Abstract
We describe the successful pediatric liver transplant for unresectable hepatoblastoma in a 4‐year‐old male with COVID‐19 prior to transplant. The first negative NP swab was documented 1 month after initial diagnosis, when SARS‐CoV‐2 antibodies were also detected. The patient was actively listed for liver transplant after completing four blocks of a SIOPEL‐4 based regimen due to his PRETEXT IV disease which remained unresectable. Following three additional negative NP swabs and resolution of symptoms for 4 weeks, he underwent a whole‐organ pediatric liver transplant. COVID‐19 positivity determined via NP swab SARS‐CoV‐2 real‐time RT‐PCR (Hologic Aptima SARS‐CoV‐2 RT‐PCR assay). IgG and IgM total SARS‐ CoV‐2 antibodies detected by Ortho Clinical Diagnostics VITROS® Immunodiagnostics Products Anti‐SARS‐CoV‐2 Test. Patient received standard prednisone and tacrolimus‐based immunosuppression without induction therapy following transplant. Post‐transplant course was remarkable for neutropenia and thrombocytopenia, with discharge home on post‐transplant day #11. Surveillance tests have remained negative with persistent SARS‐CoV‐2 IgG antibodies at 6 weeks after transplant. We describe one of the earliest, if not the first case of liver transplant following recent recovery from COVID‐19 in a pediatric patient with a lethal malignant liver tumor. A better understanding of how to balance the risk profile of transplant in the setting of COVID‐19 with disease progression if transplant is not performed is needed. We followed existing ASTS guidelines to document clearance of the viral infection and resolution of symptoms before transplant. This case highlights that pediatric liver transplantation can be safely performed upon clearance of COVID‐19.
Keywords: hepatoblastoma, pediatric liver transplantation, viral infection
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- AFP
alpha‐fetoprotein
- ANC
absolute neutrophil count
- ASTS
American Society of Transplant Surgeons
- COVID‐19
coronavirus disease 2019
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor
- MRI
magnetic resonance imaging
- NP
nasopharyngeal
- PRETEXT
preoperative evaluation of the tumor extent
- RT‐PCR
reverse transcription‐polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SIOPEL
Société Internationale d'Oncologie Pédiatrique—Epithelial Liver Tumor Study Group
1. INTRODUCTION
The novel COVID‐19 caused by SARS‐CoV‐2 has transformed innumerable aspects of clinical practice, particularly in the field of organ transplantation. Transplant recipients are at a higher risk of viral infection progression due to attenuated T cell–mediated immunity from immunosuppressive medications 1 (Figure 1). Additionally, transplant team members and the need for transportation of allografts may play a significant role in viral disease transmission. As a result, transplant centers have developed protocols 2 so life‐saving transplants can be safely provided while minimizing risks to healthcare teams during this dynamic period. As outcomes data remain scarce and a definitive treatment has yet to be realized, it is speculated that transplant of an actively or recently COVID‐19 infected patient could result in an adverse outcome. Therefore, the ASTS provided guidance in March 2020 to defer transplant in the potential recipient actively infected with COVID‐19. 3 , 4 It was recommended that recently infected patients have at least two negative SARS‐CoV‐2 RT‐PCR tests and complete resolution of symptoms for 4 weeks prior to transplant to maximize the chance of a good outcome. However, the consequences of SARS‐CoV‐2 infection before or after transplant in children remain unknown. Meanwhile, it is important to acknowledge that withholding or delaying transplant can lead to increased morbidity or death in some patients. Therefore, the risk of not transplanting must also be considered when making decisions in the actively or recently COVID‐19 infected transplant candidate, reflecting the hesitance of the AASLD to declare COVID‐19 a severe threat in the setting of immunosuppression without firm evidence. 5
FIGURE 1.

SARS‐CoV‐2 replication cycle and theorized T‐cell response with immunosuppression
Hepatoblastoma is the most common primary tumor of the liver in children. Based on the PRETEXT staging system, 6 “high risk” patients require preoperative chemotherapy followed by complete surgical resection to ideally achieve disease freedom. During the pandemic and based upon ASTS guidance, 3 , 4 a patient's candidacy for transplant may be threatened or delayed by the development of an active SARS‐CoV‐2 infection.
We report our short‐term experience navigating the indication for liver transplant amid chemotherapy, risk of viral exposure of our healthcare team during an unprecedented pandemic, and a progressive oncologic disease in a COVID‐19 infected pediatric patient.
2. CASE REPORT
Our patient is a 4‐year‐old formerly 23‐week premature male who presented to our emergency department with acute abdominal pain and hepatomegaly. Right upper quadrant ultrasound demonstrated multiple calcified lesions in all lobes of the liver. AFP was elevated at 207 000 ng/mL and liver biopsy confirmed hepatoblastoma, epithelial subtype. Due to the patient's PRETEXT IV classification without extrahepatic disease, he was deemed to be “high risk” and treated with a SIOPEL‐4 based regimen consisting of cisplatin and doxorubicin/dexrazoxane. The first block of therapy was tolerated well; however, during the second block, the patient developed neutropenia (WBC 1.70‐5.53 × 103/µL (normal 4.86‐13.38 × 103/µL)), cough, and 100.8°F fever. A comprehensive infectious disease evaluation was negative, except for a positive NP swab SARS‐CoV‐2 real‐time RT‐PCR (Hologic Aptima SARS‐CoV‐2 RT‐PCR assay). Both parents underwent SARS‐CoV‐2 NP swab and viral RNA was not detected. As the patient was admitted from home without exposure to a COVID‐19 positive healthcare provider, community‐acquired infection was presumed. As is usually performed in the febrile neutropenic patient at our institution, the child received broad‐spectrum empiric antibiotics until blood and urine cultures were finalized as negative. The patient did not require any additional supportive care for his mild respiratory symptoms and successfully completed the second block of the SIOPEL‐4 based regimen without adjustment to chemotherapy. While AFP decreased to 7761 ng/mL, the multifocal disease persisted when evaluated by MRI. Cross‐sectional imaging of the chest and abdomen did not reveal any evidence of extrahepatic metastatic disease. Therefore, he was considered for liver transplant.
Since the patient was tolerating the chemotherapeutic protocol and had only mild COVID‐19 symptoms, he received block 3 of chemotherapy without complications. He was closely followed for development of new symptoms and with serial RT‐PCR testing for SARS‐CoV‐2. As depicted in Figure 2, four additional NP swabs tested positive over the course of 3 weeks. One month following his first positive NP swab, the patient no longer had symptoms or detectable viral RNA and concurrently developed detectable IgG and IgM total SARS‐CoV‐2 antibodies (Ortho Clinical Diagnostics VITROS® Immunodiagnostics Products Anti‐SARS‐CoV‐2 Test).
FIGURE 2.
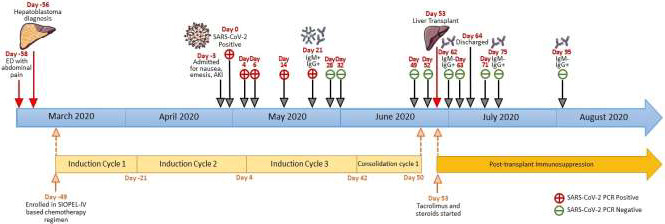
Timeline of COVID‐19 infection, resolution, and resultant liver transplant
The patient's liver lesions did not regress to a resectable level, and given clearance of the SARS‐CoV‐2 infection, the patient was listed for liver transplant. He started the 1st consolidation block (four/six blocks) of the SIOPEL‐4 based regimen and continued surveillance SARS‐CoV‐2 NP swabs, which remained negative (Figure 2). Four weeks after the first negative NP swab and documentation of presence of SARS‐CoV‐2 antibodies, the patient underwent an uncomplicated whole‐organ ABO‐compatible liver transplant with choledochocholedochostomy. Following transplant, the patient's immunosuppressive therapy consisted of a methylprednisolone taper followed by prednisolone (0.3 mg/kg) and tacrolimus (serum trough 6‐10 ng/mL). The patient's post‐transplant course was remarkable for neutropenia and thrombocytopenia secondary to chemotherapy. As his ANC never fell below 1000/mm3, GM‐CSF or other therapies were not needed. Prior to discharge on post‐transplant day #11, RT‐PCR testing was repeated twice and both tests did not detect SARS‐CoV‐2 RNA (Figure 2). Three surveillance tests remained negative following discharge. Additionally, the patient's SARS‐CoV‐2 IgG‐specific antibodies have persisted at 6 weeks following transplant while IgM antibodies remain negative indicating previous and not active infection. Post‐transplant immunosuppression was not altered compared with standard protocol.
3. DISCUSSION
As the COVID‐19 pandemic continues to unfold, it is critical to understand how infected patients tolerate surgery, namely liver transplant. In the Lei et al 7 retrospective review of 34 patients who underwent elective operative procedures during the SARS‐CoV‐2 incubation period, it was shown that 44.1% required ICU care with a mortality rate of 20.5%. Additional studies, for example, Doglietto et al 8 and Nahshon et al, 9 have revealed a high complication rate and increased mortality in adult COVID‐19 patients following surgery. Some transplant‐specific studies have demonstrated worse outcomes for adults contracting SARS‐CoV‐2 post‐transplant, 10 , 11 , 12 though other reports seemingly contradict the observation that the transplant subpopulation is at increased risk for a more severe clinical course. 13 , 14 These differing conclusions are likely the result of confounding factors such as highly associated comorbidities playing a significant role in COVID‐19 outcomes. 15
We describe one of the earliest, if not the first case of liver transplant following recent recovery from COVID‐19 in a pediatric patient with a lethal malignant liver tumor. A better understanding of how to balance the risk profile of transplant in the setting of COVID‐19 with disease progression if transplant is not performed is needed. It remains imperative that we provide life‐saving liver transplants to children amid the COVID‐19 pandemic while recognizing that transplant with any active viral infection typically portends worse outcomes. In addition, the safety of our healthcare team remains a priority. To meet these goals, we followed existing ASTS guidance to document clearance of the viral infection and resolution of symptoms and only then performed the transplant, similar to our approach with other aggressive pediatric viruses like influenza or adenovirus. The immediate post‐operative and long‐term effects of a recent SARS‐CoV‐2 infection prior to solid organ transplantation are unknown, particularly in the pediatric population. In the early post‐transplant period, our patient has not had COVID‐19 reinfection and has maintained SARS‐CoV‐2 IgG antibodies. Unfortunately, while antibodies have been detected three times post‐transplant, our testing is qualitative rather than quantitative. As such, it is possible the antibody titer has decreased to levels that are not immunoprotective, though the significance of SARS‐CoV‐2 antibodies remains unclear. This case report is limited by a relatively short‐term follow‐up period. However, there is urgency for shared clinical reports amid this extraordinary pandemic to inform evolving policies. We plan to continue to evaluate this patient through his post‐transplant period in part by assessing antibody kinetics, with testing every 2 weeks, to improve our understanding of risk for reinfection and host response. This case highlights that liver transplantation can be safely performed in a child upon documented clearance of COVID‐19 infection, while on chemotherapy, with adherence to current guidelines. Continued follow‐up will be crucial to begin discerning the potential long‐term effects of recent or peritransplant COVID‐19 infection.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose as described by Pediatric Transplantation.
AUTHORS' CONTRIBUTIONS
Matthew B. Goss, MS: Paper writing; Flor M. Munoz, MD: Contribution of drafting/research design; Wenly Ruan, MD: Production figures/contribution of drafting; Nhu Thao Nguyen Galván, MD, MPH: Contribution of drafting; Christine A. O'Mahony, MD: Participation in research design; Abbas Rana, MD: Participation in research design; Ronald T. Cotton, MD: Participation in research design; Nicolas F. Moreno, BS: Contribution of drafting/research design; Andras A. Heczey, MD: Contribution of drafting/research design; Daniel H. Leung, MD: Contribution of drafting/research design; John A. Goss, MD: Research/study design.
Goss MB, Munoz FM, Ruan W, et al. Liver transplant in a recently COVID‐19 positive child with hepatoblastoma. Pediatr Transplant 2021;25:e13880. 10.1111/petr.13880
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Khanna R, Smith C. Cellular immune therapy for viral infections in transplant patients. Indian J Med Res. 2013;138(5):796‐807. [PMC free article] [PubMed] [Google Scholar]
- 2. Galván NTN, Moreno NF, Garza JE, et al. Donor and transplant candidate selection for solid organ transplantation during the COVID‐19 pandemic. Am J Transpl. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ASTS . COVID 19 Strike Force Guidance to Members on the Evolving Pandemic. ASTS; 2020. https://asts.org/advocacy/covid-19-resources/asts-covid-19-strike-force/asts-covid-19-strike-force-initial-guidance. Accessed July 14, 2020 [Google Scholar]
- 4. Organ Retrieval for Transplantation in the COVID‐19 Era. ASTS; 2020. https://asts.org/advocacy/covid-19-resources/asts-covid-19-strike-force/asts-covid-19-strike-force-organ-retrieval-guidance. Accessed July 14, 2020 [Google Scholar]
- 5. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers during the COVID‐19 Pandemic: AASLD Expert Panel Consensus Statement. AASLD; 2020. https://www.aasld.org/sites/default/files/2020-06/AASLD-COVID19-ExpertPanelConsensusStatement-June252020-v2-FINAL.pdf. Accessed August 4, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perilongo G, Shafford E, Plaschkes J, et al. SIOPEL trials using preoperative chemotherapy in hepatoblastoma. Lancet Oncol. 2000;1:94‐100. [DOI] [PubMed] [Google Scholar]
- 7. Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID‐19 infection. EClinicalMedicine. 2020;21:100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doglietto F, Vezzoli M, Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID‐19) in Italy. JAMA Surg. 2020;155(8):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nahshon C, Bitterman A, Haddad R, Hazzan D, Lavie O. Hazardous postoperative outcomes of unexpected COVID‐19 infected patients: a call for global consideration of sampling all asymptomatic patients before surgical treatment. World J Surg. 2020;44(8):2477‐2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int. 2020;97(6):1083‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transpl. 2020;20(7):1849‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang J, Zheng KI, George J, et al. Fatal outcome in a liver transplant recipient with COVID‐19. Am J Transpl. 2020;20(7):1907‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Travi G, Rossotti R, Merli M, et al. Clinical outcome in solid organ transplant recipients with COVID‐19: a single‐center experience. Am J Transpl. 2020;20(9):2628‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tschopp J, L'Huillier AG, Mombelli M, et al. First experience of SARS‐CoV‐2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transpl. 2020;20(10):2876‐2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhoori S, Rossi RE, Citterio D, et al. COVID‐19 in long‐term liver transplant patients: preliminary experiences from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


