Abstract
During the outbreak of COVID‐19 many pernio‐like lesions have been increasingly reported. The aim of the study is to describe our management of these skin manifestations and to evaluate a possible correlation to SARS‐CoV‐2 infection. All patients underwent clinical and laboratory tests to detect a possible underlying connective disease and also to specific SARS‐CoV‐2 investigations such as oropharyngeal swab and IgG‐IgM serology. Nine patients aged between 5 and 15 years old were evaluated. Skin lesions observed were purplish, erythematous and oedematous, in some cases painful and itchy. Six out of nine had respiratory and systemic symptoms (cough, nasal congestion, chills, fever, and asthenia) that preceded cutaneous findings of approximately 2 weeks. Concerning blood exams, three out of nine had D‐dimer weakly increased, four had ANA positivity: two with a title 1:160, one with 1:320, and one with 1:5120 and a speckled pattern. The latter patient had also ENA SS‐A positive and RF positivity, confirmed at a second check, so as to allow us to make a diagnosis of connective tissue disease. Four out of nine had aPL positivity (IgM). Reactants acute phase were all negative. Oropharyngeal swabs and serology tests for SARS‐CoV‐2 was negative (borderline in one patient for IgM). No treatment was needed. Even if we do not have enough data to prove it, we hypothesize a correlation between pernio‐like lesions and SARS‐CoV‐2 infection for an increased number of these lesions described during the pandemic and also because such manifestations appeared when temperatures were mild and patients were at home in isolation for the lockdown. Many questions remain open about interaction host‐virus.
Keywords: chilblain, children, COVID‐19, management, pernio‐like, skin lesions
1. INTRODUCTION
During the outbreak of COVID‐19 many skin manifestations have been reported, and among these, in very significant numbers, newly vascular eruptions and peculiar pernio‐like skin lesions have been described in observational studies 1 , 2 , 3 , 4 . Pernio, also referred to as chilblains, is a rare inflammatory condition. Chilblains derives from two Old English words “chill” (cold) and “blegen” (sore). Most commonly, pernio affects acral skin and develops among susceptible individuals who are exposed to cold, the lesions usually appear in fall or winter and disappear in spring or early summer. It is typically idiopathic and acute, nevertheless chronic forms also exist. 5 The diagnosis of pernio is largely clinical and based on a thorough history and physical exam. The differential diagnosis must exclude diseases that can often be confused with other forms of pernio or vasculitis processes like Systemic Lupus Erythematous (SLE) or other conditions as Raynaud phenomenon, acrocyanosis, cryoglobulinemia, cold panniculitis and Interferonopathies. The prognosis of pernio is good with minimal chronic sequelae. Single or multiple erythematous, purplish, edematous lesions appear, accompanied by intense pain, itching, or burning. Usually, pernio affects the toes and dorsum of the proximal phalanges. 6 The mainstay of treatment is the avoidance of the cold and, in some cases, drugs as nifedipine and other calcium channel blockers are needed for the resolution of existing lesions. 5
Since March 2020, children with acral red and painful skin lesions, referable to chilblain have come to our attention. The increased number of cases of pernio‐like lesions compared to the cases per year we usually observe, the mild temperatures of those months in Southern Italy and the concomitant lockdown, led us to hypothesize a possible correlation with SARS‐CoV‐2 infection.
2. METHODS
We evaluated the personal history and photographs of skin lesions of 26 patients, sent to us by their pediatrician, through multidisciplinary telematic meetings with dermatologists, rheumatological pediatricians, and infectious disease specialist. We only included patients with pernio‐like skin lesions (nine patients). Patients who could not perform the oropharyngeal swab for SARS‐CoV‐2 were not admitted to the hospital. We collected informed consent to obtain clinical information and photos of patients and to perform blood chemistry sampling. Therefore, we evaluated nine cases of children who were referred to the Section of Pediatric Rheumatology, of General Pediatric Unit, department of Human Pathology in Adulthood and Childhood “G. Barresi”, University of Messina, since March to April 2020 during outbreak of COVID‐19. We created a medical record in which we have included demographic information on patients, family and personal medical history, clinical manifestations, and follow‐up. We analyzed the photos of these patients at the time of the onset of symptoms and we assessed the cutaneous manifestations when they were admitted in the outpatient setting of the hospital. They underwent blood chemistry: a complete blood count (CBC), renal, hepatic, muscle function tests, urine test, complement levels, immunoglobulins, coagulation studies (prothrombin time, activated partial thromboplastin time, thrombin time and D‐dimer test), rheumatoid factor (RF), antinuclear antibodies (ANA), extractable nuclear antigens (ENA), double stranded DNA (dsDNA), anti‐topoisomerase I (anti‐SCL70), antiphospholipid antibodies (aPL), anti‐cardiolipin (aCL), anti‐β‐2‐glycoprotein 1(β2GP1), and Lupus anticoagulant (LAC). In addition, serology was performed using a chemiluminescent microparticle immunoassay (CLIA) for detection of IgG antibodies directed against the nucleocapsid protein of SARS‐CoV‐2 and IgM.
3. RESULTS
In our group of patients, no significant difference in gender was detected (five females and four males). The median age was 11.4 years (from 5 to 15 years). Two patients were siblings. All patients were from the South of Italy coming from the town of Messina and surrounding. Their family histories and their personal histories were negative for autoimmune disorders, Raynaud's phenomenon, acrocyanosis, chilblains, or photosensitivity except for one child who had suffered from an episode of Raynaud's phenomenon a few years earlier. No family member of these patients presented symptoms attributable to SARS‐CoV‐2 infection but, for work reasons, their parents were in contact with public. Only two siblings had both parents with compatible symptoms and confirmed SARS‐CoV‐2 infection by positive nasal swab. The cutaneous manifestations observed were purplish, erythematous and edematous, four children reported subjective symptoms, painful and pruritus localized to the sole of the feet or to the toes, and/or fingers or heels (Figures 1, 2, and 3). Feet alone were mostly affected (six out of nine), hands alone (three out of nine) (Figure 1). Six patients had respiratory and systemic symptoms (cough, nasal congestion, chills, fever, and asthenia) that preceded the skin lesions by about 2 weeks (Table 1). At the time of the first visit in three out of nine patients the skin lesions were quite mild only erythema persisted. Concerning the results of blood test: three out of nine had coagulation alterations (D‐dimer weakly increased), four had ANA positivity: two with a title of 1:160, one 1:320 and the last one 1:5120 with a speckled pattern. The latter patient had also ENA SS‐A and FR positivity. Four out of nine had aPL positivity (IgM). Reactants acute phase were negative in all patients. Serology for SARS‐CoV‐2 was negative (borderline in one patient for IgM; Table 2).
FIGURE 1.
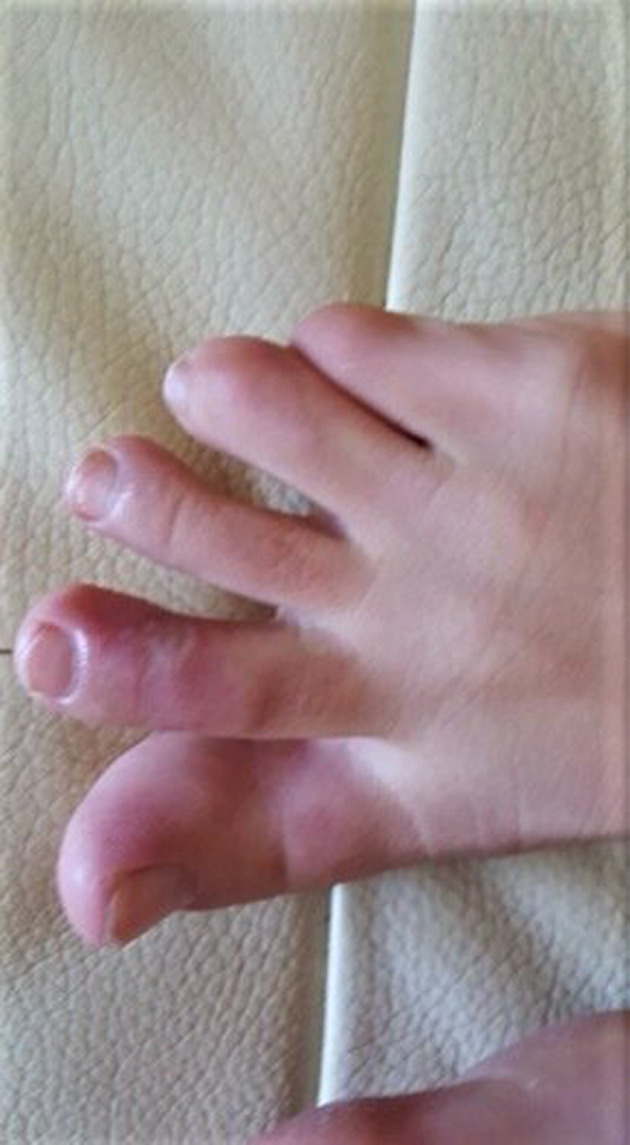
Chillblain like lesions
FIGURE 2.
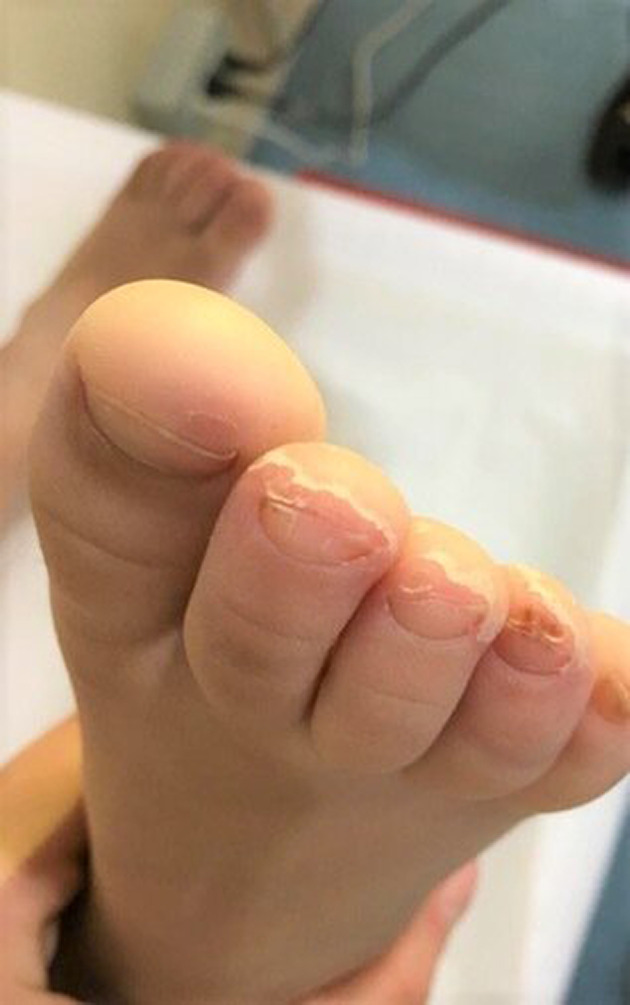
Periungual flaking following chillblain like lesions
FIGURE 3.
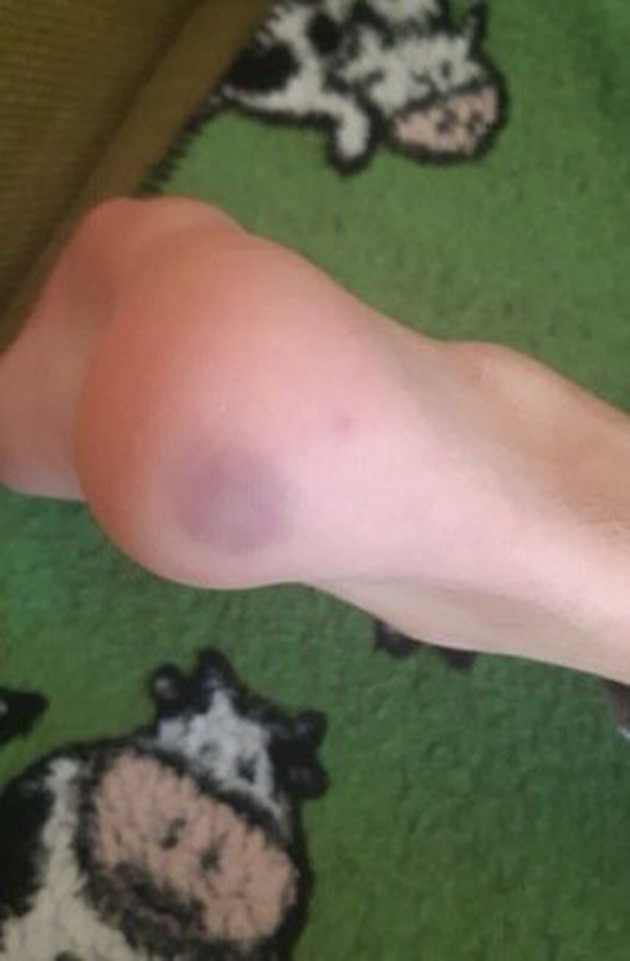
Purpuric lesion of the heels
TABLE 1.
Clinical manifestations in nine patients with chilblain‐like lesions
| Patient | Symptoms attributable to COVID‐19 | Chilblain location/symptoms | Close contact to COVID‐19 | Time between systemic symptoms and chilblain lesion |
|---|---|---|---|---|
| 1 | Fever | Itchy toes, swelling and redness followed by flaking | No | 3 weeks |
| 2 | Fever | Purple and painless hand injuries. Not itchy | No | 3 weeks |
| 3 | Fever | Purple, painless, itchy hands lesions | No | 4 days |
| 4 | Chills, asthenia | Purple skin lesions, neither painful nor itchy on the toes of both feet and heels | Yes | 3 weeks |
| 5 | Asymptomatic | Petechial skin lesions on the heels | Yes | / |
| 6 | Asymptomatic | Purple, painless, itchy skin lesions on toes and heels. | No | / |
| 7 | Nasal congestion | Itching, burning and purple manifestations with pain in the feet | No | At the same time |
| 8 | Asymtomatic | Purple, painful lesions on the fingers of both hands | No | / |
| 9 | Fever, chest pain and dyspnoea | Purple painful lesion on sole of both feet, first and second toes of both feet | No | 1 week |
TABLE 2.
Demographics, laboratory test in nine patients with chilblain‐like‐lesions
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Age/sex | 5/M | 12/F | 11/M | 11/M | 8/F | 13/F | 14/F | 15/M | 14/F |
| Nasopharyngeal swab | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Serology SARS‐CoV‐2 infection (IgM‐IgG) | Negative | Negative | Negative | Negative | IgM 1 AU/mL (N.V. <1) | Negative | Negative | Negative | Negative |
| Coagulation | D‐dimer 0.99 (N.V. 0.0‐0.5) | D‐dimer 0.60 (N.V. 0.0‐0.5) | Normal | Normal | Normal | Normal | D‐dimer 0.80 (N.V. 0.0‐0.5) | Normal | Normal |
| Immunoglobulin | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| ANA, ENA, nDNA, SCl70, RF | Negative | ANA 1:160 speckled | Negative | Negative | ANA 1:160 speckled | Negative | ANA 1:5120 speckled FR 30.2 (0‐14) | ANA 1:320 speckled | Negative |
| aPL, aCL LAC, anti‐β2GP1 | Negative | aPL IgM 27.9 U/mL (N.V. <25) | Negative | Negative | Negative | Negative | Negative | aPL IgM 36.7 U/mL (N.V. <25) | aPL IgM 51.1 U/mL (N.V. <25) |
| Complement levels | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Urine test | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| ESR/PCR | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
Abbreviations: aCL, anti‐cardiolipin; ANA, antinuclear antibodies; anti‐β2GP1, anti β‐2‐glycoprotein1; aPL, antiphospholipid antibodies (Normal values <25 U/mL); ENA, extractable nuclear antigens; FR, rheumatoid factor; LAC, lupus anticoagulant; nDNA, double stranded DNA; NV, normal value; SCL70, anti‐topoisomerase.
4. DISCUSSION
During the SARS‐CoV‐2 pandemic there have been numerous reports (case report, case series) in literature of pernio‐like lesions. In our clinical practice, we usually observe 1 to 2 cases per year of pernio‐like lesions, mainly in cold months. The increased demand for medical visits for these skin manifestations, associated to mild temperatures observed in those months in Southern Italy and the high diffusion of SARS‐CoV‐2 infection, led us to hypothesize a possible correlation with it.
We evaluated nine cases of children who presented pernio‐like lesions, since March to April 2020 during the outbreak of COVID‐19. We performed first level tests, all negative except for D‐dimer weakly increased in three of our patients. D‐dimer is a marker of activation of coagulation and fibrinolysis and it provides a rapid evaluation of thrombotic activity. Its level correlated to coagulopathy have been described as prognostic factors in the evolution of SARS‐CoV‐2 infection, especially in more severe patients. Zhang's study developed a triage, testing D‐dimer levels at the admission, on the 1st and 3rd day to predict survival in a cohort of patients and to evaluate management and follow‐up. The result was that a regulatory level of D‐dimer at the presentation is highly predictive for survival. 7 This is useful to highlight, as in our case, the D‐dimer of our patients was weakly increased, a condition perfectly correlated with the mild symptoms of SARS‐CoV‐2 putative infection presented. We have also performed autoimmunity tests, three out of nine had ANA positivity (speckled pattern): two with a title of 1:160, one 1:320 not confirmed at a subsequent check after 2 months, another one 1:5120 (speckled pattern) with FR and ENA SS‐A positivity too. Four out of nine had aPL positivity (IgM). The findings of the high title positivity of the ANA, ENA and the FR, in the seventh patient, help us to make a diagnosis of connective tissue disorder considering that a previous episode of Raynaud phenomenon had presented 1 year before. However, we cannot rule out that a possible SARS‐CoV‐2 infection acted as a trigger. In other patients with ANA positivity, we can say that either it is a completely occasional finding, or more likely, that this increase was caused by a recent viral infection (SARS‐CoV‐2?). Acute viral, bacterial or parasitic infections may in fact induce antinuclear antibody and antiphospholipid antibodies positivity. This condition is transient and disappear within 2 or 3 months like in our patients. 8 It is important to underline that the pernio‐like manifestations in our patients healed spontaneously without any treatment. The results of our study are homogeneous, describing the negativity of the oropharyngeal swab and serological tests but carried out, respectively, after about 30 and 60 days from the onset of the systemic manifestations probably related to SARS‐CoV‐2 infection (Table 1). A lack of seroconversion is hypothesized in asymptomatic or pauci‐symptomatic subjects who had SARS‐CoV‐2 infection. Zhang et al 9 showed that in a cohort of patients divided into three groups according to the severity of the symptoms (severe, mild, and asymptomatic): 100% of the serious patients had shown seroconversion, only one out five asymptomatic cases generated specific antibody responses for SARS‐CoV‐2. The case of our family is emblematic: two parents presented striking symptoms of SARS‐CoV‐2 infection, high fever, and difficulty in breathing, and three positive swabs; the father had positive serology with high IgG, such as to allow donation of his plasma for therapeutic purposes and the mother was surprisingly negative. Sons who came to our observation, both with chilblain, presented mild respiratory symptoms and they were negative for both swabs and IgG serology, while in one of the two cases, the IgM were borderline. Despite the negativities of diagnostic test for SARS‐CoV‐2, we are still convinced that there is a correlation between this infection and the development of pernio‐like lesions, as described in numerous reports. A group of Italian Dermatologists described 63 patients with pernio‐like lesions, primarily adolescents. Swab for SARS‐CoV‐2 infection was performed in 11 patients and resulted positive in two cases; serology was available in six cases and it was positive in the two patients with positive swab. 2 Also a study among 375 cutaneous manifestations in suspected SARS‐CoV‐2 patients in Spain, 71 (19%) presented with “pseudo‐chilblains.” Only one of the 71 patients had previous history of chilblain; 29 (41%) had SARS‐CoV‐2 confirmed. 3 Recalcati et al 5 described from Lombardy, 14 cases including 11 children (average age 14 years), and three young adults (average age 29 years) with pernio‐like eruptions. No systemic symptoms were reported, except a mild itch in three cases. Nasopharyngeal swab for SARS‐CoV‐2 gave negative results. In a report of 19 adolescent patients with a clinical diagnosis of pernio‐like lesions, nasopharyngeal swab and IgG serology for SARS‐CoV‐2 nucleocapsid protein were negative. Importantly, IgA serology for S1 domain of SARS‐CoV‐2 spike protein was positive in six patients and borderline in three patients. 10 In a study performed in Sicily (Italy) via teledermatology a total of 22 patients complaining of perniosis‐like lesions were screened, mainly in the pediatric age (≤18 years). All of them were tested with rhino‐pharyngeal swabs and SARS‐CoV‐2 was detected in six patients, five of whom were children. 11 The understanding of the immuno‐pathogenetic mechanisms of interaction between the SARS‐CoV‐2 infection and children is very intriguing but current knowledge does not seem to be sufficient. Why some children who come into contact with the SARS‐CoV‐2 do not develop striking respiratory symptoms but present pernio‐like lesions with negativity on diagnostic tests?
Matricardi et al 12 developed the first model of interaction between the human immune system and SARS‐CoV‐2, as an attempt to produce a synthesis of the actual knowledge. What emerges is that innate immunity represents the first line of defense against the new SARS‐CoV‐2 and this first comparison establishes the natural history of the pathology: either the infection will effectively block in the upper airways or if the virus manages to reach the lungs. Innate immunity is essential for controlling virus replication before an adaptive immune response is generated. 13 Type I Interferones (IFN‐I) are major components of the innate immune system and it represents critical antiviral molecules. 14 It is hypothesized that the IFN‐I response may induce microangiopathic changes, producing chilblains and lupus‐like erythematous eruption.
 Coronavirus infection‐induced chilblains
15
Coronavirus infection‐induced chilblains
15
A mechanism has recently been considered to explain the appearance of autoimmune phenomena following the whole SARS‐CoV‐2 infection: molecular mimicry. 16 , 17 , 18 Lucchesi and Floel 19 have hypothesized a molecular mimicry mechanism between neuronal proteins present in the brain stem respiratory pacemaker neurons (DAB1, AIFM, and SURF1) and viral epitopes of SARS‐CoV‐2 treated antigenic. The same mechanism, according to Angileri et al 20 , could be responsible of anosmia, leukopenia and multi‐organ failure caused by vascular damage, assuming they are associated with the molecular mimicry. A type 3 hypersensitivity therefore occurs with the deposition of antibody antigen complexes precipitating inside the tissues, in particular the blood vessels, inducing a serious inflammatory state by the action of the complement anaphylatoxins (C3a and C5a), which in turn stimulate the release of histamine from mast cells and the recruitment of phagocytes, first in neutrophils, the main cause of tissue damage and following “leukocytoclastic vasculitis” (LCV), also reported in the English medical literature from the term “hypersensitivity vasculitis.” 21 , 22
This pathogenic mechanism could explain the appearance of pernio‐like lesions due to SARS‐CoV‐2 infection.
In conclusion, we think there is a correlation between pernio‐like lesions and SARS‐CoV‐2 infection, but further studies are needed to prove it. This, for the increased number of these lesions described during this short time, as in our experience, and because such manifestations appeared when temperatures were mild in Southern Italy and patients were at home for the lockdown.
To our knowledge, this is one of the few studies that collects a series of pediatric patients with pernio‐like lesions, evaluating the possible association with COVID‐19 (oropharyngeal swab and serology test) but also for rheumatological diseases. Many questions remain open about interaction host‐virus.
Abbreviations
- aCL
anti‐cardiolipin antibodies
- AIFM
Apoptosis‐inducing factor 1, mitochondrial
- ANA
antinuclear antibodies
- anti‐β2GP1
anti β‐2‐glycoprotein1 antibodies
- aPL
antiphospholipid antibodies
- C3a
complement factor 3 anaphylotoxin
- C5a
complement factor 5 anaphylotoxin
- CBC
complete blood count
- CLIA
chemiluminescent microparticle immunoassay
- COVID‐19
coronavirus disease‐19
- DAB‐1
disabled homolog 1 protein
- ENA
extractable nuclear antigens autoantibodies
- RF
rheumatoid factor
- IFN‐I
interferon type I
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- LAC
Lupus anticoagulant
- LCV
leukocytoclastic vasculitis»
- nDNA
native DNA autoantibodies
- NV
normal value
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SCL70
autoantibodies against‐topoisomerase I
- SLE
systemic lupus erythematous
- SURF1
surfeit locus protein 1
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Romina Gallizzi: Conceptualization (Lead) Data curation (Lead) Investigation (Lead) Methodology (Lead) Project administration (Lead) Resources (Lead) Software (Lead) Supervision (Lead) Validation (Lead) Visualization (Lead) Writing‐original draft (Lead) Writing‐review and editing (Lead). Diana Sutera: Writing‐original draft (Lead). Alessandra Spagnolo: Writing‐original draft (Lead). Anna Maria Bagnato: Writing‐original draft (Lead). Serafinella Patrizia Cannavò: Visualization (Equal). Loredana Grasso: Methodology (Equal). Claudio Guarneri: Visualization (Equal). Giuseppe Nunnari: Visualization (Equal). Francesca Mazza: Writing‐original draft (Equal). Giovanni Battista Pajno: Supervision (Equal) Validation (Equal) Visualization (Equal).
ETHICS STATEMENT
The study was approved by our Institutional Ethical Committee and conducted in accordance with the Declaration of Helsinki.
ACKNOWLEDGMENTS
Thanks to Italian Federation of Pediatrician (FIMP) of Messina for collaboration.
Gallizzi R, Sutera D, Spagnolo A, et al. Management of pernio‐like cutaneous manifestations in children during the outbreak of COVID‐19. Dermatologic Therapy. 2020;33:e14312. 10.1111/dth.14312
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Piccolo V, Neri I, Filippeschi C, et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020;34(7):e291–e293. 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–77. 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P, Aguirre T. Chilblain‐like lesions on feet and hands during the COVID‐19 pandemic. Int J Dermatol. 2020;59:739‐743. 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venereol. 2020;34(8):e346–e347. 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whitman PA, Crane JS. Pernio (Chilblains) StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. ‐2019. [Google Scholar]
- 6. Simon TD, Soep JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116(3):e472‐e475. [DOI] [PubMed] [Google Scholar]
- 7. Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020;18(6):1324‐1329. 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berlin T, Zandman‐Goddard G, Blank M, et al. Autoantibodies in nonautoimmune individuals during infections. Ann N Y Acad Sci. 2007;1108:584‐593. 10.1196/annals.1422.061. [DOI] [PubMed] [Google Scholar]
- 9. Yongchen Z, Shen H, Wang X, et al. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):833‐836. 10.1080/22221751.2020.1756699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. el Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guarneri C, Venanzi Rullo E, Gallizzi R, Ceccarelli M, Cannavò SP, Nunnari G. Diversity of clinical appearance of cutaneous manifestations in the course of COVID‐19. J Eur Acad Dermatol Venereol. 2020;34(9):e449–e450. 10.1111/jdv.16669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matricardi PM, Dal Negro RW, Nisini R. The first, holistic immunological model of COVID‐19: implications for prevention, diagnosis, and public health measures. Pediatr Allergy Immunol. 2020;31(5):454–470. 10.1111/pai.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu JC, Khodadadi H, Malik A, et al. Innate immunity of neonates and infants. Front Immunol. 2018;9:1759. 10.3389/fimmu.2018.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (a/b) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307‐335. [DOI] [PubMed] [Google Scholar]
- 15. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cappello F. Is COVID‐19 a proteiform disease inducing also molecular mimicry phenomena? Cell Stress Chaperones. 2020;25(3):381‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID‐19 infection: a case report. J Clin Neurosci. 2020.76 233–235. 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cappello F. COVID‐19 and molecular mimicry: the Columbus' egg? J Clin Neurosci. 2020;77 246 10.1016/j.jocn.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lucchese G, Flöel A. Molecular mimicry between SARS‐CoV‐2 and respiratory pacemaker neurons. Autoimmun Rev. 2020;19:102556. 10.1016/j.autrev.2020.102556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Angileri F. Molecular mimicry may explain multi‐organ damage in COVID‐19. Autoimmun Rev. 2020;19:102591. 10.1016/j.autrev.2020.102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Janeway CA Jr, Travers P, Walport M, Shlomchik M. Immunobiology: the Immune System in Health and Disease. 5th ed. New York: Garland Science; 2001. [Google Scholar]
- 22. Baigrie D, Bansal P, Goyal A, Crane JS. Leukocytoclastic Vasculitis (Hypersensitivity Vasculitis). Treasure Island: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


