.7% of patients had abnormal chest X‐rays, 79.9% had bilateral changes on chest X‐ray or CT scans and 63.0% had ground‐glass opacities on imaging (Figure 5).
FIGURE 5.
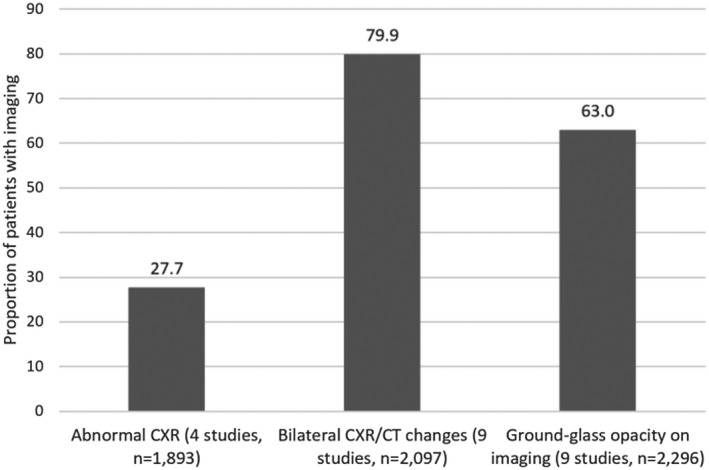
Changes on imaging reported from multiple studies of adults hospitalized with COVID‐19
The outcomes for patients with COVID‐19 are presented in Table 2. Studies had follow‐up of up to 31 days and the pooled results for outcomes are shown in Figure 6. The most common adverse outcome was acute respiratory distress syndrome (27.4%), acute cardiac injury (16.2%) and acute kidney injury (12.6%). Death occurred in 8.2% of patients and 16.3% required intensive care admission while 11.7% had mechanical ventilation. Bacterial or secondary infections affected 8.5% of patients and 6.9% developed shock.
TABLE 2.
Follow‐up and outcomes for patients with COVID‐19
| Study ID | Follow‐up | Outcomes |
|---|---|---|
| Cai 2020 | 31 days | Death 3/298 |
| Cao 2020 | 14 days | Death 17/102. Intensive care admission 18/102. Acute respiratory distress syndrome 20/102. Acute cardiac injury 15/102. Acute kidney injury 20/102. Acute liver injury 34/102. Shock 10/102. Acute infection 17/102. Arrhythmia 18/102. Lymphopenia 78/102 |
| Chen 2020a | 19 days | Death 2/249. Intensive care admission 22/298 |
| Chen 2020b | 14 days | Death 113/274. Acute respiratory distress syndrome 197/274. Type I respiratory failure 18/67. Acute cardiac injury 89/203. Heart failure 43/176. Hypoxic encephalopathy 24/274. Sepsis 179/274. Acidosis 8/67. Alkalosis 19/76. Acute kidney injury 29/274. Disseminated intravascular coagulation 21/274. Hyperkalemia 62/274. Shock 46/ 274, acute liver injury 13/274, gastrointestinal bleeding 1/274 |
| Deng 2020a | None | Death 109/225. Acute respiratory distress syndrome 108/225, acute cardiac injury 66/225, acute kidney injury 20/225, shock 123/225, disseminated intravascular coagulation 7/225 |
| Deng 2020b | 20 days | Intensive care admission 26/112. Mechanical ventilation 28/112. Extracorporeal membrane oxygenation 3/112. Death 14/112 |
| Du 2020 | None | Death 21/179 |
| Fan 2020 | 19 days | Remained severe/critically ill 10/148. Death 1/148 |
| Feng 2020 | 31 days | Remained in hospital 23/476. Death 38/476. Secondary bacterial infection 35/410 |
| Guan 2020a | None | Remained in hospital 1029/1099. Death 15/1099. Intensive care unit admission 55/1099. Mechanical ventilation 67/1099. Acute respiratory distress syndrome 37/1099, acute kidney injury 6/1099, septic shock 12/1099, disseminated intravascular coagulation 1/1099 |
| Guan 2020b | None | Death 50/1590. Intensive care admission 90/1590. Invasive ventilation 50/1590 |
| Guo 2020 | 3 days | Death 21/174 |
| Han 2020a | 18 days | No significant difference in hospital stay amongst patients with digestive only symptoms vs digestive and respiratory symptoms (24.4 ± 5.1 vs 23.9 ± 2.4, P = .868) |
| Han 2020b | 34 days | Death 24/273 |
| Lechien 2020 | None | Olfactory dysfunction persisted following clinical resolving of other symptoms in 63% patients |
| Li 2020a | 15 days | Death 2/225 |
| Li 2020b | 26 days | Death 90/545. Acute respiratory distress syndrome 210/549. Cardiac injury 119/549. Liver dysfunction 106/549. Acute kidney injury 95/549. Bacteremia infection 38/549. Disseminated intravascular coagulation 38/549. Hyperglycemia 181/549 |
| Liu 2020a | None | Remained in hospital 44/137. Death 16/137 |
| Liu 2020b | None | Death 33/245 |
| Lu 2020 | None | Seizure‐like symptoms 2/304. Brain insults or metabolic imbalances 84/304. Death 10/304. Septic shock 5/304. Hypovolemia or cardiac problems 3/304 |
| Mo 2020 | 10 days | Readmission within 10 days 70/155 |
| Pan 2020 | 28 days | Death 37/204. Intensive care admission 16/204 |
| Tian 2020 | None | Remained in hospital 214/262. Death 3/262 |
| Wan 2020 | None | Remained in hospital 120/135. Death 1/135. Acute respiratory distress syndrome 21/135. Acute cardiac injury 10/135. Acute kidney injury 5/135. Secondary infection 7/135. Shock 1/135 |
| Wang 2020a | 5 days | Remained in hospital 76/149. Death 0/149 |
| Wang 2020b | 10 days | Remained in hospital 819/1012. Death 0/1012 |
| Wang 2020c | 28 days | Remained in hospital 215/339. Death 76/339. Acute respiratory distress syndrome 71/339. Acute cardiac injury 70/339. Cardiac insufficiency 58/339. Acute kidney injury 27/339. Liver enzyme abnormalities 86/339. Bacterial infection 143/339. Shock 8/339. Arrhythmia 35/339 |
| Wang 2020d | None | Death 7/116 |
| Wang 2020f | 11 days | Remain in hospital 78/125. Death 0/125. Intensive care admission 19/125. Mechanical ventilation 4/125. Acute respiratory shock distress syndrome 6/125. Secondary infection 6/125 |
| Wang 2020g | 4 days | Remained in hospital 85/138. Death 6/138. Intensive care admission 36/138. Mechanical ventilation 6/138. Acute respiratory distress syndrome 12/138. Acute cardiac injury 10/138. Shock 12/138. Arrhythmia 23/138 |
| Xu 2020 | 4 days | Remained in hospital 45/187. Death 28/187. Bacterial infection 23/187. Fungal infection 2/187 |
| Yan 2020 | None | Admitted 26/128 |
| Yao 2020 | 21 days | Intensive care admission 17/108. Acute respiratory distress syndrome 45/108. Acute cardiac injury 8/108. Acute kidney injury 16/108. Sepsis 35/108. Septic shock 6/108 |
| Zhang 2020b | 3 days | Improvement in clinical status 251/663. Death 25/663 |
| Zhang 2020d | 5 days | Discharged 42/221. Remained in hospital 167/221. Death 12/221. Acute respiratory distress syndrome 48/221. Acute cardiac injury 16/221. Acute kidney injury 10/221. Shock 15/221. Arrhythmia 22/221. Bacterial infection 17/221. Fungal infection 7/221 |
| Zhoa 2020b | 7 days | Discharged 42/118. Remained in hospital 76/118 |
| Zhou 2020 | None | Death 54/191. Intensive care admission 50/191. Acute respiratory distress syndrome 49/191. Respiratory failure 103/191. Acute cardiac injury 33/191. Heart failure 44/191. Acute kidney injury 28/191. Septic shock 38/191. Sepsis 112/191. Coagulopathy 37/191. Secondary infection 28/191. Hypoproteinemia 22/191. Acidosis 17/191. Mechanical ventilation 33/191 |
FIGURE 6.
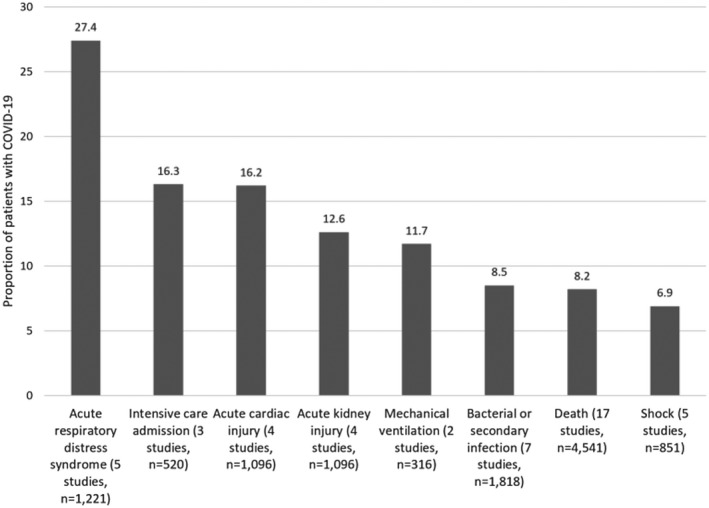
Adverse outcomes reported from multiple studies of adults hospitalized with COVID‐19
4. DISCUSSION
Our study has several key findings. First, the majority of the literature is currently dominated by reports from China and whether the results are generalisable to other countries is uncertain. Second, the symptoms of patients with COVID‐19 are very heterogeneous as there are populations with common features like fever and cough as well as those which are asymptomatic or have atypical symptoms such as loss of taste/smell. Third, cardiovascular diseases are the most common comorbidity in COVID‐19 patients while other comorbidities such as diabetes, gastrointestinal disease and respiratory disease are present in less than 1 in 10 patients. Fourth, under a third of patients have abnormal chest X‐ray and the majority of patients undergoing further imaging have bilateral changes and ground‐glass opacities on CT scan. Finally, death occurred in 8.2% of hospitalised patients but many suffered from acute respiratory distress syndrome, acute cardiac injury and acute kidney injury and required intensive care admission and mechanical ventilation.
Our review adds to what is already known from existing studies. Our review identified three key studies: Garg et al, 23 a study from the United States, Guan et al, 25 a large cohort from China and Lechien et al, 29 a multicentre European study. Consistent across these studies and our pooled results was fever being present in more than 80% of patients. However, the American cohort showed a much greater proportion of patients with shortness of breath (80% in Garg et al, 27% in pooled analysis and 24% in Guan et al) and myalgia (34.4% in Garg et al, 14.4% in the pooled analysis and 17.5% in Guan et al) while diarrhoea was much lower in the Chinese study (4.2% in Guan et al, 13.1% in pooled analysis and 26.7% in Garg et al). Collectively these findings should be compared against the large observational study only published on a preprint server by Docherty et al which included 16,749 patients with COVID‐19 at 166 hospitals in the United Kingdom. 55 They found three clusters of symptoms which include respiratory, systemic and enteric and 17% required admission to high dependency or intensive care unit and 33% of patients died. By pooling the results from many studies, we reported the relative frequencies of individual symptoms but also built by evaluating the prevalence of less common symptoms such as anosmia/loss of taste, altered consciousness/confusion, palpitations and weakness. For outcomes, admissions to intensive care was 16% from the pooled analysis which was similar to the 17% reported by Docherty et al but death rates were much lower at 8.2% (33.3% died in Docherty et al). We also highlight other outcomes such as acute cardiac injury and acute kidney injury which are not uncommon as they affect 16.2% and 12.6% of patients, respectively. Also, the pooled findings from imaging suggest that many patients have bilateral changes and the majority have ground glass opacities.
While use of the Ottawa‐Newcastle quality evaluation has classified many studies as of reasonable quality there are a few important considerations because the majority of studies are from China. Despite recent reforms to reduce healthcare inequalities, there remains a proportion of patients who require but do not receive hospital care mainly for financial reasons. 56 Therefore, the population that present and are captured in the Chinese studies may not generalisable to other countries, especially those with universal access to free healthcare. In China, patients may also receive both Western and/or traditional Chinese medications. 57 The use of alternative medicine may affect the timing of presentation and consequently the severity of symptoms when patients with COVID‐19 present to hospital. It is possible that patients who fail to improve on traditional Chinese medicine may present to hospitals thus skewing the data towards patients that are more symptomatic and have higher adverse event rates because of delay in presentation. In addition, it has been reported that information on COVID‐19 is tightly controlled on Chinese social media and censorship of COVID‐19 content started at early stages of the outbreak and continue to expand. 57 It is unclear how reflective the published literature compared with actual practice because of this regulation and censorship. Finally, there are some concerns about the rigorousness of research practices in China as many researchers felt pressure to publish articles as quickly as possible and this has led to proliferation of research malpractice. 58
Our study focused on adult patients who were admitted to hospital with COVID‐19 but is likely not reflective of the entire population with the virus. There are many people affected with the virus that are asymptomatic or less symptomatic to the extent that they can remain in the community and do not present to hospital (in the hospital population pooled only 2.8% were asymptomatic). Furthermore, factors such as lockdown, self‐isolation and community treatment are not reflected in these studies. This makes capturing the true impact of COVID‐19 on adults challenging as the hospital population only potentially represents the more severe end of patients affected.
A major challenge of the current study was to ensure that the population that was pooled did not count patients from the same hospital. Many of the included studies in this review took place in Wuhan hospitals the epicentre of the virus outbreak. We therefore had to be careful and analyse which individual hospitals contributed to the reported findings. We took the approach of including the studies with the greatest number of patients because this would most likely include the most recent data in the pooled analysis.
We observed significant heterogeneity in the follow‐up period for the included studies which ranged from only in‐hospital events to at least 34 days postdischarge. While we expect that mortality risk would be greatest at time of hospitalisation for the acute illness, there is still risk of mortality when discharged from hospital especially when patients are discharged for self‐isolation or they are discharged before complete symptom resolution. A key consideration which we are unable to capture is the discharge criteria at each hospital as this may affect mortality rates in the community after hospitalisation. This is further complicated by hospital policies that may have changed depending on the timing of the epidemic when the study took place. Mortality and outcomes for patients with COVID‐19 depend on the duration of follow‐up and the time point of the epidemic when the study occurred.
As the pandemic continues to progress, the challenge has and will remain in detecting COVID‐19 cases and identifying local outbreaks as early as possible to prevent spread and secondary outbreaks. The common symptoms of patients who present to hospital as seen in this review include fever, cough, fatigue and anorexia or loss of appetite. However, we have shown that there are many other symptoms such as anosmia/loss of taste, weakness and facial/ear pain that appear in patients affected by the virus. More understanding is needed as to the timeline of symptomatology and disease progression with COVID‐19 is not known. We expect that some patients present early to hospital while other wait for further symptoms to develop or increase in the severity of symptoms before coming to hospital. As treatments are being developed and used in practice such as dexamethasone (RECOVERY trial) 59 informing the public and clinicians of the range of symptoms of COVID‐19 is important so that patients with the virus can be identified quickly and they can undergo treatment before it progresses or spreads uncontrollably within local communities.
At the time of our search for this review, most of the literature on COVID‐19 were based on studies in China as the findings reported reflect those early in the COVID‐19 pandemic. This is reflected by the inclusion of 42 studies from China (93.3%) in the current review. A notable study from the Boston area which was subsequently published on data early in the pandemic, showed that patients hospitalised with COVID‐19 were frequently from the most vulnerable socioeconomic groups and often required intensive care. Furthermore, among those who survive COVID‐19 there is substantial need for postacute care as 10% are readmitted. 60 Recently there has been a growth of literature on COVID‐19 outside of China but among case‐series literature majority are from China (54.0%). 61
This systematic review has several limitations. First, 44 of the included studies were retrospective and observational in nature. Second, there were inconsistent reporting among the studies; this was especially true for the imaging features of COVID‐19, where findings ranged from including normal or abnormal to chest X‐rays or chest computer tomography studies to studies providing changes per lung lobe. Third, all studies included in this review were of short follow‐up duration (maximum of 34 days) thus long‐term follow‐up information is, understandably, limited at present. Fourth, these findings are only generalisable to the hospital patients who were tested positive and it is possible that some patients have a false negative test and there may also be many patients in the community who are not tested so the true incidence and prevalence of COVID‐19 is unknown. Finally, our review was searched at the end of April and the results largely reflect those from early on in the COVID‐19 pandemic. As a result, many or the atypical symptoms such as loss of smells is only reported by a few studies.
5. CONCLUSIONS
Fever, cough, fatigue and anorexia are common symptoms of COVID‐19 which frequently present in patients with existing hypertension and cardiovascular disease. Serious adverse outcomes associated with COVID‐19 infection include acute respiratory distress syndrome, acute cardiac injury, acute kidney injury and death.
DISCLOSURES
None.
Supporting information
Table S1‐S6
Bennett S, Tafuro J, Mayer J, et al. Clinical features and outcomes of adults with coronavirus disease 2019: A systematic review and pooled analysis of the literature. Int. J. Clin. Pract. 2021;75:e13725. 10.1111/ijcp.13725
REFERENCES
- 1. World Health Organization . https://www.who.int/news‐room/detail/27‐04‐2020‐who‐timeline–‐covid‐19. Accessed September 27, 2020.
- 2. Worldometer . https://www.worldometers.info/coronavirus/. Accessed September 27, 2020.
- 3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019; retrospective study. Br Med J. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID‐19: evidence from front‐line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang R, Pan M, Zhang X, et al. Epidemiological and clinical features of 125 hospitalized patients with COVID‐19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Du R, Fan G, et al. Clinical course and risk factors for mortality of adults inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amariles P, Granados J, Ceballos M, Montoya CJ. COVID‐19 in Colombia endpoints. Are we difference, like Europe? Res Social Adm Pharm. 2020. Mar 31;17:2036–2039. 10.1016/j.sapharm.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qi X, Liu C, Jiang Z, et al. Multicentre analysis of clinical characteristics and outcomes in patients with COVID‐19 who develop liver injury. J Hepatol. 2020;73:455‐458. 10.1016/j.jhep.2020/04/010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siordia JA. Epidemiology and clinical features of COVID‐19: a review of current literature. J Clin Virol. 2020;127:104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS‐CoV‐2 infection: a single arm meta‐analysis. J Med Virol. 2020;92:612‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fu L, Wnag B, Yuan T, et al. Clinical characteristics of coronavirus 2019 (COVID‐19) in China: a systematic review and meta‐analysis. J Infect. 2020;80:656‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wells GA, Shea B, O’Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 14. Kwok CS, Holland R, Gibbs G. Efficacy of topical treatments for cutaneous warts: a meta‐analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233‐246. [DOI] [PubMed] [Google Scholar]
- 15. Cai Q, Huang D, Ou P, et al. COVID‐19 in a designated infectious disease hospital outside Hubei Province, China. Allergy. 2020;75:1742‐1752. 10.1111/all.14309 [DOI] [PubMed] [Google Scholar]
- 16. Cao K, Wen‐Jun T, Cheng W, et al. Clinical features and short‐term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:748‐755 10.1093/cid/ciaa243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID‐19 in Shanghai, China. J Infect. 2020;80:e1‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID‐19) in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133:1261‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du R, Liang L, Yang C, et al. Predictors of mortality for patients with COVID‐19 pneumonia caused by SARS‐CoV‐2: a prospective cohort study. Eur Respir J. 2020;55:2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan Z, Chen L, Li J, et al. Clinical features of COVID‐10 related liver damage. Clin Gastroenterol Hepatol. 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feng Y, Ling Y, Bai T, et al. Covid‐19 with difference severity: a multicentre study of clinical features. Am J Respir Crit Care Med. 2020;201:1380‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garg S, Kim L, Whitaker M, et al. Hospitalisation rate & characteristics of patients hospitalised with laboratory confirmed coronavirus disease 2019—COVID‐NET, 14 states, March 1‐30, 2020. MMWR Morb Mortal Wkley Rep. 2019;2020:458‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guan W, Lina W, Zhoa Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression of prognosis of COVID‐19. Diabetes Metab Res Rev. 2020 Mar 31:e3319. 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han C, Duan C, Zhang S, et al. Digestive symptoms in COVID‐19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroentrol. 2020;115:916‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han H, Xie L, Liu R, et al. Analysis of heart injury laboratory parameters in 273 COVID‐19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li R, Tian J, Yang F, et al. Clinical characteristics of 225 patients with COVID‐19 in a tertiary hospital near Wuhan, China. J Clin Viorl. 2020;127:104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110‐118. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu K, Fang YY, Deng Y, et al. Clinical characteristics of normal coronavirus cases in tertiary hospital in Hubei Province. Chin Med J (Engl). 2020;133:1025‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Y, Du X, Chen J, et al. Neutrophil‐to‐lymphocyte ration as an independent risk factor for mortality in hospitalized patients with COVID‐19. J Infect. 2020;81:e6‐e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu L, Xiong W, Liu D, et al. New onset acute syndrome seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61:e49‐e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. Mar 16. 10.1093/cid/ciaa270. [Epub ahead of print] [DOI] [Google Scholar]
- 35. Pan LP, Mu M, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicentre study. Am J Gastroenterol. 2020;115:766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80:401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wan S, Xiang W, Fang W, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J Med Virol. 2020;92:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang W, Cao Q, Wang X, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID‐19): a multi‐center study in Wenzhou city, China. J Infect. 2020;80:388‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X, Fang J, Zhu Y, et al. Clinical characteristics of non‐critically ill patients with novel coronavirus infection (COVID‐19) in a Fangcang Hospital. Clin Microbiol Infect. 2020;26(8):1063‐1068. 10.1016/j.cmi/2020/03/032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4 weeks follow‐up. J Infect. 2020;80:639‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nerphol. 2020;51:343‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang K, Kang S, Tian R, Zhang X, Zhang X, Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID‐19) in the Xiaogan area. Clin Radiol. 2020;75:341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang D, Hu B, Chang H, et al. Clinical characteristics of 138 hospitalised patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu B, Fan C, Wang A, et al. Suppressed T cell‐mediated immunity in patients with COVID‐19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81:e51‐e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self‐reported olfactory loss of associates with outpatient clinical course in COVID‐19. Int Forum Allergy Rhinol. 2020;10(7):821‐831. 10.1002/alr/22592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yao Q, Wang PW, Wang X, et al. Retrospective study of risk factors for severe SARS‐Cov‐2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130:390‐399. [DOI] [PubMed] [Google Scholar]
- 48. Zhang J, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARA‐COV‐2 in Wuhan, China. Allergy. 2020;75(7):1730‐1741. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 49. Zhang K, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality of COVID‐19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26:767‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang R, Ouyang H, Fu L, et al. CT features of SARS‐CoV‐2 pneumonia according to clinical presentation: a retrospective analysis of 120 consecutive patients from Wuhan city. Eur Radiol. 2020;11:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol. 2020;127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao W, Zhong Z, Xiw X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID‐19) pneumonia: a multicenter study. Am J Roentgenol. 2020;214:1072‐1077. [DOI] [PubMed] [Google Scholar]
- 53. Zhao W, Zhong X, Xie X, Yu Q, Liu J. CT scans of patients with 2019 novel coronavirus COVID‐10) pneumonia. Theranostics. 2020;10:4606‐4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zheng F, Tang W, Li H, Huang YX, Zhou ZG. Clinical characteristics of 161 cases of coronavirus disease 2019 (COVID‐19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404‐3410. [DOI] [PubMed] [Google Scholar]
- 55. Docherty AB, Harrison EM, Green CA, et al. Features of 16,749 hospitalised UK patients with COVID‐19 using the ISARIC WHO clinical characterisation protocol. BMJ 2020;369:20076042. 10.1101/2020.04.23.20076042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ming Q, Mills A, Wang L, Han Q. What can we learn from China’s health system reform? Br Med J. 2019;365:I2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. https://citizenlab.ca/2020/03/censored‐contagion‐how‐information‐on‐the‐coronavirus‐is‐managed‐on‐chinese‐social‐media/. Accessed September 27, 2020.
- 58. Huang F. Low Quality Studies Belie Hype About Research Boom in China. Nature Index. https://www.scientificamerican.com/article/low‐quality‐studies‐belie‐hype‐about‐research‐boom‐in‐china/. Accessed September 27, 2020. [Google Scholar]
- 59. Horby P, Lim WS, Emberson J, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. 2020. 10.1101/2020.06.22.20137273 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McCarthy CP, Murphy S, Jones‐O'Connor M, et al. Early clinical and sociodemographic experience with patients hospitalized with COVID‐19 at a large American healthcare system. EClinicalMedicine 2020;26:100504.. 10.1016/j.eclinm.2020.100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Berger S. COVID‐19 Case Series: Publishing Trends in High‐Incidence Countries. https://www.gideononline.com/2020/08/14/covid‐19‐publishing‐trends/. Accessed September 27, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S6


