Abbreviations
- ALL
acute lymphoblastic leukemia
- ARDS
acute respiratory distress syndrome
- ATG
anti‐thymocyte globulin
- BDG
beta‐D‐glucan
- COVID‐19
coronavirus disease 2019
- CT
computed tomography
- GM
Galactomannan
- GvHD
graft versus host disease
- HCT
hematopoietic cell transplantation
- IPS
Idiopathic Pneumonia Syndrome
- PBSC
peripheral blood stem cells
- RT‐PCR
reverse transcriptase‐polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
To the editor,
So far, we do not have any published database regarding HCT patients during the present pandemic. We herein describe the first case of COVID‐19 presenting in the early hematopoietic cell transplantation (HCT) setting.
A previously healthy 19‐year‐old patient diagnosed and treated for ALL was then referred to our center for HCT from a full‐matched unrelated donor. On February 24, 2020, he was admitted to HCT service in good clinical condition, asymptomatic, with unremarkable laboratory findings. Conditioning regimen was myeloablative; graft versus host disease (GvHD) prophylaxis included rabbit anti‐thymocyte globulin (ATG), short‐course methotrexate, and cyclosporine. On March 4, 2020, peripheral blood stem cell (PBSC) infusion (day 0) was performed, without complications.
On day +12, the patient presented fever, nonproductive dry cough, with normal oxygen saturations; chest radiography revealed a pulmonary infiltrate in the left middle to lower lung zones. Computed tomography (CT) scan of the chest showed a diffuse consolidative lesion in the left middle to lower lung lobes and smaller consolidative opacities in the right lower lobe with surrounding minimal ground‐glass opacities, suggestive for fungal infection. Galactomannan (GM) and beta‐D‐glucan (BDG) were positive (GM assay index 1.6; BDG assay index > 500). We could not perform a bronchoalveolar lavage because of the patient's clinical condition and thrombocytopenia. Based on revised EORTC‐MSG criteria, a probable invasive aspergillosis was diagnosed. 1 Considering the previously primary prophylaxis with posaconazole, we started liposomal amphotericin B and broad‐spectrum antimicrobial therapy with good response.
Nevertheless, on day +25, along with neutrophil engraftment, the patient presented fever, cough, and required oxygen support (2‐3 L/min via nasal cannula). Chest CT showed several ground‐glass opacities mainly distributed in peripheral and juxta pleural bilateral lungs associated with a crazy‐paving pattern at the interlobular septal level and an increased dimension of known consolidative lesions (Figure 1).
Figure 1.
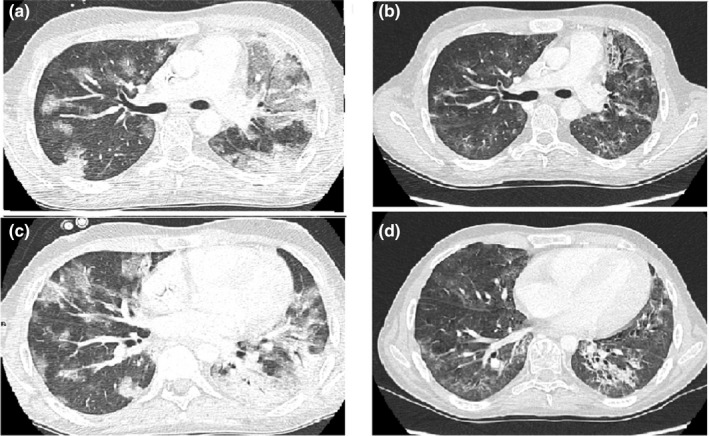
Chest CT scan evolution from SARS‐CoV‐2 diagnosis (day + 27) to resolution of symptoms (day + 75). (A‐C) Several ground‐glass opacities mainly distributed in peripheral and juxta pleural bilateral lungs and diffuse consolidative lesions in the left lung. (B‐D) Important improvement of described lesions with reduction of ground‐glass opacities and consolidative lesions
Considering the chest CT features that resembled that of patients with COVID‐19, nasopharyngeal, and oropharyngeal swab specimens were collected to detect SARS‐CoV‐2 by reverse transcriptase‐polymerase chain reaction (RT‐PCR). The results came back positive. The patient was moved to an airborne‐isolation unit, in our hospital's COVID‐19 area. He denied any contact with confirmed COVID‐19 cases or travel to high‐risk areas before HCT service admission. Healthcare workers and caregivers tested negative both for RT‐PCR and, 4 weeks later, for serology assay.
Non‐invasive ventilation was started, as indicated for HCT patients. 2
From a therapy standpoint, he was treated with hydroxychloroquine (400 mg BID for 24 hours, afterward 200 mg BID, oral) and azithromycin, according to ongoing European Group for Blood and Marrow Transplantation (EBMT) indications (March 29, 2020) and first literature suggestions. 3 We strictly monitored electrocardiogram, but no QTc prolongation was observed. From a supportive therapy standpoint we administered: immunoglobulins (0.5‐1 g/kg, once a week), maintaining IgG level >1000 mg/dL; intravenous unfractionated heparin continuous infusion, per standard guidelines, with the aim to prevent disseminated intravascular coagulopathy, 4 carefully monitoring thrombocytopenia, without observing bleeding complications. Meanwhile, we switched liposomal amphotericin B to isavuconazole.
We carefully screened the patient for cytokine storm, but a clear pathologic inflammatory profile was not distinguishable (Figure 2). In the meantime, from a graft standpoint, the patient was transfusion independent, with a good neutrophil count (neutrophils more than 1.5 × 109/L, constantly).
Figure 2.
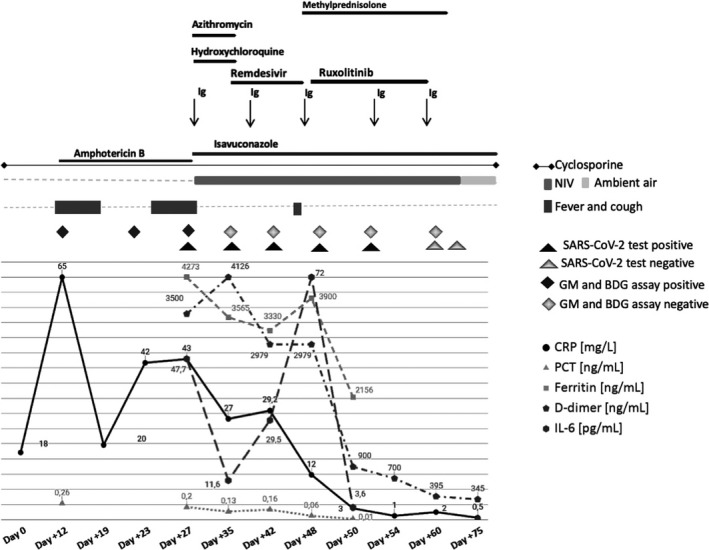
Laboratory results and their correlation with clinical picture and therapeutic approach. All data are normalized for improving visualization; procalcitonin (PCT) values are then scaled to preserve proportions wrt. the other quantities. Normal values considered as follows: C‐reactive protein (CRP) < 5 mg/L, procalcitonin (PCT) < 2 ng/mL, ferritin < 110 ng/mL, D‐dimer < 500 ng/mL, interleukin‐6 (IL‐6) < 30 pg/mL. To be noted: ferritin did not return to the normal values, albeit our patient presented higher ferritin levels even before transplant (ferritin 3000 pg/mL), as frequent in HCT patients
Besides, COVID‐19 test persisted positive. From day +35, we administered intravenous remdesivir, for 10 days, according to the ongoing recommendations, 5 pursuing its promising therapeutic effect, rather than its efficacy in clearing viral load. 6 Unfortunately, on the last day of remdesivir treatment, the patient presented fever and the first signs of ventilatory muscles' fatigue, whereas laboratory findings were nearly stable. Thus, we started methylprednisolone (1 mg/kg/day, from day +46) as in accordance with acute distress respiratory syndrome (ARDS) guidelines. For the sake of completeness, no clinical data have been reported which indicates that net benefit is derived from corticosteroids in the treatment of ARDS because of an underlying coronavirus infection. 7 , 8
Nevertheless, considering that patient's pulmonary function was still deteriorating while other viral and fungal markers were constantly negative, we could not rule out the risk of development of a subsequently COVID‐19‐related Idiopathic Pneumonia Syndrome (IPS). 9 Thus, from day +48, we pursued the compassionate use of ruxolitinib (5 mg TID, orally, 12 days), with the aim to prevent further inflammation and to dampen lung fibrotic transformation. 10 We observed a great clinical improvement and laboratory markers reduction to the baseline, even after 48 hours after the start of the therapy, thus, avoiding intubation (Figure 2).
Ultimately, two consecutive COVID‐19 tests returned negative (day +60, +61), while patient's respiratory function constantly improved (supplemental oxygen discontinued on day +70). Moreover, a follow‐up chest CT (day +75) confirmed the considerable improvement (Figure 1). Patient was discharged on day +85.
Our report highlights interesting questions to debate.
First, no clear source of exposure was identified in our patient. We do believe that our young man became infected before his hospital admission and, owing to the immunosuppressing therapy he received thereafter, he had a prolonged incubation period, such as some immune‐suppressed patients previously described. 11 , 12
Not surprisingly, our patient presented with a co‐infection. However, particularly for fungal infection, reaching a proven diagnosis could be very challenging in these vulnerable cohort of patients. Indeed, the lack of specificity of fungal biomarkers comes often with the inability to perform an invasive diagnostic test (as in our case). Nevertheless, even in the case of a probable diagnosis, a targeted treatment should be promptly provided avoiding life‐threatening complications.
Last but not least, it is worth remarking that the administration of anti‐inflammatory drugs, such as ruxolitinib, is of paramount relevance when treating such a unique cohort of severe COVID‐19 patients, especially in order to prevent additional transplant‐related complications. Furthermore, encouraging results regarding the efficacy and safety of ruxolitinib were presented from a randomized phase II trial. 13 Indeed, our report highlights the added value of a timely immunomodulatory therapy in preventing the development of cryptogenic organizing pneumonia as the ultimate result of COVID‐19 infection.
As a final remark, we strongly suggest a tailor‐made approach while handling diagnosis, management, and treatment of COVID‐19 and its related complications in transplanted patients.
To this aim, further studies are needed.
CONFLICTS OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose as described by the Transplant Infectious disease journal.
AUTHORS' CONTRIBUTION
MS and FC performed the research, collected, analyzed the data, and wrote the paper; FS, EV, CS, SG, RP, and MB collected and analyzed the data; FF critically revised the manuscript.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
ACKNOWLEDGMENTS
The authors thank all of the medical staff members involved in treating this patient.
Spadea M, Carraro F, Saglio F, et al. Successfully treated severe COVID‐19 and invasive aspergillosis in early hematopoietic cell transplantation setting. Transpl Infect Dis. 2021;23:e13470. 10.1111/tid.13470
REFERENCES
- 1. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) C. Clin Infect Dis. 2008;46(12):1813‐1821. 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azoulay E, Mokart D, Pène F, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium‐A groupe de recherche respiratoire en réanimation onco‐hématologique study. J Clin Oncol. 2013;31(22):2810‐2818. 10.1200/JCO.2012.47.2365 [DOI] [PubMed] [Google Scholar]
- 3. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid‐19. N Engl J Med. 2020;382(24):2327‐2336. 10.1056/nejmoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 — preliminary report. N Engl J Med. 2020;383(10):994. 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 7. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. 10.1371/journal.pmed.0030343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757‐767. 10.1164/rccm.201706-1172OC [DOI] [PubMed] [Google Scholar]
- 9. Panoskaltsis‐Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society Research Statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183(9):1262‐1279. 10.1164/rccm.2007-413ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malavolta M, Giacconi R, Brunetti D, Provinciali M, Maggi F. Exploring the relevance of senotherapeutics for the current SARS‐CoV‐2 emergency and similar future global health threats. Cells. 2020;9(4):909. 10.3390/cells9040909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin XH, Zheng KI, Pan KH, Xie YP, Zheng MH. COVID‐19 in a patient with chronic lymphocytic leukaemia. Lancet Haematol. 2020;7(4):e351‐e352. 10.1016/S2352-3026(20)30074-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang J, Lin H, Wu Y, et al. COVID‐19 in posttransplant patients—report of 2 cases. Am J Transplant. 2020;20(7):1879‐1881. 10.1111/ajt.15896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID‐19): a multicenter, single‐blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146(1):137–146.e3. 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


