Abstract
As of June 2020, the COVID‐19 pandemic has totaled over 9 000 000 cases and 470 000 deaths globally (ref. 1). Emerging data from COVID‐19 patients have suggested a clear role for oxidative stress in the pathogenesis of SARS‐CoV‐2, the pathogenic agent of COVID‐19. Several comorbidities, including hypertension, diabetes, obesity, and aging, have been associated with an increase in baseline oxidative stress, likely explaining why such individuals at risk for poor outcomes with SARS‐CoV‐2 infection. Similarly, the concept of oxidative stress remains one of the best supported theories to explain the mechanism behind aging. Oxidative stress through both endogenous and exogenous sources has known deleterious effects in both aging and SARS‐CoV‐2 infection. Herein, we will review the role of oxidative stress as a key player in both aging and COVID‐19 and highlight why some individuals may have better or poorer outcomes because of this. Additionally, we will discuss potential therapeutic pathways for effectively anti‐aging as we take away from our learnings on COVID‐19.
Keywords: aging, anti‐aging, COVID‐19, oxidative stress, skin aging
1. INTRODUCTION
As of June 2020, the COVID‐19 pandemic has totaled over 9 000 000 cases and 470 000 deaths globally, including over 2 290 000 and 120 000 cases and deaths, respectively, in the United States of America. 1 Infection with SARS‐CoV‐2, the pathogenic agent of COVID‐19, has been observed to cause significant oxidative stress and elevation of cytokines in the blood, with the resulting pro‐inflammatory environment predisposing individuals to severe tissue damage and poor outcomes. 2 , 3
Similarly, aging has been shown to be an oxidative process resulting in receptor‐initiated signaling, mitochondrial damage, protein oxidation, and telomere‐based DNA damage responses. 4 Both intrinsic (genetic make‐up somatic capacity, and composition) and extrinsic (nutrition, exercise, hormones, dental hygiene, stress, and environmental influences) factors contribute to the aging process. 5 The effects of oxidative stress in aging extend beyond the skin and have been linked with cancer, cardiovascular disease, diabetic complications, as well as effects on osteocytes, skeletal muscle, and adipocytes. 5 , 6 , 7 , 8 , 9
Herein, we will review the role of oxidative stress as a key player in both aging and COVID‐19 and highlight why some individuals may have better or poorer outcomes because of this. Additionally, we will discuss potential therapeutic pathways for effectively anti‐aging as we take away from our learnings on COVID‐19.
2. OXIDATIVE STRESS IN AGING
The concept of oxidative stress remains one of the best supported theories to explain the mechanism behind aging. A significant component of oxidative stress is that of free radicals and reactive oxygen and nitrogen species (RONS) produced during aerobic metabolism. As far back as 1956, Harman proposed that free radicals, highly reactive atoms or molecules with one or more unpaired electron(s) in their outermost shell, may play a crucial role in aging. 10 , 11 RONS are comprised of both free radicals and non‐free radical intermediates involving oxygen and nitrogen, respectively. RONS have important physiologic roles in extraction of energy from organic molecules, immune defense, and intracellular signaling, as well as pathologic roles in aging and degenerative diseases. 12 , 13 The tissue damage caused by most RONS occurs at a slow steady‐state and is counterbalanced by antioxidant enzymes and antioxidant molecules (see below). 12 , 13 , 14 Over time, there is progressive imbalance favoring oxidative stress, resulting in progressive loss of tissue and organ function that characterizes the aging process. 12 , 13 Oxidative stress is thought to lead to cell injury through lipid peroxidation of membranes, oxidative modification of proteins, and DNA damage. 6
Endogenous sources of RONS include nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, myeloperoxidase (MPO), lipoxygenase (LOX), and angiotensin‐II (ANG2). 7 , 12 Exogenous sources of RONS can include air and water pollution, tobacco, alcohol, heavy or transition metals, drugs, industrial solvents, cooking practices (smoked meat, waste oil, and fat), and radiation. 7 , 12 The impact of genetic predisposition suggests that some individuals will inherently age better than others (“Super Agers”), while the extrinsic influences highlight the importance of a holistic approach toward anti‐aging. 5
In aging, oxidative stress production is increased while antioxidant enzyme activity and adaptive responses are reduced. 15 This may be attributable to age‐related decline in nuclear factor E2‐related factor 2 (Nrf2), which binds to the antioxidant response element (ARE) to regulate the basal and inducible expression of hundreds of antioxidant enzymes, including superoxide dismutase (SOD), catalase, peroxiredoxins, and glutathione peroxidases. 16 , 17 Age‐related and/or oxidative stress‐induced DNA damage can involve both genomic and mitochondrial DNA, and can result in DNA mutations and genomic instability. 6 Damaged mitochondrial DNA can also enhance oxidant production and increase oxidative stress. 6 This oxidative stress causes progressive damage to mitochondrial DNA, creating an endless cycle. 6 , 18
Oxidative stress affects virtually all human cells during the aging process. The skin shows progressive age‐related breakdown of the collagen and elastin network, manifesting as xerosis, loss of elasticity, atrophy, dyschromia, and rhytides. 5 Skeletal muscle shows progressive, age‐related atrophy starting around 40 years of age, with roughly 8% loss in muscle mass per decade until 70 years of age, after which this increases to 15%. 18 Monickaraj and colleagues 15 conducted an in vitro study showing that adipocytes subjected to oxidative stress showed greater ROS production, DNA damage, and telomere shortening. Similarly, it has been shown that oxidative stress can negatively affect bone homeostasis through reducing bone formation and increasing bone resorption. 5 , 19
Oxidative stress can also play a role in inflammatory, degenerative, and neoplastic conditions. Oxidative damage and mitochondrial dysfunction can ultimately lead to age‐related neurodegenerative disorders (ex. Alzheimer's and Parkinson's diseases) and tumorigenesis. 6 There is evidence suggesting NAPDH‐oxidase‐mediated ROS signaling pathways are involved in age‐associated vascular changes and cardiovascular diseases. 7 , 20 MPO and LOX have similarly been implicated in atherogenesis. 8 Type 2 diabetes mellitus (T2DM), hypertension, atrial fibrillation, peripheral artery disease, obesity, dyslipidemia, and metabolic syndrome have all been associated with increased ROS production and oxidative stress. 8 Hyperglycemia‐induced oxidative stress has been shown to play a pivotal role in the developmental of both microvascular and macrovascular diabetic complications; furthermore, increased expression of SOD and use of antioxidant compounds in animal models were found to delay or prevent diabetic retinopathy, nephropathy, and cardiomyopathy. 9 Together, these findings support a role for oxidative stress in both normal aging and pathologic age‐related disease.
3. OXIDATIVE STRESS IN SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS‐2 (SARS‐COV‐2) INFECTION
Overproduction of ROS, through enzymes such as NADPH oxidase and xanthine oxidase, and a concurrent decrease in antioxidant mechanisms are crucial for the pathogenesis of respiratory viral infections. 2 , 16 Increased levels of ROS and oxidized metabolites correlated with poorer outcomes in the setting of chronic viral hepatitis B and C infection, including increased severity of liver damage and elevated risks of fibrosis and hepatocellular carcinoma. 16 , 20 ROSE production typically results in activation of Nrf2 and antioxidant responses, but respiratory viral infections have been shown to suppress Nrf2 pathways. 2
To date (June 2020), the COVID‐19 pandemic has totaled over 9 000 000 cases and 470 000 deaths globally, including over 2 290 000 and 120 000 cases and deaths, respectively, in the United States of America. 1 SARS‐CoV‐2 infection, the pathogenic agent of COVID‐19, is known to cause significant oxidative stress and elevation of cytokines. 2 , 3 This increase in cytokines such as IL‐1β, IL‐6, and IFN‐γ establishes a pro‐inflammatory environment associated with tissue damage and poorer outcomes with extensive pulmonary inflammation and damage. 3 It has been suggested that SARS‐CoV infection also induces oxidative stress through activation of transcription factors (ex. nuclear factor‐kB; NF‐kB) and toll‐like receptors (TLR; mainly TLR‐4), further amplifying the pro‐inflammatory state. 2 Lin et al 21 showed that the viral protease SARS‐CoV 3CLpro activated NF‐kB signaling and increased ROS production in HL‐CZ cells. Shao et al 22 found that genes encoded by mitochondrial DNA and some oxidative stress‐sensitive genes were upregulated in peripheral blood mononuclear cells of convalescent SARS‐CoV patients.
SARS‐CoV‐2 enters cells through binding of the spike (S) viral protein to the ubiquitous ACE2 (Angiotensin‐converting enzyme‐2) receptors, which can be found in the heart, vessels, gut, lung, kidney, testis, and brain. 23 Zhao et al 24 found that 83% of ACE2‐expressing cells are alveolar epithelial type II cells, which may provide insight into why the lungs can be particularly susceptible to SARS‐CoV‐2 infection. ACE2 receptors degrade ANG2 (a vasoconstrictor and inducer of inflammation) to angiotensin 1 and 7 (ANG1 and ANG7); through the latter, ACE2 counter‐balances the effects of ANG2‐induced oxidative stress. 23 , 25 ANG2 can enhance production of superoxide (O2 ‐) and other ROS directly, as well as indirectly through enhancing NADPH oxidase activity. 26 Binding of SARS‐CoV‐2 leads to down‐regulation of ACE2 receptors, resulting in increased ANG2 and NADPH oxidase‐induced inflammation. 23 Despite ACE2 being the cellular receptor for SARS‐CoV‐2 invasion, it is unlikely that ACE2 deficiency would prevent viral invasion given the intrinsically high affinity of the virus for ACE2. 23 Together, this evidence suggests a clear role for oxidative stress in SARS‐CoV‐2 pathogenesis.
4. ACE2 DEFICIENCY AND INCREASED OXIDATIVE STRESS: WORSE OUTCOMES WITH COVID‐19?
It seems reasonable to propose that individuals with elevated baseline oxidative stress may be prone to worse outcomes with SARS‐CoV‐2 infection. A review of 72 314 cases of SARS‐CoV‐2 in China revealed older age, cardiovascular disease, diabetes, and hypertension, among other comorbidities, to be associated with an increased overall case‐fatality rate. 27 Similarly, a meta‐analysis of seven studies including 1576 SARS‐CoV‐2‐infected patients found that those with severe disease were more likely to have comorbidities such as hypertension, respiratory disease, and cardiovascular disease. 28 The comorbidities and patient factors that appear to be associated with more severe SARS‐CoV‐2 infection share some degree of ACE2 deficiency (and thus, oxidative stress) at baseline. 23
It has been shown that ACE2 confers endothelial protection and slows atherosclerosis and atherothrombosis in an ANG1/ANG7‐dependent fashion. 25 Conversely, ACE2 deficiency, and resulting decreased ANG1/ANG7 levels, has been linked with hypertension and heart failure. 23 , 29 , 30 , 31 Diabetes mellitus has also been associated with reduced ACE2 expression, possibly due to glycosylation. 23 , 32 Animal studies have shown ACE2‐induced upregulation of ANG1/ANG7 to improve insulin secretion, possibly through increased release of nitric oxide. 23 , 33 Obesity, which is strongly correlated with these metabolic comorbidities, has also been linked to ACE2 deficiency in animal studies. 23 , 34 Animal studies have similarly shown that ACE2 expression in lung tissue decreases significantly with age, with a more precipitous decrease in ACE2 expression in older males. 35 Future study is needed to examine whether the elevated baseline oxidative stress in these comorbid individuals also results in poorer aging outcomes.
5. COUNTERBALANCING OXIDATIVE STRESS AND ANTI‐AGING EFFECTIVELY
Oxidative stress has critical roles in both aging and SARS‐CoV‐2 pathogenesis (Figure 1). 36 Counterbalancing oxidative stress may have therapeutic potential in improving both aging and SARS‐CoV‐2 infection outcomes.
FIGURE 1.
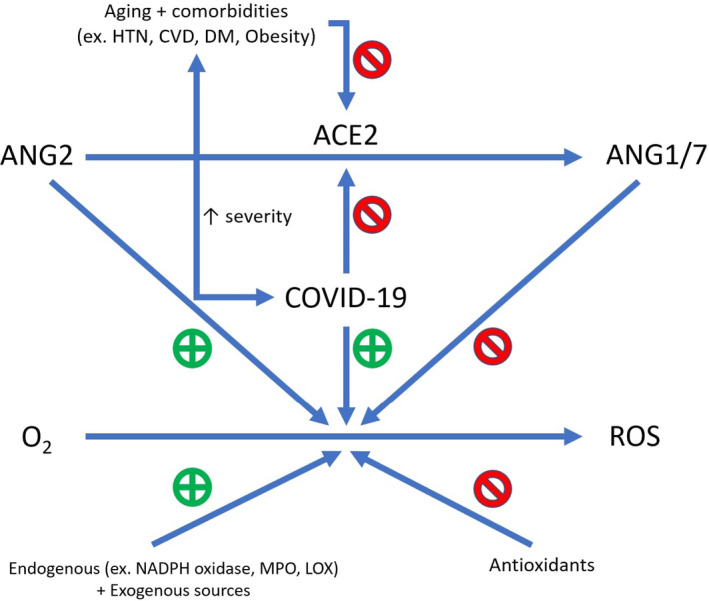
Oxidative stress. Oxidative stress is thought to play a role in both aging and COVID‐19 pathogenesis. There are both endogenous and exogenous sources of oxidative stress; one of the former is believed to be ANG2. ACE2 degrades ANG2 (a vasoconstrictor and inducer of inflammation) to ANG1/7, which helps counterbalance the effects of ANG2‐induced oxidative stress. SARS‐CoV 2, the pathogenic agent of COVID‐19, enters cells through binding and subsequently downregulating ACE2, resulting in increased ANG2‐induced inflammation. Aging and several comorbidities that have been linked to more severe cases of COVID‐19 are also thought to represent states of ACE2 deficiency (and increased ANG2 levels). ACE2, Angiotensin‐converting enzyme 2; ANG2, Angiotensin 2; ANG1/7, Angiotensin 1 and Angiotensin 7; HTN, Hypertension; CVD; Cardiovascular disease; DM, Diabetes mellitus; ROS, Reactive oxygen species; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase; MPO, myeloperoxidase; LOX, lipoxygenase. Note: Figure adapted from figure in Ref 39
Both endogenous and exogenous antioxidant defenses confer protection from free radical toxicity. Key antioxidant enzymes include SOD, catalase (CAT), glutathione peroxidase (GSH‐Px), glutathione‐S‐transferase, and glucose‐6‐phosphate dehydrogenase. 12 , 37 Bilirubin, α‐tocopherol (Vitamin E), β‐carotene, proteins (ferritin, transferrin, ceruloplasmin, albumin), and low molecular weight scavengers, like uric acid, coenzyme Q, and lipoic acid are examples of endogenous non‐enzymatic antioxidants that terminate free radical chain reactions. 12 , 37 , 38 Examples of exogenous antioxidants include ascorbic acid (Vitamin C), α‐tocopherol (Vitamin E), and phenolic antioxidants, which include stilbene derivatives (resveratrol, phenolic acids, and flavonoids), oil lecitinas, selenium, zinc, and drugs including acetylcysteine. 12
Evidence surrounding supplementation to counterbalance oxidative stress through systemic antioxidants remains somewhat controversial. A randomized double‐blind trial by Kiokias and Gordon 39 found that patients who took a carotenoid mixture (β‐catotene, α‐carotene, lycopene, bixin, lutein, and paprika carotenoids) had reduced DNA damage and oxidative stress. Carotenoids, found in many fruits and vegetables, are thought to exert these effects through inhibiting inflammatory cytokine production through the NF‐kB. 40 Vitamins C and E have antioxidant effects associated with increased and decreased activity of SOD and NADPH oxidase, respectively, in animal models. 41 Vitamin C is also thought to serve as a defense against MPO‐mediated low density lipoprotein (LDL)‐oxidation. 42 Pumpkin skin extracts have been found to scavenge free radicals and inhibit lipid peroxidation catalyzed by LOX. 43 Vitamin E and omega(n)‐3 fatty acids are thought to play a role in preventing lipid peroxidation in mice. 44 n‐3 fatty acid consumption also reduced lipid peroxidation in type‐2 diabetic patients, which animal models suggest may occur through increased SOD2 activity. 45 , 46 Conversely, excessive n‐6 fatty acid intake is thought to stimulate inflammation. 47 , 48 Additionally, telomere length was found to increase with decreasing n‐6:n‐3 fatty acid ratios; short telomeres represent cellular aging and predict earlier disease. 49
These antioxidative effects of n‐3 fatty acids were found to be protective for the olfactory system in patients following endoscopic sellar and parasellar tumor resection. 50 There has been speculation of similar therapeutic benefit with post‐viral anosmia with SARS‐CoV‐2 infection, though this has not been formally tested. 51 Other potential antioxidants, including Vitamin C, Vitamin E melatonin, and N‐acetylcysteine have been proposed as possible therapeutics for SARS‐CoV‐2 patients, but evidence is currently lacking. 52 , 53 , 54 A clinical trial is investigating the use of vitamin C infusion for COVID‐19 patients. 55 Specific recommendations regarding use of synthetic exogenous antioxidants to reduce oxidative stress remain controversial. 38 We must also consider that inappropriate overuse of exogenous synthetic antioxidants can result in unregulated neutralization of radicals, including those with a beneficial physiological role. 38
Several extrinsic factors can augment intrinsic oxidative stress; likely the most impactful of these is cumulative sun exposure. Exposure to ultraviolet radiation (UVR), visible light, infrared radiation (IR) and heat causes skin damage through enhanced production of reactive molecule species. 56 Despite the focus on UVR, visible and IR light account for approximately 50% of radical formation with respect to the complete spectrum. 56 An ideal sunscreen should be cosmetically pleasing and contain organic UV filters, physical filters, and antioxidants to ensure protection across the entire solar spectrum. 57 Pollution can also accelerate skin aging, and certain pollutants such as benzopyrene may worsen oxidative stress synergistically with UVA. 5 , 58 In a study of the impact of air pollution on skin aging, an increase in soot and particles from traffic was associated with increased pigmented spots on the forehead and cheeks. 59 It is recommend that patients avoid air pollutants as much as possible, use protective clothing (scarf, hats), implement a skin cleansing routine to remove particulate matter, and consider use of topical (Vitamins C and E, and ferulic acid) and systemic (see below) antioxidants. 5 The smoke from a single cigarette contains close to 10 18 reactive molecule species, and smoking cessation should always be addressed in the anti‐aging consultation. 56 Maintaining good oral hygiene, managing stress, and obtaining adequate sleep and exercise can help optimize the aging process. 5
Diet is thought to play a role in oxidative stress, although specific recommendations remain controversial. 47 The current evidence suggests high‐glucose, animal‐based protein diets, and excessive fat consumption may promote oxidative stress. 47 Conversely, the Mediterranean and Okinawan diets, as well as the consumption of whole grains, nuts, fruits, vegetables, fish, and legumes, appear to decrease oxidative stress. 47 , 60 Recommendations to patients may include replacing refined carbohydrates with whole grains, increasing consumption of fruits and vegetables, decreasing consumption of total and saturated fat (and replacing with unsaturated fatty acids), and consuming an overall moderate amount of calories. 47
Hormone levels have also been found to play a role in oxidative stress. Estrogen has been found to help counteract oxidative stress‐induced damage to the central nervous and cardiovascular systems. 61 Bottai et al 62 noted that human fibroblasts were protected from oxidative damage by the presence of 17β‐estradiol, and proposed that lower estrogen levels during menopause could predispose to skin damage. Progesterone has been found to reduce oxidative stress leading to a neuroprotective effect in rat models. 63 , 64 It has additionally been proposed that some physiologic hormones (estrogen, progesterone, testosterone, cholesterol) may interfere with SARS‐CoV‐2 attachment to cells. 65 A clinical trial is investigating the safety and efficacy of progesterone (100mg subcutaneous twice daily) for treatment of COVID‐19 among hospitalized men. 66 For aging, the evidence linking hormone replacement (both systemic and topical) to improved skin quality has been controversial to date. 5
6. CONCLUSION
Learning from the physiologic processes that affect one disease to help understand other present and future conditions has been a mainstay of medicine. Identifying links between various disease states can result in therapeutic developments that mitigate or minimize complications. Oxidative stress has known deleterious effects in both aging and SARS‐CoV‐2 infection and has been linked to comorbidities that appear to predispose to poor outcomes with SARS‐CoV‐2 infection. By reducing oxidative stress, not only will aging likely be slowed, but the comorbidities placing individuals at high risk of poor outcomes from SARS‐CoV‐2 may also be reduced. More research is required to discover novel ways to reduce oxidative stress and unlock both the secrets of anti‐aging and ways to reduce the morbidity and mortality of COVID‐19.
Zarbafian M, Dayan S, Fabi SG. Teachings from COVID‐19 and aging—An oxidative process. J Cosmet Dermatol. 2020;19:3171–3176. 10.1111/jocd.13751
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19) situation report – 156. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200624‐covid‐19‐sitrep‐156.pdf?sfvrsn=af42e480_2. Accessed June 25, 2020
- 2. Delgado‐Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS‐CoV) infection. Arch Med Res. 2020;51:384‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol. 2007;157(5):874‐887. [DOI] [PubMed] [Google Scholar]
- 5. Saluja SS, Fabi SG. A holistic approach to antiaging as an adjunct to antiaging procedures: a review of the literature. Dermatol Surg. 2017;43(4):475‐484. [DOI] [PubMed] [Google Scholar]
- 6. Kudryavtseva AV, Krasnov GS, Dmitriev AA, et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7(29):44879‐44905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sahoo S, Meijles DN, Pagano PJ. NADPH oxidases: key modulators in aging and age‐related cardiovascular diseases? Clin Sci. 2016;130(5):317‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pignatelli P, Menichelli D, Pastori D, Violi F. Oxidative stress and cardiovascular disease: new insights. Kardiol Pol. 2018;76:713‐722. [DOI] [PubMed] [Google Scholar]
- 9. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harraan D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298‐300. [DOI] [PubMed] [Google Scholar]
- 11. Chandrasekaran A, Idelchik MD, Melendez JA. Redox control of senescence and age‐related disease. Redox Biol. 2017;11:91‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Genestra M. Oxyl radicals, redox‐sensitive signalling cascades and antioxidants. Cell Signal. 2007;19(9):1807‐1819. [DOI] [PubMed] [Google Scholar]
- 14. Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78(2):547‐581. [DOI] [PubMed] [Google Scholar]
- 15. Monickaraj F, Aravind S, Nandhini P, et al. Accelerated fat cell aging links oxidative stress and insulin resistance in adipocytes. J Biosci. 2013;38(1):113‐122. [DOI] [PubMed] [Google Scholar]
- 16. Khomich OA, Kochetkov SN, Bartosch B, Ivanov AV. Redox biology of respiratory viral infections. Viruses. 2018;10(8):392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Davies KJ, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015;88:314‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gomes MJ, Martinez PF, Pagan LU, et al. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8(12):20428‐20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. 2015;33(4):359‐370. [DOI] [PubMed] [Google Scholar]
- 20. Ivanov AV, Valuev‐Elliston VT, Tyurina DA, et al. Oxidative stress, a trigger of hepatitis C and B virus‐induced liver carcinogenesis. Oncotarget. 2017;8(3):3895‐3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin CW, Lin KH, Hsieh TH, et al. Severe acute respiratory syndrome coronavirus 3C‐like protease‐induced apoptosis. FEMS Immunol Med Microbiol. 2006;46(3):375‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shao H, Lan D, Duan Z, et al. Upregulation of mitochondrial gene expression in PBMC from convalescent SARS patients. J Clin Immunol. 2006;26(6):546‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. Eur J Intern Med. 2020;76:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao Y, Zhao Z, Wang Y, et al. Single‐cell RNA expression profiling of ACE2, the receptor of SARS‐CoV‐2. Am J Respir Crit Care Med. 2020;202(5):756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lovren F, Pan Y, Quan A, et al. Angiotensin converting enzyme‐2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295(4):H1377‐H1384. [DOI] [PubMed] [Google Scholar]
- 26. Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005;3(2):254‐267. [DOI] [PubMed] [Google Scholar]
- 27. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 28. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID‐19) infection: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupte M, Thatcher SE, Boustany‐Kari CM, et al. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol. 2012;32(6):1392‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel VB, Basu R, Oudit GY. ACE2/Ang 1–7 axis: a critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte. 2016;5(3):306‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tikellis C, Thomas MC. Angiotensin‐converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012;2012:256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pal R, Bhansali A. COVID‐19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162:108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yuan L, Li Y, Li G, Song Y, Gong X. Ang (1–7) treatment attenuates β‐cell dysfunction by improving pancreatic microcirculation in a rat model of type 2 diabetes. J Endocrinol Invest. 2013;36(11):931‐937. [DOI] [PubMed] [Google Scholar]
- 34. Gupte M, Boustany‐Kari CM, Bharadwaj K, et al. ACE2 is expressed in mouse adipocytes and regulated by a high‐fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R781‐R788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xudong X, Junzhu C, Xingxiang W, et al. Age‐and gender‐related difference of ACE2 expression in rat lung. Life Sci. 2006;78(19):2166‐2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. MedCram [MedCram – Medical Lectures Explained CLEARLY] (2020, May 1). Coronavirus Pandemic Update 65: COVID‐19 and Oxidative Stress (Prevention & Risk Factors) [Video]. Youtube. https://youtu.be/gzx8LH4Fjic. Accessed April 14, 2020.
- 37. Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55‐74. [DOI] [PubMed] [Google Scholar]
- 38. Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kiokias S, Gordon MH. Dietary supplementation with a natural carotenoid mixture decreases oxidative stress. Eur J Clin Nutr. 2003;57(9):1135‐1140. [DOI] [PubMed] [Google Scholar]
- 40. Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. 2014;34(11):907‐929. [DOI] [PubMed] [Google Scholar]
- 41. Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke‐prone SHR. Hypertension. 2001;38(3 Pt 2):606‐611. [DOI] [PubMed] [Google Scholar]
- 42. Carr AC, McCall MR, Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: reaction pathways and antioxidant protection. Arterioscler Thromb Vasc Biol. 2000;20(7):1716‐1723. [DOI] [PubMed] [Google Scholar]
- 43. Xanthopoulou MN, Nomikos T, Fragopoulou E, Antonopoulou S. Antioxidant and lipoxygenase inhibitory activities of pumpkin seed extracts. Food Res Int. 2009;42(5–6):641‐646. [Google Scholar]
- 44. Laganiere S, Byung PY, Fernandes G. Studies on membrane lipid peroxidation in n‐3 fatty acid‐fed autoimmune mice: effect of vitamin E supplementation. Adv Exp Med Biol. 1990;262:95‐102. [DOI] [PubMed] [Google Scholar]
- 45. Garrel C, Alessandri JM, Guesnet P, Al‐Gubory KH. Omega‐3 fatty acids enhance mitochondrial superoxide dismutase activity in rat organs during post‐natal development. Int J Biochem Cell Biol. 2012;44(1):123‐131. [DOI] [PubMed] [Google Scholar]
- 46. Mori TA, Dunstan DW, Burke V, et al. Effect of dietary fish and exercise training on urinary F2‐isoprostane excretion in non—insulin‐dependent diabetic patients. Metabolism. 1999;48(11):1402‐1408. [DOI] [PubMed] [Google Scholar]
- 47. Tan BL, Norhaizan ME, Liew WP. Nutrients and oxidative stress: friend or foe? Oxid Med Cell Longev. 2018;2018:9719584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wendell SG, Baffi C, Holguin F. Fatty acids, inflammation, and asthma. J Allergy Clin Immunol. 2014;133(5):1255‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kiecolt‐Glaser JK, Epel ES, Belury MA, et al. Omega‐3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial. Brain Behav Immun. 2013;28:16‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yan CH, Rathor A, Krook K, et al. Effect of N‐3 supplementation in patients with smell dysfunction following endoscopic sellar and parasellar tumor resection: a multicenter prospective randomized controlled trial. Neurosurgery. 2020;87(2):E91–E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology. 2020;58(3):295‐298. [DOI] [PubMed] [Google Scholar]
- 52. Wang JZ, Zhang RY, Bai J. An anti‐oxidative therapy for ameliorating cardiac injuries of critically ill COVID‐19‐infected patients. Int J Cardiol. 2020;312:137‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang R, Wang X, Ni L, et al. COVID‐19: Melatonin as a potential adjuvant treatment. Life Sci. 2020;23:117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.“N‐acetylcysteine: A Rapid Review of the Evidence for Effectiveness in Treating COVID‐19.” The Centre of Evidence‐Based Medicine. https://www.cebm.net/covid‐19/n‐acetylcysteine‐a‐rapid‐review‐of‐the‐evidence‐for‐effectiveness‐in‐treating‐covid‐19/. Accessed April 14, 2020.
- 55. Carr AC. A new clinical trial to test high‐dose vitamin C in patients with COVID‐19. Crit Care. 2020;24(1):1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wölfle U, Seelinger G, Bauer G, et al. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol Physiol. 2014;27(6):316‐332. [DOI] [PubMed] [Google Scholar]
- 57. Meinke MC, Syring F, Schanzer S, et al. Radical protection by differently composed creams in the UV/VIS and IR spectral ranges. Photochem Photobiol. 2013;89(5):1079‐1084. [DOI] [PubMed] [Google Scholar]
- 58. Burke KE, Wei H. Synergistic damage by UVA radiation and pollutants. Toxicol Ind Health. 2009;25(4–5):219‐224. [DOI] [PubMed] [Google Scholar]
- 59. Vierkötter A, Schikowski T, Ranft U, et al. Airborne particle exposure and extrinsic skin aging. J Invest Dermatol. 2010;130(12):2719‐2726. [DOI] [PubMed] [Google Scholar]
- 60. Casas R, Sacanella E, Urpί‐Sardà M, et al. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS One. 2014;9(6):e100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lagranha CJ, Silva TL, Silva SC, et al. Protective effects of estrogen against cardiovascular disease mediated via oxidative stress in the brain. Life Sci. 2018;192:190‐198. [DOI] [PubMed] [Google Scholar]
- 62. Bottai G, Mancina R, Muratori M, et al. 17β‐estradiol protects human skin fibroblasts and keratinocytes against oxidative damage. J Eur Acad Dermatol Venereol. 2013;27(10):1236‐1243. [DOI] [PubMed] [Google Scholar]
- 63. Ozacmak VH, Sayan H. The effects of 17β estradiol, 17α estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemic. Physiol Res. 2009;58(6):909. [DOI] [PubMed] [Google Scholar]
- 64. Webster KM, Wright DK, Sun M, et al. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long‐term outcomes in a rat model of repeated mild traumatic brain injury. J Neruoinflammation. 2015;12(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Elfiky AA. Natural products may interfere with SARS‐CoV‐2 attachment to the host cell. J Biomol Struct Dyn. 2020;1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.“Progesterone for the treatment of COVID‐19 in hospitalized men”. ClinicalTrials.gov. clinicaltrials.gov/ct2/show/NCT04365127. Accessed April 28, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


