Coronavirus disease 2019 (COVID‐19) was initially identified by clusters of pneumonia of an unknown cause in Wuhan, China. 1 Later, extensive extra‐pulmonary manifestations of COVID‐19 were recognised. Haematological manifestations of COVID‐19 have gained special attention due to the high frequency of laboratory detected abnormalities such as lymphopenia and thromboembolic complications. 2 Additionally, cases of autoimmune haemolytic anaemia and immune thrombocytopenic purpura have also been documented. 3 , 4 , 5
Sickle cell disease (SCD) is a one of the commonest genetic diseases. 6 Although patients with SCD are not clearly vulnerable to COVID‐19 infection, COVID‐19 may trigger serious complications in these patients. 7 To our knowledge, we describe the first case of SCD diagnosed due to concomitant COVID‐19 infection.
In early June, a 22‐year‐old female presented to the emergency room after a few days of fever and generalised bony aches, tachycardia and tachypnoea. Her prior history was splenectomy at the age 8 years for anaemia and long‐standing bony aches that recurred every 3–4 months, which had been diagnosed as rheumatic fever without extensive investigations. She was non‐compliant with the long‐acting penicillin prescribed.
Her laboratory evaluation revealed leucocytosis, a white blood cell (WBC) count of 14·1 × 103/µl; neutrophilia, with a neutrophil count of 10·32 × 103/µl; a haemoglobin (Hb) level of 45 g/l; a mean corpuscular volume of 63·2 fl and platelet count of 100 × 103/µl. The lactate dehydrogenase level was 1026 iu/l; the C‐reactive protein (CRP) level was 62.7 mg/l; and the ferritin level was 1047 ng/ml. Oxygen saturation was 93%. She was transfused two packs of red blood cells (RBCs) due to impending heart failure, and reverse transcriptase‐polymerase chain reaction confirmed that she was positive for severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). The direct Coombs test was positive, the erythrocyte sedimentation rate was 90 mm/h and the anti‐nuclear antibody test was negative (titre <1/40). High‐resolution computed tomography (HRCT) of the chest showed a single patch of sub‐segmental consolidation in the right middle lobe, and she was admitted as a case of moderate COVID‐19 pneumonia complicated by autoimmune haemolytic anaemia and thrombocytopenia. At follow‐up the full blood count revealed a Hb level of 56 g/l and repeat packed RBCs transfusion was given.
Medical treatment prescribed was 2 l/min of supplemental oxygen, hydroxychloroquine, azithromycin and prophylactic anticoagulation. Corticosteroids were prescribed at a dose of 2 mg/kg for 5 days for haemolytic anaemia. The patient’s fever and bony aches persisted, principally localised to the right hip, knee and back with pain, tenderness and marked limitation of movement.
At follow‐up laboratory evaluation 1 week later, the WBC count was 14·2 × 103/µl, the Hb level was 98 g/l, the platelet count was 351 × 103/µl, the CRP titre was 357 mg/l and the ferritin level was 3157 ng/ml. Repeat HRCT of the chest revealed a new consolidation in the anterior segment of the right lower lobe and the medial segment of the right lower lobe with minimal right pleural effusion suggestive of multilobar pneumonia. Linezolide and quinolones were added. Plain X‐ray of the pelvic bones showed right femoral head dense sclerosis with collapse denoting osteonecrosis. At this point and because of agonising bony aches, re‐reading of the bone and mediastinal windows of the HRCT chest revealed osteonecrosis suggestive of SCD (Fig 1) and the characteristic ‘fish‐shaped’ vertebrae in the thoracic and lumbar regions (Fig 2).
Fig 1.
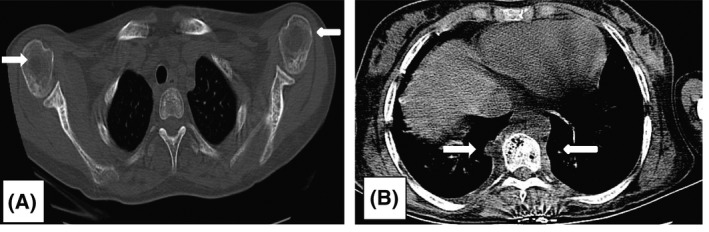
High‐resolution computed tomography (HRCT) of the chest. (A) Bone window of chest CT showing exaggerated marrow trabeculae of both scapulae and ribs. Both humeral heads show lytic lesions suggestive of osteonecrosis. (B) Mediastinal window of CT chest showing paraspinal soft tissue mass bilaterally in the thoracic region, denoting extramedullary haemopoiesis.
Fig 2.
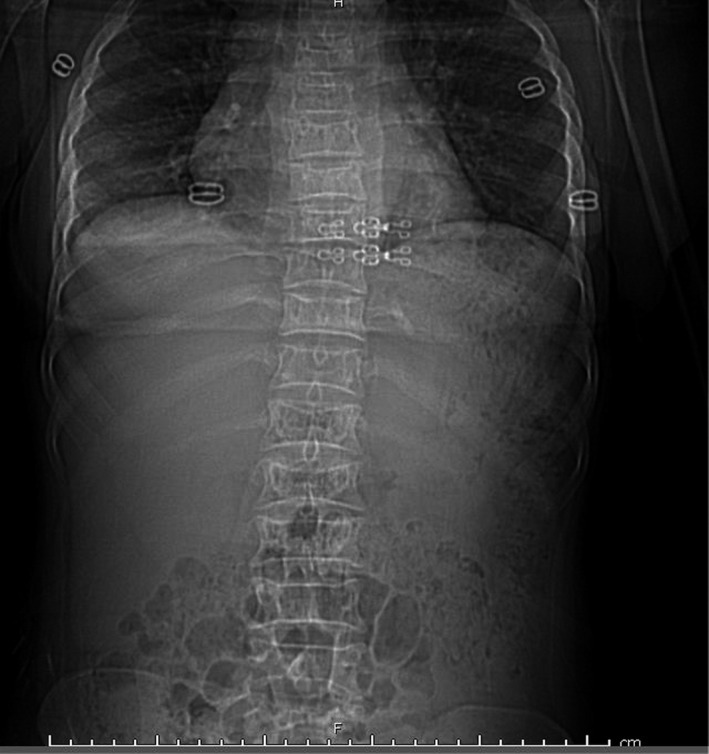
Scout image for chest computed tomography showing bilateral paraspinal shadows due to extra‐medullary haemopoiesis. Vertebral bodies in the thoracic and lumbar region are ‘H’‐shaped with exaggerated marrow trabeculae, suggestive of sickle cell disease.
Vaso‐occlusive crisis (VOC) is the most common bone pathology affecting patients with SCD causing significant morbidity. Sickling of RBCs causes chronic tissue ischaemia with subsequent pain and oedema. 8 Acute painful VOC most commonly affects the spine, knee and femur. Approximately two‐thirds of patients have involvement of the lumbosacral level of the spine, and 20% have involvement of the thoracic spine. The ‘Fish vertebra’ sign and ‘vanishing’ of the vertebra in patients with SCD are due to ischaemia in the middle part of the vertebral growth plate. 9
Haemoglobin electrophoresis was requested for suspicion of SCD at 1 week after the second blood transfusion. The patient was later given a repeat blood transfusion as her Hb level was 72 g/l, and the results of Hb electrophoresis a few days later were positive for sickle cell trait (SCT). The patient had been transfused multiple times prior to electrophoresis. It could be argued that the patient might have sickle cell anaemia rather than SCT that was masked by multiple blood transfusions; however, the patient was lost to follow‐up so this hypothesis remains unconfirmed.
There are numerous forms of SCD including Hb SS, Hb SC, Hb S β‐thalassaemia, all of which tend to cause VOC. SCT is described as benign with limited clinical implications, a better quality of life and mortality risk almost similar to the general population and unlikely to have VOC. However, adverse events have been reported in patients with SCT. Hence, SCT is not always benign and these patients ought to be aggressively managed if patients develop complications. 10
To our knowledge, this is the first case of SCD to present after COVID‐19 infection. Secondary bacterial infection precipitated painful VOC in the patient, which revealed SCD. Patients with SCD are listed amongst individuals with a high risk of severe illness from COVID‐19 partly due to impaired splenic function. Typical VOC triggered by COVID‐19 was reported in patients with SCD with COVID‐19 with favourable outcomes, and it was suggested that the chronic anaemic and haemolytic state might have protected the patients from fatal COVID‐19 infection. 11 , 12 , 13
The female patient described here had severe anaemia, with secondary bacterial infection following corticosteroids prescription for presumed autoimmune haemolysis. Her symptoms abated gradually with repeated RBC transfusion and CRP normalised before discharge. Persistent fever with hyperferritinaemia was worrisome for cytokine storm; however, the HRCT of the chest taken at follow‐up did not support this grave complication of COVID‐19, and no treatment was initiated. The patient’s quality of life was poor due to frequent bony aches that were evidenced by osteonecrosis, and she was undiagnosed for more than two decades of her life. She remains liable for complications such as renal papillary necrosis, renal medullary carcinoma, asymptomatic bacteriuria and chronic kidney disease.
To conclude, we report what is to our knowledge the first interesting case of SCD that was revealed due to concomitant COVID‐19 infection. The patient’s painful crises were misdiagnosed for a long time and she had osteonecrosis of the thoracic and lumbar spine, ribs, scapulae and avascular hip necrosis. She is liable to have complications and her condition warrants medical treatment of SCD.
Authors’ contributions
D. Sheha wrote the paper. M. El‐Shayeb contributed to the medical treatment decisions. Y. Eid advised the treatment and follow‐up plan. M. Amin shared in treatment decisions. A. Saeed advised the investigations. D. Abdou, T. Aly, S. Samy, R. Elziaty, S. Aboelyazed were the treating physicians on the frontline who implemented the treatment plan and followed‐up the patient along the course of admission in isolation. A. Osman and A. Sheha were involved in interpretation of the imaging of the patient.
References
- 1. Zhu Na, Zhang D, Wang W, Li X, Yang Bo, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;26:1017–32. [DOI] [PubMed] [Google Scholar]
- 3. Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, et al. Autoimmune haemolytic anaemia associated with COVID‐19 infection. Br J Haematol. 2020;190:29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capes A, Bailly S, Hantson P, Gerard L, Laterre PF. COVID‐19 infection associated with autoimmune hemolytic anaemia. Ann Hematol. 2020;99:1679–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zulfiqar AA, Lorenzo‐Villalba N, Hassler P, Andrès E. Immune thrombocytopenic Purpura in a patient with Covid‐19. N Engl J Med. 2020;382:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390:311–23. [DOI] [PubMed] [Google Scholar]
- 7. Vives Corrons JL, De Sanctis V. Rare anaemias, sickle‐cell disease and COVID‐19. Acta Biomed. 2020;91:216–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashorobi D, Ramsey A, Bhatt R. Sickle Cell Trait. [Updated 2019 May 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537130/ [Accessed 8 August 2020].
- 9. Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol. 2005;129:482–90. [DOI] [PubMed] [Google Scholar]
- 10. Rudy HL, Yang D, Nam AD, Cho W. Review of sickle cell disease and spinal pathology. Global Spine J. 2018;9:761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Sanctis V, Canatan D, Vives Corrons JL, Karimi M, Daar S, Kattamis C, et al. Preliminary Data on COVID‐19 in Patients with Hemoglobinopathies: A Multicentre ICET‐A Study. Mediterr J Hematol Infect Dis. 2020;12:e2020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCloskey KA, Meenan J, Hall R, Tsitsikas DA. COVID‐19 infection and sickle cell disease: a UK centre experience. Br J Haematol. 2020;190;e57–e58. [DOI] [PubMed] [Google Scholar]
- 13. Hussain FA, Njoku FU, Saraf SL, Molokie RE, Gordeuk VR, Han J. COVID‐19 infection in patients with sickle cell disease. Br J Haematol. 2020;189:851–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


